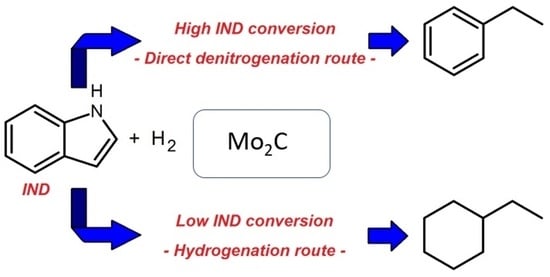
On Catalytic Behavior of Bulk Mo2C in the Hydrodenitrogenation of Indole over a Wide Range of Conversion Thereof
Abstract
1. Introduction
2. Results and Discussion
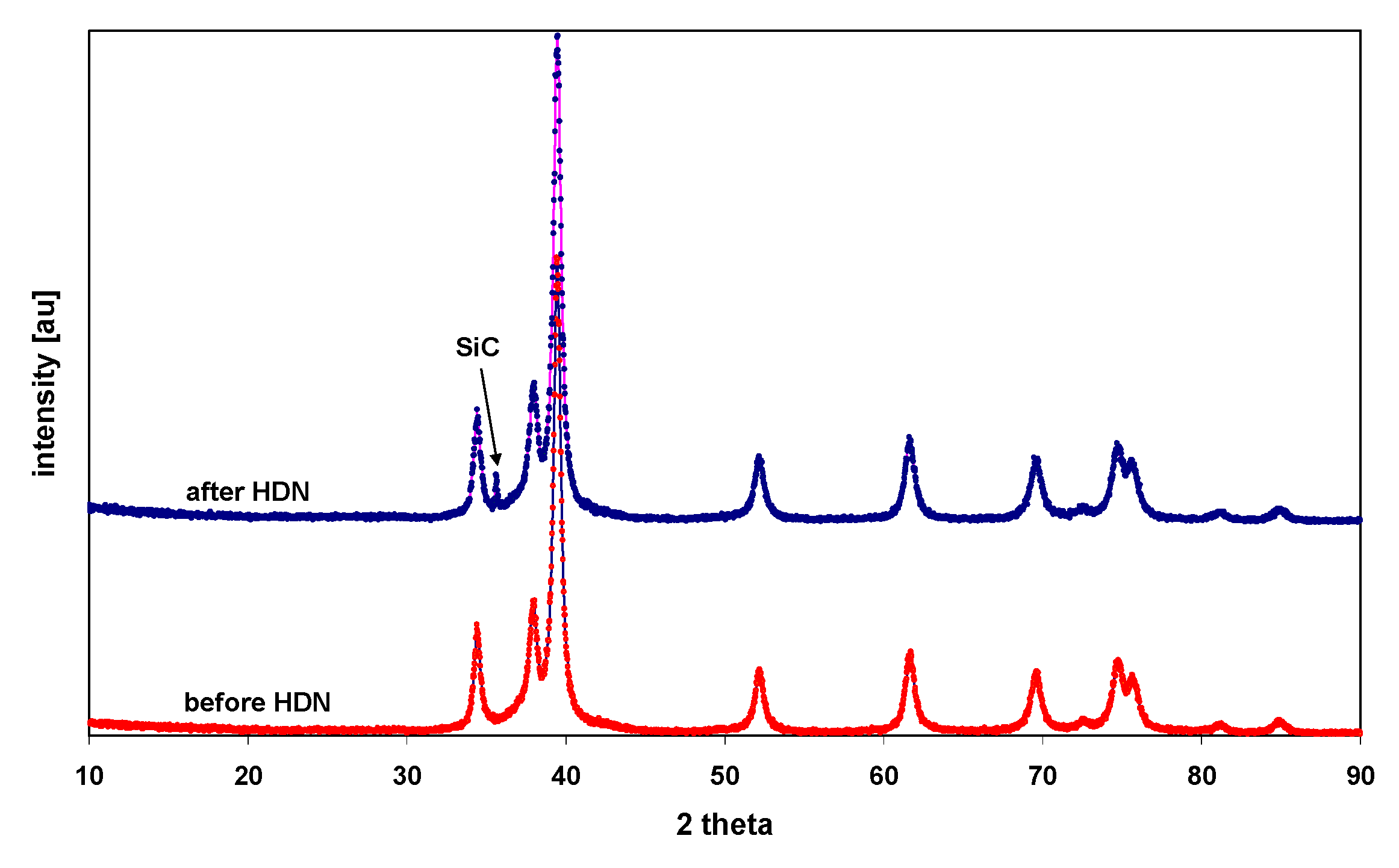
2.1. Characterization of the Catalyst
2.2. HDN of Indole over Mo2C
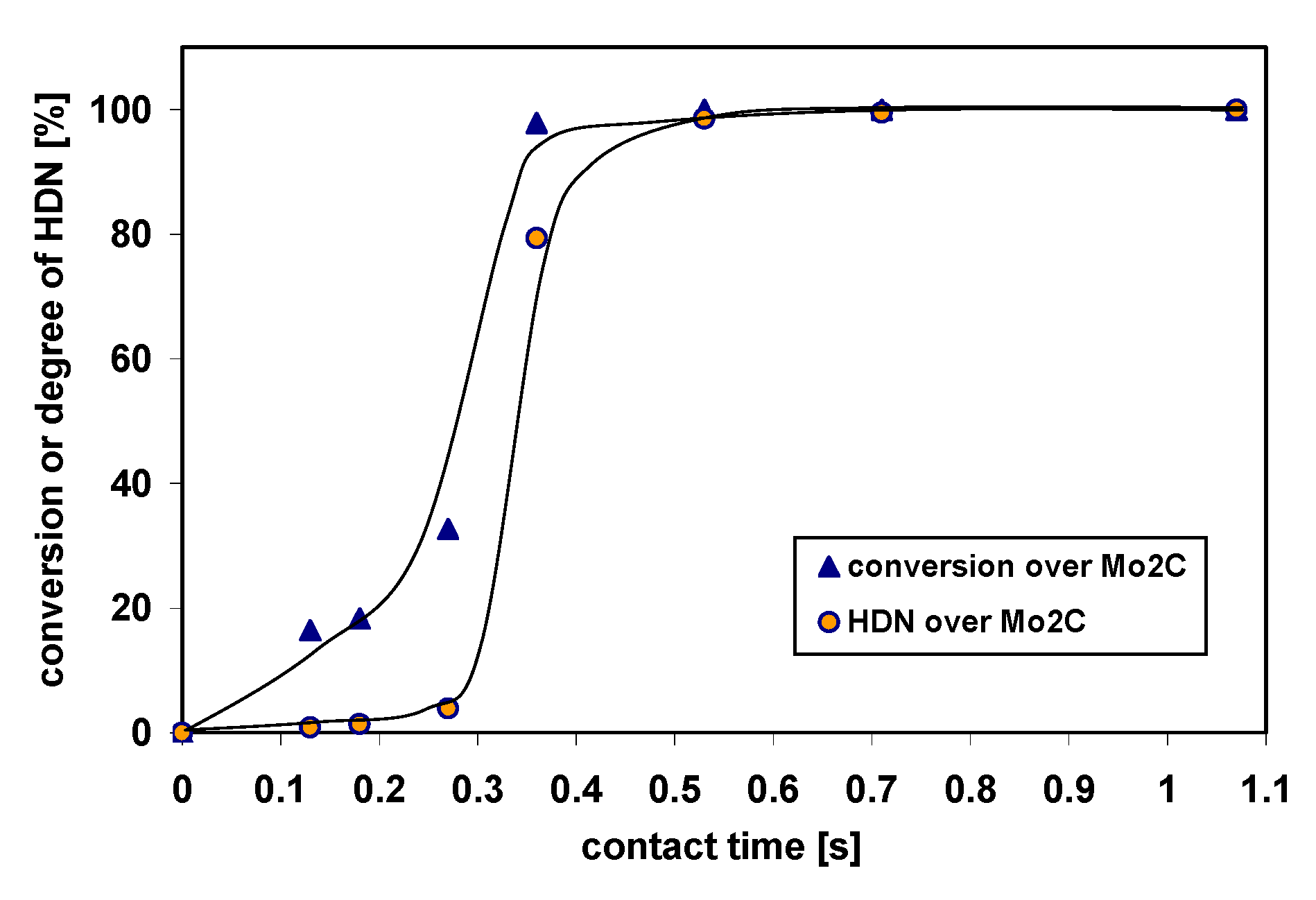
2.3. Kinetic Study of the HDN of Indole over Bulk Mo2C
3. Materials and Methods
3.1. Materials
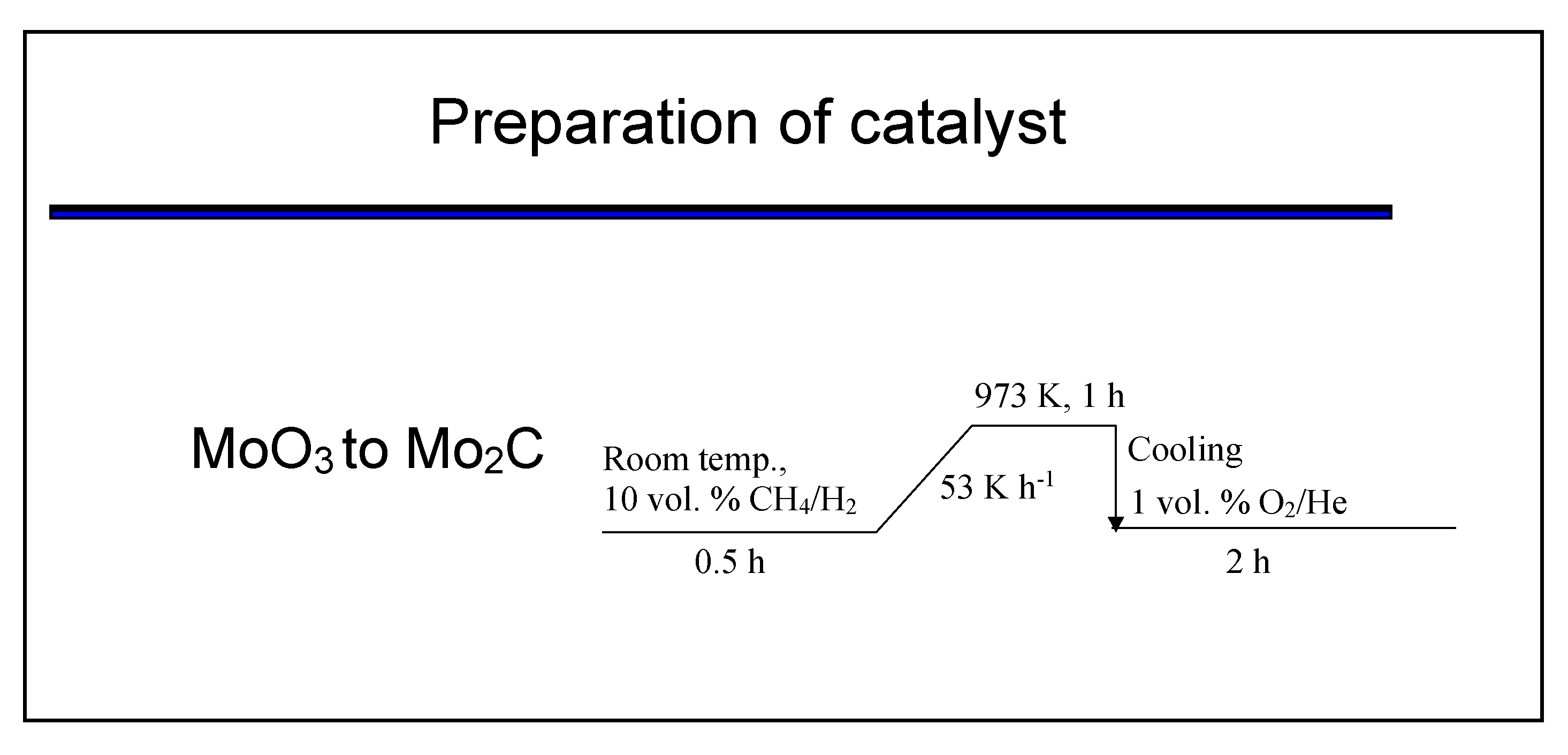
3.2. Synthesis of Bulk Mo2C
3.3. Characterization Techniques
3.4. Kinetic Study of HDN of Indole
- NN is the mole % of all products of decomposition of indole (including N-containing compounds except indole);
- Nindole is the mole % of indole in the liquid product after reaction;
- NH is the mole % of non-nitrogen products from the decomposition of indole;
- EB + T + B are mole % of ethylbenzene, toluene, and benzene, respectively;
- ECH means mole % of ethylcyclohexane.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Clark, P.; Oyama, S.T. Synthesis, characterization, and hydrotreating activity of several iron group transition metal phosphides. J. Catal. 2002, 208, 321–331. [Google Scholar] [CrossRef]
- Breysse, M.; Djéga-Mariadassou, G.; Pessayre, S.; Geantet, C.; Vrinat, M.; Perot, G.; Lemaire, M. Deep desulfurization: Reactions, catalysts and technological challenges. Catal. Today 2003, 84, 129–138. [Google Scholar] [CrossRef]
- Ishihara, A.; Dumeignil, F.; Lee, J.; Mitsuhashi, K.; Qian, E.W.; Kabe, T. Hydrodesulfurization of sulfur-containing polyaromatic compounds in light gas oil using noble metal catalysts. Appl. Catal. A 2005, 289, 163–173. [Google Scholar] [CrossRef]
- Sano, Y.; Choi, K.-H.; Korai, Y.; Mochida, I. Effects of nitrogen and refractory sulfur species removal on the deep HDS of gas oil. Appl. Catal. B 2004, 53, 169–174. [Google Scholar] [CrossRef]
- Sano, Y.; Choi, K.-H.; Korai, Y.; Mochida, I. Adsorptive removal of sulfur and nitrogen species from a straight run gas oil over activated carbons for its deep hydrodesulfurization. Appl. Catal. B 2004, 49, 219–225. [Google Scholar] [CrossRef]
- Rana, M.S.; Al-Barood, A.; Brouresli, R.; Al-Hendi, A.W.; Mustafa, N. Effect of organic nitrogen compounds on deep hydrodesulfurization of middle distillate. Fuel Proc. Technol. 2018, 177, 170–178. [Google Scholar] [CrossRef]
- Szymańska-Kolasa, A.; Lewandowski, M.; Sayag, C.; Brodzki, D.; Djéga-Mariadassou, G. Comparison between tungsten carbide and molybdenum carbide for the hydrodenitrogenation of carbazole. Catal. Today 2007, 119, 35–38. [Google Scholar] [CrossRef]
- Li, S.; Lee, J.S.; Hyeon, T.; Suslick, K. Catalytic hydrodenitrogenation of indole over molybdenum nitride and carbides with different structures. Appl. Catal. A 1999, 184, 1–9. [Google Scholar] [CrossRef]
- Dhandapani, B.; Clair, T.S.; Oyama, S.T. Simultaneous hydrodesulfurization, hydrodeoxygenation, and hydrogenation with molybdenum carbide. Appl. Catal. A 1998, 168, 219–228. [Google Scholar] [CrossRef]
- Nagai, M.; Goto, Y.; Irisawa, A.; Omi, S. Catalytic activity and surface properties of nitrided molybdena–alumina for carbazole hydrodenitrogenation. J. Catal. 2000, 191, 128–137. [Google Scholar] [CrossRef]
- Schwartz, V.; Oyama, S.T. Reaction network of pyridine hydrodenitrogenation over carbide and sulfide catalysts. J. Mol. Catal. A Chem. 2000, 163, 269–282. [Google Scholar] [CrossRef]
- Szymańska, A.; Lewandowski, M.; Sayag, C.; Djéga-Mariadassou, G. Kinetic study of the hydrodenitrogenation of carbazole over bulk molybdenum carbide. J. Catal. 2003, 218, 24–31. [Google Scholar] [CrossRef]
- Da Costa, P.; Potvin, C.; Manoli, J.-M.; Breysse, B.; Djéga-Mariadassou, G. Supported molybdenum carbides lie between metallic and sulfided catalysts for deep HDS. Catal. Lett. 2003, 86, 133–138. [Google Scholar] [CrossRef]
- McCrea, K.R.; Logan, J.W.; Tarbuck, T.L.; Heiser, J.L.; Bussell, M.E. Thiophene hydrodesulfurization over alumina-supported molybdenum carbide and nitride catalysts: Effect of Mo loading and phase. J. Catal. 1997, 171, 255–267. [Google Scholar] [CrossRef]
- Hynaux, A.; Sayag, C.; Suppan, S.; Trawczyński, J.; Lewandowski, M.; Szymańska-Kolasa, A.; Djéga-Mariadassou, G. Kinetic study of the deep hydrodesulfurization of dibenzothiophene over molybdenum carbide supported on a carbon black composite: Existence of two types of active sites. Catal. Today 2007, 119, 3–6. [Google Scholar] [CrossRef]
- Szymańska-Kolasa, A.; Lewandowski, M.; Sayag, C.; Djéga-Mariadassou, G. Comparison of molybdenum carbide and tungsten carbide for the hydrodesulfurization of dibenzothiophene. Catal. Today 2007, 119, 7–12. [Google Scholar] [CrossRef]
- Lewandowski, M.; Szymańska-Kolasa, A.; Da Costa, P.; Sayag, C. Catalytic performances of platinum doped molybdenum carbide for simultaneous hydrodenitrogenation and hydrodesulfurization. Catal. Today 2007, 119, 31–34. [Google Scholar] [CrossRef]
- Diaz, B.; Sawhill, S.J.; Bale, D.H.; Main, R.; Phillips, D.C.; Korlann, S.; Self, R.; Bussell, M.E. Hydrodesulfurization over supported monometallic, bimetallic and promoted carbide and nitride catalysts. Catal. Today 2003, 86, 191–209. [Google Scholar] [CrossRef]
- Manoli, J.-M.; Da Costa, P.; Brun, M.; Vrinat, M.; Mauge, F.; Potvin, C. Hydrodesulfurization of 4,6-dimethyldibenzothiophene over promoted (Ni,P) alumina-supported molybdenum carbide catalysts: Activity and characterization of active sites. J. Catal. 2004, 221, 365–377. [Google Scholar] [CrossRef]
- Al-Megren, H.A.; González-Cortés, S.L.; Xiao, T.; Green, M.L.H. A comparative study of the catalytic performance of Co-Mo and Co(Ni)-W carbide catalysts in the hydrodenitrogenation (HDN) reaction of pyridine. Appl. Catal. A 2007, 329, 36–45. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Govindarajan, R.; Smith, K.J. Preparation of Ni-Mo2C/carbon catalysts and their stability in the HDS of dibenzothiophene. Appl. Catal. A 2017, 539, 114–127. [Google Scholar] [CrossRef]
- Nagai, M.; Tung, N.T.; Adachi, Y.; Kobayashi, K. New approach to active sites analysis of molybdenum-containing catalysts for hydrodesulfurization and hydrodenitrogenation based on inverse problem, fractal and site-type analyses. Catal. Today 2016, 271, 91–101. [Google Scholar] [CrossRef]
- Lewandowski, M.; Szymańska-Kolasa, A.; Sayag, C.; Beaunier, P.; Djéga-Mariadassou, G. Atomic level characterization and sulfur resistance of unsupported W2C during dibenzothiophene hydrodesulfurization. Classical kinetics simulation of the reaction. Appl. Catal. B 2014, 144, 750–759. [Google Scholar] [CrossRef]
- Neylon, M.K.; Choi, S.; Kwon, H.; Curry, K.E.; Thompson, L.T. Catalytic properties of early transition metal nitrides and carbides: N-butane hydrogenolysis, dehydrogenation and isomerization. Appl. Catal. A 1999, 183, 253–263. [Google Scholar] [CrossRef]
- Rocha, A.S.; Rocha, A.B.; Teixeira da Silva, V. Benzene adsorption on Mo2C: A theoretical and experimental study. Appl. Catal. A 2010, 379, 54–60. [Google Scholar] [CrossRef]
- Monjardin, E.; Cruz-Reyes, J.; Del Valle-Granados, M.; Flores-Aquino, E.; Avalos-Borja, M.; Fuentes-Moyado, S. Synthesis, characterization and catalytic activity in the hydrogenation of cyclohexene with molybdenum carbide. Catal. Lett. 2008, 120, 137–142. [Google Scholar] [CrossRef]
- Tominaga, H.; Aoki, Y.; Nagai, M. Hydrogenation of CO on molybdenum and cobalt molybdenum carbides. Appl. Catal. A 2012, 423–424, 192–204. [Google Scholar] [CrossRef]
- Mehdad, A.; Jentoft, R.E.; Jentoft, F.C. Passivation agents and conditions for Mo2C and W2C: Effect on catalytic activity for toluene hydrogenation. J. Catal. 2017, 347, 89–101. [Google Scholar] [CrossRef]
- Lewandowski, M.; Szymańska-Kolasa, A.; Sayag, C.; Djéga-Mariadassou, G. Activity of molybdenum and tungsten oxycarbides in hydrodenitrogenation of carbazole leading to isomerization secondary reaction of bicyclohexyl. Results using bicyclohexyl as feedstock. Appl. Catal. B 2020, 261, 118239. [Google Scholar] [CrossRef]
- Delannoy, L.; Giraudon, J.-M.; Granger, P.; Leclercq, L.; Leclercq, G. Group VI transition metal carbides as alternatives in the hydrodechlorination of chlorofluorocarbons. Catal. Today 2000, 59, 231–240. [Google Scholar] [CrossRef]
- Jujjuri, S.; Cárdenas-Lizana, F.; Keane, M.A. Synthesis of group VI carbides and nitrides: Application in catalytic hydrodechlorination. J. Mater. Sci. 2014, 49, 5406–5417. [Google Scholar] [CrossRef]
- Nagai, M.; Matsuda, K. Low-temperature water–gas shift reaction over cobalt–molybdenum carbide catalyst. J. Catal. 2006, 238, 489–496. [Google Scholar] [CrossRef]
- Barthos, R.; Solymosi, F. Hydrogen production in the decomposition and steam reforming of methanol on Mo2C/carbon catalysts. J. Catal. 2007, 249, 289–299. [Google Scholar] [CrossRef]
- Lausche, A.C.; Schaidle, J.A.; Thompson, L.T. Understanding the effects of sulfur on Mo2C and Pt/Mo2C catalysts: Methanol steam reforming. Appl. Catal. A 2011, 401, 29–36. [Google Scholar] [CrossRef]
- Sabnis, K.D.; Cui, Y.; Akatay, M.C.; Shekhar, M.; Lee, W.-S.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Water–gas shift catalysis over transition metals supported on molybdenum carbide. J. Catal. 2015, 331, 162–171. [Google Scholar] [CrossRef]
- Sousa, L.A.; Zotin, J.L.; Teixeira da Silva, V. Hydrotreatment of sunflower oil using supported molybdenum carbide. Appl. Catal. A 2012, 449, 105–111. [Google Scholar] [CrossRef]
- Patel, M.A.; Baldanza, M.A.S.; Teixeira da Silva, V.; Bridgwater, A.V. In situ catalytic upgrading of bio-oil using supported molybdenum carbide. Appl. Catal. A 2013, 458, 48–54. [Google Scholar] [CrossRef]
- Chen, C.-J.; Lee, W.-S.; Bhan, A. Mo2C catalyzed vapor phase hydrodeoxygenation of lignin-derived phenolic compound mixtures to aromatics under ambient pressure. Appl. Catal. A 2016, 510, 42–48. [Google Scholar] [CrossRef]
- Rocha, A.S.; Souza, L.A.; Oliveira, R.R.; Rocha, A.B.; Teixeira da Silva, V. Hydrodeoxygenation of acrylic acid using Mo2C/Al2O3. Appl. Catal. A 2017, 531, 69–78. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Smith, K.J. Synthesis and hydrodeoxygenation activity of carbon supported molybdenum carbide and oxycarbide catalysts. Energy Fuels 2016, 30, 6039–6049. [Google Scholar] [CrossRef]
- Machado, M.A.; He, S.; Davies, T.E.; Seshan, K.; Teixeira da Silva, V. Renewable fuel production from hydropyrolysis of residual biomass using molybdenum carbide-based catalysts. Catal. Today 2018, 302, 161–168. [Google Scholar] [CrossRef]
- Huo, X.; Wang, Z.; Huang, J.; Zhang, R.; Fang, Y. Bulk Mo and Co–Mo carbides as catalysts for methanation. Catal. Commun. 2016, 79, 39–44. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, G.; Hao, X.; Cao, J.; Abudula, A. Molybdenum carbide as alternative catalyst for hydrogen production—A review. Renew. Sustain. Energy Rev. 2017, 75, 1101–1129. [Google Scholar] [CrossRef]
- Ardakani, S.J.; Liu, X.; Smith, K.J. Hydrogenation and ring opening of naphthalene on bulk and supported Mo2C catalysts. Appl. Catal. A 2007, 324, 9–19. [Google Scholar] [CrossRef]
- Volpe, L.; Boudart, M. Compounds of molybdenum and tungsten with high specific surface area: I. Nitrides. J. Solid State Chem. 1985, 59, 332–347. [Google Scholar] [CrossRef]
- Volpe, L.; Boudart, M. Compounds of molybdenum and tungsten with high specific surface area: II. Carbides. J. Solid State Chem. 1985, 59, 348–356. [Google Scholar] [CrossRef]
- Miga, K.; Stańczyk, K.; Sayag, C.; Brodzki, D.; Djéga-Mariadassou, G. Bifunctional behavior of bulk MoOxNy and nitrided supported NiMo catalyst in hydrodenitrogenation of indole. J. Catal. 1999, 183, 63–68. [Google Scholar] [CrossRef]
- Georginia, C.; Laredo, S.; De los Reyes, H.J.A.; Luis Cano, D.J.; Jesús Castillo, M.J. Inhibition effects of nitrogen compounds on the hydrodesulfurization of dibenzothiophene. Appl. Catal. A 2001, 207, 103–112. [Google Scholar]
- Topsøe, H.; Clausen, B.S.; Massoth, F.E. Hydrotreating Catalysis Science and Technology; Springer: New York, NY, USA, 1996. [Google Scholar]
- Abe, H.; Bell, A.T. Catalytic hydrotreating of indole, benzothiophene, and benzofuran over Mo2N. Catal. Lett. 1993, 18, 1–8. [Google Scholar] [CrossRef]
- Nagai, M.; Miyao, T.; Tuboi, T. Hydrodesulfurization of dibenzothiophene on alumina-supported molybdenum nitride. Catal. Lett. 1993, 18, 9–14. [Google Scholar] [CrossRef]
- Senzi, L.; Lee, J.S. Molybdenum nitride and carbide prepared from heteropolyacid: II. Hydrodenitrogenation of indole. J. Catal. 1998, 173, 134–144. [Google Scholar] [CrossRef]
- Bunch, A.; Zhang, L.; Karakas, G.; Ozkan, U.S. Reaction network of indole hydrodenitrogenation over NiMoS/γ-Al2O3 catalysts. Appl. Catal. A 2000, 190, 51–60. [Google Scholar] [CrossRef]
- Ozkan, U.S.; Zhang, L.; Clark, P.A. Performance and postreaction characterization of γ-Mo2N catalysts in simultaneous hydrodesulfurization and hydrodenitrogenation reactions. J. Catal. 1997, 172, 294–306. [Google Scholar] [CrossRef]
- Sayag, C.; Suppan, S.; Trawczyński, J.; Djéga-Mariadassou, G. Effect of support activation on the kinetics of indole hydrodenitrogenation over mesoporous carbon black composites-supported molybdenum carbide. Fuel Proc. Technol. 2002, 77–78, 261–267. [Google Scholar] [CrossRef]
- Adamski, G.; Dyrek, K.; Kotarba, A.; Sojka, Z.; Sayag, C.; Djéga-Mariadassou, G. Kinetic model of indole HDN over molybdenum carbide: Influence of potassium on early and late denitrogenation pathways. Catal. Today 2004, 90, 115–119. [Google Scholar] [CrossRef]
- Sayag, C.; Benkhaleda, M.; Suppan, S.; Trawczyński, J.; Djéga-Mariadassou, G. Comparative kinetic study of the hydrodenitrogenation of indole over activated carbon black composites (CBC) supported molybdenum carbides. Appl. Catal. A 2004, 275, 15–24. [Google Scholar] [CrossRef]
- Ledesma, B.C.; Anunziata, O.A.; Beltramone, A.R. HDN of indole over Ir-modified Ti-SBA-15. Appl. Catal. B 2016, 192, 220–233. [Google Scholar] [CrossRef]
- Ledesma, B.C.; Juárez, J.M.; Valles, V.A.; Anunziata, O.A.; Beltramone, A.R. Novel preparation of titania-modified CMK-3 nanostructured material as support for Ir catalyst applied in hydrodenitrogenation of indole. Catal Lett. 2017, 147, 1029–1103. [Google Scholar] [CrossRef]
- Choi, J.-S.; Bugli, G.; Djéga-Mariadassou, G. Influence of the degree of carburization on the density of sites and hydrogenating activity of molybdenum carbides. J. Catal. 2000, 193, 238–247. [Google Scholar] [CrossRef]
- Schlatter, J.C.; Oyama, S.T.; Metcalfe III, J.E.; Lambert, J.M., Jr. Catalytic behavior of selected transition metal carbides, nitrides, and borides in the hydrodenitrogenation of quinoline. Ind. Eng. Chem. Res. 1988, 27, 1648–1653. [Google Scholar] [CrossRef]
- Sajkowski, D.J.; Oyama, S.T. Catalytic hydrotreating by molybdenum carbide and nitride: Unsupported Mo2N and Mo2CAl2O3. Appl. Catal. A 1996, 134, 339–349. [Google Scholar] [CrossRef]
- Prins, R. Catalytic hydrodenitrogenation. Adv. Catal. 2001, 46, 399–464. [Google Scholar]
- Da Costa, P.; Potvin, C.; Manoli, J.-M.; Lemberton, J.L.; Pérot, G.; Djéga-Mariadassou, G. New catalysts for deep hydrotreatment of diesel fuel: Kinetics of 4,6-dimethyldibenzothiophene hydrodesulfurization over alumina-supported molybdenum carbide. J. Mol. Catal. A Chem. 2002, 184, 323–333. [Google Scholar] [CrossRef]
- Rota, F.; Prins, R. Mechanism of the hydrodenitrogenation of o-toluidine and methylcyclohexylamine over NiMo/γ-Al2O3. Top. Catal. 2000, 11/12, 327–333. [Google Scholar] [CrossRef]
- Callant, M.; Holder, K.A.; Grange, P.; Delmon, B. Effect of the H2S and H2 partial pressure on the hydrodenitrogenation (HDN) of aniline and indole over a NiMoP-γ-Al2O3 catalyst. Bull. Soc. Chim. Belg. 1995, 104, 245–251. [Google Scholar] [CrossRef]
- Laredo, G.C.; Altamirano, E.; De los Reyes, J.A. Self-inhibition observed during indole and o-ethylaniline hydrogenation in the presence of dibenzothiophene. Appl. Catal. A 2003, 242, 311–320. [Google Scholar] [CrossRef]
- Piskorz, W.; Adamski, G.; Kotarba, A.; Sojka, Z.; Sayag, C.; Djéga-Mariadassou, G. Hydrodenitrogenation of indole over Mo2C catalyst: Insights into mechanistic events through DFT modeling. Catal. Today 2007, 119, 39–43. [Google Scholar] [CrossRef]
- Mordenti, D.; Brodzki, D.; Djéga-Mariadassou, G. New synthesis of Mo2C 14 nm in average size supported on a high specific surface area carbon material. J. Solid State Chem. 1998, 141, 114–120. [Google Scholar] [CrossRef]
- Sayag, C. Reactivite de l’oxynitrure de Molybdene dans l’hydrodeazotation de la 1,2,3,4-tetrahydroquinoleine: Adaptation de la Composition Chimique Superficielle du Solide a Celle du Melange Reactionnel. Ph.D. Thesis, University of Pierre and Marie Curie, Paris, France, 1993. [Google Scholar]
- Mamède, A.S.; Giraudon, J.-M.; Löfberg, A.; Leclercq, L.; Leclercq, G. Hydrogenation of toluene over β-Mo2C in the presence of thiophene. Appl. Catal. A 2002, 227, 73–82. [Google Scholar] [CrossRef]
- Xiang, C.; Chai, Y.-M.; Liu, Y.-Q.; Liu, C.-G. Mutual influences of hydrodesulfurization of dibenzothiophene and hydrodenitrogenation of indole over NiMoS/γ-Al2O3 catalyst. J. Fuel Chem. Technol. 2008, 36, 684–690. [Google Scholar] [CrossRef]
- Kim, S.C.; Massoth, F.E. Kinetics of the hydrodenitrogenation of indole. Ind. Eng. Chem. Res. 2000, 39, 1705–1712. [Google Scholar] [CrossRef]
- Hynaux, A. Synthèse et Caractérisation de Carbures de Molybdène Supportés sur Composite de noir de Carbone Mésoporeux: Application en Hydrodésulfuration du Dibenotiophène et en Hydrodésazotation de l’indole. PhD. Thesis, University of Pierre and Marie Curie, Paris, France, 2006. [Google Scholar]
- Mordenti, D. Nouvelle Methode de Preparation des Carbures de Molybdene et de Tungstene Supportes sur Charbon Actif et Reactivite en Hydrodesazotation de l’indole. PhD. Thesis, University of Pierre and Marie Curie, Paris, France, 1998. [Google Scholar]
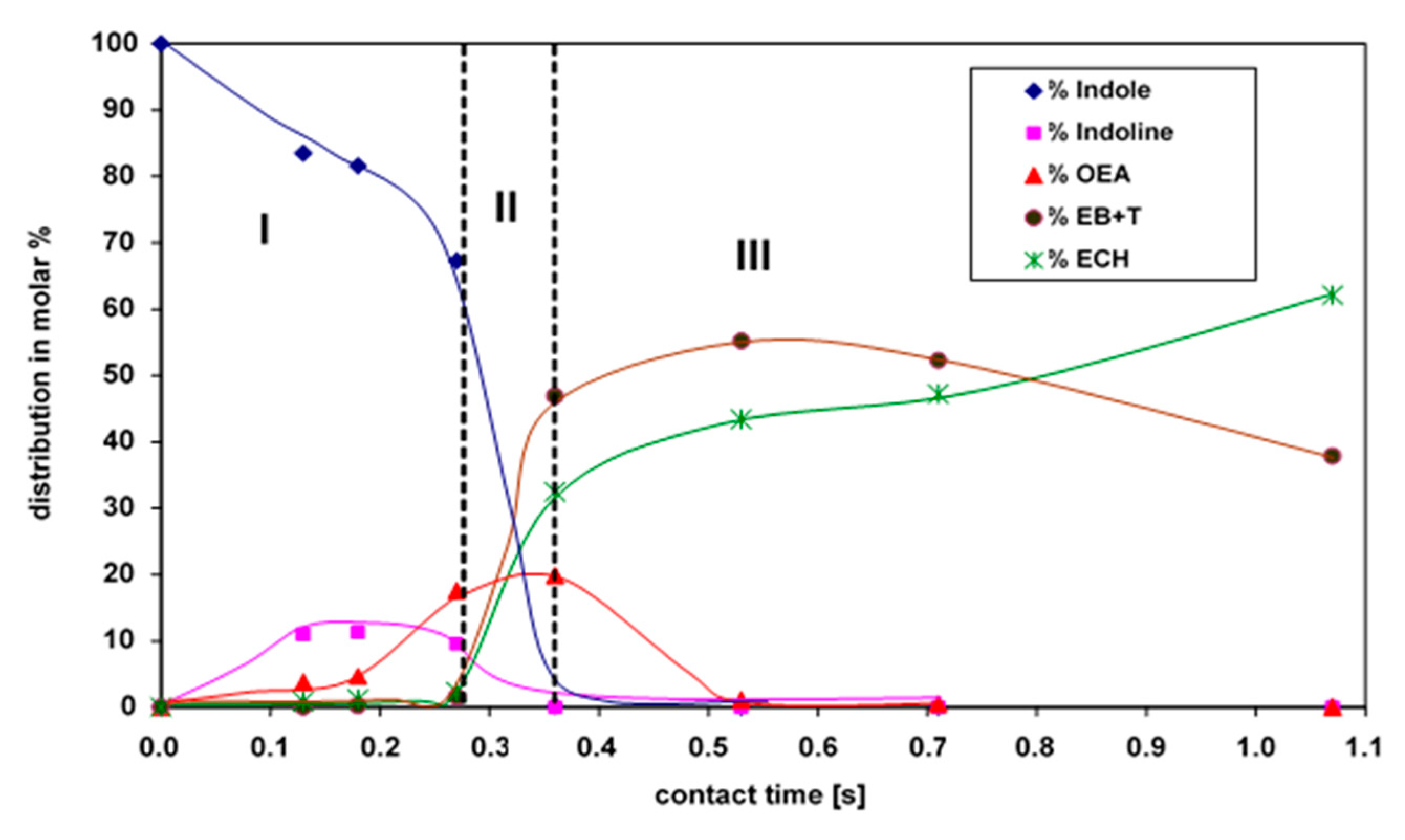
| HDN Products | Share [Mole %] |
|---|---|
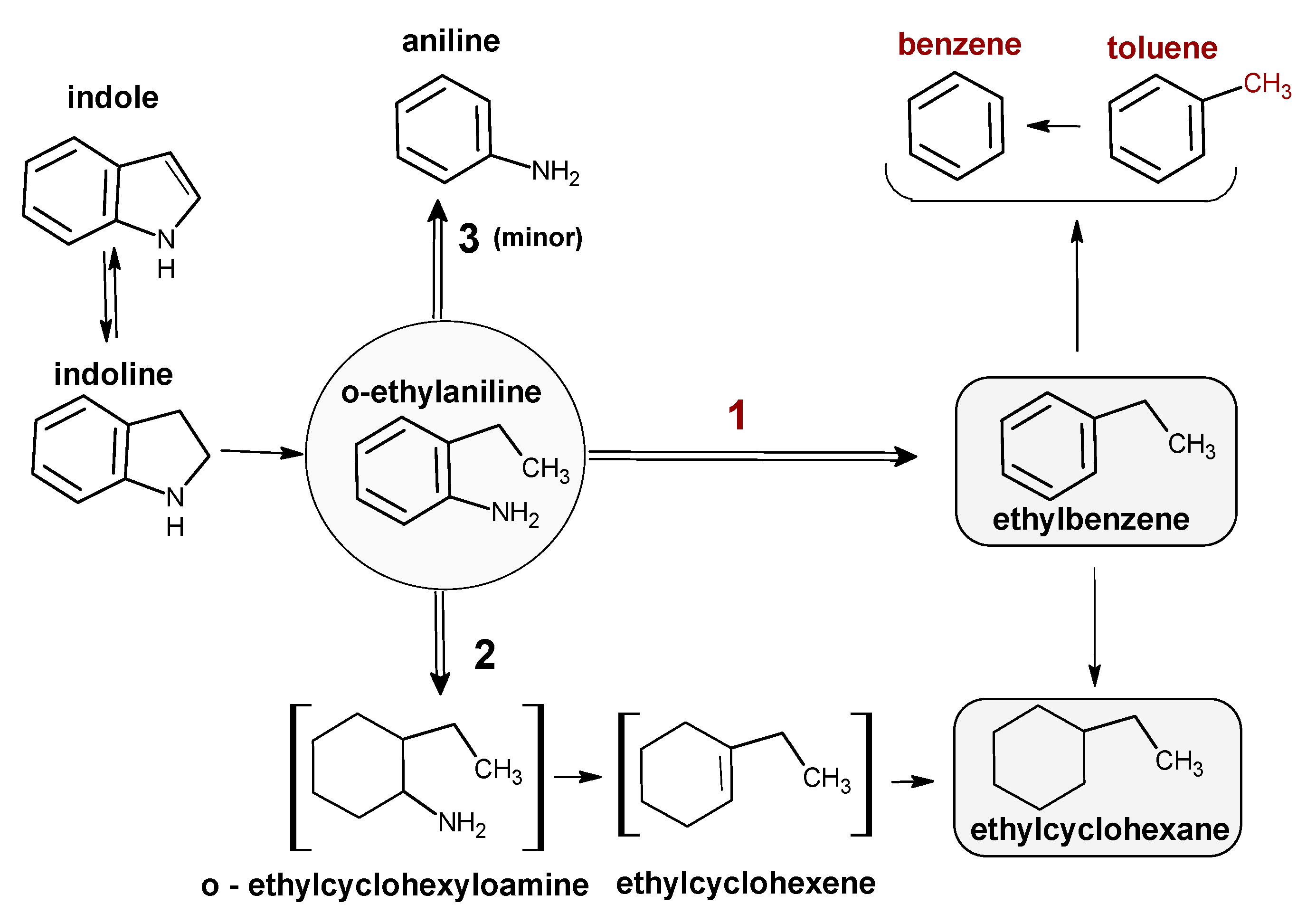
| 1—Nitrogen compounds [%]: Indole Indoline o-ethylaniline (OEA) Aniline (A) | 0 Traces 20 Traces |
| 2—Direct denitrogenation (DDN, route #1, Scheme 1) [%]: Ethylbenzene (EB) Toluene (T) Benzene (B) | 34 11 2 |
| 3—Hydrogenation (HYD, route #2, Scheme 1) [%]: Ethylcyclohexane (ECH) Ethylcyclohexene (ECHen) | 32 Traces |
| Total conversion of indole [%] (1 + 2 + 3) | 99 |
| HDN conversion [%] (2 + 3) | 79 |
| Total Conversion over Mo2C [Mole %] | |
|---|---|
| 99 | 1.4 |
| 33 | 0.7 |
| 18 | 0.2 |
| Catalyst/Test Conditions | Temperature of Reaction [K] | Pressure [bar] | Selectivity ECH/EB | Reactor | Sulfidation Conditions | Ref. |
|---|---|---|---|---|---|---|
| CoMoS/Al2O3 | 593 | 50 | 300 | Autoclave | – | [67] |
| NiMoS/Al2O3 | 593 | 70 | 15 | Fixed-bed reactor | 673 K; 10% H2S; 10 h | [53] |
| NiMoS/Al2O3 | 593 | 70 | 25 | Fixed-bed reactor | 673 K; 10% H2S; 10 h | [53] |
| NiMoS/Al2O3 | 593 | 70 | 40 | Fixed-bed reactor | 673 K; 10% H2S; 10 h | [53] |
| NiMoS CoMoS | 613 | 35 | 7.5 | Fixed-bed reactor | 673 K; 10% H2S; 2 h | [73] |
| Mo2C/CBC no sulfur H2/feed = 600 | 623 | 50 | 1.25 | Fixed-bed reactor | – | [74] |
| Mo2C/CBC H2/feed = 200 | 623 | 50 | 2 | Fixed-bed reactor | – | [74] |
| Mo2C/C in situ | 613 | 50 | 2 | Fixed-bed reactor | – | [69,75] |
| Bulk Mo2C with 50 ppm of S | 613 | 60 | 0.7 | Fixed-bed reactor | – | This paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowski, M.; Janus, R.; Wądrzyk, M.; Szymańska-Kolasa, A.; Sayag, C.; Djéga-Mariadassou, G. On Catalytic Behavior of Bulk Mo2C in the Hydrodenitrogenation of Indole over a Wide Range of Conversion Thereof. Catalysts 2020, 10, 1355. https://doi.org/10.3390/catal10111355
Lewandowski M, Janus R, Wądrzyk M, Szymańska-Kolasa A, Sayag C, Djéga-Mariadassou G. On Catalytic Behavior of Bulk Mo2C in the Hydrodenitrogenation of Indole over a Wide Range of Conversion Thereof. Catalysts. 2020; 10(11):1355. https://doi.org/10.3390/catal10111355
Chicago/Turabian StyleLewandowski, Marek, Rafał Janus, Mariusz Wądrzyk, Agnieszka Szymańska-Kolasa, Céline Sayag, and Gérald Djéga-Mariadassou. 2020. "On Catalytic Behavior of Bulk Mo2C in the Hydrodenitrogenation of Indole over a Wide Range of Conversion Thereof" Catalysts 10, no. 11: 1355. https://doi.org/10.3390/catal10111355
APA StyleLewandowski, M., Janus, R., Wądrzyk, M., Szymańska-Kolasa, A., Sayag, C., & Djéga-Mariadassou, G. (2020). On Catalytic Behavior of Bulk Mo2C in the Hydrodenitrogenation of Indole over a Wide Range of Conversion Thereof. Catalysts, 10(11), 1355. https://doi.org/10.3390/catal10111355