Combined Reforming of Clean Biogas over Nanosized Ni–Rh Bimetallic Clusters
Abstract
:1. Introduction
2. Results and Discussion
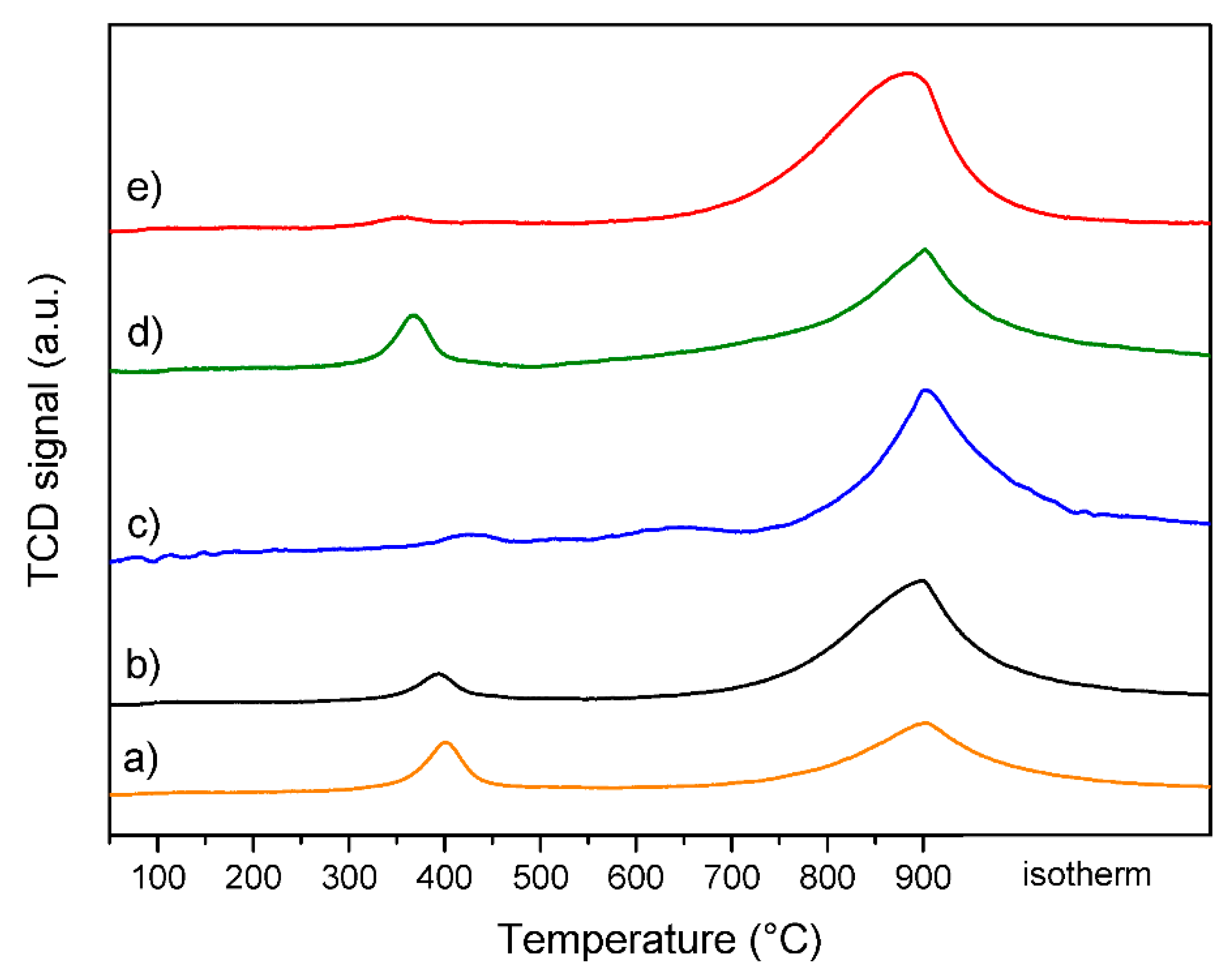
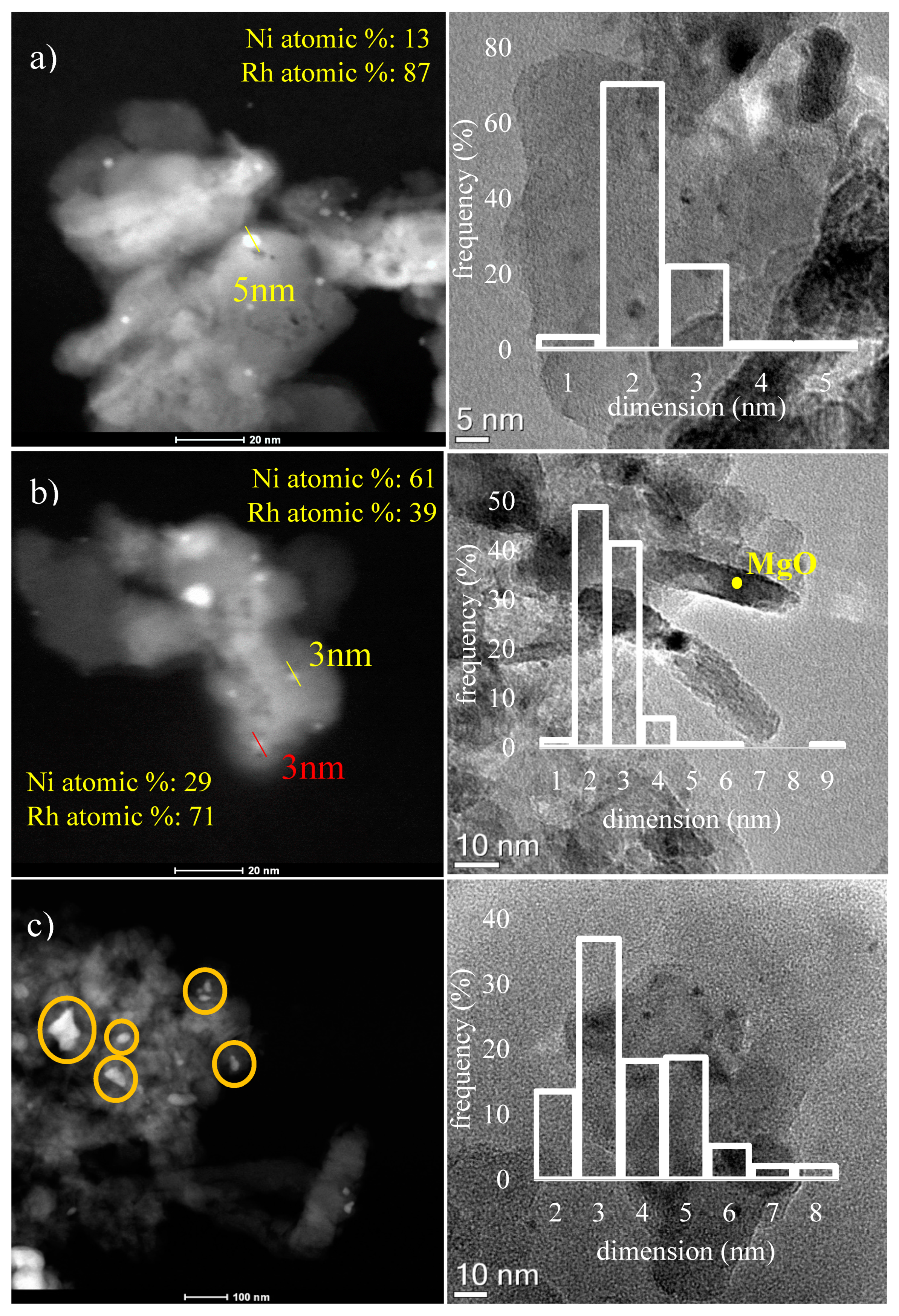
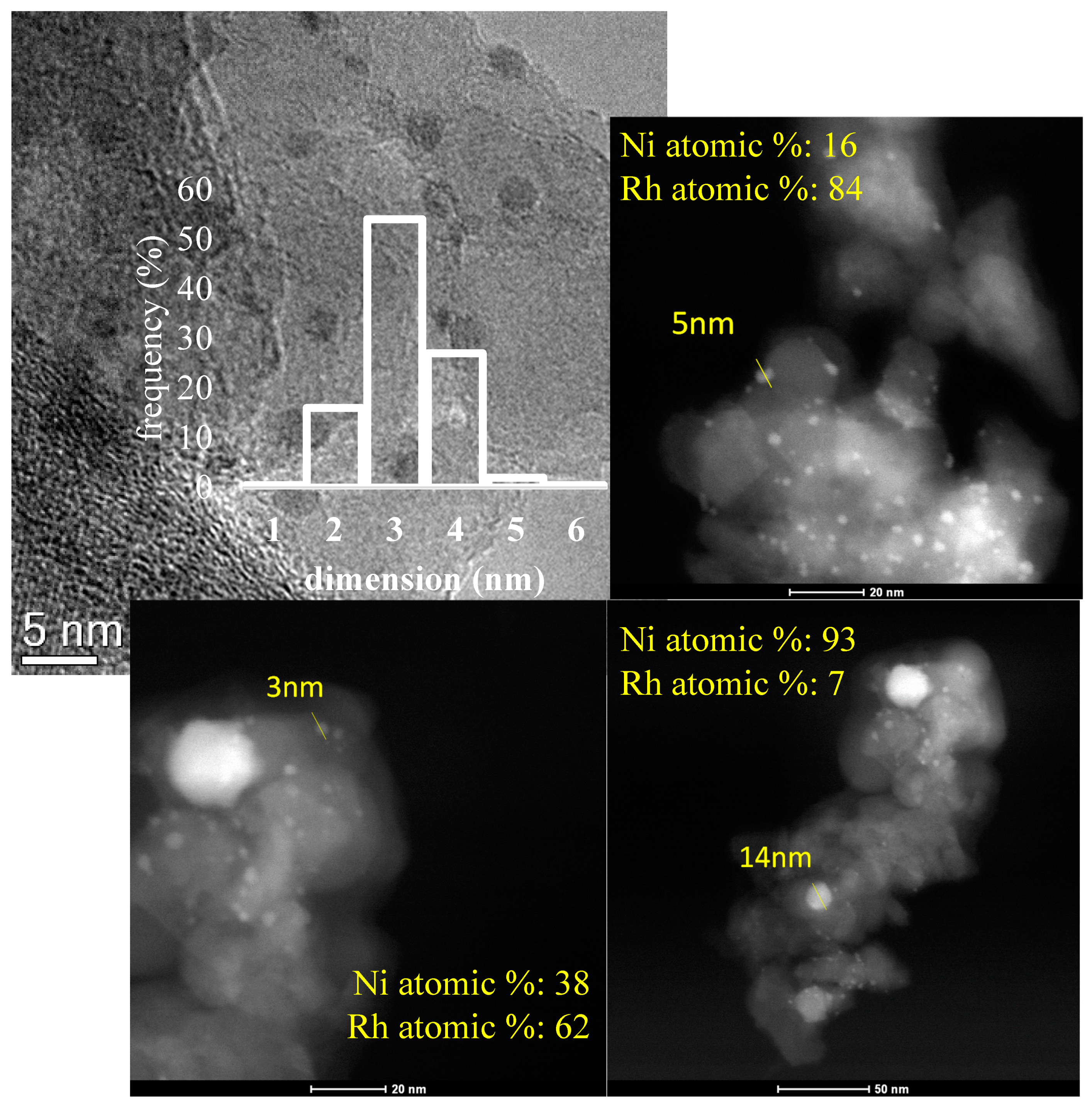
2.1. Characterization of Fresh Catalysts
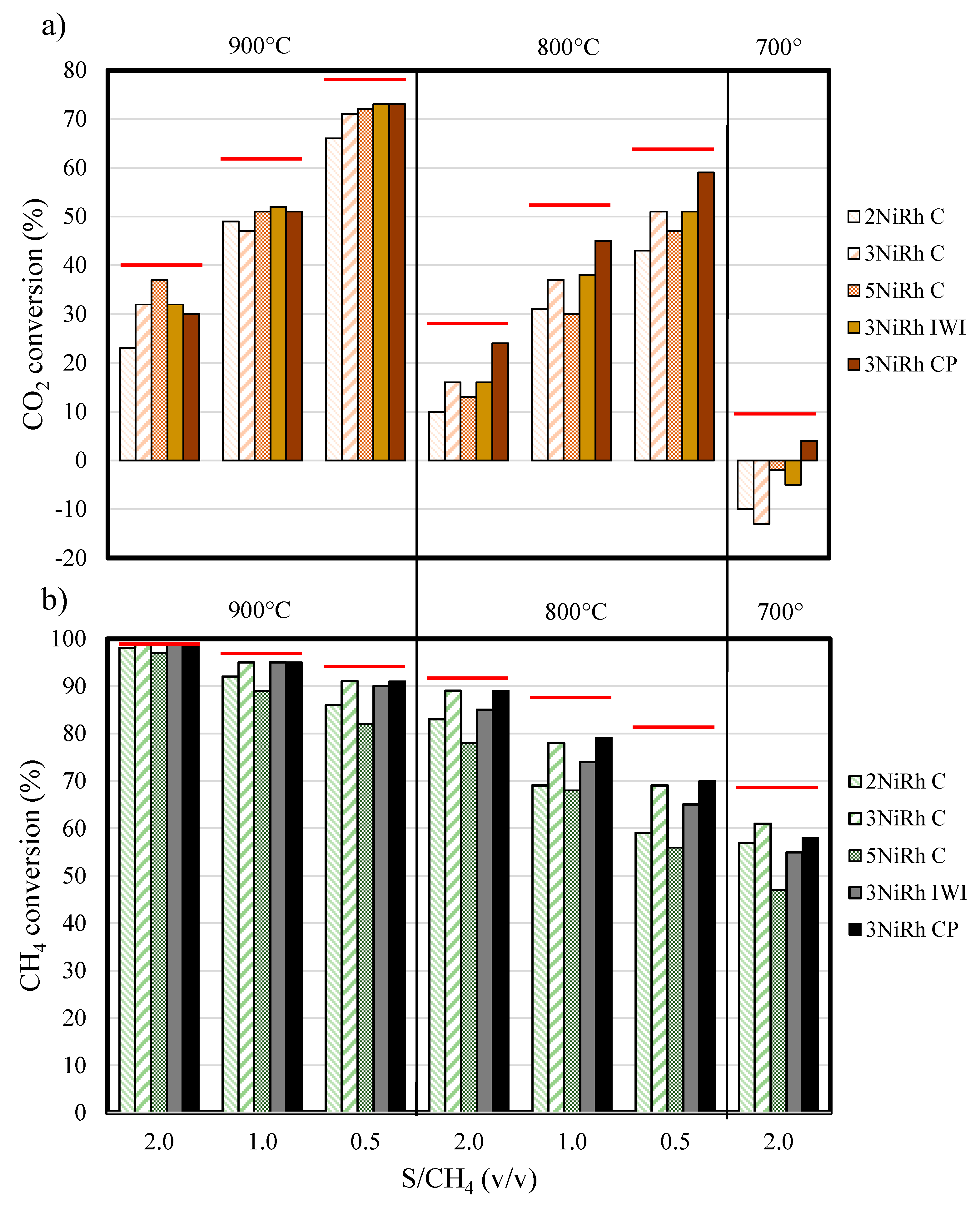
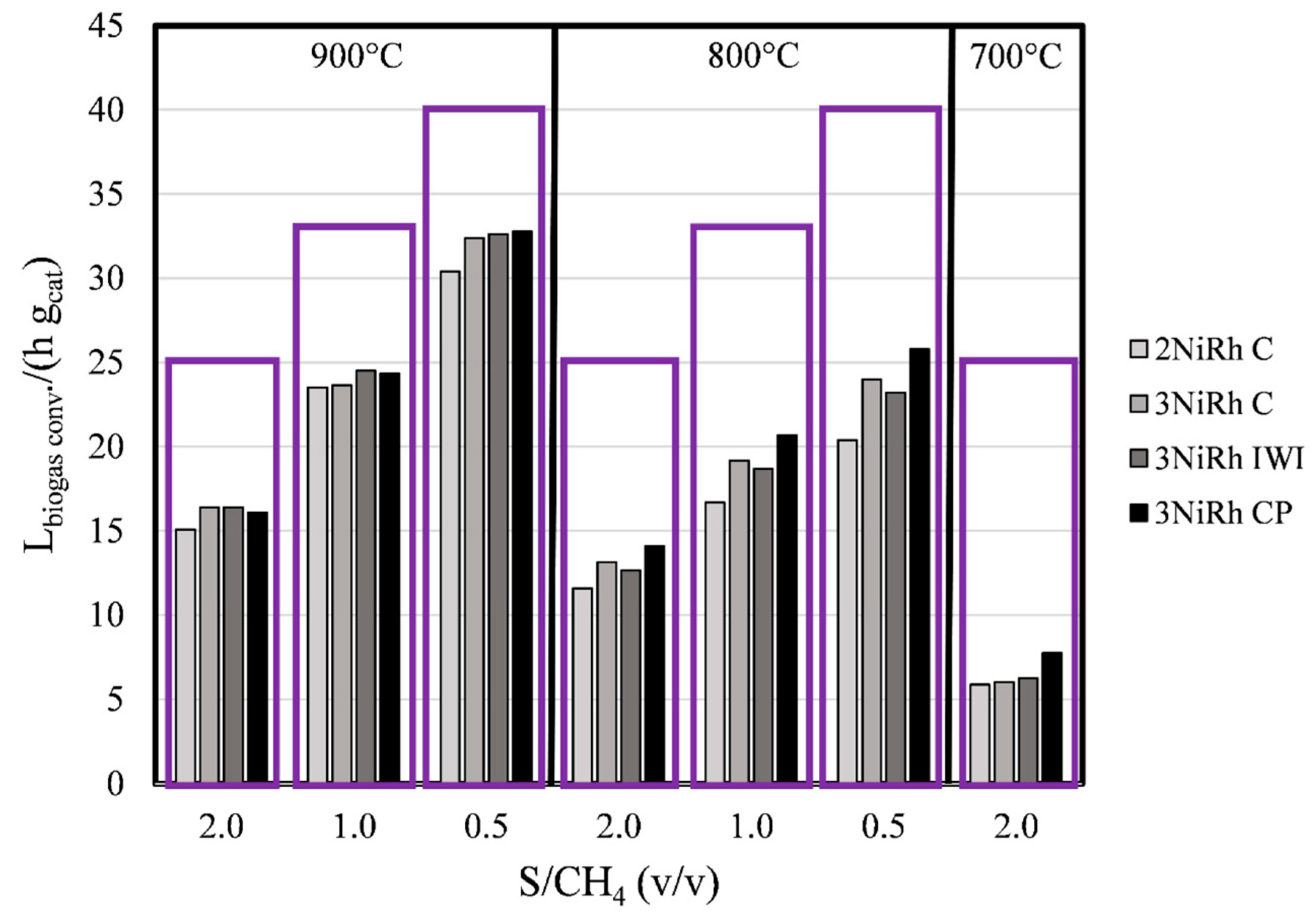
2.2. Combined Steam and Dry Reforming of Clean Biogas
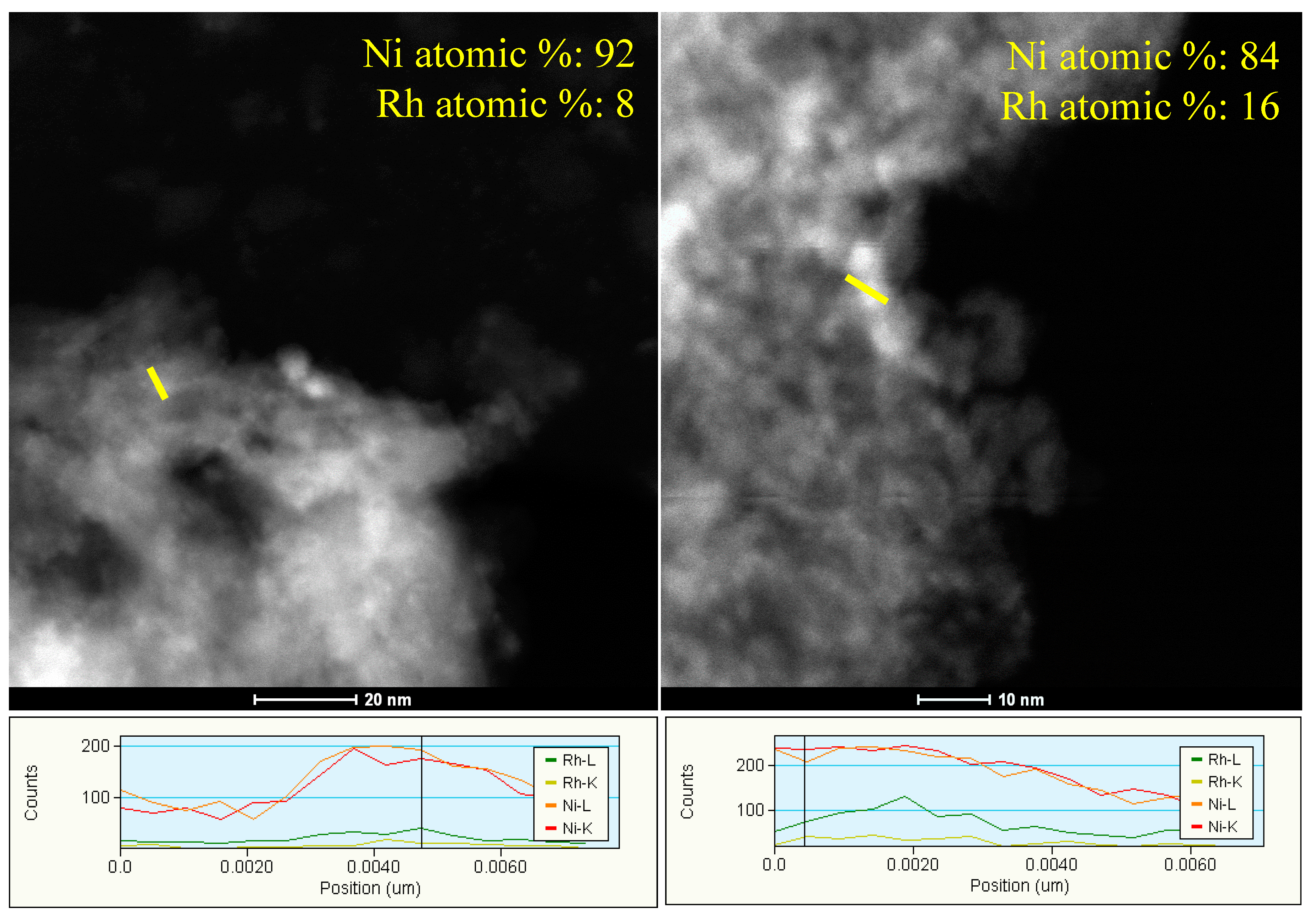
2.3. Characterization of Spent Catalysts
3. Material and Methods
3.1. Preparation of the Catalysts
3.2. Characterization Techniques
3.3. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stürmer, S.; Leiers, D.; Anspach, V.; Brügging, E.; Scharfy, D.; Wissel, T. Agricultural biogas production: A regional comparison of technical parameters. Renew. Energy 2021, 164, 171–182. [Google Scholar] [CrossRef]
- Naverro-Puyuelo, A.; Reyero, I.; Moral, A.; Bimbela, F.; Bañares, M.A.; Gandìa, L.M. Effect of oxygen addition, reaction temperature and thermal treatments on syngas production from biogas combined reforming using Rh/alumina catalysts. J. Ind. Eng. Chem. 2019, 80, 217–226. [Google Scholar] [CrossRef]
- Rasi, S.; Veijanen, A.; Rintala, J. Trace compounds of biogas from different biogas production plants. Energy 2007, 32, 1375–1380. [Google Scholar] [CrossRef]
- Rasi, S.; Lantela, J.; Rintala, J. Trace compounds affecting biogas energy utilisation, A review. Energy Convers. Manag. 2011, 52, 3369–3375. [Google Scholar] [CrossRef]
- Sosnowski, P.; Wieczorek, A.; Ledakowicz, S. Anaerobic co-digestion of sewage sludge and organic fraction of municipal solid wastes. Adv. Environ. Res. 2003, 7, 609–616. [Google Scholar] [CrossRef]
- Luostarinen, S.; Luste, S.; Sillanpaa, M. Increased biogas production at wastewater treatment plants through co-digestion of sewage sludge with grease trap sludge from a meat processing plant. Bioresour. Technol. 2009, 100, 79–85. [Google Scholar] [CrossRef]
- Johnsonn, O.; Pullman, E.; Jensen, J.K.; Eklund, R.; Schyl, H.; Ivarsson, S. Sustainable Gas Enters the European Gas Distribution System; Danish Gas Technology Centre: Hørsholm, Denmark, 2003. [Google Scholar]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of Anaerobic Digestion Process: A Review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- Neto, J.G.; Ozorio, L.V.; de Abreu, T.C.C.; dos Santos, B.F.; Pradelle, F. Modeling of biogas production from food, fruits and vegetables wastes using artificial neural network (ANN). Fuel 2021, 285, 119081. [Google Scholar] [CrossRef]
- Renewables 2017 Global Status Report; Renewable Energy Policy Network for the 21thCentury REN21; REN21: Paris, France, 2017.
- Van Nes, W.J.; Nhete, T.D. Biogas for a better life: An African initiative. Renew. Energy World 2007, 10, 184–187. [Google Scholar]
- Scarlat, N.; Dallemand, J.F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129A, 457–472. [Google Scholar] [CrossRef]
- Andriani, D.; Wresta, A.; Atmaja, T.D.; Saepudin, A. A review on Optimization Production and Upgrading Biogas Through CO2 Removal Using Various Techniques. Appl. Biochem. Biotechnol. 2014, 172, 1909–1928. [Google Scholar] [CrossRef] [PubMed]
- Akinyele, D.O.; Rayudu, R.K. Review of energy storage technologies for sustainable power networks. Sustain. Energy Technol. Assess. 2014, 8, 74–91. [Google Scholar] [CrossRef]
- Smith, A.D.; Mago, P.J.; Fumo, N. Benefits of thermal energy storage option combined with CHP system for different commercial building types. Sustain. Energy Technol. Assess. 2013, 1, 3–12. [Google Scholar] [CrossRef]
- Yang, L.; Ge, X.; Wan, C.; Yu, F.; Li, Y. Progress and perspectives in converting biogas to transportation fuels. Renew. Sustain. Energy Rev. 2014, 40, 1133–1152. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current biodiesel production technologies: A comparative review. Energy Convers. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Hahn, H.; Hartmann, K.; Bühle, L.; Wachendorf, M. Comparative life cycle assessment of biogas plant configurations for a demand oriented biogas supply for flexible power generation. Bioresour. Technol. 2015, 179, 348–358. [Google Scholar] [CrossRef]
- Kapdi, S.S.; Vijay, V.K.; Rajesh, S.K.; Prasad, R. Biogas scrubbing, compression and storage: Perspective and prospectus in Indian context. Renew. Energy 2005, 30, 1195–1202. [Google Scholar] [CrossRef]
- Subramanian, K.A.; Mathad, V.C.; Vijay, V.K.; Subbarao, P.M.V. Comparative Evaluation of Emission and Fuel Economy of an Automotive Spark Ignition Vehicle Fuelled with Methane Enriched Biogas and CNG Using Chassis Dynamometer. Appl. Energy 2013, 105, 17–29. [Google Scholar] [CrossRef]
- Shiga, H.; Shinda, K.; Hagiwara, K.; Tsutsumi, A.; Sakurai, M.; Yoshida, K.; Bilgen, E. Large-scale hydrogen production from biogas. Int. J. Hydrogen Energy 1998, 23, 631–640. [Google Scholar] [CrossRef]
- Wei, Q.; Gao, X.; Wang, L.; Ma, Q. Rational design of nickel-based catalyst coupling with combined methane reforming to steadily produce syngas. Fuel 2020, 271, 117631–117642. [Google Scholar] [CrossRef]
- Parente, M.; Soria, M.A.; Madeira, L.M. Hydrogen and/or syngas production through combined dry and steam reforming of biogas in a membrane reactor: A thermodynamic study. Renew. Energy 2020, 157, 1254–1264. [Google Scholar] [CrossRef]
- Simpson, A.P.; Lutz, A.E. Exergy analysis of hydrogen production via steam methane reforming. Int. J. Hydrogen Energy 2007, 32, 4811–4820. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Gallorini, F.; Servili, S. Hydrogen production through sorption-enhanced steam methane reforming and membrane technology: A Review. Energy 2008, 33, 554–570. [Google Scholar] [CrossRef]
- Lunsford, J.H. Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century. Catal. Today 2000, 63, 165–174. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. A review on coke management during dry reforming of methane. Int. J. Energy Res. 2015, 39, 1196–1216. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Nikoo, M.N.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Tech. 2011, 92, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Istadi; Amin, N.A.S. Co-generation of synthesis gas and C2+ hydrocarbons from methane and carbon dioxide in a hybrid catalytic-plasma reactor: A review. Fuel 2006, 85, 577–592. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q.; Millar, G.J. Carbon Dioxide Reforming of Methane To Produce Synthesis Gas over Metal-Supported Catalysts: State of the Art. Energy Fuels 1996, 10, 896–904. [Google Scholar] [CrossRef]
- Zeppieri, M.; Villa, P.L.; Verdone, N.; Scarsella, M.; Filippis, P.D. Kinetic of methane steam reforming reaction over nickel- and rhodium-based catalysts. Appl. Catal. A Gen. 2010, 387, 147–154. [Google Scholar] [CrossRef]
- Richardson, J.T.; Garrait, M.; Hung, J.K. Carbon Dioxide Reforming with Rh and Pt-Re Catalysts Dispersed on Ceramic Foam Supports. Appl. Catal. A Gen. 2003, 255, 69–82. [Google Scholar] [CrossRef]
- Papurello, D.; Chiodo, V.; Maisano, S.; Lanzini, A.; Santarelli, M. Catalytic stability of a Ni-Catalyst towards biogas reforming in the presence of deactivating trace compounds. Renew. Energy 2018, 127, 481–494. [Google Scholar] [CrossRef]
- Dan, M.; Mihet, M.; Borodi, G.; Lazar, M.D. Combined steam and dry reforming of methane for syngas production from biogas using bimodal pore catalyst. Catal. Today 2020, in press corrected proof. [Google Scholar] [CrossRef]
- Pashchenko, D. Combined methane reforming with a mixture of methane combustion products and steam over a Ni-based catalyst: An experimental and thermodynamic study. Energy 2019, 185, 573–584. [Google Scholar] [CrossRef]
- Dan, M.; Mihet, M.; Lazar, M.D. Hydrogen and/or syngas production by combined steam and dry reforming of methane on nickel catalysts. Int. J. Hydrogen Energy 2020, 45, 26254–26264. [Google Scholar] [CrossRef]
- Horn, R.; Williams, K.A.; Degenstein, N.J.; Schmidt, L.D. Syngas by catalytic partial oxidation of methane on rhodium: Mechanistic conclusions from spatially resolved measurements and numerical simulations. J. Catal. 2006, 242, 92–102. [Google Scholar] [CrossRef]
- Jakobsen, J.G.; Jørgensen, T.L.; Chorkendorff, I.; Sehested, J. Steam and CO2 reforming of methane over Ru/ZrO2 catalyst. Appl. Catal. A Gen. 2010, 377, 158–166. [Google Scholar] [CrossRef]
- Souza, M.M.V.M.; Neto, O.R.M.; Schmal, M. Synthesis Gas Production from Natural Gas on Supported Pt Catalysts. J. Nat. Gas. Chem. 2006, 15, 21–27. [Google Scholar] [CrossRef]
- Hou, Z.; Chen, P.; Fang, H.; Zheng, X.; Yashima, T. Production of synthesis gas via methane reforming with CO2 on noble metals and small amount of noble-(Rh-) promoted Ni caltaysts. Int. J. Hydrogen Energy 2006, 31, 555–561. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–291. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Rives, V. Layered Double Hydroxides: Present and Future; Nova Publ.: New York, NY, USA, 2001. [Google Scholar]
- Zhang, F.; Xiang, X.; Li, F.; Duan, X. Layered Double Hydroxides as Catalytic Materials: Recent Development. Catal. Surv. Asia 2008, 12, 253–265. [Google Scholar] [CrossRef]
- Carrado, K.A.; Kostapapas, A.; Suib, S.L. Layered double hydroxides (LDHs). Solid State Ion. 2008, 26, 77–86. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zhang, J.; Adebajo, M.O.; Zhang, H.; Zhou, C. Catalytic applications of layered double hydroxides and derivatives. Appl. Clay Sci. 2011, 53, 139–150. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Hernandez, W.Y.; Lauwaert, J.; Van Der Voort, P.; Verberckmoes, A. Recent advances on the utilization of layered double hydroxides (LDHs) and related heterogeneous catalysts in a lignocellulosic-feedstock biorefinery scheme. Green Chem. 2017, 19, 5269–5302. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Yu, F.; Altaf, N.; Zhu, M.; Li, J.; Dai, B.; Wang, Q. Two-Dimensional Layered Double Hydroxides for Reactions of Methanation and Methane Reforming in C1 Chemistry. Materials 2018, 11, 221. [Google Scholar] [CrossRef] [Green Version]
- Schiaroli, N.; Lucarelli, C.; Sanghez de Luna, G.; Fornasari, G.; Vaccari, A. Ni-based catalysts to produce synthesis gas by combined reforming of clean biogas. Appl. Catal. Ageneral 2019, 582, 117087–117099. [Google Scholar] [CrossRef]
- Lucrédio, A.F.; Assaf, J.M.; Assaf, E.M. Reforming of a model sulfur-free biogas on Ni catalysts supported on Mg(Al)O derived from hydrotalcite precursors: Effect of La and Rh addition. Biomass Bioenergy 2014, 60, 8–17. [Google Scholar] [CrossRef]
- Diez, V.; Ferretti, C.; Torresi, P.; Apesteguia, C.R.; Di Cosimo, J.I. Effect of MgO activation conditions on its catalytic properties for base-catalyzed reactions. Catal. Today 2017, 173, 21–27. [Google Scholar] [CrossRef]
- Selvamani, T.; Sinhamahapatra, A.; Bhattacharjya, D.; Mukhopadhyay, I. Rectangular MgO microsheets with strong catalytic activity. Mater. Chem. Phys. 2011, 129, 853–861. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Jiménez-González, C.; de Rivas, B.; Gutiérrez-Ortiz, J.I. Partial oxidation of methane to syngas on bulk NiAl2O4 catalyst. Comparison with alumina supported nickel, platinum and rhodium catalysts. Appl. Catal. A Gen. 2012, 437–438, 53–62. [Google Scholar]
- le Saché, E.; Pastor-Pérez, L.; Garcilaso, V.; Watson, D.J.; Centeno, M.A.; Odriozola, J.A.; Reina, T.R. Flexible syngas production using a La2Zr2-xNixO7-δ pyrochlore-double perovskite catalyst: Towards a direct route for gas phase CO2 recycling. Catal. Today 2020, 357, 583–589. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Czaun, M.; Prakash, G.K.S. Bi-reforming of methane from any source with steam and carbon dioxide exclusively to metgas (CO-2H2) for methanol and hydrocarbon synthesis. J. Am. Chem. Soc. 2013, 135, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Garcìa-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Alemany, L.J. RhNi nanocatalysts for the CO2 and CO2+H2O reforming of methane. Catal. Today 2011, 175, 136–142. [Google Scholar] [CrossRef]
- Jabbour, K.; El Hassan, N.; Davidson, A.; Casale, S.; Massiani, P. Factors affecting the long-term stability of mesoporous nickel-based catalysts in cobined steam and dry reforming of methane. Catal. Sci. Technol. 2016, 6, 4616–4631. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, A.; Centeno, M.Á.; Odriozola, J.A. Ru-Ni Catalyst in the Combined Dry-Steam Reforming of Methane: The Importance in the Metal Order Addition. Top. Catal. 2016, 59, 303–313. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Z.; Ren, P.; Wang, G. Effect of molybdenum carbide concentration on the Ni/ZrO2 catalyst for steam-CO2 bi-reforming of methane. RSC Adv. 2015, 5, 100865–100872. [Google Scholar] [CrossRef]
- Lucarelli, C.; Molinari, C.; Faure, R.; Fornasari, G.; Gary, D.; Schiaroli, N.; Vaccari, A. Novel Cu-Zn-Al catalysts obtained from hydrotalcite-type precursors for middle-temperature water-gas shift applications. Appl. Clay Sci. 2018, 155, 103–110. [Google Scholar] [CrossRef]
- Femoni, C.; Demartin, F.; Iapalucci, M.C.; Lombardi, A.; Longoni, G.; Marin, C.; Svensson, P.H. New Ni-Rh carbonyl clusters with unprecedented structural and electronic features: Synthesis and X-ray structure of [NEt4]3[Ni10Rh(CO)19]∙C2H5CN, [NMe3CH2Ph]3[Ni6Rh3(CO)17] and [NEt4]3[Ni9Rh3(CO)22]. J. Organomet. Chem. 2000, 614–615, 294–303. [Google Scholar] [CrossRef]
- Tabanelli, T.; Cocchi, S.; Gumina, B.; Izzo, L.; Mella, M.; Passeri, S.; Cavani, F.; Lucarelli, C.; Schutz, J.; Bonrath, W.; et al. Mg/Ga mixed-oxide catalysts for phenol methylation: Outstanding performance in 2,4,6-trimethylphenol synthesis with co-feeding of water. Appl. Catal. Agen. 2018, 552, 86–97. [Google Scholar] [CrossRef]
- Lucarelli, C.; Lolli, A.; Giugni, A.; Grazia, L.; Albonetti, S.; Monticelli, D.; Vaccari, A. Efficient and ecofriendly route for the solvent-free synthesis of piperonal and aromatic aldehydes using Au/CeO2 catalyst. Appl. Catal. B Environ. 2017, 203, 314–323. [Google Scholar] [CrossRef]
- Lucarelli, C.; Pavarelli, G.; Molinari, C.; Albonetti, S.; Mista, W.; Di Domenico, D.; Vaccari, A. Catalyst deactivation in on-board H2 production by fuel dehydrogenation. Int. J. Hydrog. Energy 2014, 39, 1336–1349. [Google Scholar] [CrossRef]
- Lucarelli, C.; Moggi, P.; Cavani, F.; Devillers, M. Sol-gel synthesis and characterization of transition metal based mixed oxides and their application as catalysts in selective oxidation of propane. Appl. Catal. A Gen. 2007, 325, 244–250. [Google Scholar] [CrossRef]
- Chemical Equilibrium with Applications. Available online: http://www.grc.nasa.gov/www/CEAWeb/ (accessed on 10 November 2020).



| SSBET | 2NiRh C | 3NiRh C | 5NiRh C | 3NiRh IWI | 3NiRh CP |
|---|---|---|---|---|---|
| Before reaction (m2/g) | 73 | 80 | 48 | 60 | 99 |
| After reaction (m2/g) | 43 | 39 | 39 | 26 | 46 |
| Catalyst Active Phase/Promoter/Support | GHSV (mL h−1 g−1cat) | P (Mpa) | T (°C) | S/CH4 (v/v) | Biogas Converted (L h−1 gactive phase−1) | Ref. |
|---|---|---|---|---|---|---|
| 3NiRh C | 50,000 | 0.5 | 900 | 0.50 | 926 | this work |
| 800 | 0.50 | 686 | ||||
| 700 | 2.00 | 172 | ||||
| Ni (10 wt %)/La2Zr2O7 | 20,000 | 0.1 | 700 | 1.00 | 68 | [57] |
| Ni (10 wt %)/La2Zr2O7 | 60,000 | 0.1 | 700 | 1.00 | 94 | [57] |
| Ni (10 wt %)/Al2O3 | 12,000 | 0.1 | 700 | 1.20 | 17 | [38] |
| Ni (15 wt %)/MgO | 60,000 | 0.7 | 830 | 0.80 | 181 | [58] |
| Ni (4 wt %)/ Rh2O3 (0.04 wt %)/γ-Al2O3 | 6000 | 0.1 | 750 | 0.67 | 76 | [59] |
| Ni (5 wt %)/γ-Al2O3 | 69,000 | 0.1 | 800 | 0.80 | 731 | [60] |
| Ni (15 wt %)/ RuO2 (0.5 wt %)/HDL | 120,000 | 0.1 | 750 | 0.14 | 231 | [61] |
| Ni (10 wt %)/ Mo-carbide (0.5 wt %)/ZrO2 | 60,000 | 0.1 | 850 | 0.80 | 308 | [62] |
| Catalyst | Precursor/Synthesis | Ni (wt %) | Rh (wt %) |
|---|---|---|---|
| 2NiRh C | Ni10Rh(CO)19/WI | 2.0 | 0.3 |
| 3NiRh C | Ni10Rh(CO)19/WI | 3.0 | 0.5 |
| 5NiRh C | Ni10Rh(CO)19/WI | 5.0 | 0.9 |
| 3NiRh IWI | Ni(NO3)2, Rh(NO3)3/IWI | 3.0 | 0.5 |
| 3NiRh CP | Ni(NO3)2, Rh(NO3)3/CP | 3.0 | 0.5 |
| Mg/Al/O (support) | Mg(NO3)2, Al(NO3)3/CP | / | / |
| Temperature (°C) | S/CH4 (v/v) | P (MPa) | GHSV (mL h−1 gcat−1) |
|---|---|---|---|
| 900 | 2.0 | 0.5 | 50,000 |
| 1.0 | |||
| 0.5 | |||
| 800 | 2.0 | ||
| 1.0 | |||
| 0.5 | |||
| 700 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiaroli, N.; Lucarelli, C.; Iapalucci, M.C.; Fornasari, G.; Crimaldi, A.; Vaccari, A. Combined Reforming of Clean Biogas over Nanosized Ni–Rh Bimetallic Clusters. Catalysts 2020, 10, 1345. https://doi.org/10.3390/catal10111345
Schiaroli N, Lucarelli C, Iapalucci MC, Fornasari G, Crimaldi A, Vaccari A. Combined Reforming of Clean Biogas over Nanosized Ni–Rh Bimetallic Clusters. Catalysts. 2020; 10(11):1345. https://doi.org/10.3390/catal10111345
Chicago/Turabian StyleSchiaroli, Nicola, Carlo Lucarelli, Maria Carmela Iapalucci, Giuseppe Fornasari, Antonio Crimaldi, and Angelo Vaccari. 2020. "Combined Reforming of Clean Biogas over Nanosized Ni–Rh Bimetallic Clusters" Catalysts 10, no. 11: 1345. https://doi.org/10.3390/catal10111345
APA StyleSchiaroli, N., Lucarelli, C., Iapalucci, M. C., Fornasari, G., Crimaldi, A., & Vaccari, A. (2020). Combined Reforming of Clean Biogas over Nanosized Ni–Rh Bimetallic Clusters. Catalysts, 10(11), 1345. https://doi.org/10.3390/catal10111345









