Investigating Light-Induced Processes in Covalent Dye-Catalyst Assemblies for Hydrogen Production
Abstract
1. Introduction
2. Results
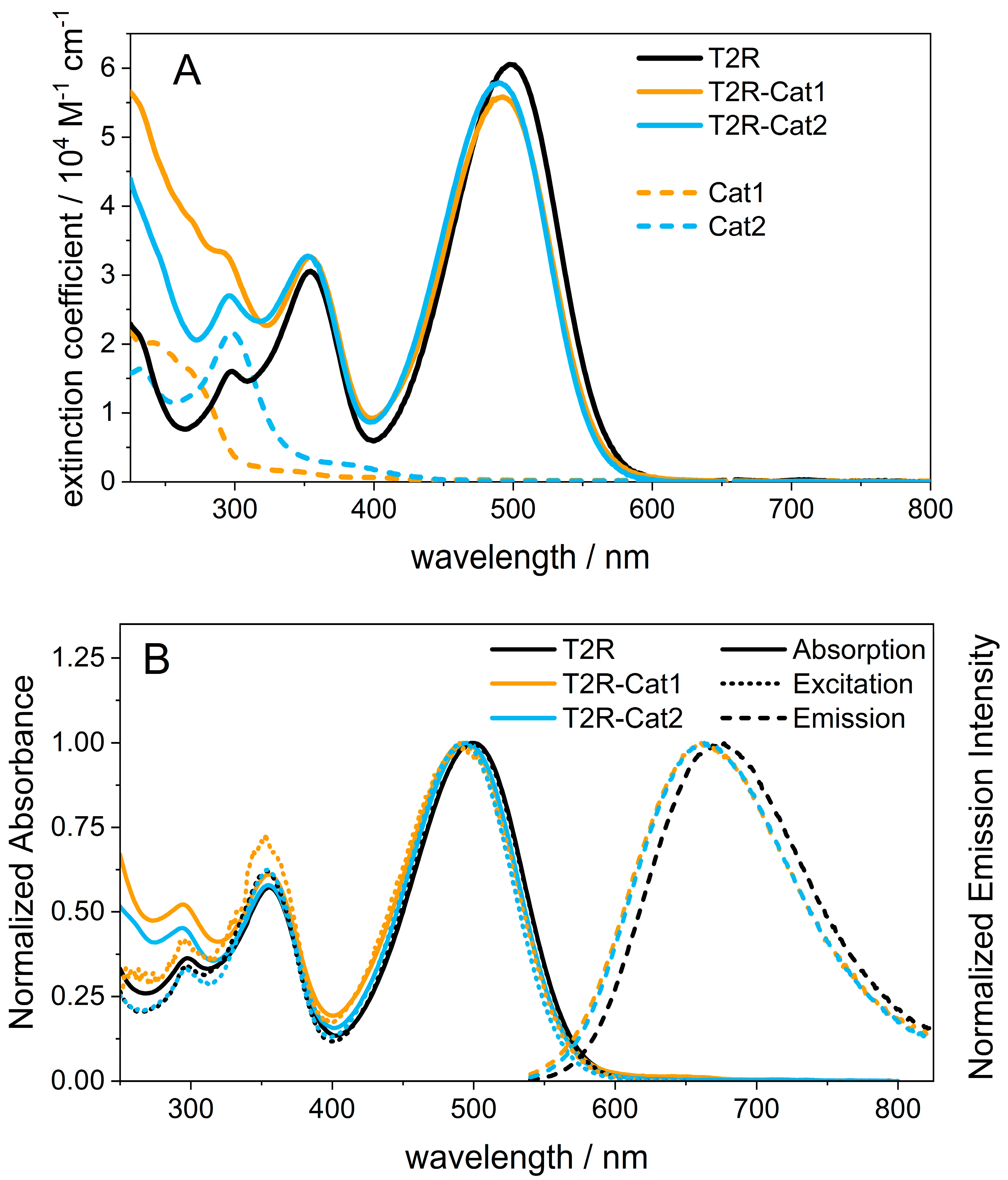
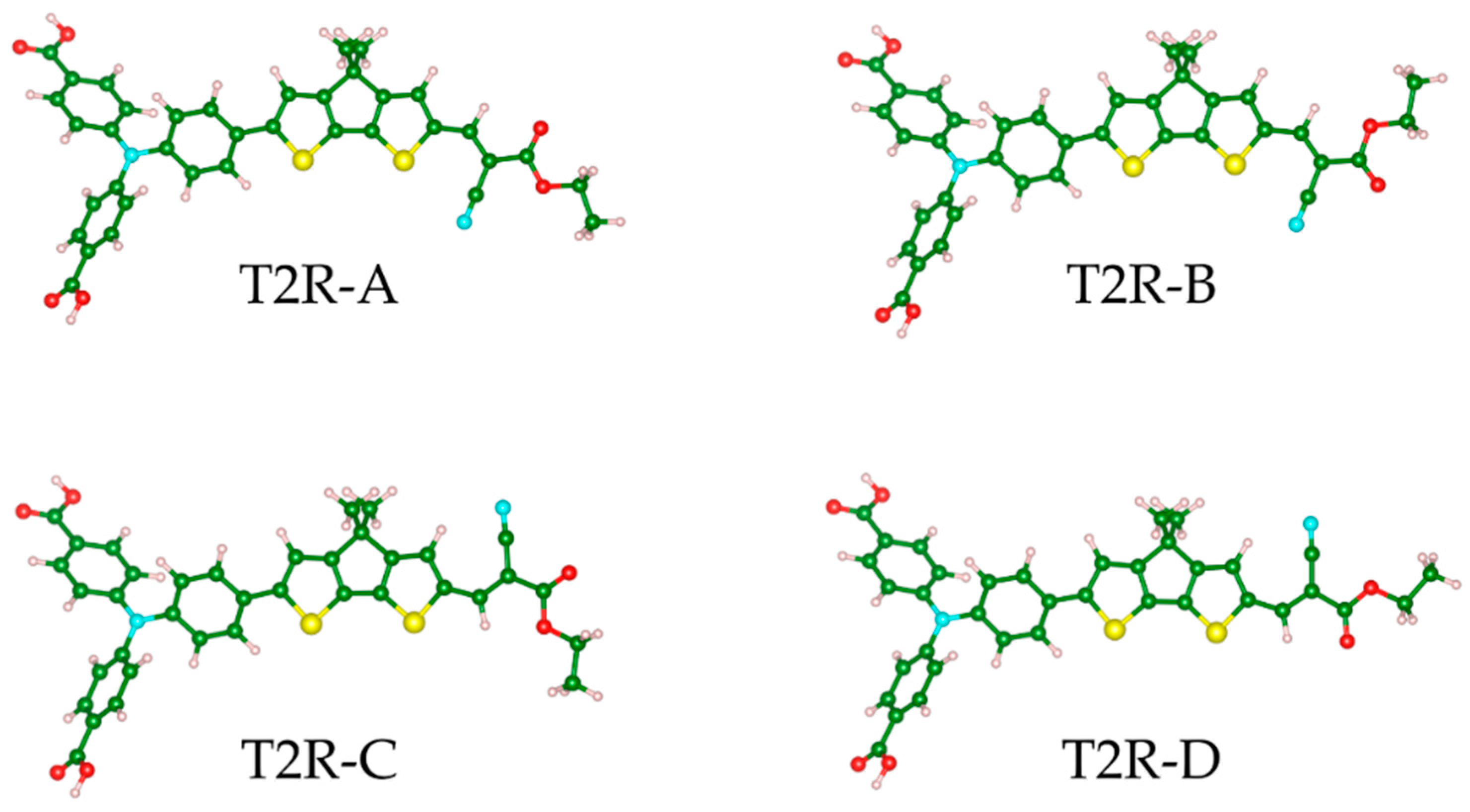
2.1. Steady-State Spectroscopy and Density Functional Theory (DFT) Calculations
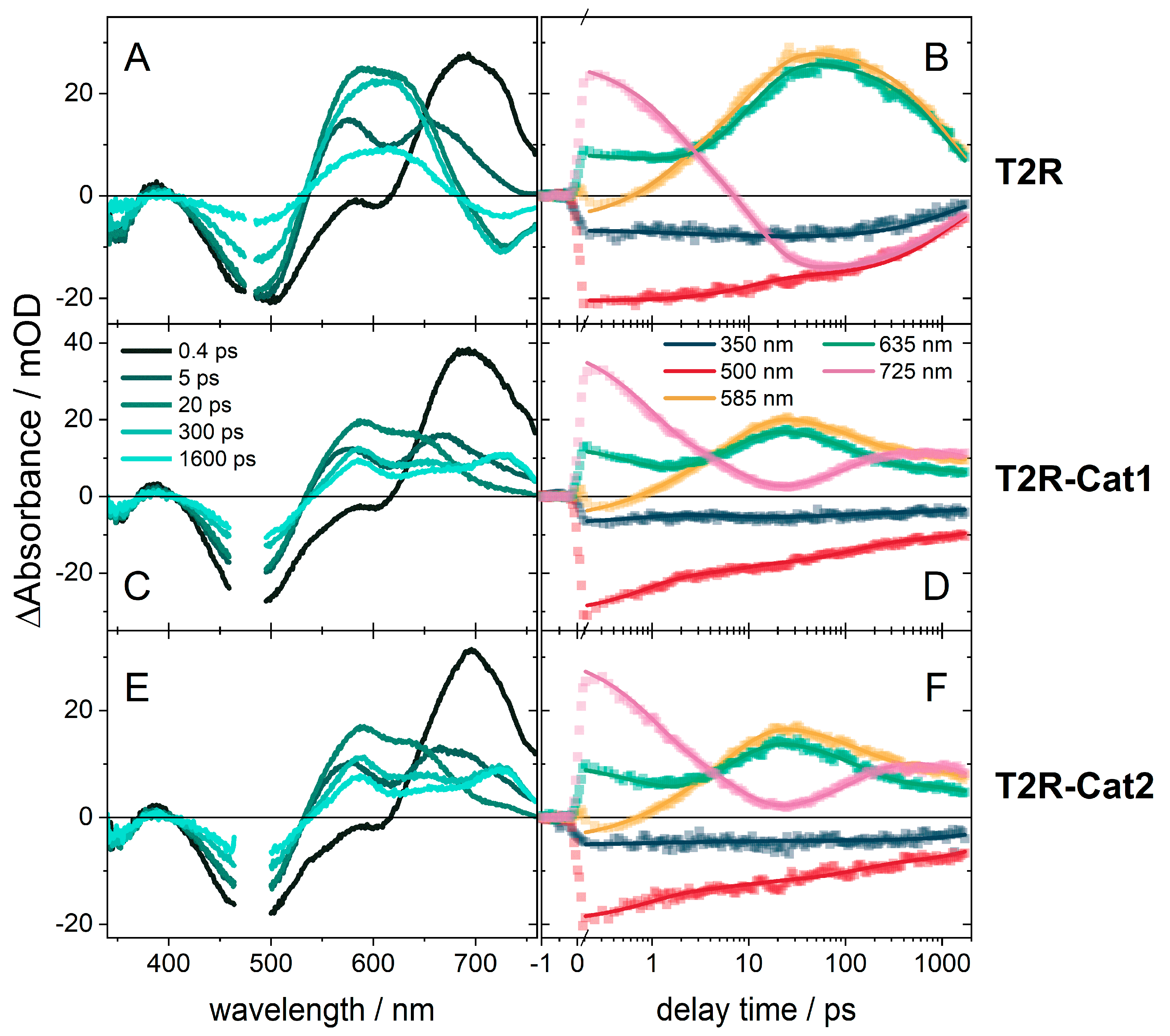
2.2. Transient Absorption (TA) Spectroscopy of T2R
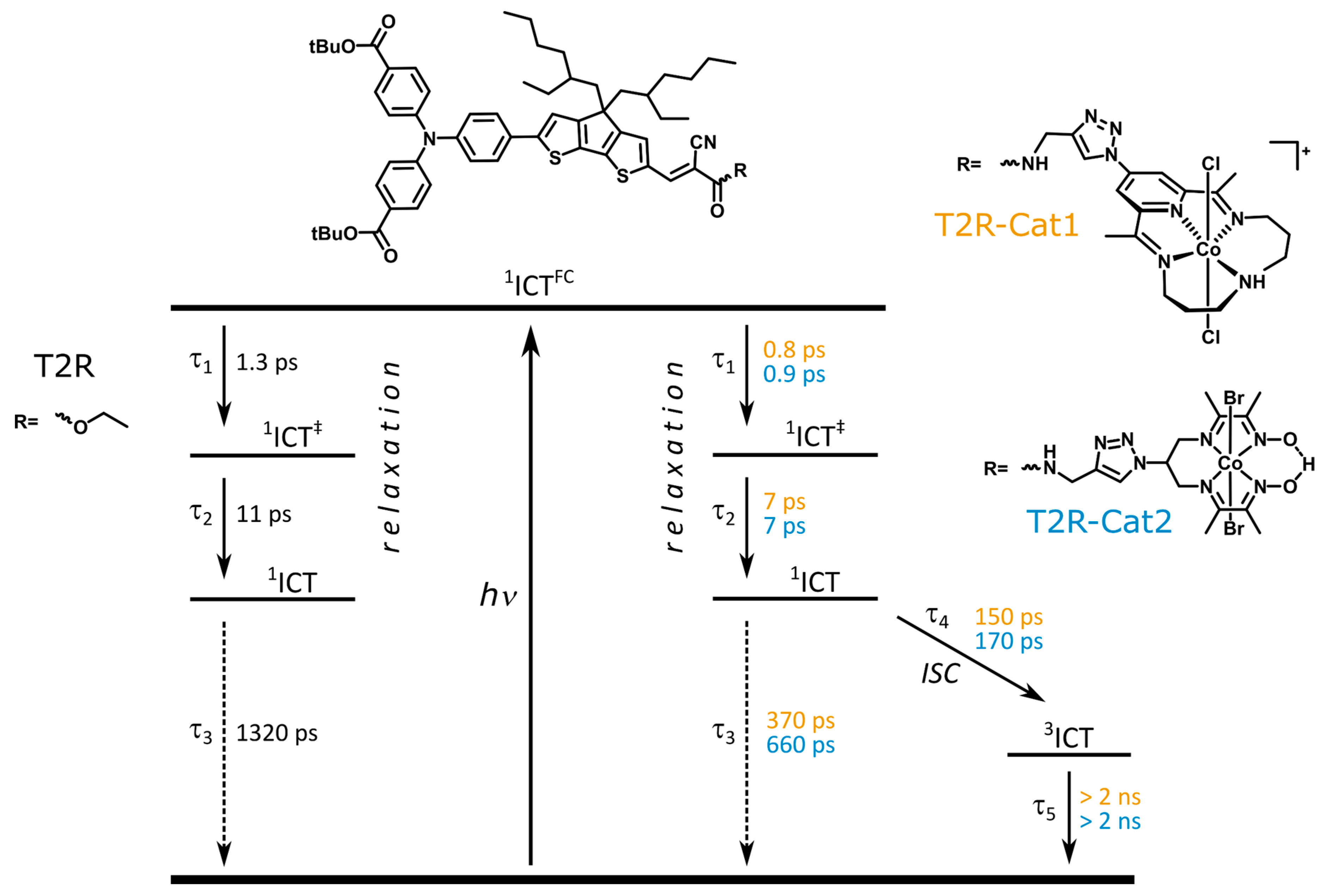
2.3. Transient Absorption Spectroscopy of the Dyads
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Dalle, K.E.; Warnan, J.; Leung, J.J.; Reuillard, B.; Karmel, I.S.; Reisner, E. Electro- and Solar-Driven Fuel Synthesis with First Row Transition Metal Complexes. Chem. Rev. 2019, 119, 2752–2875. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.G.; Schäfer, B.; Smolentsev, G.; Uhlig, J.; Nazarenko, E.; Guthmuller, J.; Kuhnt, C.; Wächtler, M.; Dietzek, B.; Sundström, V.; et al. Palladium versus Platinum: The Metal in the Catalytic Center of a Molecular Photocatalyst Determines the Mechanism of the Hydrogen Production with Visible Light. Angew. Chem. Int. Ed. 2015, 54, 5044–5048. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.L.; Brunschwig, B.S.; Winkler, J.R.; Gray, H.B. Hydrogen Evolution Catalyzed by Cobaloximes. ACC Chem. Res. 2009, 42, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Das, A.K.; Marquard, S.; Farnum, B.H.; Wang, D.; Bullock, R.M.; Meyer, T.J. Photogeneration of hydrogen from water by a robust dye-sensitized photocathode. Energy Environ. Sci. 2016, 9, 3693–3697. [Google Scholar] [CrossRef]
- Mengele, A.K.; Kaufhold, S.; Streb, C.; Rau, S. Generation of a stable supramolecular hydrogen evolving photocatalyst by alteration of the catalytic center. Dalton Trans. 2016, 45, 6612–6618. [Google Scholar] [CrossRef]
- Luo, G.-G.; Pan, Z.-H.; Lin, J.; Sun, D. Tethered sensitizer–catalyst noble-metal-free molecular devices for solar-driven hydrogen generation. Dalton Trans. 2018, 47, 15633–15645. [Google Scholar] [CrossRef]
- Braumüller, M.; Schulz, M.; Sorsche, D.; Pfeffer, M.; Schaub, M.; Popp, J.; Park, B.-W.; Hagfeldt, A.; Dietzek, B.; Rau, S. Synthesis and characterization of an immobilizable photochemical molecular device for H2-generation. Dalton Trans. 2015, 44, 5577–5586. [Google Scholar] [CrossRef]
- Põldme, N.; O’Reilly, L.; Fletcher, I.; Portoles, J.; Sazanovich, I.V.; Towrie, M.; Long, C.; Vos, J.G.; Pryce, M.T.; Gibson, E.A. Photoelectrocatalytic H2 evolution from integrated photocatalysts adsorbed on NiO. Chem. Sci. 2019, 10, 99–112. [Google Scholar] [CrossRef]
- Karnahl, M.; Kuhnt, C.; Ma, F.; Yartsev, A.; Schmitt, M.; Dietzek, B.; Rau, S.; Popp, J. Tuning of Photocatalytic Hydrogen Production and Photoinduced Intramolecular Electron Transfer Rates by Regioselective Bridging Ligand Substitution. ChemPhysChem 2011, 12, 2101–2109. [Google Scholar] [CrossRef]
- Shan, B.; Nayak, A.; Brennaman, M.K.; Liu, M.; Marquard, S.L.; Eberhart, M.S.; Meyer, T.J. Controlling Vertical and Lateral Electron Migration Using a Bifunctional Chromophore Assembly in Dye-Sensitized Photoelectrosynthesis Cells. J. Am. Chem. Soc. 2018, 140, 6493–6500. [Google Scholar] [CrossRef] [PubMed]
- Windle, C.D.; Massin, J.; Chavarot-Kerlidou, M.; Artero, V. A protocol for quantifying hydrogen evolution by dye-sensitized molecular photocathodes and its implementation for evaluating a new covalent architecture based on an optimized dye-catalyst dyad. Dalton Trans. 2018, 47, 10509–10516. [Google Scholar] [CrossRef] [PubMed]
- Kaeffer, N.; Massin, J.; Lebrun, C.; Renault, O.; Chavarot-Kerlidou, M.; Artero, V. Covalent Design for Dye-Sensitized H2 -Evolving Photocathodes Based on a Cobalt Diimine–Dioxime Catalyst. J. Am. Chem. Soc. 2016, 138, 12308–12311. [Google Scholar] [CrossRef] [PubMed]
- McTavish, H. Hydrogen Evolution by Direct Electron Transfer from Photosystem I to Hydrogenases. J. Biochem. 1998, 123, 644–649. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gibson, E.A. Dye-sensitized photocathodes for H2 evolution. Chem. Soc. Rev. 2017, 46, 6194–6209. [Google Scholar] [CrossRef]
- Lyu, S.; Massin, J.; Pavone, M.; Muñoz-García, A.B.; Labrugère, C.; Toupance, T.; Chavarot-Kerlidou, M.; Artero, V.; Olivier, C. H2-Evolving Dye-Sensitized Photocathode Based on a Ruthenium–Diacetylide/Cobaloxime Supramolecular Assembly. ACS Appl. Energy Mater. 2019, 2, 4971–4980. [Google Scholar] [CrossRef]
- Bold, S.; Zedler, L.; Zhang, Y.; Massin, J.; Artero, V.; Chavarot-Kerlidou, M.; Dietzek, B. Electron transfer in a covalent dye–cobalt catalyst assembly–a transient absorption spectroelectrochemistry perspective. Chem. Commun. 2018, 54, 10594–10597. [Google Scholar] [CrossRef]
- Bold, S.; Massin, J.; Giannoudis, E.; Koepf, M.; Artero, V.; Dietzek, B.; Chavarot-Kerlidou, M. Spectroscopic investigations provide a rational for the hydrogen-evolving activity of dye-sensitized photocathodes based on a cobalt tetraazamacrocyclic catalyst. 2020. Submitted for publication. [Google Scholar]
- Kaeffer, N.; Chavarot-Kerlidou, M.; Artero, V. Hydrogen evolution catalyzed by cobalt diimine-dioxime complexes. Acc. Chem. Res. 2015, 48, 1286–1295. [Google Scholar] [CrossRef]
- Varma, S.; Castillo, C.E.; Stoll, T.; Fortage, J.; Blackman, A.G.; Molton, F.; Deronzier, A.; Collomb, M.-N. Efficient photocatalytic hydrogen production in water using a cobalt(III) tetraaza-macrocyclic catalyst: Electrochemical generation of the low-valent Co(I) species and its reactivity toward proton reduction. Phys. Chem. Chem. Phys. 2013, 15, 17544. [Google Scholar] [CrossRef]
- Grau, S.; Schilling, M.; Moonshiram, D.; Benet-Buchholz, J.; Luber, S.; Llobet, A.; Gimbert-Suriñach, C. Electrochemically and Photochemically Induced Hydrogen Evolution Catalysis with Cobalt Tetraazamacrocycles Occurs Through Different Pathways. ChemSusChem 2020, 13, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Bacchi, M.; Berggren, G.; Artero, V. A Systematic Comparative Study of Hydrogen-Evolving Molecular Catalysts in Aqueous Solutions. ChemSusChem 2015, 8, 3632–3638. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.-S.; Chen, W.-T.; Hsu, C.-Y.; Chou, H.-H.; Lin, J.T.; Yeh, M.-C.P. Arylamine-Based Dyes for p-Type Dye-Sensitized Solar Cells. Org. Lett. 2011, 13, 4930–4933. [Google Scholar] [CrossRef]
- Cai, Y.; Meng, L.; Gao, H.; Guo, Z.; Zheng, N.; Xie, Z.; Zhang, H.; Li, C.; Wan, X.; Chen, Y. Achieving organic solar cells with efficiency over 14% based on a non-fullerene acceptor incorporating a cyclopentathiophene unit fused backbone. J. Mater. Chem. A 2020, 8, 5194–5199. [Google Scholar] [CrossRef]
- Fujitsuka, M.; Sato, T.; Sezaki, F.; Tanaka, K.; Watanabe, A.; Ito, O. Laser flash photolysis study on the photoinduced reactions of 3,3′-bridged bithiophenes. J. Chem. Soc. Faraday Trans. 1998, 94, 3331–3337. [Google Scholar] [CrossRef]
- Paa, W.; Yang, J.-P.; Rentsch, S. Intersystem crossing in oligothiophenes studied by fs time-resolved spectroscopy. Appl. Phys. B 2000, 71, 443–449. [Google Scholar] [CrossRef]
- Massin, J.; Bräutigam, M.; Kaeffer, N.; Queyriaux, N.; Field, M.J.; Schacher, F.H.; Popp, J.; Chavarot-Kerlidou, M.; Dietzek, B.; Artero, V. Dye-sensitized PS-b-P2VP-templated nickel oxide films for photoelectrochemical applications. Interface Focus 2015, 5, 20140083. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Appendix A. Properties, Purification, and Use of Organic Solvents. In Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; Volume 16, pp. 549–586. ISBN 9783527324736. [Google Scholar]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. 9-Solvent Properties. In Handbook of Photochemistry; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2006; Volume 3, pp. 535–559. [Google Scholar]
- Ishow, E.; Guillot, R.; Buntinx, G.; Poizat, O. Photoinduced intramolecular charge-transfer dynamics of a red-emitting dicyanovinyl-based triarylamine dye in solution. J. Photochem. Photobiol. A Chem. 2012, 234, 27–36. [Google Scholar] [CrossRef]
- Ishow, E.; Clavier, G.; Miomandre, F.; Rebarz, M.; Buntinx, G.; Poizat, O. Comprehensive investigation of the excited-state dynamics of push–pull triphenylamine dyes as models for photonic applications. Phys. Chem. Chem. Phys. 2013, 15, 13922. [Google Scholar] [CrossRef]
- Flender, O.; Scholz, M.; Klein, J.R.; Oum, K.; Lenzer, T. Excited-state relaxation of the solar cell dye D49 in organic solvents and on mesoporous Al2O3 and TiO2 thin films. Phys. Chem. Chem. Phys. 2016, 18, 26010–26019. [Google Scholar] [CrossRef]
- Oum, K.; Lohse, P.W.; Klein, J.R.; Flender, O.; Scholz, M.; Hagfeldt, A.; Boschloo, G.; Lenzer, T. Photoinduced ultrafast dynamics of the triphenylamine-based organic sensitizer D35 on TiO2, ZrO2 and in acetonitrile. Phys. Chem. Chem. Phys. 2013, 15, 3906. [Google Scholar] [CrossRef] [PubMed]
- Massin, J.; Bräutigam, M.; Bold, S.; Wächtler, M.; Pavone, M.; Muñoz-García, A.B.; Dietzek, B.; Artero, V.; Chavarot-Kerlidou, M. Investigating Light-Driven Hole Injection and Hydrogen Evolution Catalysis at Dye-Sensitized NiO Photocathodes: A Combined Experimental–Theoretical Study. J. Phys. Chem. C 2019, 123, 17176–17184. [Google Scholar] [CrossRef]
- Muresan, N.M.; Willkomm, J.; Mersch, D.; Vaynzof, Y.; Reisner, E. Immobilization of a Molecular Cobaloxime Catalyst for Hydrogen Evolution on a Mesoporous Metal Oxide Electrode. Angew. Chem. Int. Ed. 2012, 51, 12749–12753. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, S.; Yang, J.P.; Paa, W.; Birckner, E.; Schiedt, J.; Weinkauf, R. Size dependence of triplet and singlet states of α-oligothiophenes. Phys. Chem. Chem. Phys. 1999, 1, 1707–1714. [Google Scholar] [CrossRef]
- Grebner, D.; Helbig, M.; Rentsch, S. Size-Dependent Properties of Oligothiophenes by Picosecond Time-Resolved Spectroscopy. J. Phys. Chem. 1995, 99, 16991–16998. [Google Scholar] [CrossRef]
- Paa, W.; Yang, J.P.; Helbig, M.; Hein, J.; Rentsch, S. Femtosecond time-resolved measurements of terthiophene: Fast singlet-triplet intersystem crossing. Chem. Phys. Lett. 1998, 292, 607–614. [Google Scholar] [CrossRef]
- Janssen, R.A.J.; Smilowitz, L.; Sariciftci, N.S.; Moses, D. Triplet-state photoexcitations of oligothiophene films and solutions. J. Chem. Phys. 1994, 101, 1787–1798. [Google Scholar] [CrossRef]
- Volchkov, V.V.; Ivanov, V.L.; Uzhinov, B.M. Induced intersystem crossing at the fluorescence quenching of laser dye 7-amino-1,3-naphthalenedisulfonic acid by paramagnetic metal ions. J. Fluoresc. 2010, 20, 299–303. [Google Scholar] [CrossRef]
- Takashima, H.; Kawahara, H.; Kitano, M.; Shibata, S.; Murakami, H.; Tsukahara, K. Metal ion-dependent fluorescent dynamics of photoexcited zinc-porphyrin and zinc-myoglobin modified with ethylenediaminetetraacetic acid. J. Phys. Chem. B 2008, 112, 15493–15502. [Google Scholar] [CrossRef]
- Ji, S.; Zhao, J.; Donato, M.D.; Xing, Y.H.; Mahmood, Z.; Taddei, M.; Rehmat, N.; Bussotti, L.; Doria, S.; Guan, Q.; et al. Color-tunable delayed fluorescence and efficient spin−orbit charge transfer intersystem crossing in compact carbazole-anthracene-BODIPY triads employing the sequential electron transfer approach. J. Phys. Chem. C 2020, 124, 5944–5957. [Google Scholar] [CrossRef]
- Weiss, E.A.; Ratner, M.A.; Wasielewski, M.R. Direct measurement of singlet-triplet splitting within rodlike photogenerated radical ion pairs using magnetic field effects: Estimation of the electronic coupling for charge recombination. J. Phys. Chem. A 2003, 107, 3639–3647. [Google Scholar] [CrossRef]
- Dance, Z.E.X.; Mi, Q.; McCamant, D.W.; Ahrens, M.J.; Ratner, M.A.; Wasielewski, M.R. Time-resolved EPR studies of photogenerated radical ion pairs separated by p-phenylene oligomers and of triplet states resulting from charge recombination. J. Phys. Chem. B 2006, 110, 25163–25173. [Google Scholar] [CrossRef] [PubMed]
- Colvin, M.T.; Ricks, A.B.; Scott, A.M.; Co, D.T.; Wasielewski, M.R. Intersystem crossing involving strongly spin exchange-coupled radical ion pairs in donor-bridge-acceptor molecules. J. Phys. Chem. A 2012, 116, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhao, J.; Sun, J.; Guo, S. Light-harvesting fullerene dyads as organic triplet photosensitizers for triplet-triplet annihilation upconversions. J. Org. Chem. 2012, 77, 5305–5312. [Google Scholar] [CrossRef]
- Huang, L.; Cui, X.; Therrien, B.; Zhao, J. Energy-funneling-based broadband visible-light-absorbing Bodipy-C 60 triads and tetrads as dual functional heavy-atom-free organic triplet photosensitizers for photocatalytic organic reactions. Chem. A Eur. J. 2013, 19, 17472–17482. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhao, J.; Wu, W.; Yi, X.; Yang, P.; Ma, J. Visible-light-harvesting triphenylamine ethynyl C60-BODIPY dyads as heavy-atom-free organic triplet photosensitizers for triplet-triplet annihilation Upconversion. Asian J. Org. Chem. 2012, 1, 264–273. [Google Scholar] [CrossRef]
- Marroux, H.J.B.; Curchod, B.F.E.; Faradji, C.A.; Shuttleworth, T.A.; Sparkes, H.A.; Pringle, P.G.; Orr-Ewing, A.J. Spin Changes Accompany Ultrafast Structural Interconversion in the Ground State of a Cobalt Nitrosyl Complex. Angew. Chem. Int. Ed. 2017, 56, 13713–13716. [Google Scholar] [CrossRef]
- McCusker, J.K.; Walda, K.N.; Magde, D.; Hendrickson, D.N. Picosecond Excited-State Dynamics in Octahedral Cobalt(III) Complexes: Intersystem Crossing versus Internal Conversion. Inorg. Chem. 1993, 32, 394–399. [Google Scholar] [CrossRef]
- Jacques, P.-A.; Artero, V.; Pecaut, J.; Fontecave, M. Cobalt and nickel diimine-dioxime complexes as molecular electrocatalysts for hydrogen evolution with low overvoltages. Proc. Natl. Acad. Sci. USA 2009, 106, 20627–20632. [Google Scholar] [CrossRef]
- Dietzek, B.; Pascher, T.; Sundström, V.; Yartsev, A. Appearance of coherent artifact signals in femtosecond transient absorption spectroscopy in dependence on detector design. Laser Phys. Lett. 2007, 4, 38–43. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Runge, E.; Gross, E.K.U. Density-Functional Theory for Time-Dependent Systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the first row transition elements. J. Chem. Phys. 1987, 86, 866–872. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; He, G.; Zhu, H.; Wang, X.; Guo, Q.; Xia, A.; Lin, Y.; Wang, J.; Zhan, X. Influence of Thiophene Moiety on the Excited State Properties of Push–Pull Chromophores. J. Phys. Chem. C 2016, 120, 13922–13930. [Google Scholar] [CrossRef]
- Labrunie, A.; Gorenflot, J.; Babics, M.; Alévêque, O.; Dabos-Seignon, S.; Balawi, A.H.; Kan, Z.; Wohlfahrt, M.; Levillain, E.; Hudhomme, P.; et al. Triphenylamine-Based Push–Pull σ–C 60 Dyad As Photoactive Molecular Material for Single-Component Organic Solar Cells: Synthesis, Characterizations, and Photophysical Properties. Chem. Mater. 2018, 30, 3474–3485. [Google Scholar] [CrossRef]
- Oum, K.; Flender, O.; Lohse, P.W.; Scholz, M.; Hagfeldt, A.; Boschloo, G.; Lenzer, T. Electron and hole transfer dynamics of a triarylamine-based dye with peripheral hole acceptors on TiO2 in the absence and presence of solvent. Phys. Chem. Chem. Phys. 2014, 16, 8019. [Google Scholar] [CrossRef]
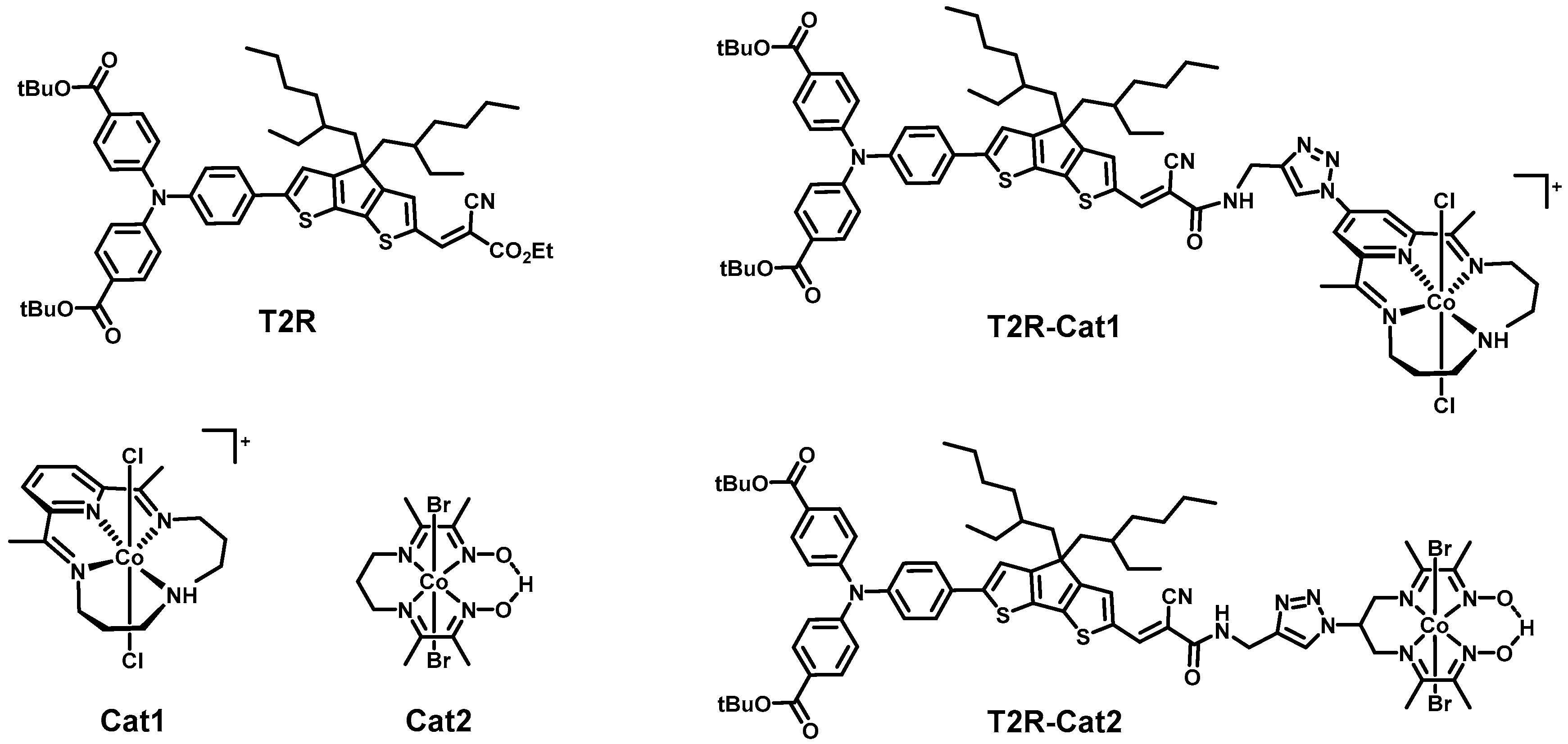
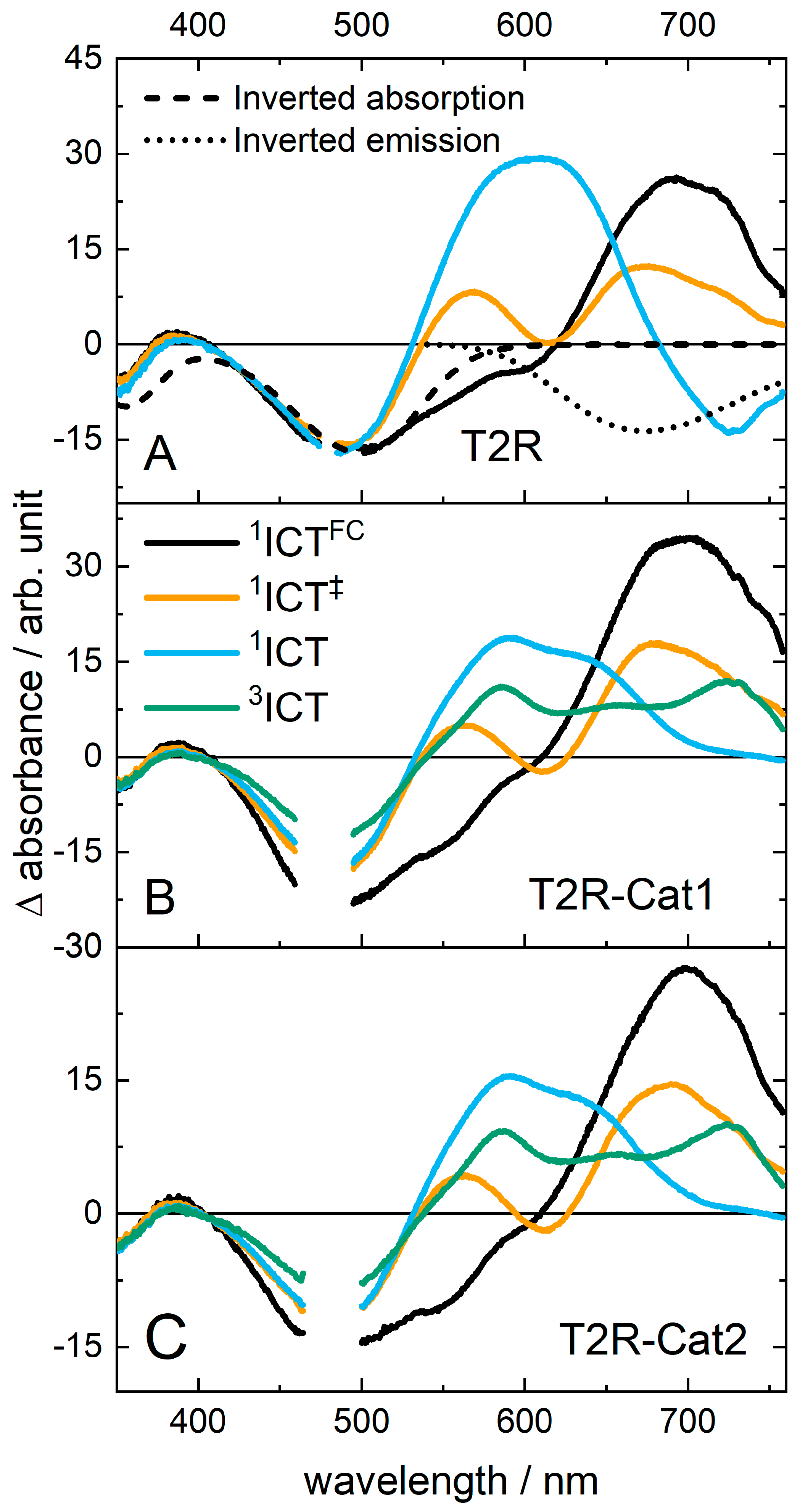
| - | Time Constants1/ps | ||||
|---|---|---|---|---|---|
| τ1 | τ2 | τ3 | τ4 | τ5 | |
| T2R | 1.3 | 11 | 1320 | ||
| T2R-Cat1 | 0.8 | 7 | 370 | 150 | >> 2 ns |
| T2R-Cat2 | 0.9 | 7 | 660 | 170 | >> 2 ns |
| T2R-A | T2R-B | T2R-C | T2R-D | |
|---|---|---|---|---|
| ∆E/eV | 0.014 | 0.107 | 0.084 | 0.0 |
| λ(ICT)/nm | 473 (1.793) | 472 (1.784) | 471 (1.900) | 472 (1.929) |
| λ(TPA)/nm | 331 (0.618) | 331 (0.619) | 331 (0.615) | 331 (0.616) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bold, S.; Straistari, T.; Muñoz-García, A.B.; Pavone, M.; Artero, V.; Chavarot-Kerlidou, M.; Dietzek, B. Investigating Light-Induced Processes in Covalent Dye-Catalyst Assemblies for Hydrogen Production. Catalysts 2020, 10, 1340. https://doi.org/10.3390/catal10111340
Bold S, Straistari T, Muñoz-García AB, Pavone M, Artero V, Chavarot-Kerlidou M, Dietzek B. Investigating Light-Induced Processes in Covalent Dye-Catalyst Assemblies for Hydrogen Production. Catalysts. 2020; 10(11):1340. https://doi.org/10.3390/catal10111340
Chicago/Turabian StyleBold, Sebastian, Tatiana Straistari, Ana B. Muñoz-García, Michele Pavone, Vincent Artero, Murielle Chavarot-Kerlidou, and Benjamin Dietzek. 2020. "Investigating Light-Induced Processes in Covalent Dye-Catalyst Assemblies for Hydrogen Production" Catalysts 10, no. 11: 1340. https://doi.org/10.3390/catal10111340
APA StyleBold, S., Straistari, T., Muñoz-García, A. B., Pavone, M., Artero, V., Chavarot-Kerlidou, M., & Dietzek, B. (2020). Investigating Light-Induced Processes in Covalent Dye-Catalyst Assemblies for Hydrogen Production. Catalysts, 10(11), 1340. https://doi.org/10.3390/catal10111340





