Characterization of Anaerobic Biofilms Growing on Carbon Felt Bioanodes Exposed to Air
Abstract
1. Introduction
2. Results and Discussion
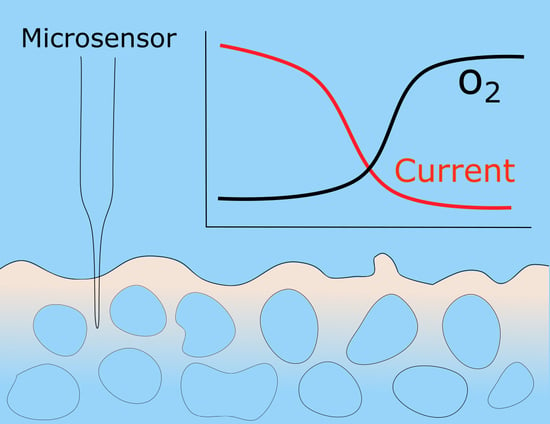
2.1. The Interplay between DO and CD
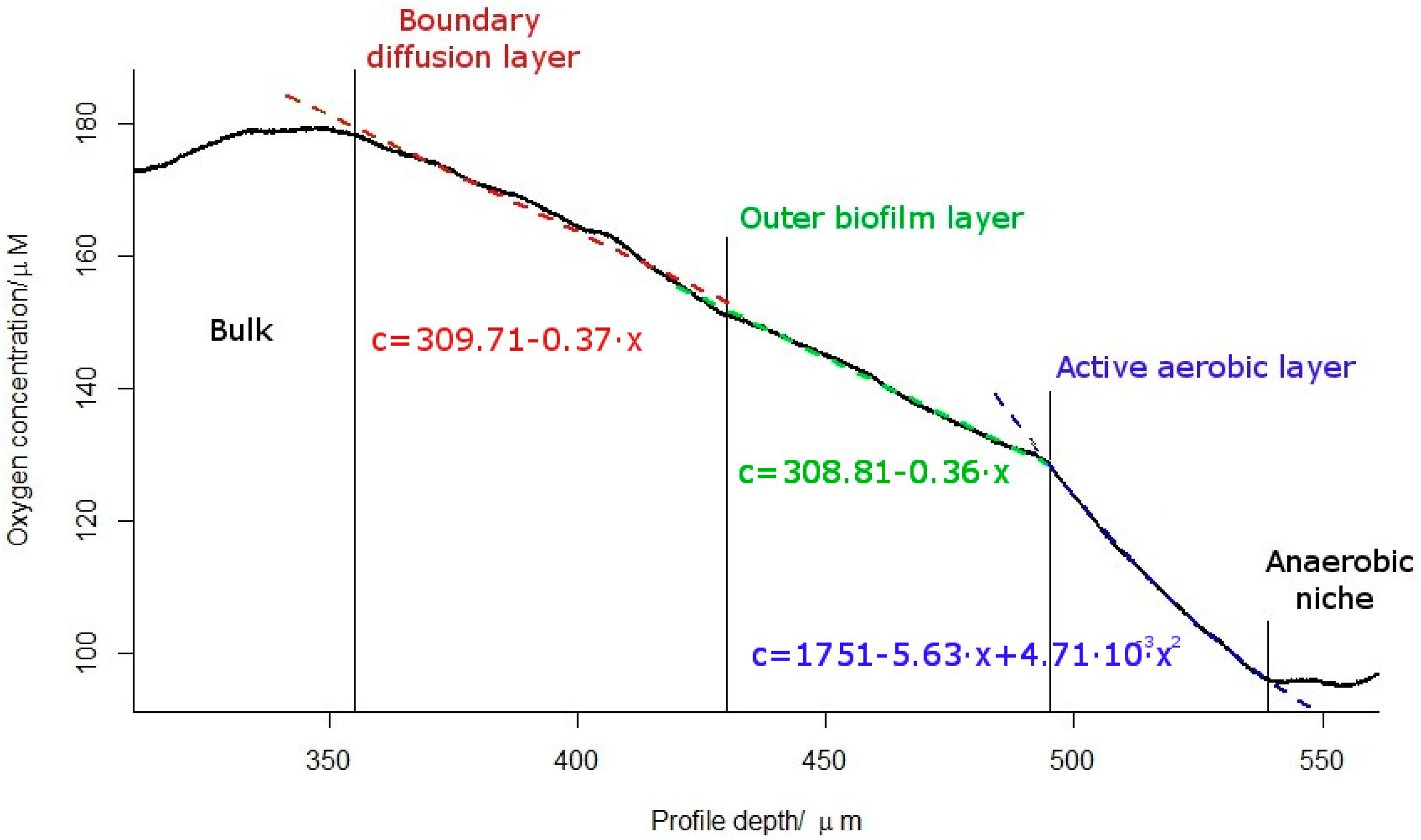
2.2. Oxygen Concentration Profile within the Biofilm
2.3. The Biofilm under Substrate Limited Conditions
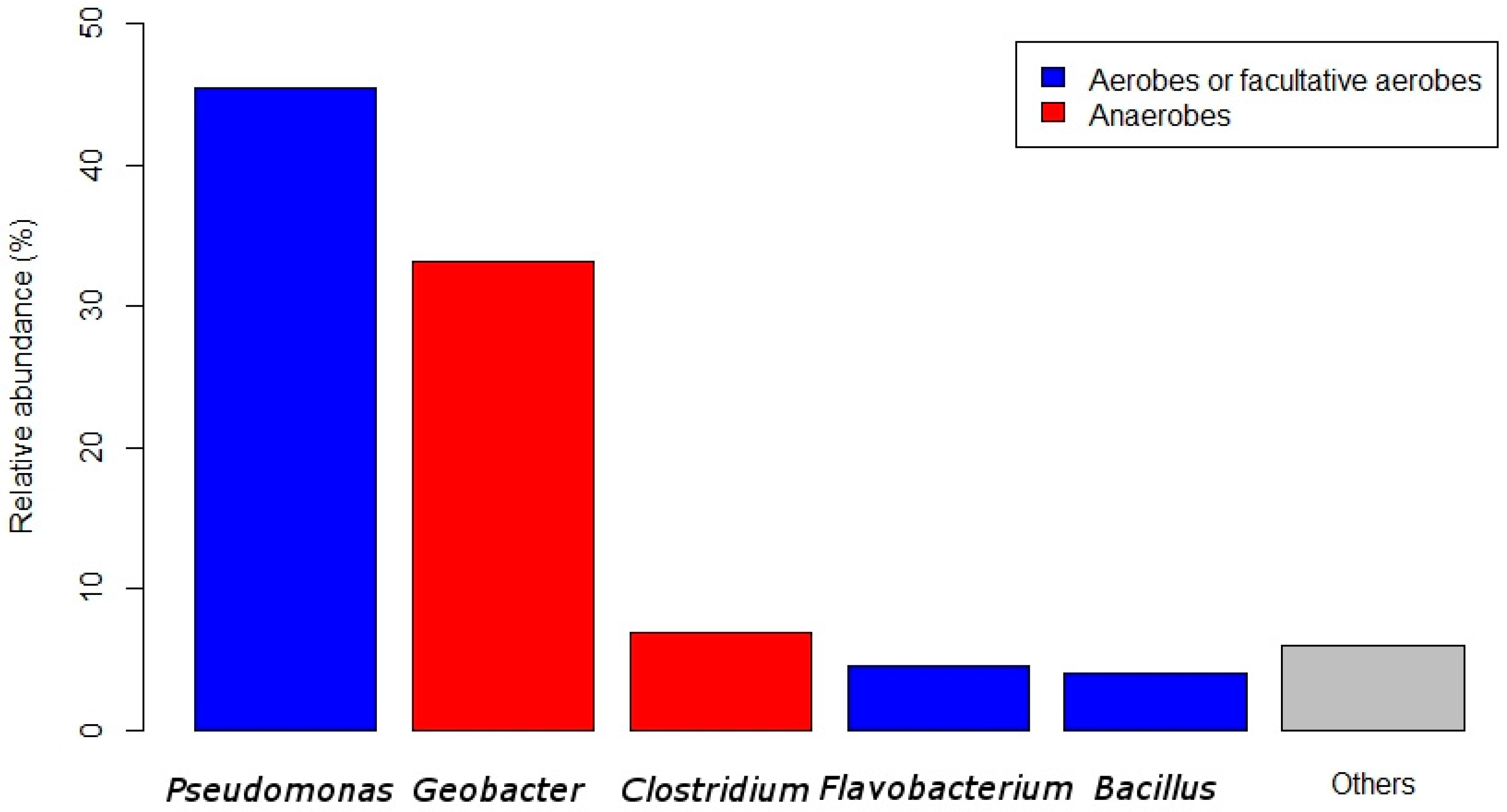
2.4. Biofilm Composition
3. Experimental
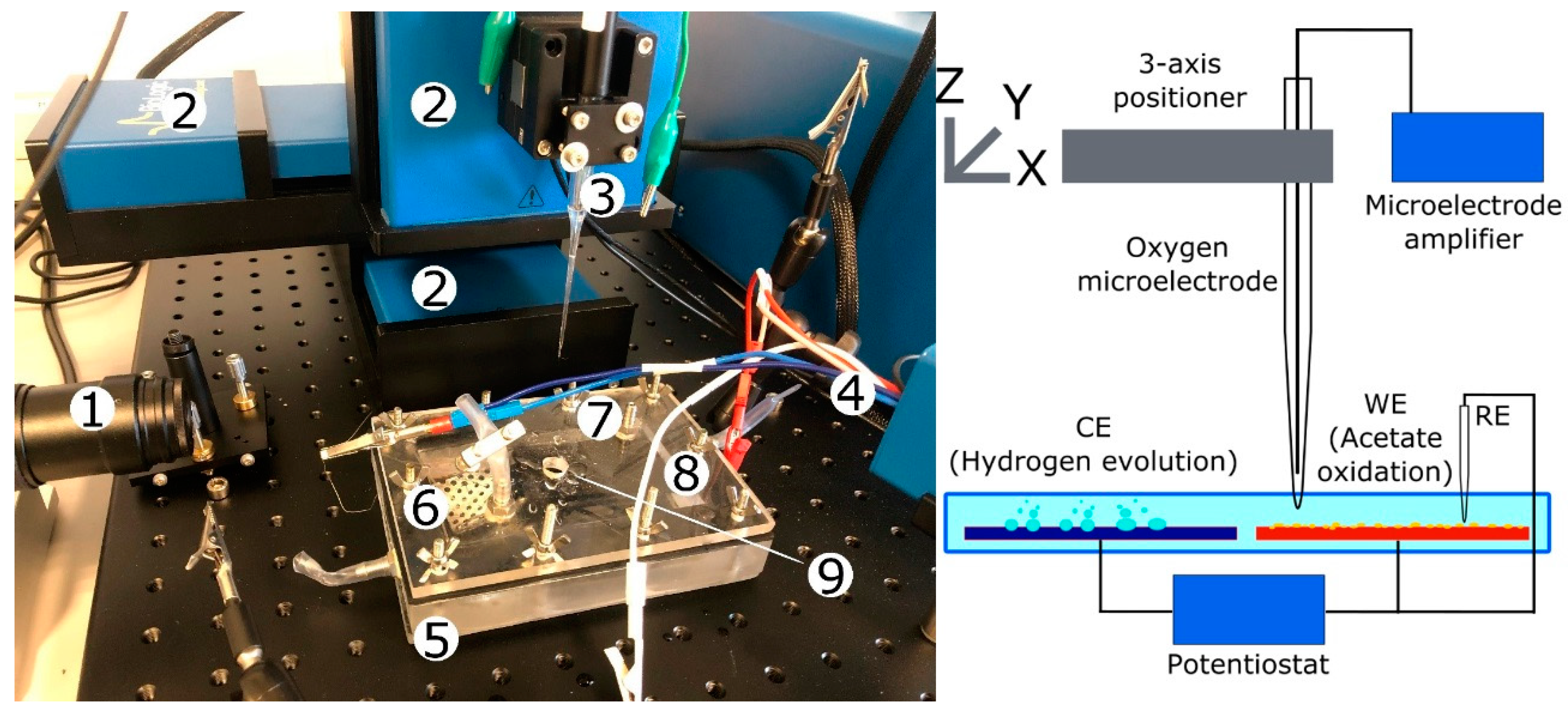
3.1. Reactor Set-Up and Biofilm Growth
3.2. DO Concentration Measurement
3.3. Experimental Procedure
3.4. Microbial Community Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Escapa, A.; Mateos, R.; Martínez, E.J.; Blanes, J. Microbial electrolysis cells: An emerging technology for wastewater treatment and energy recovery. From laboratory to pilot plant and beyond. Renew. Sustain. Energy Rev. 2016, 55, 942–956. [Google Scholar] [CrossRef]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Harnisch, F. Is there a specific ecological niche for electroactive microorganisms? ChemElectroChem 2016, 3, 1282–1295. [Google Scholar] [CrossRef]
- Wang, C.; Shen, J.; Chen, Q.; Ma, D.; Zhang, G.; Cui, C.; Xin, Y.; Zhao, Y.; Hu, C. The inhibiting effect of oxygen diffusion on the electricity generation of three-chamber microbial fuel cells. J. Power Sources 2020, 453, 227889. [Google Scholar] [CrossRef]
- Yang, L.-H.; Zhu, T.-T.; Cai, W.-W.; Haider, M.R.; Wang, H.-C.; Cheng, H.-Y.; Wang, A.-J. Micro-oxygen bioanode: An efficient strategy for enhancement of phenol degradation and current generation in mix-cultured MFCs. Bioresour. Technol. 2018, 268, 176–182. [Google Scholar] [CrossRef]
- Yong, X.-Y.; Yan, Z.-Y.; Shen, H.-B.; Zhou, J.; Wu, X.-Y.; Zhang, L.-J.; Zheng, T.; Jiang, M.; Wei, P.; Jia, H.-H.; et al. An integrated aerobic-anaerobic strategy for performance enhancement of Pseudomonas aeruginosa-inoculated microbial fuel cell. Bioresour. Technol. 2017, 241, 1191–1196. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, S.; Wang, X.; Huang, X.; Logan, B.E. Separator characteristics for increasing performance of microbial fuel cells. Environ. Sci. Technol. 2009, 43, 8456–8461. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Joshi, Y.; Rawat, K.; Bisht, R. Biofilms: Architecture, resistance, quorum sensing and control mechanisms. Indian J. Microbiol. 2019, 59, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, Z.; Beyenal, H. Fundamentals of Biofilm Research; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Beyenal, H.; Babauta, J.T. Biofilms in Bioelectrochemical Systems: From Laboratory Practice to Data Interpretation; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Maegaard, K.; Garcia-Robledo, E.; Kofoed, M.V.W.; Agneessens, L.M.; de Jonge, N.; Nielsen, J.L.; Ottosen, L.D.M.; Nielsen, L.P.; Revsbech, N.P. Biogas upgrading with hydrogenotrophic methanogenic biofilms. Bioresour. Technol. 2019, 287, 121422. [Google Scholar] [CrossRef]
- Atci, E.; Babauta, J.T.; Sultana, S.T.; Beyenal, H. Microbiosensor for the detection of acetate in electrode-respiring biofilms. Biosens. Bioelectron. 2016, 81, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Wahman, D.G.; Pressman, J.G. Amperometric carbon fiber nitrite microsensor for in situ biofilm monitoring. Sens. Actuators B Chem. 2013, 188, 1263–1269. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, X.; Yan, Y.; Li, T.; An, J.; Liao, C.; Li, N.; Wang, X. Electrode potential regulates phenol degradation pathways in oxygen-diffused microbial electrochemical system. Chem. Eng. J. 2020, 381, 122663. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, H.; Chen, L.; Xiao, Y.; Zhao, F. In situ measurements of dissolved oxygen, pH and redox potential of biocathode microenvironments using microelectrodes. Bioresour. Technol. 2013, 132, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Montpart, N.; Rago, L.; Baeza, J.A.; Guisasola, A. Oxygen barrier and catalytic effect of the cathodic biofilm in single chamber microbial fuel cells. J. Chem. Technol. Biotechnol. 2018, 93, 2199–2207. [Google Scholar] [CrossRef]
- San-Martín, M.I. Bioelectrochemical Systems for Energy Valorization of Waste Streams; Leicester, D.D., Ed.; IntechOpen: Rijeka, Yugoslavia, 2018; Chapter 8; ISBN 978-1-78923-711-5. [Google Scholar]
- Qu, Y.; Feng, Y.; Wang, X.; Logan, B.E. Use of a coculture to enable current production by geobacter sulfurreducens. Appl. Environ. Microbiol. 2012, 78, 3484–3487. [Google Scholar] [CrossRef]
- Kragh, K.N.; Hutchison, J.B.; Melaugh, G.; Rodesney, C.; Roberts, A.E.L.; Irie, Y.; Jensen, P.Ø.; Diggle, S.P.; Allen, R.J.; Gordon, V.; et al. Role of multicellular aggregates in biofilm formation. MBio 2016, 7, e00237-16. [Google Scholar] [CrossRef]
- Lewandowski, Z.; Walser, G.; Characklis, W.G. Reaction kinetics in biofilms. Biotechnol. Bioeng. 1991, 38, 877–882. [Google Scholar] [CrossRef]
- De Beer, D.; Stoodley, P.; Roe, F.; Lewandowski, Z. Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol. Bioeng. 1994, 43, 1131–1138. [Google Scholar] [CrossRef]
- Mclamore, E.S.; Zhang, W.; Porterfield, D.M.; Banks, M.K. Membrane-aerated biofilm proton and oxygen flux during chemical toxin exposure. Environ. Sci. Technol. 2010, 44, 7050–7057. [Google Scholar] [CrossRef]
- Berg, P.; Risgaard-Petersen, N.; Rysgaard, S. Interpretation of measured concentration profiles in sediment pore water. Limnol. Oceanogr. 1998, 43, 1500–1510. [Google Scholar] [CrossRef]
- Ning, Y.-F.; Chen, Y.-P.; Shen, Y.; Zeng, N.; Liu, S.-Y.; Guo, J.-S.; Fang, F. A new approach for estimating aerobic–anaerobic biofilm structure in wastewater treatment via dissolved oxygen microdistribution. Chem. Eng. J. 2014, 255, 171–177. [Google Scholar] [CrossRef]
- Stewart, P.S. Diffusion in biofilms. J. Bacteriol. 2003, 185, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G.; Kashefi, K. The electrifying physiology of Geobacter bacteria, 30 years on. Adv. Microb. Physiol. 2019, 74, 1–96. [Google Scholar]
- Sun, D.; Chen, J.; Huang, H.; Liu, W.; Ye, Y.; Cheng, S. The effect of biofilm thickness on electrochemical activity of geobacter sulfurreducens. Int. J. Hydrogen Energy 2016, 41. [Google Scholar] [CrossRef]
- Sun, D.; Cheng, S.; Wang, A.; Li, F.; Logan, B.E.; Cen, K. Temporal-spatial changes in viabilities and electrochemical properties of anode biofilms. Environ. Sci. Technol. 2015, 49, 5227–5235. [Google Scholar] [CrossRef]
- Banerjee, R.; Bevilacqua, N.; Eifert, L.; Zeis, R. Characterization of carbon felt electrodes for vanadium redox flow batteries—A pore network modeling approach. J. Energy Storage 2019, 21, 163–171. [Google Scholar] [CrossRef]
- Döner, A.; Karcı, İ.; Kardaş, G. Effect of C-felt supported Ni, Co and NiCo catalysts to produce hydrogen. Int. J. Hydrogen Energy 2012, 37, 9470–9476. [Google Scholar] [CrossRef]
- González, Z.; Sánchez, A.; Blanco, C.; Granda, M.; Menéndez, R.; Santamaría, R. Enhanced performance of a Bi-modified graphite felt as the positive electrode of a vanadium redox flow battery. Electrochem. Commun. 2011, 13, 1379–1382. [Google Scholar] [CrossRef]
- Chong, P.; Erable, B.; Bergel, A. Effect of pore size on the current produced by 3-dimensional porous microbial anodes: A critical review. Bioresour. Technol. 2019, 289, 121641. [Google Scholar] [CrossRef]
- Blanchet, E.; Erable, B.; De Solan, M.-L.; Bergel, A. Two-dimensional carbon cloth and three-dimensional carbon felt perform similarly to form bioanode fed with food waste. Electrochem. Commun. 2016. [Google Scholar] [CrossRef]
- Brunschweiger, S.; Ojong, E.T.; Weisser, J.; Schwaferts, C.; Elsner, M.; Ivleva, N.P.; Haseneder, R.; Hofmann, T.; Glas, K. The effect of clogging on the long-term stability of different carbon fiber brushes in microbial fuel cells for brewery wastewater treatment. Bioresour. Technol. Rep. 2020, 11, 100420. [Google Scholar] [CrossRef]
- Flexer, V.; Jourdin, L. Purposely designed hierarchical porous electrodes for high rate microbial electrosynthesis of acetate from carbon dioxide. Acc. Chem. Res. 2020, 53, 311–321. [Google Scholar] [CrossRef]
- Ma, P.; Ma, H.; Galia, A.; Sabatino, S.; Scialdone, O. Reduction of oxygen to H2O2 at carbon felt cathode in undivided cells. Effect of the ratio between the anode and the cathode surfaces and of other operative parameters. Sep. Purif. Technol. 2019, 208, 116–122. [Google Scholar] [CrossRef]
- Smith, R.E.G.; Davies, T.J.; Baynes, N.d.B.; Nichols, R.J. The electrochemical characterisation of graphite felts. J. Electroanal. Chem. 2015, 747, 29–38. [Google Scholar] [CrossRef]
- Marozava, S.; Röling, W.F.M.; Seifert, J.; Küffner, R.; von Bergen, M.; Meckenstock, R.U. Physiology of geobacter metallireducens under excess and limitation of electron donors. Part II. Mimicking environmental conditions during cultivation in retentostats. Syst. Appl. Microbiol. 2014, 37, 287–295. [Google Scholar] [CrossRef][Green Version]
- Lin, B.; Westerhoff, H.V.; Röling, W.F.M. How geobacteraceae may dominate subsurface biodegradation: Physiology of geobacter metallireducens in slow-growth habitat-simulating retentostats. Environ. Microbiol. 2009, 11, 2425–2433. [Google Scholar] [CrossRef]
- Commault, A.S.; Barrière, F.; Lapinsonnière, L.; Lear, G.; Bouvier, S.; Weld, R.J. Influence of inoculum and anode surface properties on the selection of Geobacter-dominated biofilms. Bioresour. Technol. 2015, 195, 265–272. [Google Scholar] [CrossRef]
- Nevin, K.P.; Zhang, P.; Franks, A.E.; Woodard, T.L.; Lovley, D.R. Anaerobes unleashed: Aerobic fuel cells of Geobacter sulfurreducens. J. Power Sources 2011, 196, 7514–7518. [Google Scholar] [CrossRef]
- Peix, A.; Ramírez-Bahena, M.-H.; Velázquez, E. Historical evolution and current status of the taxonomy of genus Pseudomonas. Infect. Genet. Evol. 2009, 9, 1132–1147. [Google Scholar] [CrossRef]
- Su, W.; Zhang, L.; Li, D.; Zhan, G.; Qian, J.; Tao, Y. Dissimilatory nitrate reduction by Pseudomonas alcaliphila with an electrode as the sole electron donor. Biotechnol. Bioeng. 2012, 109, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.W.; Ross, D.E.; Fichot, E.B.; Norman, R.S.; May, H.D. Electrosynthesis of commodity chemicals by an autotrophic microbial community. Appl. Environ. Microbiol. 2012, 78, 8412–8420. [Google Scholar] [CrossRef] [PubMed]
- Revsbech, N.P. An oxygen microsensor with a guard cathode. Limnol. Oceanogr. 1989, 34, 474–478. [Google Scholar] [CrossRef]
- Stewart, P.S. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol. Bioeng. 1998, 59, 261–272. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Reeder, J.; Knight, R. Rapidly denoising pyrosequencing amplicon reads by exploiting rank-abundance distributions. Nat. Methods 2010, 7, 668–669. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso, R.M.; Pelaz, G.; San-Martín, M.I.; Morán, A.; Escapa, A. Characterization of Anaerobic Biofilms Growing on Carbon Felt Bioanodes Exposed to Air. Catalysts 2020, 10, 1341. https://doi.org/10.3390/catal10111341
Alonso RM, Pelaz G, San-Martín MI, Morán A, Escapa A. Characterization of Anaerobic Biofilms Growing on Carbon Felt Bioanodes Exposed to Air. Catalysts. 2020; 10(11):1341. https://doi.org/10.3390/catal10111341
Chicago/Turabian StyleAlonso, Raúl M., Guillermo Pelaz, María Isabel San-Martín, Antonio Morán, and Adrián Escapa. 2020. "Characterization of Anaerobic Biofilms Growing on Carbon Felt Bioanodes Exposed to Air" Catalysts 10, no. 11: 1341. https://doi.org/10.3390/catal10111341
APA StyleAlonso, R. M., Pelaz, G., San-Martín, M. I., Morán, A., & Escapa, A. (2020). Characterization of Anaerobic Biofilms Growing on Carbon Felt Bioanodes Exposed to Air. Catalysts, 10(11), 1341. https://doi.org/10.3390/catal10111341