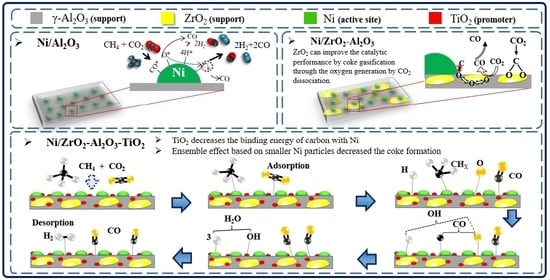
Preparation and Characterization of Ni/ZrTiAlOx Catalyst via Sol-Gel and Impregnation Methods for Low Temperature Dry Reforming of Methane
Abstract
1. Introduction
2. Results and Discussion
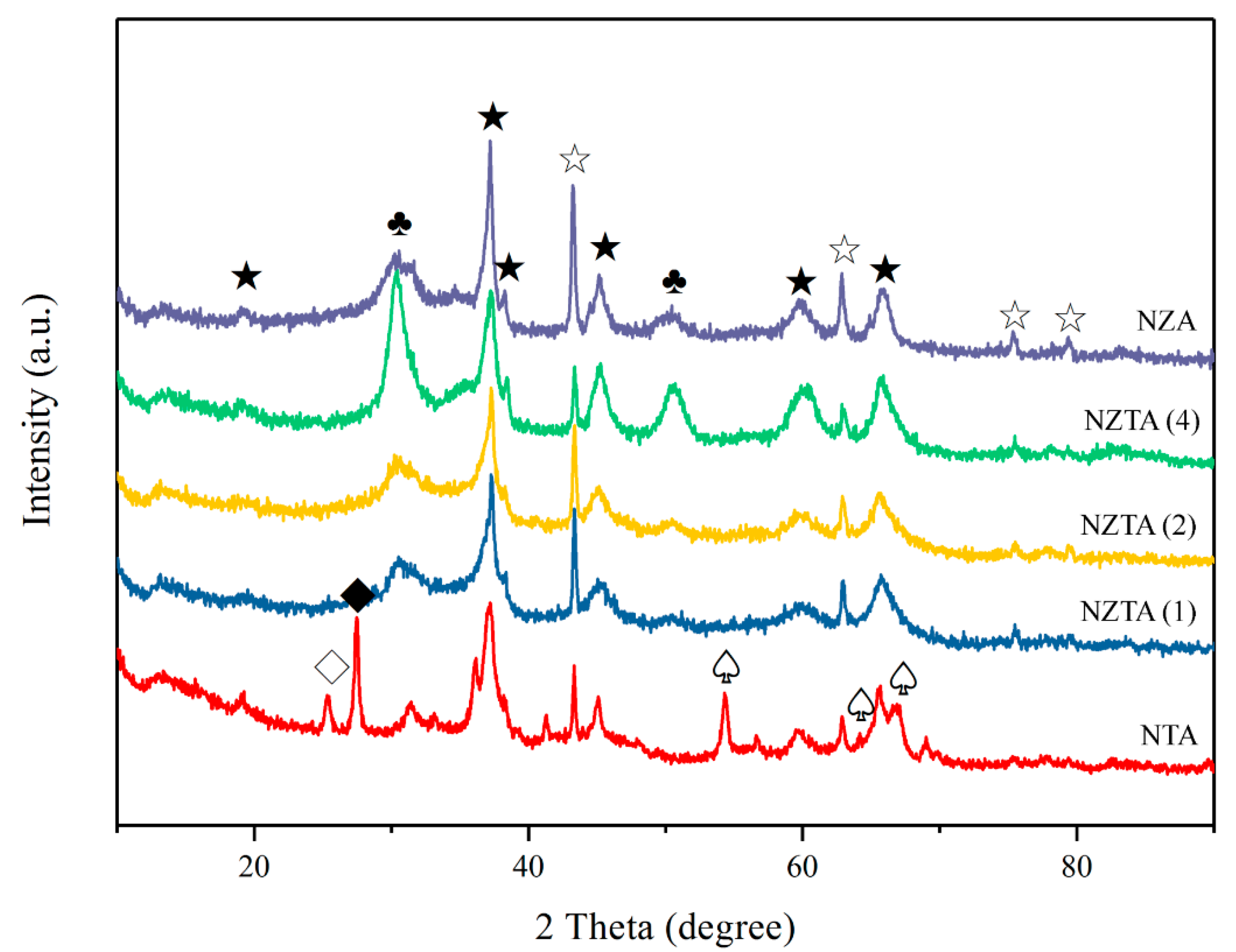
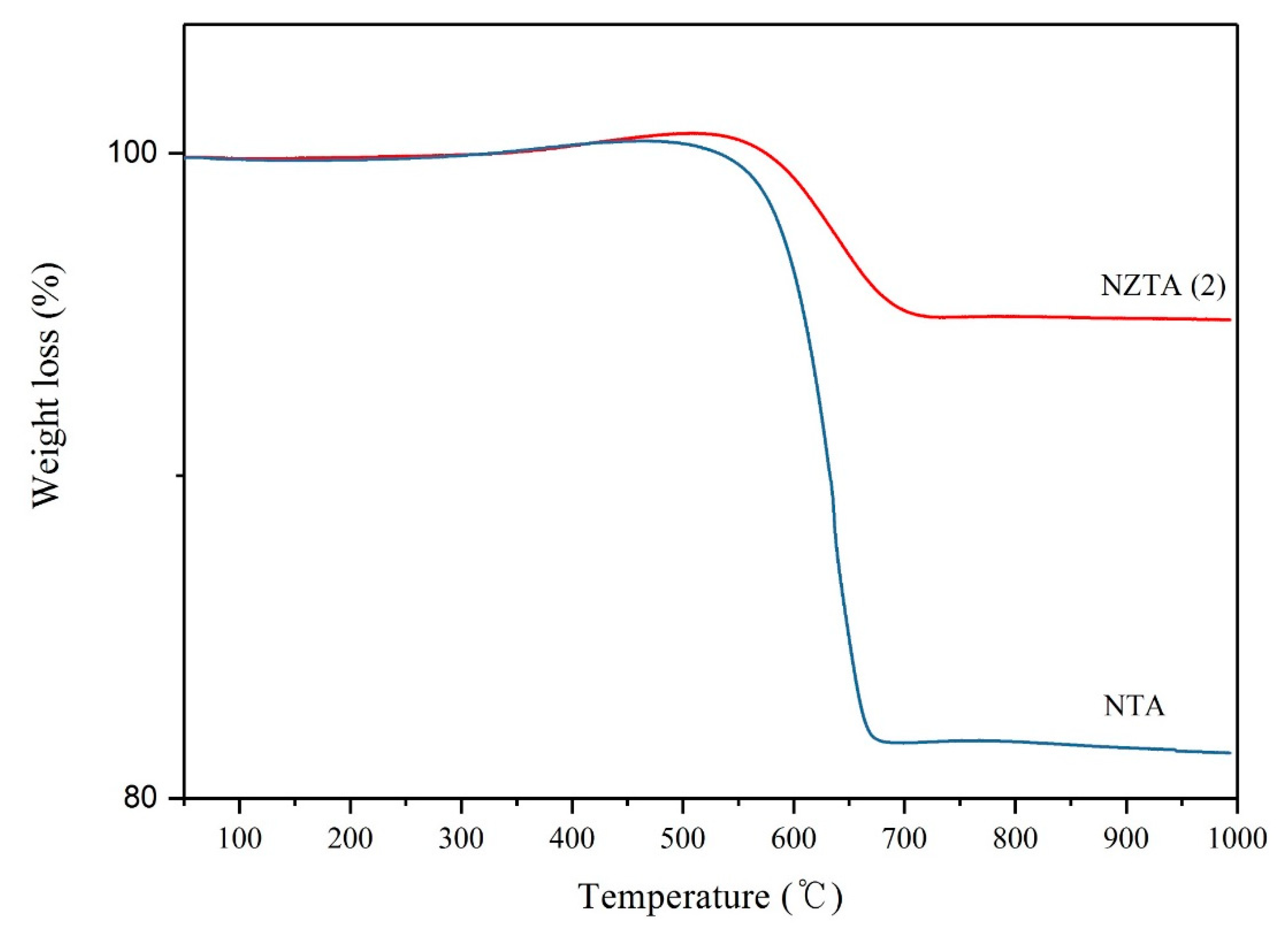
2.1. Physico-Chemical Analysis
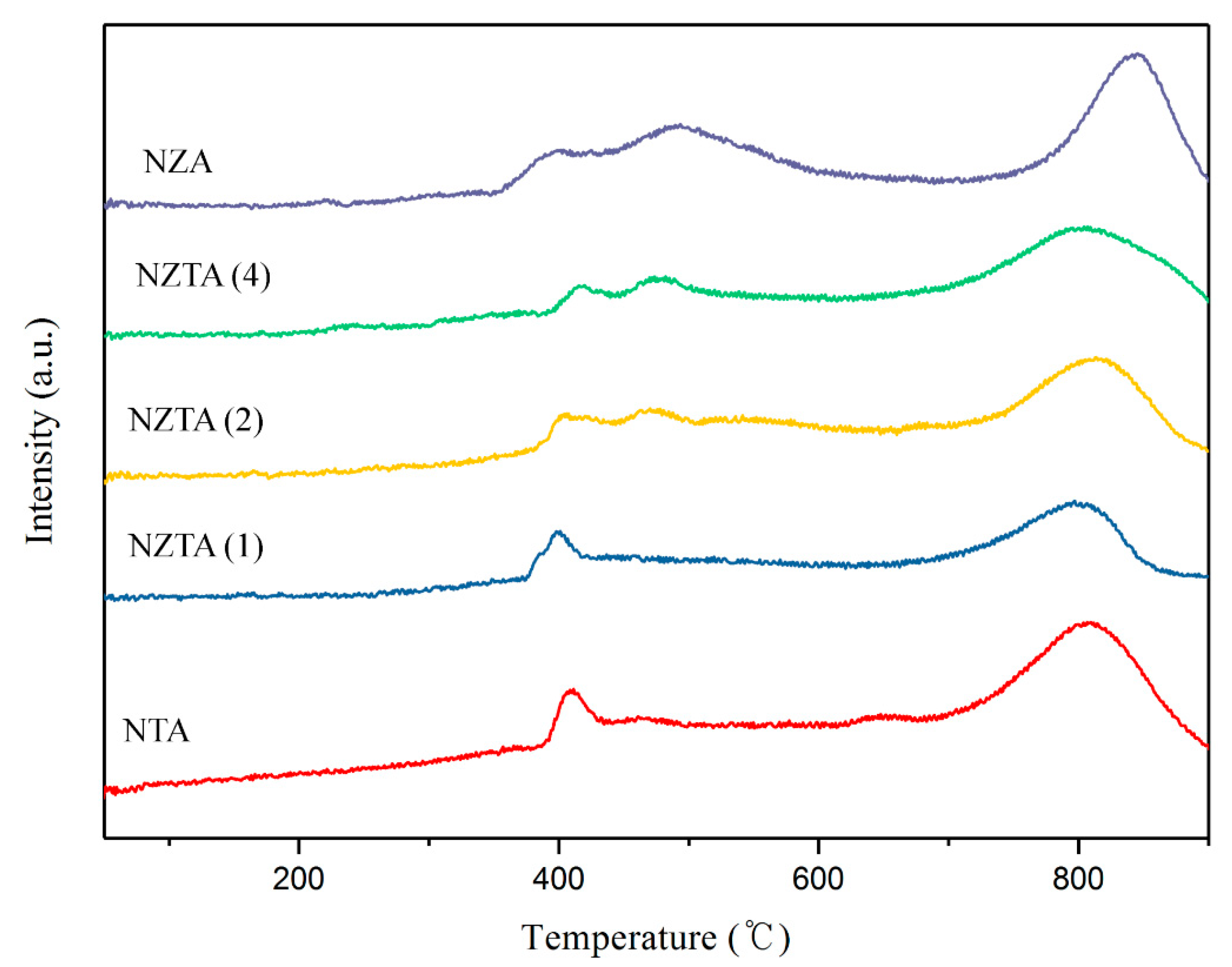
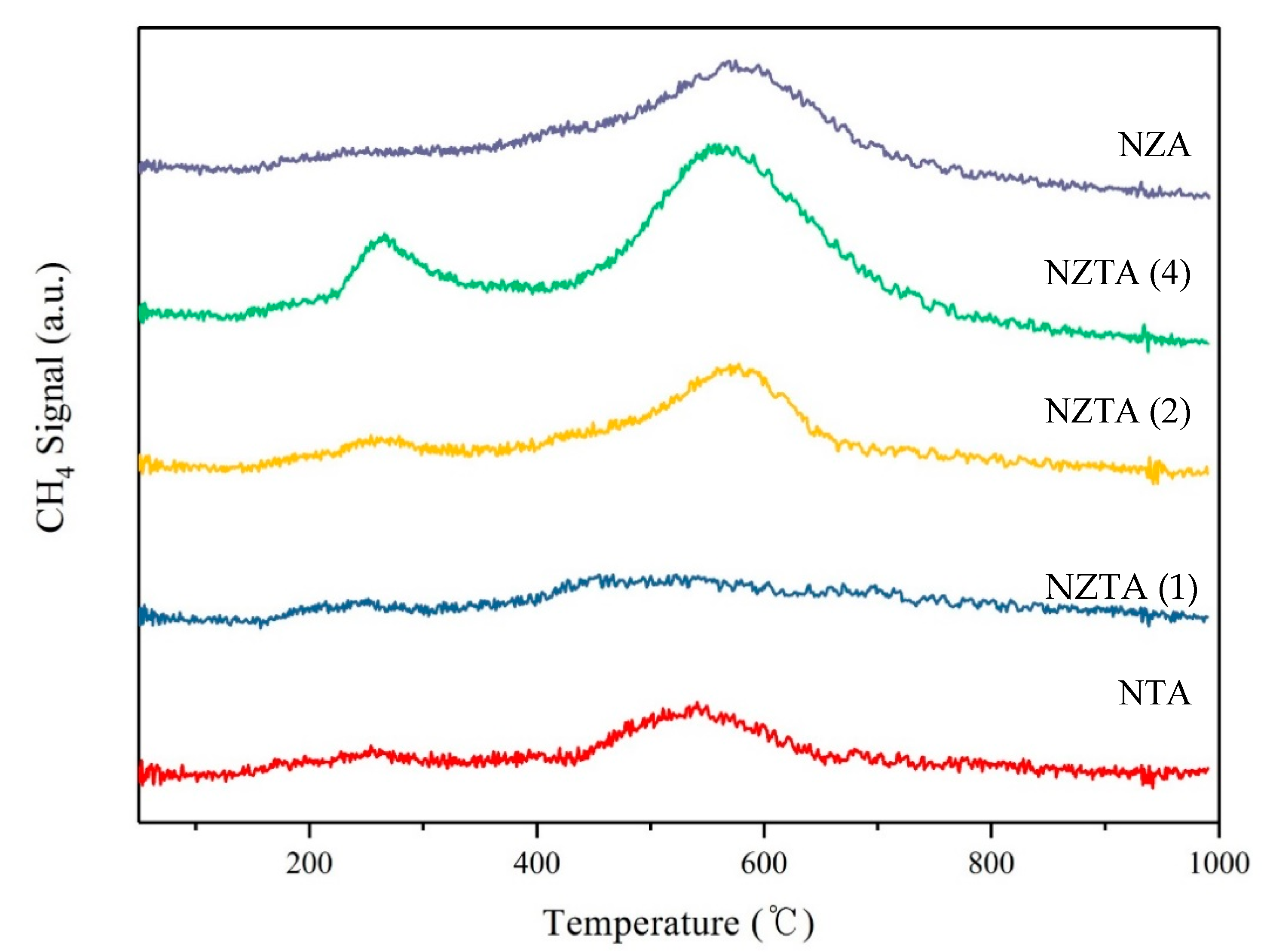
2.1.1. Temperature-Programmed Surface Reaction (TPSR)
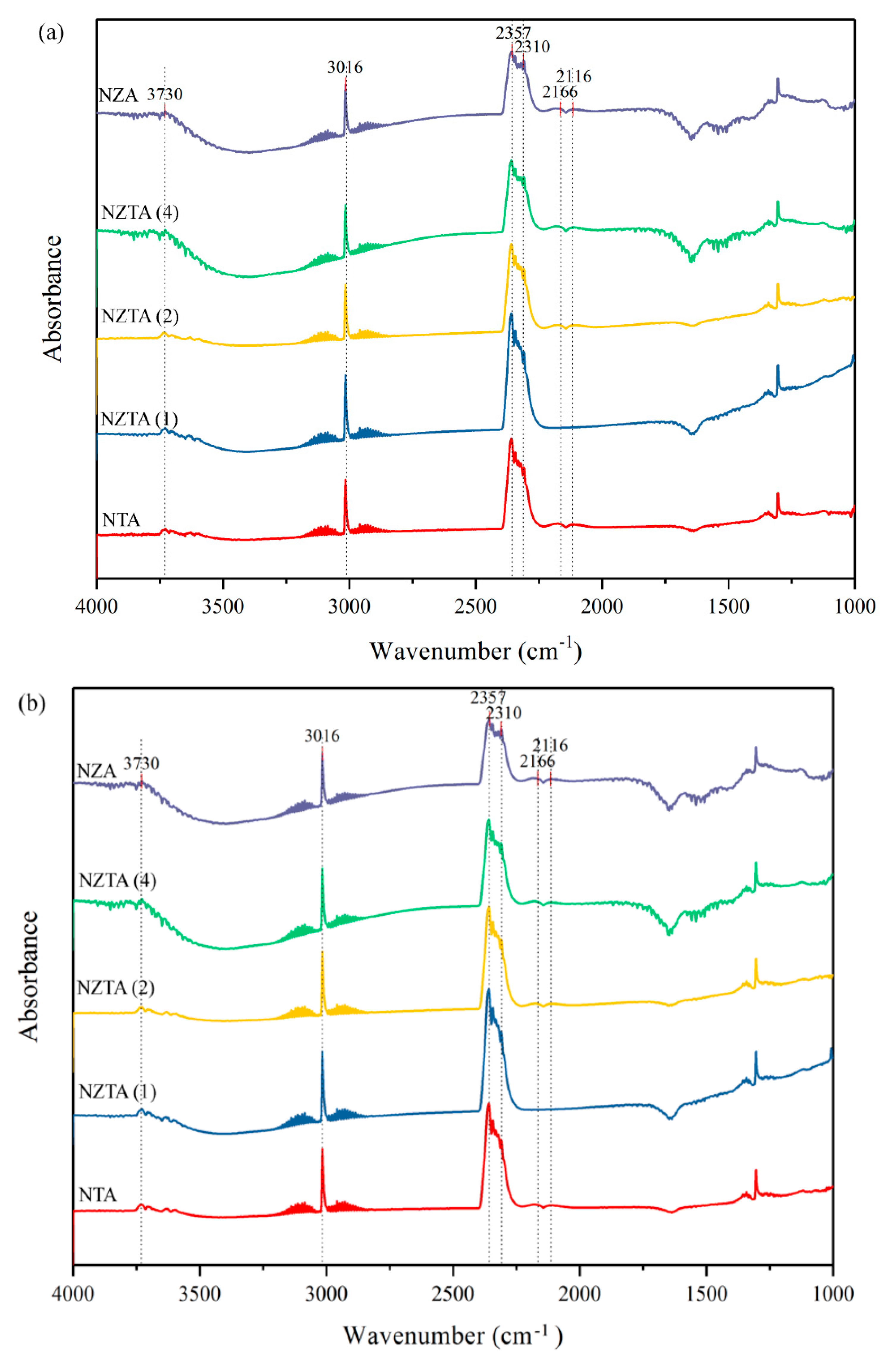
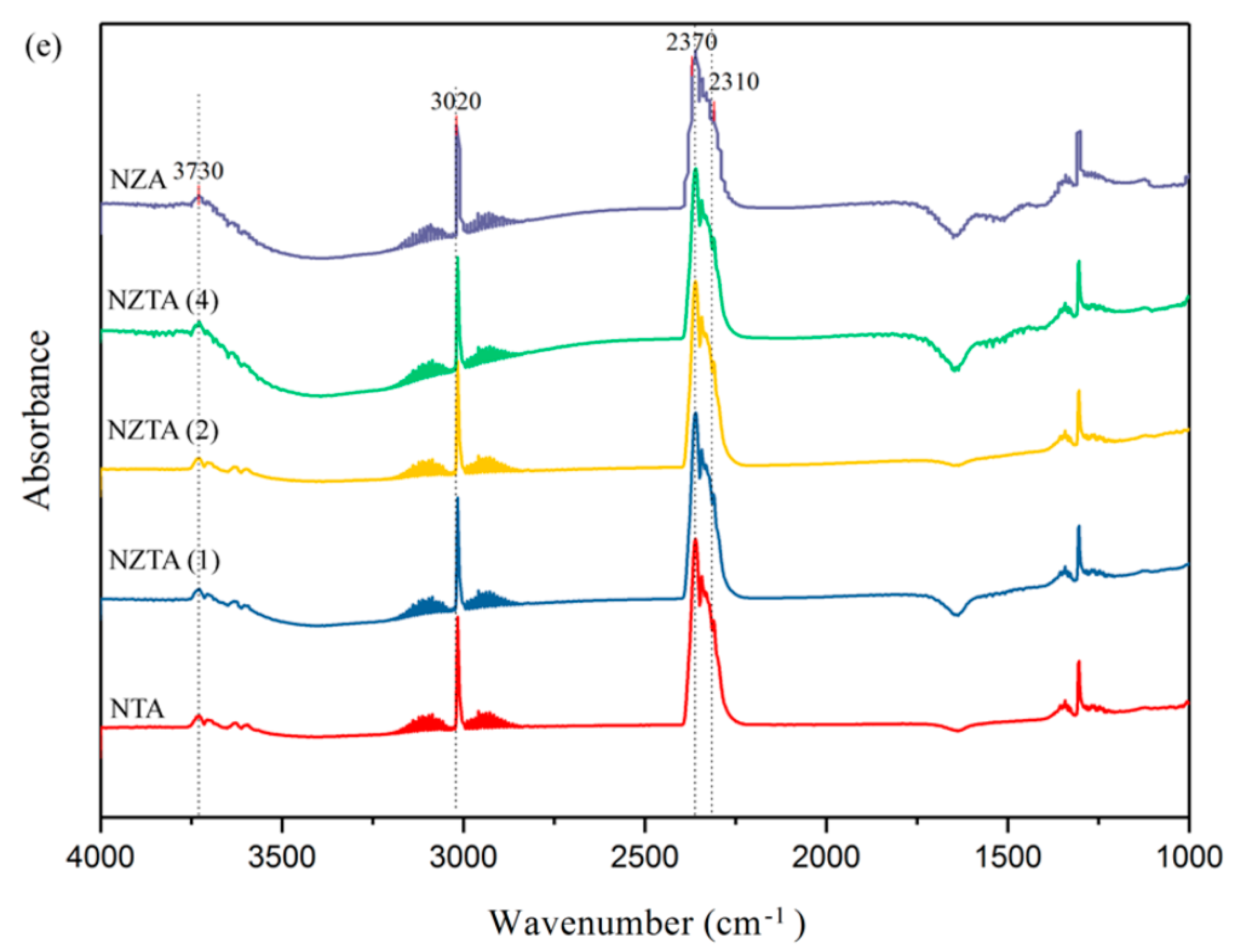
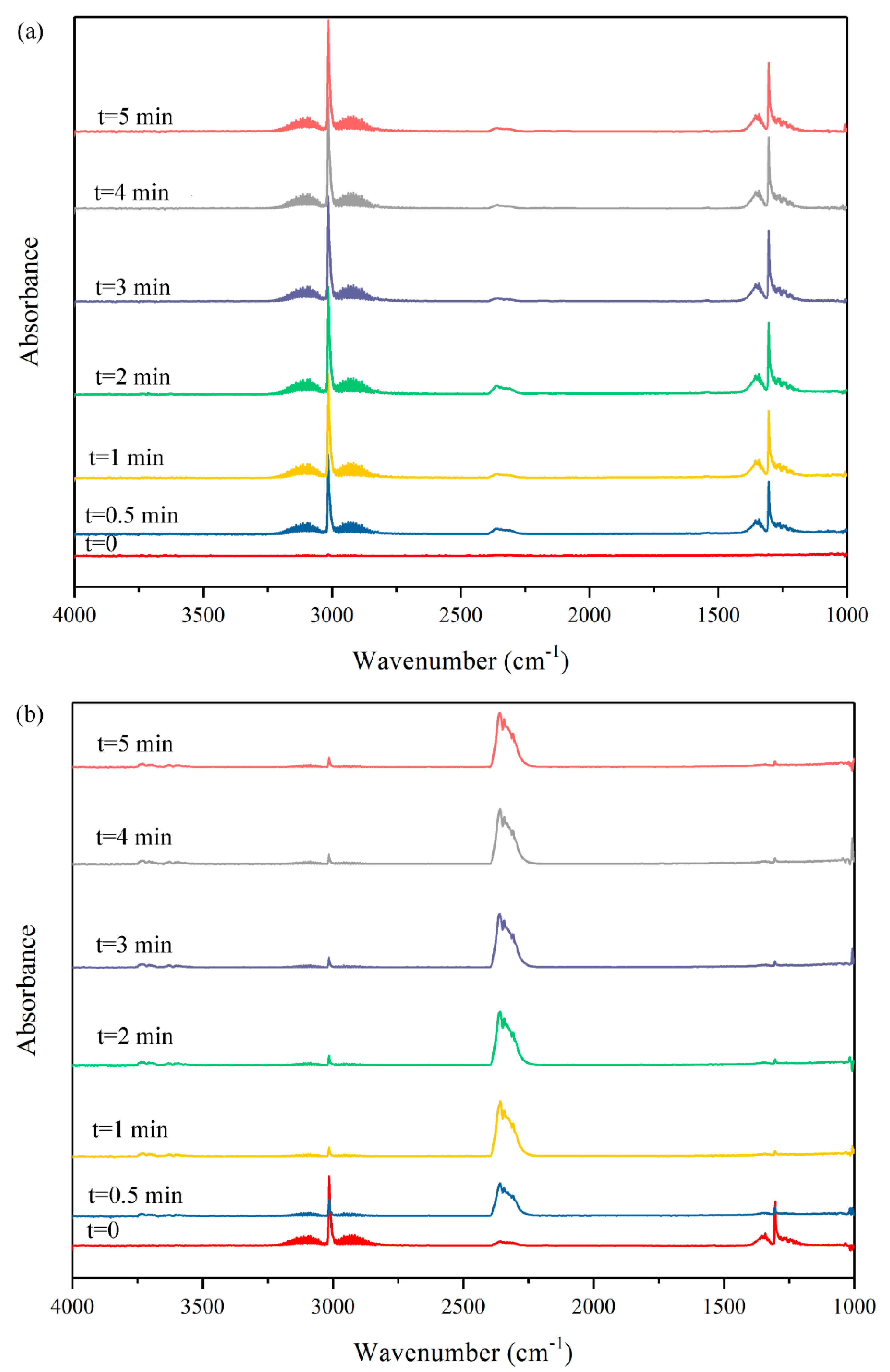
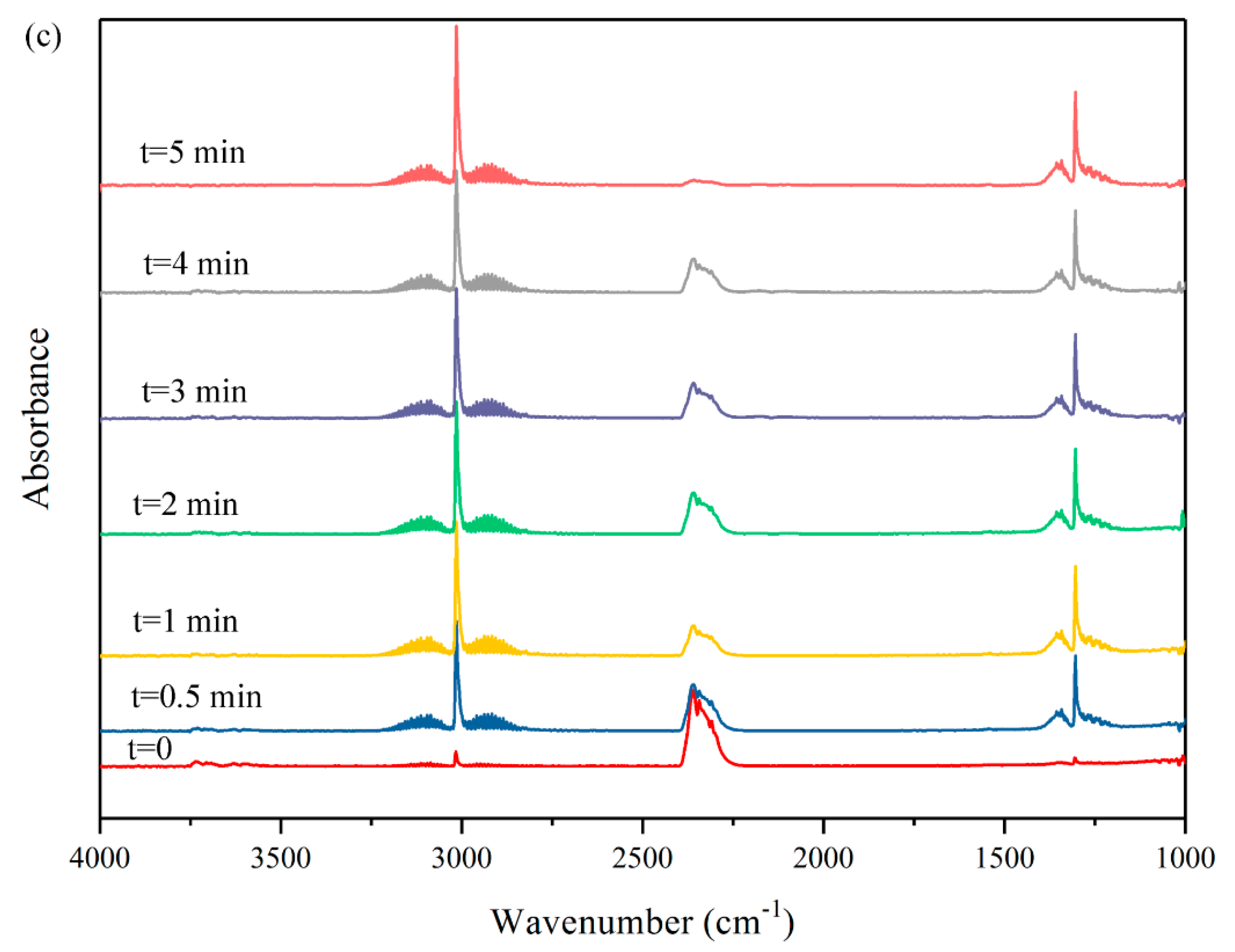
2.1.2. In Situ DRIFT Study on Dry Reforming of Methane
2.2. Catalyst Evaluation in the Dry Reforming of Methane
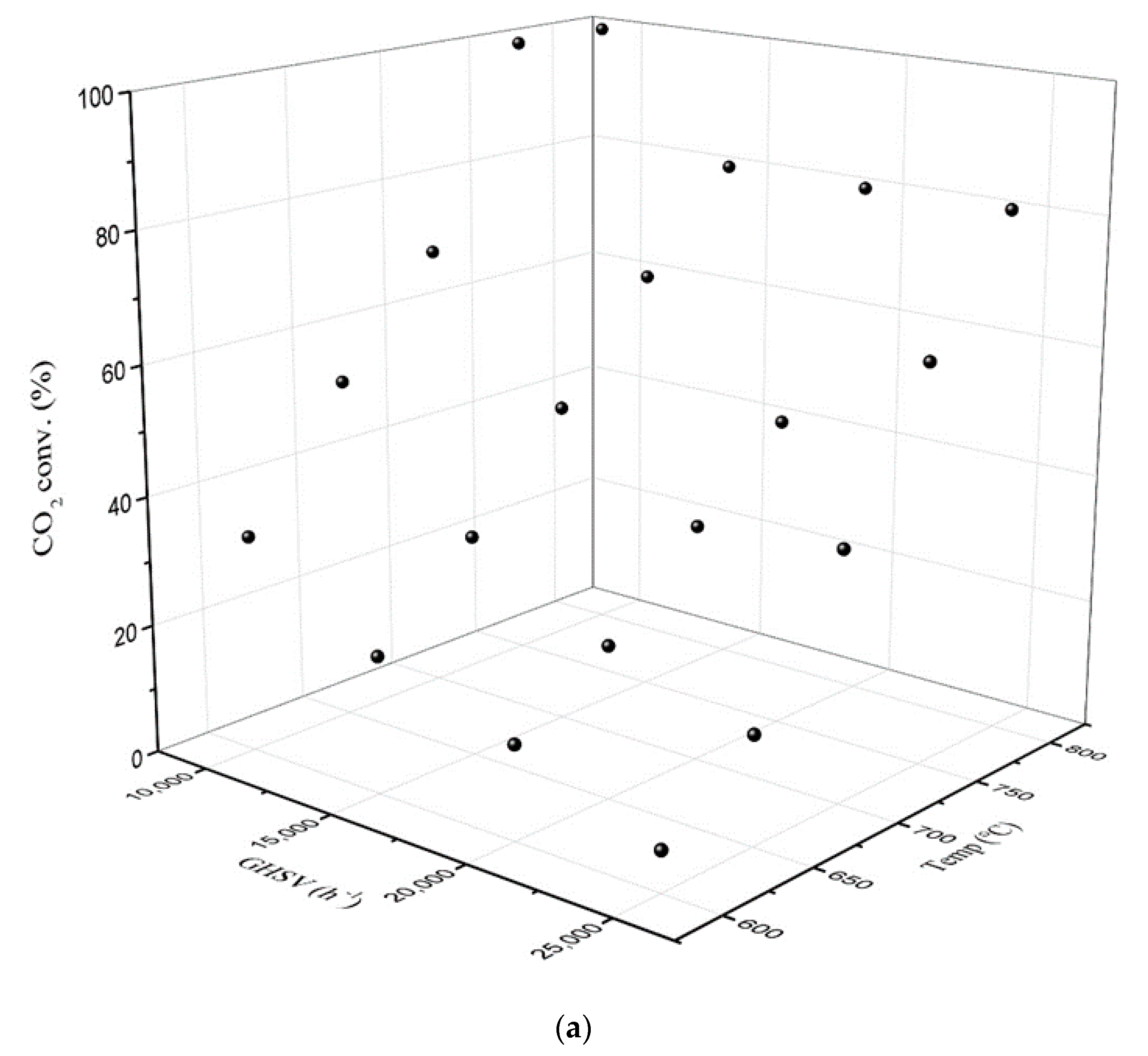
2.2.1. Dry Reforming over the Prepared Catalyst
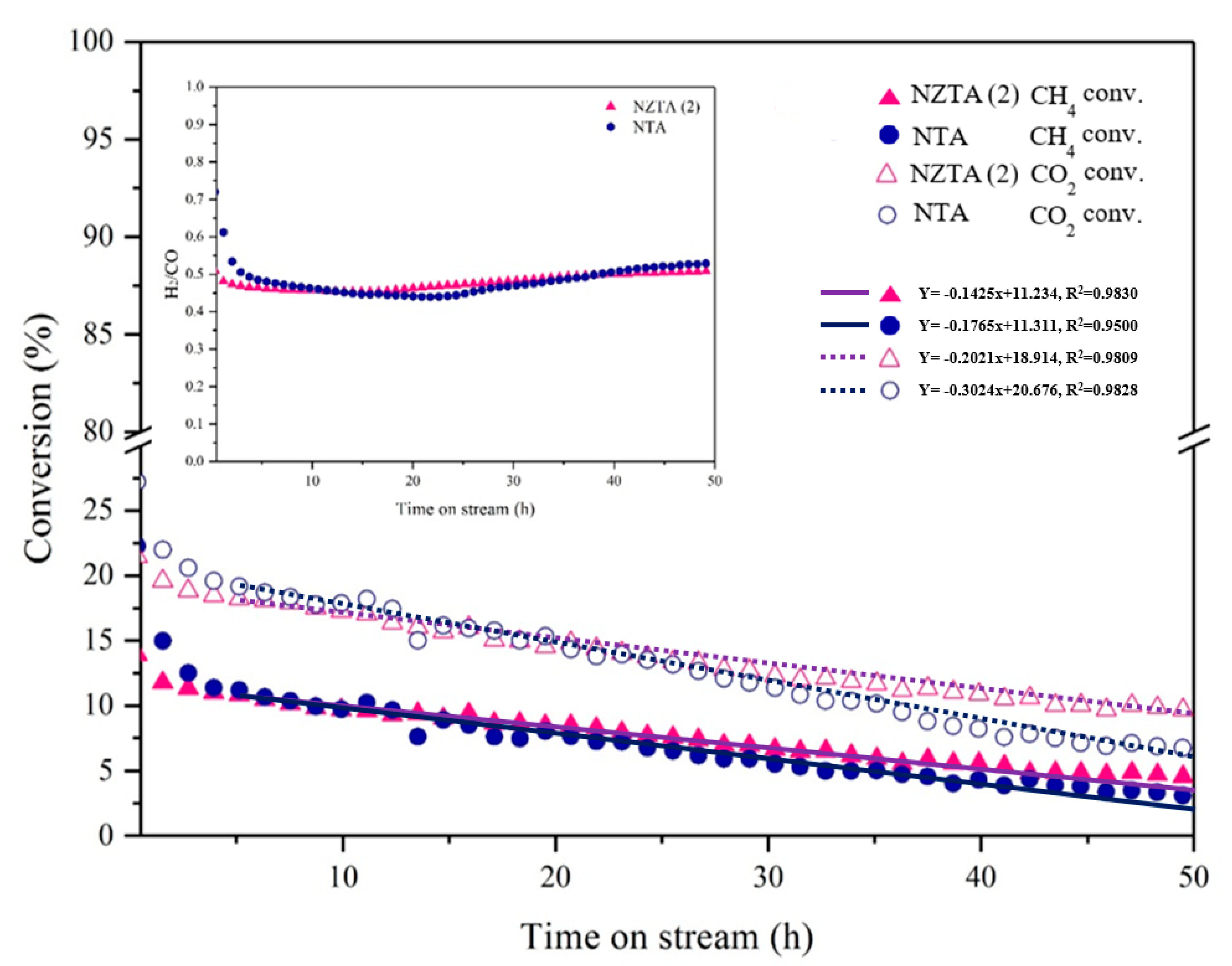
2.2.2. Long-Term Stability of Catalyst
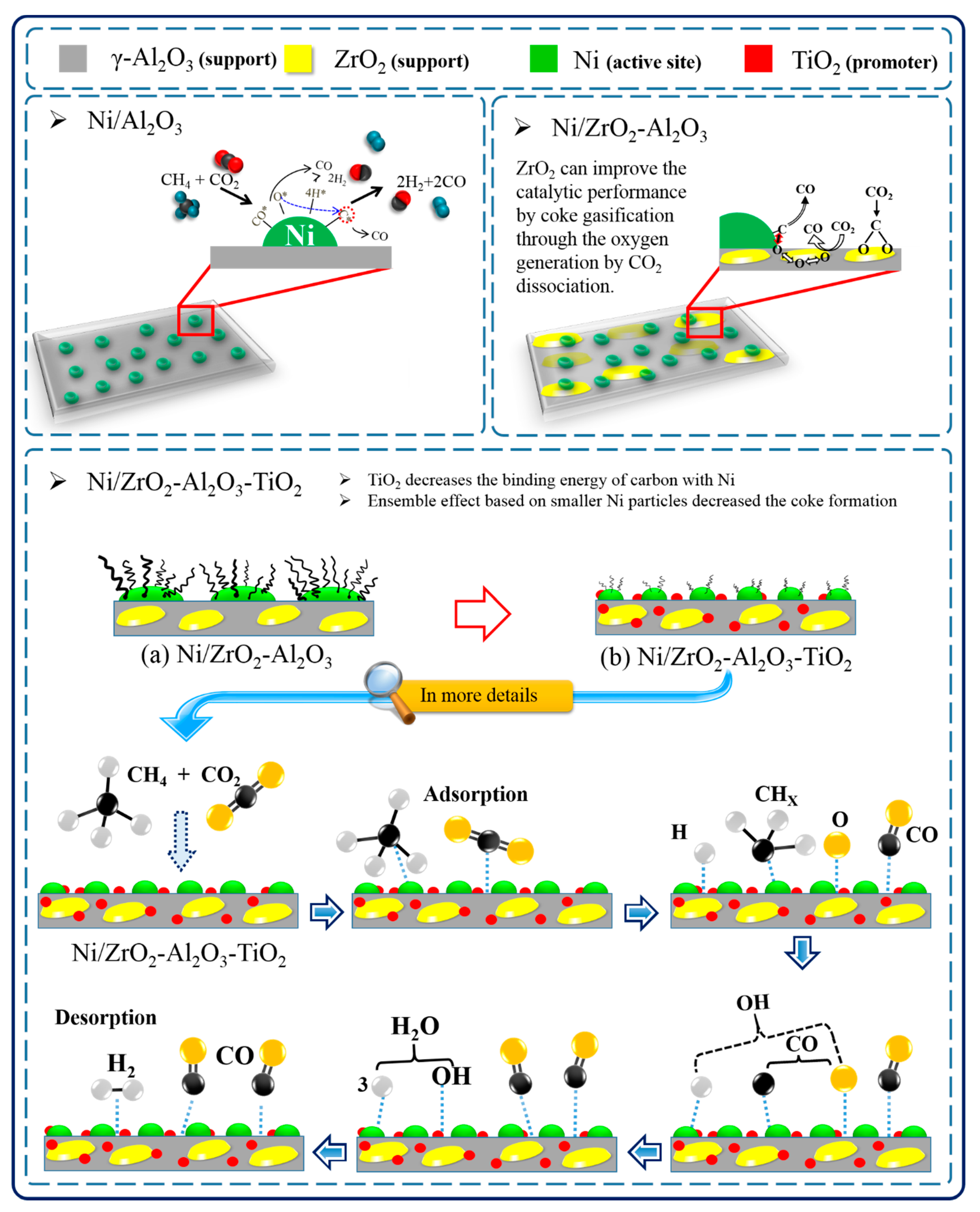
2.3. The Role of ZrO2 and TiO2 over Ni/ZrO2-Al2O3-TiO2 Catalyst in the Dry Reforming of Methane
3. Experiment
3.1. Preparation of Supports
3.2. Characterization of Catalysts
3.3. Catalytic Performance Test under the Dry Reforming of Methane
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rostrup-Nielsen, J.R. New aspects of syngas production and use. Catal. Today 2000, 63, 159–164. [Google Scholar] [CrossRef]
- Sharifi, M.; Haghighi, M.; Eslami, A.A.; Rahmani, F.; Rahemi, N. Synthesis and Characterization of Ni-Cu/Al2O3-ZrO2 Nanocatalyst via Sequential Impregnation and Sol-Gel Methods Used in CH4/CO2 Conversion to Syngas. Pet. Res. 2016, 25, 142–157. [Google Scholar]
- Eslami, A.A.; Haghighi, M.; Rahemi, N.; Laheghi, S.N. Sol-Gel Synthesis and Characterization of Ni/Al2O3 Nanocatalysts Doped with Co and Cu. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2011; Volume 1315, pp. 1297–1302. [Google Scholar]
- Baltrusaitis, J.; Luyben, W.L. Methane Conversion to Syngas for Gas-to-Liquids (GTL): Is Sustainable CO2 Reuse via Dry Methane Reforming (DMR) Cost Competitive with SMR and ATR Processes? ACS Sustain. Chem. Eng. 2015, 3, 2100–2111. [Google Scholar] [CrossRef]
- Gangadharan, P.; Kanchi, K.C.; Lou, H.H. Evaluation of the economic and environmental impact of combining dry reforming with steam reforming of methane. Chem. Eng. Res. Des. 2012, 90, 1956–1968. [Google Scholar] [CrossRef]
- Hong, G.H.; Shin, D.; Moon, D.J. Development of fixed bed reactor for applications in GTL-FPSO: The effect of dilution material for control of reaction heat. Catal. Today 2020, in press. [Google Scholar] [CrossRef]
- Kim, H.D.; Song, H.-T.; Fazeli, A.; Eslami, A.A.; Noh, Y.S.; Saeidabad, N.G.; Lee, K.-Y.; Moon, D.J. CO/CO2 hydrogenation for the production of lighter hydrocarbons over SAPO-34 modified hybrid FTS catalysts. Catal. Today 2020, in press. [Google Scholar] [CrossRef]
- Bepari, S.; Kuila, D. Steam reforming of methanol, ethanol and glycerol over nickel-based catalysts-A review. Int. J. Hydrog. Energy 2020, 45, 18090–18113. [Google Scholar] [CrossRef]
- Saeidabad, N.G.; Noh, Y.S.; Eslami, A.A.; Song, H.-T.; Kim, H.D.; Fazeli, A.; Moon, D.J. A Review on Catalysts Development for Steam Reforming of Biodiesel Derived Glycerol; Promoters and Supports. Catalysts 2020, 10, 910. [Google Scholar] [CrossRef]
- Zhu, Y.-A.; Chen, D.; Zhou, X.-G.; Yuan, W.-K. DFT studies of dry reforming of methane on Ni catalyst. Catal. Today 2009, 148, 260–267. [Google Scholar] [CrossRef]
- Yusuf, M.; Farooqi, A.S.; Keong, L.K.; Hellgardt, K.; Abdullah, B. Contemporary trends in composite Ni-based catalysts for CO2 reforming of methane. Chem. Eng. Sci. 2020, 229, 116072. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of alkaline earth promoters (MgO, CaO, and BaO) on the activity and coke formation of Ni catalysts supported on nanocrystalline Al2O3 in dry reforming of methane. J. Ind. Eng. Chem. 2014, 20, 2858–2863. [Google Scholar] [CrossRef]
- Barroso-Quiroga, M.M.; Castro-Luna, A.E. Catalytic activity and effect of modifiers on Ni-based catalysts for the dry reforming of methane. Int. J. Hydrog. Energy 2010, 35, 6052–6056. [Google Scholar] [CrossRef]
- Xu, B.-Q.; Wei, J.-M.; Yu, Y.-T.; Li, J.-L.; Zhu, Q.-M. Carbon Dioxide Reforming of Methane Over Nanocomposite Ni/ZrO2 Catalysts. Top. Catal. 2003, 22, 77–85. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.C.; Navarro, R.M.; Fierro, J.L.G. Ethanol steam reforming over Ni/MxOy–Al2O3 (M=Ce, La, Zr and Mg) catalysts: Influence of support on the hydrogen production. Int. J. Hydrog. Energy 2007, 32, 1462–1471. [Google Scholar] [CrossRef]
- Brungs, A.J.; York, A.P.E.; Claridge, J.B.; Márquez-Alvarez, C.; Green, M.L.H. Dry reforming of methane to synthesis gas over supported molybdenum carbide catalysts. Catal. Lett. 2000, 70, 117–122. [Google Scholar] [CrossRef]
- Guo, J.; Hou, Z.; Gao, J.; Zheng, X. DRIFTS Study on Adsorption and Activation of CH4 and CO2 over Ni/SiO2 Catalyst with Various Ni Particle Sizes. Chin. J. Catal. 2007, 28, 22–26. [Google Scholar] [CrossRef]
- Awadallah, A.E.; Mostafa, M.S.; Aboul-Enein, A.A.; Hanafi, S.A. Hydrogen production via methane decomposition over Al2O3–TiO2 binary oxides supported Ni catalysts: Effect of Ti content on the catalytic efficiency. Fuel 2014, 129, 68–77. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Zhang, J.; Chang, L.; Zhou, R.; Duan, Z. Bound-state Ni species—A superior form in Ni-based catalyst for CH4/CO2 reforming. Appl. Catal. A Gen. 2001, 210, 45–53. [Google Scholar] [CrossRef]
- Dong, W.-S.; Roh, H.-S.; Jun, K.-W.; Park, S.-E.; Oh, Y.-S. Methane reforming over Ni/Ce-ZrO2 catalysts: Effect of nickel content. Appl. Catal. A Gen. 2002, 226, 63–72. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. Catalytic reforming of methane with carbon dioxide over nickel catalysts I. Catalyst characterization and activity. Appl. Catal. A Gen. 1996, 142, 73–96. [Google Scholar] [CrossRef]
- Yan, Q.G.; Weng, W.Z.; Wan, H.L.; Toghiani, H.; Toghiani, R.K.; Pittman, C.U. Activation of methane to syngas over a Ni/TiO2 catalyst. Appl. Catal. A Gen. 2003, 239, 43–58. [Google Scholar] [CrossRef]
- Chauke, H.R.; Murovhi, P.; Ngoepe, P.E.; de Leeuw, N.H.; Grau-Crespo, R. Electronic Structure and Redox Properties of the Ti-Doped Zirconia (111) Surface. J. Phys. Chem. C 2010, 114, 15403–15409. [Google Scholar] [CrossRef]
- Gallino, F.; di Valentin, C.; Pacchioni, G. Band gap engineering of bulk ZrO2 by Ti doping. Phys. Chem. Chem. Phys. 2011, 13, 17667–17675. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, K.; Fu, H.; Pan, K.; Zhang, L.; Wang, L.; Sun, C.C. Multi-modal mesoporous TiO(2)-ZrO(2) composites with high photocatalytic activity and hydrophilicity. Nanotechnology 2008, 19, 035610. [Google Scholar] [CrossRef]
- Shin, S.A.; Noh, Y.S.; Hong, G.H.; Park, J.I.; Song, H.T.; Lee, K.-Y.; Moon, D.J. Dry reforming of methane over Ni/ZrO2-Al2O3 catalysts: Effect of preparation methods. J. Taiwan Inst. Chem. Eng. 2018, 90, 25–32. [Google Scholar] [CrossRef]
- Gonçalves, A.A.S.; Faustino, P.B.; Assaf, J.M.; Jaroniec, M. One-Pot Synthesis of Mesoporous Ni–Ti–Al Ternary Oxides: Highly Active and Selective Catalysts for Steam Reforming of Ethanol. ACS Appl. Mater. Interfaces 2017, 9, 6079–6092. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, S.M.; Won, J.M.; Yang, H.J.; Hong, S.C. Effect of Ce/Ti ratio on the catalytic activity and stability of Ni/CeO2–TiO2 catalyst for dry reforming of methane. Chem. Eng. J. 2015, 280, 433–440. [Google Scholar] [CrossRef]
- Damyanova, S.; Spojakina, A.; Jiratova, K. Effect of mixed titania-alumina supports on the phase composition of NiMo/TiO2Al2O3 catalysts. Appl. Catal. A Gen. 1995, 125, 257–269. [Google Scholar] [CrossRef]
- Bitter, J.H.; Seshan, K.; Lercher, J.A. Deactivation and Coke Accumulation during CO2/CH4 Reforming over Pt Catalysts. J. Catal. 1999, 183, 336–343. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.H.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica-Ceria sandwiched Ni core-shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights. Appl. Catal. B-Environ. 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Tomishige, K.; Chen, Y.-G.; Fujimoto, K. Studies on Carbon Deposition in CO2 Reforming of CH4 over Nickel–Magnesia Solid Solution Catalysts. J. Catal. 1999, 181, 91–103. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Yan, Z.-F. Syngas Production by Methane Reforming with Carbon Dioxide on Noble Metal Catalysts. J. Nat. Gas Chem. 2006, 15, 327–334. [Google Scholar] [CrossRef]
- Takatani, S.; Chung, Y.-W. Strong metal-support interaction in NiTiO2: Auger and vibrational spectroscopy evidence for the segregation of TiOx (x ≈ −1) on Ni and its effects on CO chemisorption. J. Catal. 1984, 90, 75–83. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Yang, E.H.; Noh, Y.S.; Lim, S.S.; Ahn, B.S.; Moon, D.J. The Effect of Fe in Perovskite Catalysts for Steam CO2 Reforming of Methane. J. Nanosci. Nanotechnol. 2016, 16, 1938–1941. [Google Scholar] [CrossRef]
- Noh, Y.S.; Lee, K.Y.; Moon, D.J. Hydrogen production by steam reforming of methane over nickel based structured catalysts supported on calcium aluminate modified SiC. Int. J. Hydrog. Energy. 2019, 44, 21010–21019. [Google Scholar] [CrossRef]
- Wang, C.Z.; Sun, N.N.; Wei, W.; Zhao, Y.X. Carbon intermediates during CO2 reforming of methane over Ni-CaO-ZrO2 catalysts: A temperature-programmed surface reaction study. Int. J. Hydrog. Energy 2016, 41, 19014–19024. [Google Scholar] [CrossRef]
- Yang, E.H.; Noh, Y.S.; Hong, G.H.; Moon, D.J. Combined steam and CO2 reforming of methane over La1-xSrxNiO3 perovskite oxides. Catal. Today 2018, 299, 242–250. [Google Scholar] [CrossRef]








| Catalysts Code | Support Component Molar Ratio | Zr/Ti Molar Ratio | Surface Area (m2/g) | Pore Volume 1 (cm3/g) | Average Pore Diameter (nm) | NiO Size (nm) | ||
|---|---|---|---|---|---|---|---|---|
| Ti | Zr | Al | ||||||
| NTA | 1 | - | 3 | - | 4.9 | 0.028 | 23.5 | 8.9 |
| NZTA (1) | 0.5 | 0.5 | 3 | 1 | 20.6 | 0.065 | 12.7 | 10.7 |
| NZTA (2) | 0.3 | 0.7 | 3 | 2 | 7.5 | 0.022 | 12.3 | 10.0 |
| NZTA (4) | 0.2 | 0.8 | 3 | 4 | 6.9 | 0.060 | 7.9 | 7.4 |
| NZA | - | 1 | 3 | - | 17.3 | 0.037 | 8.5 | 15.2 |
| Parameters | Conditions |
|---|---|
| Packing type | Carbosphere packed column 60/80 meshes 2.5 m × 2.16 mm I.D. |
| Injector temp. | 200 °C |
| Oven temp. | 120 °C |
| TCD temp. | 250 °C |
| Ar flow rate (carrier gas) | 20 mL/min |
| recording intervals | ~20 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.A.; Alizadeh Eslami, A.; Noh, Y.S.; Song, H.-t.; Kim, H.D.; Ghaffari Saeidabad, N.; Moon, D.J. Preparation and Characterization of Ni/ZrTiAlOx Catalyst via Sol-Gel and Impregnation Methods for Low Temperature Dry Reforming of Methane. Catalysts 2020, 10, 1335. https://doi.org/10.3390/catal10111335
Shin SA, Alizadeh Eslami A, Noh YS, Song H-t, Kim HD, Ghaffari Saeidabad N, Moon DJ. Preparation and Characterization of Ni/ZrTiAlOx Catalyst via Sol-Gel and Impregnation Methods for Low Temperature Dry Reforming of Methane. Catalysts. 2020; 10(11):1335. https://doi.org/10.3390/catal10111335
Chicago/Turabian StyleShin, Seol A, Ali Alizadeh Eslami, Young Su Noh, Hyun-tae Song, Hyun Dong Kim, Nasim Ghaffari Saeidabad, and Dong Ju Moon. 2020. "Preparation and Characterization of Ni/ZrTiAlOx Catalyst via Sol-Gel and Impregnation Methods for Low Temperature Dry Reforming of Methane" Catalysts 10, no. 11: 1335. https://doi.org/10.3390/catal10111335
APA StyleShin, S. A., Alizadeh Eslami, A., Noh, Y. S., Song, H.-t., Kim, H. D., Ghaffari Saeidabad, N., & Moon, D. J. (2020). Preparation and Characterization of Ni/ZrTiAlOx Catalyst via Sol-Gel and Impregnation Methods for Low Temperature Dry Reforming of Methane. Catalysts, 10(11), 1335. https://doi.org/10.3390/catal10111335