Immobilized Cell Physiology Imaging and Stabilization of Enzyme Cascade Reaction Using Recombinant Cells Escherichia coli Entrapped in Polyelectrolyte Complex Beads by Jet Break-Up Encapsulator
Abstract
1. Introduction
2. Results
2.1. Production of PEC Beads with Entrapped E. coli Containing Enzyme Cascade ADH, XenB, and CHMO Using Jet Break-Up Technique
2.2. Operational Stability of PEC Beads with Entrapped E. coli Containing Enzyme Cascade
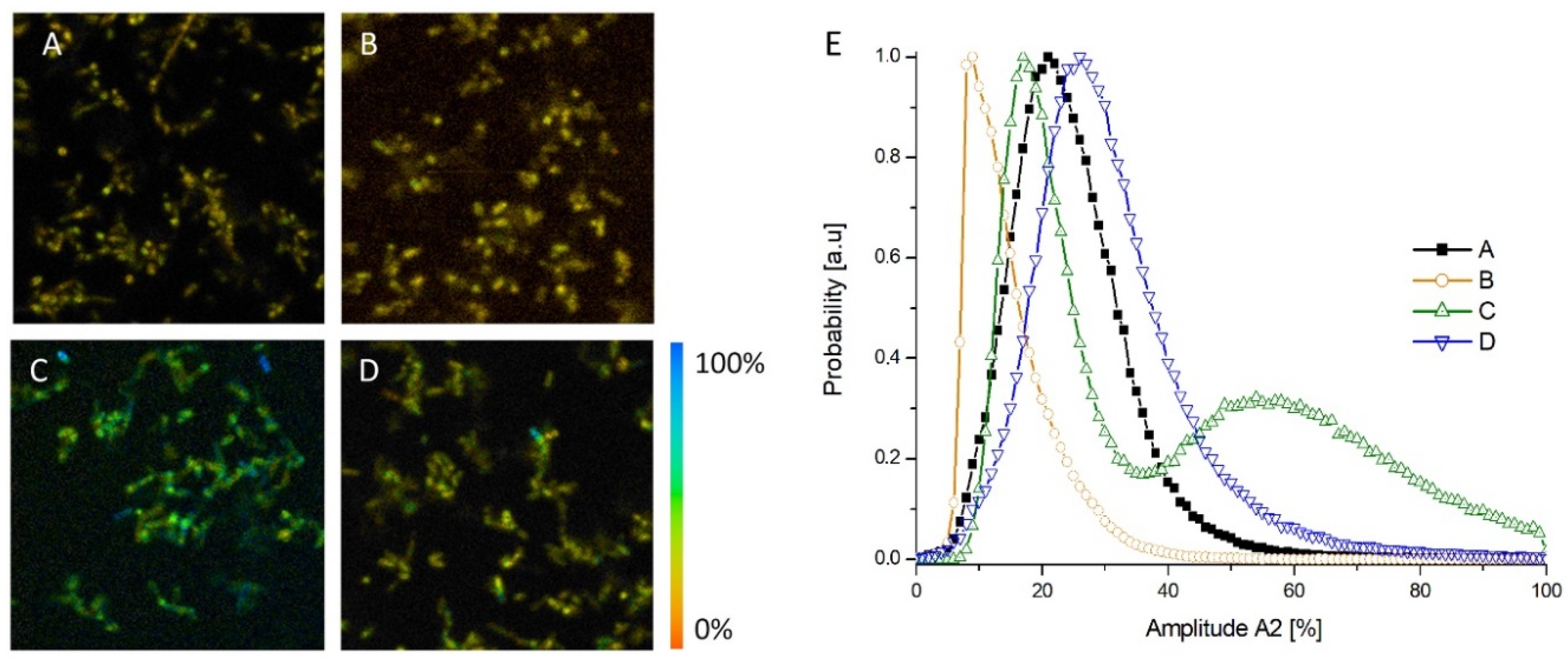
2.3. Metabolic Assessment of E. coli Using Multi-Modal Optical Imaging
2.4. Influence of Cell Immobilization on MWCO of PEC Beads
3. Materials and Methods
3.1. Immobilization Polymers, Media and Chemicals
3.2. Cultivation of Cells
3.3. Preparation of Polyelectrolyte Complex Beads
3.4. Repeated Biotransformations
3.5. Multi-Modal Optical Imaging
3.6. Inverse Size Exclusion Chromatography (iSEC)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bornscheuer, U.T. The fourth wave of biocatalysis is approaching. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 376, 20170063. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, F. Whole-cell based synthetic enzyme cascades—light and shadow of a promising technology. Curr. Opin. Chem. Biol. 2019, 49, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bučko, M.; Gemeiner, P.; Schenkmayerová, A.; Krajčovič, T.; Rudroff, F.; Mihovilovič, M.D. Baeyer-Villiger oxidations: Biotechnological approach. Appl. Microbiol. Biotechnol. 2016, 100, 6585–6599. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, D.A.; Morán-Ramallal, R.; Iqbal, N.; Rudroff, F.; Mihovilovic, M.D. Enantiocomplementary access to carba-analogs of C-nucleoside derivatives by recombinant baeyer–villiger monooxygenases. Bioorg. Med. Chem. Lett. 2013, 23, 2718–2720. [Google Scholar] [CrossRef] [PubMed]
- Seley-Radtke, K.L.; Yates, M.K. The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antivir. Res. 2018, 154, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.K.; DiPaola, R.S.; Romanelli, F.; Dutch, R.E. Rapid repurposing of drugs for COVID-19. Science 2020, 368, 829–830. [Google Scholar] [CrossRef]
- Bong, Y.K.; Clay, M.D.; Collier, S.J.; Mijts, B.; Vogel, M.; Zhang, X.; Hang, X.; Zhu, J.; Nazor, J.; Smith, D. Synthesis of Pyrazole Compounds. U.S. Patent WO2011/071982 A2, 16 June 2011. [Google Scholar]
- Schmidt, S.; Bornscheuer, U.T. Baeyer-Villiger monooxygenases: From protein engineering to biocatalytic applications. In Peptidomics of Cancer-Derived Enzyme Products; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 187–6047. [Google Scholar]
- Kisukuri, C.M.; Andrade, L.H. Production of chiral compounds using immobilized cells as a source of biocatalysts. Org. Biomol. Chem. 2015, 13, 10086–10107. [Google Scholar] [CrossRef]
- Guzzon, R.; Carturan, G.; Krieger-Weber, S.; Cavazza, A. Use of organo-silica immobilized bacteria produced in a pilot scale plant to induce malolactic fermentation in wines that contain lysozyme. Ann. Microbiol. 2011, 62, 381–390. [Google Scholar] [CrossRef]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skjåk-BraeK, G. Effect of Ca2+, Ba2+, and Sr2+ on Alginate Microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Liu, H.; Duan, W.-D.; De Souza, F.Z.R.; Liu, L.; Chen, B.-S. Asymmetric Ketone Reduction by Immobilized Rhodotorula mucilaginosa. Catalysts 2018, 8, 165. [Google Scholar] [CrossRef]
- Buchholz, K.; Kasche, V.; Bornscheuer, U.T. Immobilization of microorganisms and cells: In Biocatalysts and Enzyme Technology; Wiley-VCH: Weinheim, Germany, 2005; pp. 283–284. ISBN 3-527-30497-5. [Google Scholar]
- Auriemma, G.; Russo, P.; Del Gaudio, P.; García-González, C.; Landín, M.; Aquino, R.P. Technologies and formulation design of polysaccharide-based hydrogels for drug delivery. Molecules 2020, 25, 3156. [Google Scholar] [CrossRef] [PubMed]
- Prüsse, U.; Bilancetti, L.; Bučko, M.; Bugarski, B.; Bukowski, J.; Gemeiner, P.; Lewińska, D.; Manojlovic, V.; Massart, B.; Nastruzzi, C. Comparison of different technologies for alginate beads production. Chem. Pap. 2008, 62, 364–374. [Google Scholar] [CrossRef]
- Krajčovič, T.; Bučko, M.; Vikartovska, A.; Lacik, I.; Uhelská, L.; Chorvát, D.; Neděla, V.; Tihlaříková, E.; Gericke, M.; Heinze, T.J. Polyelectrolyte Complex Beads by Novel Two-Step Process for Improved Performance of Viable Whole-Cell Baeyer-Villiger Monoxygenase by Immobilization. Catalysts 2017, 7, 353. [Google Scholar] [CrossRef]
- Polakovič, M.; Švitel, J.; Bučko, M.; Filip, J.; Neděla, V.; Ansorge-Schumacher, M.B.; Gemeiner, P. Progress in biocatalysis with immobilized viable whole cells: Systems development, reaction engineering and applications. Biotechnol. Lett. 2017, 39, 667–683. [Google Scholar] [CrossRef]
- Silva, A.L.P.; Caridade, T.N.D.S.; Magalhães, R.R.; De Sousa, K.T.; De Sousa, C.C.; Vale, J.A. Biocatalytic production of Ɛ-caprolactone using Geotrichum candidum cells immobilized on functionalized silica. Appl. Microbiol. Biotechnol. 2020, 1–9. [Google Scholar] [CrossRef]
- Oberleitner, N.; Peters, C.; Muschiol, J.; Kadow, M.; Saß, S.; Bayer, T.; Schaaf, P.; Iqbal, N.; Rudroff, F.; Mihovilovic, M.D. An enzymatic toolbox for cascade reactions: A showcase for an in vivo redox sequence in asymmetric synthesis. ChemCatChem 2013, 5, 3524–3528. [Google Scholar] [CrossRef]
- Bučko, M.; Vikartovská, A.; Schenkmayerová, A.; Tkáč, J.; Filip, J.; Chorvát, D.; Neděla, V.; Ansorge-Schumacher, M.B.; Gemeiner, P. Progress in emerging techniques for characterization of immobilized viable whole-cell biocatalysts. Chem. Pap. 2017, 71, 2309–2324. [Google Scholar] [CrossRef]
- Neděla, V.; Tihlaříková, E.; Maxa, J.; Imrichová, K.; Bučko, M.; Gemeiner, P. Simulation-based optimisation of thermodynamic conditions in the ESEM for dynamical in-situ study of spherical polyelectrolyte complex particles in their native state. Ultramicroscopy 2020, 211, 112954. [Google Scholar] [CrossRef]
- Becker, W. Errata to: Advanced Time-Correlated Single Photon Counting Applications. Vibronic Interact. Mol. Cryst. 2015, 111. [Google Scholar] [CrossRef]
- Chorvat, D.; Chorvatova, A. Multi-wavelength fluorescence lifetime spectroscopy: A new approach to the study of endogenous fluorescence in living cells and tissues. Laser Phys. Lett. 2009, 6, 175–193. [Google Scholar] [CrossRef]
- Brissova, M.; Petro, M.; Lacık, I.; Powers, A.C.; Wang, T. Evaluation of Microcapsule Permeability via Inverse Size Exclusion Chromatography. Anal. Biochem. 1996, 242, 104–111. [Google Scholar] [CrossRef]
- Briššová, M.; Lacík, I.; Powers, A.C.; Anilkumar, A.V.; Wang, T. Control and measurement of permeability for design of microcapsule cell delivery system. J. Biomed. Mater. Res. 1998, 39, 61–70. [Google Scholar] [CrossRef]
- Schenkmayerová, A.; Bucko, M.; Gemeiner, P.; Treľová, D.; Lacik, I.; Chorvát, D.; Ačai, P.; Polakovič, M.; Lipták, L.; Rebroš, M. Physical and bioengineering properties of polyvinyl alcohol lens-shaped particles versus spherical polyelectrolyte complex microcapsules as immobilisation matrices for a whole-cell baeyer–villiger monooxygenase. Appl. Biochem. Biotechnol. 2014, 174, 1834–1849. [Google Scholar] [CrossRef]
- Kroneková, Z.; Pelach, M.; Mazancová, P.; Uhelská, L.; Treľová, D.; Rázga, F.; Némethová, V.; Szalai, S.; Chorvát, D.; McGarrigle, J.J. Structural changes in alginate-based microspheres exposed to in vivo environment as revealed by confocal raman microscopy. Sci. Rep. 2018, 8, 1637. [Google Scholar] [CrossRef]
- Melgarejo-Torres, R.; Pérez-Vega, S.; Rivera-Arredondo, V.M.; Che-Galicia, G. Multiphase bioreactors in the pharmaceutical industry. In Modeling and Simulation of Heterogeneous Catalytic Processes; Elsevier BV: Amsterdam, The Netherlands, 2019; Volume 54, pp. 195–237. [Google Scholar]


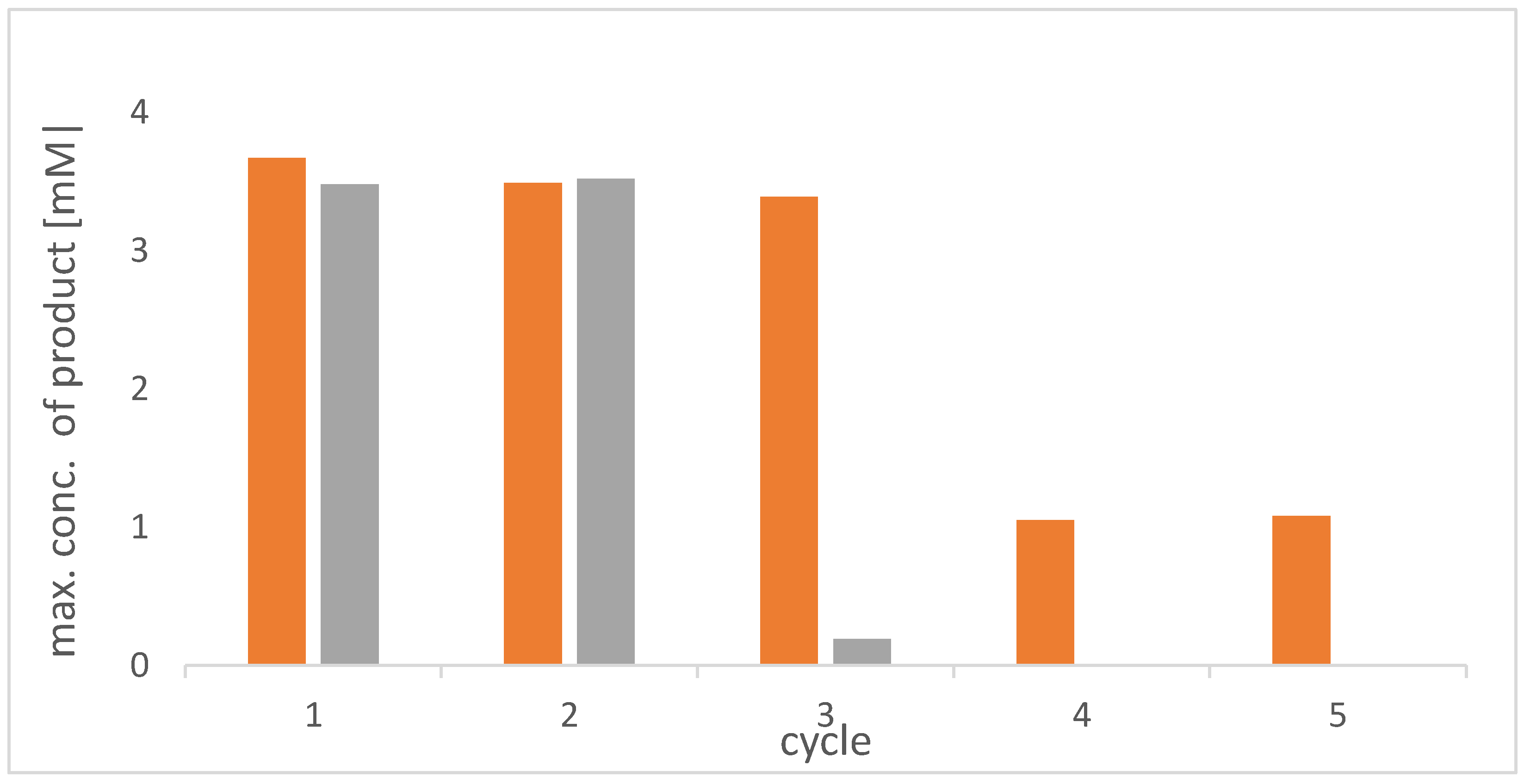


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bučko, M.; Gemeiner, P.; Krajčovič, T.; Hakarová, M.; Chorvát, D.; Chorvátová, A.M.; Lacík, I.; Rudroff, F.; Mihovilovič, M.D. Immobilized Cell Physiology Imaging and Stabilization of Enzyme Cascade Reaction Using Recombinant Cells Escherichia coli Entrapped in Polyelectrolyte Complex Beads by Jet Break-Up Encapsulator. Catalysts 2020, 10, 1288. https://doi.org/10.3390/catal10111288
Bučko M, Gemeiner P, Krajčovič T, Hakarová M, Chorvát D, Chorvátová AM, Lacík I, Rudroff F, Mihovilovič MD. Immobilized Cell Physiology Imaging and Stabilization of Enzyme Cascade Reaction Using Recombinant Cells Escherichia coli Entrapped in Polyelectrolyte Complex Beads by Jet Break-Up Encapsulator. Catalysts. 2020; 10(11):1288. https://doi.org/10.3390/catal10111288
Chicago/Turabian StyleBučko, Marek, Peter Gemeiner, Tomáš Krajčovič, Marietta Hakarová, Dušan Chorvát, Alžbeta Marček Chorvátová, Igor Lacík, Florian Rudroff, and Marko D. Mihovilovič. 2020. "Immobilized Cell Physiology Imaging and Stabilization of Enzyme Cascade Reaction Using Recombinant Cells Escherichia coli Entrapped in Polyelectrolyte Complex Beads by Jet Break-Up Encapsulator" Catalysts 10, no. 11: 1288. https://doi.org/10.3390/catal10111288
APA StyleBučko, M., Gemeiner, P., Krajčovič, T., Hakarová, M., Chorvát, D., Chorvátová, A. M., Lacík, I., Rudroff, F., & Mihovilovič, M. D. (2020). Immobilized Cell Physiology Imaging and Stabilization of Enzyme Cascade Reaction Using Recombinant Cells Escherichia coli Entrapped in Polyelectrolyte Complex Beads by Jet Break-Up Encapsulator. Catalysts, 10(11), 1288. https://doi.org/10.3390/catal10111288




