Advances in Clean Fuel Ethanol Production from Electro-, Photo- and Photoelectro-Catalytic CO2 Reduction
Abstract
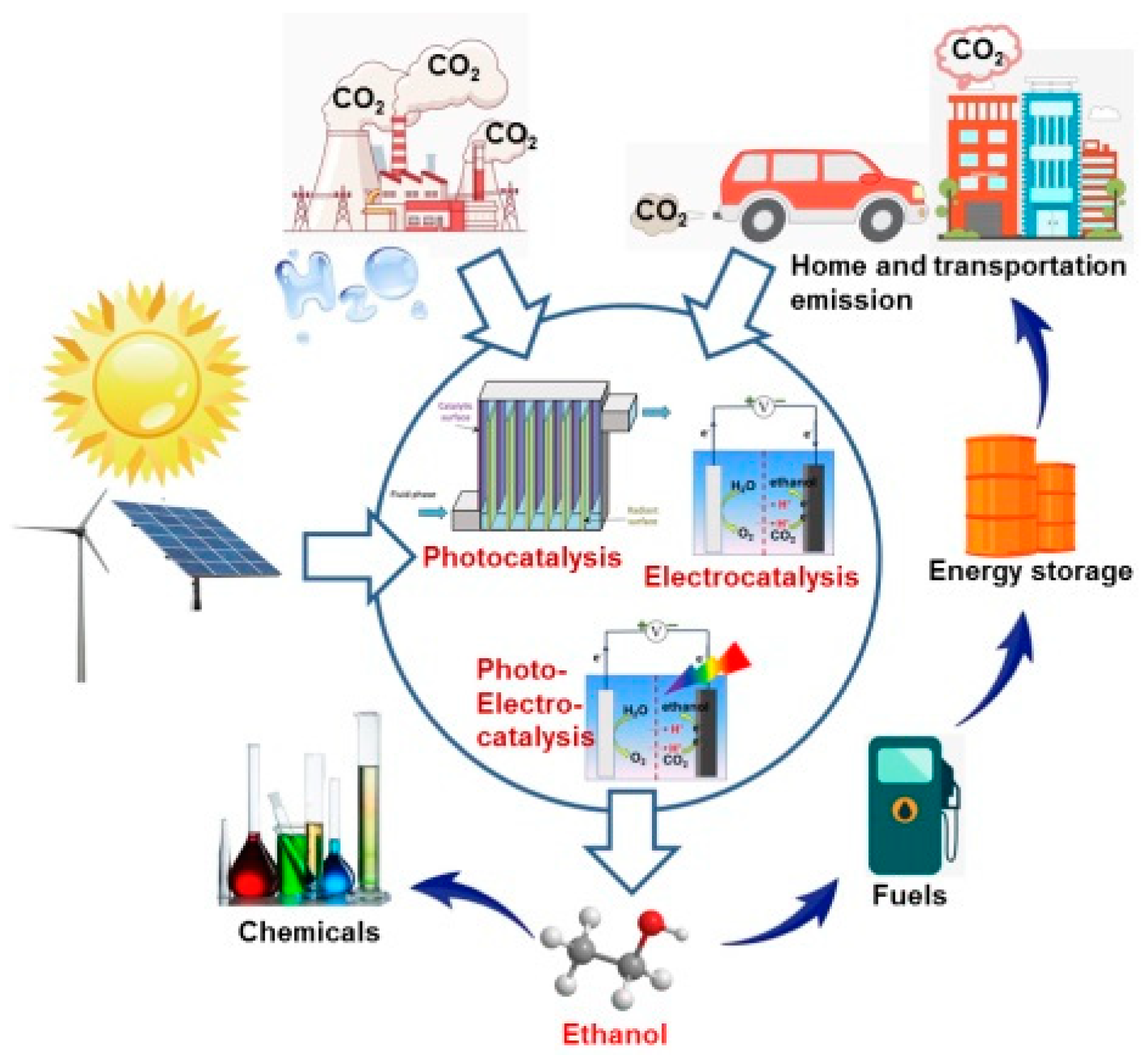
1. Introduction
2. Basic Principles of Clean Fuel Ethanol Production from CO2
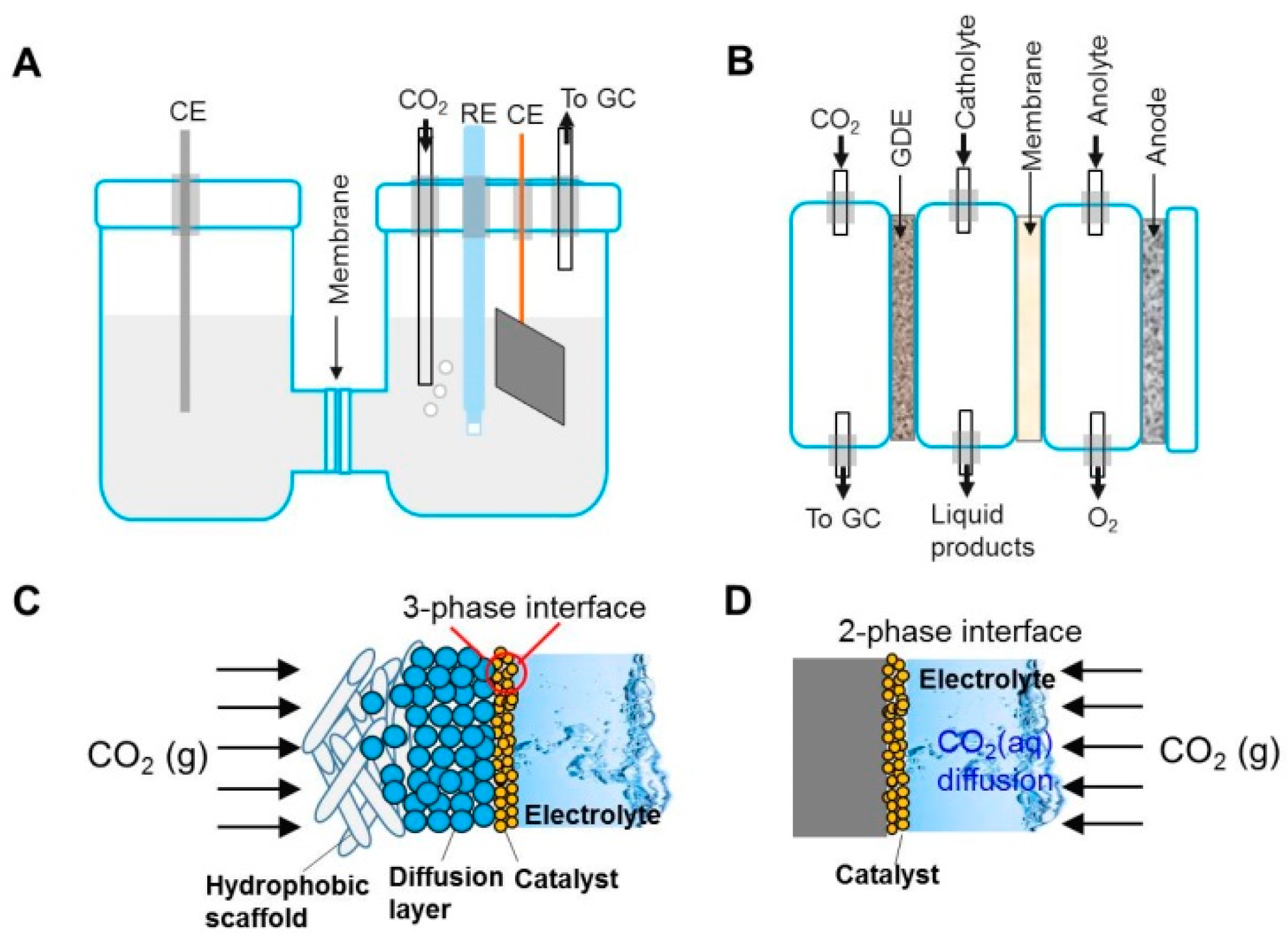
2.1. CO2 Electroreduction
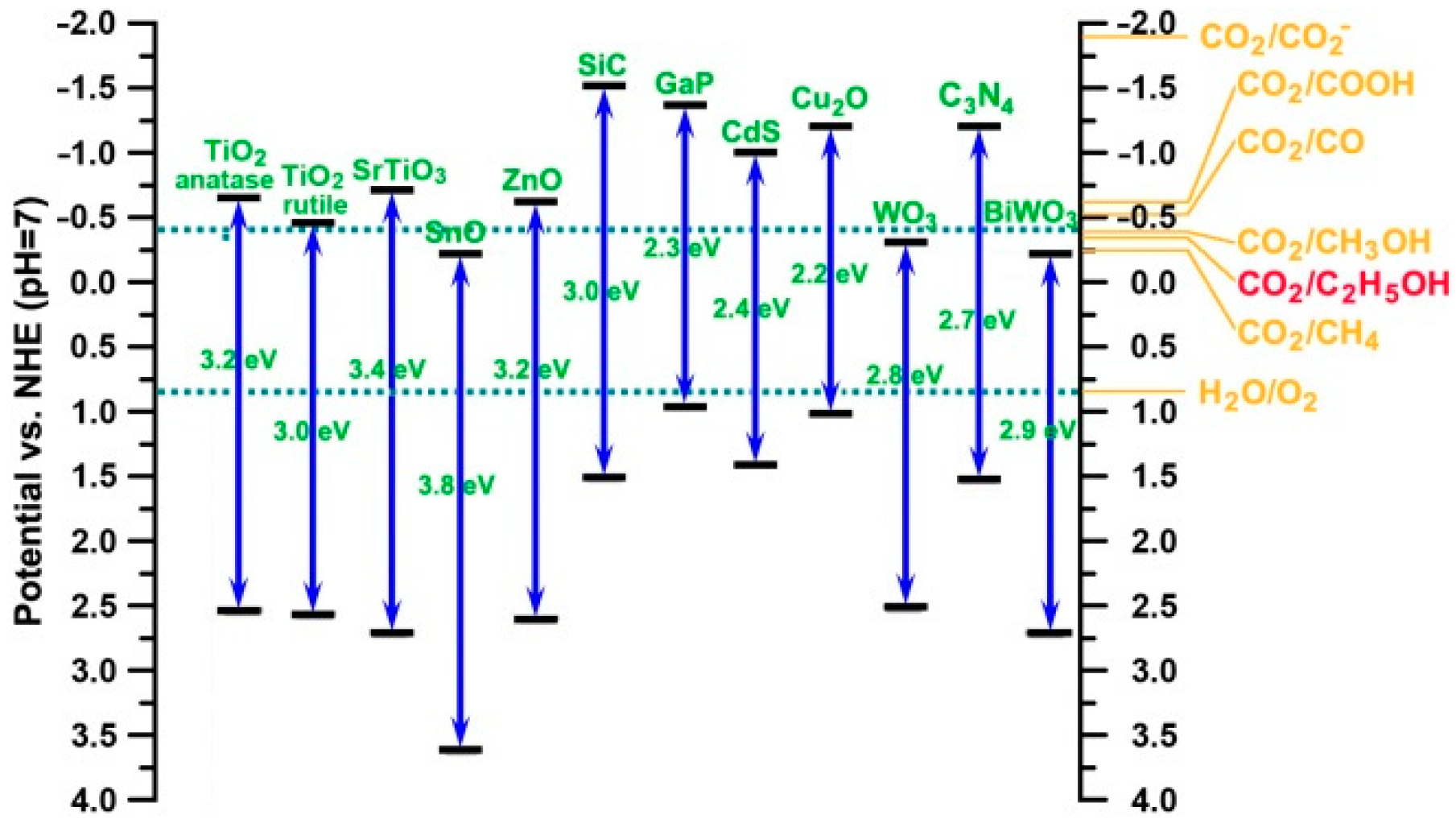
2.2. CO2 Photoreduction
2.3. CO2 Photoelectroreduction
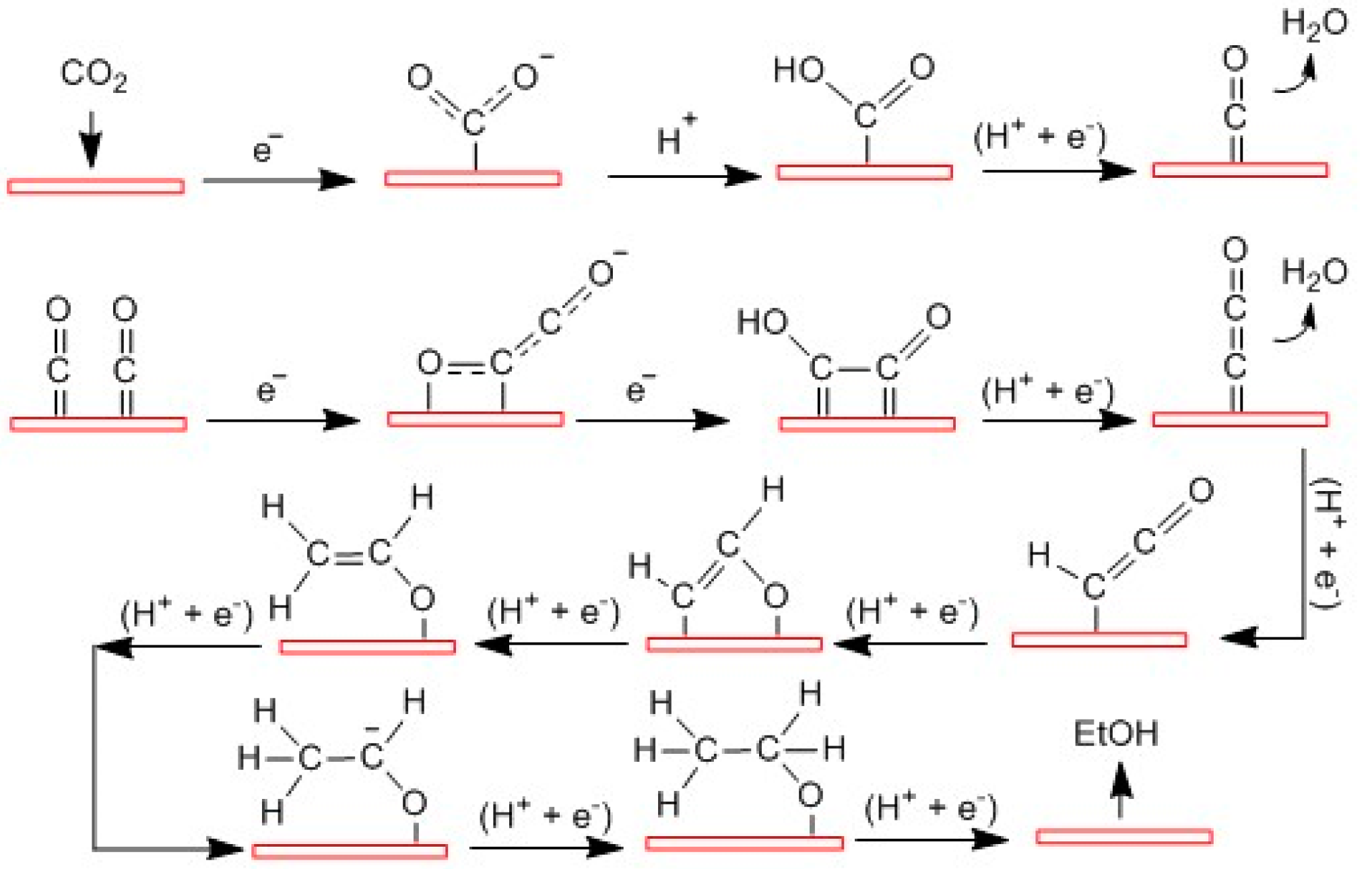
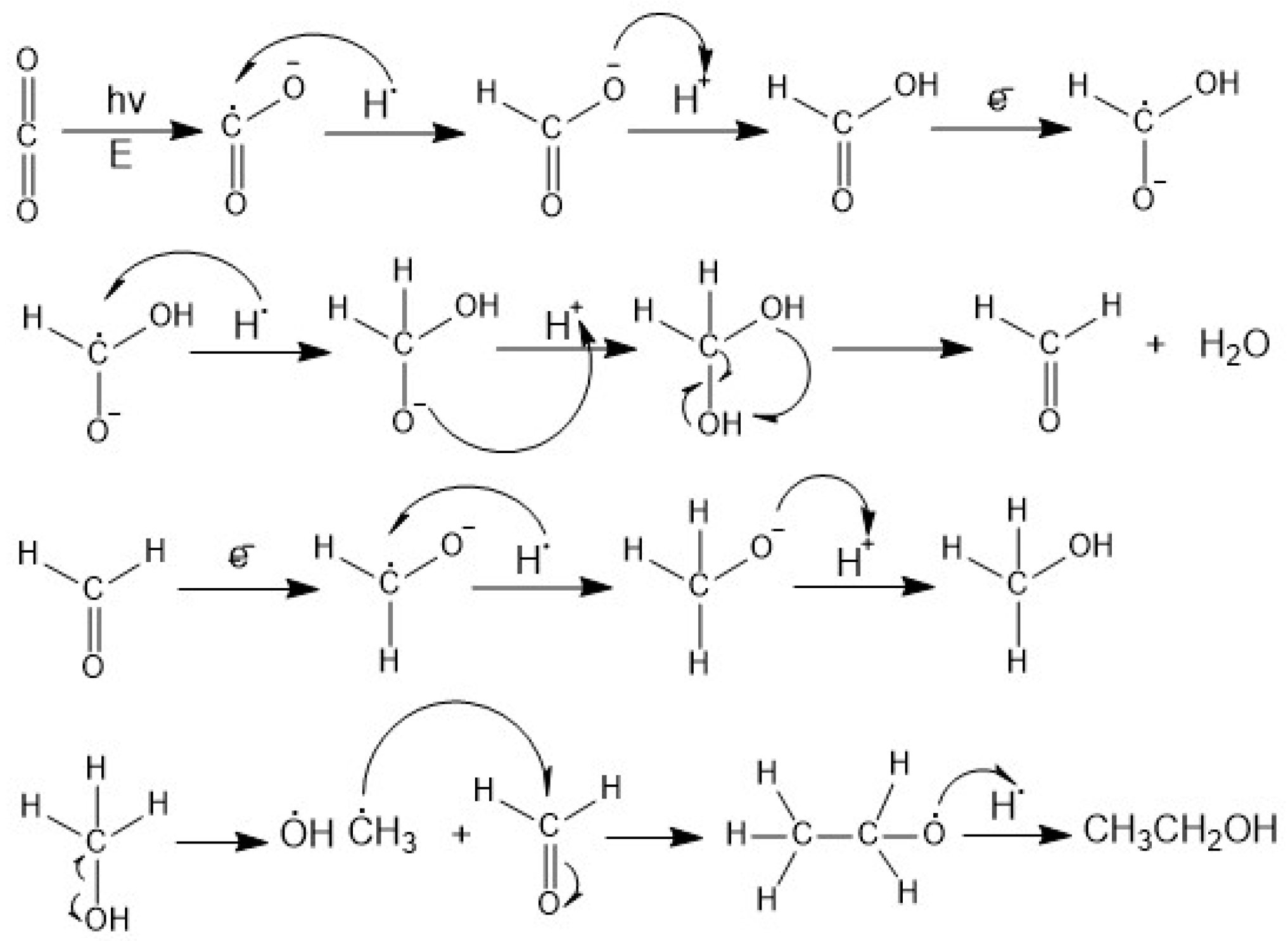
2.4. Mechanisms of Ethanol Production from CO2
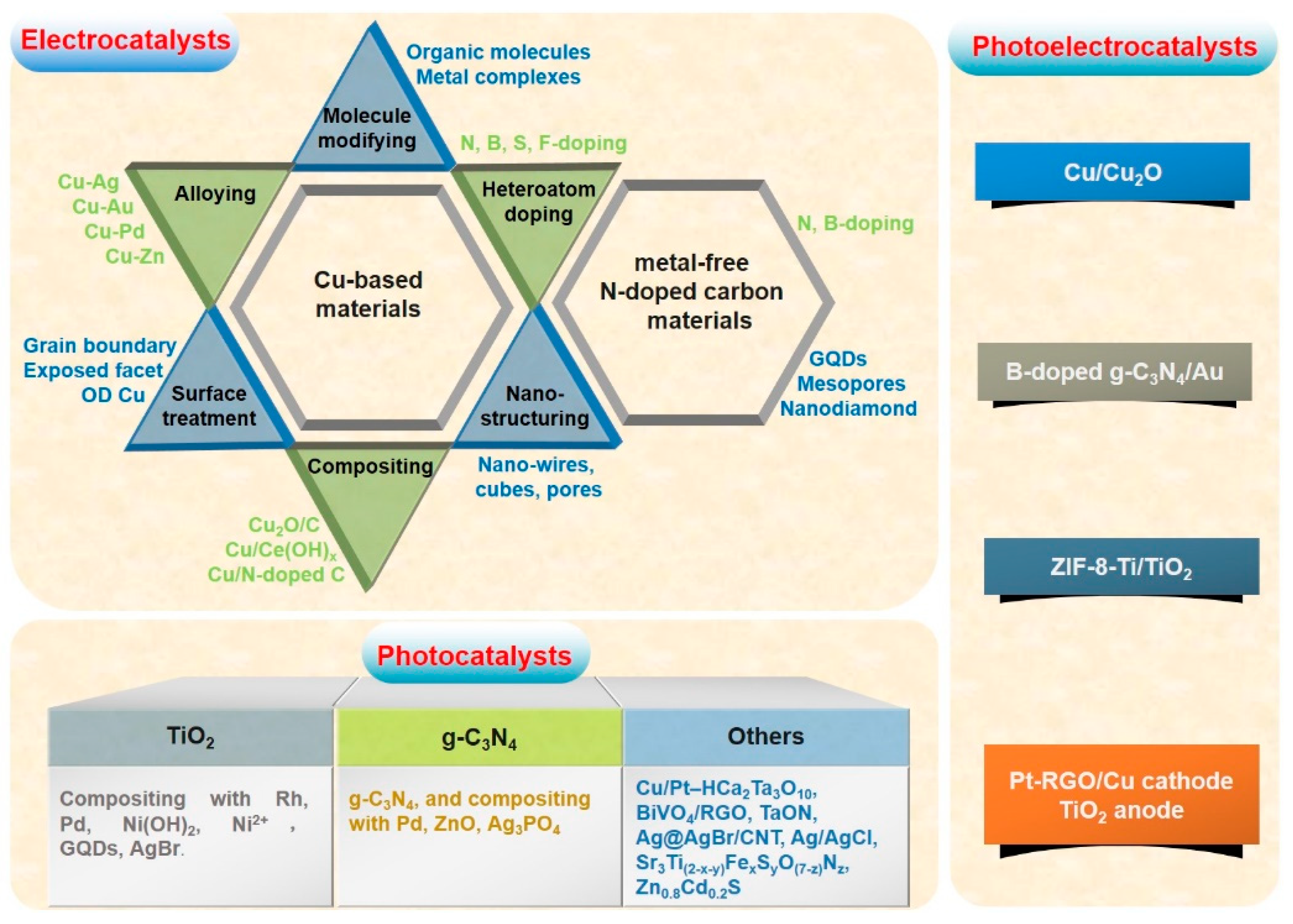
3. The Advances of CO2 Reduction to Clean Fuel Ethanol
3.1. Electrocatalytic CO2 Reduction to Ethanol
3.1.1. Modified Cu
3.1.2. Cu Alloy
3.1.3. Cu/Carbon Composites
3.1.4. Cu MOF
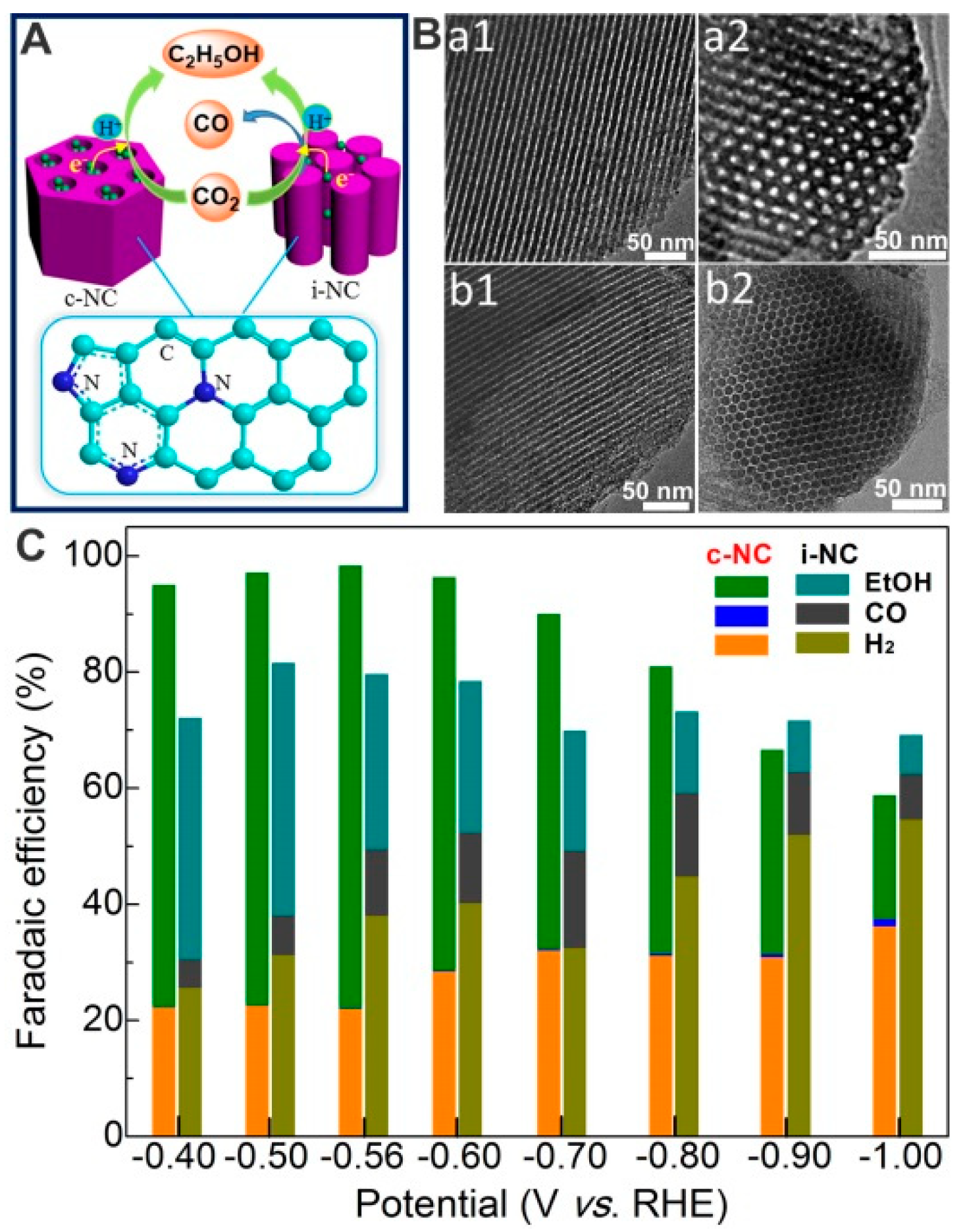
3.1.5. N-doped Carbon Materials
3.2. Photocatalytic CO2 Reduction to Ethanol
3.2.1. TiO2
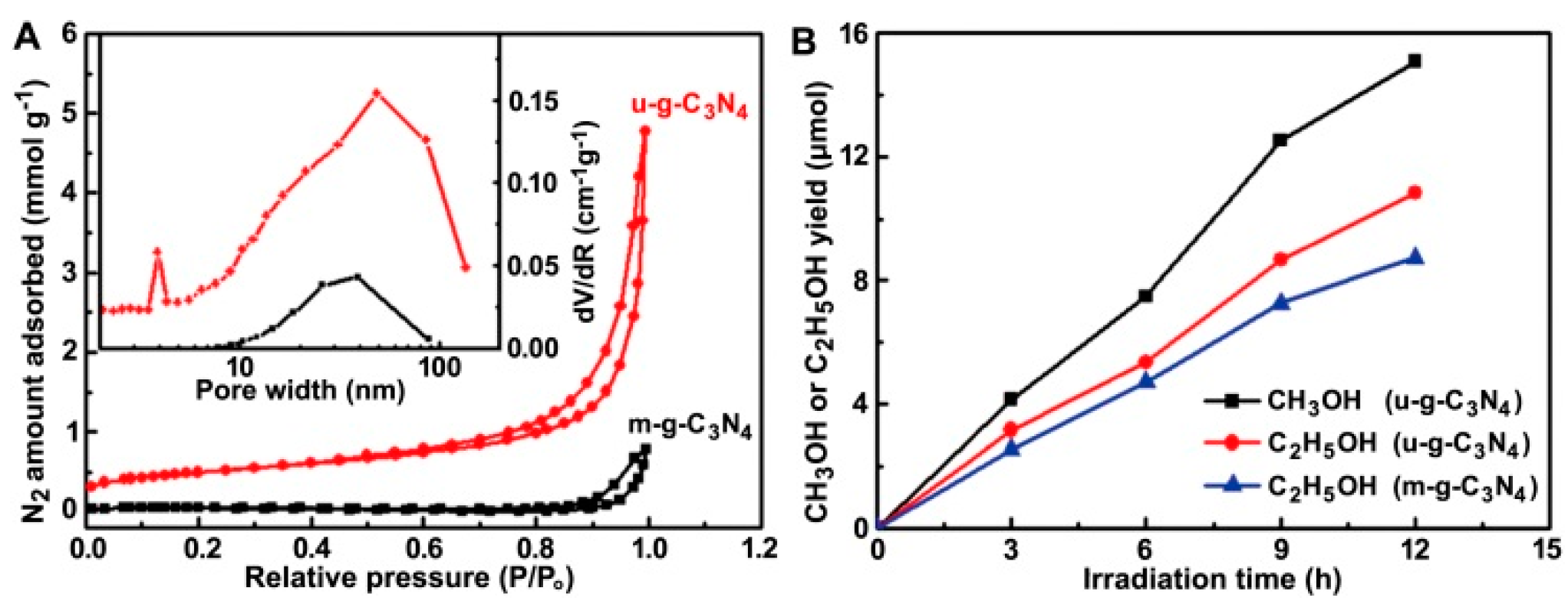
3.2.2. G-C3N4
3.2.3. Others
3.3. Photoelectrocatalytic CO2 Reduction to Ethanol
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Duan, X.; Xu, J.; Wei, Z.; Ma, J.; Guo, S.; Wang, S.; Liu, H.; Dou, S. Metal-Free Carbon Materials for CO2 Electrochemical Reduction. Adv. Mater. 2017, 29, 1701784. [Google Scholar] [CrossRef]
- Vasileff, A.; Zheng, Y.; Qiao, S.Z. Carbon Solving Carbon’s Problems: Recent Progress of Nanostructured Carbon-Based Catalysts for the Electrochemical Reduction of CO2. Adv. Energy Mater. 2017, 7, 1700759. [Google Scholar] [CrossRef]
- Tans, P.; Keeling, R. NORR/ESRL. Available online: http://www.esrl.noaa.gov/gmd/ccgg/trends/ (accessed on 25 May 2020).
- Li, K.; Peng, B.; Peng, T. Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- National Renewable Energy Information Management Platform. Available online: http://djfj.renewable.org.cn/ (accessed on 25 May 2020).
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R.; et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016, 353, 467–470. [Google Scholar]
- Gao, S.; Lin, Y.; Jiao, X.; Sun, Y.; Luo, Q.; Zhang, W.; Li, D.; Yang, J.; Xie, Y. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 2016, 529, 68–71. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.J.; Gong, J. Nanostructured Materials for Heterogeneous Electrocatalytic CO2 Reduction and Related Reaction Mechanisms. Angew. Chem. Int. Ed. 2017, 56, 11326–11353. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Q. Recent advances in breaking scaling relations for effective electrochemical conversion of CO2. Adv. Energy Mater. 2016, 6, 1600463. [Google Scholar] [CrossRef]
- Shih, C.F.; Zhang, T.; Li, J.; Bai, C. Powering the Future with Liquid Sunshine. Joule 2018, 2, 1925–1949. [Google Scholar] [CrossRef]
- Kumar, B.; Llorente, M.; Froehlich, J.; Dang, T.; Sathrum, A.; Kubiak, C.P. Photochemical and photoelectrochemical reduction of CO2. Annu. Rev. Phys. Chem. 2012, 63, 541–569. [Google Scholar] [CrossRef]
- Jones, J.-P.; Prakash, G.K.S.; Olah, G.A. Electrochemical CO2 Reduction: Recent Advances and Current Trends. Isr. J. Chem. 2014, 54, 1451–1466. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, X.; Wang, X.; Wang, X. Progress in catalyst exploration for heterogeneous CO2 reduction and utilization: A critical review. J. Mater. Chem. A 2017, 5, 21625–21649. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef]
- Tang, Q.; Lee, Y.; Li, D.Y.; Choi, W.; Liu, C.W.; Lee, D.; Jiang, D. Lattice-Hydride Mechanism in Electrocatalytic CO2 Reduction by Structurally Precise Copper-Hydride Nanoclusters. J. Am Chem. Soc. 2017, 139, 9728–9736. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef]
- Lim, R.J.; Xie, M.; Sk, M.A.; Lee, J.-M.; Fisher, A.; Wang, X.; Lim, K.H. A review on the electrochemical reduction of CO2 in fuel cells, metal electrodes and molecular catalysts. Catal. Today 2014, 233, 169–180. [Google Scholar] [CrossRef]
- Yang, K.D.; Ha, Y.; Sim, U.; An, J.; Lee, C.W.; Jin, K.; Kim, Y.; Park, J.; Hong, J.S.; Lee, J.H.; et al. Graphene Quantum Sheet Catalyzed Silicon Photocathode for Selective CO2 Conversion to CO. Adv. Funct. Mater. 2016, 26, 233–242. [Google Scholar] [CrossRef]
- Schneider, J.; Jia, H.; Muckerman, J.T.; Fujita, E. Thermodynamics and kinetics of CO2, CO, and H+ binding to the metal centre of CO2 reduction catalysts. Chem. Soc. Rev. 2012, 41, 2036–2051. [Google Scholar] [CrossRef]
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev. 2009, 38, 89–99. [Google Scholar] [CrossRef]
- Burdyny, T.; Smith, W.A. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy Environ. Sci. 2019, 12, 1442–1453. [Google Scholar] [CrossRef]
- Chen, C.; Khosrowabadi Kotyk, J.F.; Sheehan, S.W. Progress toward Commercial Application of Electrochemical Carbon Dioxide Reduction. Chem 2018, 4, 2571–2586. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, W.; Züttel, A. Self-supported copper-based gas diffusion electrodes for CO2 electrochemical reduction. J. Mater. Chem. A 2019, 7, 26285–26292. [Google Scholar] [CrossRef]
- Mao, J.; Li, K.; Peng, T. Recent advances in the photocatalytic CO2 reduction over semiconductors. Catal. Sci. Technol. 2013, 3, 2481–2498. [Google Scholar] [CrossRef]
- Li, K.; An, X.; Park, K.H.; Khraisheh, M.; Tang, J. A critical review of CO2 photoconversion: Catalysts and reactors. Catal. Today 2014, 224, 3–12. [Google Scholar] [CrossRef]
- Das, S.; Wan Daud, W.M.A. A review on advances in photocatalysts towards CO2 conversion. RSC Adv. 2014, 4, 20856. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Photocatalytic reduction of CO2 for fuel production: Possibilities and challenges. J. Catal. 2013, 308, 168–175. [Google Scholar] [CrossRef]
- Van Gerven, T.; Mul, G.; Moulijn, J.; Stankiewicz, A. A review of intensification of photocatalytic processes. Chem. Eng. Process. 2007, 46, 781–789. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Advances in visible light responsive titanium oxide-based photocatalysts for CO2 conversion to hydrocarbon fuels. Energy Convers. Manag. 2013, 76, 194–214. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Ran, J.; Jaroniec, M.; Qiao, S.Z. Cocatalysts in semiconductor-based photocatalytic CO2 reduction: Achievements, challenges, and opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef] [PubMed]
- Barton, E.E.; Rampulla, D.M.; Bocarsly, A.B. Selective solar-driven reduction of CO2 to methanol using a catalyzed p-GaP based photoelectrochemical cell. J. Am. Chem. Soc. 2008, 130, 6342–6344. [Google Scholar] [CrossRef]
- Ba, X.; Yan, L.-L.; Huang, S.; Yu, J.; Xia, X.-J.; Yu, Y. New Way for CO2 Reduction under Visible Light by a Combination of a Cu Electrode and Semiconductor Thin Film: Cu2O Conduction Type and Morphology Effect. J. Phys. Chem. C 2014, 118, 24467–24478. [Google Scholar] [CrossRef]
- Arai, T.; Sato, S.; Kajino, T.; Morikawa, T. Solar CO2 reduction using H2O by a semiconductor/metal-complex hybrid photocatalyst: Enhanced efficiency and demonstration of a wireless system using SrTiO3 photoanodes. Energy Environ. Sci. 2013, 6, 1274–1282. [Google Scholar] [CrossRef]
- Ganesh, I. Conversion of carbon dioxide into methanol–a potential liquid fuel: Fundamental challenges and opportunities (a review). Renew. Sustain. Energy Rev. 2014, 31, 221–257. [Google Scholar] [CrossRef]
- Indrakanti, V.P.; Kubicki, J.D.; Schobert, H.H. Photoinduced activation of CO2 on Ti-based heterogeneous catalysts: Current state, chemical physics-based insights and outlook. Energy Environ. Sci. 2009, 2, 745–758. [Google Scholar] [CrossRef]
- Indrakanti, V.P.; Schobert, H.H.; Kubicki, J.D. Quantum Mechanical Modeling of CO2 Interactions with Irradiated Stoichiometric and Oxygen-Deficient Anatase TiO2 Surfaces: Implications for the Photocatalytic Reduction of CO2. Energy Fuels 2009, 23, 5247–5256. [Google Scholar] [CrossRef]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef]
- Song, Y.; Peng, R.; Hensley, D.K.; Bonnesen, P.V.; Liang, L.; Wu, Z.; Meyer, H.M.; Chi, M.; Ma, C.; Sumpter, B.G.; et al. High-Selectivity Electrochemical Conversion of CO2 to Ethanol using a Copper Nanoparticle/N-Doped Graphene Electrode. ChemistrySelect 2016, 1, 6055–6061. [Google Scholar] [CrossRef]
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Norskov, J.K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010, 3, 1311–1315. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Koper, M.T. Theoretical considerations on the electroreduction of CO to C2 species on Cu(100) electrodes. Angew. Chem. Int. Ed. 2013, 52, 7282–7285. [Google Scholar] [CrossRef]
- Ren, D.; Ang, B.S.-H.; Yeo, B.S. Tuning the Selectivity of Carbon Dioxide Electroreduction toward Ethanol on Oxide-Derived CuxZn Catalysts. ACS Catal. 2016, 6, 8239–8247. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper(I) Oxide Catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- Yin, Z.; Yu, C.; Zhao, Z.; Guo, X.; Shen, M.; Li, N.; Muzzio, M.; Li, J.; Liu, H.; Lin, H.; et al. Cu3N Nanocubes for Selective Electrochemical Reduction of CO2 to Ethylene. Nano Lett. 2019, 19, 8658–8663. [Google Scholar] [CrossRef]
- De Brito, J.F.; Araujo, A.R.; Rajeshwar, K.; Zanoni, M.V.B. Photoelectrochemical reduction of CO2 on Cu/Cu2O films: Product distribution and pH effects. Chem. Eng. J. 2015, 264, 302–309. [Google Scholar] [CrossRef]
- Teeter, T.E.; Van Rysselberghe, P. Reduction of Carbon Dioxide on Mercury Cathodes. J. Chem. Phys. 1954, 22, 759–760. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, Q.; Xia, R.; Meyer, T.J. CO2 reduction: From homogeneous to heterogeneous electrocatalysis. Acc. Chem. Res. 2020, 53, 255–264. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019. [Google Scholar] [CrossRef]
- Popovic, S.; Smiljanic, M.; Jovanovic, P.; Vavra, J.; Buonsanti, R.; Hodnik, N. Stability and degradation mechanisms of copper-based catalysts for electrochemical CO2 reduction. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Ma, M.; Djanashvili, K.; Smith, W.A. Controllable Hydrocarbon Formation from the Electrochemical Reduction of CO2 over Cu Nanowire Arrays. Angew. Chem. Int. Ed. 2016, 55, 6680–6684. [Google Scholar] [CrossRef]
- Lv, J.J.; Jouny, M.; Luc, W.; Zhu, W.; Zhu, J.J.; Jiao, F. A Highly Porous Copper Electrocatalyst for Carbon Dioxide Reduction. Adv. Mater. 2018, 30, e1803111. [Google Scholar] [CrossRef]
- Lum, Y.; Yue, B.; Lobaccaro, P.; Bell, A.T.; Ager, J.W. Optimizing C–C Coupling on Oxide-Derived Copper Catalysts for Electrochemical CO2 Reduction. J. Phys. Chem. C 2017, 121, 14191–14203. [Google Scholar] [CrossRef]
- De Luna, P.; Quintero-Bermudez, R.; Dinh, C.-T.; Ross, M.B.; Bushuyev, O.S.; Todorović, P.; Regier, T.; Kelley, S.O.; Yang, P.; Sargent, E.H. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 2018, 1, 103–110. [Google Scholar] [CrossRef]
- Loiudice, A.; Lobaccaro, P.; Kamali, E.A.; Thao, T.; Huang, B.H.; Ager, J.W.; Buonsanti, R. Tailoring Copper Nanocrystals towards C2 Products in Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2016, 55, 5789–5792. [Google Scholar] [CrossRef]
- Jiang, K.; Sandberg, R.B.; Akey, A.J.; Liu, X.; Bell, D.C.; Nørskov, J.K.; Chan, K.; Wang, H. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. Nat. Catal. 2018, 1, 111–119. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, T.; Liu, B.; Cheng, D.; Hu, C.; Zhang, G.; Zhu, W.; Wang, H.; Zhao, Z.J.; Gong, J. Grain-Boundary-Rich Copper for Efficient Solar-Driven Electrochemical CO2 Reduction to Ethylene and Ethanol. J. Am. Chem.Soc. 2020, 142, 6878–6883. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, X.; Yang, D.; Ma, J.; Kang, X.; Zheng, L.; Zhang, J.; Wu, Z.; Han, B. Carbon dioxide electroreduction to C2 products over copper-cuprous oxide derived from electrosynthesized copper complex. Nat. Commun. 2019, 10, 3851. [Google Scholar] [CrossRef]
- Yang, P.P.; Zhang, X.L.; Gao, F.Y.; Zheng, Y.R.; Niu, Z.Z.; Yu, X.; Liu, R.; Wu, Z.Z.; Qin, S.; Chi, L.P.; et al. Protecting Copper Oxidation State via Intermediate Confinement for Selective CO2 Electroreduction to C2+ Fuels. J. Am. Chem. Soc. 2020, 142, 6400–6408. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.Q.; Zhuang, T.T.; Seifitokaldani, A.; Li, J.; Huang, C.W.; Tan, C.S.; Li, Y.; De Luna, P.; Dinh, C.T.; Hu, Y.; et al. Copper-on-nitride enhances the stable electrosynthesis of multi-carbon products from CO2. Nat. Commun. 2018, 9, 3828. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Lu, L.; Yang, D.; Ma, J.; Zhu, Q.; Qian, Q.; Han, B. Efficient electroreduction of CO2 to C2 products over B-doped oxide-derived copper. Green Chem. 2018, 20, 4579–4583. [Google Scholar] [CrossRef]
- Zhou, Y.; Che, F.; Liu, M.; Zou, C.; Liang, Z.; De Luna, P.; Yuan, H.; Li, J.; Wang, Z.; Xie, H.; et al. Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat. Chem. 2018, 10, 974–980. [Google Scholar] [CrossRef]
- Zhuang, T.-T.; Liang, Z.-Q.; Seifitokaldani, A.; Li, Y.; De Luna, P.; Burdyny, T.; Che, F.; Meng, F.; Min, Y.; Quintero-Bermudez, R.; et al. Steering post-C–C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi-carbon alcohols. Nat. Catal. 2018, 1, 421–428. [Google Scholar] [CrossRef]
- Ma, W.; Xie, S.; Liu, T.; Fan, Q.; Ye, J.; Sun, F.; Jiang, Z.; Zhang, Q.; Cheng, J.; Wang, Y. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat. Catal. 2020. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Z.; Li, Y.C.; Li, J.; Li, F.; Lum, Y.; Nam, D.H.; Chen, B.; Wicks, J.; Xu, A.; et al. Hydroxide promotes carbon dioxide electroreduction to ethanol on copper via tuning of adsorbed hydrogen. Nat. Commun. 2019, 10, 5814. [Google Scholar] [CrossRef]
- Han, Z.; Kortlever, R.; Chen, H.Y.; Peters, J.C.; Agapie, T. CO2 Reduction Selective for C≥2 Products on Polycrystalline Copper with N-Substituted Pyridinium Additives. ACS Cent. Sci. 2017, 3, 853–859. [Google Scholar] [CrossRef]
- Thevenon, A.; Rosas-Hernandez, A.; Peters, J.C.; Agapie, T. In-Situ Nanostructuring and Stabilization of Polycrystalline Copper by an Organic Salt Additive Promotes Electrocatalytic CO2 Reduction to Ethylene. Angew. Chem. Int. Ed. 2019, 58, 16952–16958. [Google Scholar] [CrossRef]
- Wakerley, D.; Lamaison, S.; Ozanam, F.; Menguy, N.; Mercier, D.; Marcus, P.; Fontecave, M.; Mougel, V. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface. Nat. Mater. 2019, 18, 1222–1227. [Google Scholar] [CrossRef]
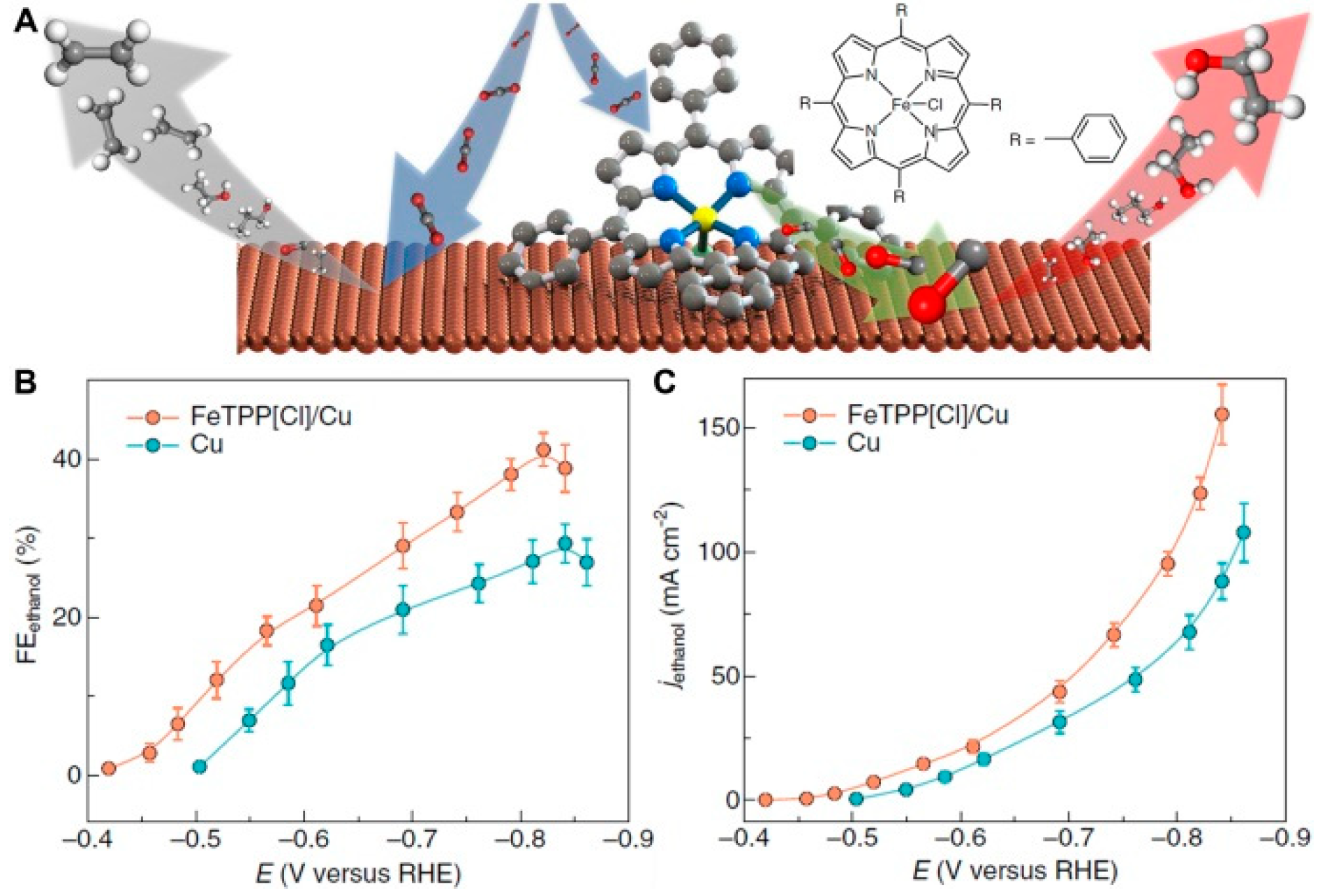
- Li, F.; Li, Y.C.; Wang, Z.; Li, J.; Nam, D.-H.; Lum, Y.; Luo, M.; Wang, X.; Ozden, A.; Hung, S.-F.; et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule–metal catalyst interfaces. Nat. Catal. 2019, 3, 75–82. [Google Scholar] [CrossRef]
- Jia, F.; Yu, X.; Zhang, L. Enhanced selectivity for the electrochemical reduction of CO2 to alcohols in aqueous solution with nanostructured Cu–Au alloy as catalyst. J. Power Sources 2014, 252, 85–89. [Google Scholar] [CrossRef]
- Ishimaru, S.; Shiratsuchi, R.; Nogami, G. Pulsed Electroreduction of CO2 on Cu-Ag Alloy Electrodes. J. Electrochem. Soc. 2000, 147, 1864. [Google Scholar] [CrossRef]
- Hoang, T.T.H.; Verma, S.; Ma, S.; Fister, T.T.; Timoshenko, J.; Frenkel, A.I.; Kenis, P.J.A.; Gewirth, A.A. Nanoporous Copper-Silver Alloys by Additive-Controlled Electrodeposition for the Selective Electroreduction of CO2 to Ethylene and Ethanol. J. Am. Chem. Soc. 2018, 140, 5791–5797. [Google Scholar] [CrossRef]
- Dutta, A.; Montiel, I.Z.; Erni, R.; Kiran, K.; Rahaman, M.; Drnec, J.; Broekmann, P. Activation of bimetallic AgCu foam electrocatalysts for ethanol formation from CO2 by selective Cu oxidation/reduction. Nano Energy 2020, 68. [Google Scholar] [CrossRef]
- Ma, S.; Sadakiyo, M.; Heima, M.; Luo, R.; Haasch, R.T.; Gold, J.I.; Yamauchi, M.; Kenis, P.J. Electroreduction of Carbon Dioxide to Hydrocarbons Using Bimetallic Cu-Pd Catalysts with Different Mixing Patterns. J. Am. Chem. Soc. 2017, 139, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Gao, J.; Pan, L.; Wang, Z.; Luo, J.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Atomic Layer Deposition of ZnO on CuO Enables Selectiveand Efficient Electroreduction of Carbon Dioxide to Liquid Fuels. Angew. Chem. Int. Ed. 2019, 58, 15036–15040. [Google Scholar] [CrossRef]
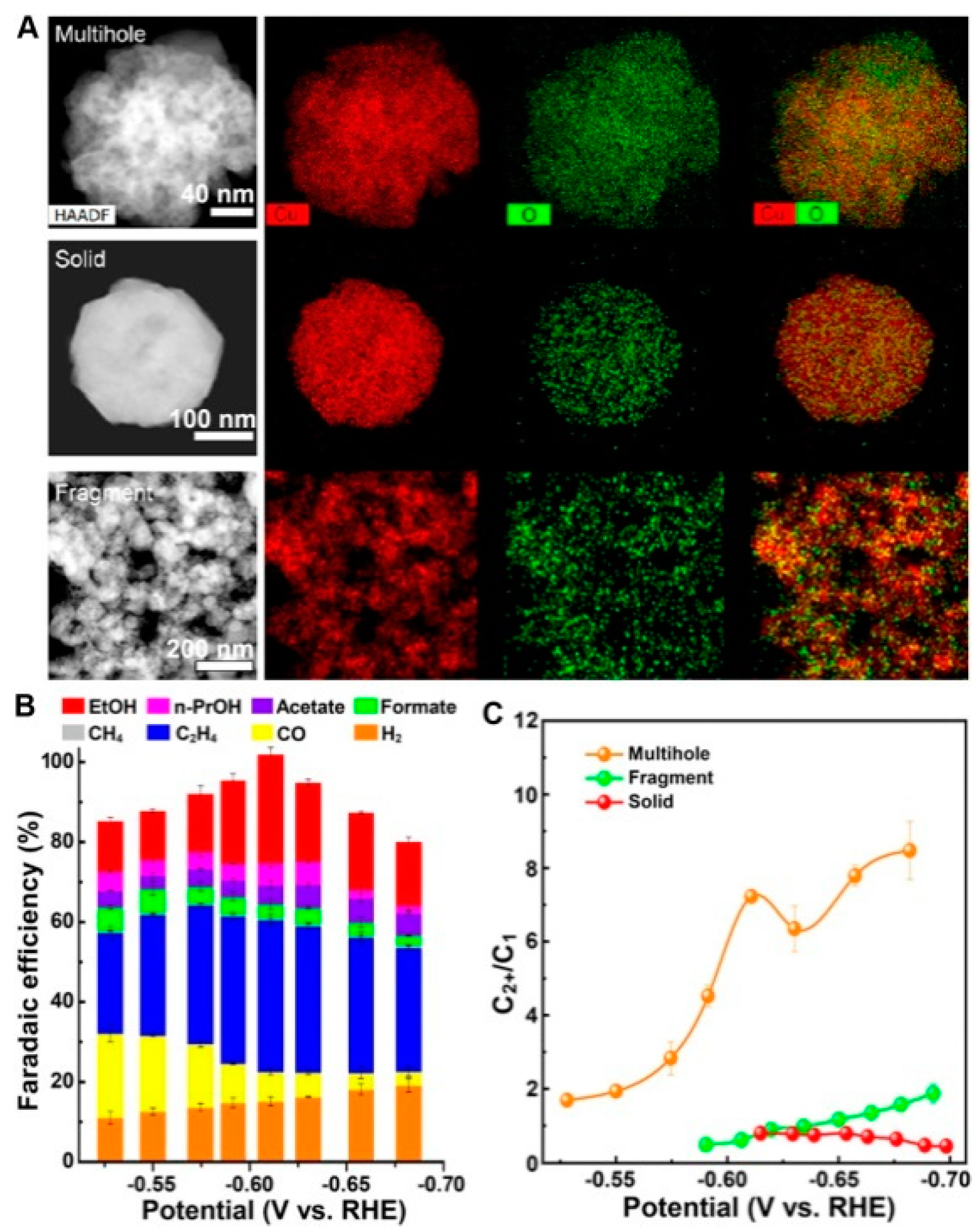
- Jung, H.; Lee, S.Y.; Lee, C.W.; Cho, M.K.; Won, D.H.; Kim, C.; Oh, H.S.; Min, B.K.; Hwang, Y.J. Electrochemical Fragmentation of Cu2O Nanoparticles Enhancing Selective C-C Coupling from CO2 Reduction Reaction. J. Am. Chem. Soc. 2019, 141, 4624–4633. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, Y.; Quan, X.; Chen, S.; Yu, H. CO2 Electroreduction at Low Overpotential on Oxide-Derived Cu/Carbons Fabricated from Metal Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 5302–5311. [Google Scholar] [CrossRef]
- Han, H.; Noh, Y.; Kim, Y.; Park, S.; Yoon, W.; Jang, D.; Choi, S.M.; Kim, W.B. Selective electrochemical CO2 conversion to multicarbon alcohols on highly efficient N-doped porous carbon-supported Cu catalysts. Green Chem. 2020, 22, 71–84. [Google Scholar] [CrossRef]
- Wu, J.; Ma, S.; Sun, J.; Gold, J.I.; Tiwary, C.; Kim, B.; Zhu, L.; Chopra, N.; Odeh, I.N.; Vajtai, R.; et al. A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates. Nat. Commun. 2016, 7, 13869. [Google Scholar] [CrossRef]
- Song, Y.; Chen, W.; Zhao, C.; Li, S.; Wei, W.; Sun, Y. Metal-Free Nitrogen-Doped Mesoporous Carbon for Electroreduction of CO2 to Ethanol. Angew. Chem. Int. Ed. 2017, 56, 10840–10844. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, S.; Chen, W.; Li, S.; Feng, G.; Wei, W.; Sun, Y. Enhanced Ethanol Production from CO2 Electroreduction at Micropores in Nitrogen-Doped Mesoporous Carbon. ChemSusChem 2020, 13, 293–297. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Cheng, K.; Quan, X.; Fan, X.; Su, Y.; Chen, S.; Zhao, H.; Zhang, Y.; Yu, H.; et al. Selective electrochemical reduction of CO2 to ethanol on B and N codoped nanodiamond. Angew. Chem. Int. Ed. 2017, 56, 15607–15611. [Google Scholar] [CrossRef]
- Reller, C.; Krause, R.; Volkova, E.; Schmid, B.; Neubauer, S.; Rucki, A.; Schuster, M.; Schmid, G. Selective Electroreduction of CO2 toward Ethylene on Nano Dendritic Copper Catalysts at High Current Density. Adv. Energy Mater. 2017. [Google Scholar] [CrossRef]
- Li, C.W.; Kanan, M.W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef]
- Handoko, A.D.; Chan, K.W.; Yeo, B.S. –CH3 Mediated Pathway for the Electroreduction of CO2 to Ethane and Ethanol on Thick Oxide-Derived Copper Catalysts at Low Overpotentials. ACS Energy Lett. 2017, 2, 2103–2109. [Google Scholar] [CrossRef]
- Lei, Q.; Zhu, H.; Song, K.; Wei, N.; Liu, L.; Zhang, D.; Yin, J.; Dong, X.; Yao, K.; Wang, N.; et al. Investigating the Origin of Enhanced C2+ Selectivity in Oxide-/Hydroxide-Derived Copper Electrodes during CO2 Electroreduction. J. Am. Chem. Soc. 2020, 142, 4213–4222. [Google Scholar] [CrossRef]
- Nam, D.H.; De Luna, P.; Rosas-Hernandez, A.; Thevenon, A.; Li, F.; Agapie, T.; Peters, J.C.; Shekhah, O.; Eddaoudi, M.; Sargent, E.H. Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 2020, 19, 266–276. [Google Scholar] [CrossRef]
- Vasileff, A.; Xu, C.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Surface and Interface Engineering in Copper-Based Bimetallic Materials for Selective CO2 Electroreduction. Chem 2018, 4, 1809–1831. [Google Scholar] [CrossRef]
- Lee, C.W.; Yang, K.D.; Nam, D.H.; Jang, J.H.; Cho, N.H.; Im, S.W.; Nam, K.T. Defining a materials database for the design of copper binary alloy catalysts for electrochemical CO2 conversion. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Albo, J.; Vallejo, D.; Beobide, G.; Castillo, O.; Castano, P.; Irabien, A. Copper-Based Metal-Organic Porous Materials for CO2 Electrocatalytic Reduction to Alcohols. ChemSusChem 2017, 10, 1100–1109. [Google Scholar] [CrossRef]
- Vu, N.N.; Kaliaguine, S.; Do, T.O. Critical Aspects and Recent Advances in Structural Engineering of Photocatalysts for Sunlight-Driven Photocatalytic Reduction of CO2 into Fuels. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Whang, D.R.; Apaydin, D.H. Artificial Photosynthesis: Learning from Nature. ChemPhotoChem 2018, 2, 148–160. [Google Scholar] [CrossRef]
- Zhao, T.-T.; Feng, G.-H.; Chen, W.; Song, Y.-F.; Dong, X.; Li, G.-H.; Zhang, H.-J.; Wei, W. Artificial bioconversion of carbon dioxide. Chin. J. Catal. 2019, 40, 1421–1437. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, B.; Ran, J.; Davey, K.; Qiao, S.Z. Atomic-Level Reactive Sites for Semiconductor-Based Photocatalytic CO2 Reduction. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Huang, H.; Zhang, Y.; Ma, T. Surface sites engineering on semiconductors to boost photocatalytic CO2 reduction. Nano Energy 2020, 75. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Z.; Lang, Q.; Jiang, W.; Yin, Q.; Zhong, S.; Bai, S. Grain boundary engineered metal nanowire cocatalysts for enhanced photocatalytic reduction of carbon dioxide. Appl. Catal. B Environ. 2017, 206, 282–292. [Google Scholar] [CrossRef]
- Meng, A.; Wu, S.; Cheng, B.; Yu, J.; Xu, J. Hierarchical TiO2/Ni(OH)2 composite fibers with enhanced photocatalytic CO2 reduction performance. J. Mater. Chem. A 2018, 6, 4729–4736. [Google Scholar] [CrossRef]
- Ola, O.; Mercedes Maroto-Valer, M. Role of catalyst carriers in CO2 photoreduction over nanocrystalline nickel loaded TiO2-based photocatalysts. J. Catal. 2014, 309, 300–308. [Google Scholar] [CrossRef]
- Zou, J.-P.; Wu, D.-D.; Luo, J.; Xing, Q.-J.; Luo, X.-B.; Dong, W.-H.; Luo, S.-L.; Du, H.-M.; Suib, S.L. A Strategy for One-Pot Conversion of Organic Pollutants into Useful Hydrocarbons through Coupling Photodegradation of MB with Photoreduction of CO2. ACS Catal. 2016, 6, 6861–6867. [Google Scholar] [CrossRef]
- Abou Asi, M.; He, C.; Su, M.; Xia, D.; Lin, L.; Deng, H.; Xiong, Y.; Qiu, R.; Li, X.-Z. Photocatalytic reduction of CO2 to hydrocarbons using AgBr/TiO2 nanocomposites under visible light. Catal. Today 2011, 175, 256–263. [Google Scholar] [CrossRef]
- Mao, J.; Peng, T.; Zhang, X.; Li, K.; Ye, L.; Zan, L. Effect of graphitic carbon nitride microstructures on the activity and selectivity of photocatalytic CO2 reduction under visible light. Catal. Sci. Technol. 2013, 3, 1253. [Google Scholar] [CrossRef]
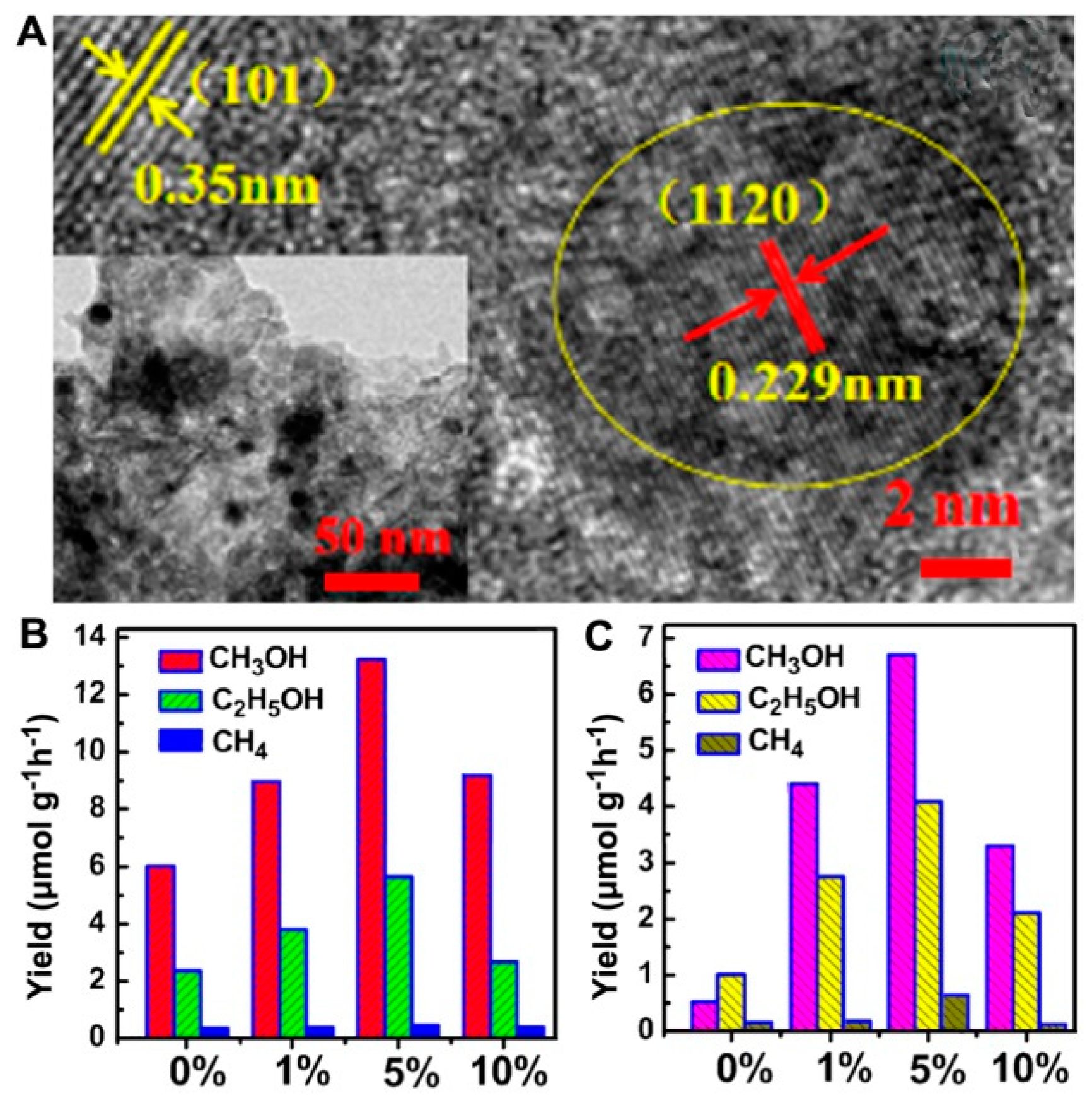
- He, Y.; Wang, Y.; Zhang, L.; Teng, B.; Fan, M. High-efficiency conversion of CO2 to fuel over ZnO/g-C3N4 photocatalyst. Appl. Catal. B Environ. 2015, 168, 1–8. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Teng, B.; Fan, M. New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel. Environ. Sci. Technol. 2015, 49, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wang, X.; Hu, C.; Xie, M.; Jiang, J.; Xiong, Y. Two-dimensional g-C3N4: An ideal platform for examining facet selectivity of metal co-catalysts in photocatalysis. Chem. Commun. 2014, 50, 6094–6097. [Google Scholar] [CrossRef]
- Vu, N.-N.; Nguyen, C.-C.; Kaliaguine, S.; Do, T.-O. Reduced Cu/Pt-HCa2Ta3O10 Perovskite Nanosheets for Sunlight-Driven Conversion of CO2 into Valuable Fuels. Adv. Sustain. Syst. 2017, 1. [Google Scholar] [CrossRef]
- Wang, A.; Shen, S.; Zhao, Y.; Wu, W. Preparation and characterizations of BiVO4/reduced graphene oxide nanocomposites with higher visible light reduction activities. J. Colloid Interface Sci. 2015, 445, 330–336. [Google Scholar] [CrossRef]
- Han, Q.; Zhou, Y.; Tang, L.; Li, P.; Tu, W.; Li, L.; Li, H.; Zou, Z. Synthesis of single-crystalline, porous TaON microspheres toward visible-light photocatalytic conversion of CO2 into liquid hydrocarbon fuels. RSC Adv. 2016, 6, 90792–90796. [Google Scholar] [CrossRef]
- Abou Asi, M.; Zhu, L.; He, C.; Sharma, V.K.; Shu, D.; Li, S.; Yang, J.; Xiong, Y. Visible-light-harvesting reduction of CO2 to chemical fuels with plasmonic Ag@AgBr/CNT nanocomposites. Catal. Today 2013, 216, 268–275. [Google Scholar] [CrossRef]
- Cai, B.; Wang, J.; Gan, S.; Han, D.; Wu, Z.; Niu, L. A distinctive red Ag/AgCl photocatalyst with efficient photocatalytic oxidative and reductive activities. J. Mater. Chem. A 2014, 2, 5280–5286. [Google Scholar] [CrossRef]
- Jeyalakshmi, V.; Mahalakshmy, R.; Krishnamurthy, K.R.; Viswanathan, B. Strontium titanates with perovskite structure as photo catalysts for reduction of CO2 by water: Influence of co-doping with N, S & Fe. Catal. Today 2018, 300, 152–159. [Google Scholar] [CrossRef]
- Tang, L.; Kuai, L.; Li, Y.; Li, H.; Zhou, Y.; Zou, Z. ZnxCd1-xS tunable band structure-directing photocatalytic activity and selectivity of visible-light reduction of CO2 into liquid solar fuels. Nanotechnology 2018, 29. [Google Scholar] [CrossRef]
- Halmann, M. Photoelectrochemical reduction of aqueous carbon dioxide on p-type gallium phosphide in liquid junction solar cells. Nature 1978, 275, 115–116. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, M.; Wu, G.; Wang, X.; Zhou, J.; Cen, K. Photoelectrocatalytic Reduction of CO2 into Chemicals Using Pt-Modified Reduced Graphene Oxide Combined with Pt-Modified TiO2 Nanotubes. Environ. Sci. Technol. 2014, 48, 7076–7084. [Google Scholar] [CrossRef]
- Sagara, N.; Kamimura, S.; Tsubota, T.; Ohno, T. Photoelectrochemical CO2 reduction by a p-type boron-doped g-C3N4 electrode under visible light. Appl. Catal. B Environ. 2016, 192, 193–198. [Google Scholar] [CrossRef]
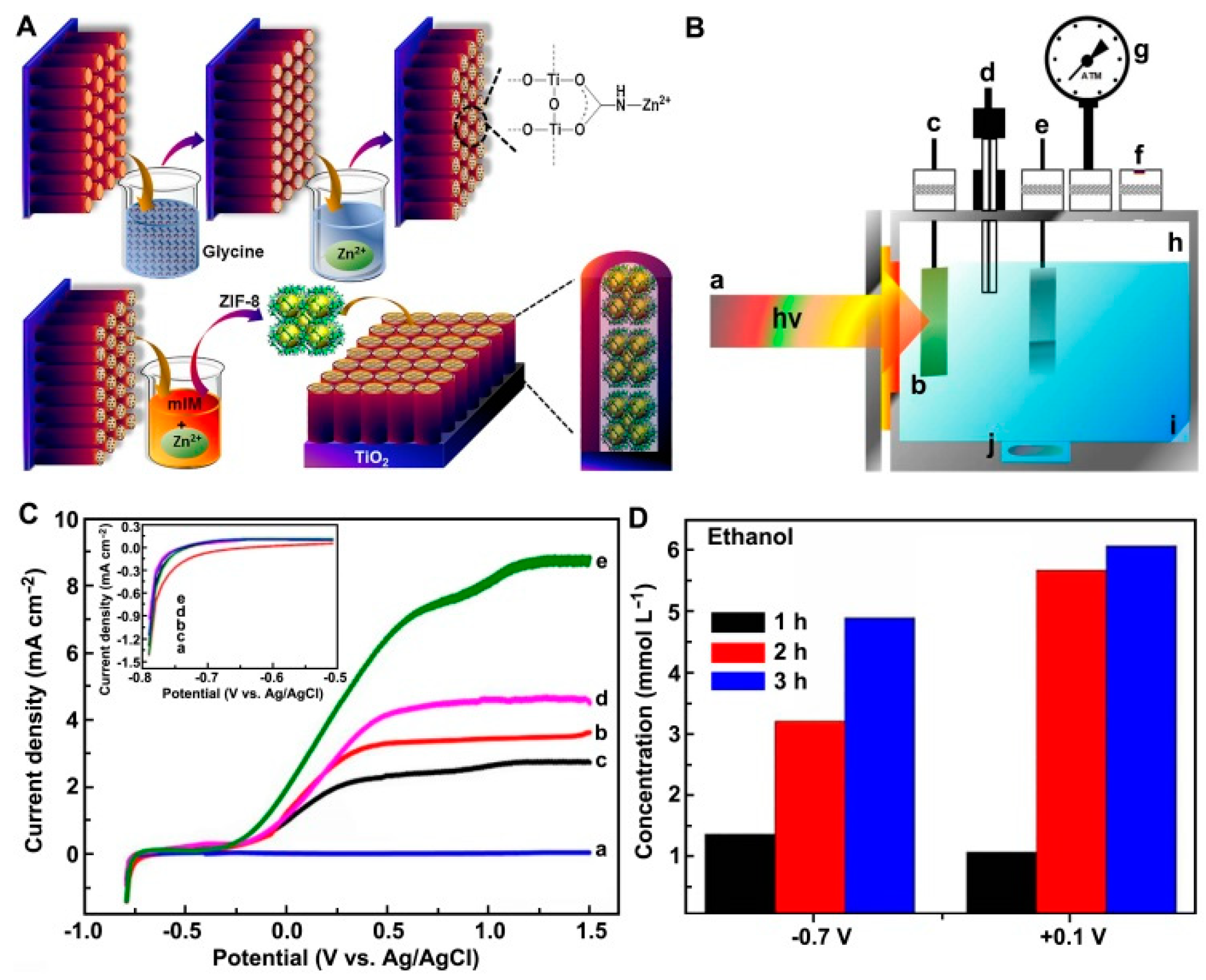
- Cardoso, J.C.; Stulp, S.; de Brito, J.F.; Flor, J.B.S.; Frem, R.C.G.; Zanoni, M.V.B. MOFs based on ZIF-8 deposited on TiO2 nanotubes increase the surface adsorption of CO2 and its photoelectrocatalytic reduction to alcohols in aqueous media. Appl. Catal. B Environ. 2018, 225, 563–573. [Google Scholar] [CrossRef]
| Half-Reactions | E0 (V vs. NHE) |
|---|---|
| CO2 + 2H+ + 2e− = CO + H2O | −0.53 |
| CO2 + 2H+ + 2e− = HCOOH | −0.61 |
| CO2 + 4H+ + 4e− = HCHO + H2O | −0.48 |
| CO2 + 6H+ + 6e− = CH3OH + H2O | −0.38 |
| CO2 + 8H+ + 8e− = CH4 + 2H2O | −0.24 |
| 2CO2 + 8H+ + 8e− = CH3COOH + H2O | −0.29 |
| 2CO2 + 12H+ + 12e− = C2H5OH + H2O | −0.33 |
| 2H+ + 2e− = H2 | −0.41 |
| Electrocatalyst | Electrolyte | Potential (V vs. RHE) | EtOH FE (%) | Ref. |
|---|---|---|---|---|
| Cu nanowire (7 μm in length) | 0.1 M KHCO3 | −1.1 | 4 | [52] |
| Nanoporous Cu | 1 M KOH (flow cell) | −0.67 | 17 | [53] |
| Oxide-derived Cu foil | 0.1 M CsHCO3 | −1.0 | 18 | [54] |
| Electro-redeposited Cu | 0.1 M KHCO3 | −1.1 | 12 | [55] |
| Cu nanocubes | 0.1 M KHCO3 | −1.1 | 10 | [56] |
| Cu nanocubes with exposed (100) facets | 0.25 M KHCO3 | −0.95 | 13 | [57] |
| Grain-boundary-rich Cu | 1 M KOH (flow cell) | −1.3 | 32 | [58] |
| Cu2O film | 0.1 M KHCO3 | −0.99 | 16 | [45] |
| 3D dendritic Cu-Cu2O | 0.1 M KCl | −0.4 | 32 | [59] |
| Multihollow Cu2O | 2 M KOH (flow cell) | −0.61 | 27 | [60] |
| Cu-on-Cu3N | 0.1 M KHCO3 | −0.95 | 19 | [61] |
| B-doped oxide-derived-Cu | 0.1 M KHCO3 | −1.05 | 20 | [62] |
| B-doped Cu | 0.1 M KCl | −1.1 | 27 | [63] |
| Cu2S-Cu-V core-shell nanoparticles | 1 M KOH (flow cell) | −0.92 | 25 | [64] |
| F-modified Cu | 1 M KOH (flow cell) | −0.54 | 16 | [65] |
| Ce(OH)x-doped-Cu | 1 M KOH (flow cell) | −0.7 | 43 | [66] |
| Polycrystalline Cu electrode with N-tolylpyridinium chloride additive | 0.1 M KHCO3 | −1.1 | 31 | [67] |
| Cu electrode with N,N’-ethylene-phenanthrolinium dibromide | 0.1 M KHCO3 | −1.07 | 15 | [68] |
| 1-octadecanethiol-modified dentritic Cu electrode | 0.1 M CsHCO3 | −1.1 | 17 | [69] |
| FeTPP[Cl]-functionalized Cu electrode | 1 M KHCO3 (flow cell) | −0.82 | 41 | [70] |
| Cu63.9Au36.1 | 0.5 M KHCO3 | −0.41 | 12 | [71] |
| Cu55Ag45 | 0.1 M KHCO3 | −1.4 | 25.5 | [72] |
| CuAg alloy wire | 1 M KOH (flow cell) | −0.7 | 25 | [73] |
| CuAg poly | 1 M KOH (flow cell) | −0.75 | 20 | [73] |
| Cu wire | 1 M KOH (flow cell) | −0.7 | 27 | [73] |
| Cu85Ag15 foam | 0.5 M KHCO3 | −1.0 | 33.7 | [74] |
| CuPd | 1 M KOH (flow cell) | −0.75 | 15 | [75] |
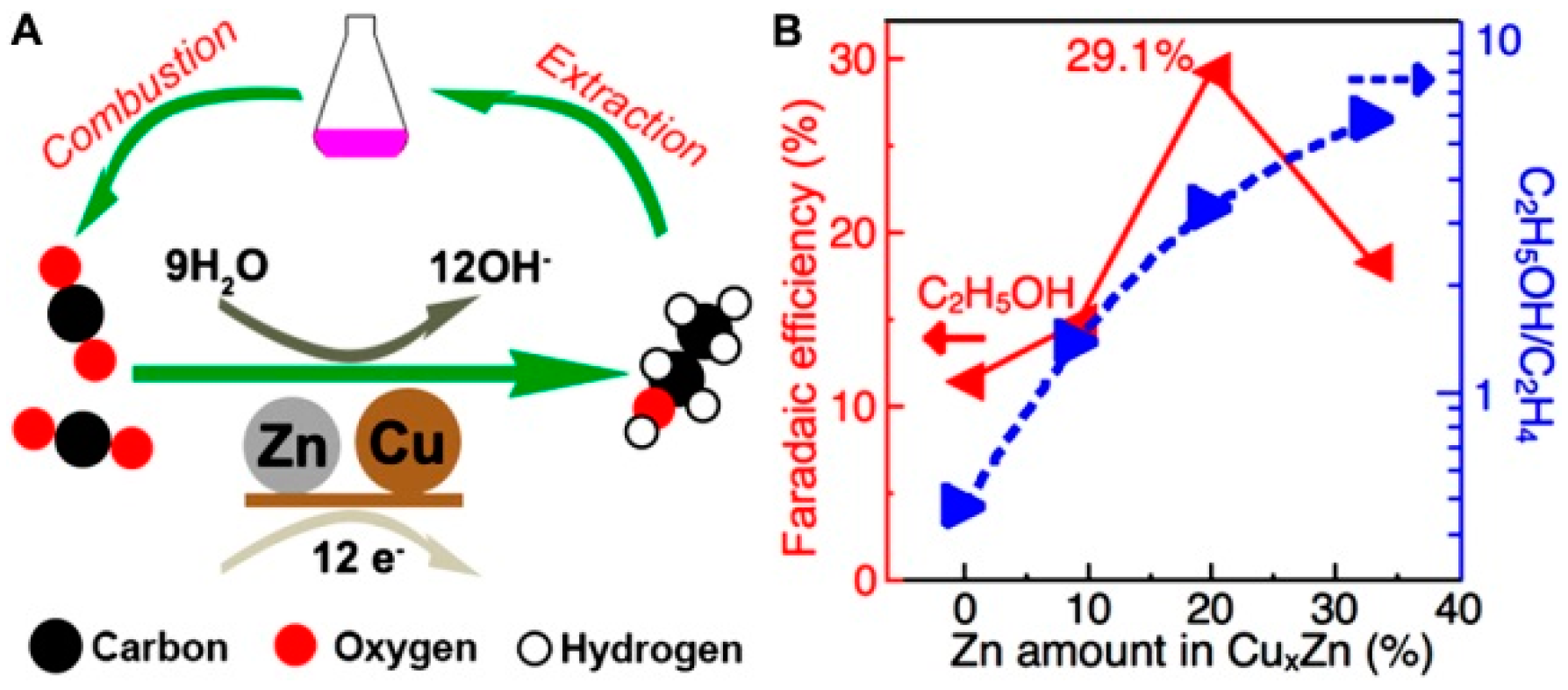
| Cu4Zn | 0.1 M KHCO3 | −1.05 | 29.1 | [44] |
| ZnO@CuO-derived CuZn | 1 M KOH (flow cell) | −0.68 V | 41.4 | [76] |
| ZnO@CuO-derived CuZn | 0.1 M KHCO3 | −1.15 | 32 | [76] |
| Cu2O nanoparticles/carbon | 0.1 M KHCO3 | −1.1 | 12 | [77] |
| Cu nanoparticles/N-doped carbon | 0.1 M KHCO3 | −1.2 | 63 | [41] |
| HKUST-1-derived Cu/C | 0.1 M KHCO3 | −0.5 | 35 | [78] |
| N-doped porous carbon-supported Cu nanoparticles | 0.2 M KHCO3 | −1.05 | 64.6 | [79] |
| N-doped graphene quantum dots | 1 M KOH (flow cell) | −0.75 | 16 | [80] |
| Cylindrical mesoporous N-doped carbon | 0.1 M KHCO3 | −0.56 | 77 | [81] |
| Hierarchical porous N-doped carbon | 0.1 M KHCO3 | −0.56 | 78 | [82] |
| B, N-co-doped nanodimond | 0.1 M NaHCO3 | −1.0 | 93.2 | [83] |
| Photocatalyst | Light Source | Reaction Medium | EtOH Yield (μmol·g−1·h−1) | Ref. |
|---|---|---|---|---|
| TiO2-Rh long nanowires | UV light (λ < 400 nm) | 0.5 M Na2SO4 | 12.1 | [97] |
| TiO2-Pd nanowires | UV light (λ < 400 nm) | 0.5 M Na2SO4 | 13 | [97] |
| TiO2/Ni(OH)2 composite nanofibers | Simulated sunlight | H2O vapor | 0.37 | [98] |
| 1.5 wt%Ni2+–TiO2 | UV light (λ < 400 nm) | H2O vapor | 13.2 | [99] |
| 5%GQDs/V-TiO2 | Simulated sunlight | 8 H2O mg/L MB and 0.01 M NaOH | 5.65 | [100] |
| 23.2% AgBr/TiO2 | Visible light λ > 420 nm | 0.2 M KHCO3 | 13.28 | [101] |
| g-C3N4 derived from urea | Visible light λ > 420 nm | 1.0 M NaOH | 4.5 | [102] |
| g-C3N4 derived from melamine | Visible light λ > 420 nm | 1.0 M NaOH | 3.6 | [102] |
| ZnO/g-C3N4 | Simulated sunlight | H2O | 1.5 | [103] |
| Ag3PO4/g-C3N4 | Simulated sunlight | 0.5 M Na2SO4 | 1.3 | [104] |
| 5.8wt%Pd/g-C3N4 | Visible light λ > 420 nm | H2O vapor | 2.18 | [105] |
| Reduced Cu/Pt–HCa2Ta3O10 | Simulated sunlight | H2O vapor | 113 | [106] |
| BiVO4/RGO | Simulated sunlight | 0.1 M NaOH | 5.15 | [107] |
| TaON microspheres | Visible light λ > 420 nm | 1.0 M NaHCO3 | 2.03 | [108] |
| Ag@AgBr/CNT | Visible light λ > 420 nm | 0.2 M KHCO3 | 2.94 | [109] |
| Red Ag/AgCl | Visible light λ > 420 nm | 0.1 M NaHCO3 | 44.6 | [110] |
| Sr3Ti(2−x−y)FexSyO(7−z)Nz | UV visible region (300–700 nm) | 0.2 M NaOH | 9.9 | [111] |
| Zn0.8Cd0.2S | Visible light λ > 400 nm | 1.0 M NaHCO3 | 6 | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Chen, W.; Wei, W.; Sun, Y. Advances in Clean Fuel Ethanol Production from Electro-, Photo- and Photoelectro-Catalytic CO2 Reduction. Catalysts 2020, 10, 1287. https://doi.org/10.3390/catal10111287
Song Y, Chen W, Wei W, Sun Y. Advances in Clean Fuel Ethanol Production from Electro-, Photo- and Photoelectro-Catalytic CO2 Reduction. Catalysts. 2020; 10(11):1287. https://doi.org/10.3390/catal10111287
Chicago/Turabian StyleSong, Yanfang, Wei Chen, Wei Wei, and Yuhan Sun. 2020. "Advances in Clean Fuel Ethanol Production from Electro-, Photo- and Photoelectro-Catalytic CO2 Reduction" Catalysts 10, no. 11: 1287. https://doi.org/10.3390/catal10111287
APA StyleSong, Y., Chen, W., Wei, W., & Sun, Y. (2020). Advances in Clean Fuel Ethanol Production from Electro-, Photo- and Photoelectro-Catalytic CO2 Reduction. Catalysts, 10(11), 1287. https://doi.org/10.3390/catal10111287





