A Discussion on the Unique Features of Electrochemical Promotion of Catalysis (EPOC): Are We in the Right Path Towards Commercial Implementation?
Abstract
Contents
- Introduction
- 1.1.
- EPOC: Opportunities for Catalytic Processes
- 1.2.
- Bridging the Gap to Practical Application of EPOC
- 1.2.1.
- Promotion of Metal/Metal Oxide Nanoparticles
- 1.2.2.
- Scale-Up EPOC Reactors
- 1.2.3.
- Suggested Catalytic and Electro-Catalytic Processes Suitable for EPOC
- The Case of Ethylene Epoxidation
- 2.1.
- Industrial Catalysts for Ethylene Epoxidation: Issues and Challenges
- 2.2.
- Discussion on EPOC Studies on Ethylene Epoxidation with O2− Promoters
- The Case of NOx Storage and Reduction (NSR)
- 3.1.
- Industrial Catalysts for NSR: Issues and Challenges
- 3.2.
- Discussion on Dynamic EPOC Studies on NSR with Alkaline Promoters
- The Case of H2 Production by Catalytic Reforming
- 4.1.
- Industrial Catalysts for CH4 Steam Reforming: Issues and Challenges
- 4.2.
- Discussion on Dynamic EPOC Studies on Catalytic Steam Reforming with Alkaline Promoters
- Coupling Electrolysis and EPOC
- 5.1.
- EPOC in Solid Oxide Electrolysers
- 5.2.
- EPOC in Depolarized PEM Electrolysers
1. Introduction
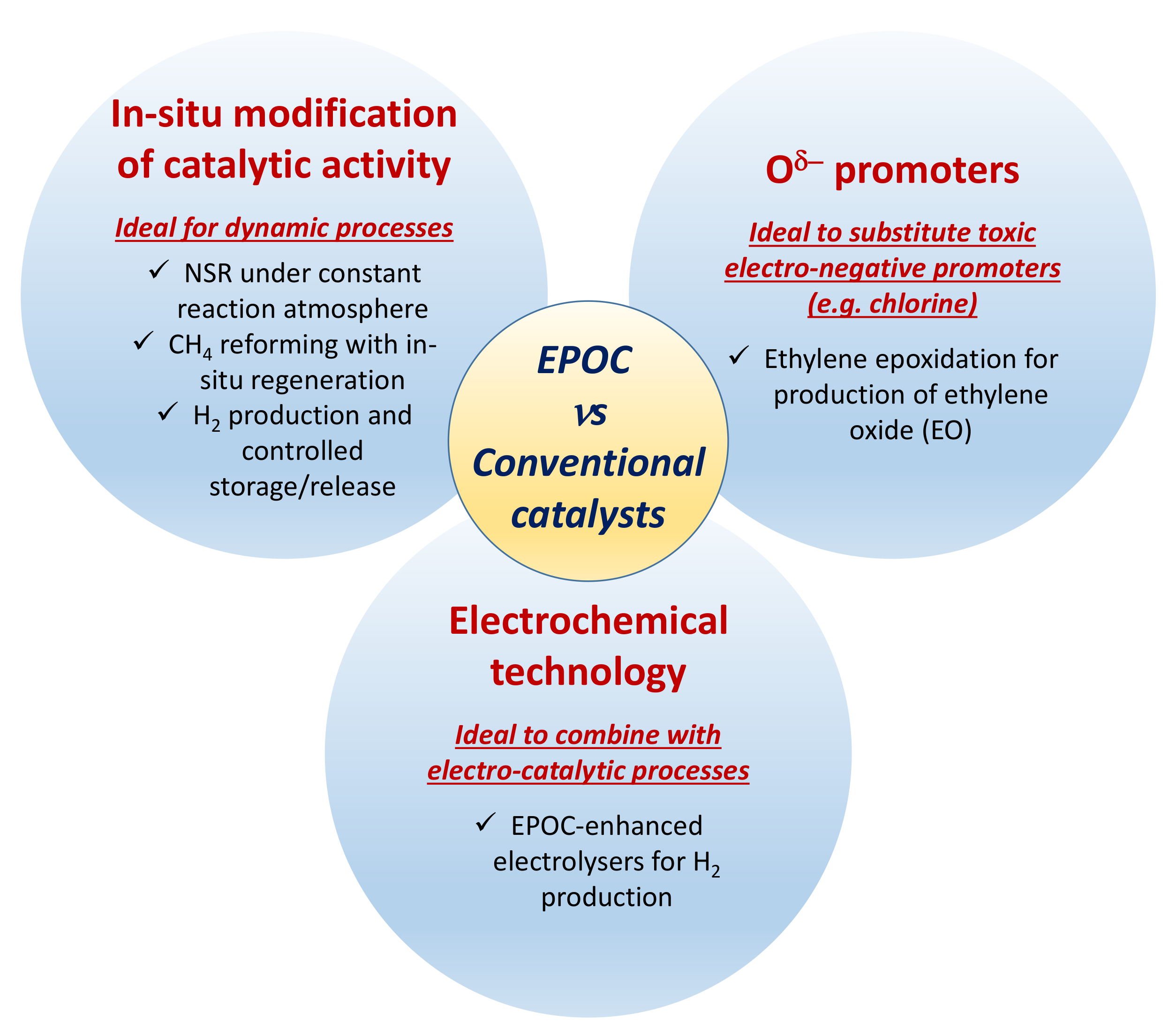
1.1. EPOC: Opportunities for Catalytic Processes
- The activity/selectivity of a heterogeneous catalyst in a given reaction can be “in-operando” modified and optimized by tuning the applied potential/current. This process is potentially reversible since the applied polarization can be easily reversed, allowing for the removal of promoter species. These features could be significantly useful for dynamic chemical processes, such as activation/regeneration of catalysts, storage/desorption of species, etc. On the contrary, this is absolutely not possible when working with conventional promotion of catalysis, where the amount of promoter is fixed for a given catalytic process from the catalyst synthesis stage.
- It allows to supply electro-negative Oδ- promoter species, as previously described for the ethylene epoxidation reaction. This is not possible in conventional systems since Oδ- cannot be formed via gaseous adsorption and cannot be easily dosed by chemical ways. These promoter species could potentially substitute highly toxic and hazardous electro-negative promoters commonly used in industrial catalysts (e.g., chlorine species).
- The EPOC phenomenon could be used to enhance the performance of electro-catalytic processes (e.g., fuel cells and electrolysers) where the state-of-the-art electrochemical reactors (commercially available) are already featured with electrical connections. In view of the potential industrialization of EPOC, the currently available electro-catalytic reaction devices could be used straightforward for that purpose.
1.2. Bridging the Gap to Practical Application of EPOC
- (a)
- “The use of less expensive catalysts and electrolyte systems needs to be put in focus of further development” [18].
- (b)
- The importance of having the “ability to promote finely dispersed metal or metal oxide nanoparticles” [12].
- (c)
- The need for “ease of electrical connection” and “efficient and compact reactor design” [20].
- (d)
- “To boost NEMCA commercial application it is necessary to focus appropriate research and development on right targets, right chemical processes” [18].
1.2.1. Promotion of Metal/Metal Oxide Nanoparticles
- ✓ Some studies proposed the possibility to disperse the metallic active phase on electronically conductor materials, including Pt nanoparticles supported on a Au electrode [21], Pt supported on carbon (Vulcan) electrodes [22], Ni and Ru supported on carbon nanofibers [23] and Pt or Ni nanoparticles dispersed on a diamond-like carbon matrix [24,25]. In these cases, the metallic nanoparticles were successfully electrochemically promoted for different catalytic reactions including CO2 hydrogenation, CO + C3H6 oxidation, methanol steam reforming and partial oxidation, respectively.
- ✓ It was also possible to disperse the metallic nanoparticles on mixed ionic-electronic (O2−, e−) conductors, including Pt/LSCF-GDC (LSCF = La0.6Sr0.4Co0.2Fe0.8O3) where Pt was prepared by impregnation [26], Pt/LSM-GDC (LSM = (La0.8Sr0.2)0.95MnO3-δ) where Pt was prepared by atomic layer deposition (ALD) [27], and the electrodes prepared by the polyol method developed by the Baranova group, like Ru/CeO2 [28] and Pd/Co3O4 [29]. These highly dispersed materials were supported on YSZ solid electrolytes and tested, under EPOC conditions, for propylene, propane, ethylene and methane oxidation, respectively.
- ✓ Another strategy was to increase the active surface area of the electrodes by developing nanoporous catalyst films by physical vapour deposition (PVD) techniques performed in oblique angle. This technique was optimized by the González-Elipe group for the development of an electrode formed by Cu nanocolumns tested for EPOC-assisted methanol partial oxidation [30] and an analogous Ni nanoporous catalyst film used for EPOC-assisted H2 production and storage [31].
1.2.2. Scale-Up EPOC Reactors
- ✓ EPOC multi-pellet reactor design for NH3 synthesis: This reactor design by Yiokari et al. constituted one of the first attempts to scale-up the EPOC catalysts, and was used for the NH3 synthesis process on commercial Fe-based catalyst (BASF S6-10RED) deposited on CaZr0.9In0.1O3-α, a proton conducting solid electrolyte [32]. One of the most interesting technical features of this reactor is the possibility to operate at high pressure (50 bar), with 24 cell-pellets electrically connected in parallel.
- ✓ EPOC with monolithic electrochemically promoted reactors (MEPR): This reactor developed by the Vayenas group consists of up to 22 electrocatalytic plate cells (5 × 5 cm2) in parallel exposed to the same reaction atmosphere [33]. The plate cells are composed of dense square solid electrolytes (e.g., YSZ) with a catalytic film deposited on each side. The reactor is equipped of two external connecting wires for polarization purposes. This reactor has been used, for instance, for the oxidation of ethylene [33,34] and SO2 [35] on Pt electrodes and reduction of CO2 on Rh and Cu/TiO2 electrodes [36]. Also, a MEPR with 22 Rh/YSZ/Pt plates was successfully tested under real conditions for the treatment of an automotive exhaust gas of a diesel engine [37,38].
1.2.3. Suggested Catalytic and Electro-Catalytic Processes Suitable for EPOC
2. The Case of Ethylene Epoxidation
2.1. Industrial Catalysts for Ethylene Epoxidation: Issues and Challenges
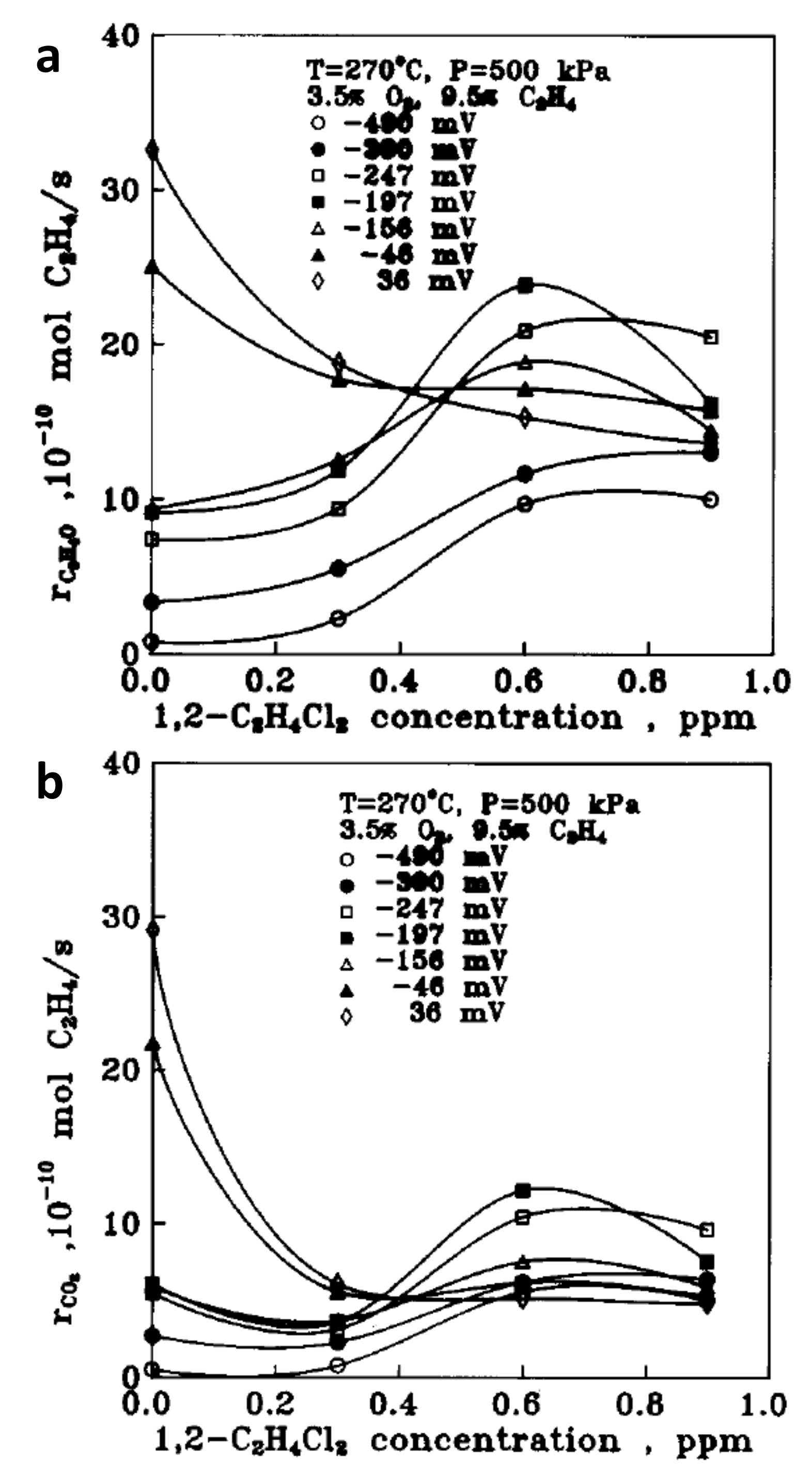
2.2. Discussion on EPOC Studies on Ethylene Epoxidation with O2− Promoters
- ✓ The Ag catalysts used in all these EPOC studies were prepared by the thermal decomposition of organometallic paste precursors. This procedure results in a catalyst with a poor metallic dispersion compared to that of commercial materials. This implies that, to achieve similar reaction rates, a much higher amount of Ag should be used in the EPOC catalysts, which is not sustainable from an economic point of view.
- ✓ These studies were performed in reactors usually designed for electrochemical (rather than catalytic) purposes, e.g., solid oxide fuel cells.
- ✓ Based on the premises of the “sacrificial promoter” mechanism, after a limited period of time, the Oδ- promoter species may react with ethylene through a purely electro-catalytic process (C2H4 + O2− → C2H4O + 2 e−) or evolve in form of molecular oxygen (2 O2− → O2 + 2 e−). Therefore, a continuous supply of promoting oxygen species is required via applied potential to maintain the promoted state of the catalyst. Hence, even though the required currents are generally rather small (of the order of a few μA-mA), constant or periodic electrical currents should be applied.
3. The Case of NOx Storage and Reduction (NSR)
3.1. Industrial Catalysts for NSR: Issues and Challenges
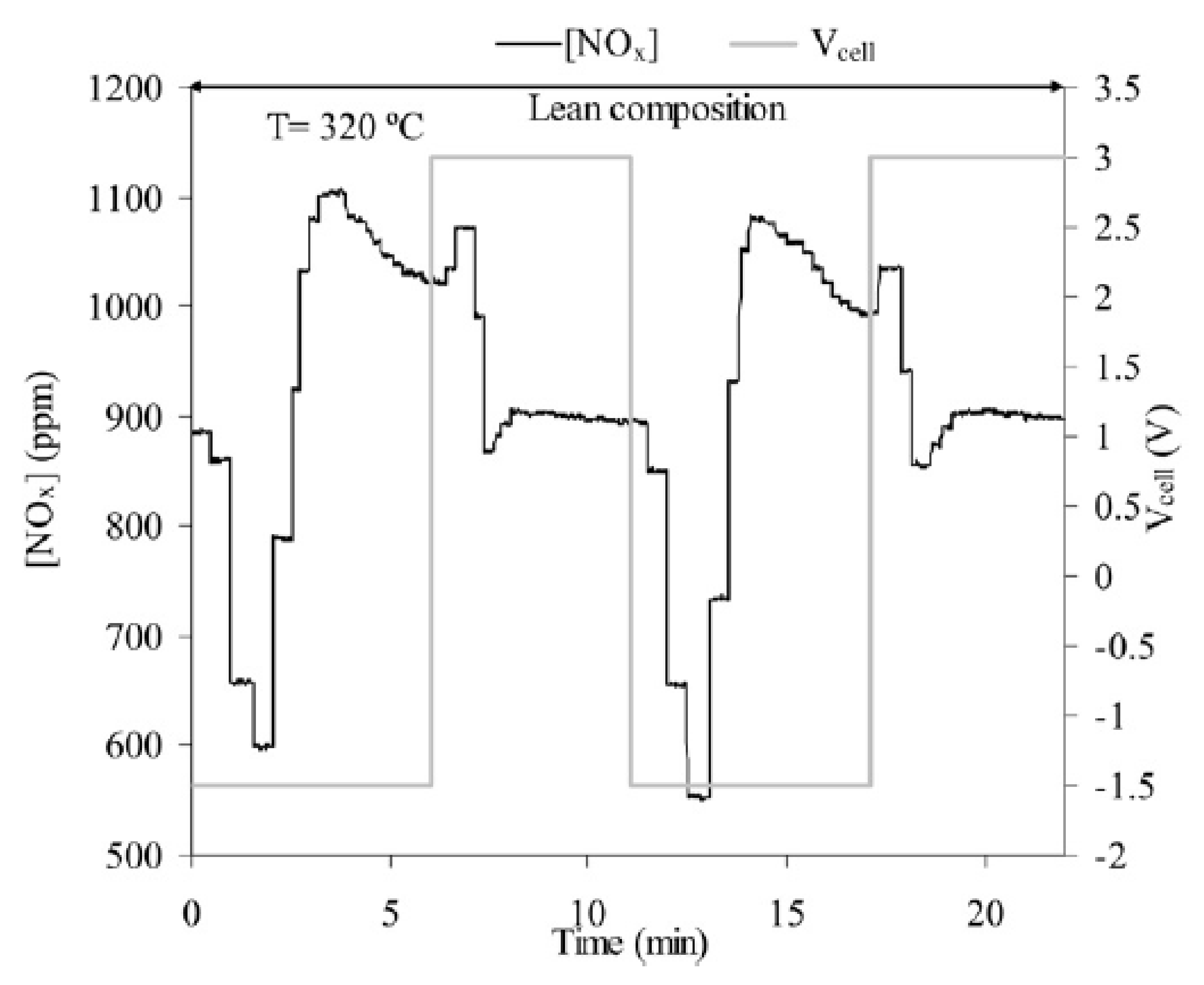
3.2. Discussion on Dynamic EPOC Studies on NSR with Alkaline Promoters
- ✓ As for the Ag catalysts previously described for the EO production, the Pt catalyst consists of a thick layer prepared by the thermal decomposition of organometallic paste precursors with a poor metallic dispersion (and therefore much lower overall catalytic activity) compared to that of commercial catalysts.
- ✓ Even if a tubular configuration with higher exposed catalyst area was used in the second study [87], the catalytic reactor should be further improved and optimized, considering this catalyst would be ultimately introduced in the exhaust of a vehicle (with limited space).
4. The Case of H2 Production by Catalytic Reforming
4.1. Industrial Catalysts for CH4 Steam Reforming: Issues and Challenges
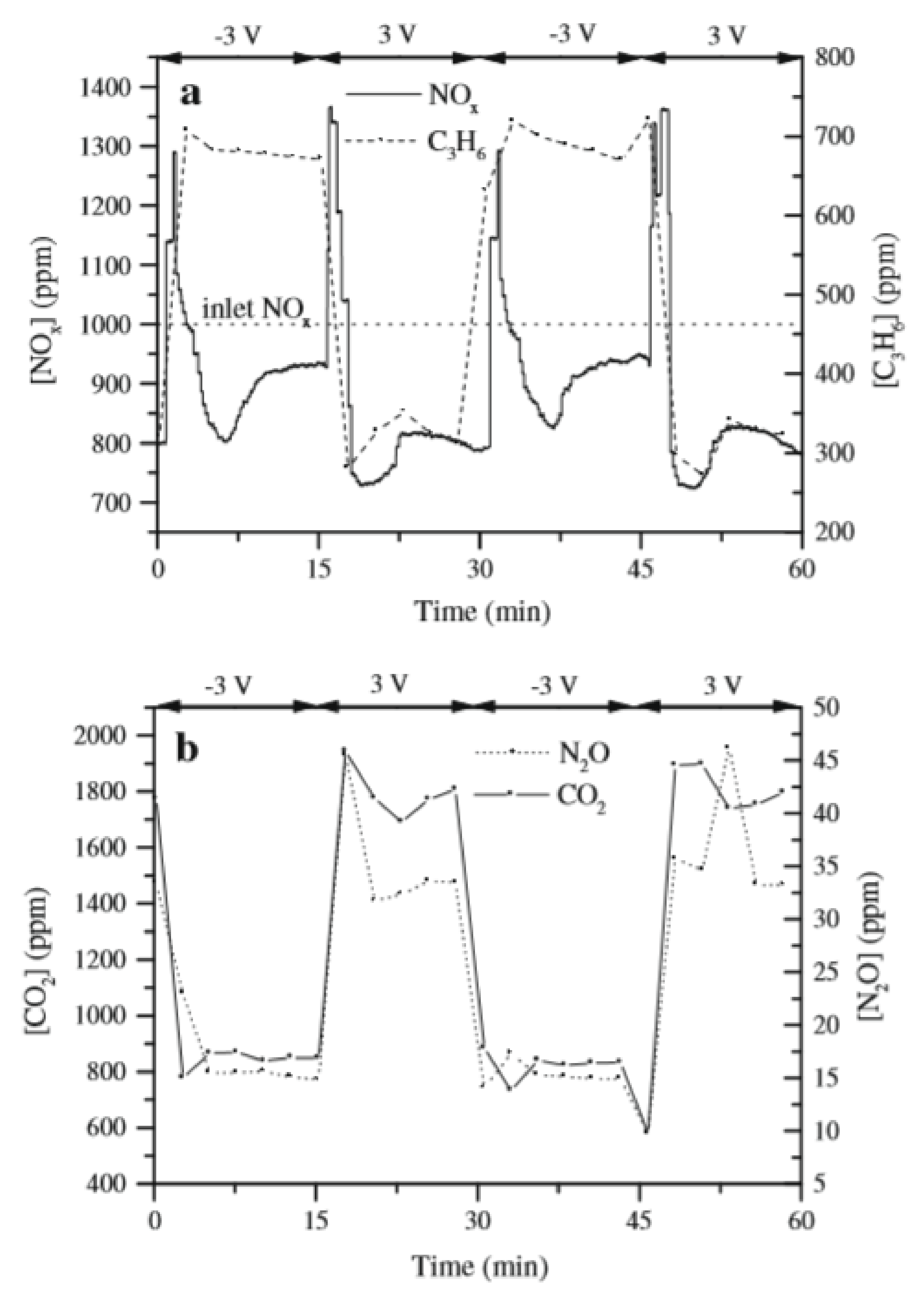
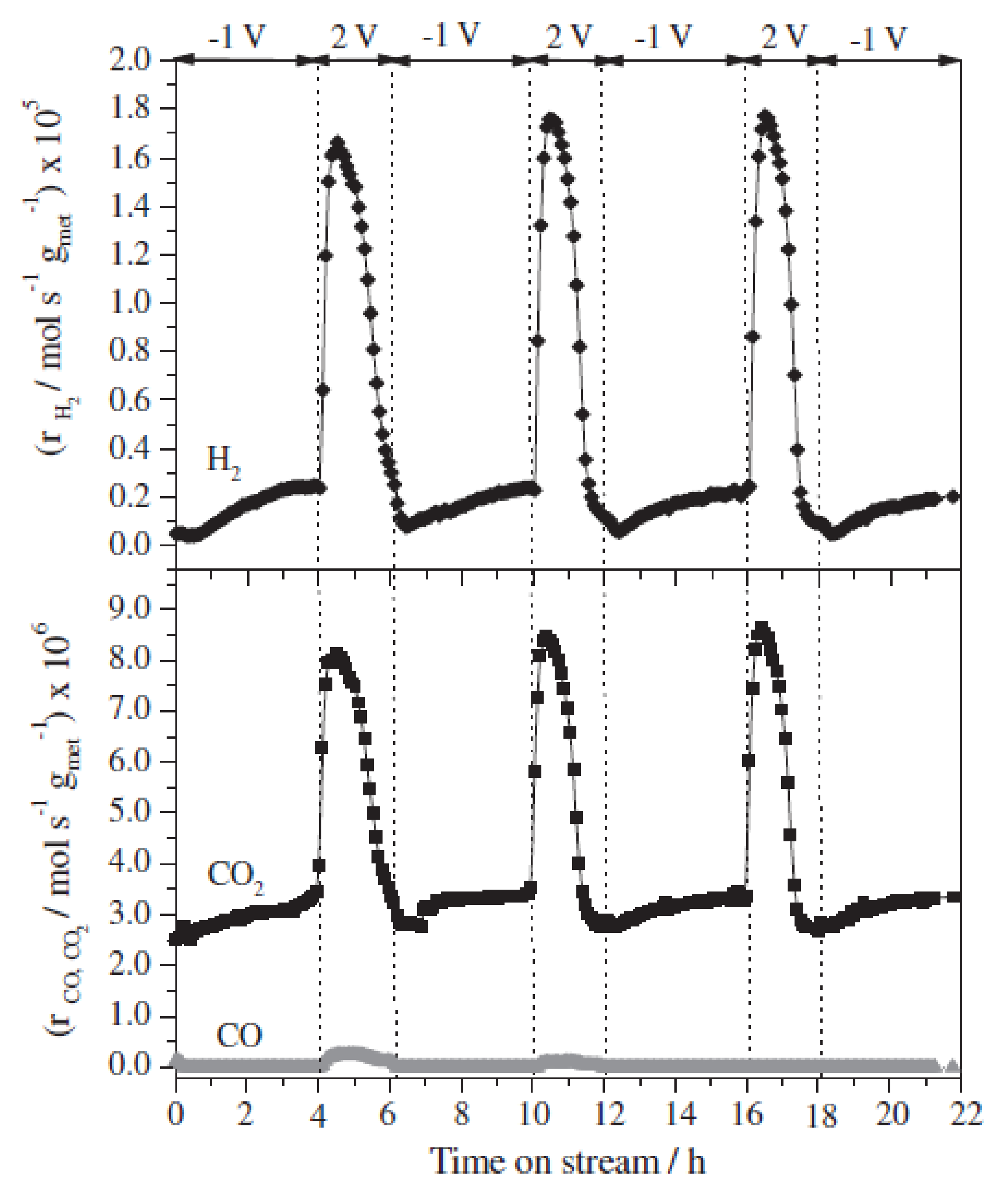
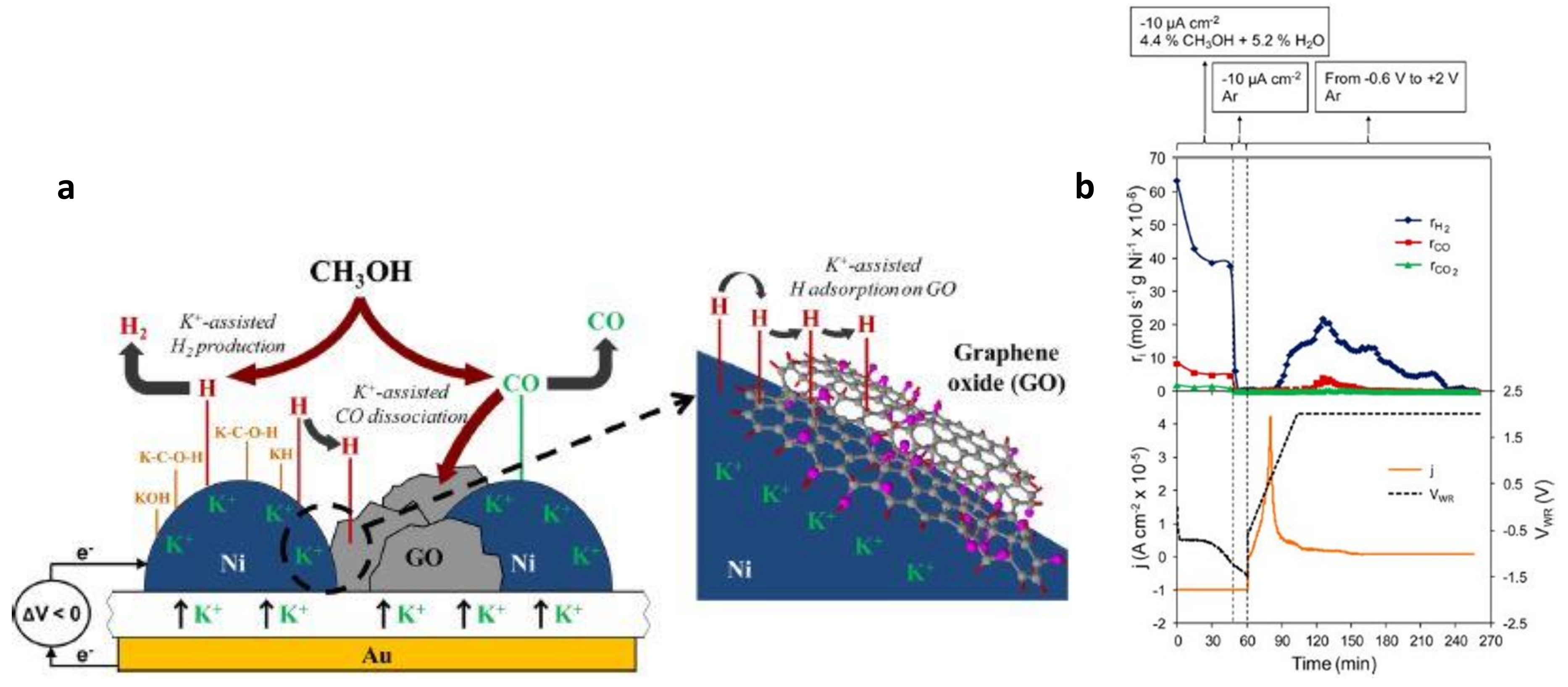
4.2. Discussion on Dynamic EPOC Studies on Catalytic Steam Reforming with Alkaline Promoters
- ✓ A Pt-based composite layer was used as a catalyst. This metal exhibits high cost compared to Ni and a much lower dispersion than conventional catalysts.
- ✓ A proper reactor design should be developed to substitute the current methane reformers by EPOC technologies.
5. Coupling Electrolysis and EPOC
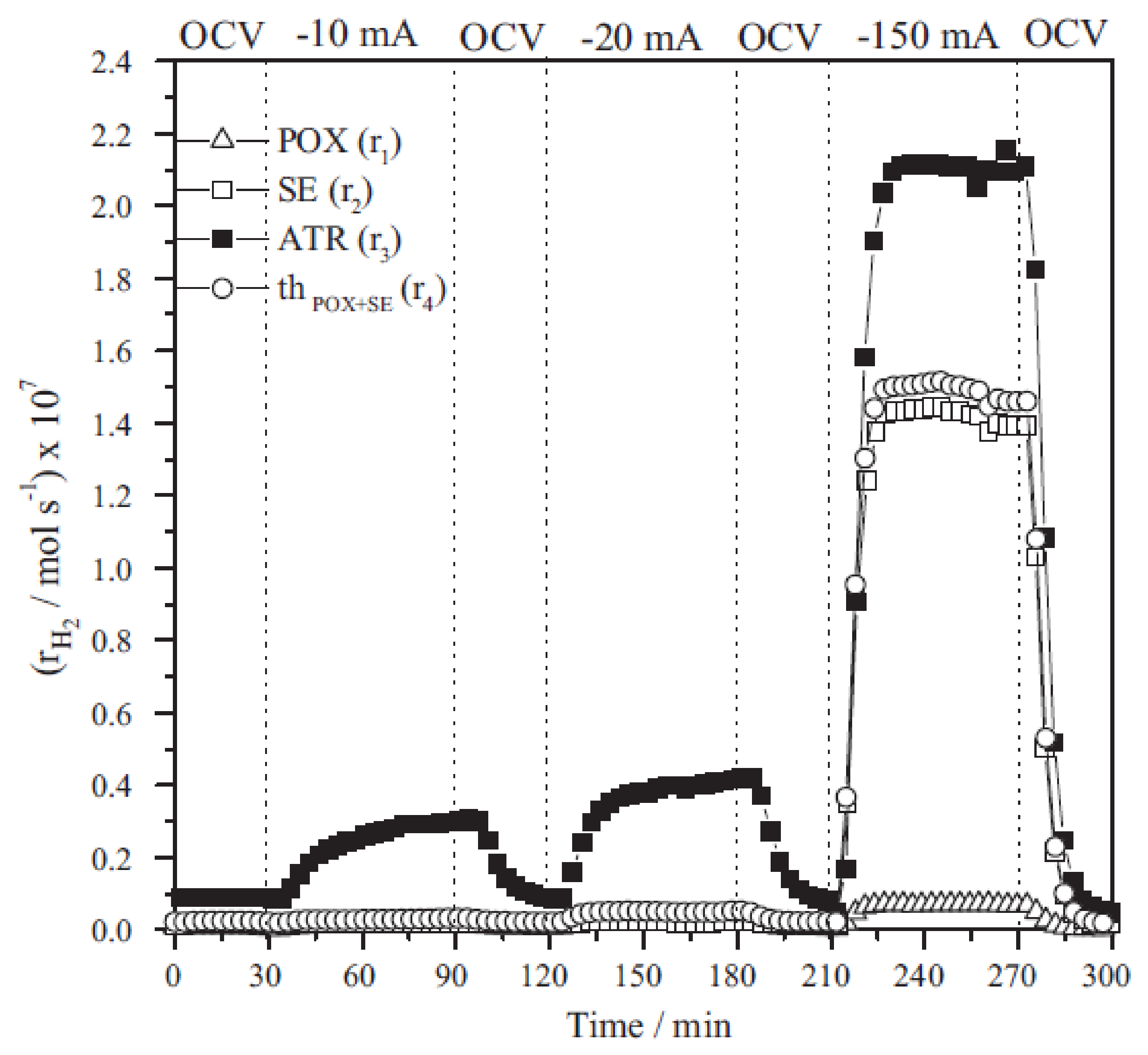
5.1. EPOC in Solid Oxide Electrolysers
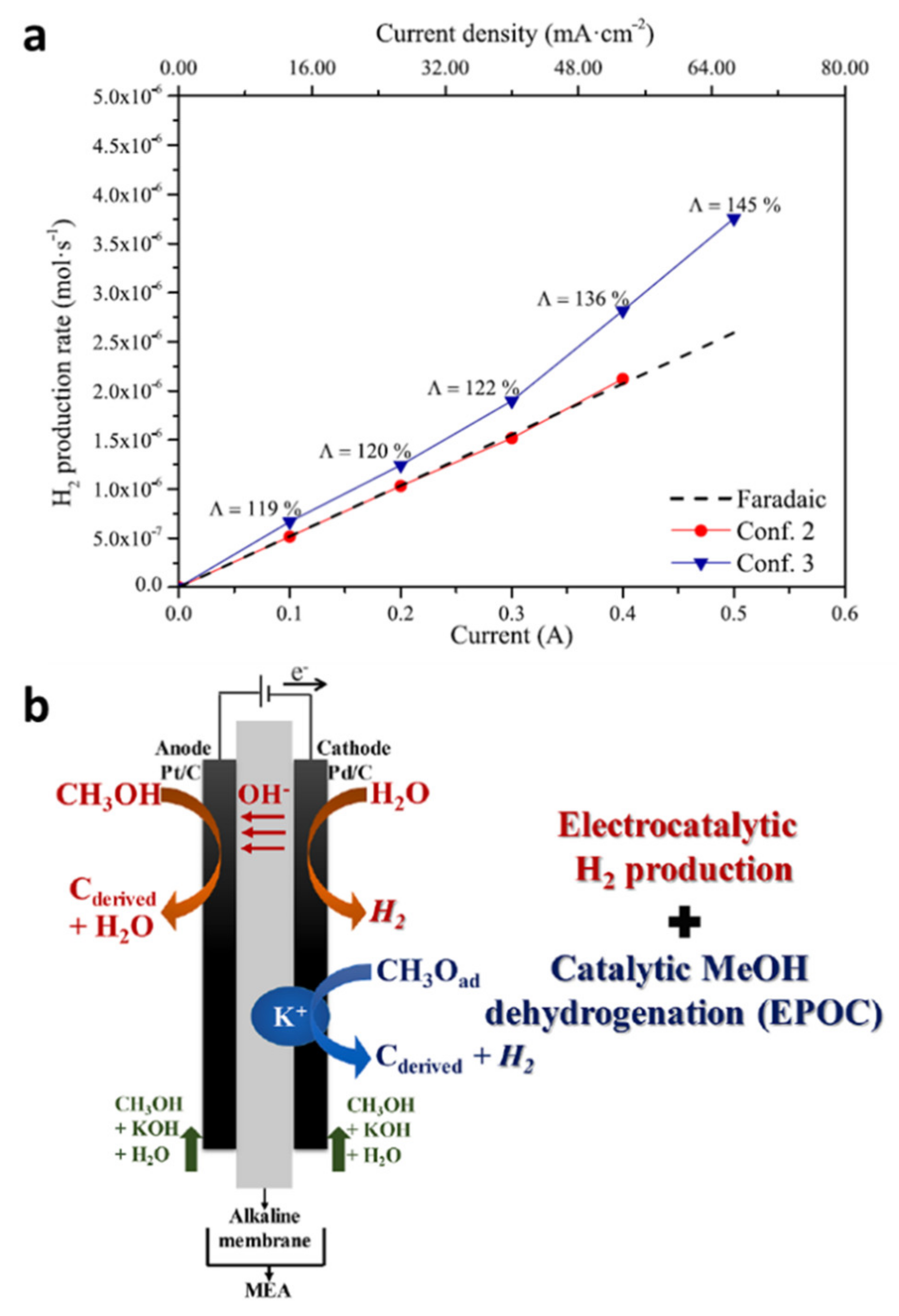
5.2. EPOC in Depolarized PEM Electrolysers
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nielsen, A. Review of Ammonia Catalysis. Catal. Rev. 1971, 4, 1–26. [Google Scholar] [CrossRef]
- Jahangiri, H.; Bennett, J.; Mahjoubi, P.; Wilson, K.; Gu, S. A review of advanced catalyst development for Fischer-Tropsch synthesis of hydrocarbons from biomass derived syn-gas. Catal. Sci. Technol. 2014, 4, 2210–2229. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, L.; Nan, Y.; Xie, Y.; Cheng, D. Revisit the Role of Chlorine in Selectivity Enhancement of Ethylene Epoxidation. Ind. Eng. Chem. Res. 2019, 58, 21403–21412. [Google Scholar] [CrossRef]
- Stoukides, M.; Vayenas, C.G. The effect of electrochemical oxygen pumping on the rate and selectivity of ethylene oxidation on polycrystalline silver. J. Catal. 1981, 70, 137–146. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Bebelis, S.; Neophytides, S. Non-faradaic electrochemical modification of catalytic activity. J. Phys. Chem. 1988, 92, 5083–5085. [Google Scholar] [CrossRef]
- Vayenas, C.G. Thermodynamic analysis of the electrochemical promotion of catalysis. Solid State Ionics 2004, 168, 321–326. [Google Scholar] [CrossRef]
- Tsiplakides, D.; Balomenou, S.; Katsaounis, A.; Archonta, D.; Koutsodontis, C.; Vayenas, C.G. Electrochemical promotion of catalysis: Mechanistic investigations and monolithic electropromoted reactors. Catal. Today 2005, 100, 133–144. [Google Scholar] [CrossRef]
- Lintz, H.-G.; Vayenas, C.G. Solid Ion Conductors in Heterogeneous Catalysis. Angew. Chemie Int. Ed. English 1989, 28, 708–715. [Google Scholar] [CrossRef]
- Vayenas, C.G. Bridging electrochemistry and heterogeneous catalysis. J. Solid State Electrochem. 2011, 15, 1425–1435. [Google Scholar] [CrossRef]
- González-Cobos, J.; de Lucas-Consuegra, A. A review of surface analysis techniques for the investigation of the phenomenon of electrochemical promotion of catalysis with alkaline ionic conductors. Catalysts 2016, 6. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A. New trends of alkali promotion in heterogeneous catalysis: Electrochemical promotion with alkaline ionic conductors. Catal. Surv. Asia 2015, 19, 25–37. [Google Scholar] [CrossRef]
- Vernoux, P. Recent advances in electrochemical promotion of catalysis. In Catalysis; The Royal Society of Chemistry: London, UK, 2017; Volume 29, pp. 29–59. ISBN 9781782629566. [Google Scholar]
- Vayenas, C.G. Promotion, electrochemical promotion and metal-support interactions: Their common features. Catal. Lett. 2013, 143, 1085–1097. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Vernoux, P.; Goula, G.; Caravaca, A. Electropositive promotion by Alkalis or Alkaline earths of Pt-group metals in emissions control catalysis: A status report. Catalysts 2019, 9. [Google Scholar] [CrossRef]
- Vayenas, C.; Bebeli, S.; Pliangos, C.; Brosda, S.; Tsiplakides, D. Electrochemical Activation of Catalysis: Promotion, Electrochemical Promotion, and Metal-support Interactions; Springer Science & Business Media: New York, NY, USA, 2001. [Google Scholar]
- Vernoux, P.; Lizarraga, L.; Tsampas, M.N.; Sapountzi, F.M.; De Lucas-Consuegra, A.; Valverde, J.-L.; Souentie, S.; Vayenas, C.G.; Tsiplakides, D.; Balomenou, S.; et al. Ionically conducting ceramics as active catalyst supports. Chem. Rev. 2013, 113, 8192–8260. [Google Scholar] [CrossRef]
- González-Cobos, J.; Valverde, J.L.; de Lucas-Consuegra, A. Electrochemical vs. chemical promotion in the H2 production catalytic reactions. Int. J. Hydrogen Energy 2017, 42, 13712–13723. [Google Scholar] [CrossRef]
- Anastasijevic, N.A. NEMCA-From discovery to technology. Catal. Today 2009, 146, 308–311. [Google Scholar] [CrossRef]
- Tsiplakides, D.; Balomenou, S. Milestones and perspectives in electrochemically promoted catalysis. Catal. Today 2009, 146, 312–318. [Google Scholar] [CrossRef]
- Tsiplakides, D.; Balomenou, S. Electrochemical promoted catalysis: Towards practical utilization. Chem. Ind. Chem. Eng. Q. 2008, 14, 97–105. [Google Scholar] [CrossRef]
- Marwood, M.; Vayenas, C.G. Electrochemical Promotion of a Dispersed Platinum Catalyst. J. Catal. 1998, 178, 429–440. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A.; Princivalle, A.; Caravaca, A.; Dorado, F.; Marouf, A.; Guizard, C.; Valverde, J.L.; Vernoux, P. Preparation and characterization of a low particle size Pt/C catalyst electrode for the simultaneous electrochemical promotion of CO and C3H6 oxidation. Appl. Catal. A Gen. 2009, 365. [Google Scholar] [CrossRef]
- Jiménez, V.; Jiménez-Borja, C.; Sánchez, P.; Romero, A.; Papaioannou, E.I.; Theleritis, D.; Souentie, S.; Brosda, S.; Valverde, J.L. Electrochemical promotion of the CO2 hydrogenation reaction on composite Ni or Ru impregnated carbon nanofiber catalyst-electrodes deposited on YSZ. Appl. Catal. B Environ. 2011, 107, 210–220. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A.; González-Cobos, J.; Carcelén, V.; Magén, C.; Endrino, J.L.; Valverde, J.L. Electrochemical promotion of Pt nanoparticles dispersed on a diamond-like carbon matrix: A novel electrocatalytic system for H2 production. J. Catal. 2013, 307, 18–26. [Google Scholar] [CrossRef]
- González-Cobos, J.; Ruiz-López, E.; Valverde, J.L.; de Lucas-Consuegra, A. Electrochemical promotion of a dispersed Ni catalyst for H2 production via partial oxidation of methanol. Int. J. Hydrogen Energy 2016, 41, 19418–19429. [Google Scholar] [CrossRef]
- Kambolis, A.; Lizarraga, L.; Tsampas, M.N.; Burel, L.; Rieu, M.; Viricelle, J.-P.; Vernoux, P. Electrochemical promotion of catalysis with highly dispersed Pt nanoparticles. Electrochem. Commun. 2012, 19, 5–8. [Google Scholar] [CrossRef]
- Hajar, Y.; Di Palma, V.; Kyriakou, V.; Verheijen, M.A.; Baranova, E.A.; Vernoux, P.; Kessels, W.M.M.; Creatore, M.; van de Sanden, M.C.M.; Tsampas, M.N. Atomic layer deposition of highly dispersed Pt nanoparticles on a high surface area electrode backbone for electrochemical promotion of catalysis. Electrochem. Commun. 2017, 84, 40–44. [Google Scholar] [CrossRef]
- Dole, H.A.E.; Safady, L.F.; Ntais, S.; Couillard, M.; Baranova, E.A. Electrochemically enhanced metal-support interaction of highly dispersed Ru nanoparticles with a CeO2 support. J. Catal. 2014, 318, 85–94. [Google Scholar] [CrossRef]
- Zagoraios, D.; Athanasiadi, A.; Kalaitzidou, I.; Ntais, S.; Katsaounis, A.; Caravaca, A.; Vernoux, P.; Vayenas, C.G. Electrochemical promotion of methane oxidation over nanodispersed Pd/Co3O4 catalysts. Catal. Today 2019. [Google Scholar] [CrossRef]
- González-Cobos, J.; Rico, V.J.; González-Elipe, A.R.; Valverde, J.L.; De Lucas-Consuegra, A. Electrochemical activation of an oblique angle deposited Cu catalyst film for H2 production. Catal. Sci. Technol. 2015, 5, 2203–2214. [Google Scholar] [CrossRef]
- González-Cobos, J.; Rico, V.J.; González-Elipe, A.R.; Valverde, J.L.; de Lucas-Consuegra, A. Electrocatalytic System for the Simultaneous Hydrogen Production and Storage from Methanol. ACS Catal. 2016, 6, 1942–1951. [Google Scholar] [CrossRef]
- Yiokari, C.G.; Pitselis, G.E.; Polydoros, D.G.; Katsaounis, A.D.; Vayenas, C.G. High-Pressure Electrochemical Promotion of Ammonia Synthesis over an Industrial Iron Catalyst. J. Phys. Chem. A 2000, 104, 10600–10602. [Google Scholar] [CrossRef]
- Balomenou, S.; Tsiplakides, D.; Katsaounis, A.; Thiemann-Handler, S.; Cramer, B.; Foti, G.; Comninellis, C.; Vayenas, C.G. Novel monolithic electrochemically promoted catalytic reactor for environmentally important reactions. Appl. Catal. B Environ. 2004, 52, 181–196. [Google Scholar] [CrossRef]
- Hammad, A.; Souentie, S.; Balomenou, S.; Tsiplakides, D.; Figueroa, J.C.; Cavalca, C.; Pereira, C.J.; Vayenas, C.G. Tailor-structured skeletal Pt catalysts employed in a monolithic electropromoted reactor. J. Appl. Electrochem. 2008, 38, 1171–1176. [Google Scholar] [CrossRef]
- Hammad, A.; Souentie, S.; Papaioannou, E.I.; Balomenou, S.; Tsiplakides, D.; Figueroa, J.C.; Cavalca, C.; Pereira, C.J. Electrochemical promotion of the SO2 oxidation over thin Pt films interfaced with YSZ in a monolithic electropromoted reactor. Appl. Catal. B Environ. 2011, 103, 336–342. [Google Scholar] [CrossRef]
- Papaioannou, E.I.; Souentie, S.; Hammad, A.; Vayenas, C.G. Electrochemical promotion of the CO2 hydrogenation reaction using thin Rh, Pt and Cu films in a monolithic reactor at atmospheric pressure. Catal. Today 2009, 146, 336–344. [Google Scholar] [CrossRef]
- Balomenou, S.P.; Tsiplakides, D.; Vayenas, C.G.; Poulston, S.; Houel, V.; Collier, P.; Konstandopoulos, A.G.; Agrafiotis, C. Electrochemical promotion in a monolith electrochemical plate reactor applied to simulated and real automotive pollution control. Top. Catal. 2007, 44, 481–486. [Google Scholar] [CrossRef]
- Souentie, S.; Hammad, A.; Brosda, S.; Foti, G.; Vayenas, C.G. Electrochemical promotion of NO reduction by C2H4 in 10% O2 using a monolithic electropromoted reactor with Rh/YSZ/Pt elements. J. Appl. Electrochem. 2008, 38, 1159–1170. [Google Scholar] [CrossRef]
- TechNavio. Global Ethylene Oxide and Ethylene Glycol Market 2016–2020; TechNavio: London, UK, 2016. [Google Scholar]
- Rebsdat, S.; Mayer, D. Ethylene Oxide. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2001; Vol. Organic Chemicals. [Google Scholar]
- Van Santen, R.A.; Kuipers, H.P.C.E. The Mechanism of Ethylene Epoxidation. In Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B.B.T.-A., Eds.; Academic Press: Cambridge, MA, USA, 1987; Volume 35, pp. 265–321. ISBN 0360-0564. [Google Scholar]
- Kenge, N.; Pitale, S.; Joshi, K. The nature of electrophilic oxygen: Insights from periodic density functional theory investigations. Surf. Sci. 2019, 679, 188–195. [Google Scholar] [CrossRef]
- Christopher, P.; Linic, S. Engineering Selectivity in Heterogeneous Catalysis: Ag Nanowires as Selective Ethylene Epoxidation Catalysts. J. Am. Chem. Soc. 2008, 130, 11264–11265. [Google Scholar] [CrossRef]
- Campbell, C.T.; Paffett, M.T. The role of chlorine promoters in catalytic ethylene epoxidation over the Ag(110) surface. Appl. Surf. Sci. 1984, 19, 28–42. [Google Scholar] [CrossRef]
- Van Hoof, A.J.F.; Filot, I.A.W.; Friedrich, H.; Hensen, E.J.M. Reversible Restructuring of Silver Particles during Ethylene Epoxidation. ACS Catal. 2018, 8, 11794–11800. [Google Scholar] [CrossRef]
- Rocha, T.C.R.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R. Promoters in heterogeneous catalysis: The role of Cl on ethylene epoxidation over Ag. J. Catal. 2014, 312, 12–16. [Google Scholar] [CrossRef]
- Özbek, M.O.; van Santen, R.A. The Mechanism of Ethylene Epoxidation Catalysis. Catal. Lett. 2013, 143, 131–141. [Google Scholar] [CrossRef]
- Sass, J.B.; Castleman, B.; Wallinga, D. Vinyl chloride. A case study of data suppression and misrepresentation. Environ. Health Perspect. 2005, 113, 809–812. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boghosian, S.; Bebelis, S.; Vayenas, C.G.; Papatheodorou, G.N. In situ high temperature SERS study of Ag catalysts and electrodes during ethylene epoxidation. J. Catal. 1989, 117, 561–565. [Google Scholar] [CrossRef]
- Kondarides, D.L.; Papatheodorou, G.N.; Vayenas, C.G.; Verykios, X.E. In situ high temperature SERS study of oxygen adsorbed on Ag. Support and electrochemical promotion effects. Berichte der Bunsengesellschaft/Physical Chem. Chem. Phys. 1993, 97, 709–720. [Google Scholar] [CrossRef]
- Bebelis, S.; Vayenas, C.G. Non-faradaic electrochemical modification of catalytic activity 6. Ethylene epoxidation on Ag deposited on stabilized ZrO2. J. Catal. 1992, 138, 588–610. [Google Scholar] [CrossRef]
- Karavasilis, C.; Bebelis, S.; Vayenas, C.G. Selectivity maximization of ethylene epoxidation via NEMCA with zirconia and β″-Al2O3 solid electrolytes. Ionics (Kiel) 1995, 1, 85–91. [Google Scholar] [CrossRef]
- Karavasilis, C.; Bebelis, S.; Vayenas, C.G. Non-Faradaic Electrochemical Modification of Catalytic Activity: X. Ethylene Epoxidation on Ag Deposited on Stabilized ZrO2 in the Presence of Chlorine Moderators. J. Catal. 1996, 160, 190–204. [Google Scholar] [CrossRef]
- Dole, H.A.E.; Isaifan, R.J.; Sapountzi, F.M.; Lizarraga, L.; Aubert, D.; Princivalle, A.; Vernoux, P.; Baranova, E.A. Low Temperature Toluene Oxidation Over Pt Nanoparticles Supported on Yttria Stabilized-Zirconia. Catal. Lett. 2013, 143, 996–1002. [Google Scholar] [CrossRef]
- Hajar, Y.M.; Houache, M.S.E.; Tariq, U.; Vernoux, P.; Baranova, E.A. Nanoscopic Ni Interfaced with Oxygen Conductive Supports: Link between Electrochemical and Catalytic Studies. ECS Trans. 2017, 77, 51–66. [Google Scholar] [CrossRef]
- Tsampas, M.N.; Sapountzi, F.M.; Vernoux, P. Applications of yttria stabilized zirconia (YSZ) in catalysis. Catal. Sci. Technol. 2015, 5, 4884–4900. [Google Scholar] [CrossRef]
- Nicole, J.; Tsiplakides, D.; Pliangos, C.; Verykios, X.; Comninellis, C.; Vayenas, C. Electrochemical Promotion and Metal–Support Interactions. J. Catal. 2001, 204, 23–34. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, H.; Liu, H.; Zhou, X. Self-sustained electrochemical promotion catalysts for partial oxidation reforming of heavy hydrocarbons. Int. J. Hydrogen Energy 2012, 37, 17928–17935. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, H.; Liu, H. Study of partial oxidation reforming of methane to syngas over self-sustained electrochemical promotion catalyst. Int. J. Hydrogen Energy 2013, 38, 6391–6396. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Liu, H.; Sun, H.; Wei, Y.; Zhou, X. A kinetic model for analyzing partial oxidation reforming of heavy hydrocarbon over a novel self-sustained electrochemical promotion catalyst. Int. J. Hydrogen Energy 2012, 37, 15125–15134. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Zhou, X.; Liu, H.; Wei, Y.; Pramuanjaroenkij, A.; Bordas, A.; Page, M.; Cai, S.; Zhang, X. Development and Study of Self-Sustained Electrochemical Promotion Catalysts for Hydrocarbon Reforming. ECS Trans. 2013, 58, 243–254. [Google Scholar] [CrossRef]
- Poulidi, D.; Thursfield, A.; Metcalfe, I.S. Electrochemical promotion of catalysis controlled by chemical potential difference across a mixed ionic-electronic conducting ceramic membrane - An example of wireless NEMCA. Top. Catal. 2007, 44, 435–449. [Google Scholar] [CrossRef]
- Stavrakakis, E.; West, M.; Johnston, S.; McIllwaine, R.; Poulidi, D. Hydration, CO2 stability and wireless electrochemical promotion studies on yttria-doped Ba(Ce,Zr)O3 perovskites. Ionics (Kiel) 2019, 25, 1243–1257. [Google Scholar] [CrossRef]
- Poulidi, D.; Rivas, M.E.; Zydorczak, B.; Wu, Z.; Li, K.; Metcalfe, I.S. Electrochemical promotion of a Pt catalyst supported on La0.6Sr0.4Co0.2Fe0.8O3-δ hollow fibre membranes. In Proceedings of the Solid State Ionics; Elsevier: Amsterdam, The Netherlands, 2012; Volume 225, pp. 382–385. [Google Scholar] [CrossRef]
- Miyoshi, N.; Matsumoto, S.; Katoh, K.; Tanaka, T.; Harada, J.; Takahashi, N.; Yokota, K.; Sugiura, M.; Kasahara, K. Development of new concept three-way catalyst for automotive lean-burn engines. SAE Tech. Pap. 1995. [Google Scholar] [CrossRef]
- Matsumoto, S. DeNOx catalyst for automotive lean-burn engine. Catal. Today 1996, 29, 43–45. [Google Scholar] [CrossRef]
- Takahashi, N.; Shinjoh, H.; Iijima, T.; Suzuki, T.; Yamazaki, K.; Yokota, K.; Suzuki, H.; Miyoshi, N.; Matsumoto, S.; Tanizawa, T.; et al. The new concept 3-way catalyst for automotive lean-burn engine: NOx storage and reduction catalyst. Catal. Today 1996, 27, 63–69. [Google Scholar] [CrossRef]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks II, J.E. Overview of the fundamental reactions and degradation mechanisms of NOx storage/reduction catalysts. Catal. Rev. Sci. Eng. 2004, 46, 163–245. [Google Scholar] [CrossRef]
- Liu, G.; Gao, P.-X. A review of NOx storage/reduction catalysts: Mechanism, materials and degradation studies. Catal. Sci. Technol. 2011, 1, 552–568. [Google Scholar] [CrossRef]
- Kim, D.H. Sulfation and Desulfation Mechanisms on Pt–BaO/Al2O3 NOx Storage-Reduction (NSR) Catalysts. Catal. Surv. Asia 2014, 18, 13–23. [Google Scholar] [CrossRef]
- Pancharatnam, S. Catalytic Decomposition of Nitric Oxide on Zirconia by Electrolytic Removal of Oxygen. J. Electrochem. Soc. 1975, 122, 869. [Google Scholar] [CrossRef]
- Bredikhin, S.; Maeda, K.; Awano, M. NO decomposition by an electrochemical cell with mixed oxide working electrode. Solid State Ionics 2001, 144, 1–9. [Google Scholar] [CrossRef]
- Awano, M.; Bredikhin, S.; Aronin, A.; Abrosimova, G.; Katayama, S.; Hiramatsu, T. NOx decomposition by electrochemical reactor with electrochemically assembled multilayer electrode. Solid State Ionics 2004, 175, 605–608. [Google Scholar] [CrossRef]
- Hamamoto, K.; Fujishiro, Y.; Awano, M. Low-Temperature NOx Decomposition Using an Electrochemical Reactor. J. Electrochem. Soc. 2008, 155, E109. [Google Scholar] [CrossRef]
- Hadjar, A.; Hernández, W.Y.; Princivalle, A.; Tardivat, C.; Guizard, C.; Vernoux, P. Electrochemical activation of Pt-Ba/YSZ NOxTRAP catalyst under lean-burn conditions. Electrochem. Commun. 2011, 13, 924–927. [Google Scholar] [CrossRef]
- Wang, X.; Westermann, A.; Shi, Y.X.; Cai, N.S.; Rieu, M.; Viricelle, J.-P.; Vernoux, P. Electrochemical removal of NOx on ceria-based catalyst-electrodes. Catalysts 2017, 7. [Google Scholar] [CrossRef]
- Tsampas, M.N.; Vernoux, P. Electrochemical Promotion of Catalysis for Automotive Post-Treatment and Air Cleaning. In New and Future Developments in Catalysis; Ch. 11; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780444538703. [Google Scholar]
- Tang, X.; Xu, X.; Yi, H.; Chen, C.; Wang, C. Recent Developments of Electrochemical Promotion of Catalysis in the Techniques of DeNOx. Sci. World J. 2013, 2013, 463160. [Google Scholar] [CrossRef]
- Williams, F.J.; Palermo, A.; Tikhov, M.S.; Lambert, R.M. First Demonstration of in Situ Electrochemical Control of a Base Metal Catalyst: Spectroscopic and Kinetic Study of the CO + NO Reaction over Na-Promoted Cu. J. Phys. Chem. B 1999, 103, 9960–9966. [Google Scholar] [CrossRef]
- Williams, F.J.; Tikhov, M.S.; Palermo, A.; Macleod, N.; Lambert, R.M. Electrochemical promotion of rhodium-catalyzed NO reduction by CO and by propene in the presence of oxygen. J. Phys. Chem. B 2001, 105, 2800–2808. [Google Scholar] [CrossRef]
- Williams, F.J.; Palermo, A.; Tikhov, M.S.; Lambert, R.M. Mechanism of alkali promotion in heterogeneous catalysis under realistic conditions: Application of electron spectroscopy and electrochemical promotion to the reduction of NO by CO and by propene over rhodium. Surf. Sci. 2001, 482–485, 177–182. [Google Scholar] [CrossRef]
- Williams, F.J.; Palermo, A.; Tikhov, M.S.; Lambert, R.M. Electrochemical promotion by sodium of the rhodium-catalyzed NO + CO reaction. J. Phys. Chem. B 2000, 104, 11883–11890. [Google Scholar] [CrossRef]
- Lambert, R.M.; Tikhov, M.; Palermo, A.; Yentekakis, I.V.; Vayenas, C.G. Electrochemical promotion of environmentally important catalytic reactions. Ionics (Kiel) 1995, 1, 366–376. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Palermo, A.; Filkin, N.C.; Tikhov, M.S.; Lambert, R.M. In situ electrochemical promotion by sodium of the platinum-catalyzed reduction of NO by propene. J. Phys. Chem. B 1997, 101, 3759–3768. [Google Scholar] [CrossRef]
- Williams, F.J.; Palermo, A.; Tikhov, M.S.; Lambert, R.M. Electrochemical promotion by sodium of the rhodium-catalyzed reduction of NO by propene: Kinetics and spectroscopy. J. Phys. Chem. B 2001, 105, 1381–1388. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A.; Caravaca, Á.; Sánchez, P.; Dorado, F.; Valverde, J.L. A new improvement of catalysis by solid-state electrochemistry: An electrochemically assisted NOx storage/reduction catalyst. J. Catal. 2008, 259, 54–65. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A.; Caravaca, A.; Martín de Vidales, M.J.; Dorado, F.; Balomenou, S.; Tsiplakides, D.; Vernoux, P.; Valverde, J.L. An electrochemically assisted NOx storage/reduction catalyst operating under fixed lean burn conditions. Catal. Commun. 2009, 11, 247–251. [Google Scholar] [CrossRef]
- Christensen, H.; Dinesen, J.; Engell, H.H.; Hansen, K.K. Electrochemical reactor for exhaust gas purification. SAE Tech. Pap. 1999. [Google Scholar] [CrossRef]
- Christensen, H.; Dinesen, J.; Engell, H.H.; Larsen, L.C.; Hansen, K.K.; Skou, E.M. Electrochemical exhaust gas purification. SAE Tech. Pap. 2000. [Google Scholar] [CrossRef]
- Jena, P. Materials for hydrogen storage: Past, present, and future. J. Phys. Chem. Lett. 2011, 2, 206–211. [Google Scholar] [CrossRef]
- Klingmann, J.; Andersson, M. Hydrogen and Hydrogen-Rich Fuels: Production and Conversion to Electricity. In Innovations in Sustainable Energy and Cleaner Environment; Ch. 10; Gupta, A.K., De, A., Aggarwal, S.K., Kushari, A., Runchal, A., Eds.; Springer: Singapore, 2020; pp. 219–233. ISBN 978-981-13-9012-8. [Google Scholar]
- Dodds, P.E.; Staffell, I.; Hawkes, A.D.; Li, F.; Grünewald, P.; McDowall, W.; Ekins, P. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrogen Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef]
- Apostolou, D.; Xydis, G. A literature review on hydrogen refuelling stations and infrastructure. Current status and future prospects. Renew. Sustain. Energy Rev. 2019, 113, 109292. [Google Scholar] [CrossRef]
- Cipriani, G.; Di Dio, V.; Genduso, F.; La Cascia, D.; Liga, R.; Miceli, R.; Ricco Galluzzo, G. Perspective on hydrogen energy carrier and its automotive applications. Int. J. Hydrogen Energy 2014, 39, 8482–8494. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Busca, G.; Costantino, U.; Montanari, T.; Ramis, G.; Resini, C.; Sisani, M. Nickel versus cobalt catalysts for hydrogen production by ethanol steam reforming: Ni-Co-Zn-Al catalysts from hydrotalcite-like precursors. Int. J. Hydrogen Energy 2010, 35, 5356–5366. [Google Scholar] [CrossRef]
- Megía, P.J.; Calles, J.A.; Carrero, A.; Vizcaíno, A.J. Effect of the incorporation of reducibility promoters (Cu, Ce, Ag) in Co/CaSBA-15 catalysts for acetic acid steam reforming. Int. J. Energy Res. 2020, 1, 1–18. [Google Scholar] [CrossRef]
- Chen, S.; Zaffran, J.; Yang, B. Dry reforming of methane over the cobalt catalyst: Theoretical insights into the reaction kinetics and mechanism for catalyst deactivation. Appl. Catal. B Environ. 2020, 270, 118859–118868. [Google Scholar] [CrossRef]
- Budiman, A.W.; Song, S.-H.; Chang, T.-S.; Shin, C.-H.; Choi, M.-J. Dry Reforming of Methane Over Cobalt Catalysts: A Literature Review of Catalyst Development. Catal. Surv. Asia 2012, 16, 183–197. [Google Scholar] [CrossRef]
- Osman, A.I. Catalytic hydrogen production from methane partial oxidation: Mechanism and kinetic study. Chem. Eng. Technol. 2020, 43, 641–648. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Charpentier, P.A. Hydrogen production by gasification of biomass and opportunity fuels. In Compendium of Hydrogen Energy; Ch. 6; Subramani, V., Basile, A., Veziroglu, T.N., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 137–175. [Google Scholar]
- Summa, P.; Samojeden, B.; Motak, M. Dry and steam reforming of methane. Comparison and analysis of recently investigated catalytic materials. A short review. Polish J. Chem. Technol. 2019, 21, 31–37. [Google Scholar] [CrossRef]
- De Souza, V.P.; Costa, D.; dos Santos, D.; Sato, A.G.; Bueno, J.M.C. Pt-promoted α-Al2O3-supported Ni catalysts: Effect of preparation conditions on oxi-reduction and catalytic properties for hydrogen production by steam reforming of methane. Int. J. Hydrogen Energy 2012, 37, 9985–9993. [Google Scholar] [CrossRef]
- Borowiecki, T.; Denis, A.; Rawski, M.; Gołębiowski, A.; Stołecki, K.; Dmytrzyk, J.; Kotarba, A. Studies of potassium-promoted nickel catalysts for methane steam reforming: Effect of surface potassium location. Appl. Surf. Sci. 2014, 300, 191–200. [Google Scholar] [CrossRef]
- Borowiecki, T.; Gołębiowski, A.; Ryczkowski, J.; Stasmska, B. The influence of promoters on the coking rate of nickel catalysts in the steam reforming of hydrocarbons. Stud. Surf. Sci. Catal. 1998, 119, 711–716. [Google Scholar] [CrossRef]
- Alstrup, I.; Clausen, B.S.; Olsen, C.; Smits, R.H.H.; Rostrup-Nielsen, J.R. Promotion of Steam Reforming Catalysts. Stud. Surf. Sci. Catal. 1998, 119, 5–14. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A.; Caravaca, A.; Martínez, P.J.; Endrino, J.L.; Dorado, F.; Valverde, J.L. Development of a new electrochemical catalyst with an electrochemically assisted regeneration ability for H2 production at low temperatures. J. Catal. 2010, 274, 251–258. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Mukainakano, Y.; Kado, S.; Miyazawa, T.; Okumura, K.; Miyao, T.; Naito, S.; Suzuki, K.; Fujimoto, K.-I.; Kunimori, K.; et al. Oxidative steam reforming of methane under atmospheric and pressurized conditions over Pd/NiO–MgO solid solution catalysts. Appl. Catal. A Gen. 2006, 308, 1–12. [Google Scholar] [CrossRef]
- González-Cobos, J.; López-Pedrajas, D.; Ruiz-López, E.; Valverde, J.L.; de Lucas-Consuegra, A. Applications of the Electrochemical Promotion of Catalysis in Methanol Conversion Processes. Top. Catal. 2015, 58, 1290–1302. [Google Scholar] [CrossRef]
- Deng, W.-Q.; Xu, X.; Goddard, W.A. New Alkali Doped Pillared Carbon Materials Designed to Achieve Practical Reversible Hydrogen Storage for Transportation. Phys. Rev. Lett. 2004, 92, 166103. [Google Scholar] [CrossRef]
- Espinós, J.P.; Rico, V.J.; González-Cobos, J.; Sánchez-Valencia, J.R.; Pérez-Dieste, V.; Escudero, C.; de Lucas-Consuegra, A.; González-Elipe, A.R. In situ monitoring of the phenomenon of electrochemical promotion of catalysis. J. Catal. 2018, 358, 27–34. [Google Scholar] [CrossRef]
- Espinós, J.P.; Rico, V.J.; González-Cobos, J.; Sánchez-Valencia, J.R.; Pérez-Dieste, V.; Escudero, C.; De Lucas-Consuegra, A.; González-Elipe, A.R. Graphene Formation Mechanism by the Electrochemical Promotion of a Ni Catalyst. ACS Catal. 2019, 11447–11454. [Google Scholar] [CrossRef]
- Van Den Berg, A.W.C.; Areán, C.O. Materials for hydrogen storage: Current research trends and perspectives. Chem. Commun. 2008, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Dalebrook, A.F.; Gan, W.; Grasemann, M.; Moret, S.; Laurenczy, G. Hydrogen storage: Beyond conventional methods. Chem. Commun. 2013, 49, 8735–8751. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Weststrate, C.J.K.-J.; Fredriksson, H.O.A.; Niemantsverdriet, J.W.H. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Technological development of hydrogen production by solid oxide electrolyzer cell (SOEC). Int. J. Hydrogen Energy 2008, 33, 2337–2354. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, J.; Yuan, Z.; Liu, J.; Ni, M.; Chen, F. Progress Report on Proton Conducting Solid Oxide Electrolysis Cells. Adv. Funct. Mater. 2019. [Google Scholar] [CrossRef]
- Sasikumar, G.; Muthumeenal, A.; Pethaiah, S.S.; Nachiappan, N.; Balaji, R. Aqueous methanol eletrolysis using proton conducting membrane for hydrogen production. Int. J. Hydrogen Energy 2008, 33, 5905–5910. [Google Scholar] [CrossRef]
- Caravaca, A.; Sapountzi, F.M.; De Lucas-Consuegra, A.; Molina-Mora, C.; Dorado, F.; Valverde, J.L. Electrochemical reforming of ethanol-water solutions for pure H2 production in a PEM electrolysis cell. Int. J. Hydrogen Energy 2012, 37, 9504–9513. [Google Scholar] [CrossRef]
- Caravaca, A.; De Lucas-Consuegra, A.; Calcerrada, A.B.; Lobato, J.; Valverde, J.L.; Dorado, F. From biomass to pure hydrogen: Electrochemical reforming of bio-ethanol in a PEM electrolyser. Appl. Catal. B Environ. 2013, 134–135. [Google Scholar] [CrossRef]
- Calcerrada, A.B.; de la Osa, A.R.; Llanos, J.; Dorado, F.; de Lucas-Consuegra, A. Hydrogen from electrochemical reforming of ethanol assisted by sulfuric acid addition. Appl. Catal. B Environ. 2018, 231, 310–316. [Google Scholar] [CrossRef]
- Calcerrada, A.B.; de la Osa, A.R.; Lopez-Fernandez, E.; Dorado, F.; de Lucas-Consuegra, A. Influence of the carbon support on the Pt–Sn anodic catalyst for the electrochemical reforming of ethanol. Int. J. Hydrogen Energy 2019, 44, 10616–10626. [Google Scholar] [CrossRef]
- Calcerrada, A.B.; de la Osa, A.R.; Dole, H.A.E.; Dorado, F.; Baranova, E.A.; de Lucas-Consuegra, A. Stability Testing of PtxSn1 − x/C Anodic Catalyst for Renewable Hydrogen Production Via Electrochemical Reforming of Ethanol. Electrocatalysis 2018, 9, 293–301. [Google Scholar] [CrossRef]
- Gutiérrez-Guerra, N.; Jiménez-Vázquez, M.; Serrano-Ruiz, J.C.; Valverde, J.L.; de Lucas-Consuegra, A. Electrochemical reforming vs. catalytic reforming of ethanol: A process energy analysis for hydrogen production. Chem. Eng. Process. Process Intensif. 2015, 95, 9–16. [Google Scholar] [CrossRef]
- Ruiz-López, E.; Amores, E.; Raquel de la Osa, A.; Dorado, F.; de Lucas-Consuegra, A. Electrochemical reforming of ethanol in a membrane-less reactor configuration. Chem. Eng. J. 2020. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A.; De La Osa, A.R.; Calcerrada, A.B.; Linares, J.J.; Horwat, D. A novel sputtered Pd mesh architecture as an advanced electrocatalyst for highly efficient hydrogen production. J. Power Sources 2016, 321, 248–256. [Google Scholar] [CrossRef]
- Simões, M.; Baranton, S.; Coutanceau, C. Electrochemical valorisation of glycerol. ChemSusChem 2012, 5, 2106–2124. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S.; Kouamé, R.S.B. Selective electrooxidation of glycerol into value-added chemicals: A short overview. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Haisch, T.; Kubannek, F.; Baranton, S.; Coutanceau, C.; Krewer, U. The influence of adsorbed substances on alkaline methanol electro-oxidation. Electrochim. Acta 2019, 295, 278–285. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S. Electrochemical conversion of alcohols for hydrogen production: A short overview. Wiley Interdiscip. Rev. Energy Environ. 2016, 5, 388–400. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Jensen, S.H.; Hauch, A.; Mogensen, M.B. High Temperature Electrolysis in Alkaline Cells, Solid Proton Conducting Cells, and Solid Oxide Cells. Chem. Rev. 2014, 114, 10697–10734. [Google Scholar] [CrossRef]
- Caravaca, A.; De Lucas-Consuegra, A.; Molina-Mora, C.; Valverde, J.L.; Dorado, F. Enhanced H2 formation by electrochemical promotion in a single chamber steam electrolysis cell. Appl. Catal. B Environ. 2011, 106. [Google Scholar] [CrossRef]
- Ruiz-López, E.; Caravaca, A.; Vernoux, P.; Dorado, F.; de Lucas-Consuegra, A. Over-faradaic hydrogen production in methanol electrolysis cells. Chem. Eng. J. 2020, 396, 125217. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Yubero, F.; Espinós, J.P.; González-Elipe, A.R.; Lambert, R.M. Synthesis, characterization and performance of robust poison-resistant ultrathin film yttria stabilized zirconia - Nickel anodes for application in solid electrolyte fuel cells. J. Power Sources 2016, 324, 679–686. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Beltrán, A.M.; Yubero, F.; González-Elipe, A.R.; Lambert, R.M. High performance novel gadolinium doped ceria/yttria stabilized zirconia/nickel layered and hybrid thin film anodes for application in solid oxide fuel cells. J. Power Sources 2017, 363, 251–259. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Yubero, F.; González-Elipe, A.R.; Balomenou, S.P.; Tsiplakides, D.; Petrakopoulou, I.; Lambert, R.M. Porous, robust highly conducting Ni-YSZ thin film anodes prepared by magnetron sputtering at oblique angles for application as anodes and buffer layers in solid oxide fuel cells. Int. J. Hydrogen Energy 2015, 40, 7382–7387. [Google Scholar] [CrossRef]
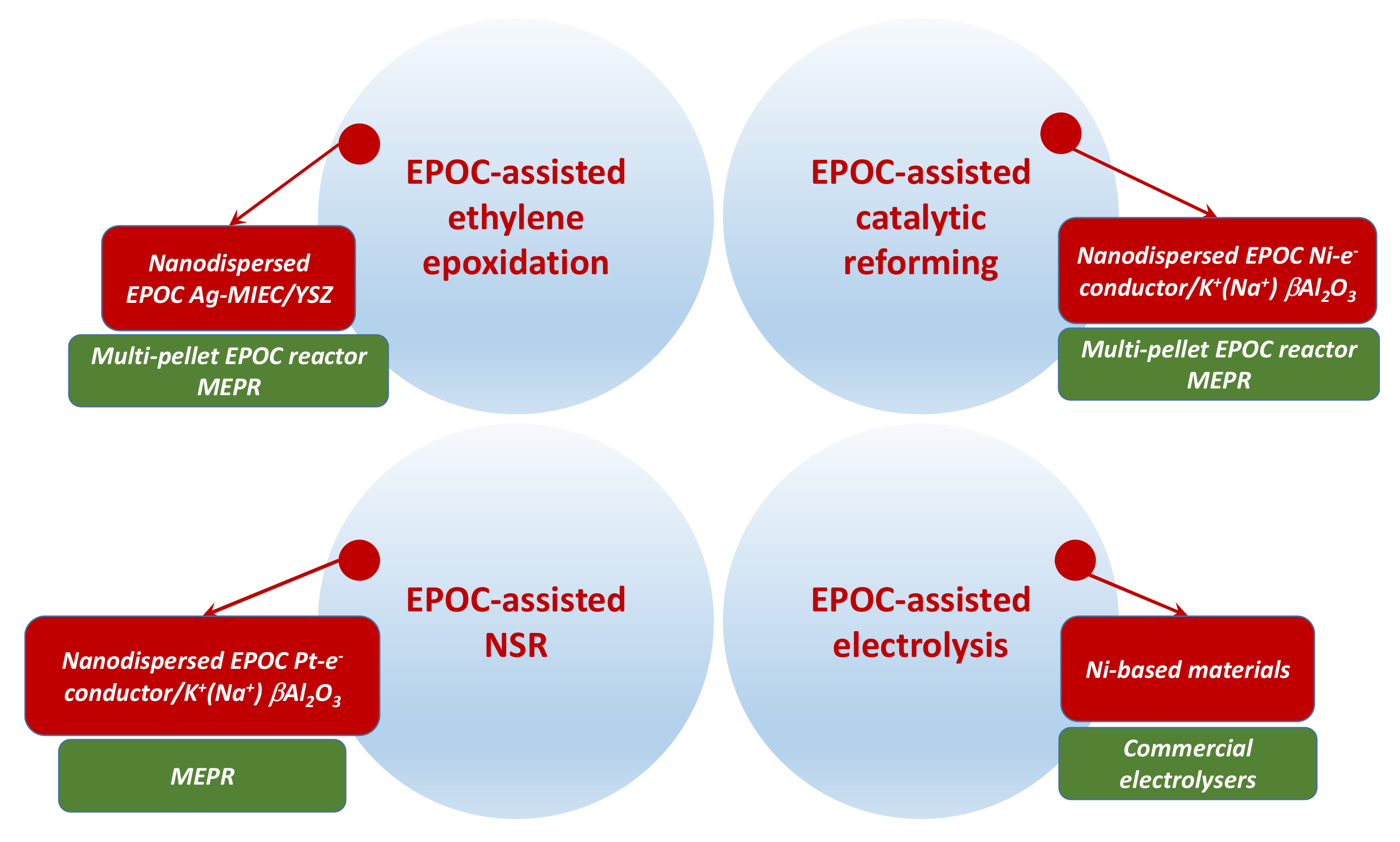
| The Case of EPOC-Assisted Ethylene Epoxidation | |
|---|---|
| Conventional catalysts | Issues |
| Use of toxic chlorine promoters | |
| EPOC approach | Benefits |
| Substitution of chlorine promoters by Oδ- (non-toxic) | |
| in-situ optimization of promoted state by applied polarization | |
| Issues | |
| Non-optimized Ag catalyst: low dispersion = low activity | |
| Oδ- sacrificial promoter: constant current application | |
| Reactor design | |
| The Case of EPOC-Assisted NOx Storage and Reduction (NSR) | |
|---|---|
| Conventional catalysts | Issues |
| Need of rich cycles for NOx reduction: fuel penalty | |
| EPOC approach | Benefits |
| NSR under constant lean-burn conditions: no fuel penalty | |
| Low power-input | |
| in-situ optimization of promoted state by applied polarization | |
| Issues | |
| Non-optimized Pt catalyst: low dispersion = low activity | |
| Reactor design | |
| The Case of EPOC-Assisted Catalytic Reforming | |
|---|---|
| Conventional catalysts | Issues |
| No promotion: carbon deposition by coking | |
| Promotion by alkalis: low activity | |
| Benefits | |
| Cyclic operation by step changes in applied polarization | |
| Unpromoted conditions = high activity + coking (deactivation) | |
| EPOC approach | Promoted conditions = low activity + regeneration |
| Methane reforming | in-situ optimization of promoted/unpromoted states in cyclic polarisation mode |
| Issues | |
| Non-optimized Pt catalyst: low dispersion = low activity | |
| Use of Pt, while conventional catalysts are based on Ni | |
| Reactor design | |
| Benefits | |
| Capability of simultaneous H2 production and storage via graphene-like surface compounds | |
| Promoted conditions = high activity + controlled H2 storage | |
| EPOC approach | Unpromoted conditions = controlled H2 release |
| Methanol reforming | Cyclic operation via applied polarization at mild temperature and atmospheric pressure |
| H2 storage/release | Development of advanced Ni catalysts with high dispersion |
| Issues | |
| Reactor design | |
| Complexity of the H2 storage system | |
| The Case of EPOC-Assisted Electrolysis | |
|---|---|
| Conventional catalysts | Issues |
| Catalyst efficiency | |
| Material durability and energy consumption costs | |
| Benefits | |
| Coupling of steam electrolysis (electrocatalysis) + methane reforming (EPOC) | |
| Additional H2 production with the same power input = higher energy efficiency | |
| EPOC approach | Easy to scale-up by the use of state-of-the art SOE stack-electrolysers |
| Solid oxide electrolysis | in-situ optimization of promoted states by applied polarization |
| (Water electrolysis) | Issues |
| Non-optimized Pt catalyst: low dispersion = low activity | |
| Use of Pt, while conventional electrolysers are based on Ni | |
| Use of single chamber reactors instead of state-of-the art double chamber configurations | |
| Benefits | |
| Coupling of methanol electrolysis (electrocatalysis) + methanol decomposition (EPOC) | |
| EPOC approach | Additional H2 production with the same power input = higher energy efficiency |
| Depolarized PEM electrolysers | Easy to scale-up by the use of state-of-the art PEM stack-electrolysers |
| (Methanol electrolysis) | in-situ optimization of promoted states by applied polarization |
| Issues | |
| Use of noble metals, while conventional water electrolysers are based on Ni | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caravaca, A.; González-Cobos, J.; Vernoux, P. A Discussion on the Unique Features of Electrochemical Promotion of Catalysis (EPOC): Are We in the Right Path Towards Commercial Implementation? Catalysts 2020, 10, 1276. https://doi.org/10.3390/catal10111276
Caravaca A, González-Cobos J, Vernoux P. A Discussion on the Unique Features of Electrochemical Promotion of Catalysis (EPOC): Are We in the Right Path Towards Commercial Implementation? Catalysts. 2020; 10(11):1276. https://doi.org/10.3390/catal10111276
Chicago/Turabian StyleCaravaca, Angel, Jesús González-Cobos, and Philippe Vernoux. 2020. "A Discussion on the Unique Features of Electrochemical Promotion of Catalysis (EPOC): Are We in the Right Path Towards Commercial Implementation?" Catalysts 10, no. 11: 1276. https://doi.org/10.3390/catal10111276
APA StyleCaravaca, A., González-Cobos, J., & Vernoux, P. (2020). A Discussion on the Unique Features of Electrochemical Promotion of Catalysis (EPOC): Are We in the Right Path Towards Commercial Implementation? Catalysts, 10(11), 1276. https://doi.org/10.3390/catal10111276






