Baeyer-Villiger-Including Domino Two-Step Oxidations of β-O-Substituted Primary Alcohols: Reflection of the Migratory Aptitudes of O-Substituted Alkyl Group in the Outcome of the Reaction
Abstract
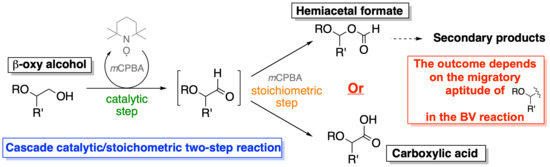
1. Introduction
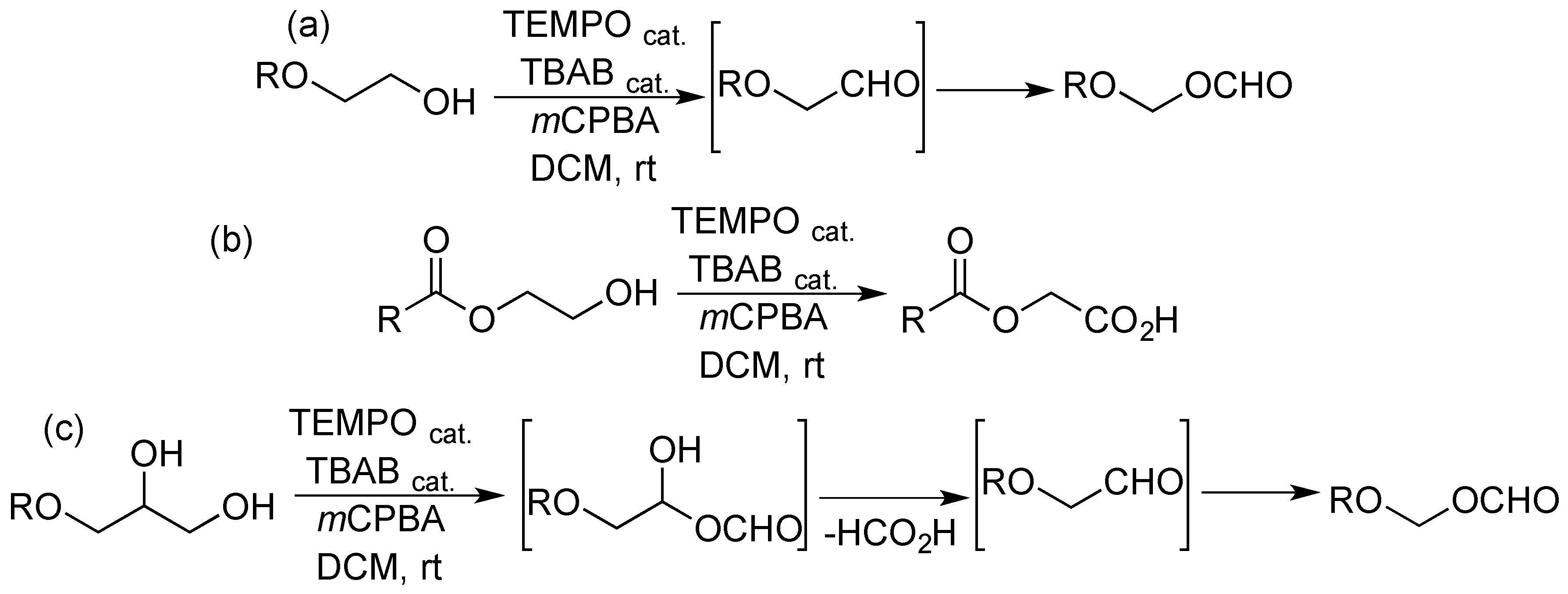
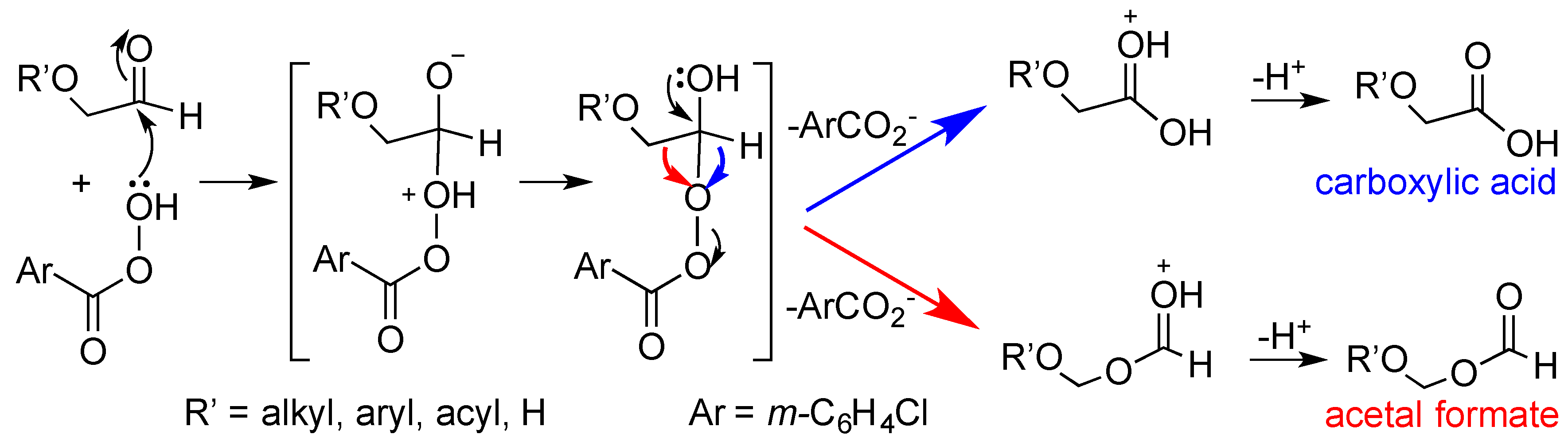
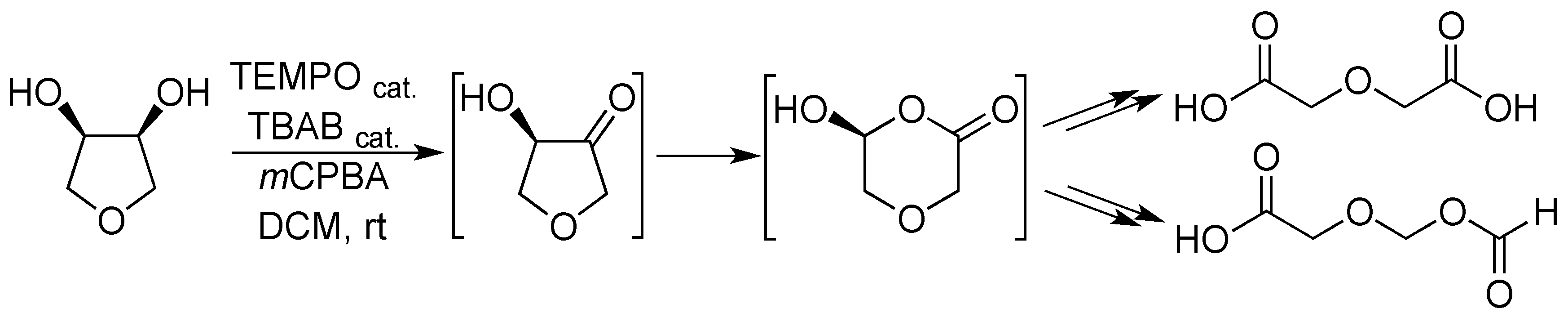
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Techniques
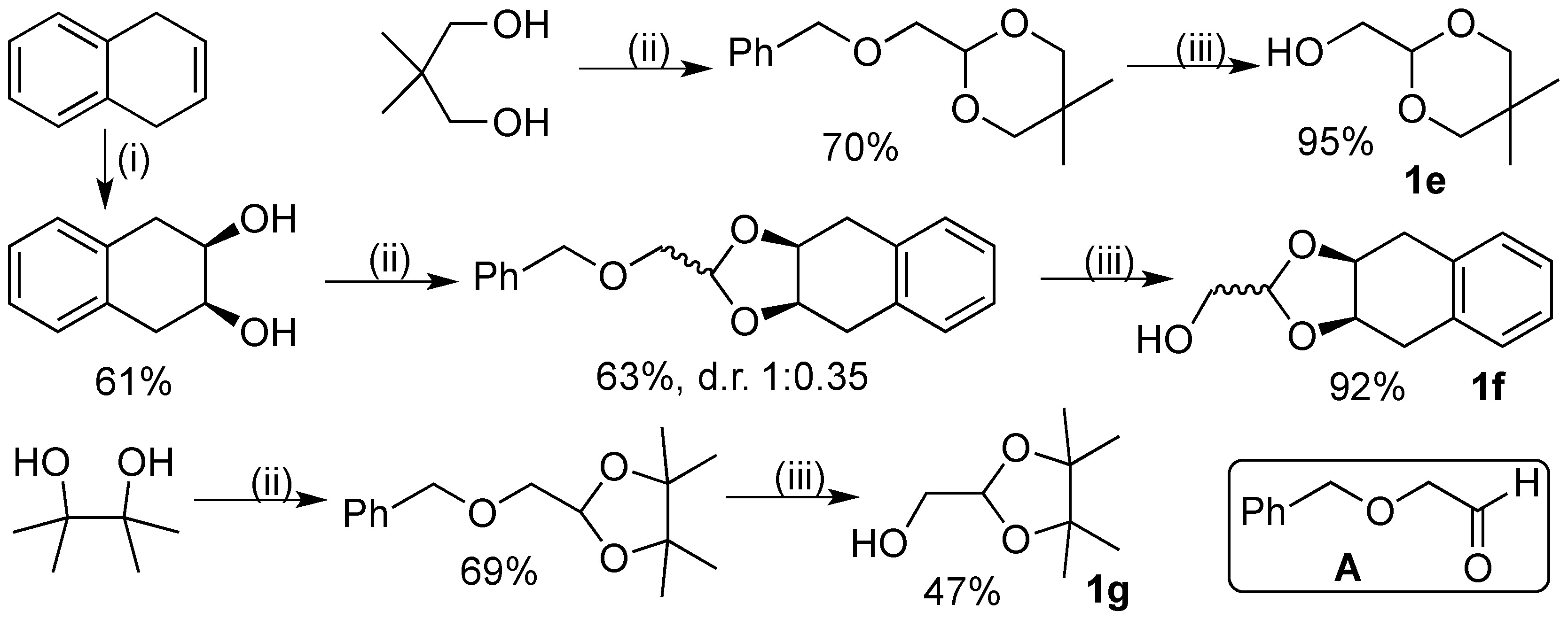
3.2. Synthesis of Substrates
3.2.1. Synthesis of [(2S,4S)-2-Phenyl-1,3-Dioxan-4-yl]Methanol (1b):
3.2.2. Synthesis of (5,5-Dimethyl-1,3-Dioxan-2-yl)Methanol (1e):
3.2.3. Synthesis of ((3ar,9as)-3a,4,9,9a-Tetrahydronaphtho[2,3-d][1,3]dioxol-2-yl)methanol (1f)
3.2.4. Synthesis of (4,4,5,5-Tetramethyl-1,3-dioxolan-2-yl)methanol (1g):
3.3. Oxidations and Characterization of Products
3.3.1. Typical Procedure for TEMPO/mCPBA Oxidation:
3.3.2. Oxidation of 2-Phenoxyethan-2-ol (1a):
3.3.3. Oxidation of [(2S,4S)-2-Phenyl-1,3-dioxan-4-yl]methanol (1b):
3.3.4. Oxidation of (2R,3R,4S,5R,6S)-2-(hydroxymethyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triyl tribenzoate (1c):
3.3.5. Oxidation of (4R-cis)-6-Hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetic acid 1,1-dimethylethyl ester (1d):
3.3.6. Oxidation of (5,5-Dimethyl-1,3-dioxan-2-yl)methanol (1e):
3.3.7. Oxidation of ((3aR,9aS)-3a,4,9,9a-Tetrahydronaphtho[2,3-d][1,3]dioxol-2-yl)methanol (1f):
3.3.8. Oxidation of (4,4,5,5-Tetramethyl-1,3-dioxolan-2-yl)methanol (1g):
3.3.9. Oxidation of Methyl 3,5-di-O-(2,4-dichlorobenzyl)- α-D-Ribofuranoside (1h):
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Tojo, G.; Fernandez, M. Oxidation of Alcohols to Aldehydes and Ketones; Springer: New York, NY, USA, 2006. [Google Scholar]
- Krow, G.R. The Baeyer-Villiger oxidation of ketones and aldehydes. Org. React. 1993, 43, 251–798. [Google Scholar]
- ten Brink, G.J.; Arends, I.W.C.E.; Sheldon, R.A. The Baeyer-Villiger reaction: New developments toward greener procedures. Chem. Rev. 2004, 104, 4105–4123. [Google Scholar] [CrossRef]
- Strukul, G. Transition metal catalysis in the Baeyer-Villiger oxidation of ketones. Angew. Chem. Int. Ed. 1998, 37, 1199–1209. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Vil, V.A.; Demchuk, D.V.; Terent’ev, A.O. Rearrangements of organic peroxides and related processes. Beilstein. J. Org. Chem. 2016, 12, 1647–1748. [Google Scholar] [CrossRef] [PubMed]
- For examples of oxidation of secondary alcohols to esters in two separate steps, see subsequent references 7-15, while for infrequent examples of one-pot oxidation of secondary alcohols to esters, see references 16-19.
- Tsang, R.; Fraser-Reid, B. Pyranose alpha-enones provide ready access to functionalized trans-decalins via bis-annulated pyranosides obtained by intramolecular Diels-Alder reactions—a key intermediate for Forskolin. J. Org. Chem. 1992, 57, 1065–1067. [Google Scholar] [CrossRef]
- Chida, N.; Tobe, T.; Ogawa, S. Regioselective Baeyer-Villiger reaction of polyhydroxycyclohexanone derivatives. Tetrahedron Lett. 1994, 35, 7249–7252. [Google Scholar] [CrossRef]
- Mecerreyes, D.; Atthoff, B.; Boduch, K.A.; Trollsas, M.; Hedrick, J.L. Unimolecular combination of an atom transfer radical polymerization initiator and a lactone monomer as a route to new graft copolymers. Macromolecules 1999, 32, 5175–5182. [Google Scholar] [CrossRef]
- Ishmuratov, G.Y.; Yakovleva, M.P.; Ganieva, V.A.; Gareeva, G.R.; Muslukhov, R.R.; Tolstikov, G.A. Synthesis of optically pure 3R-methylcyclopentan-1-one from L-(-)-menthol. Chem. Nat. Compd. 2005, 41, 549–551. [Google Scholar] [CrossRef]
- Rainbolt, E.A.; Washington, K.E.; Biewer, M.C.; Stefan, M.C. Towards smart polymeric drug carriers: Self-assembling γ-substituted polycaprolactones with highly tunable thermoresponsive behavior. J. Mater. Chem. B 2013, 1, 6532–6537. [Google Scholar] [CrossRef]
- Surnar, B.; Jayakannan, M. Stimuli-responsive poly(caprolactone) vesicles for dual drug delivery under the gastrointestinal tract. Biomacromolecules 2013, 14, 4377–4387. [Google Scholar] [CrossRef]
- Ercole, F.; Rodda, A.E.; Meagher, L.; Forsythe, J.S.; Dove, A.P. Surface grafted poly(ε-caprolactone) prepared using organocatalysed ring-opening polymerisation followed by SI-ATRP. Polym. Chem. 2014, 5, 2809–2815. [Google Scholar] [CrossRef]
- Yamauchi, S.; Nishimura, H.; Nishiwaki, H. Stereoselective syntheses of cryptocarya diacetate and all its stereoisomers in optically pure forms. Biosci. Biotechnol. Biochem. 2015, 79, 16–24. [Google Scholar] [CrossRef]
- Malhotra, M.; Surnar, B.; Jayakannan, M. Polymer topology driven enzymatic biodegradation in polycaprolactone block and random copolymer architectures for drug delivery to cancer cells. Macromolecules 2016, 49, 8098–8112. [Google Scholar] [CrossRef]
- Cella, J.A.; McGrath, J.P.; Kelley, J.A.; El Soukkary, O.; Hilpert, L. Applications of the peracid-mediated oxidation of alcohols. J. Org. Chem. 1977, 42, 2077–2080. [Google Scholar] [CrossRef]
- Chrobok, A. An efficient tandem oxidation of cyclohexanol to ε-caprolactone with peroxyacids and TEMPO catalyst in ionic liquids as solvents. Synlett 2011, 391–395. [Google Scholar] [CrossRef]
- Weisser, F.; Stevens, H.; Klein, J.; van der Meer, M.; Hohloch, S.; Sarkar, B. Tailoring Ru(II) pyridine/triazole oxygenation catalysts and using photoreactivity to probe their electronic properties. Chem. Eur. J. 2015, 21, 8926–8938. [Google Scholar] [CrossRef]
- Dijkmans, J.; Schutyser, W.; Dusselier, M.; Sels, B.F. Snβ-zeolite catalyzed oxido-reduction cascade chemistry with biomass-derived molecules. Chem. Commun. 2016, 52, 6712–6715. [Google Scholar] [CrossRef]
- Figadere, B.; Franck, X. Carboxylic Acids: Synthesis from alcohols. In Science of Synthesis; Panek, J.S., Ed.; Georg Thieme Verlag: Stuttgart, Germany, 2006; Volume 20a, pp. 173–204. [Google Scholar]
- For reactions of electron-rich benzaldehydes (Dakin reaction), see subsequent references 22–23, while for reactions of aldehydes with a nitrogen- or an oxygen-substituted α-carbon, see references 24–31.
- Hocking, M.B.; Bhandari, K.; Shell, B.; Smyth, T.A. Steric and pH effects on the rate of Dakin oxidation of acylphenols. J. Org. Chem. 1982, 47, 4208–4215. [Google Scholar] [CrossRef]
- Saikia, B.; Borah, P. A new avenue to the Dakin reaction in H2O2–WERSA. RSC Adv. 2015, 5, 105583–105586. [Google Scholar] [CrossRef]
- Alcaide, B.; Aly, M.F.; Sierra, M.A. Stereoselective synthesis of 3-substituted 4-(formyloxy)-2-azetidinones by the unusual Baeyer-Villiger reaction of beta-lactam aldehydes. Scope and synthetic applications. J. Org. Chem. 1996, 61, 8819–8825. [Google Scholar] [CrossRef]
- Deboer, A.; Ellwanger, R.E. Baeyer-Villiger oxidation of Δ1(9)-Octalone-2 and Δ1(8)-Indanone-2. J. Org. Chem. 1974, 39, 77–83. [Google Scholar] [CrossRef]
- Labadie, G.R.; Luna, L.E.; Gonzalez-Sierra, M.; Cravero, R.M. Synthesis of the tetracyclic bis(acetal) lactone portion of Saudin. Eur. J. Org. Chem. 2003, 3429–3434. [Google Scholar] [CrossRef]
- Jeso, V.; Iqbal, S.; Hernandez, P.; Cameron, M.D.; Park, H.; LoGrasso, P.V.; Micalizio, G.C. Synthesis of benzoquinone Ansamycin-inspired macrocyclic lactams from Shikimic acid. Angew. Chem. Int. Ed. 2013, 52, 4800–4804. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Frederick, M.O.; Burtoloso, A.C.B.; Denton, R.M.; Rivas, F.; Cole, K.P.; Aversa, R.J.; Gibe, R.; Umezawa, T.; Suzuki, T. Chemical synthesis of the GHIJKLMNO ring system of maitotoxin. J. Am. Chem. Soc. 2008, 130, 7466–7476. [Google Scholar] [CrossRef]
- Chaubet, G.; Bourgeois, D.; Périgaud, C. Synthetic studies towards new nucleoside analogues: Preparation of (±)-1′,4′-dimethyladenosine. Eur. J. Org. Chem. 2011, 319–326. [Google Scholar] [CrossRef]
- Himmelbauer, M.; Farcet, J.B.; Gagnepain, J.; Mulzer, J. Palladium-catalyzed carbo-oxygenation: The Bielschowskysin case. Org. Lett. 2013, 15, 3098–3101. [Google Scholar] [CrossRef]
- Urabe, F.; Nagashima, S.; Takahashi, K.; Ishihara, J.; Hatakeyama, S. Total synthesis of (-)-Cinatrin C1 based on an In(OTf)3-catalyzed Conia-ene reaction. J. Org. Chem. 2013, 78, 3847–3857. [Google Scholar] [CrossRef]
- Targel, T.; Ramesh, P.; Portnoy, M. Domino two-step oxidation of β-alkoxy alcohols to hemiacetal esters: Linking a stoichiometric step to an organocatalytic step with a common organic oxidant. Eur. J. Org. Chem. 2018, 23, 3017–3021. [Google Scholar] [CrossRef]
- For cases when such transformation was conducted in two successive, but separate, steps, see refs. 7,8.
- For similar comparison of some of these migratory aptitudes in ketones, see ref. 8.
- The endocyclic character of the alkoxyalkyl should not be a factor in this comparison, since the related 3-hydroxytetrahydrofurane undergoes a rapid cascade reaction with the insertion of oxygen near this group.
- Jimenez, F.; del Carmen Cruz, M.; Zuniga, C.; Martinez, M.A.; Chamorro, G.; Diza, F.; Tamariz, J. Aryloxyacetic esters structurally related to α-Asarone as potential antifungal agents. Med. Chem. Res. 2010, 19, 33–57. [Google Scholar] [CrossRef]
- 1b was prepared from a triol precursor by trans-acetalization with benzaldehyde dimethylacetal, see Materials and Methods.
- 38. See, for instance: Xie, Y.; Zhang, J.; Tian, G.; Xu, M.; Hu, T.; Jiang, X.; Shen, J. A neighboring group participation strategy: Facile synthesis of 3,5-di-O-benzoyl-2-C-methyl-d-arabino-γ-lactone. Tetrahedron Lett. 2015, 56, 4345–4348. [Google Scholar] [CrossRef]
- In order to drive the reaction to completion, excess of mCPBA was applied.
- Lehtinen, C.; Nevalainen, V.; Brunow, G. Experimental and computational studies on solvent effects in reactions of peracid-aldehyde adducts. Tetrahedron 2001, 57, 4741–4751. [Google Scholar] [CrossRef]
- Ogata, Y.; Sawaki, Y. Kinetics of the Baeyer-Villiger reaction of benzaldehydes with perbenzoic acid in aquoorganic solvents. J. Org. Chem. 1969, 34, 3985–3991. [Google Scholar] [CrossRef]
- Flogel, O.; Okala Amombo, M.G.; Reissig, H.U.; Zahn, G.; Brudgam, I.; Hartl, H. A stereoselective and short total synthesis of the polyhydroxylated γ-amino acid (-)-detoxinine, based on stereoselective preparation of dihydropyrrole derivatives from lithiated alkoxyallenes. Chem. Eur. J. 2003, 9, 1405–1415. [Google Scholar] [CrossRef]
- Miyazawa, S.; Shinoda, M.; Kawahara, T.; Watanabe, N.; Harada, H.; Iida, D.; Terauchi, H.; Nagakawa, J.; Fujisaki, H.; Kubota, A.; et al. Preparation of Benzimidazole Derivatives as Gastric Acid Secretion Inhibitors. U.S. Patent Appl. Publ. US 2007/0010542 A1, 11 January 2007. [Google Scholar]
- Huo, C.; Yang, H.; Cui, Q.C.; Dou, Q.P.; Chan, T.H. Proteasome inhibition in human breast cancer cells with high catechol-O-methyltransferase activity by green tea polyphenol EGCG analogs. Bioorg. Med. Chem. 2010, 18, 1252–1258. [Google Scholar] [CrossRef]
- Lei, P.-s.; Ogawa, Y.; Kovac, P. New N-acylating reagent derived from 3-deoxy-L-glycero-tetronic acid. J. Carbohydr. Chem. 1996, 15, 485–500. [Google Scholar] [CrossRef]
- Esmurziev, A.M.; Reimers, A.; Andreassen, T.; Simic, N.; Sundby, E.; Hoff, B.H. Benzoylated uronic acid building blocks and synthesis of N-uronate conjugates of Lamotrigine. Molecules 2012, 17, 820–835. [Google Scholar] [CrossRef]
- 47. For characterization of 3d, see: Edwards, J.T.; Merchant, R.R.; McClymont, K.S.; Knouse, K.W.; Qin, T.; Malins, L.R.; Vokits, B.; Shaw, S.A.; Bao, D.H.; Wei, F.-L.; et al. Decarboxylative alkenylation. Nature 2017, 545, 213–219. [Google Scholar]
- For characterization of 4e, see: Filliatre, C.; Brigand, G.; Lalande, R. Peroxidation of oxygenated heterocyclic compounds. Bull. Soc. Chim. Fr. 1971, 170–176. [Google Scholar]
- Goosen, A.; McCleland, C.W. Reaction of 1,3-dioxolans with iodine monochloride: The scope and mechanism of formation of 1,3-dioxolan-2-ylium dichloroiodates(I). J. Chem. Soc. Perkin Trans. I 1981, 977–983. [Google Scholar] [CrossRef]
- Suzuki, M.; Sugai, T. Mechanistic studies on nitrosation–deaminocyclization of mono-carbamoylated vicinal amino alcohols and diols: A new preparative in situ formation of ethanediazo hydroxide for the ethylation of carboxylates under mild conditions. Bull. Chem. Soc. Jpn. 2004, 77, 1217–1227. [Google Scholar] [CrossRef]
- Pihlaja, K.; Rossi, K. Conformational analysis. Part XLIII. 13C Chemical shifts and coupling constants as proof of the nonplanarity of the 2-oxo-1,3-dioxalane ring. Acta Chem. Scand. B, 1977; 31, 899–902. [Google Scholar]
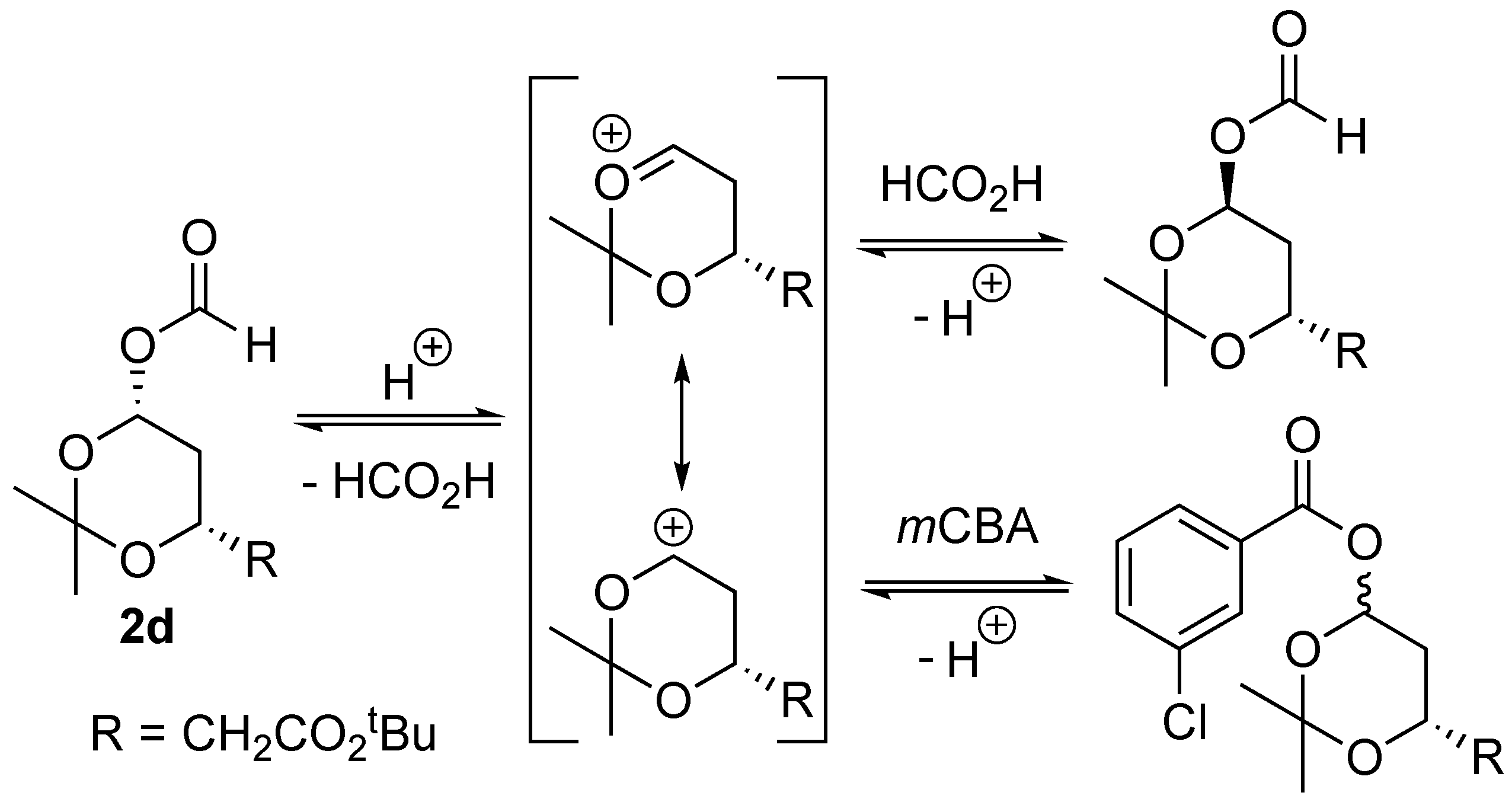

| Entry | Substrate | Time (h) | Consumption (%) | Products (Product Ratio) |
|---|---|---|---|---|
| 1 |  | 3 | 100 |  (1: 0.4) (1: 0.4) |
| 2 | 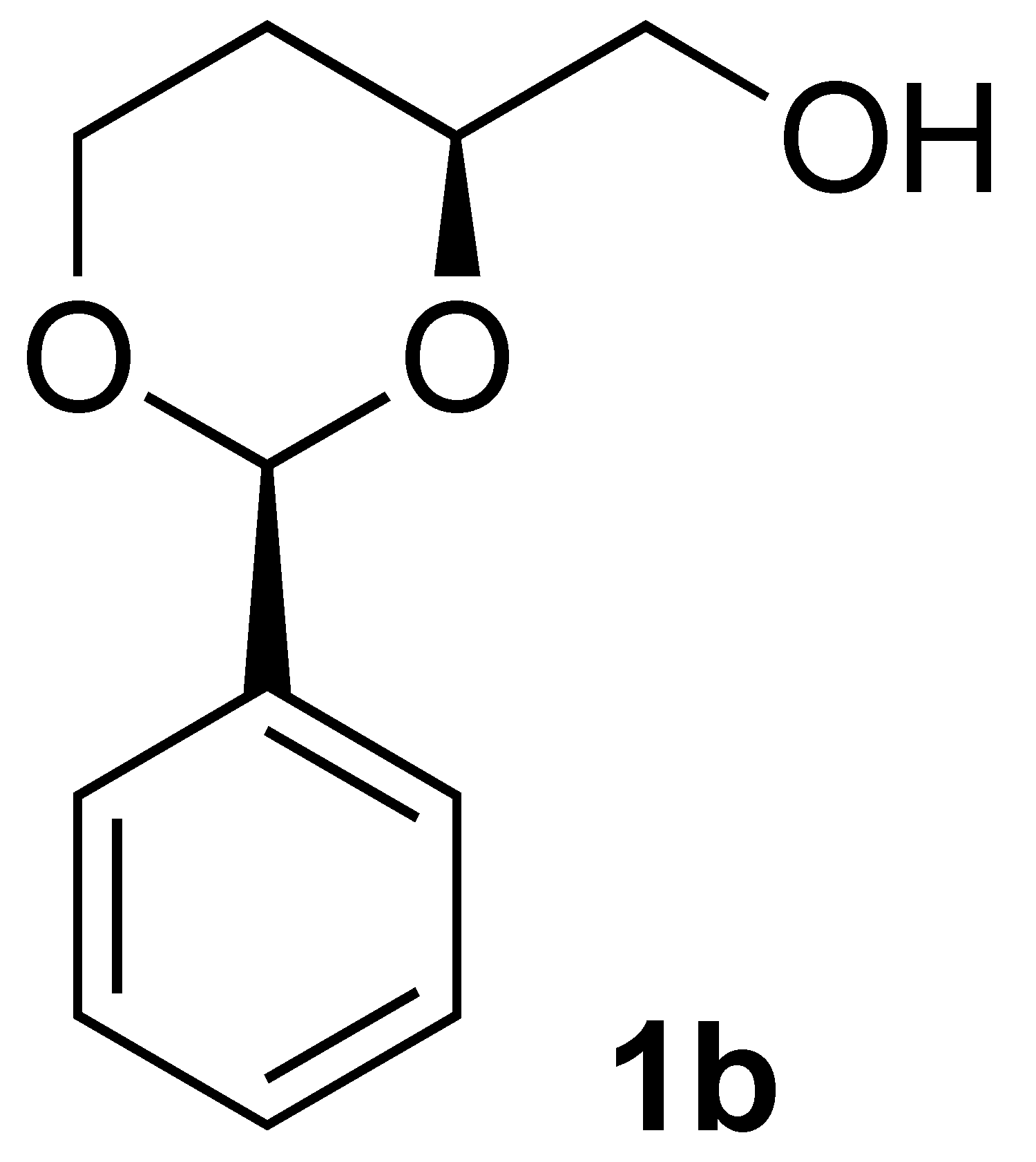 | 17 | 82 |  |
| 3 | 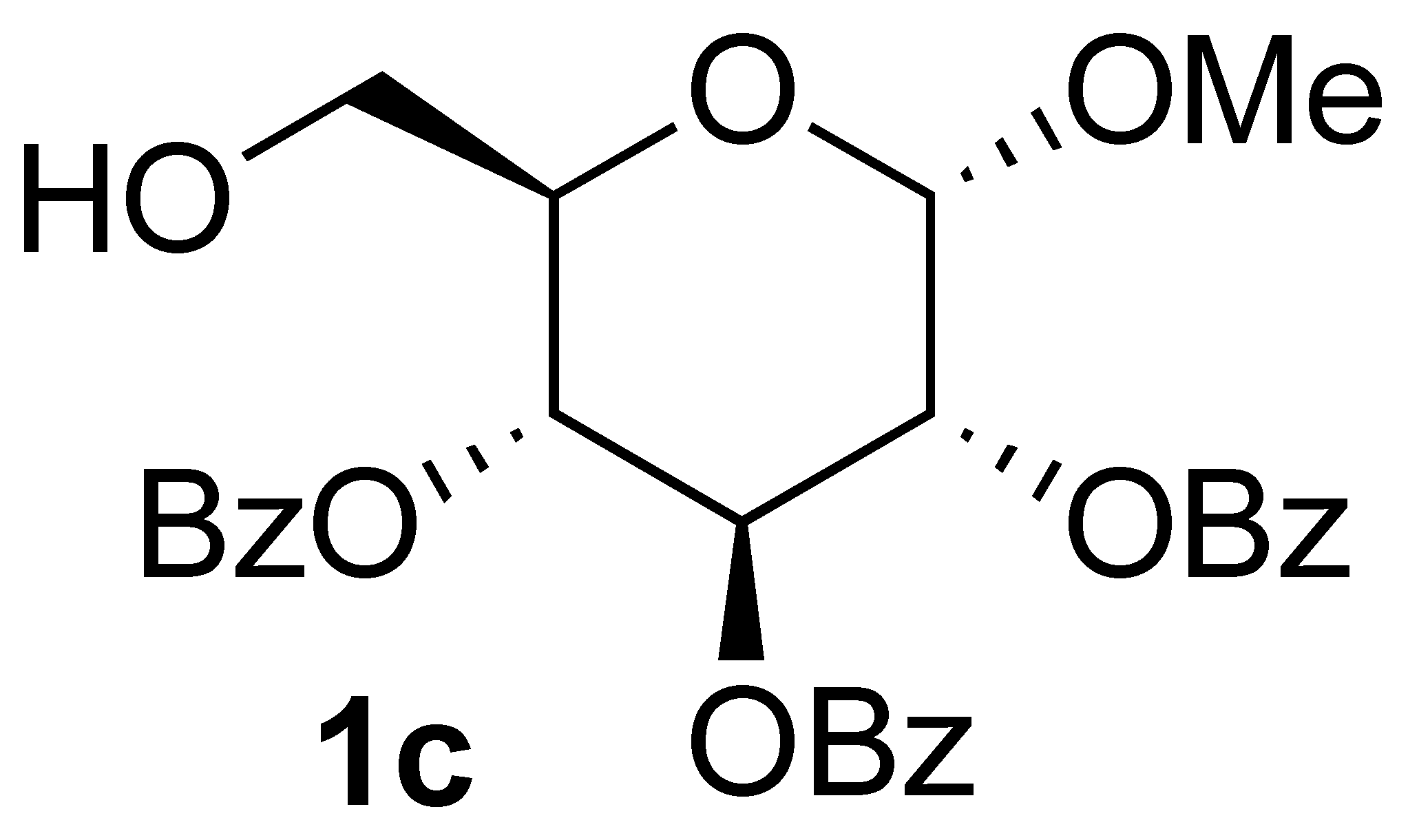 | 3 | 100 | 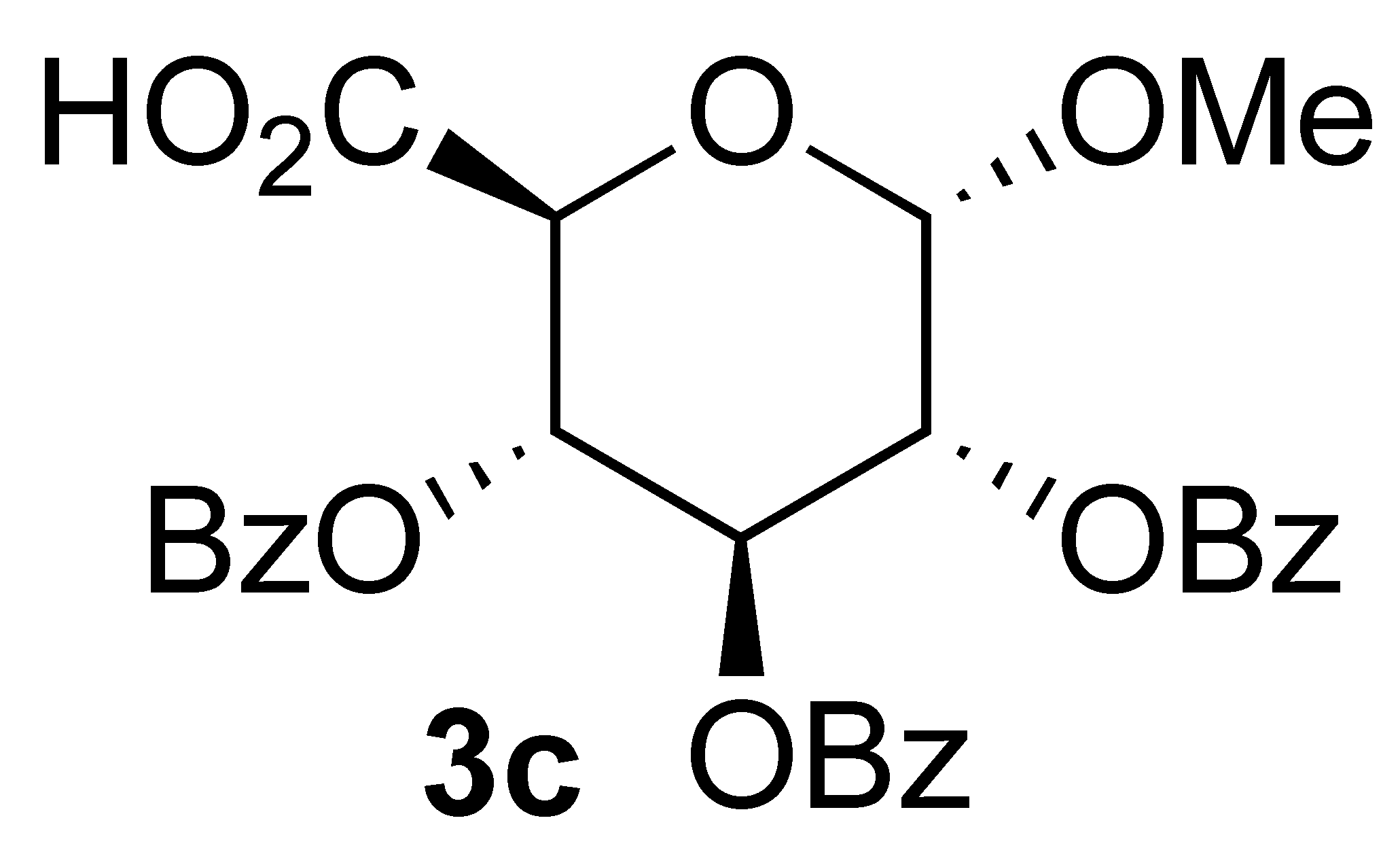 |
| 4 | 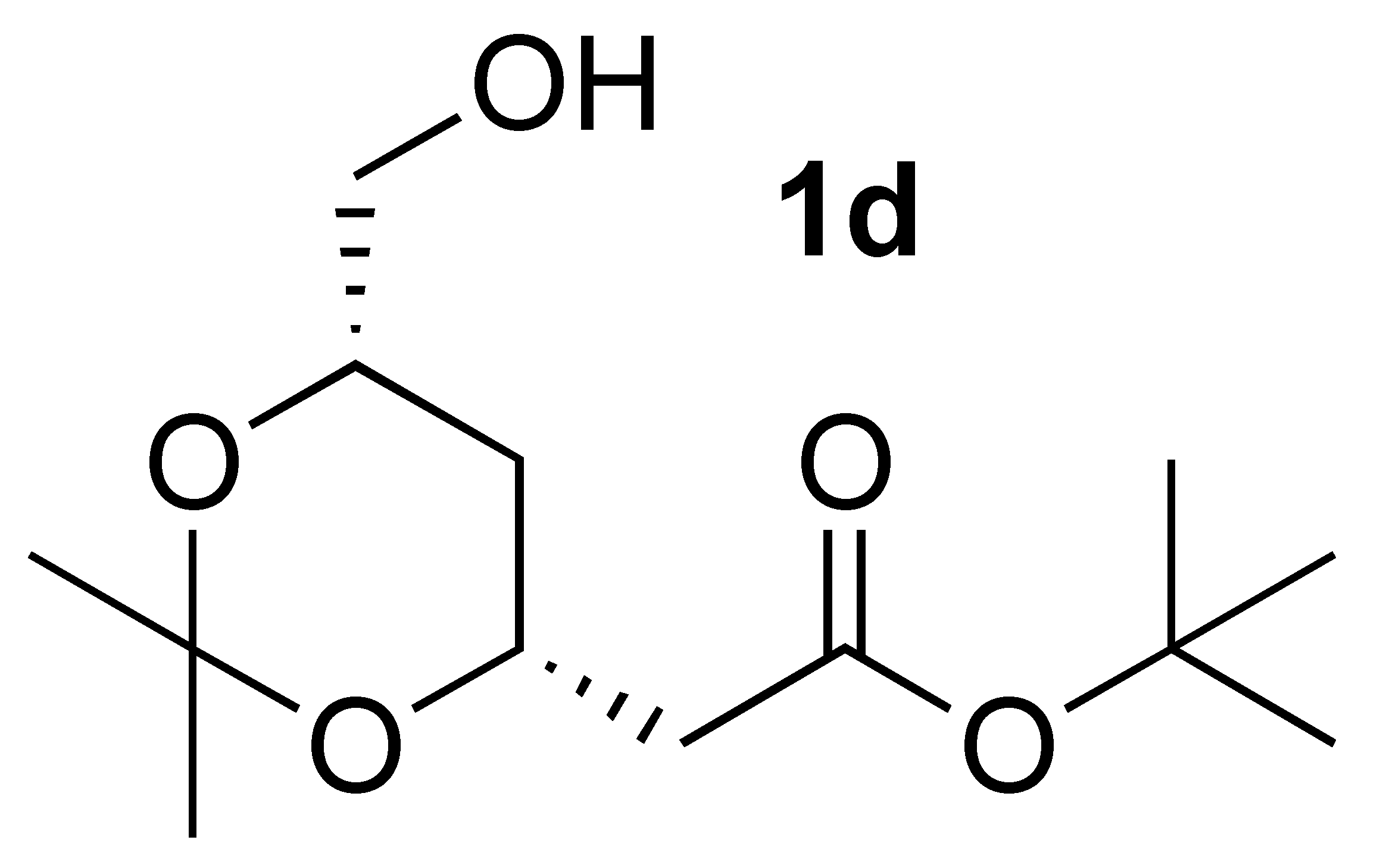 | 3 | 87 | 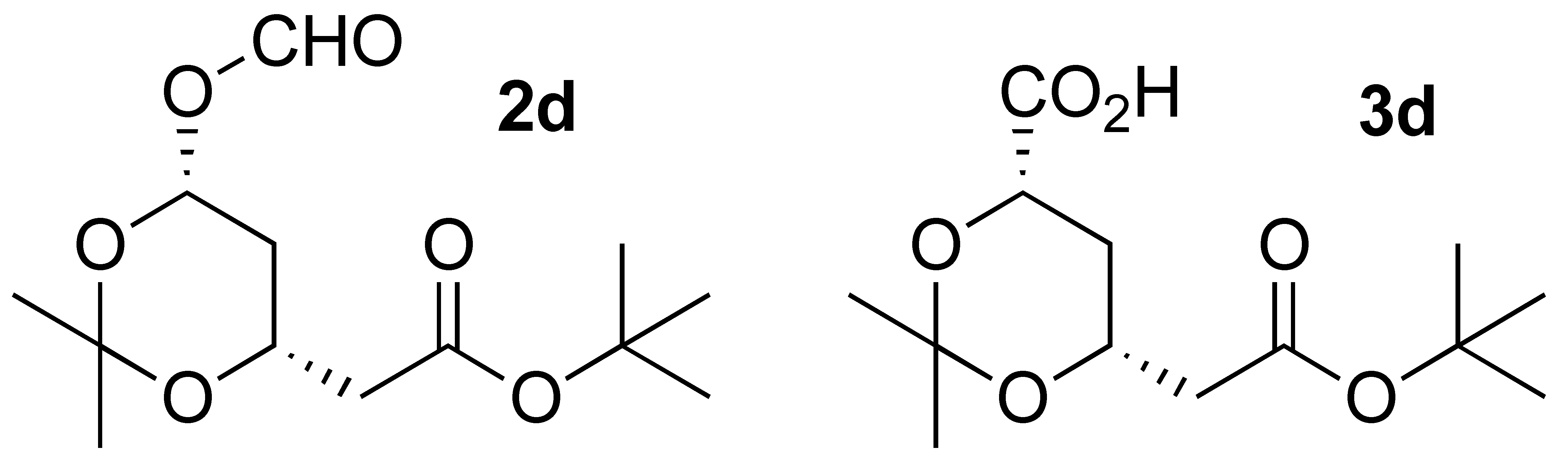 (1: 6.7) (1: 6.7) |
| 5 | 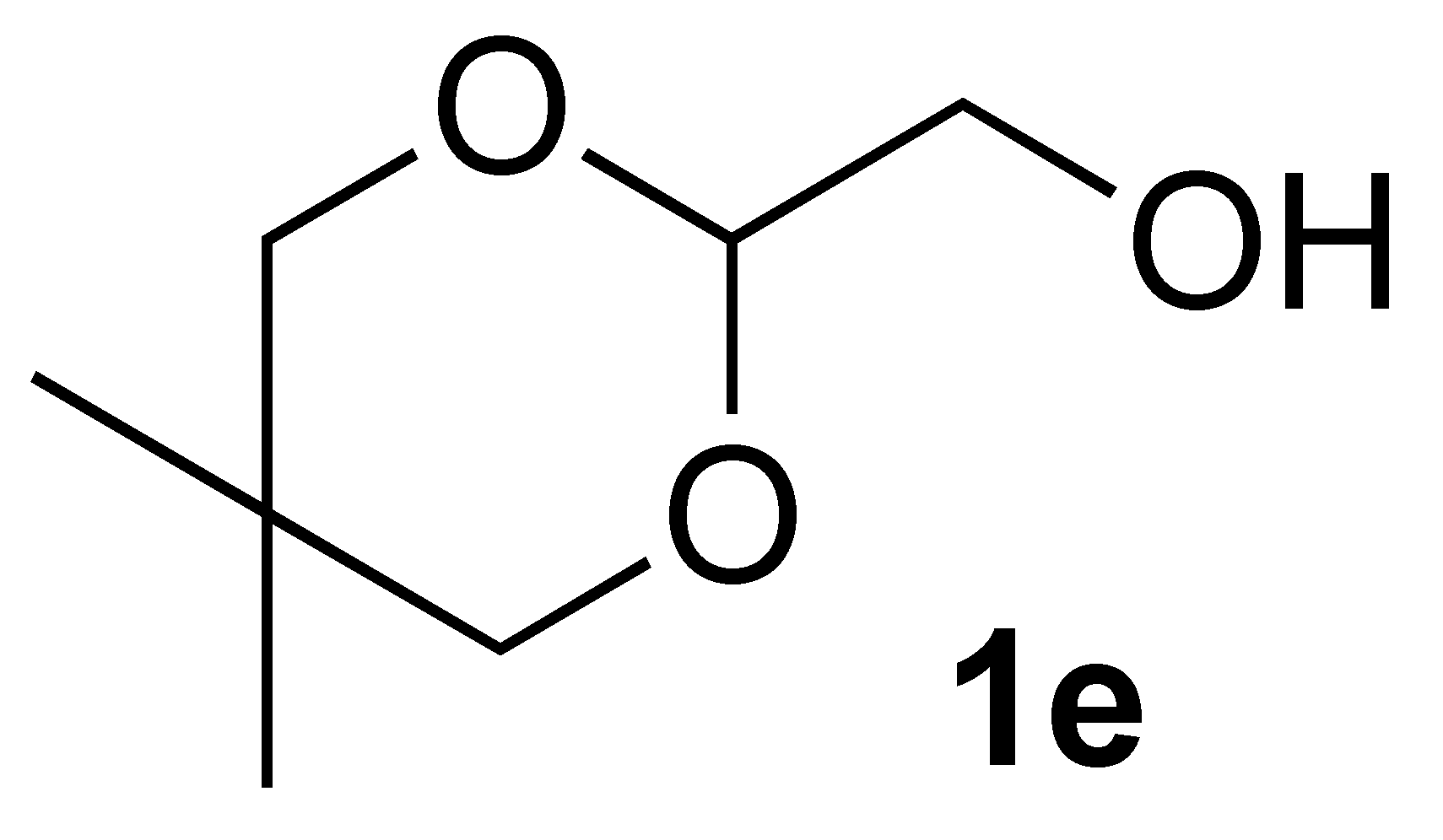 | 1 | 100 | 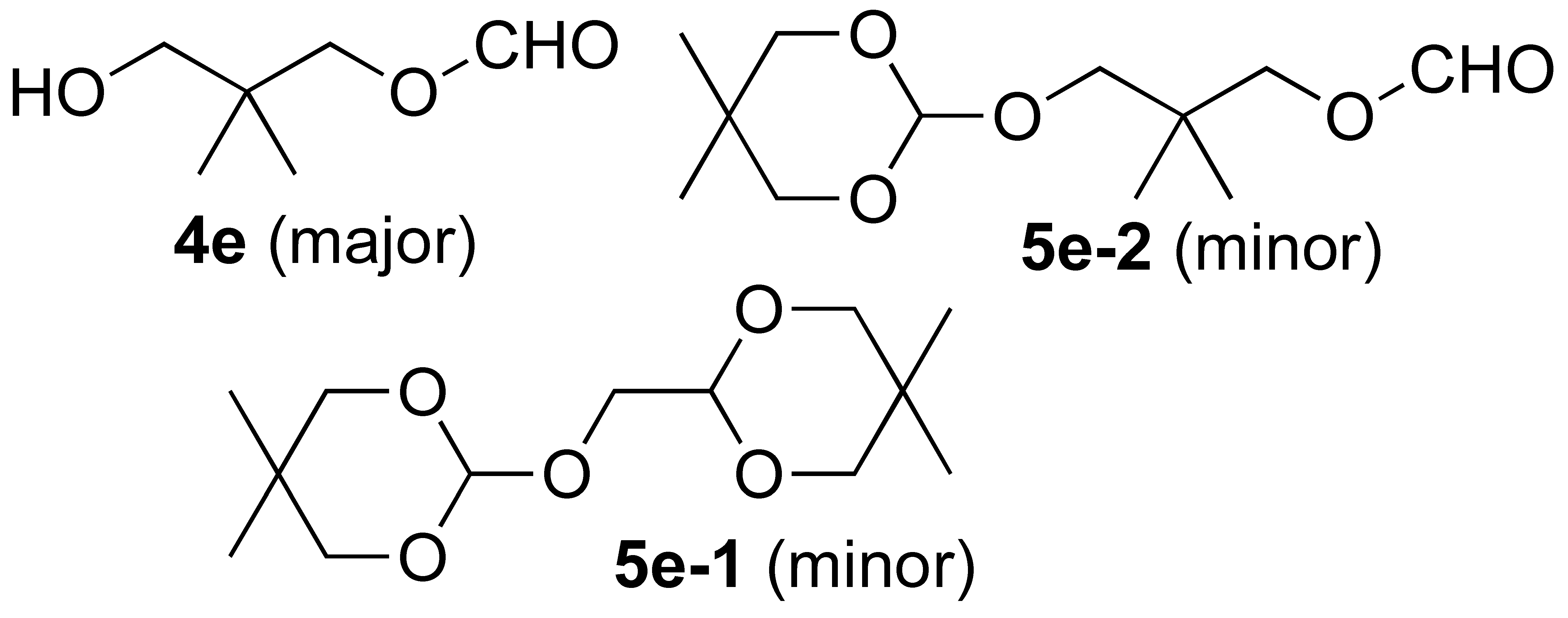 |
| 6 | 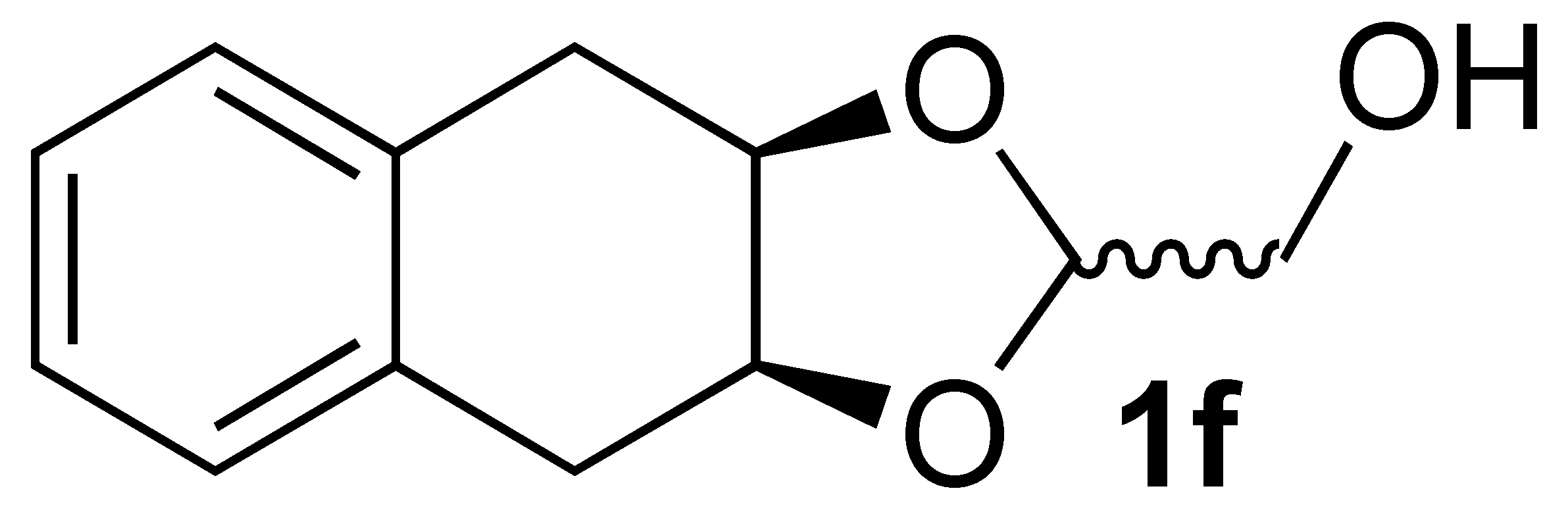 | 1 | 100 | 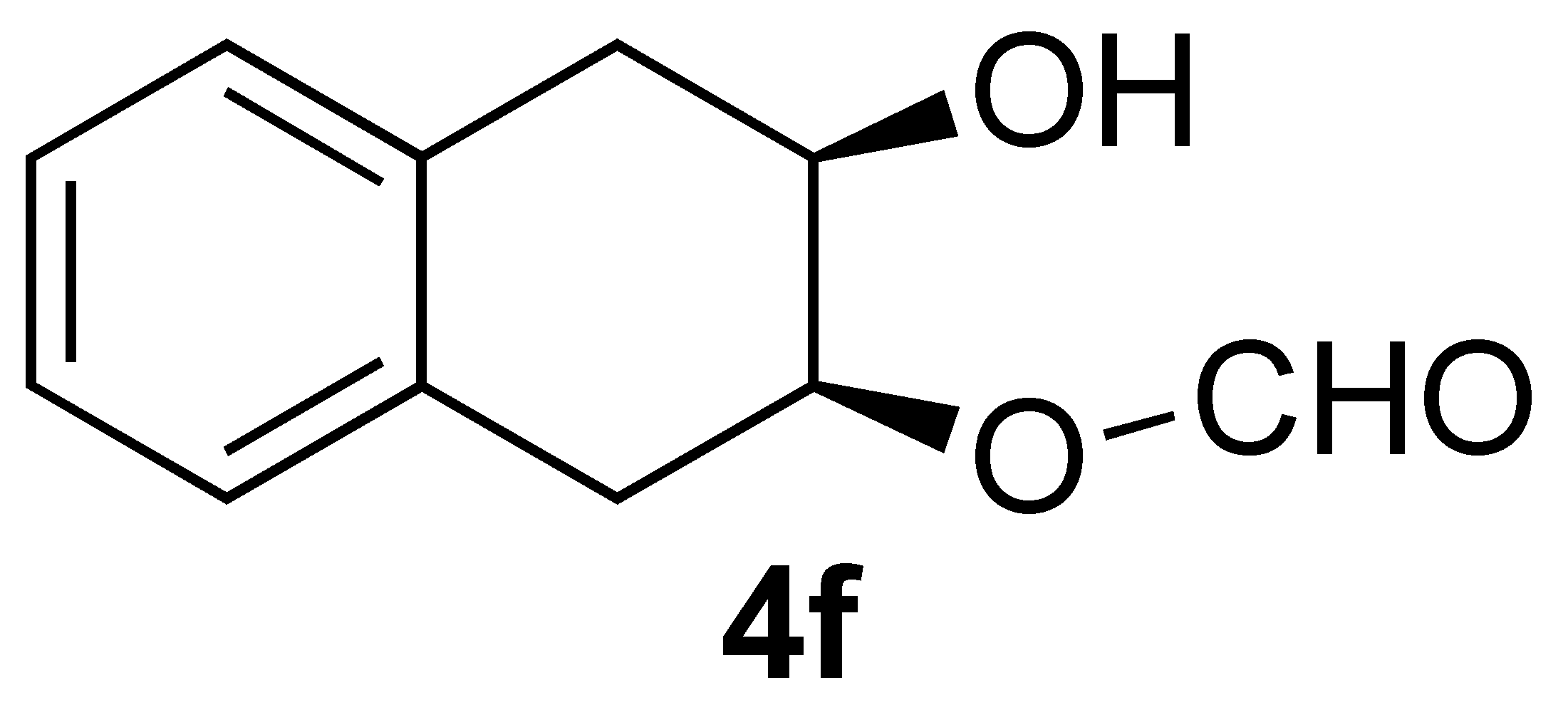 |
| 7 | 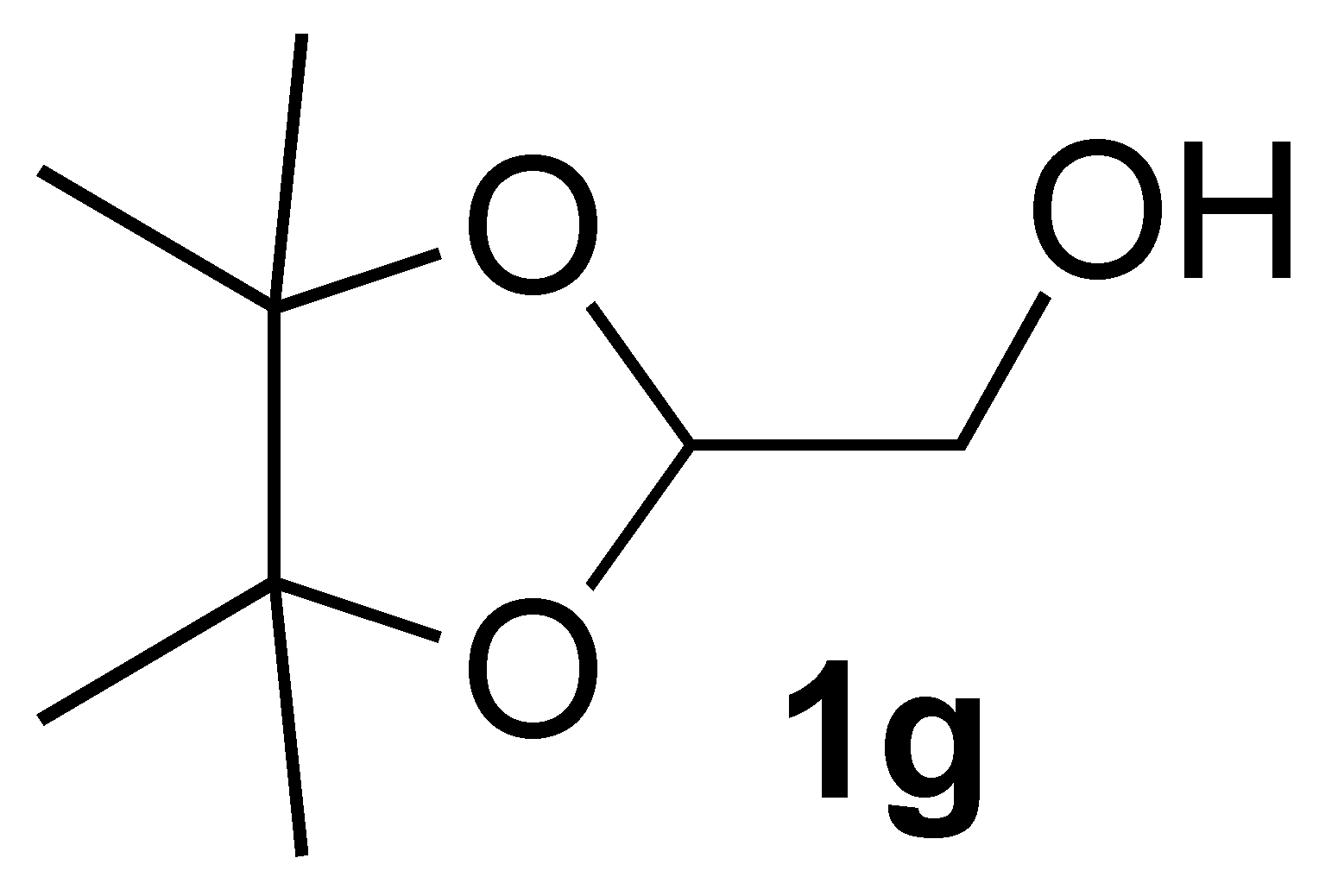 | 1 | 100 | 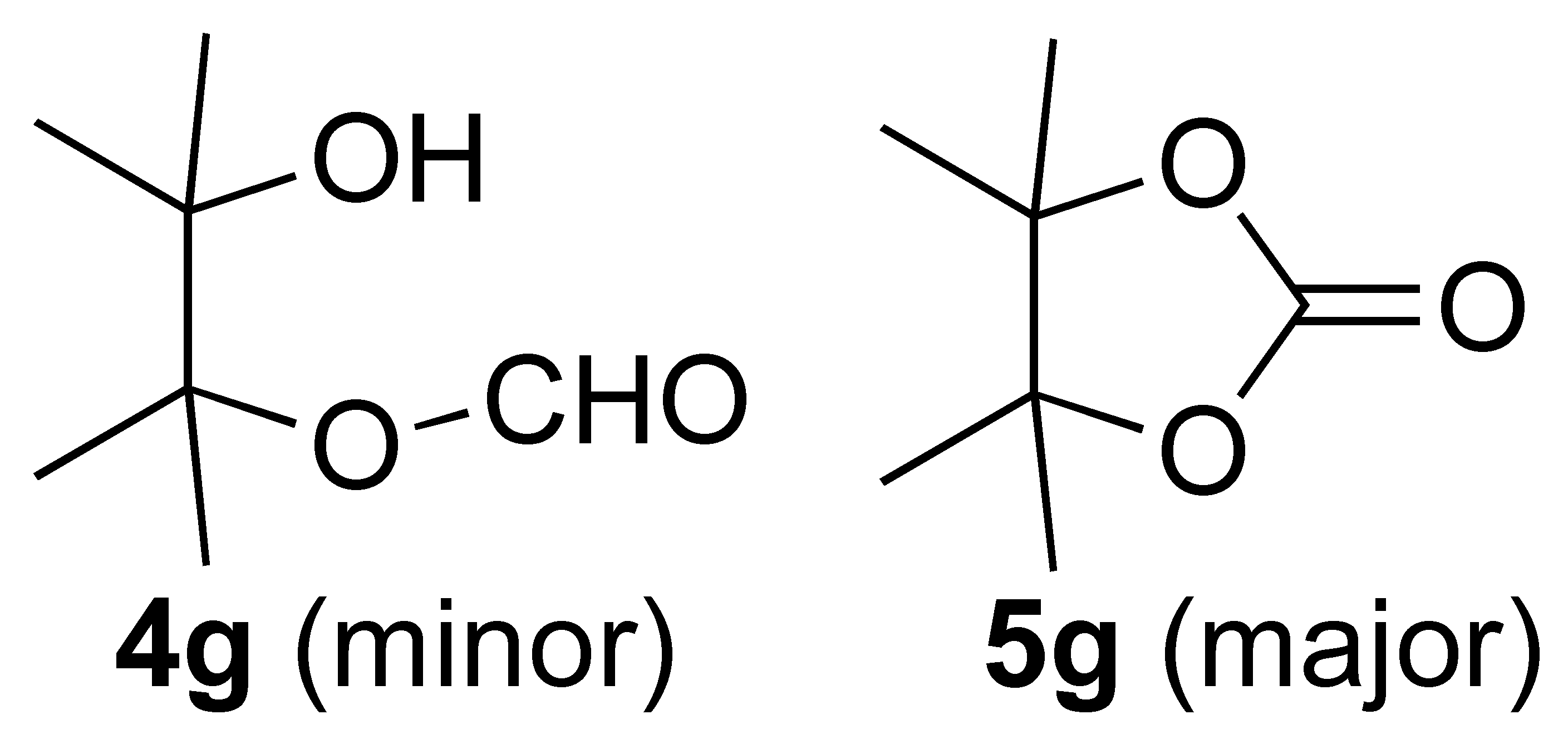 |
| 8 |  | 4 | 100 | 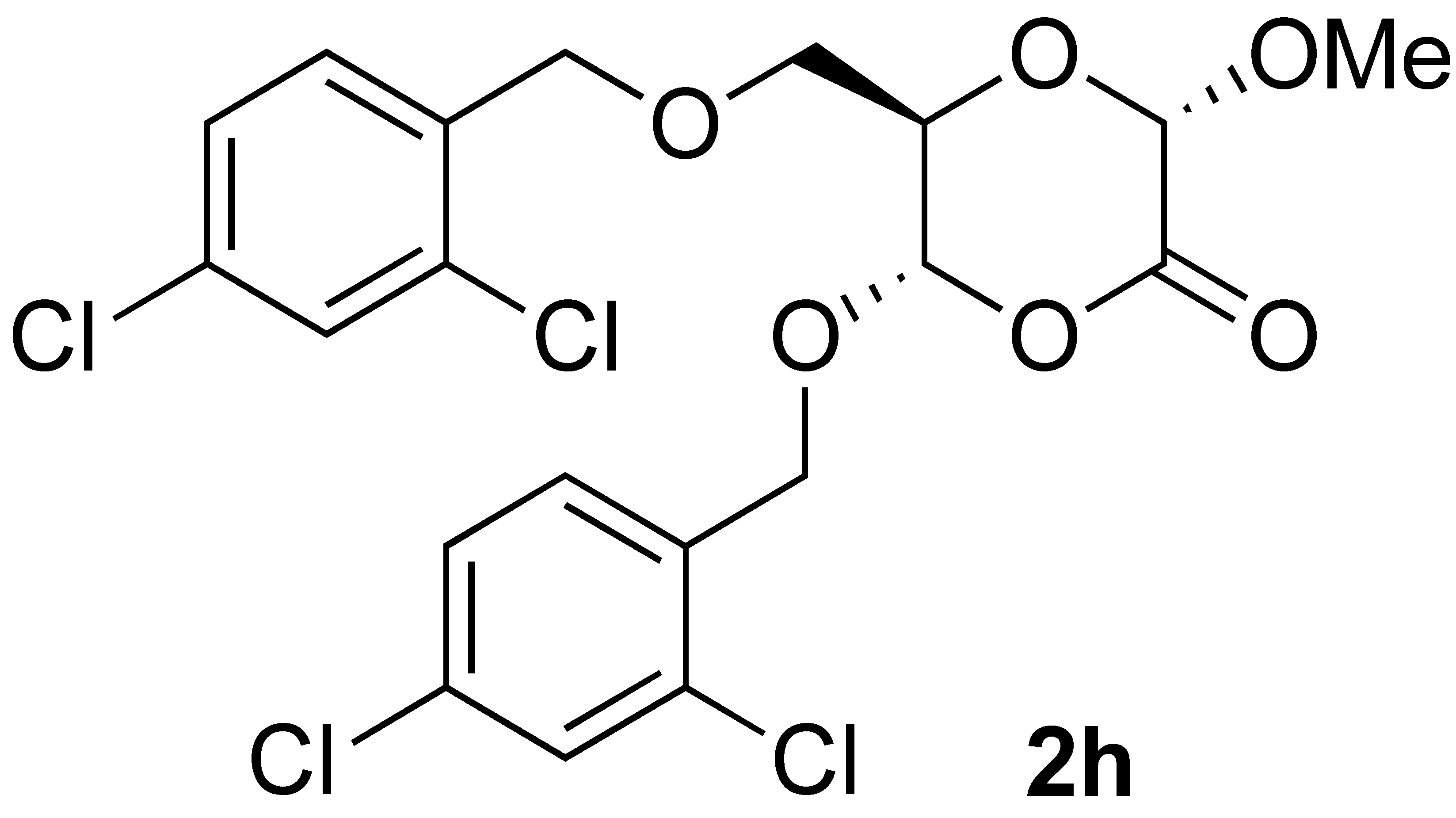 |
| Entry | Substrate | Solvent | Consumption (%) | Product Ratio 2 |
|---|---|---|---|---|
| 1 | 1i | benzene | 100 | 14:1 |
| 2 | 1i | DCM | 100 | 11:1 |
| 3 3 | 1i | EtOAc | 47 | 10:1 |
| 4 | 1i | ACN | 96 | 3.1:1 |
| 5 | 1d | benzene | 86 | 1:4.9 |
| 6 | 1d | DCM | 87 | 1:6.7 |
| 7 3 | 1d | EtOAc | 79 | 1:24 |
| 8 | 1d | ACN | 100 | Acid only |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Targel, T.; Portnoy, M. Baeyer-Villiger-Including Domino Two-Step Oxidations of β-O-Substituted Primary Alcohols: Reflection of the Migratory Aptitudes of O-Substituted Alkyl Group in the Outcome of the Reaction. Catalysts 2020, 10, 1275. https://doi.org/10.3390/catal10111275
Targel T, Portnoy M. Baeyer-Villiger-Including Domino Two-Step Oxidations of β-O-Substituted Primary Alcohols: Reflection of the Migratory Aptitudes of O-Substituted Alkyl Group in the Outcome of the Reaction. Catalysts. 2020; 10(11):1275. https://doi.org/10.3390/catal10111275
Chicago/Turabian StyleTargel, Tom, and Moshe Portnoy. 2020. "Baeyer-Villiger-Including Domino Two-Step Oxidations of β-O-Substituted Primary Alcohols: Reflection of the Migratory Aptitudes of O-Substituted Alkyl Group in the Outcome of the Reaction" Catalysts 10, no. 11: 1275. https://doi.org/10.3390/catal10111275
APA StyleTargel, T., & Portnoy, M. (2020). Baeyer-Villiger-Including Domino Two-Step Oxidations of β-O-Substituted Primary Alcohols: Reflection of the Migratory Aptitudes of O-Substituted Alkyl Group in the Outcome of the Reaction. Catalysts, 10(11), 1275. https://doi.org/10.3390/catal10111275