Catalytic Formation of Lactic and Levulinic Acids from Biomass Derived Monosaccarides through Sn-Beta Formed by Impregnation
Abstract
:1. Introduction
- Advance the conversion efficiency of the reaction for both glucose and xylose into lactic and levulinic acids through a careful study of the effect of residence time, reaction temperature, and substrate sources on catalyst selectivity and product yields.
- Evaluate the optimum reaction conditions for monomeric sugar mixtures that simulate the typical composition of sugars extracted from: (1) corn stover and (2) forage sorghum.
- Apply the optimum reaction conditions to actual sugar mixtures derived from corn stover and forage sorghum to evaluate effectiveness.
2. Results and Discussion
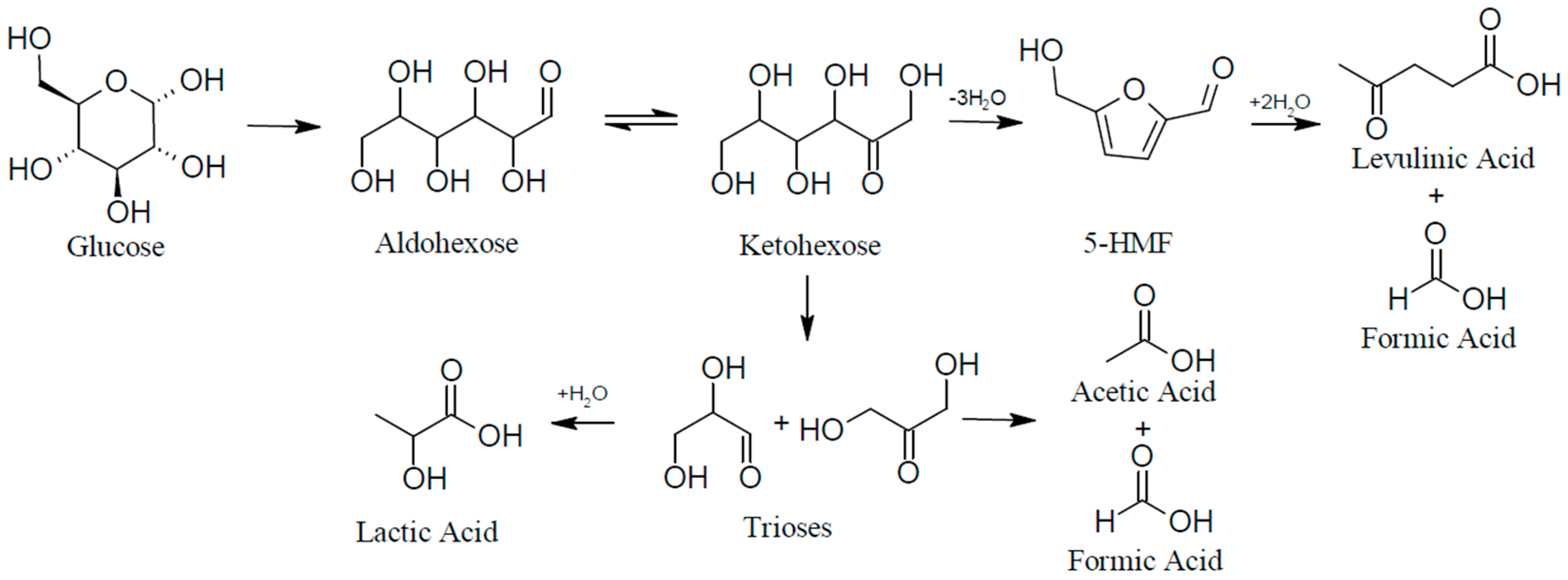
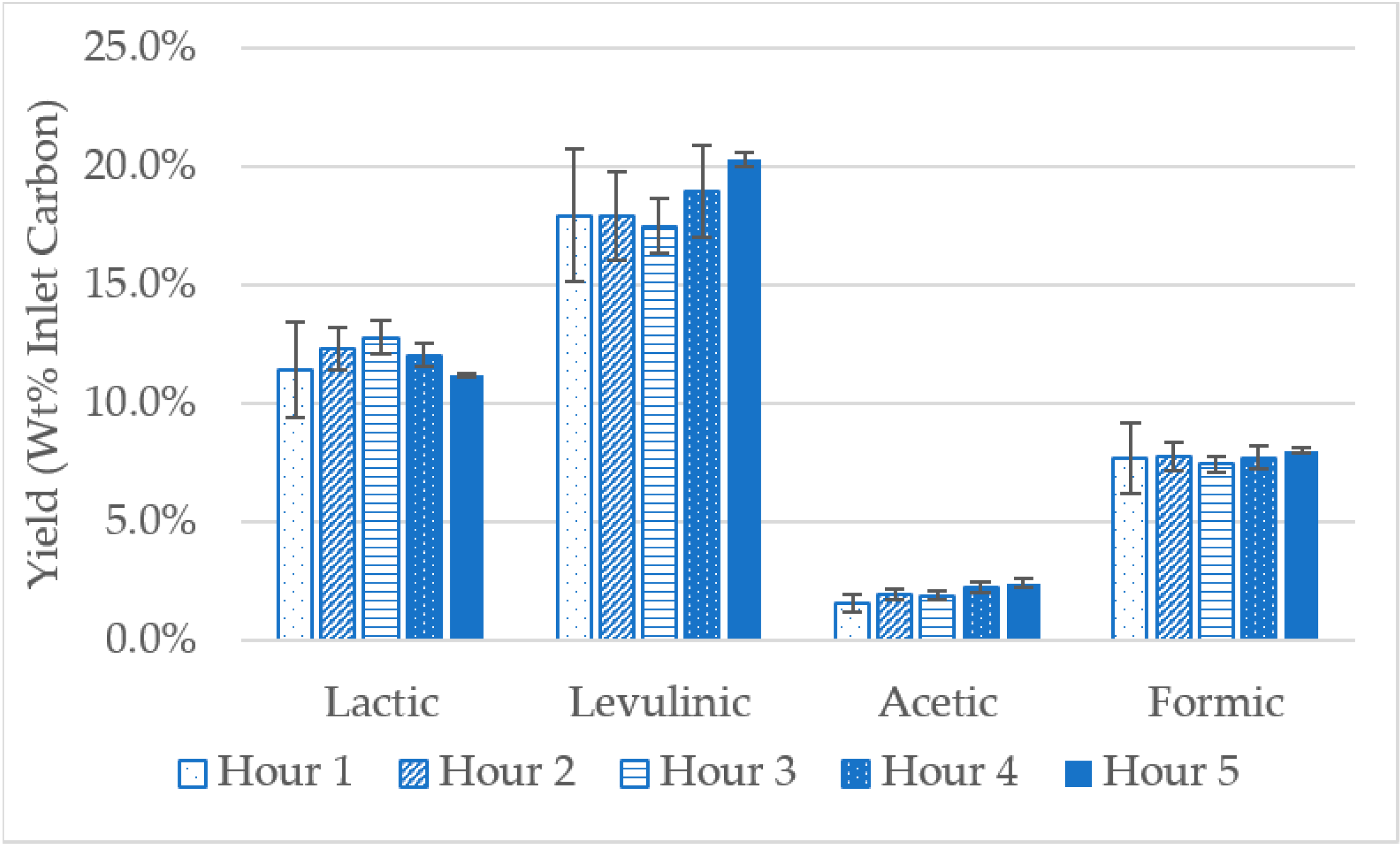
2.1. Catalyst Synthesis and the Evaluation of Reaction Yields from Glucose Model Solutions
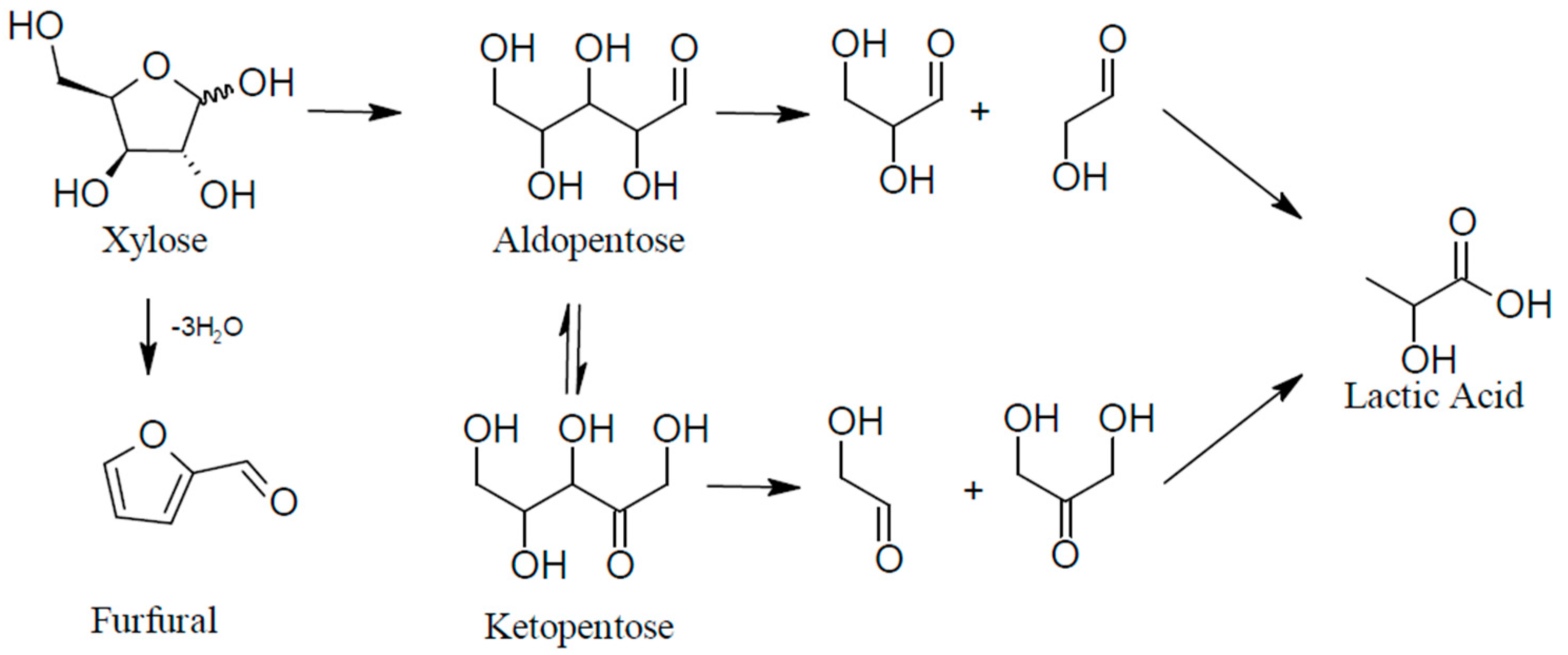
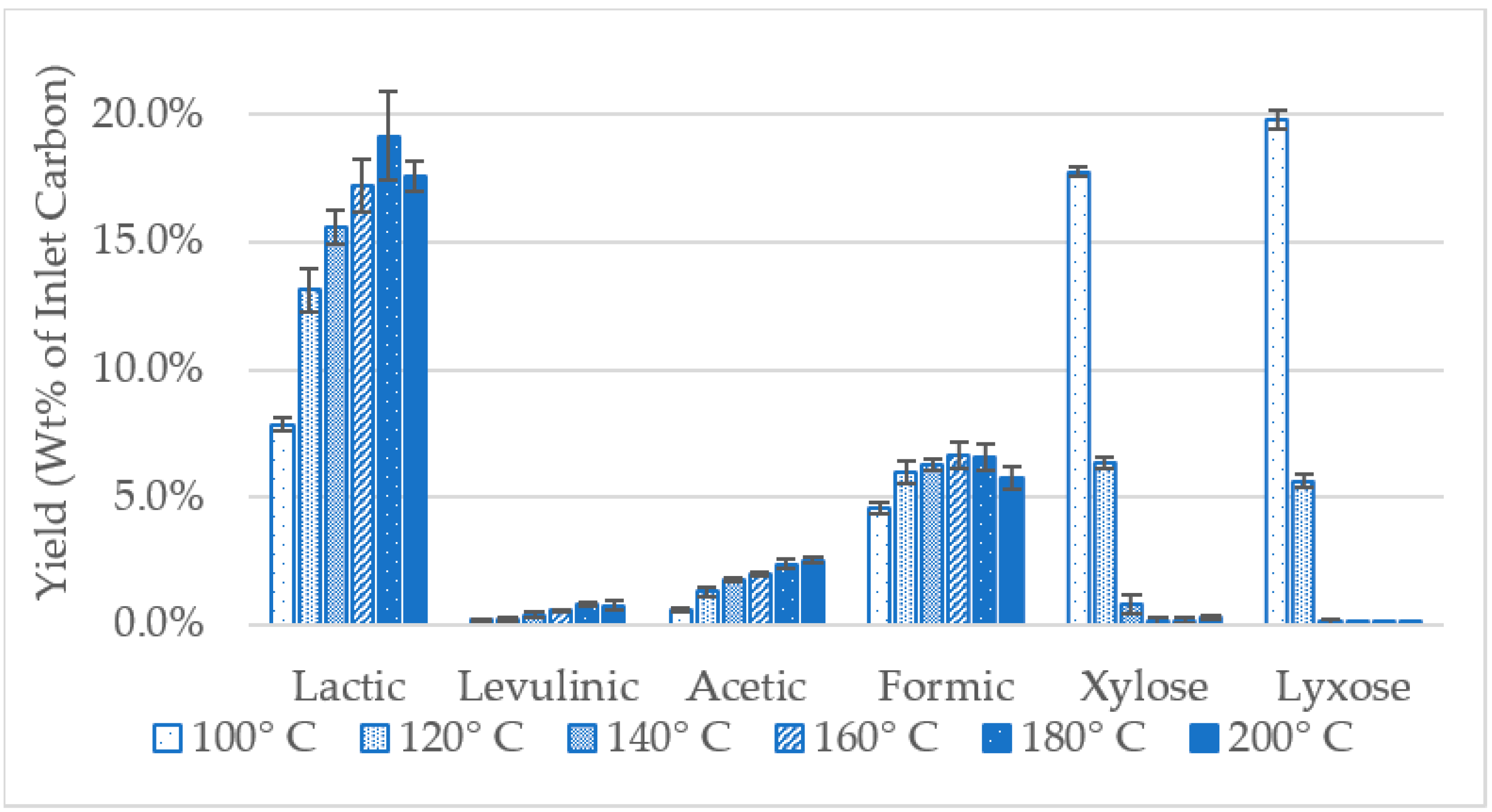
2.2. Reaction Yields from Xylose Model Solutions
2.3. Biomass Application
2.4. Acid Hydrolysis Neutralization and the Effect of CaSO4 on the Conversion Reaction
3. Materials and Methods
3.1. Reactants and Catalysts Materials
3.2. Catalyst Preparation
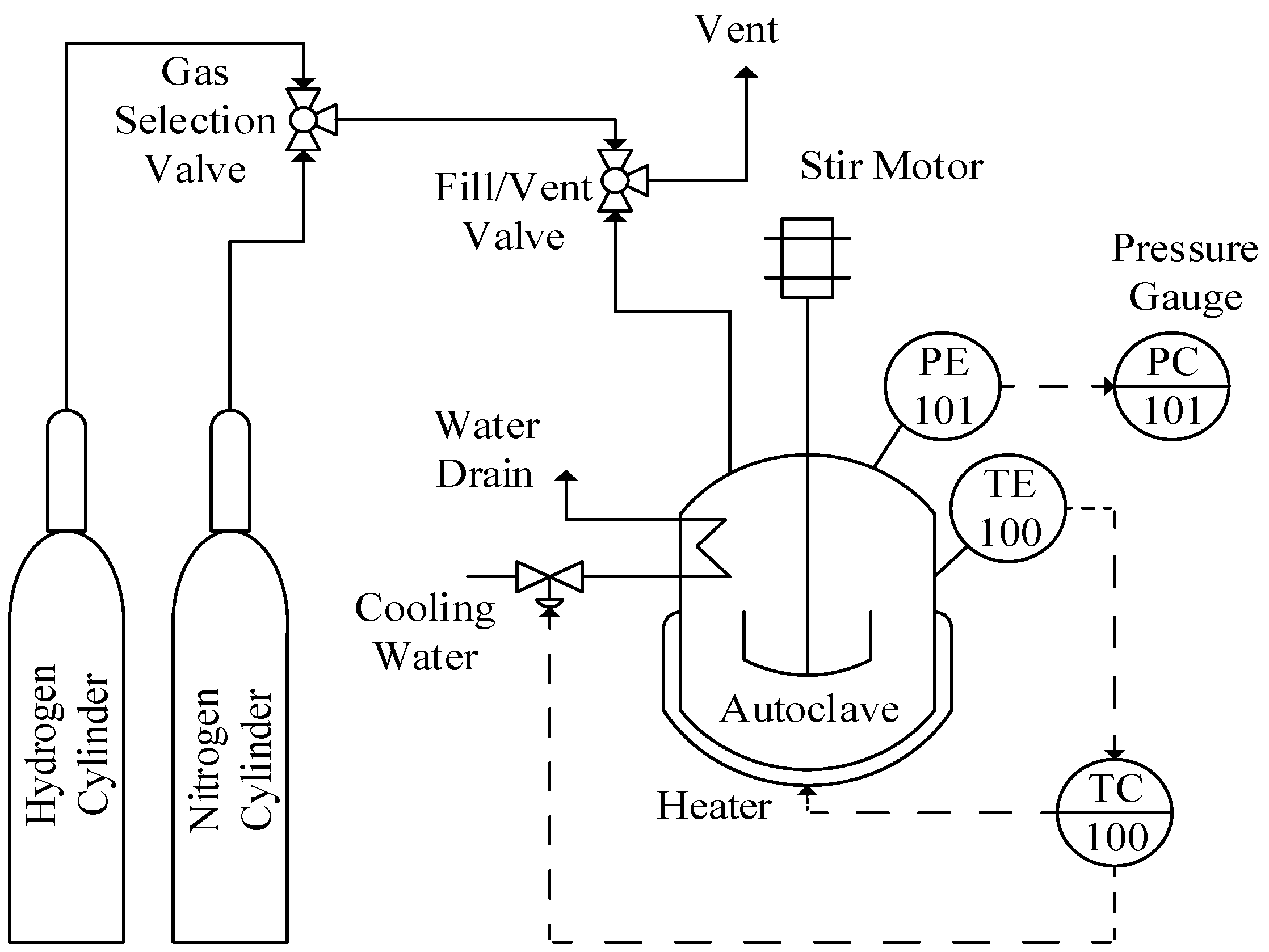
3.3. Batch Reactor
3.4. Reaction
3.5. Biomass Hydrolysis
3.6. Analysis
4. Conclusions and Recommendations for Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and technologies—A review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Hara, M.; Nakajima, K.; Kamata, K. Recent progress in the development of solid catalysts for biomass conversion into high value-added chemicals. Sci. Technol. Adv. Mater. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Conn, R.E.; Kolstad, J.J.; Borzelleca, J.F.; Dixler, D.S.; Filer, L.J.; Ladu, B.N.; Pariza, M.W. Safety assessment of polylactide (PLA) for use as a food-contact polymer. Food Chem. Toxicol. 1995, 33, 273–283. [Google Scholar] [CrossRef]
- Lim, L.T.; Cink, K.; Vanyo, T. Processing of Poly(Lactic Acid). In Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; Auras, R., Lim, L.T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Okano, K.; Tanaka, T.; Ogino, C.; Fukuda, H.; Kondo, A. Biotechnological production of enantiomeric pure lactic acid from renewable resources: Recent achievements, perspectives, and limits. Appl. Microbiol. Biotechnol. 2010, 85, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts. Science 2010, 328, 602–605. [Google Scholar] [CrossRef] [Green Version]
- Holm, M.S.; Pagan-Torres, Y.J.; Saravanamurugan, S.; Riisager, A.; Dumesic, J.A.; Taarning, E. Sn-βcatalysed conversion of hemicellulosic sugars. Green Chem. 2012, 14, 702–706. [Google Scholar] [CrossRef]
- Tolborg, S.; Sadaba, I.; Osmundsen, C.M.; Fristrup, P.; Holm, M.S.; Taarning, E. Tin-containing silicates: Alkali salts improve methyl lactate yield from sugars. Chemsuschem 2015, 8, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Werpy, T.; Pertersen, G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Tehnical Report for US Department of Energy: Washington, DC, USA, 2004. [Google Scholar]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Zhou, L.; Gao, B.; Lu, T.; Su, Y.; Xu, J. Production of lactic acid derivatives from sugars over post-synthesized Sn-Beta zeolite promoted by WO3. Food Chem. 2019, 289, 285–291. [Google Scholar] [CrossRef]
- Cordon, M.J.; Hall, J.N.; Harris, J.W.; Bates, J.S.; Hwang, S.J.; Gounder, R. Deactivation of Sn-Beta zeolites caused by structural transformation of hydrophobic to hydrophilic micropores during aqueous-phase glucose isomerization. Catal. Sci. Technol. 2019, 9, 1654–1668. [Google Scholar] [CrossRef] [Green Version]
- Moliner, M.; Roman-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc. Natl. Acad. Sci. USA 2010, 107, 6164–6168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taarning, E.; Saravanamurugan, S.; Holm, M.S.; Xiong, J.M.; West, R.M.; Christensen, C.H. Zeolite-catalyzed isomerization of triose sugars. Chemsuschem 2009, 2, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, T.C.; Lee, Y.Y. Production of Levulinic Acid from Glucose by Dual Solid-Acid Catalysts. Environ. Prog. Sustain. Energy 2018, 37, 471–480. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem. A Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.M.R.; Alonso, D.M.; Mellmer, M.A.; Yeap, J.H.; Wong, H.C.; Dumesic, J.A. Production of furfural from lignocellulosic biomass using beta zeolite and biomass-derived solvent. Top. Catal. 2013, 56, 1775–1781. [Google Scholar] [CrossRef]
- Paulino, P.N.; Reis, O.C.; Licea, Y.E.; Albuquerque, E.M.; Fraga, M.A. Valorisation of xylose to lactic acid on morphology-controlled ZnO catalysts. Catal. Sci. Technol. 2018, 8, 4945–4956. [Google Scholar] [CrossRef]
- Hammond, C.; Conrad, S.; Hermans, I. Simple and scalable preparation of highly active lewis acidic Sn-β. Angew. Chem. Int. Ed. 2012, 51, 11736–11739. [Google Scholar] [CrossRef]
- Li, P.; Liu, G.Q.; Wu, H.H.; Liu, Y.M.; Jiang, J.G.; Wu, P. Postsynthesis and selective oxidation properties of nanosized Sn-Beta zeolite. J. Phys. Chem. C 2011, 115, 3663–3670. [Google Scholar] [CrossRef]
- Dong, W.J.; Shen, Z.; Peng, B.Y.; Gu, M.Y.; Zhou, X.F.; Xiang, B.; Zhang, Y.L. Selective chemical conversion of sugars in aqueous solutions without alkali to lactic acid over a Zn-Sn-β lewis Acid-base catalyst. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Kong, L.; Shen, Z.; Zhang, W.; Xia, M.; Gu, M.Y.; Zhou, X.F.; Zhang, Y.L. Conversion of sucrose into lactic acid over functionalized sn-beta zeolite catalyst by 3-Aminopropyltrimethoxysilane. ACS Omega 2018, 3, 17430–17438. [Google Scholar] [CrossRef]
- Xia, M.; Dong, W.J.; Gu, M.Y.; Chang, C.; Shen, Z.; Zhang, Y.L. Synergetic effects of bimetals in modified beta zeolite for lactic acid synthesis from biomass-derived carbohydrates. RSC Adv. 2018, 8, 8965–8975. [Google Scholar] [CrossRef]
- Nemoto, K.; Hirano, Y.; Hirata, K.; Takahashi, T.; Tsuneki, H.; Tominaga, K.; Sato, K. Cooperative In-Sn catalyst system for efficient methyl lactate synthesis from biomass-derived sugars. Appl. Catal. B Environ. 2016, 183, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Koundinya, V. Corn Stover: What Is Its Worth? Available online: https://www.canr.msu.edu/news/corn_stover_what_is_its_worth (accessed on 26 September 2020).
- Humbird, D. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol; U.S. Department of Energy: Washington, DC, USA, 2011. [Google Scholar]
- Kadrmas, C. Synthesis, Selection, and Optimization of Doped Zeolite Catalyst for the Nonbiolgical Production of Lactic Acid Derivatives from Biomass Derived Carbohydrates. Doctoral Dissertation, University of North Dakota, Grand Forks, ND, USA, 2014. [Google Scholar]
- Kruger, J.S.; Nikolakis, V.; Vlachos, D.G. Aqueous-phase fructose dehydration using Bronsted acid zeolites: Catalytic activity of dissolved aluminosilicate species. Appl. Catal. General 2014, 469, 116–123. [Google Scholar] [CrossRef]
- Lange, J.P. Renewable feedstocks: The problem of catalyst deactivation and its mitigation. Angew. Chem. Int. Ed. 2015, 54, 13186–13197. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Q.; Fang, Z.H.; Zhang, J.; Li, X.L.; Bao, J. De-ashing treatment of corn stover improves the efficiencies of enzymatic hydrolysis and consequent ethanol fermentation. Bioresour. Technol. 2014, 169, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Tao, L.; Scarlata, C.; Tan, E.C.D.; Ross, J.; Lukas, J.; Sexton, D. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons- Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Catalytic Conversion of Sugars to Hydrocarbons; National Renewable Energy Laboratory: Golden, CO, USA, 2015. [Google Scholar]
- St’avova, J.; Beranek, J.; Nelson, E.P.; Diep, B.A.; Kubatova, A. Limits of detection for the determination of mono- and dicarboxylic acids using gas and liquid chromatographic methods coupled with mass spectrometry. J. Chromatogr. B 2011, 879, 1429–1438. [Google Scholar] [CrossRef] [Green Version]
- Padovan, D.; Tolborg, S.; Botti, L.; Taarning, E.; Sadaba, I.; Hammond, C. Overcoming catalyst deactivation during the continuous conversion of sugars to chemicals: Maximising the performance of Sn-β with a little drop of water. React. Chem. Eng. 2018, 3, 155–163. [Google Scholar] [CrossRef] [Green Version]

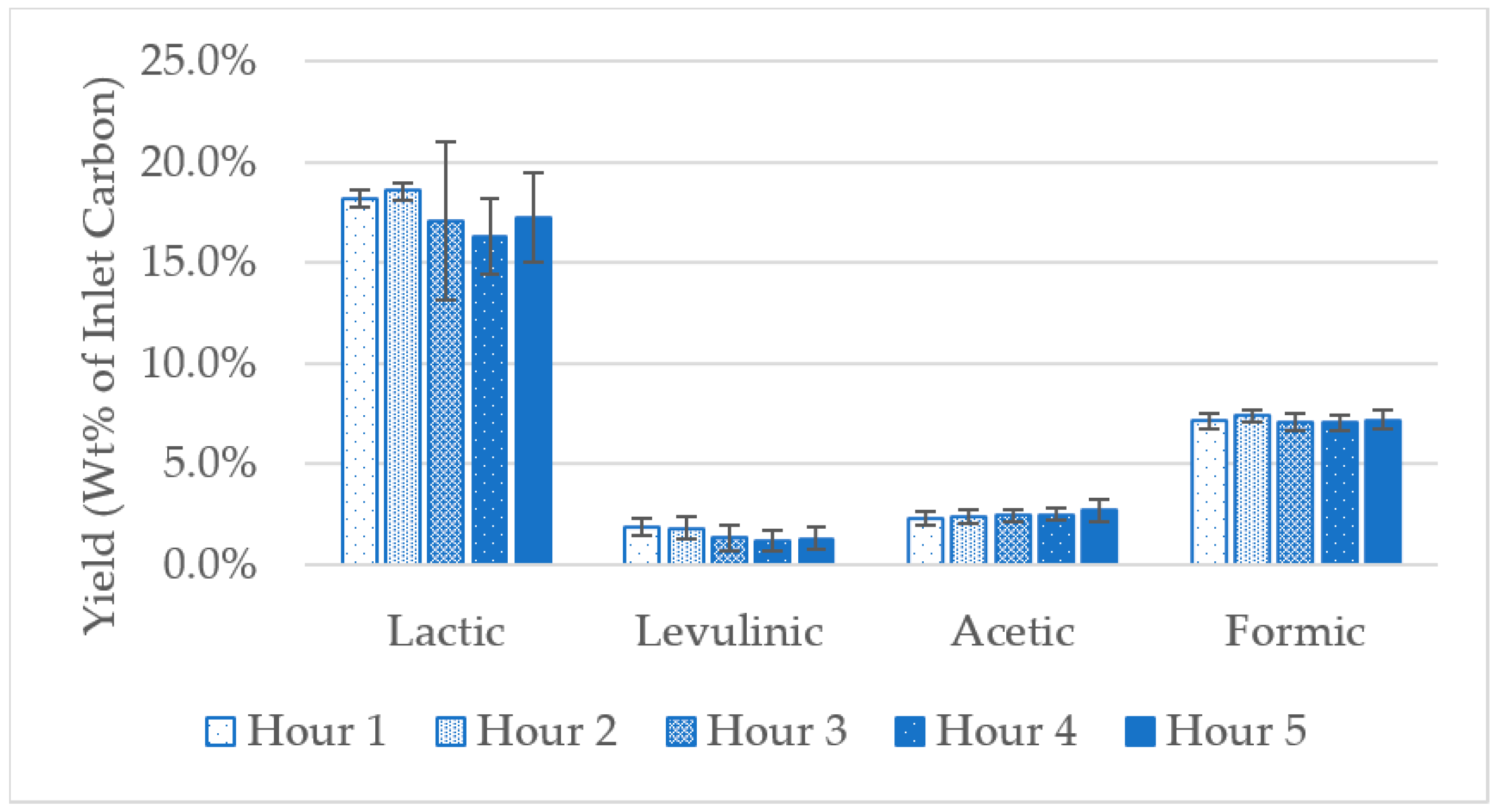


| Product | Preliminary | Kong 2018 | Xia 2018 |
|---|---|---|---|
| Lactic Acid | 7.6% | 22% | 22.4% |
| Levulinic Acid | 19.0% | NR | 14.4% |
| Product | Model Lignocellulose Solution | Corn Stover Solution | Forage Sorghum Solution |
|---|---|---|---|
| Initial pH | 2.7 | 4.5 | 5.8 |
| Final pH | 2.3 | 2.9 | 3.7 |
| Lactic Acid | 12% | 34% | 34% |
| Levulinic Acid | 15% | 1.4% | 4.0% |
| Acetic Acid | 2.1% | 10% | 8.7% |
| Formic Acid | 8.3% | 5.7% | 2.2% |
| Catalyst | Salt | Initial pH | Final pH | Lactic Acid | Levulinic Acid | Acetic Acid | Formic Acid |
|---|---|---|---|---|---|---|---|
| - | -- | 8.04 | 2.89 | 0.5 ± 0.03% | 8.4 ± 0.2% | 1.2 ± 0.15% | 3.6 ± 0.06% |
| -- | CaSO4 | 8.82 | 3.49 | 2.8 ± 0.15% | 2.1 ± 0.13% | 4.4 ± 0.7% | 2.7 ± 0.16% |
| Beta | CaSO4 | 5.49 | 3.06 | 2.9 ± 0.2% | 3.5 ± 0.03% | 2.9 ± 0.15% | 2.5 ± 0.02% |
| Sn-Beta | -- | 2.33 | 2.30 | 11 ± 0.11% | 20 ± 0.3% | 2.4 ± 0.2% | 8.0 ± 0.09% |
| Sn-Beta | CaSO4 | 5.41 | 2.59 | 68 ± 0.08% | 2.0 ± 0.2% | 3.6 ± 0.5% | 3.2 ± 0.3% |
| Catalyst | Salt | Initial pH | Final pH | Lactic Acid | Levulinic Acid | Acetic Acid | Formic Acid |
|---|---|---|---|---|---|---|---|
| Sn-Beta | -- | 2.33 | 2.30 | 11 ± 0.11% | 20 ± 0.3% | 2.4 ± 0.2% | 8.0 ± 0.09% |
| Sn-Beta | CaCl2 | 2.63 | 2.08 | 21 ± 0.99% | 16 ± 0.03% | 2.4 ± 0.18% | 6.4 ± 0.01% |
| Sn-Beta | NaOH | 9.40 | 2.48 | 49 ± 0.14% | 4.0 ± 0.08% | 3.0 ± 0.2% | 4.0 ± 0.13% |
| Sn-Beta | CaCl2 + NaOH | 6.98 | 2.30 | 56 ± 0.4% | 3.4 ± 1.1% | 2.9 ± 0.7% | 0.9 ± 0.5% |
| Sn-Beta | CaCO3 | 5.25 | 3.17 | 58 ± 0.6% | 2.6 ± 1.3% | 5.1 ± 0.4% | 2.2 ± 0.9% |
| Sn-Beta | MgSO4 | 2.88 | 2.39 | 64 ± 0.13% | 3.5 ± 2.2% | 3.6 ± 0.3% | 3.1 ± 0.13% |
| Sn-Beta | CaSO4 | 5.41 | 2.59 | 68 ± 0.08% | 2.0 ± 0.21% | 3.6 ± 0.5% | 3.2 ± 0.3% |
| Sugar | Lactic Acid | Levulinic Acid | Acetic Acid | Formic Acid |
|---|---|---|---|---|
| Fructose | 66 ± 0.3% | 2.2 ± 2.05% | 4.0 ± 0.8% | 2.7 ± 0.3% |
| Galactose | 55 ± 0.05% | 1.6 ± 0.2% | 3.6 ± 0.15% | 3.0 ± 0.08% |
| Glucose | 68 ± 0.08% | 2.0 ± 1.7% | 3.6 ± 0.2% | 3.2 ± 0.2% |
| Mannose | 65 ± 0.01% | 2.3 ± 0.02% | 3.9 ± 0.05% | 2.5 ± 0.08% |
| Xylose | 50 | 2.5 | 5.2 | 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohler, A.; Seames, W.; Foerster, I.; Kadrmas, C. Catalytic Formation of Lactic and Levulinic Acids from Biomass Derived Monosaccarides through Sn-Beta Formed by Impregnation. Catalysts 2020, 10, 1219. https://doi.org/10.3390/catal10101219
Kohler A, Seames W, Foerster I, Kadrmas C. Catalytic Formation of Lactic and Levulinic Acids from Biomass Derived Monosaccarides through Sn-Beta Formed by Impregnation. Catalysts. 2020; 10(10):1219. https://doi.org/10.3390/catal10101219
Chicago/Turabian StyleKohler, Andrew, Wayne Seames, Ian Foerster, and Clancy Kadrmas. 2020. "Catalytic Formation of Lactic and Levulinic Acids from Biomass Derived Monosaccarides through Sn-Beta Formed by Impregnation" Catalysts 10, no. 10: 1219. https://doi.org/10.3390/catal10101219
APA StyleKohler, A., Seames, W., Foerster, I., & Kadrmas, C. (2020). Catalytic Formation of Lactic and Levulinic Acids from Biomass Derived Monosaccarides through Sn-Beta Formed by Impregnation. Catalysts, 10(10), 1219. https://doi.org/10.3390/catal10101219





