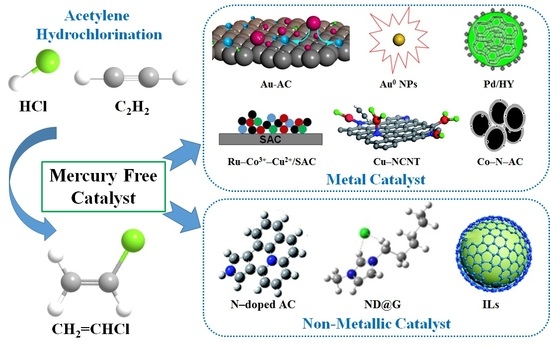
Progress and Challenges of Mercury-Free Catalysis for Acetylene Hydrochlorination
Abstract
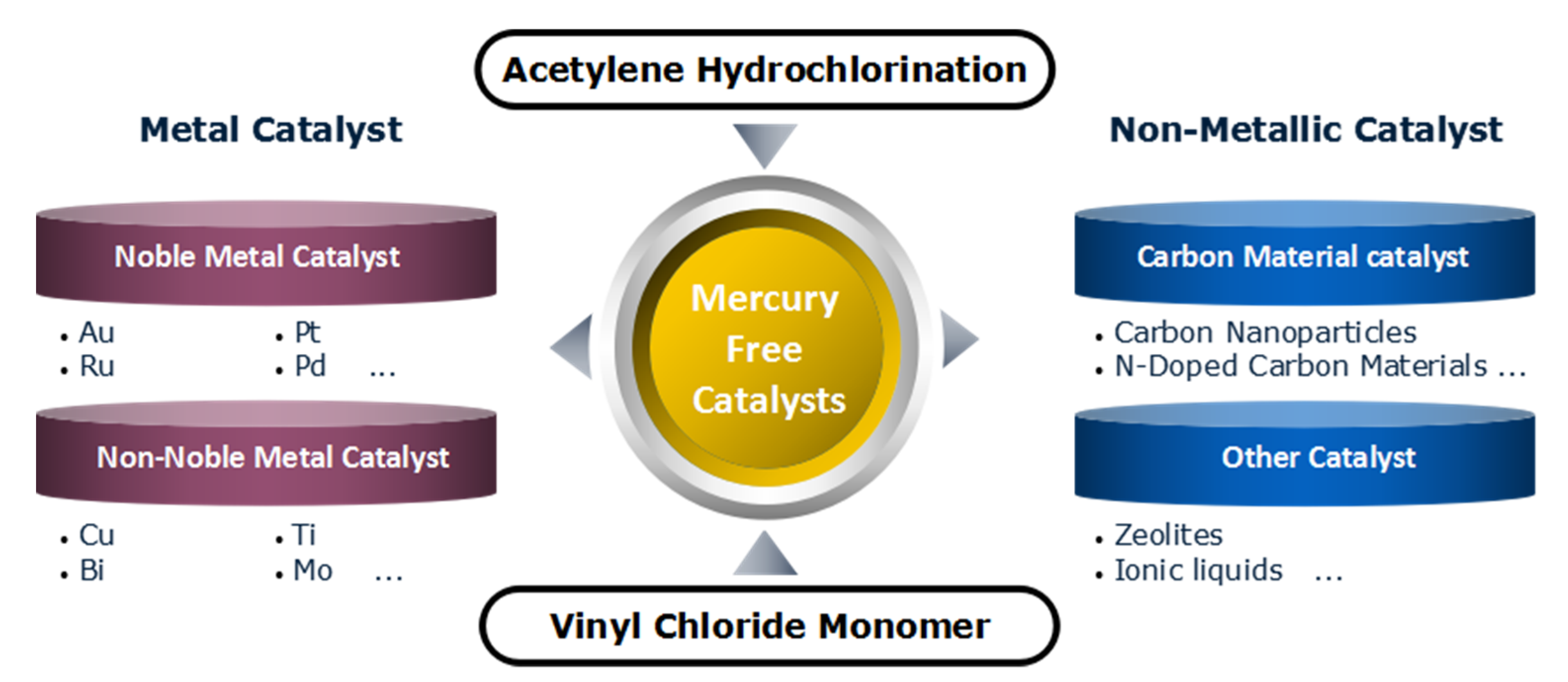
:1. Introduction
2. Noble Metal Catalysts
2.1. Au Catalysts
2.1.1. Aun+ Catalysts
2.1.2. Au0 Catalysts
2.1.3. Modification of Au Catalysts
Addition Synergistic Metal
Ligand Coordination
Support Modification
2.1.4. Deactivation and Regeneration of Au Catalysts
2.2. Ru Catalysts
2.3. Pt Catalysts
2.4. Pd Catalysts
2.5. Challenge of Noble Metal Catalysts
3. Non-Noble Metal Catalysts
3.1. Cu Catalysts
3.2. Other Non-Noble Metal Catalysts
3.3. Challenge of Non-Noble Metal Catalysts
4. Non-Metallic Catalysts
4.1. Carbon Material Catalysts
4.2. Other Non-Metallic Catalysts
4.3. Challenge of Non-Metallic Catalysts
5. Future Prospects
Funding
Conflicts of Interest
References
- Xu, H.; Luo, G.H. Green production of PVC from laboratory to industrialization: State–of–the–art review of heterogeneous non–mercury catalysts for acetylene hydrochlorination. J. Ind. Eng. Chem. 2018, 65, 13–25. [Google Scholar] [CrossRef]
- He, W.; Zhu, G.Q.; Gao, Y.; Wu, H.; Fang, Z.; Guo, K. Green plasticizers derived from epoxidized soybean oil for poly (vinyl chloride): Continuous synthesis and evaluation in PVC films. Chem. Eng. J. 2020, 380, 122532. [Google Scholar] [CrossRef]
- Shi, D.Z.; Hu, R.S.; Zhou, Q.H.; Yang, L.R. Catalytic activities of supported perovskite promoter catalysts La2NiMnO6–CuCl2/γ–Al2O3 and La1.7K0.3NiMnO6–CuCl2/γ–Al2O3 for ethane oxychlorination. Chem. Eng. J. 2016, 288, 588–595. [Google Scholar] [CrossRef]
- Ye, L.; Duan, X.P.; Wu, S.; Wu, T.S.; Zhao, Y.X.; Robertson, A.W.; Chou, H.L.; Zheng, J.W.; Ayvali, T.; Day, S.; et al. Self–regeneration of Au/CeO2 based catalysts with enhanced activity and ultra–stability for acetylene hydrochlorination. Nat. Commun. 2019, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Carley, A.F.; Heirene, C.; Willock, D.J.; Johnston, P.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Hydrochlorination of acetylene using a supported gold catalyst: A study of the reaction mechanism. J. Catal. 2007, 250, 231–239. [Google Scholar] [CrossRef]
- Zhang, J.L.; Liu, N.; Li, W.; Dai, B. Progress on cleaner production of vinyl chloride monomers over non–mercury catalysts. Front. Chem. Sci. Eng. 2011, 5, 514–520. [Google Scholar] [CrossRef]
- Malta, G.; Freakley, S.J.; Kondrat, S.A.; Hutchings, G.J. Acetylene hydrochlorination using Au/carbon: A journey towards single site catalysis. Chem. Commun. 2017, 53, 11733–11746. [Google Scholar] [CrossRef] [Green Version]
- Johnston, P.; Carthey, N.; Hutchings, G.J. Discovery, development, and commercialization of gold catalysts for acetylene hydrochlorination. J. Am. Chem. Soc. 2015, 137, 14548–14557. [Google Scholar] [CrossRef]
- Ren, W.; Duan, L.; Zhu, Z.W.; Du, W.; An, Z.Y.; Xu, L.J.; Zhang, C.; Zhuo, Y.Q.; Chen, C.H. Mercury transformation and distribution across a polyvinyl chloride (PVC) production line in China. Environ. Sci. Technol. 2014, 48, 2321–2327. [Google Scholar] [CrossRef]
- Hutchings, G.J. Gold catalysis in chemical processing. Catal. Today 2002, 72, 11–17. [Google Scholar] [CrossRef]
- Zhou, K.; Jia, J.C.; Li, X.G.; Pang, X.D.; Li, C.H.; Zhou, J.; Luo, G.H.; Wei, F. Continuous vinyl chloride monomer production by acetylene hydrochlorination on Hg–free bismuth catalyst: From lab–scale catalyst characterization, catalytic evaluation to a pilot–scale trial by circulating regeneration in coupled fluidized beds. Fuel Process. Technol. 2013, 108, 12–18. [Google Scholar] [CrossRef]
- Smith, D.M.; Walsh, P.M.; Slager, T.L. Studies of silica–supported metal chloride catalysts for the vapor–phase hydrochlorination of acetylene. J. Catal. 1968, 11, 113–130. [Google Scholar] [CrossRef]
- Shinoda, K. The vapor–phase hidrochlorination of acetylene over metal chlorides supported on activated carbon. Chem. Lett. 1975, 4, 219–220. [Google Scholar] [CrossRef]
- Hutchings, G.J. Vapor phase hydrochlorination of acetylene: Correlation of catalytic activity of supported metal chloride catalysts. J. Catal. 1985, 96, 292–295. [Google Scholar] [CrossRef]
- Nkosi, B.; Coville, N.J.; Hutchings, G.J. Reactivation of a supported gold catalyst for acetylene hydrochlorination. J. Chem. Soc. Chem. Commun. 1988, 71–72. [Google Scholar] [CrossRef]
- Nkosi, B.; Coville, N.J.; Hutchings, G.J. Vapour phase hydrochlorination of acetylene with group VIII and IB metal chloride catalysts. Appl. Catal. 1988, 43, 33–39. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, W.; Zhao, Z.; Luo, G.; Miller, J.T.; Wong, M.S.; Wei, F. Synergistic gold–bismuth catalysis for non–mercury hydrochlorination of acetylene to vinyl chloride monomer. ACS Catal. 2014, 4, 3112–3116. [Google Scholar] [CrossRef] [Green Version]
- Hashmi, A.S.K. Homogeneous gold catalysts and alkynes: A successful liaison. Gold Bull. 2003, 36, 3–9. [Google Scholar] [CrossRef]
- Schmidbaur, H. Gold: Progress in Chemistry, Biochemistry, and Technology; John Wiley & Sons Inc: New York, NY, USA, 1999; p. 894. [Google Scholar]
- Krasnyakova, T.V.; Zhikharev, I.V.; Mitchenko, R.S.; Burkhovetski, V.I.; Korduban, A.M.; Kryshchuk, T.V.; Mitchenko, S.A. Acetylene catalytic hydrochlorination over mechanically pre–activated K2PdCl4 salt: A study of the reaction mechanism. J. Catal. 2012, 288, 33–43. [Google Scholar] [CrossRef]
- Mitchenko, S.A.; Ananikov, V.P.; Beletskaya, I.P. Mechanoactivation of acetylene hydrochlorination in the presence of K2PtCl6. Zhurnal. Org. Khimii. 1998, 34, 1859–1860. [Google Scholar] [CrossRef]
- Mitchenko, S.A. Acetylene hydrochlorination by gaseous hydrogen chloride on the surface of mechanically activated K2PtCl6 salt. Kinet. Catal. 1998, 39, 859–862. [Google Scholar] [CrossRef]
- Mitchenko, S.A.; Krasnyakova, T.V.; Mitchenko, R.S.; Korduban, A.N. Acetylene catalytic hydrochlorination over powder catalyst prepared by pre–milling of K2PtCl4 salt. J. Mol. Catal. A–Chem. 2007, 275, 101–108. [Google Scholar] [CrossRef]
- Conte, M.; Davies, C.J.; Morgan, D.J.; Carley, A.F.; Johnston, P.; Hutchings, G.J. Characterization of Au3+ species in Au/C catalysts for the hydrochlorination reaction of acetylene. Catal. Lett. 2014, 144, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Conte, M.; Elias, D.; Lu, L.; Morgan, D.J.; Freakley, S.J.; Johnston, P.; Kiely, C.J.; Hutchings, G.J. Investigation of the active species in the carbon–supported gold catalyst for acetylene hydrochlorination. Catal. Sci. Technol. 2016, 6, 5144–5153. [Google Scholar] [CrossRef]
- Nkosi, B.; Adams, M.D.; Coville, N.J.; Hutchings, G.J. Hydrochlorination of acetylene using carbon–supported gold catalysts: A study of catalyst reactivation. J. Catal. 1991, 128, 378–386. [Google Scholar] [CrossRef]
- Conte, M.; Davies, C.J.; Morgan, D.J.; Davies, T.E.; Elias, D.J.; Carley, A.F.; Johnston, P.; Hutchings, G.J. Aqua regia activated Au/C catalysts for the hydrochlorination of acetylene. J. Catal. 2013, 297, 128–136. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.L.; Xu, X.L.; Yu, Y.; Di, S.X.; Xu, H.; Zhai, Y.Y.; He, H.H.; Guo, L.L.; Pan, Z.Y.; et al. Alternative solvent to aqua regia to activate Au/AC catalysts for the hydrochlorination of acetylene. J. Catal. 2017, 350, 149–158. [Google Scholar] [CrossRef]
- Huang, C.F.; Zhu, M.Y.; Kang, L.H.; Dai, B. A novel high–stability Au(III)/schiff–based catalyst for acetylene hydrochlorination reaction. Catal. Commun. 2014, 54, 61–65. [Google Scholar] [CrossRef]
- Chao, S.L.; Guan, Q.X.; Li, W. Study of the active site for acetylene hydrochlorination in AuCl3/C catalysts. J. Catal. 2015, 330, 273–279. [Google Scholar] [CrossRef]
- Zhang, C.M.; Zhang, H.Y.; Man, B.C.; Li, X.; Dai, H.; Zhang, J.L. Hydrochlorination of acetylene catalyzed by activated carbon supported highly dispersed gold nanoparticles. Appl. Catal. A Gen. 2018, 566, 15–24. [Google Scholar] [CrossRef]
- Wittanadecha, W.; Laosiripojana, N.; Ketcong, A.; Ningnuek, N.; Praserthdam, P.; Monnier, J.R.; Assabumrungrat, S. Preparation of Au/C catalysts using microwave–assisted and ultrasonic–assisted methods for acetylene hydrochlorination. Appl. Catal. A Gen. 2014, 475, 292–296. [Google Scholar] [CrossRef]
- Tian, X.H.; Hong, G.T.; Jiang, B.B.; Lu, F.P.; Liao, Z.W.; Wang, J.D.; Yang, Y.R. Efficient Au0/C catalyst synthesized by a new method for acetylene hydrochlorination. RSC Adv. 2015, 5, 46366–46371. [Google Scholar] [CrossRef]
- Conte, M.; Carley, A.F.; Attard, G.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Hydrochlorination of acetylene using supported bimetallic Au–based catalysts. J. Catal. 2008, 257, 190–198. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Wang, Q.Q.; Chen, K.; Wang, Y.; Huang, C.F.; Dai, H.; Yu, F.; Kang, L.H.; Dai, B. Development of a heterogeneous non–mercury catalyst for acetylene hydrochlorination. ACS Catal. 2015, 5, 5306–5316. [Google Scholar] [CrossRef]
- Li, G.B.; Li, W.; Zhang, J.L. Non–mercury catalytic acetylene hydrochlorination over activated carbon–supported Au catalysts promoted by CeO2. Catal. Sci. Technol. 2016, 6, 1821–1828. [Google Scholar] [CrossRef]
- Ke, J.H.; Zhao, Y.X.; Yin, Y.; Chen, K.; Duan, X.P.; Ye, L.M.; Yuan, Y.Z. Yttrium chloride–modified Au/AC catalysts for acetylene hydrochlorination with improved activity and stability. J. Rare Earths 2017, 35, 1083–1091. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Zhang, H.Y.; Li, W.; Sun, M.X.; Guo, C.L.; Zhang, J.L. Bimetallic Au–Sn/AC catalysts for acetylene hydrochlorination. J. Ind. Eng. Chem. 2016, 35, 177–184. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, Y.; Xu, X.L.; Di, S.X.; Wang, B.L.; Xu, H.; Ni, J.; Guo, L.L.; Pan, Z.Y.; Li, X.N. Stabilizing Au(III) in supported–ionic–liquid–phase (SILP) catalyst using CuCl2 via a redox mechanism. Appl. Catal. B Environ. 2017, 206, 175–183. [Google Scholar] [CrossRef]
- Du, Y.F.; Hu, R.S.; Jia, Y.; Zhou, Q.H.; Meng, W.W.; Yang, J. CuCl2 promoted low–gold–content Au/C catalyst for acetylene hydrochlorination prepared by ultrasonic–assisted impregnation. J. Ind. Eng. Chem. 2016, 37, 32–41. [Google Scholar] [CrossRef]
- Zhao, J.G.; Zeng, J.J.; Cheng, X.G.; Wang, L.; Yang, H.H.; Shen, B.X. An Au–Cu bimetal catalyst for acetylene hydrochlorination with renewable γ–Al2O3 as the support. RSC Adv. 2015, 5, 16727–16734. [Google Scholar] [CrossRef]
- Wang, L.; Shen, B.X.; Zhao, J.G.; Bi, X.T. Trimetallic Au–Cu–K/AC for acetylene hydrochlorination. Can. J. Chem. Eng. 2017, 95, 1069–1075. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Dai, B.; Li, W.; Wang, X.G.; Zhang, J.L.; Zhu, M.Y.; Gu, J.J. Non–mercury catalytic acetylene hydrochlorination over spherical activated–carbon–supported Au–Co(III)–Cu(II) catalysts. J. Catal. 2014, 316, 141–148. [Google Scholar] [CrossRef]
- Li, G.B.; Li, W.; Zhang, J.L.; Zhang, W.; Zhou, H.; Si, C.L. The effect of N–doping in activated carbon–supported Au–Sr catalysts for acetylene hydrochlorination to vinyl chloride. ChemistrySelect 2018, 3, 3561–3569. [Google Scholar] [CrossRef]
- Li, G.B.; Li, W.; Zhang, J.L. Strontium promoted activated carbon–supported gold catalysts for non–mercury catalytic acetylene hydrochlorination. Catal. Sci. Technol. 2016, 6, 3230–3237. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Li, W.; Li, X.Q.; Zhao, W.; Gu, J.J.; Qi, X.Y.; Dong, Y.Z.; Dai, B.; Zhang, J.L. Non–mercury catalytic acetylene hydrochlorination over bimetallic Au–Ba(ii)/AC catalysts. Catal. Sci. Technol. 2015, 5, 1870–1877. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.T.; Xu, J.H.; Ni, J.; Zhang, T.T.; Xu, X.L.; Li, X.N. Activated–carbon–supported gold–cesium(I) as highly effective catalysts for hydrochlorination of acetylene to vinyl chloride. ChemPlusChem 2015, 80, 196–201. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, T.T.; Di, X.X.; Xu, J.T.; Xu, J.; Feng, F.; Ni, J.; Li, X.N. Nitrogen–modified activated carbon supported bimetallic gold–cesium(i) as highly active and stable catalyst for the hydrochlorination of acetylene. RSC Adv. 2015, 5, 6925–6931. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, T.T.; Di, X.X.; Xu, J.T.; Gu, S.C.; Zhang, Q.F.; Ni, J.; Li, X.N. Activated carbon supported ternary gold–cesium(i)–indium(iii) catalyst for the hydrochlorination of acetylene. Catal. Sci. Technol. 2015, 5, 4973–4984. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, X.; Wang, L.; Ren, R.; Zeng, J.; Yang, H.; Shen, B. Free–mercury catalytic acetylene hydrochlorination over bimetallic Au–Bi/γ–Al2O3:A low gold content catalyst. Catal. Lett. 2014, 144, 2191–2197. [Google Scholar] [CrossRef]
- Huang, C.F.; Zhu, M.Y.; Kang, L.H.; Li, X.Y.; Dai, B. Active carbon supported TiO2–AuCl3/AC catalyst with excellent stability for acetylene hydrochlorination reaction. Chem. Eng. J. 2014, 242, 69–75. [Google Scholar] [CrossRef]
- Pu, Y.F.; Zhang, J.L.; Wang, X.; Zhang, H.Y.; Yu, L.; Dong, Y.Z.; Li, W. Bimetallic Au–Ni/CSs catalysts for acetylene hydrochlorination. Catal. Sci. Technol. 2014, 4, 4426–4432. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Dai, B.; Wang, X.G.; Li, W.; Han, Y.; Gu, J.J.; Zhang, J.L. Non–mercury catalytic acetylene hydrochlorination over bimetallic Au–Co(iii)/SAC catalysts for vinyl chloride monomer production. Green Chem. 2013, 15, 829–836. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Dai, B.; Wang, X.G.; Xu, L.L.; Zhu, M.Y. Hydrochlorination of acetylene to vinyl chloride monomer over bimetallic Au–La/SAC catalysts. J. Ind. Eng. Chem. 2012, 18, 49–54. [Google Scholar] [CrossRef]
- Goguet, A.; Hardacre, C.; Harvey, I.; Narasimharao, K.; Saih, Y.; Sa, J. Increased dispersion of supported gold during methanol carbonylation conditions. J. Am. Chem. Soc. 2009, 131, 6973–6975. [Google Scholar] [CrossRef]
- Zhong, J.W.; Xu, Y.P.; Liu, Z.M. Heterogeneous non–mercury catalysts for acetylene hydrochlorination: Progress, challenges, and opportunities. Green Chem. 2018, 20, 2412–2427. [Google Scholar] [CrossRef]
- Zhou, K.; Jia, J.C.; Li, C.H.; Xu, H.; Zhou, J.; Luo, G.H.; Wei, F. A low content Au–based catalyst for hydrochlorination of C2H2 and its industrial scale–up for future PVC processes. Green Chem. 2015, 17, 356–364. [Google Scholar] [CrossRef]
- Li, X.B.; Wang, H.Y.; Yang, X.D.; Zhu, Z.H.; Tang, Y.J. Size dependence of the structures and energetic and electronic properties of gold clusters. J. Chem. Phys. 2007, 126, 084505. [Google Scholar] [CrossRef]
- Li, Y.F.; Mao, A.J.; Li, Y.; Kuang, X.Y. Density functional study on size–dependent structures, stabilities, electronic and magnetic properties of AunM (M = Al and Si, n = 1–9) clusters: Comparison with pure gold clusters. J. Mol. Model. 2012, 18, 3061–3072. [Google Scholar] [CrossRef]
- Bürgel, C.; Reilly, N.M.; Johnson, G.E.; Mitrić, R.; Kimble, M.L.; Castleman, A.W.; Bonačić–Koutecký, V. Influence of charge state on the mechanism of co oxidation on gold clusters. J. Am. Chem. Soc. 2008, 130, 1694–1698. [Google Scholar] [CrossRef]
- Socaciu, L.D.; Hagen, J.; Bernhardt, T.M.; Wöste, L.; Heiz, U.; Häkkinen, H.; Landman, U. Catalytic co oxidation by free Au2–: Experiment and theory. J. Am. Chem. Soc. 2003, 125, 10437–10445. [Google Scholar] [CrossRef]
- Gao, M.; Lyalin, A.; Taketsugu, T. Role of the support effects on the catalytic activity of gold clusters: A density functional theory study. Catalysts 2011, 1, 18–39. [Google Scholar] [CrossRef]
- Spivey, K.; Williams, J.I.; Wang, L. Structures of undecagold clusters: Ligand effect. Chem. Phys. Lett. 2006, 432, 163–166. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, F.; Kang, L.H. Catalysis of the acetylene hydrochlorination reaction by Si–doped Au clusters: A DFT study. J. Mol. Model. 2018, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhou, K.; Si, J.; Li, C.; Luo, G. A ligand coordination approach for high reaction stability of an Au–Cu bimetallic carbon–based catalyst in the acetylene hydrochlorination process. Catal. Sci. Technol. 2016, 6, 1357–1366. [Google Scholar] [CrossRef]
- Li, X.; Zhu, M.; Dai, B. AuCl3 on polypyrrole–modified carbon nanotubes as acetylene hydrochlorination catalysts. Appl. Catal. B Environ. 2013, 142, 234–240. [Google Scholar] [CrossRef]
- Chen, K.; Kang, L.H.; Zhu, M.Y.; Dai, B. Mesoporous carbon with controllable pore sizes as a support of the AuCl3 catalyst for acetylene hydrochlorination. Catal. Sci. Technol. 2015, 5, 1035–1040. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, R.S.; Zhou, Q.H.; Wang, H.Y.; Gao, X.; Zhang, J. Boron–modified activated carbon supporting low–content Au–based catalysts for acetylene hydrochlorination. J. Catal. 2017, 348, 223–232. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, Y.; Kang, L.H.; Zhu, M.Y.; Dai, B. A novel, non–metallic graphitic carbon nitride catalyst for acetylene hydrochlorination. J. Catal. 2014, 311, 288–294. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, G.J.; Wang, X.L.; Li, Y. Direct synthesis of nitrogen–doped mesoporous carbons for acetylene hydrochlorination. Chin. J. Catal. 2016, 37, 1242–1248. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Xu, J.; Zhang, T.; Di, X.; Ni, J.; Li, X. Enhancement of Au/AC acetylene hydrochlorination catalyst activity and stability via nitrogen–modified activated carbon support. Chem. Eng. J. 2015, 262, 1152–1160. [Google Scholar] [CrossRef]
- Zhao, J.; Gu, S.C.; Xu, X.L.; Zhang, T.T.; Yu, Y.; Di, X.X.; Ni, J.; Pan, Z.Y.; Li, X.N. Supported ionic–liquid–phase–stabilized Au(iii) catalyst for acetylene hydrochlorination. Catal. Sci. Technol. 2016, 6, 3263–3270. [Google Scholar] [CrossRef]
- Tian, X.H.; Hong, G.T.; Liu, Y.; Jiang, B.B.; Yang, Y.R. Catalytic performance of AuIII supported on SiO2 modified activated carbon. RSC Adv. 2014, 4, 36316–36324. [Google Scholar] [CrossRef]
- Dai, B.; Li, X.; Zhang, J.; Yu, F.; Zhu, M. Application of mesoporous carbon nitride as a support for an Au catalyst for acetylene hydrochlorination. Chem. Eng. Sci. 2015, 135, 472–478. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Kang, L.; Dai, B. Neutral Aun (n = 3–10) clusters catalyze acetylene hydrochlorination: A density functional theory study. RSC Adv. 2014, 4, 38466–38473. [Google Scholar] [CrossRef]
- Gong, W.Q.; Zhao, F.; Kang, L.H. Novel nitrogen–doped Au–embedded graphene single–atom catalysts for acetylene hydrochlorination: A density functional theory study. Comput. Theor. Chem. 2018, 1130, 83–89. [Google Scholar] [CrossRef]
- Zhang, J.L.; He, Z.H.; Li, W.; Han, Y. Deactivation mechanism of AuCl3 catalyst in acetylene hydrochlorination reaction: A DFT study. RSC Adv. 2012, 2, 4814–4821. [Google Scholar] [CrossRef]
- Nkosi, B.; Coville, N.J.; Hutchings, G.J.; Adams, M.D.; Friedl, J.; Wagner, F.E. Hydrochlorination of acetylene using gold catalysts: A study of catalyst deactivation. J. Catal. 1991, 128, 366–377. [Google Scholar] [CrossRef]
- Conte, M.; Davies, C.J.; Morgan, D.J.; Davies, T.E.; Carley, A.F.; Johnston, P.; Hutchings, G.J. Modifications of the metal and support during the deactivation and regeneration of Au/C catalysts for the hydrochlorination of acetylene. Catal. Sci. Technol. 2013, 3, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Hashmi, A.S.K.; Blanco, M.C.; Fischer, D.; Bats, J.W. Gold catalysis: Evidence for the in–situ reduction of gold(iii) during the cyclization of allenyl carbinols. Eur. J. Org. Chem. 2006, 2006, 1387–1389. [Google Scholar] [CrossRef]
- Conte, M.; Carley, A.F.; Hutchings, G.J. Reactivation of a carbon–supported gold catalyst for the hydrochlorination of acetylene. Catal. Lett. 2008, 124, 165–167. [Google Scholar] [CrossRef]
- Dai, B.; Wang, Q.Q.; Yu, F.; Zhu, M.Y. Effect of Au nano–particle aggregation on the deactivation of the AuCl3/AC catalyst for acetylene hydrochlorination. Sci Rep 2015, 5, 10553. [Google Scholar] [CrossRef] [Green Version]
- Malta, G.; Kondrat, S.A.; Freakley, S.J.; Davies, C.J.; Dawson, S.; Liu, X.; Lu, L.; Dymkowski, K.; Fernandez–Alonso, F.; Mukhopadhyay, S.; et al. Deactivation of a single–site gold–on–carbon acetylene hydrochlorination catalyst: An X–ray absorption and inelastic neutron scattering study. ACS Catal. 2018, 8, 8493–8505. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.Y.; Kang, L.H.; Su, Y.; Zhang, S.Z.; Dai, B. MClx (M = Hg, Au, Ru; x = 2, 3) catalyzed hydrochlorination of acetylene—A density functional theory study. Can. J. Chem. 2013, 91, 120–125. [Google Scholar] [CrossRef]
- Li, H.; Wu, B.T.; Wang, F.M.; Zhang, X.B. Efficient and stable Ru(III)/choline chloride catalyst system with low Ru content for non–mercury acetylene hydrochlorination. Chin. J. Catal. 2018, 39, 1770–1781. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Man, B.; Zhang, C.; Dai, H.; Dai, B.; Zhang, J. Synthesis of vinyl chloride monomer over carbon–supported tris–(triphenylphosphine) ruthenium dichloride catalysts. Catalysts 2018, 8, 276. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lan, G.; Liu, H.; Zhu, Y.; Li, Y. Effect of acidity and ruthenium species on catalytic performance of ruthenium catalysts for acetylene hydrochlorination. Catal. Sci. Technol. 2018, 8, 6143–6149. [Google Scholar] [CrossRef]
- Gu, J.; Gao, Y.; Zhang, J.; Li, W.; Dong, Y.; Han, Y. Hydrochlorination of acetylene catalyzed by an activated carbon–supported ammonium hexachlororuthenate complex. Catalysts 2017, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, H.; Man, B.; Hou, L.; Zhang, C.; Dai, H.; Zhu, M.; Dai, B.; Dong, Y.; Zhang, J. Activated carbon–supported tetrapropylammonium perruthenate catalysts for acetylene hydrochlorination. Catalysts 2017, 7, 311. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Li, W.; Zhang, J. Ru/N–AC catalyst to produce vinyl chloride from acetylene and 1,2–dichloroethane. Catal. Sci. Technol. 2016, 6, 1402–1409. [Google Scholar] [CrossRef]
- Zhang, J.L.; Sheng, W.; Guo, C.L.; Li, W. Acetylene hydrochlorination over bimetallic Ru–based catalysts. RSC Adv. 2013, 3, 21062–21068. [Google Scholar] [CrossRef]
- Pu, Y.F.; Zhang, J.L.; Yu, L.; Jin, Y.H.; Li, W. Active ruthenium species in acetylene hydrochlorination. Appl. Catal. A Gen. 2014, 488, 28–36. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Li, W.; Jin, Y.H.; Sheng, W.; Hu, M.C.; Wang, X.Q.; Zhang, J.L. Ru–Co(III)–Cu(II)/SAC catalyst for acetylene hydrochlorination. Appl. Catal. B–Environ. 2016, 189, 56–64. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Y.Z.; Li, W.; Han, Y.; Zhang, J.L. Improvement of imidazolium–based ionic liquids on the activity of ruthenium catalyst for acetylene hydrochlorination. Mol. Catal. 2017, 443, 220–227. [Google Scholar] [CrossRef]
- Mitchenko, S.A.; Khomutov, E.V.; Shubin, A.A.; Shul’ga, Y.M. Mechanochemical activation of K2PtCl6: Heterogeneous catalyst for gas–phase hydrochlorination of acetylene. Theor. Exp. Chem. 2003, 39, 255–258. [Google Scholar] [CrossRef]
- Mitchenko, S.A.; Khomutov, E.V.; Shubin, A.A.; Shul’ga, Y.M. Catalytic hydrochlorination of acetylene by gaseous HCl on the surface of mechanically pre–activated K2PtCl6 salt. J. Mol. Catal. A–Chem. 2004, 212, 345–352. [Google Scholar] [CrossRef]
- Mitchenko, S.A.; Krasnyakova, T.V. Acetylene hydrochlorination over mechanically activated K2MCl4 (M = Pt, Pd) and K2PtCl6 catalysts: The HCl/DCl kinetic isotope effect and reaction mechanisms. Kinet. Catal. 2014, 55, 722–728. [Google Scholar] [CrossRef]
- Strebelle, M.; Devos, A. Catalytic Hydrochlorination System and Process for the Manufacture of Vinyl Chloride from Acetylene and Hydrogen Chloride in the Presence of this Catalytic System. U.S. Patent NO. 5,254,777, 17 October 1993. [Google Scholar]
- Wang, L.; Wang, F.; Wang, J. Enhanced stability of hydrochlorination of acetylene using polyaniline–modified Pd/HY catalysts. Catal. Commun. 2016, 74, 55–59. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Wang, J. Effect of K promoter on the stability of Pd/NFY catalysts for acetylene hydrochlorination. Catal. Commun. 2016, 83, 9–13. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Wang, J.D. Catalytic properties of Pd/HY catalysts modified with NH4F for acetylene hydrochlorination. Catal. Commun. 2015, 65, 41–45. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Wang, J.D.; Tang, X.L.; Zhao, Y.L.; Yang, D.; Jia, F.M.; Hao, T. Hydrochlorination of acetylene to vinyl chloride over Pd supported on zeolite Y. React. Kinet. Mech. Catal. 2013, 110, 187–194. [Google Scholar] [CrossRef]
- Song, Q.L.; Wang, S.J.; Shen, B.X.; Zhao, J.G. Palladium–based catalysts for the hydrochlorination of acetylene: Reasons for deactivation and its regeneration. Pet. Sci. Technol. 2010, 28, 1825–1833. [Google Scholar] [CrossRef]
- Li, P.; Ding, M.Z.; He, L.M.; Tie, K.; Ma, H.; Pan, X.L.; Bao, X.H. The activity and stability of PdCl2/C–N catalyst for acetylene hydrochlorination. Sci. China Chem. 2018, 61, 444–448. [Google Scholar] [CrossRef]
- Yang, L.F.; Yang, Q.W.; Hu, J.Y.; Bao, Z.B.; Su, B.G.; Zhang, Z.G.; Ren, Q.L.; Xing, H.B. Metal nanoparticles in ionic liquid–cosolvent biphasic systems as active catalysts for acetylene hydrochlorination. Aiche. J. 2018, 64, 2536–2544. [Google Scholar] [CrossRef]
- Zhao, J.; Yue, Y.X.; Sheng, G.F.; Wang, B.L.; Lai, H.X.; Di, S.X.; Zhai, Y.Y.; Guo, L.L.; Li, X.N. Supported ionic liquid–palladium catalyst for the highly effective hydrochlorination of acetylene. Chem. Eng. J. 2019, 360, 38–46. [Google Scholar] [CrossRef]
- Perkins, G.A. Preparation of Vinyl Chloride. U.S. Patent No. 1,934,324, 7 November 1933. [Google Scholar]
- Zhai, Y.Y.; Zhao, J.; Di, X.X.; Di, S.X.; Wang, B.L.; Yue, Y.X.; Sheng, G.F.; Lai, H.X.; Guo, L.L.; Wang, H.; et al. Carbon–supported perovskite–like CsCuCl3 nanoparticles: A highly active and cost–effective heterogeneous catalyst for the hydrochlorination of acetylene to vinyl chloride. Catal. Sci. Technol. 2018, 8, 2901–2908. [Google Scholar] [CrossRef]
- Zhao, W.L.; Zhu, M.Y.; Dai, B. The preparation of Cu–g–C3N4/AC catalyst for acetylene hydrochlorination. Catalysts 2016, 6, 193. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, F.M.; Cai, W.F.; Zhang, J.L.; Zhang, X.B. Hydrochlorination of acetylene using supported phosphorus–doped Cu–based catalysts. Catal. Sci. Technol. 2015, 5, 5174–5184. [Google Scholar] [CrossRef]
- Xu, J.T.; Zhao, J.; Zhang, T.T.; Di, X.X.; Gu, S.C.; Ni, J.; Li, X.N. Ultra–low Ru–promoted CuCl2 as highly active catalyst for the hydrochlorination of acetylene. RSC Adv. 2015, 5, 38159–38163. [Google Scholar] [CrossRef]
- Zhou, K.; Si, J.K.; Jia, J.C.; Huang, J.Q.; Zhou, J.; Luo, G.H.; Wei, F. Reactivity enhancement of N–CNTs in green catalysis of C2H2 hydrochlorination by a Cu catalyst. RSC Adv. 2014, 4, 7766–7769. [Google Scholar] [CrossRef]
- Hu, D.; Wang, L.; Wang, F.; Wang, J.D. Active carbon supported S–promoted Bi catalysts for acetylene hydrochlorination reaction. Chin. Chem. Lett. 2018, 29, 1413–1416. [Google Scholar] [CrossRef]
- Dai, H.; Zhu, M.Y.; Zhang, H.Y.; Yu, F.; Wang, C.; Dai, B. Activated carbon supported Mo–Ti–N binary transition metal nitride as catalyst for acetylene hydrochlorination. Catalysts 2017, 7, 200. [Google Scholar] [CrossRef] [Green Version]
- Dai, H.; Zhu, M.Y.; Zhang, H.Y.; Yu, F.; Wang, C.; Dai, B. Activated carbon supported VN, Mo2N, and W2N as catalysts for acetylene hydrochlorination. J. Ind. Eng. Chem. 2017, 50, 72–78. [Google Scholar] [CrossRef]
- Dai, H.; Zhu, M.Y.; Zhao, D.; Yu, F.; Dai, B. Effective catalytic performance of plasma–enhanced W2N/AC as catalysts for acetylene hydrochlorination. Top. Catal. 2017, 60, 1016–1023. [Google Scholar] [CrossRef]
- Zhao, W.L.; Zhu, M.Y.; Dai, B. Cobalt–nitrogen–activated carbon as catalyst in acetylene hydrochlorination. Catal. Commun. 2017, 98, 22–25. [Google Scholar] [CrossRef]
- Lan, G.; Qiu, Y.; Fan, J.; Wang, X.; Tang, H.; Han, W.; Liu, H.; Liu, H.; Song, S.; Li, Y. Defective graphene@diamond hybrid nanocarbon material as an effective and stable metal–free catalyst for acetylene hydrochlorination. Catal. Commun. 2019, 55, 1430–1433. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Han, Y.; Zhu, M.; Shang, S.; Li, W. MOF–derived various morphologies of N–doped carbon composites for acetylene hydrochlorination. J. Mater. Sci. 2018, 53, 4913–4926. [Google Scholar] [CrossRef]
- Dong, X.B.; Chao, S.L.; Wan, F.F.; Guan, Q.X.; Wang, G.C.; Li, W. Sulfur and nitrogen co–doped mesoporous carbon with enhanced performance for acetylene hydrochlorination. J. Catal. 2018, 359, 161–170. [Google Scholar] [CrossRef]
- Li, P.; Li, H.B.; Pan, X.L.; Tie, K.; Cui, T.T.; Ding, M.Z.; Bao, X.H. Catalytically active boron nitride in acetylene hydrochlorination. ACS Catal. 2017, 7, 8572–8577. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhang, J.L.; Li, W. MOF–derived nitrogen–doped porous carbon as metal–free catalysts for acetylene hydrochlorination. J. Ind. Eng. Chem. 2016, 44, 146–154. [Google Scholar] [CrossRef]
- Dai, B.; Chen, K.; Wang, Y.; Kang, L.H.; Zhu, M.Y. Boron and nitrogen doping in graphene for the catalysis of acetylene hydrochlorination. ACS Catal. 2015, 5, 2541–2547. [Google Scholar] [CrossRef]
- Li, X.Y.; Pan, X.L.; Bao, X.H. Nitrogen doped carbon catalyzing acetylene conversion to vinyl chloride. J. Energy Chem. 2014, 23, 131–135. [Google Scholar] [CrossRef]
- Zhou, K.; Li, B.; Zhang, Q.; Huang, J.Q.; Tian, G.L.; Jia, J.C.; Zhao, M.Q.; Luo, G.H.; Su, D.S.; Wei, F. The catalytic pathways of hydrohalogenation over metal–free nitrogen–doped carbon nanotubes. ChemSusChem 2014, 7, 723–728. [Google Scholar] [CrossRef]
- Wang, X.G.; Dai, B.; Wang, Y.; Yu, F. Nitrogen–doped pitch–based spherical active carbon as a nonmetal catalyst for acetylene hydrochlorination. ChemCatChem 2014, 6, 2339–2344. [Google Scholar] [CrossRef]
- Qiao, X.L.; Zhou, Z.Q.; Liu, X.Y.; Zhao, C.Y.; Guan, Q.X.; Li, W. Constructing of fragmentary g–C3N4 framework with rich nitrogen defects as highly efficient metal–free catalyst for acetylene hydrochlorination. Catal. Sci. Technol. 2019, 9, 3753–3762. [Google Scholar] [CrossRef]
- Zhang, C.L.; Kang, L.H.; Zhu, M.Y.; Dai, B. Nitrogen–doped active carbon as a metal–free catalyst for acetylene hydrochlorination. RSC Adv. 2015, 5, 7461–7468. [Google Scholar] [CrossRef]
- Zhao, F.; Kang, L.H. The neglected significant role for graphene–based acetylene hydrochlorination catalysts — intrinsic graphene defects. Chem. Sel. 2017, 2, 6016–6022. [Google Scholar] [CrossRef]
- Li, X.Y.; Pan, X.L.; Yu, L.; Ren, P.J.; Wu, X.; Sun, L.T.; Jiao, F.; Bao, X.H. Silicon carbide–derived carbon nanocomposite as a substitute for mercury in the catalytic hydrochlorination of acetylene. Nat. Commun. 2014, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, Y.; Zhu, M.Y.; Kang, L.H. C–doped boron nitride fullerene as a novel catalyst for acetylene hydrochlorination: A DFT study. RSC Adv. 2015, 5, 56348–56355. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, P.; Pan, X.L.; Ma, H.; Bao, X.H. Deactivation mechanism and regeneration of carbon nanocomposite catalyst for acetylene hydrochlorination. Appl. Catal. B–Environ. 2017, 210, 116–120. [Google Scholar] [CrossRef]
- Chao, S.L.; Zou, F.; Wan, F.F.; Dong, X.B.; Wang, Y.L.; Wang, Y.X.; Guan, Q.X.; Wang, G.C.; Li, W. Nitrogen–doped carbon derived from ZIF–8 as a high–performance metal–free catalyst for acetylene hydrochlorination. Sci. Rep. 2017, 7, 39789. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.J.; Liu, G.Y.; He, D.W.; Pang, X.D.; Tong, Y.S.; Wu, Y.Q.; Yuan, D.H.; Liu, Z.M.; Xu, Y.P. Acetylene hydrochlorination over 13X zeolite catalysts at high temperature. Green Chem. 2016, 18, 5994–5998. [Google Scholar] [CrossRef]
- Li, X.Y.; Nian, Y.; Shang, S.S.; Zhang, H.Y.; Zhang, J.L.; Han, Y.; Li, W. Novel nonmetal catalyst of supported tetraphenylphosphonium bromide for acetylene hydrochlorination. Catal. Sci. Technol. 2019, 9, 188–198. [Google Scholar] [CrossRef]
- Wang, B.L.; Lai, H.X.; Yue, Y.X.; Sheng, G.F.; Deng, Y.Q.; He, H.H.; Guo, L.L.; Zhao, J.; Li, X.N. Zeolite supported ionic liquid catalysts for the hydrochlorination of acetylene. Catalysts 2018, 8, 351. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.F.; Xu, S.G.; Liu, Y.L.; Cao, S.K. Mechanistic study on metal–free acetylene hydrochlorination catalyzed by imidazolium–based ionic liquids. Mol. Catal. 2018, 461, 73–79. [Google Scholar] [CrossRef]























| Year | Catalyst | Synergistic Metal | Au (wt %) | GHSV (h−1) | Temp (°C) | Acetylene Conv. (%) | Running Time (h) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2019 | AuCe | CeO2 | 0.1 | 60 | 180 | 99.9 | 70 | [4] |
| 2016 | AuCe | CeO2 | 1.0 | 852 | 180 | 98.4 | 20 | [36] |
| 2017 | AuY | YCl3 | 1.0 | 800 | 180 | 87.8 | 10 | [37] |
| 2016 | AuSn | SnCl2 | 0.9 | 720 | 170 | 95 | 48 | [38] |
| 2017 | AuCu | CuCl2 | 0.1 | 740 | 180 | 98.5 | 500 | [39] |
| 2016 | AuCu | CuCl2 | 0.1 | 120 | 150 | 97 | 4 | [40] |
| 2015 | AuCu | CuCl2 | 0.25 | 120 | 150 | 97 | 2 | [41] |
| 2017 | AuCuK | CuCl2/KCl | 0.2 | 40 | 165 | 89 | 1600 | [42] |
| 2014 | AuCoCu | Co(NH3)6Cl3/CuCl2 | 1.0 | 720 | 150 | 99 | 5 | [43] |
| 2018 | AuSr | SrCl2 | 1.0 | 1806 | 180 | 99.7 | 180 | [44] |
| 2016 | AuSr | SrCl2 | 1.0 | 762 | 180 | 87.7 | 20 | [45] |
| 2015 | AuBa | BaCl2 | 1.0 | 360 | 200 | 98.4 | 50 | [46] |
| 2015 | AuCs | CsCl | 1.0 | 740 | 180 | 94 | 50 | [47] |
| 2015 | AuCs | CsCl | 1.0 | 1480 | 180 | 90.1 | 50 | [48] |
| 2015 | AuInCs | CsCl/InCl3 | 1.0 | 1480 | 180 | 92.8 | 50 | [49] |
| 2014 | AuBi | BiCl3 | 1.0 | 600 | 180 | 85 | 10 | [17] |
| 2014 | AuBi | BiCl3 | 1.0 | 120 | 150 | 96 | 10 | [50] |
| 2014 | AuTi | TiO2 | 1.0 | 870 | 180 | 92 | 10 | [51] |
| 2014 | AuNi | NiCl2 | 1.5 | 900 | 170 | 95.4 | 46 | [52] |
| 2013 | AuCo | Co(NH3)6Cl3 | 1.0 | 360 | 150 | 99.9 | 36 | [53] |
| 2012 | AuLa | LaCl3 | 1.0 | 360 | 150 | 98 | 50 | [54] |
| Year | Catalyst | Ligand | Au (wt %) | GHSV (h−1) | Temp (°C) | Acetylene Conv. (%) | Running Time (h) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2016 | Au/TCCA | Trichloroisocyanuric acid | 0.2 | 90 | 180 | 98 | 24 | [65] |
| 2015 | HAu(C3Cl3-N3O3)3Cl | Trichloroisocyanuric acid | 1.0 | 500 | 130 | 52 | 24 | [8] |
| 2015 | Au(CS(NH2)2 | Thiourea | 0.1 | 500 | 130 | 95 | 24 | [8] |
| 2015 | Au/SCN | KSCN | 0.25 | 1200 | 180 | 99 | 10 | [57] |
| 2014 | [AuCl2(phen)]Cl | 1,10–phenanthroline | 0.49 | 603 | 180 | 90 | 40 | [29] |
| 2013 | AuCl3/PPy-MWCNT | Pyrrole | 1.47 | 120 | 150 | 90 | 10 | [66] |
| Year | Catalyst | Material | Ru (wt %) | GHSV (h−1) | Temp (°C) | Acetylene Conv. (%) | Running Time (h) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2018 | Ru(III)-ChCl/AC | RuCl3 + ChCl | 0.2 | 900 | 170 | 99.3 | 25 | [85] |
| 2018 | Φ-P-Ru/AC-HNO3 | RuCl3 + tris-(triphenylphosphine) ruthenium dichloride | 1 | 180 | 180 | 99.2 | 48 | [86] |
| 2018 | RuCl3-A/AC | RuCl3 + NH3·H2O | 2 | 100 | 180 | 95.8 | 8 | [87] |
| 2017 | (NH4)2RuCl6/AC | (NH4)2RuCl6 | 1 | 180 | 170 | 90.5 | 12 | [88] |
| 2017 | TPAP/AC-HCl | C12H28NO4Ru | 1 | 180 | 180 | 97 | 48 | [89] |
| 2016 | Ru/N-AC | RuCl3 | 1 | 57 | 250 | 95.2 | 180 | [90] |
| 2013 | Ru1Co3/SAC | RuCl3 + CoCl2 | 1 | 180 | 170 | 95 | 48 | [91] |
| 2014 | Ru/SAC-C300 | RuCl3 | 1 | 180 | 170 | 96.5 | 48 | [92] |
| 2016 | Ru-Co(III)-Cu(II)/SAC | RuCl3 + CuCl2+ Co(NH3)6Cl3 | 0.1 | 180 | 170 | 99 | 48 | [93] |
| 2017 | Ru10%[BMIM]BF4/AC | RuCl3 + 1-Butyl-3-methylimidazolium tetrafluoroborate | 1 | 180 | 170 | 98.9 | 24 | [94] |
| Year | Catalyst | Material | Pd (wt %) | GHSV (h−1) | Temp (°C) | Acetylene Conv. (%) | Running Time | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2016 | Pd/PANI-HY | H2PdCl4 + polyaniline | 0.9 | 110 | 160 | 95 | 300 h | [99] |
| 2016 | Pd-K/NFY | PdCl2 + KCl + NH4F | 0.9 | 110 | 160 | 99 | 50 h | [100] |
| 2015 | Pd/NH4F-HY | PdCl2 + NH4F | 0.9 | 110 | 160 | 99.92 | 400 min | [101] |
| 2013 | Pd/HY | H2PdCl4 | 0.5 | 110 | 160 | 95 | 160 min | [102] |
| 2010 | PdCl2-KCl-LaCl3/C | PdCl2 + KCl + LaCl3 | 0.9 | 120 | 160 | 99 | 3 h | [103] |
| Year | Catalyst | Material | Cu (wt %) | GHSV (h−1) | Temp (°C) | Acetylene Conv. (%) | Running Time | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2018 | Cu-Cs/AC | CuCl2 + CsCl | 1 | 50 | 200 | 92 | 200 | [108] |
| 2016 | Cu-g-C3N4/AC | CuCl2 + dicyan-diamide | 4.15 | 72 | 180 | 79 | 450 min | [109] |
| 2015 | Cu2P2O7/SAC | Cu2P2O7 | 15 | 180 | 140 | 40.5 | 500 min | [110] |
| 2015 | Cu400Ru/MWCNTs | CuCl2 + RuCl3 | 4.24 | 180 | 180 | 51.6 | 6 h | [111] |
| 2014 | Cu-NCNT | CuCl2 + N-doped carbon nanotubes | 5 | 180 | 180 | 45.8 | 4 h | [112] |
| Year | Catalyst | Material | GHSV (h−1) | Temp (°C) | Acetylene Conv. (%) | Running Time (h) | Ref. |
|---|---|---|---|---|---|---|---|
| 2019 | ND@G | Nanodiamond + graphene | 300 | 220 | 50 | 10 | [118] |
| 2018 | ZIF-8/SAC | ZIF-8 + spherical activated carbon | 30 | 220 | 81 | 2 | [119] |
| 2018 | NS-C-NH3 | S + N-doped carbon + NH3 | 35 | 220 | 80 | 9 | [120] |
| 2017 | p-BN | H3BO3 + melamine + NH3 | - | 280 | 99 | 50 | [121] |
| 2016 | Z4M1 | ZIF-8 + melamine | 50 | 180 | 60 | 20 | [122] |
| 2015 | B,N-G | Graphene oxide + H3BO3 + NH3 | 360 | 150 | 94.87 | 4 | [123] |
| 2014 | N-OMC-700 | N-doped ordered mesoporous carbon | - | 200 | 77 | 100 | [124] |
| 2014 | N-CNTs | C2H4 + NH3 | 180 | 180 | 7.2 | 3 | [125] |
| 2014 | g-C3N4 | Activated carbon + cyanamide | 50 | 180 | 76.52 | 7 | [69] |
| 2014 | PSAC-N | Pitch-based spherical activated carbon + melamine | 120 | 250 | 68 | - | [126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhao, L.; Zhang, Y.; Zhang, L.; Zan, X. Progress and Challenges of Mercury-Free Catalysis for Acetylene Hydrochlorination. Catalysts 2020, 10, 1218. https://doi.org/10.3390/catal10101218
Liu Y, Zhao L, Zhang Y, Zhang L, Zan X. Progress and Challenges of Mercury-Free Catalysis for Acetylene Hydrochlorination. Catalysts. 2020; 10(10):1218. https://doi.org/10.3390/catal10101218
Chicago/Turabian StyleLiu, Yanxia, Lin Zhao, Yagang Zhang, Letao Zhang, and Xingjie Zan. 2020. "Progress and Challenges of Mercury-Free Catalysis for Acetylene Hydrochlorination" Catalysts 10, no. 10: 1218. https://doi.org/10.3390/catal10101218
APA StyleLiu, Y., Zhao, L., Zhang, Y., Zhang, L., & Zan, X. (2020). Progress and Challenges of Mercury-Free Catalysis for Acetylene Hydrochlorination. Catalysts, 10(10), 1218. https://doi.org/10.3390/catal10101218