Selective Hydrogenation of Naphthalene over γ-Al2O3-Supported NiCu and NiZn Bimetal Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
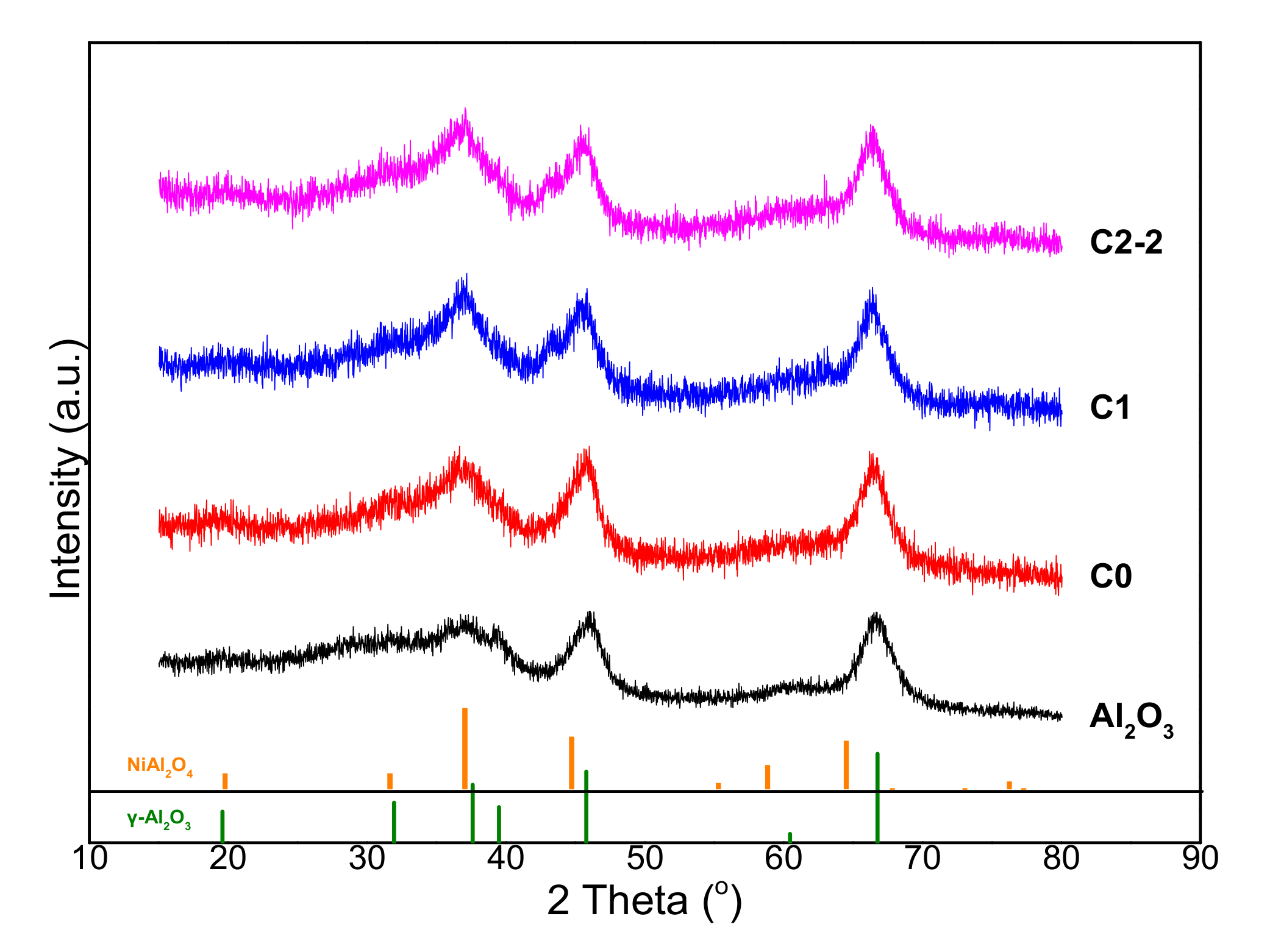
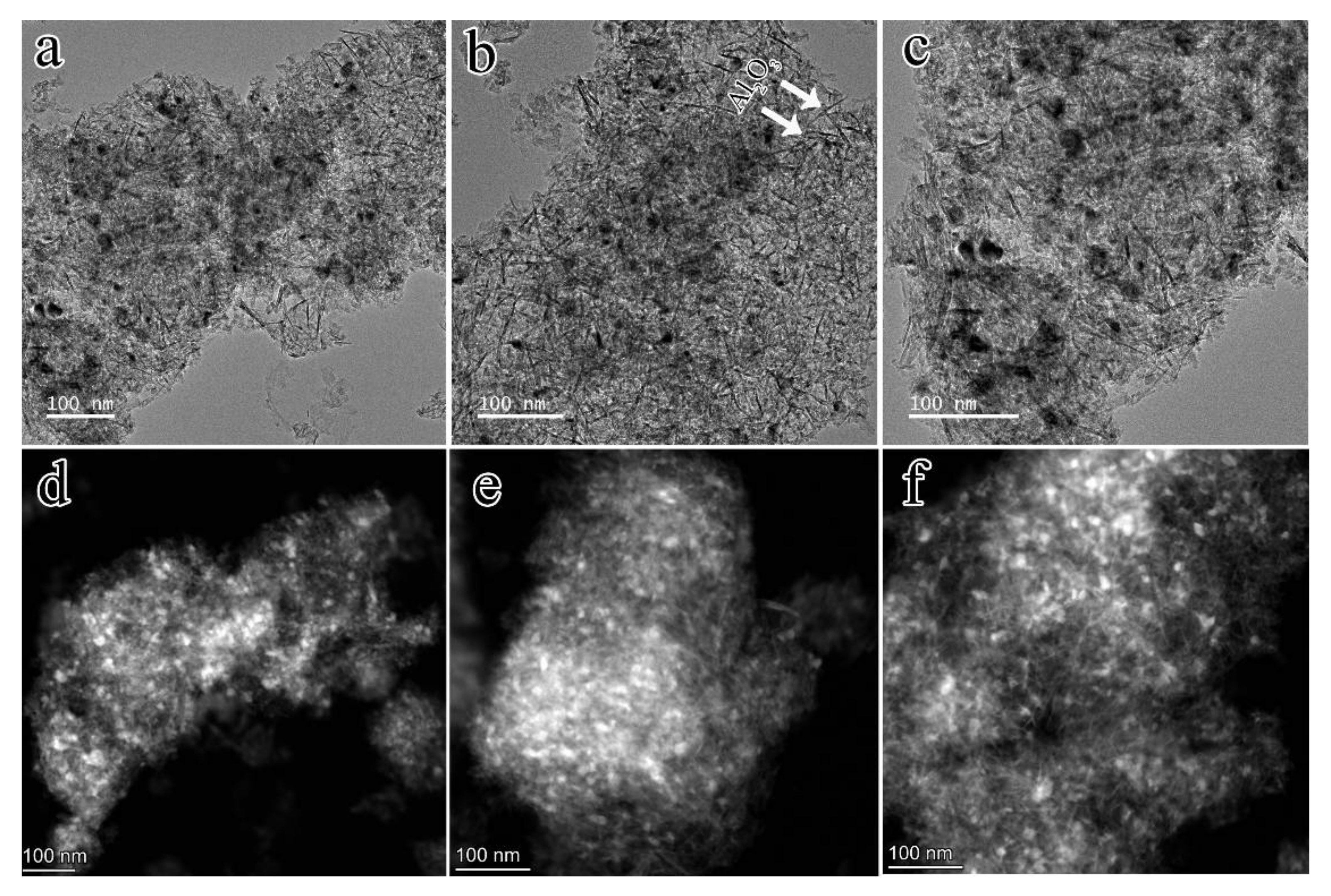
2.1.1. Textural and Structural Properties of Catalysts
2.1.2. The Reducibility of Catalysts
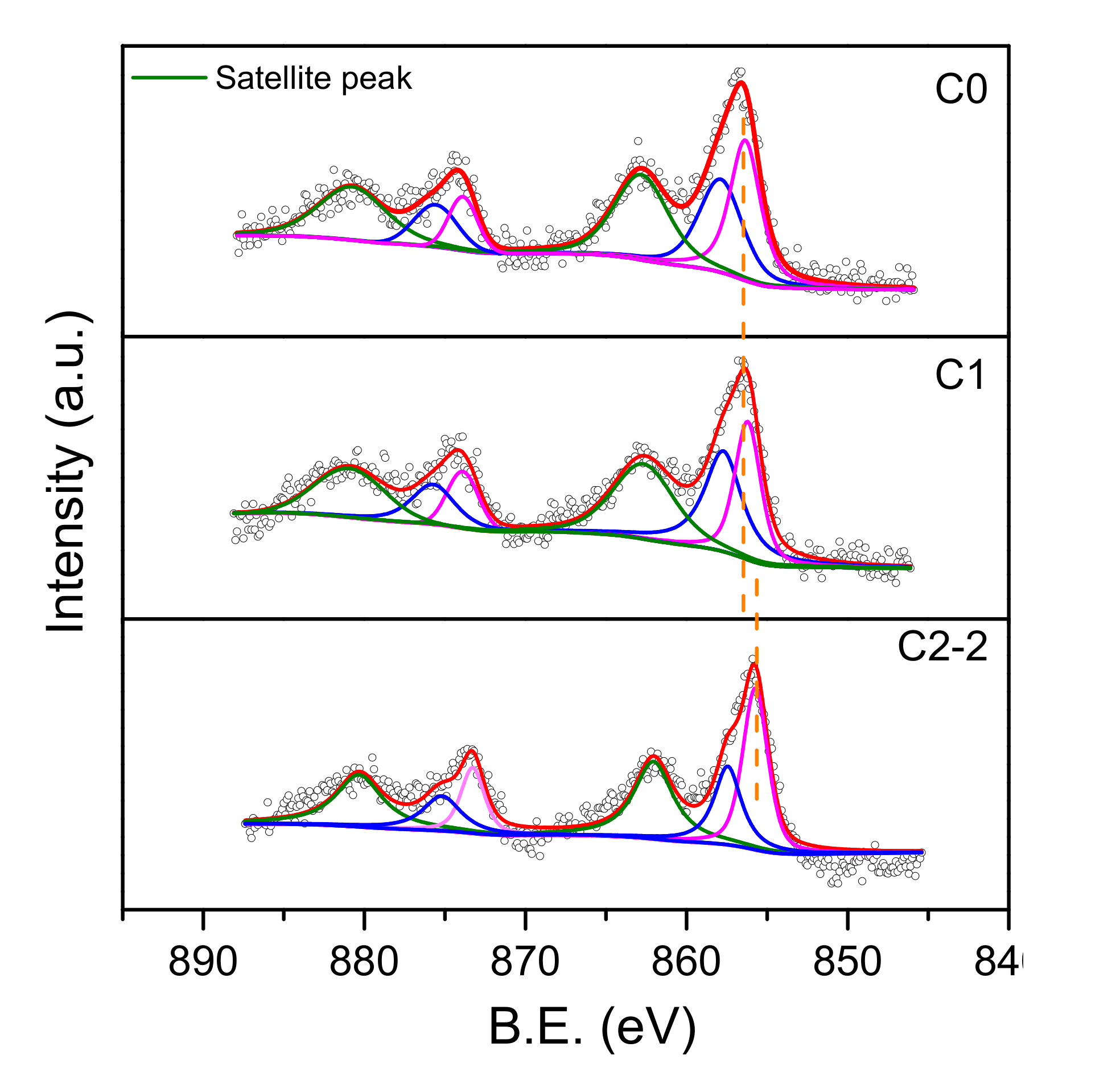
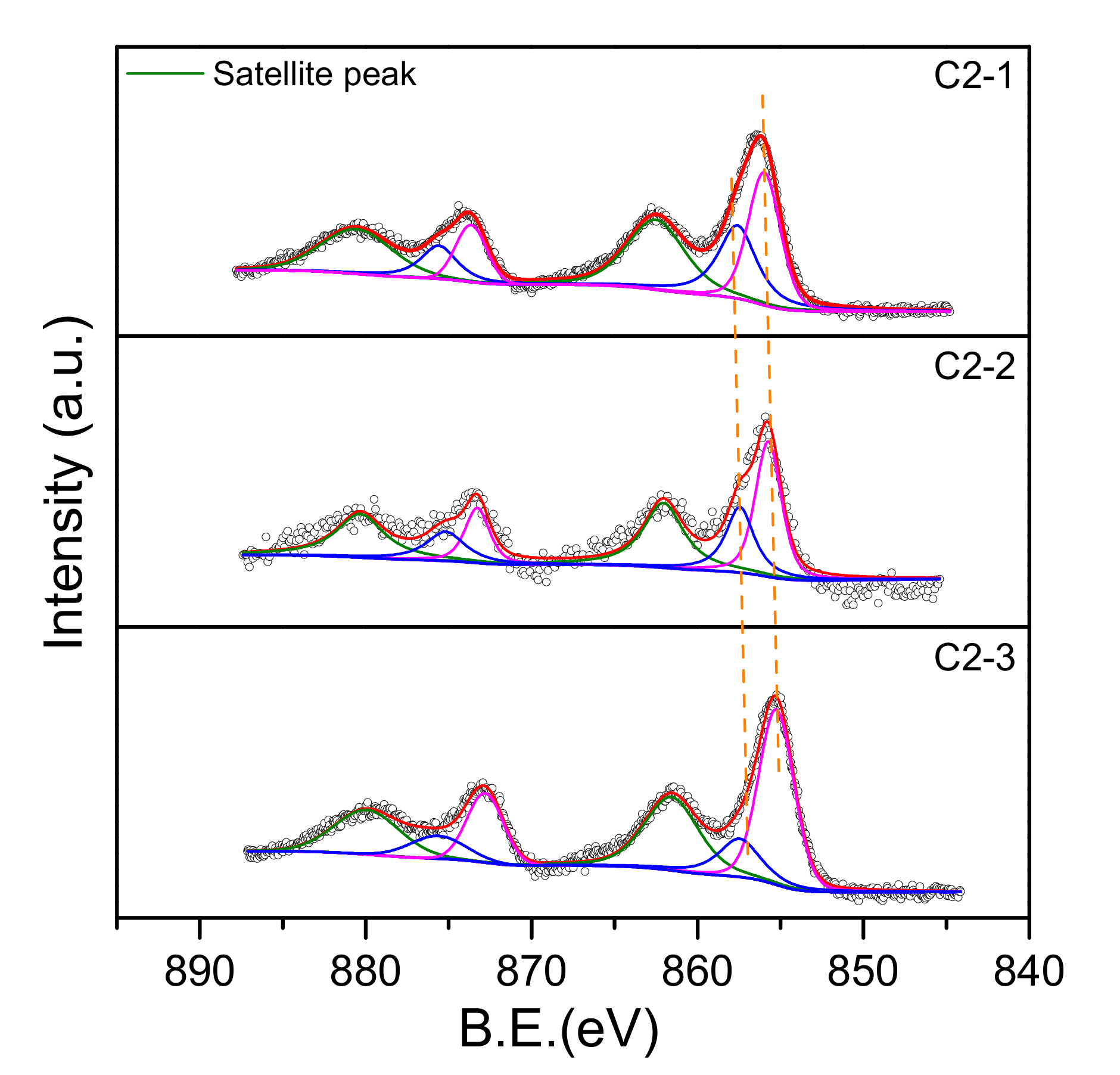
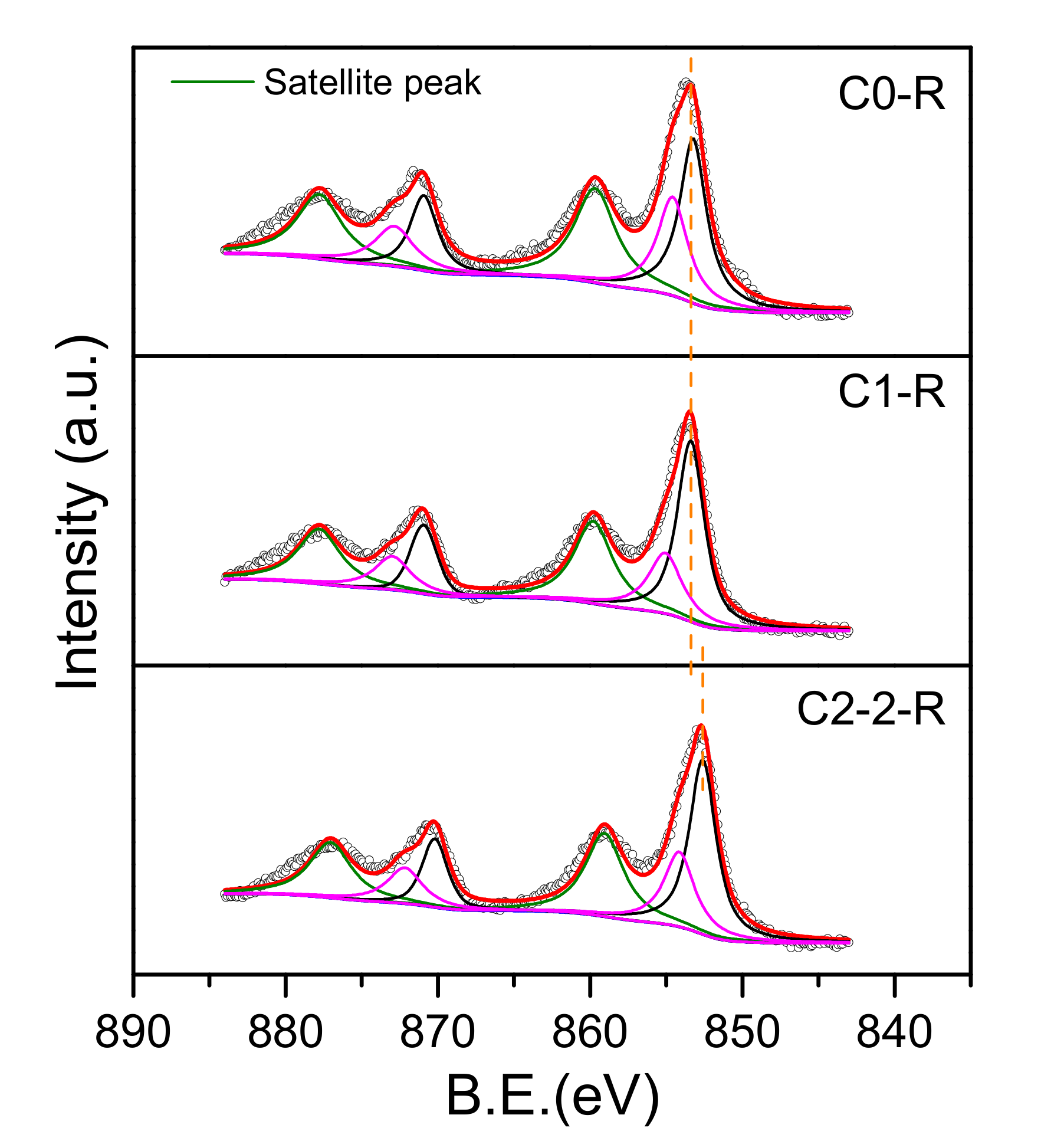
2.1.3. XPS Analysis
2.2. Catalytic Performance
2.3. Computational Results
2.3.1. Gas Phase Clusters
2.3.2. The Adsorption of Metal Clusters on the Alumina Surface and Molecules on the Model Catalysts
3. Conclusions
4. Materials and Methods
4.1. Experimental Details
4.1.1. Reagents
4.1.2. Catalysts Preparation
4.1.3. Catalysts Characterization
4.1.4. Catalysts Evaluation
4.2. Computational Details
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Junlin, Z.; Xuan, X.; Xiaolan, Q.; Dejin, K. Low quality heavy aromatic resources and relevant processing technology to produce fundamental petrochemicals. Chem. Ind. Eng. Prog. 2017, 36, 3665–3673. [Google Scholar]
- Chareonpanich, M.; Boonfueng, T.; Limtrakul, J. Production of aromatic hydrocarbons from Mae-Moh lignite. Fuel Process. Technol. 2002, 79, 171–179. [Google Scholar] [CrossRef]
- Chareonpanich, M.; Zhang, Z.-G.; Nishijima, A.; Tomita, A. Effect of catalysts on yields of monocyclic aromatic hydrocarbons in hydrocracking of coal volatile matter. Fuel 1995, 74, 1636–1640. [Google Scholar] [CrossRef]
- Shin, J.; Oh, Y.; Choi, Y.; Lee, J.; Lee, J.K. Design of selective hydrocracking catalysts for BTX production from diesel-boiling-range polycyclic aromatic hydrocarbons. Appl. Catal. A 2017, 547, 12–21. [Google Scholar] [CrossRef]
- Park, J.-I.; Lee, J.-K.; Miyawaki, J.; Kim, Y.-K.; Yoon, S.-H.; Mochida, I. Hydro-conversion of 1-methyl naphthalene into (alkyl)benzenes over alumina-coated USY zeolite-supported NiMoS catalysts. Fuel 2011, 90, 182–189. [Google Scholar] [CrossRef]
- Upare, D.P.; Park, S.; Kim, M.S.; Kim, J.; Lee, D.; Lee, J.; Chang, H.; Choi, W.; Choi, S.; Jeon, Y.P.; et al. Cobalt promoted Mo/beta zeolite for selective hydrocracking of tetralin and pyrolysis fuel oil into monocyclic aromatic hydrocarbons. J. Ind. Eng. Chem. 2016, 35, 99–107. [Google Scholar] [CrossRef]
- Jin, N.; Wang, G.; Yao, L.; Hu, M.; Gao, J. Synergistic process for FCC light cycle oil efficient conversion to produce high-octane number gasoline. Ind. Eng. Chem. Res. 2016, 55, 5108–5115. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J.; Shin, J.; Lee, S.; Kim, D.; Lee, J.K. Selective hydroconversion of naphthalenes into light alkyl-aromatic hydrocarbons. Appl. Catal. A 2015, 492, 140–150. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.; Shin, J.; Lee, J.K. Selective hydrocracking of tetralin for light aromatic hydrocarbons. Catal. Today 2016, 265, 144–153. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Yun, G.-N.; Lee, Y.-K. Novel Ni2P/zeolite catalysts for naphthalene hydrocracking to BTX. Catal. Commun. 2014, 45, 133–138. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Ring opening of hydrocarbons for diesel and aromatics production: Design of heterogeneous catalytic systems. Fuel 2016, 181, 618–629. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X. Influence of Pt on naphthalene conversion over nickel-based catalysts. Chin. J. Catal. 2003, 24, 253–258. [Google Scholar]
- Santikunaporn, M.; Herrera, J.E.; Jongpatiwut, S.; Resasco, D.E.; Alvarez, W.E.; Sughrue, E.L. Ring opening of decalin and tetralin on HY and Pt/HY zeolite catalysts. J. Catal. 2004, 228, 100–113. [Google Scholar] [CrossRef]
- Corma, A.; González-Alfaro, V.; Orchillés, A.V. Decalin and tetralin as probe molecules for cracking and hydrotreating the light cycle oil. J. Catal. 2001, 200, 34–44. [Google Scholar] [CrossRef]
- Zhao, M.; Wei, X.; Zong, Z. Preparation of a new solid acid and its catalytic performance in di (1-naphthyl) methane hydrocracking. Chin. J. Catal. 2016, 37, 1324–1330. [Google Scholar] [CrossRef]
- McVicker, G.B.; Daage, M.; Touvelle, M.S.; Hudson, C.W.; Klein, D.P.; Baird, W.C.; Cook, B.R.; Chen, J.G.; Hantzer, S.; Vaughan, D.E.W.; et al. Selective ring opening of naphthenic molecules. J. Catal. 2002, 210, 137–148. [Google Scholar] [CrossRef]
- Mi, X.; Yang, S.; He, G.; Luo, G.; Xu, X.; Jin, H. Effects of preparation conditions for Ni/γ-Al2O3 catalyst on saturated hydrogenation of naphthalene. Pet. Technol. 2017, 46(4), 414–421. [Google Scholar]
- Zhu, H.; Zhang, Y.; Qiu, Z.; Cui, H.; Zhao, L. Effects of Reaction Conditions on Saturated Hydrogenation of Naphthalene. Fine Chem. 2009, 26(5): 512–516.
- Chen, Z.; Chen, M.; Li, C.; Xu, Y. Effect of impregnantion-hydrothermal method on the structure and performance of Ni/Al2O3 catalyst. Shandong Chem. Ind. 2018, 47, 24–25. [Google Scholar]
- Mi, X.; He, G.; Guo, X.; Yang, S.; Luo, G.; Xu, X.; Jin, H. Effect of reaction conditions on the hydrogenation of naphthalene to decalin over Ni/γ-Al2O3 catalyst. J. Fuel. Chem. Technol. 2018, 46, 879–885. [Google Scholar]
- Ren, J.; Tang, Z.; Guo, Y.; Wang, D. Ni-M/Al2O3 catalysts performance for selective hydrogenation of heavy reforming aromatics. Chem. React. Eng. Technol. 2016, 32, 211–216. [Google Scholar]
- Park, J.-I.; Ali, S.A.; Alhooshani, K.; Azizi, N.; Miyawaki, J.; Kim, T.; Lee, Y.; Kim, H.-S.; Yoon, S.-H.; Mochida, I. Mild hydrocracking of 1-methyl naphthalene (1-MN) over alumina modified zeolite. J. Ind. Eng. Chem. 2013, 19, 627–632. [Google Scholar] [CrossRef]
- Kirumakki, S.R.; Shpeizer, B.G.; Sagar, G.V.; Chary, K.V.R.; Clearfield, A. Hydrogenation of naphthalene over NiO/SiO2–Al2O3 catalysts: Structure–activity correlation. J. Catal. 2006, 242, 319–331. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Liu, C.; Cui, M. Hydrogenation of naphthalene on HY/MCM-41/γ-Al2O3-supported sulfided Ni-Mo-P catalyst. Chin. J. Catal. 2004, 25, 537–541. [Google Scholar]
- Lylykangas, M.S.; Rautanen, P.A.; Krause, A.O.I. Liquid-phase hydrogenation kinetics of multicomponent aromatic mixtures on Ni/Al2O3. Ind. Eng. Chem. Res. 2002, 41, 5632–5639. [Google Scholar] [CrossRef]
- Rynkowski, J.; Paryjczak, T.; Lenik, M. On the nature of oxidic nickel phases in NiO/γ-Al2O3 catalysts. Appl. Catal. A 1993, 106, 73–82. [Google Scholar] [CrossRef]
- Guimon, C.; Auroux, A.; Romero, E.; Monzon, A. Acetylene hydrogenation over Ni–Si–Al mixed oxides prepared by sol–gel technique. Appl. Catal. A 2003, 251, 199–214. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Jin, X.; Ge, Q.; Li, W. Characterizations and activities of the nano-sized Ni/Al2O3 and Ni/La–Al2O3 catalysts for NH3 decomposition. Appl. Catal. A 2005, 290, 87–96. [Google Scholar] [CrossRef]
- Feng, G.; Ganduglia-Pirovano, M.V.; Huo, C.-F.; Sauer, J. Hydrogen spillover to copper clusters on hydroxylated γ-Al2O3. J. Phys. Chem. C 2018, 122, 18445–18455. [Google Scholar] [CrossRef]
- Hierl, R.; Knözinger, H.; Urbach, H.-P. Surface properties and reduction behavior of calcined CuOAl2O3 and CuO-NiOAl2O3 catalysts. J. Catal. 1981, 69, 475–486. [Google Scholar] [CrossRef]
- Ma, Z.; Jiang, Q.-Z.; Wang, X.; Zhang, W.-G.; Ma, Z.-F. CO2 reforming of dimethyl ether over Ni/γ-Al2O3 catalyst. Catal. Commun. 2012, 17, 49–53. [Google Scholar] [CrossRef]
- Poncelet, G.; Centeno, M.A.; Molina, R. Characterization of reduced α-alumina-supported nickel catalysts by spectroscopic and chemisorption measurements. Appl. Catal. A 2005, 288, 232–242. [Google Scholar] [CrossRef]
- Yan-peng, L.; Da-peng, L.; Chen-guang, L. Pt-K/Al2O3 dehydrogenation catalyst for synthesis of o-phenyl phenol(I): Promotion effect of potassium species. J. China U Petrol. Nat. Sci. 2012, 36, 165–174. [Google Scholar]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Digne, M.; Sautet, P.; Raybaud, P.; Euzen, P.; Toulhoat, H. Use of DFT to achieve a rational understanding of acid–basic properties of γ-alumina surfaces. J. Catal. 2012, 226, 54–68. [Google Scholar] [CrossRef]
- Krokidis, X.; Raybaud, P.; Gobichon, A.E.; Rebours, B.; Euzen, P.; Toulhoat, H. Theoretical study of the dehydration process of boehmite to γ-alumina. J. Phys. Chem. B 2001, 105, 5121–5130. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E.; Först, C.J.; Schimpl, J. Projector augmented wave method: Ab initio molecular dynamics with full wave functions. Bull. Mater. Sci. 2003, 26, 33–41. [Google Scholar] [CrossRef]
- PE, B. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar]





| Catalyst | Conversion (wt.%) | Selectivity (wt.%) | Yield (wt.%) |
|---|---|---|---|
| 10Ni/Al2O3 | 79.6 | 72.6 | 57.8 |
| 10Ni4Cu/Al2O3 | 84.9 | 58.3 | 49.5 |
| 10Ni2Zn/Al2O3 | 86.7 | 75.4 | 65.4 |
| 10Ni4Zn/Al2O3 | 86.1 | 82.0 | 70.6 |
| 10Ni8Zn/Al2O3 | 84.5 | 87.4 | 73.8 |
| Catalyst | Compositions | Content of Ni (wt.%) a | Content of M (wt.%) a | BET Surface Area (m2/g) b | Pore Volume (cm3/g) c |
|---|---|---|---|---|---|
| C0 | 10Ni/Al2O3 | 9.3 | / | 230 | 0.61 |
| C1 | 10Ni4Cu/Al2O3 | 9.5 | 4.1 | 226 | 0.55 |
| C2-1 | 10Ni2Zn/Al2O3 | 9.3 | 1.9 | 225 | 0.56 |
| C2-2 | 10Ni4Zn/Al2O3 | 9.7 | 3.9 | 221 | 0.54 |
| C2-3 | 10Ni8Zn/Al2O3 | 9.7 | 7.8 | 215 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Shi, L.; Feng, G.; Shi, Z.; Sun, C.; Kong, D. Selective Hydrogenation of Naphthalene over γ-Al2O3-Supported NiCu and NiZn Bimetal Catalysts. Catalysts 2020, 10, 1215. https://doi.org/10.3390/catal10101215
Li J, Shi L, Feng G, Shi Z, Sun C, Kong D. Selective Hydrogenation of Naphthalene over γ-Al2O3-Supported NiCu and NiZn Bimetal Catalysts. Catalysts. 2020; 10(10):1215. https://doi.org/10.3390/catal10101215
Chicago/Turabian StyleLi, Jingqiu, Liu Shi, Gang Feng, Zhangping Shi, Chenglin Sun, and Dejin Kong. 2020. "Selective Hydrogenation of Naphthalene over γ-Al2O3-Supported NiCu and NiZn Bimetal Catalysts" Catalysts 10, no. 10: 1215. https://doi.org/10.3390/catal10101215





