Combination of CTec2 and GH5 or GH26 Endo-Mannanases for Effective Lignocellulosic Biomass Degradation
Abstract
1. Introduction
2. Results and Discussion
2.1. Enzyme Specificity Studies
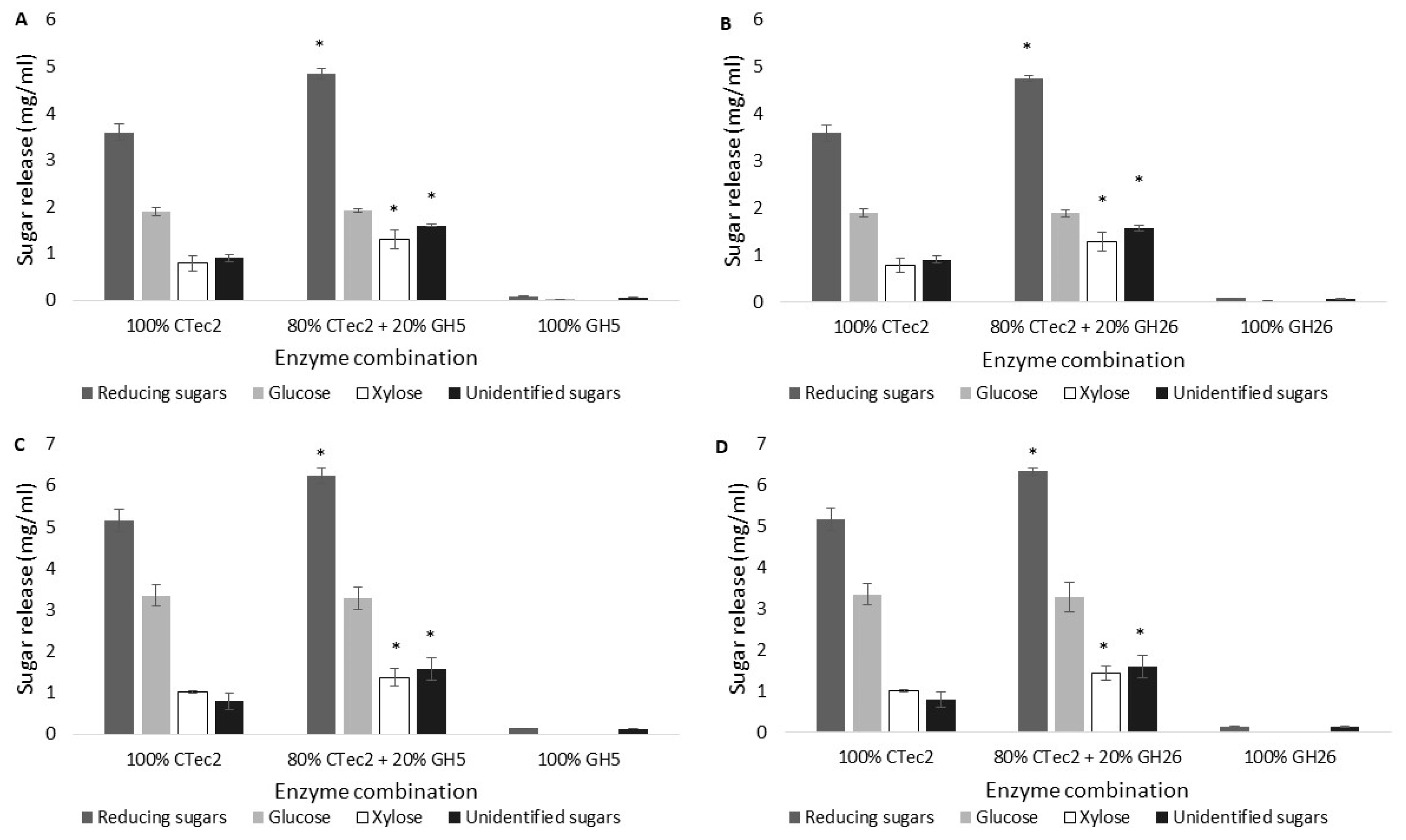
2.2. Enzyme Synergy Studies
2.3. Endo-Mannanase Binding Affinity on Various Substrates
2.4. Endo-Mannanase Thermo-Stability
2.5. Endo-Mannanase Activity on Galactomannan–Cellulose Complexes
3. Materials and Methods
3.1. Materials
3.2. Lignocellulosic Biomass Pre-Treatment and Composition Analysis
3.3. Protein Content
3.4. Enzyme Hydrolysis of Lignocellulosic Biomass
3.5. Analytical Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, J.; Saddler, J.N. Why does GH10 xylanase have better performance than GH11 xylanase for the deconstruction of pretreated biomass? Biomass Bioenergy 2018, 110, 13–16. [Google Scholar] [CrossRef]
- Pletschke, B.I.; Malgas, S.; Bhattacharya, A.; Bhattacharya-Shrivastava, A.; Clarke, M.D.; Mafa, M.S.; Morake, S.; Thoresen, M. Enzyme synergism: A powerful tool for decreasing enzyme loading for efficient biomass conversion. In Proceedings of the 24th European Biomass Conference and Exhibition, Amsterdam, The Netherlands, 6–9 June 2016; Volume 2016. [Google Scholar]
- Malgas, S.; Thoresen, M.; van Dyk, J.S.; Pletschke, B.I. Time dependence of enzyme synergism during the degradation of model and natural lignocellulosic substrates. Enzym. Microb. Technol. 2017, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Malgas, S.; van Dyk, J.S.; Pletschke, B.I. A review of the enzymatic hydrolysis of mannans and synergistic interactions between β-mannanase, β-mannosidase and α-galactosidase. World J. Microbiol. Biotechnol. 2015, 31, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Olver, B.; Dyk, J.S.; Beukes, N.; Pletschke, B.I. Synergy between EngE, XynA and ManA from Clostridium cellulovorans on corn stalk, grass and pineapple pulp substrates. 3 Biotech 2011, 1, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Beukes, N.; Chan, H.; Doi, R.H.; Pletschke, B.I. Synergistic associations between Clostridium cellulovorans enzymes XynA, ManA and EngE against sugarcane bagasse. Enzym. Microb. Technol. 2008, 42, 492–498. [Google Scholar] [CrossRef]
- Beukes, N.; Pletschke, B.I. Effect of alkaline pre-treatment on enzyme synergy for efficient hemicellulose hydrolysis in sugarcane bagasse. Bioresour. Technol. 2011, 102, 5207–5213. [Google Scholar] [CrossRef]
- Ma, L.; Ma, Q.; Cai, R.; Zong, Z.; Du, L.; Guo, G.; Zhang, Y.; Xiao, D. Effect of β-mannanase domain from Trichoderma reesei on its biochemical characters and synergistic hydrolysis of sugarcane bagasse. J. Sci. Food Agric. 2017, 98, 2540–2547. [Google Scholar] [CrossRef]
- Várnai, A.; Huikko, L.; Pere, J.; Siika-aho, M.; Viikari, L. Synergistic action of xylanase and mannanase improves the total hydrolysis of softwood. Bioresour. Technol. 2011, 102, 9096–9104. [Google Scholar] [CrossRef]
- Agrawal, P.; Verma, D.; Daniell, H. Expression of Trichoderma reesei β-mannanase in tobacco chloroplasts and its utilization in lignocellulosic woody biomass hydrolysis. PLoS ONE 2011, 6, e29302. [Google Scholar] [CrossRef]
- Malgas, S.; van Dyk, S.J.; Pletschke, B.I. β-Mannanase (Man26A) and α-galactosidase (Aga27A) synergism—A key factor for the hydrolysis of galactomannan substrates. Enzym. Microb. Technol. 2015, 70, 1–8. [Google Scholar] [CrossRef]
- Von Freiesleben, P.; Spodsberg, N.; Blicher, T.H.; Anderson, L.; Jørgensen, H.; Stålbrand, H.; Meyer, A.S.; Krogh, K.B.R.M. An Aspergillus nidulans GH26 endo-β-mannanase with a novel degradation pattern on highly substituted galactomannans. Enzym. Microb. Technol. 2016, 83, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Tailford, L.E.; Ducros, V.M.-A.; Flint, J.E.; Roberts, S.M.; Morland, C.; Zechel, D.L.; Smith, N.; Bjørnvad, M.E.; Borchert, T.V.; Wilson, K.S.; et al. Understanding how diverse β-mannanases recognize heterogeneous substrates. Biochemistry 2009, 48, 7009–7018. [Google Scholar] [CrossRef] [PubMed]
- Von Freiesleben, P.; Spodsberg, N.; Stenbæk, A.; Stålbrand, H.; Krogh, K.B.R.M.; Meyer, A.S. Boosting of enzymatic softwood saccharification by fungal GH5 and GH26 endomannanases. Biotechnol. Biofuels 2018, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Lin, M.; Zeng, M.; Cai, C.; Pang, Y.; Yang, D.; Qiu, X. Effect of Urea on the Enzymatic Hydrolysis of Lignocellulosic Substrate and Its Mechanism. Bioenergy Res. 2018, 11, 456–465. [Google Scholar] [CrossRef]
- Ko, J.K.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Adsorption of enzyme onto lignins of liquid hot water pretreated hardwoods. Biotechnol. Bioeng. 2015, 112, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Jeoh, T.; Wilson, D.B.; Walker, L.P. Effect of cellulase mole fraction and cellulose recalcitrance on synergism in cellulose hydrolysis and binding. Biotechnol. Prog. 2006, 22, 270–277. [Google Scholar] [CrossRef]
- Liang, D.; Gong, L.; Yao, B.; Xue, X.; Qin, X.; Ma, R.; Luo, H.; Xie, X.; Su, X. Implication of a galactomannan-binding GH2 β-mannosidase in mannan utilization by Caldicellulosiruptor bescii. Biochem. Biophys. Res. Commun. 2015, 467, 334–340. [Google Scholar] [CrossRef]
- Li, R.; Kibblewhite, R.; Orts, W.J.; Lee, C.C. Molecular cloning and characterization of multidomain xylanase from manure library. World J. Microbiol. Biotechnol. 2009, 25, 2071–2078. [Google Scholar] [CrossRef]
- Hägglund, P.; Eriksson, T.; Collén, A.; Nerinckx, W.; Claeyssens, M.; Stålbrand, H. A cellulose-binding module of the Trichoderma reesei β-mannanase Man5A increases the mannan-hydrolysis of complex substrates. J. Biotechnol. 2003, 101, 37–48. [Google Scholar] [CrossRef]
- Hogg, D.; Pell, G.; Dupree, P.; Goubet, F.; Martin-Orue, S.M.; Armand, S.; Gilbert, H.J. The modular architecture of Cellvibrio japonicus mannanases in glycoside hydrolase families 5 and 26 points to differences in their role in mannan degradation. Biochem. J. 2003, 371, 1027–1043. [Google Scholar] [CrossRef]
- Wang, X.; Li, K.; Yang, M.; Wang, J.; Zhang, J. Hydrolyzability of mannan after adsorption on cellulose. Cellulose 2016, 24, 35–47. [Google Scholar] [CrossRef]
- Couturier, M.; Roussel, A.; Rosengren, A.; Leone, P.; Stålbrand, H.; Berrin, J.G. Structural and biochemical analyses of glycoside hydrolase families 5 and 26 β-(1,4)-mannanases from Podospora anserina reveal differences upon manno-oligosaccharide catalysis. J. Biol. Chem. 2013, 288, 14624–14635. [Google Scholar] [CrossRef] [PubMed]
- Malgas, S.; Chandra, R.; Van Dyk, J.S.; Saddler, J.N.; Pletschke, B.I. Formulation of an optimized synergistic enzyme cocktail, HoloMix, for effective degradation of various pre-treated hardwoods. Bioresour. Technol. 2017, 245, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef] [PubMed]
- Normark, M.; Winestrand, S.; Lestander, T.A.; Jönsson, L.J. Analysis, pretreatment and enzymatic saccharification of different fractions of Scots pine. BMC Biotechnol. 2014, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Enzyme | Model Mannan Substrates | Model Cellulosic Substrates | ||||
|---|---|---|---|---|---|---|
| Ivory Nut | Konjak | Locust Bean Gum | CMC-Na | pNPC | pNPG | |
| Cellic® CTec2 | 0.00 | 0.00 | 0.00 | 16.34 | 10.41 | 12.53 |
| GH5 mannanase | 38.21 | 45.58 | 29.26 | 0.00 | 0.00 | 0.00 |
| GH26 mannanase | 61.44 | 60.58 | 77.22 | 0.00 | 0.00 | 0.00 |
| Enzyme | Avicel PH101 | Locust Bean Gum | Pine Apple Pulp | Sugarcane Bagasse |
|---|---|---|---|---|
| GH5 mannanase | 30.40 | 47.98 | 54.87 | 86.50 |
| GH26 mannanase | 32.77 | 58.04 | 37.76 | 53.61 |
| Substrate | Carbohydrates (%) | Lignin (%) | Ash | |||||
|---|---|---|---|---|---|---|---|---|
| Glucan | Xylan | Arabinan | Mannan | Galactan | KL | ASL | ||
| Pineapple pulp | 32.5 | 22.8 | 3.5 | 1.5 | 2.5 | 4.6 | 4.7 | 2.5 |
| Sugarcane bagasse | 38.2 | 27.6 | 3.1 | 3.4 | 1.2 | 16.0 | 6.6 | 4.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malgas, S.; Pletschke, B.I. Combination of CTec2 and GH5 or GH26 Endo-Mannanases for Effective Lignocellulosic Biomass Degradation. Catalysts 2020, 10, 1193. https://doi.org/10.3390/catal10101193
Malgas S, Pletschke BI. Combination of CTec2 and GH5 or GH26 Endo-Mannanases for Effective Lignocellulosic Biomass Degradation. Catalysts. 2020; 10(10):1193. https://doi.org/10.3390/catal10101193
Chicago/Turabian StyleMalgas, Samkelo, and Brett I. Pletschke. 2020. "Combination of CTec2 and GH5 or GH26 Endo-Mannanases for Effective Lignocellulosic Biomass Degradation" Catalysts 10, no. 10: 1193. https://doi.org/10.3390/catal10101193
APA StyleMalgas, S., & Pletschke, B. I. (2020). Combination of CTec2 and GH5 or GH26 Endo-Mannanases for Effective Lignocellulosic Biomass Degradation. Catalysts, 10(10), 1193. https://doi.org/10.3390/catal10101193






