CO2 Methanation of Biogas over 20 wt% Ni-Mg-Al Catalyst: on the Effect of N2, CH4, and O2 on CO2 Conversion Rate
Abstract
:1. Introduction
2. Results and Discussion
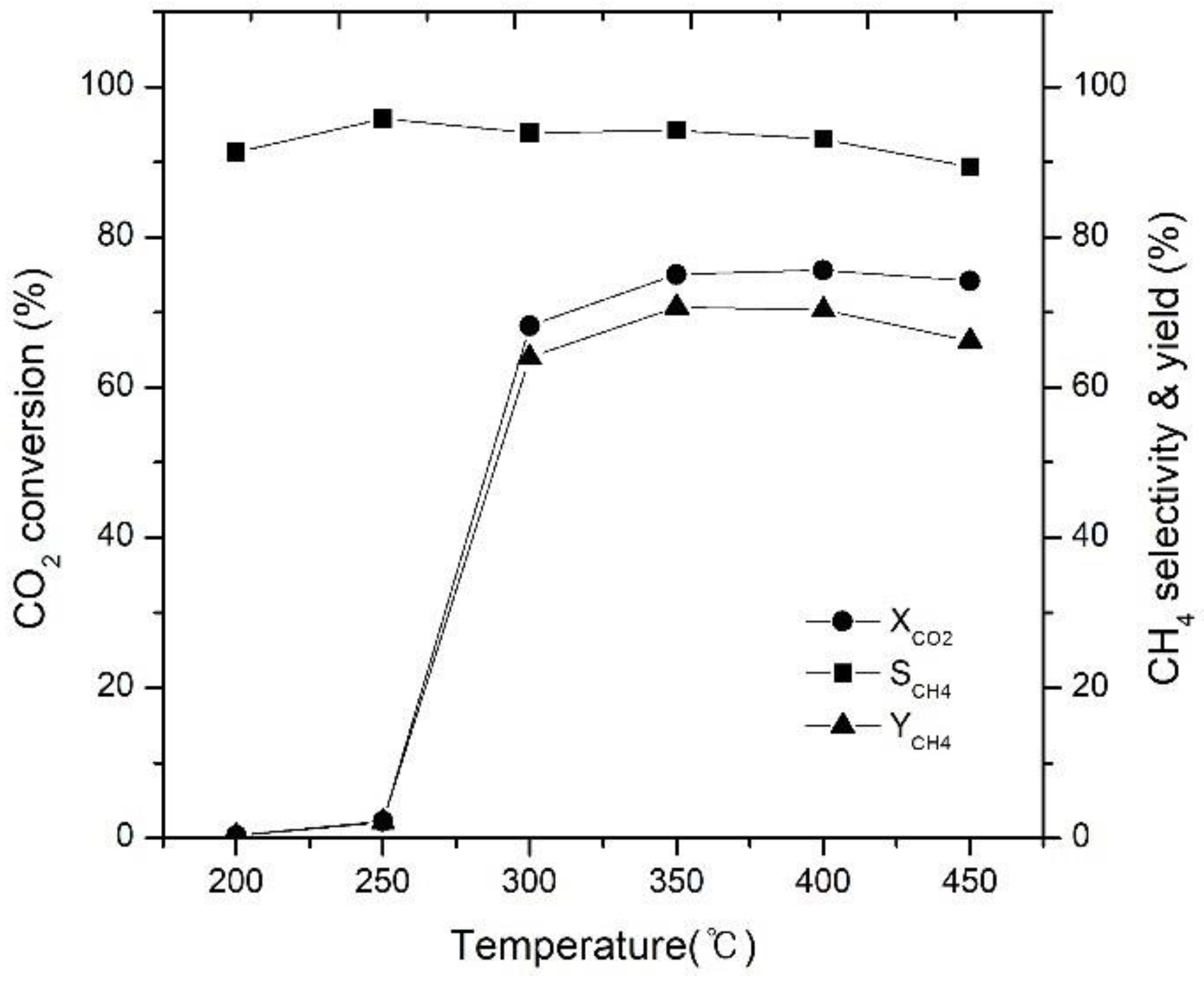
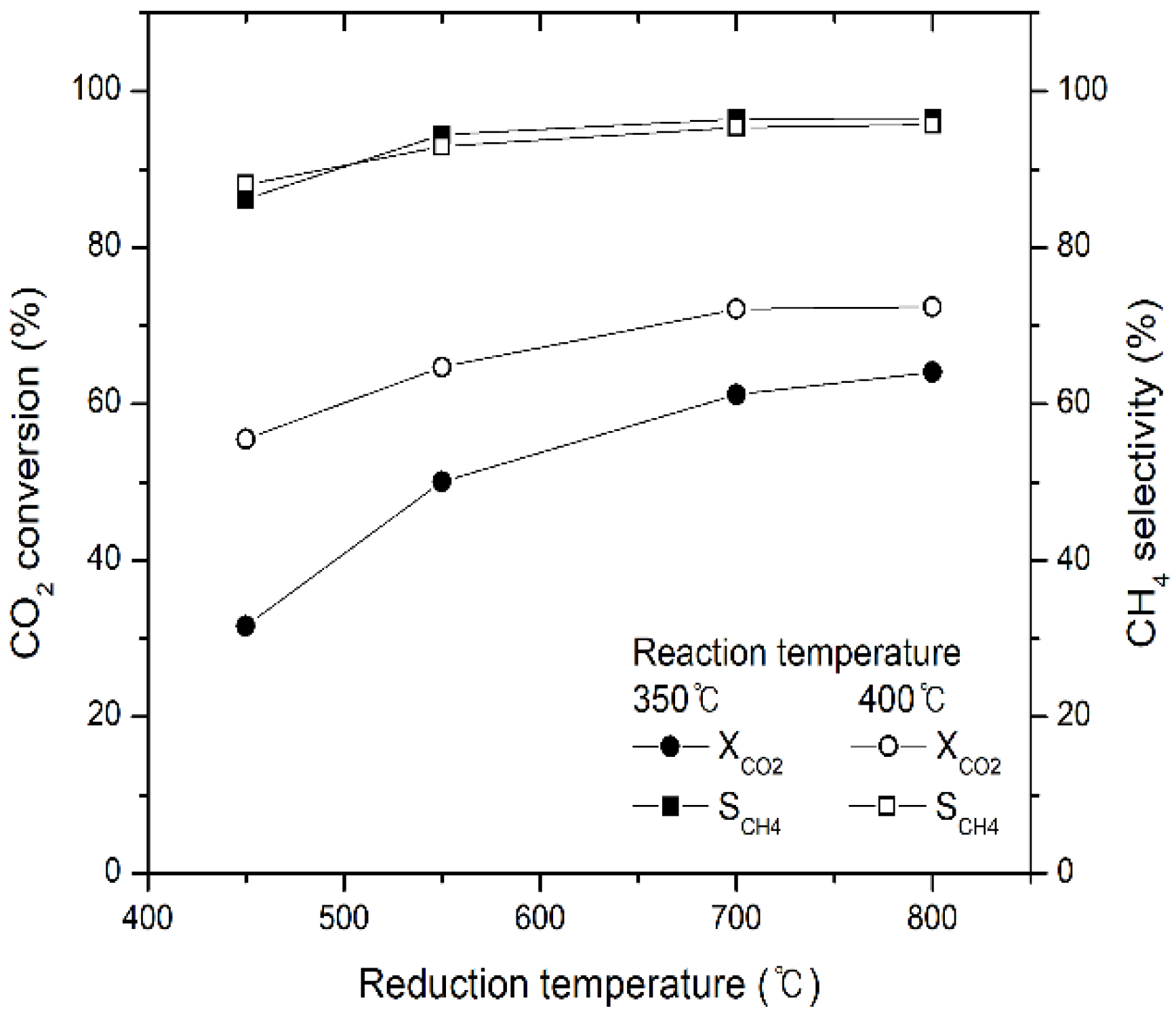
2.1. Effect of Reaction Temperature on CO2 Conversion
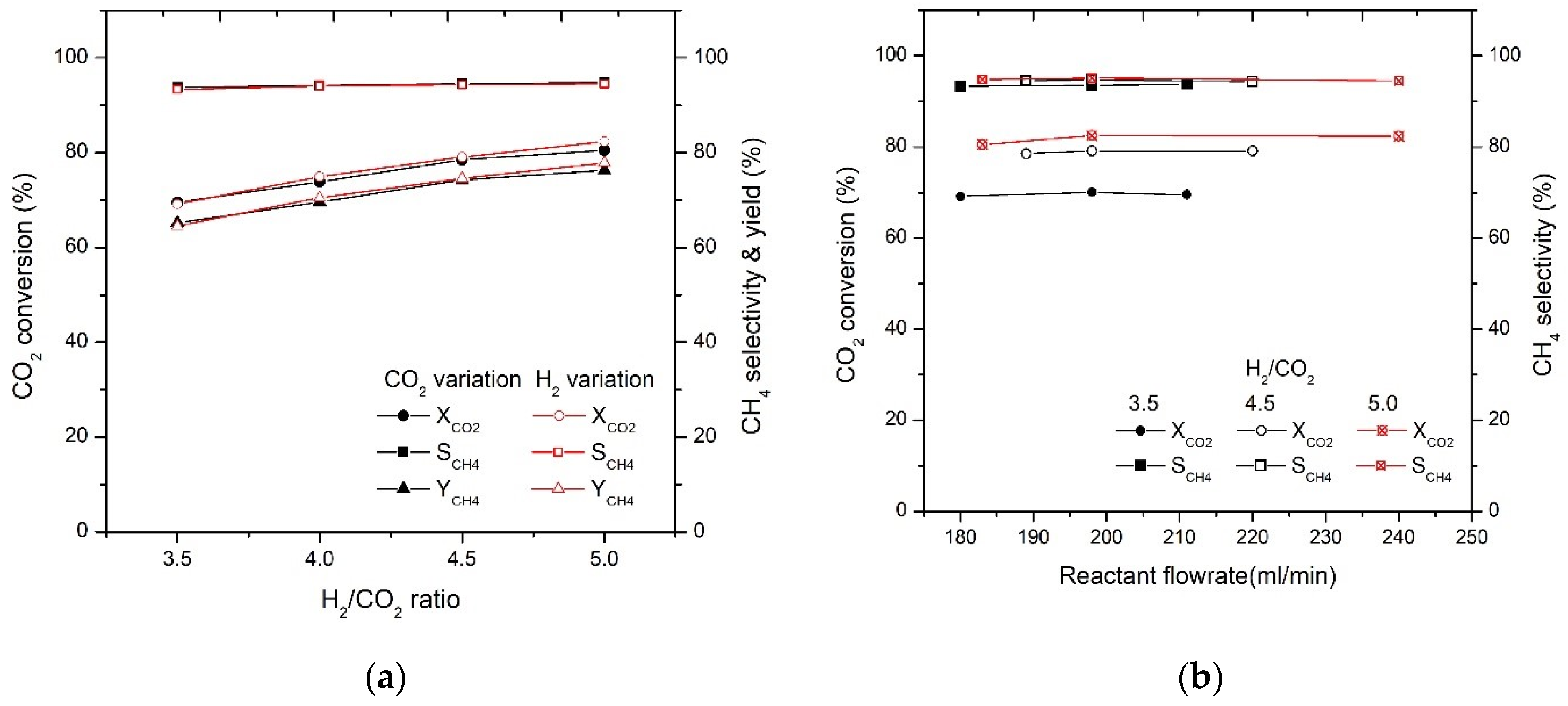
2.2. Effect of H2/CO2 Ratio on CO2 Conversion
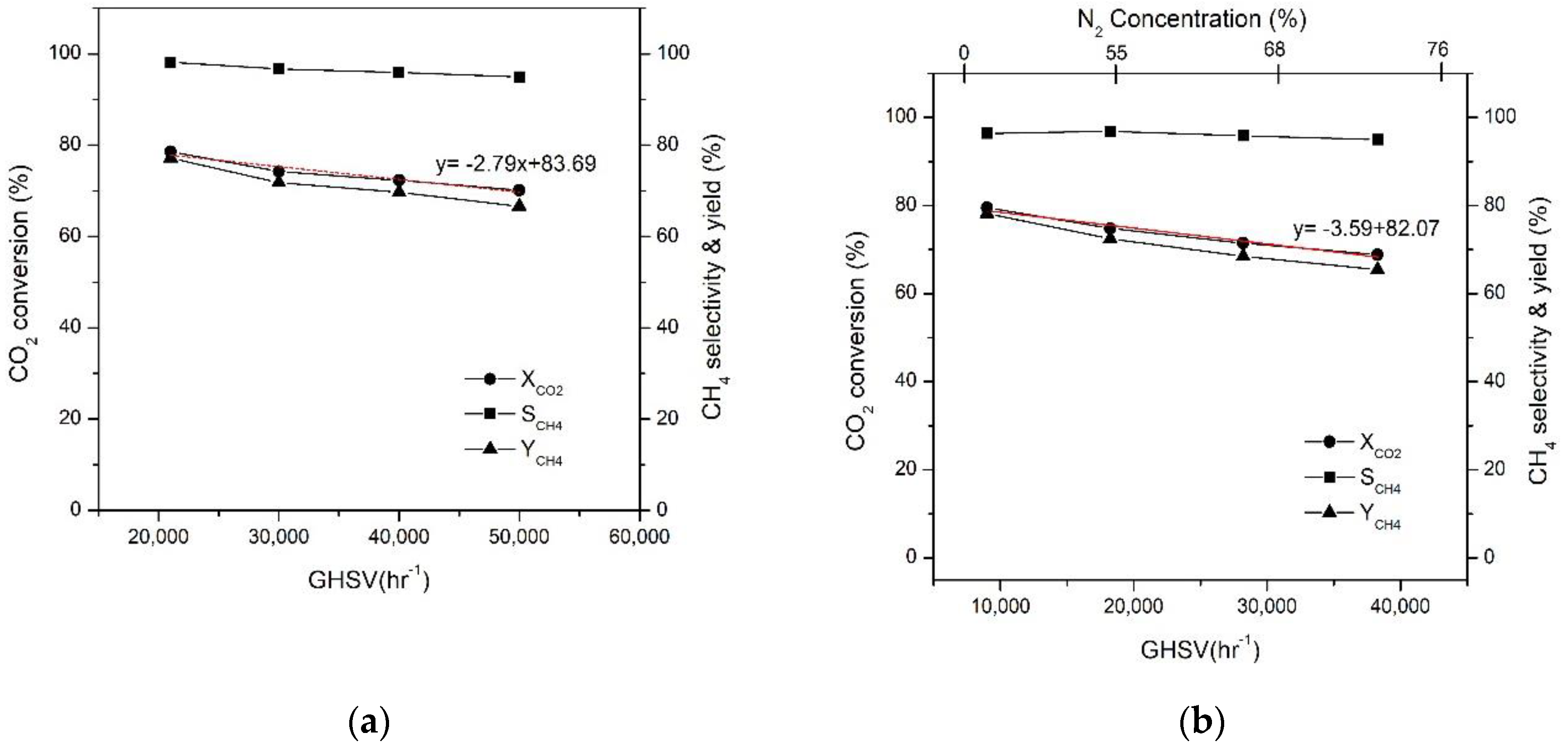
2.3. Effect of GHSV on CO2 Conversion
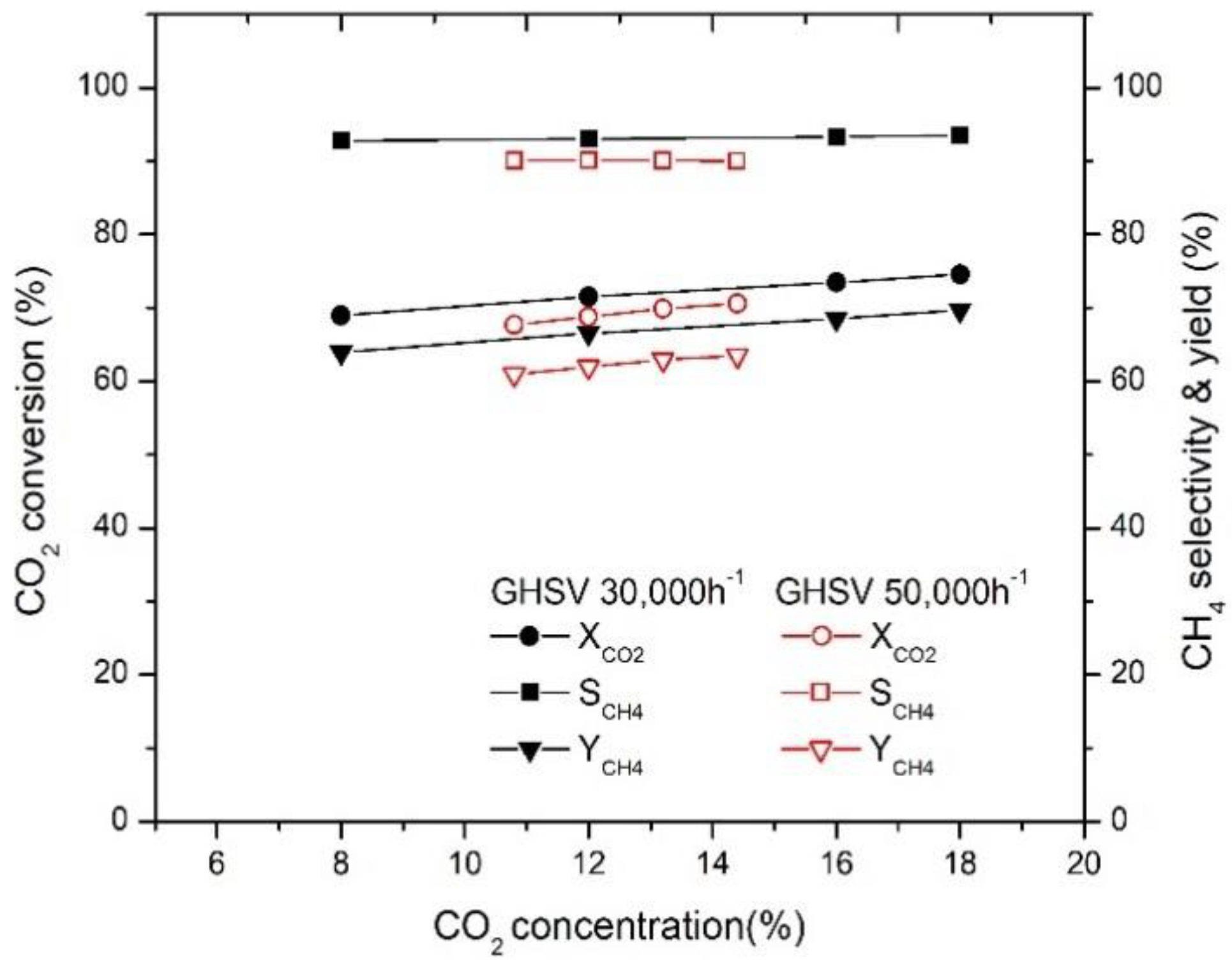
2.4. Effect of Initial Concentration of Biogas Components on CO2 Conversion
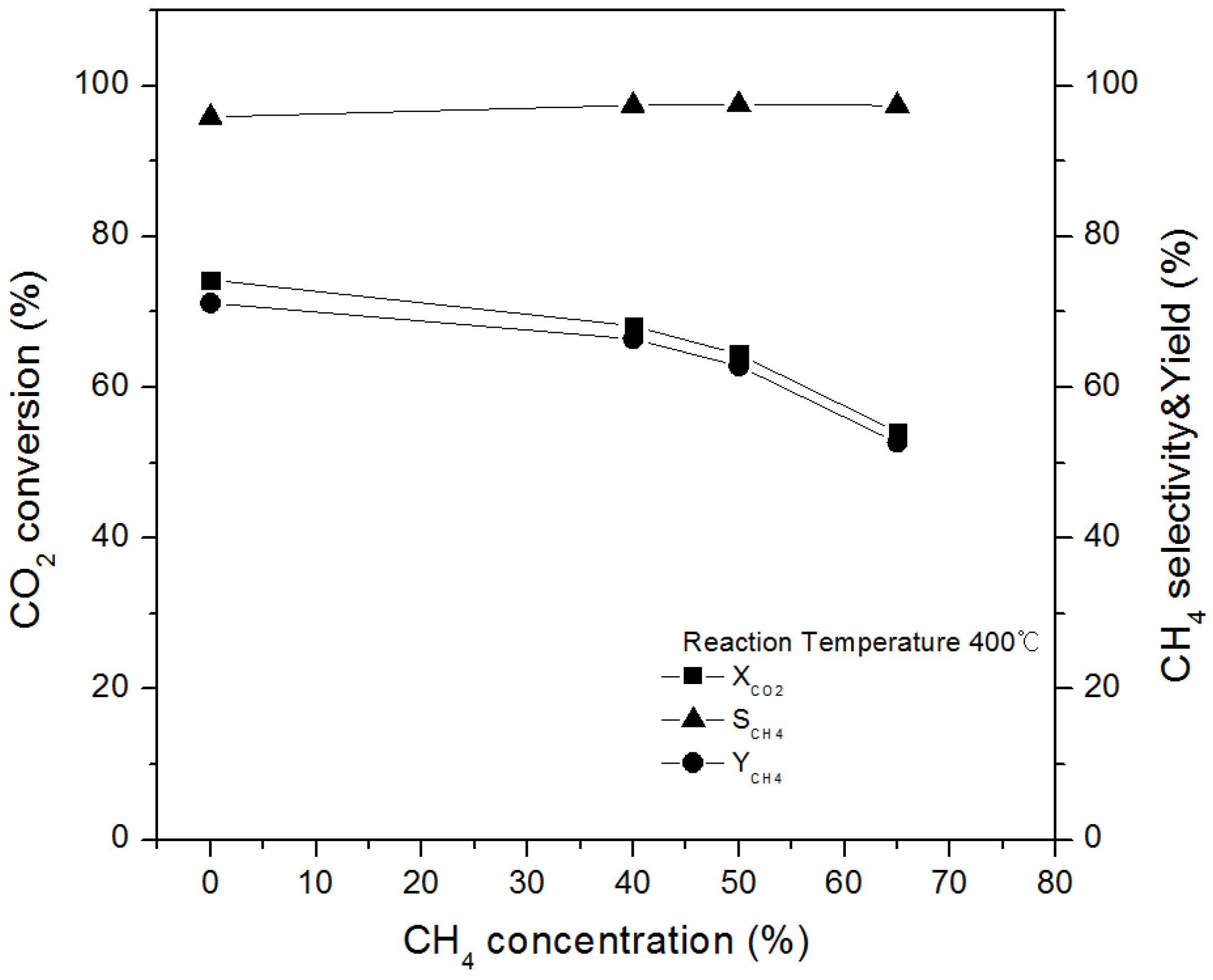
2.4.1. Effect of Initial CH4 Concentration on CO2 Conversion
2.4.2. Effect of Initial CO2 Concentration on CO2 Conversion
2.4.3. Effect of Initial N2 Concentration on CO2 Conversion
2.4.4. Effect of Initial Oxygen Concentration on CO2 Conversion
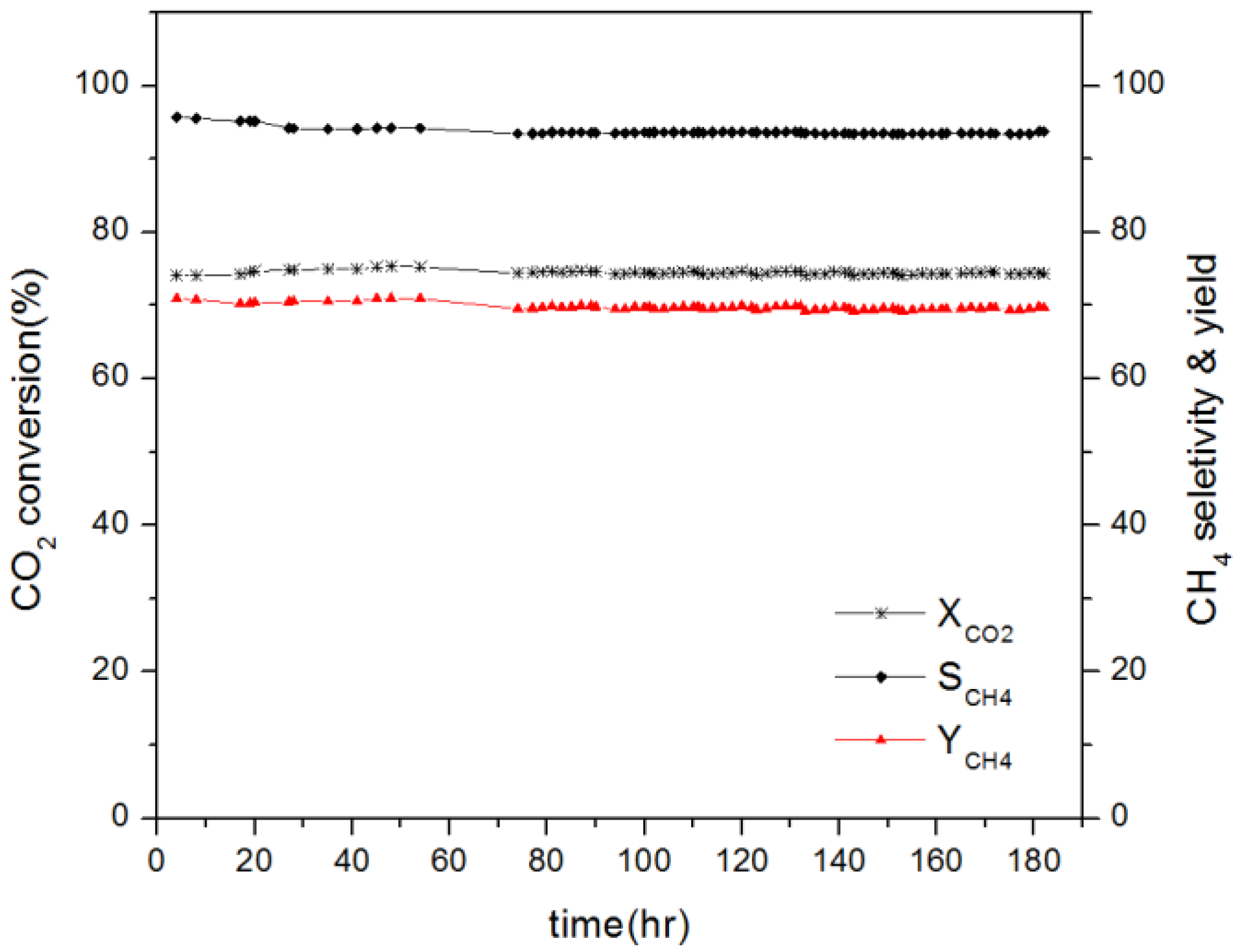
2.5. Stability and Activity of Catalyst Test
3. Materials and Methods
3.1. Catalyst Preparation
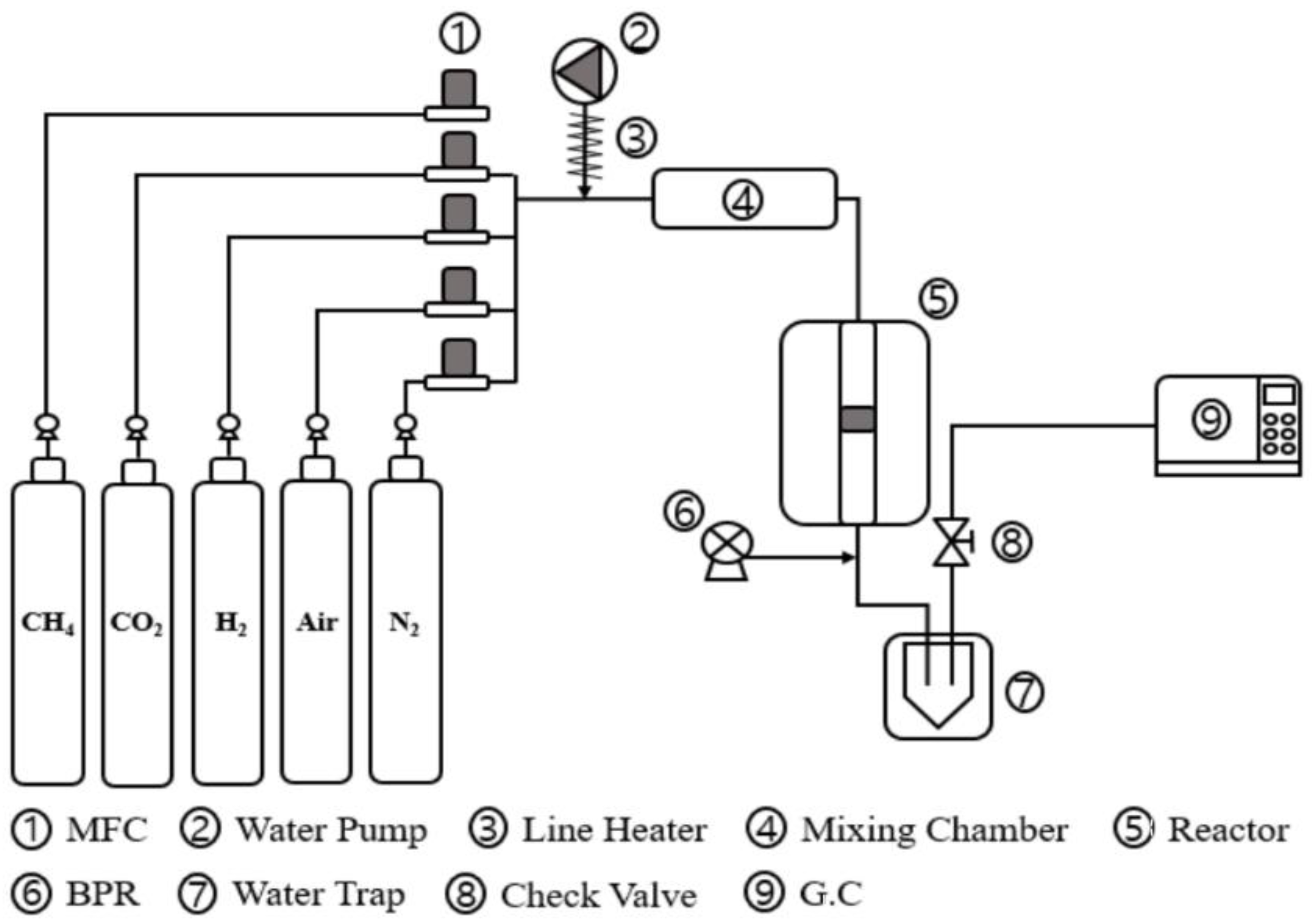
3.2. CO2 Methanation Apparatus and Activity Test
3.3. Catalyst Characterization
3.3.1. Brunauer–Emmett–Teller (BET) Measurement
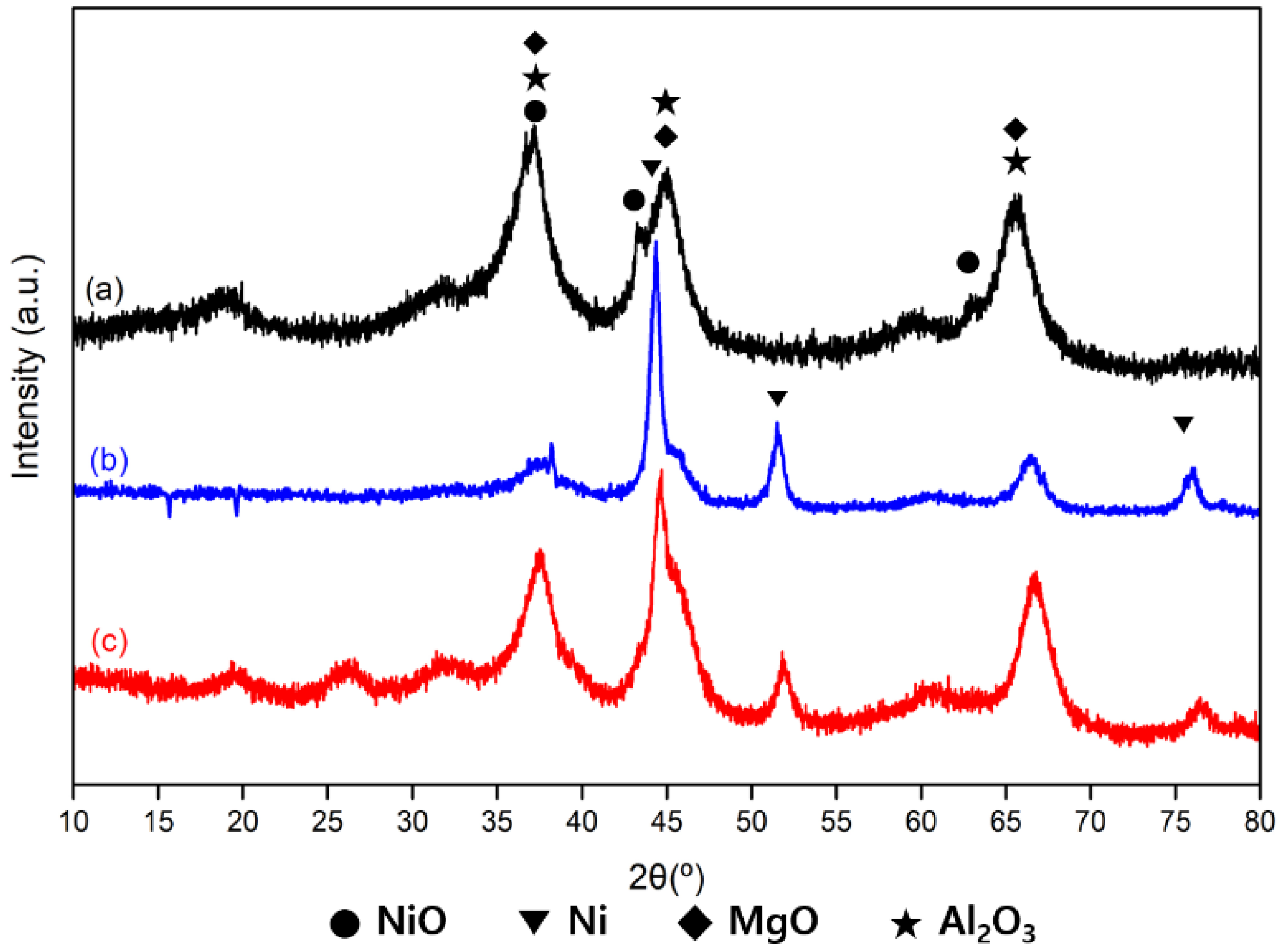
3.3.2. X-ray Diffraction (XRD) Characterization
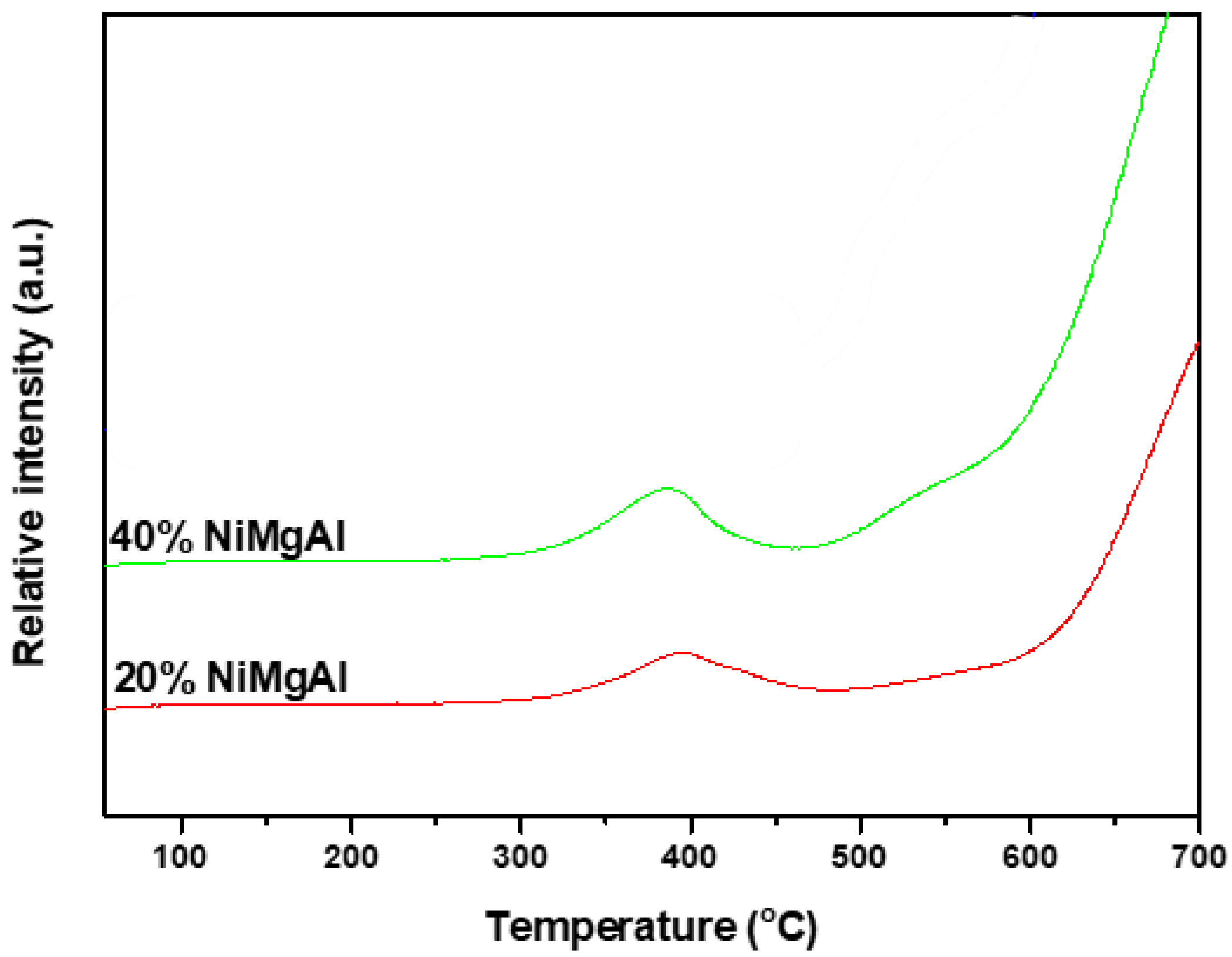
3.3.3. H2-TPR Analysis
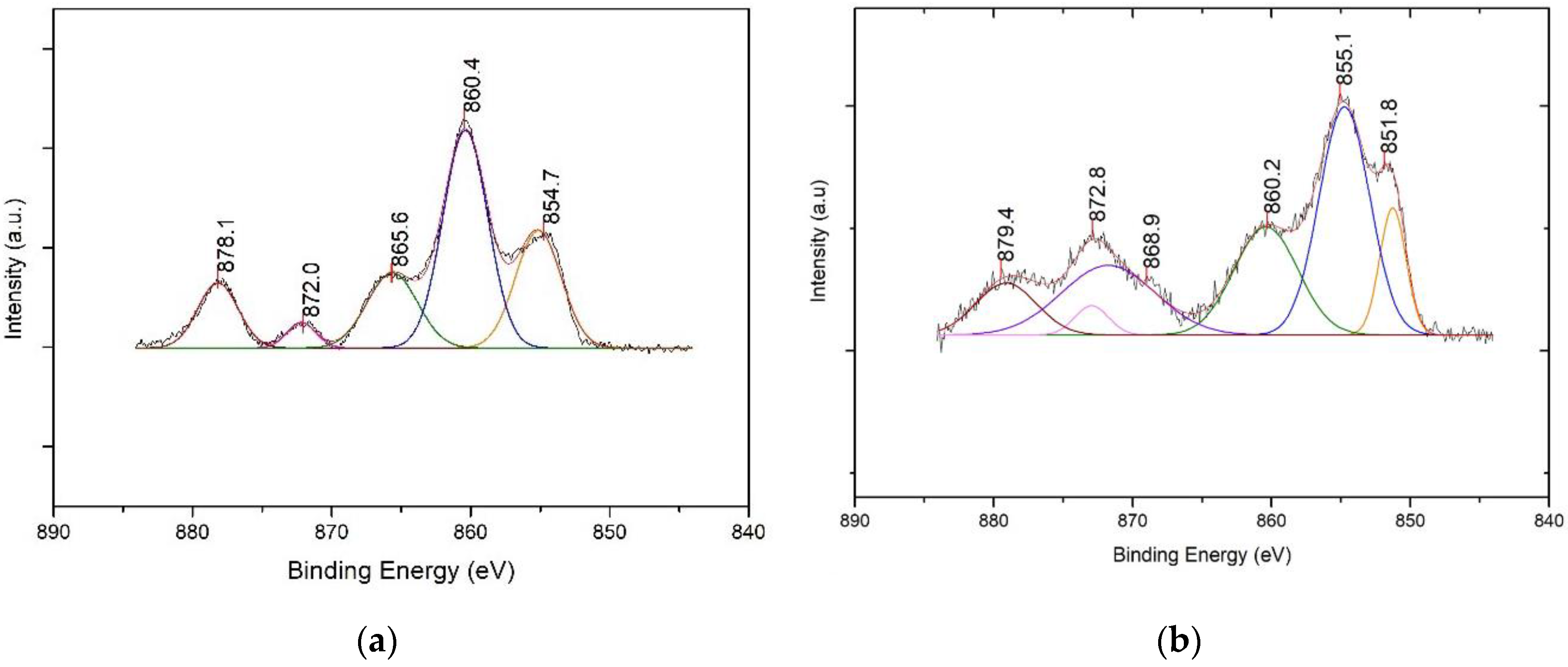
3.3.4. X-ray Photoelectron Spectroscopy (XPS) Characterization
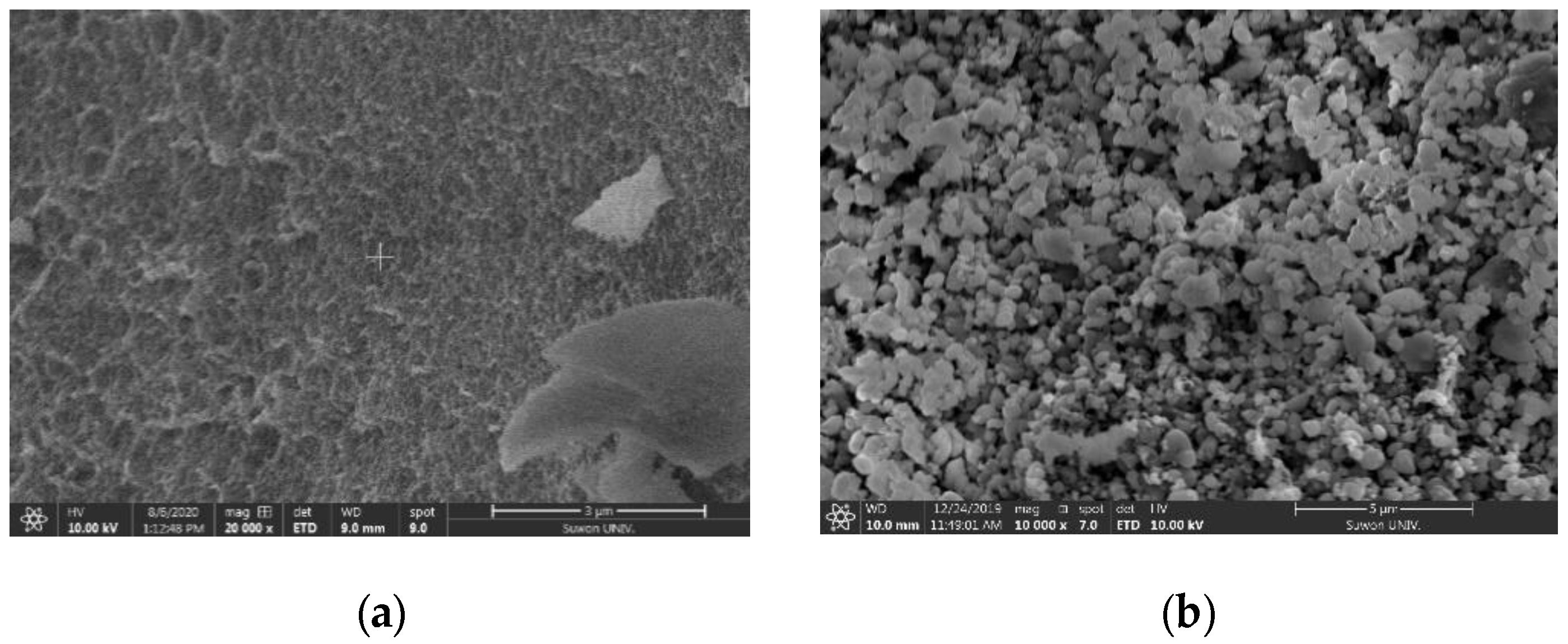
3.3.5. Scanning Electron Microscopy (SEM)–Energy-Dispersive X-ray Spectroscopy (EDX) Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.H. The Role of Hydrogen Energy for Renewable Energy 3020; KDB Research: Seoul, Korea, 2018; Volume 749. [Google Scholar]
- Nam, J.J.; Shin, J.D.; Hong, S.G.; Hahm, H.S.; Park, W.K.; So, K.H. Biomethanol conversion from biogas produced by anaerobic digestion. J. Korea Org. Resour. Recycl. Assoc. 2006, 14, 93–103. [Google Scholar]
- Petersson, A.; Wellinger, A. Biogas Upgrading Technologies–Developments and Innovations; Peterssson, A., Wellinger, A., Eds.; IEA Bioenergy Report: Paris, France, 2009. [Google Scholar]
- Li, W.; Wang, H.; Jiang, Z.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef] [Green Version]
- Shayan, E.; Zare, V.; Mirzaee, I. Hydrogen production from biomass gasification; a theoretical comparison of using different gasification agents. Energy Convers. Manag. 2018, 159, 30–41. [Google Scholar] [CrossRef]
- Sternberg, A.; Jensa, C.M.; Bardow, A. Life cycle assessment of CO2-based C1-chemicals. Green Chem. 2017, 19, 2244–2259. [Google Scholar] [CrossRef]
- Jessica, L.E.; Kovarik, L.; Kenvina, C.K.; Sievers, C. Effect of preparation methods on the performance of Co/Al2O3 catalysts for dry reforming of methane. Green Chem. 2014, 2, 885–896. [Google Scholar]
- Dimitriou, I.; Garcia-Gutierrez, P.; Elder, R.H.; Cueller-Franca, R.M.; Azapagic, A.; Allen, R.W.K. Carbon dioxide utilisation for production of transport fuel: Process and economic analysis. Energy Environ. Sci. 2015, 8, 1775–1789. [Google Scholar] [CrossRef] [Green Version]
- Affar, M.M.; Nahil, M.A.; Williams, P.T. Parametric study of CO2 methanation for synthetic natural gas production. Energy Technol. 2019, 7, 190075. [Google Scholar]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Sidik, S.M. Methanation of carbon dioxide on metal-promoted mesostructured silica nanoparticles. Appl. Catal. A 2014, 486, 115–122. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P.L. Supported Catalysts for CO2 Methanation: A Review. Catalyst 2017, 7, 59. [Google Scholar] [CrossRef]
- Daroughegi, R.; Meshjani, F.; Rezaei, M. Enhanced activity of CO2 methanation over mesoporous nanocrystalline Ni-Al2O3 catalysts prepared by ultrasound-assisted co-precipitation method. Int. J. Hydrogen Energy 2017, 42, 15115–15125. [Google Scholar] [CrossRef]
- Cho, E.H.; Kim, W.H.; Ko, C.H.; Yoon, W.L. Enhanced CO2 methanation reaction in C1 chemistry over a highly dispersed nickel nanocatalyst prepared using the one-step melt-infiltration method. Catalysts 2020, 10, 643. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jali, A.A.; Triwahyono, S.; Saad, M.W.A. CO2 methanation over Ni-promoted mesostructured silica nanoparticles: Influence of Ni loading and water vapor on activity and response surface methodology studies. Chem. Eng. J. 2015, 260, 757–764. [Google Scholar] [CrossRef]
- Vertivel, S.; Stefan, N.; Ralf, Z.; Helmut, P.; Gunther, K. Effect of support and chelating ligand on the synthesis of Ni catalysts with high activity and stability for CO2 methanation. Catalysts 2020, 10, 493. [Google Scholar]
- Wang, L.; Hu, J.; Liu, H.; Wei, Q.; Gong, D.; Mo, L.; Tao, H.; Zhang, C. Three-Dimensional Mesoporous Ni-CeO2 Catalysts with Ni Embedded in the Pore Walls for CO2 Methanation. Catalysts 2020, 10, 523. [Google Scholar] [CrossRef]
- Hu, D.; Gao, J.; Ping, Y.; Jia, L.; Gunawan, P.; Gu, F.; Su, F. Enhanced investigation of CO methanation over Ni/Al2O3 for synthetic natural gas production. Ind. Eng. Chem. Res. 2012, 51, 4875–4886. [Google Scholar] [CrossRef]
- Fan, M.-T.; Miao, K.-P.; Lin, J.-D. Mg-Al oxide supported Ni catalysts with enhanced stability for efficient synthetic natural gas from syngas. Appl. Surf Sci. 2014, 307, 682–688. [Google Scholar] [CrossRef]
- Tan, J.; Wang, J.; Zhang, Z.; Ma, Z.; Wang, L.; Liu, Y. Highly dispersed and stable Ni nanoparticles confined by MgO on ZrO2 for CO2 methanation. Appl. Surf. Sci. 2019, 281, 1538–1548. [Google Scholar] [CrossRef]
- Yan, Y.; Dai, Y.; He, H.; Yu, Y.; Yang, Y. A novel W-doped Ni-Mg mixed oxide catalyst for CO2 methanation. Appl. Catal. 2016, 196, 108–116. [Google Scholar] [CrossRef]
- Lin, J.; Ma, C.; Wang, Q.; Xu, Y.; Ma, G.; Wang, J.; Wang, H.; Dong, C.; Zhang, C.; Ding, M. Enhanced low-temperature performance of CO2 methanation over mesoporous Ni/Al2O3-ZrO2 catalysts. Appl. Catal. B 2019, 243, 262–272. [Google Scholar] [CrossRef]
- Petala, A.; Panagiotoppuluo, P. Methanation of CO2 over alkali-promoted Ru/TiO2 catalysts: I. Effect of alkali additives on catalytic activity and selectivity. Appl. Catal. B 2018, 224, 919–927. [Google Scholar] [CrossRef]
- Guo, M.; Lu, G. The effect of impregnation strategy on structural characters and CO2 methanation properties over MgO modified Ni/SiO2 catalysts. Cat. Commun. 2014, 54, 55–60. [Google Scholar] [CrossRef]
- Thien, A.L.; Jieum, K.; Park, E.D. CO and CO2 methanation over M(=Mn,Ce,Zr,Mg,K,Zn, or V)-promoted Ni/Al@Al2O3 catalysts. Catal. Today 2020, 348, 80–88. [Google Scholar]
- Bette, N.; Thielemann, J.; Schreiner, M.; Mertens, F. Methanation of CO2 over a (Mg, Al)Ox supported nickel catalyst derived from a Ni, Mg, Al-hydrotalcite-like precursor. ChemCatChem 2016, 8, 2903. [Google Scholar] [CrossRef]
- Duyar, M.S.; Wang, S.; Arellano-Trevirio, M.A.; Farrauto, R.J. CO2 utilization with a novel dual function material (DFM) for capture and catalytic conversion to synthetic natural gas: An update. J. CO2 Util. 2016, 15, 65–71. [Google Scholar] [CrossRef]
- Fisher, J.C.; Siriwardane, R.V. Mg(OH)2 for CO2 capture from high-pressure, moderate-temperature gas streams. Energy Fuels 2014, 28, 5936–5941. [Google Scholar] [CrossRef]
- Loder, A.; Siebenhofer, M.; Lux, S. The reaction kinetics of CO2 methanation on a bifunctional Ni/MgO catalyst. J. Ind. Eng. Chem. 2020, 85, 196–207. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Chang, J.L. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl. Catal. B 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Carbarino, G.; Riani, P.; Magistri, L.; Busca, G. A study of the methanation of carbon dioxide on Ni/Al2O3 catalysts at atmospheric pressure. Int. J. Hydrogen Energy 2014, 39, 11557–11565. [Google Scholar] [CrossRef]
- Marocco, P.; Morosanu, E.A.; Giglio, E.; Ferrero, D.; Mebrahtu, C.; Lanzini, A.; Abate, S.; Bensaid, S.; Perathoner, S.; Santarelli, M.; et al. CO2 methanation Ni/Al hydrotalcite-derived catalyst: Experimental characterization and kinetic study. Fuel 2018, 225, 230–242. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Rahmani, S.; Rezaei, M.; Meshkani, F. Preparation of highly active nickel catalysts supported on mesoporous nanocrystalline Al2O3 for CO2 methanation. J. Ind. Eng. Chem. 2014, 20, 1346–1352. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Mukti, R.R.; Taufiq-Yap, Y.H.; Sazegar, M.R. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B 2014, 147, 359–368. [Google Scholar] [CrossRef]
- Abate, S.; Barbera, K.; Giglio, E.; Deorsola, F.; Bensaid, S.; Perathoner, S.; Pirone, R.; Centi, G. Synthesis, Characterization, and activity pattern of Ni-Al Hydrotalcite catalysts in CO2 methanation. Ind. Eng. Chem. Res. 2016, 55, 8299–8308. [Google Scholar] [CrossRef]
- Jürgensen, L.; Ehimen, E.A.; Born, J.; Holm-Nielsen, J.B. Dynamic biogas upgrading based on the Sabatier process: Thermodynamic and dynamic process simulation. Bioresour. Technol. 2015, 178, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Muroyama, H.; Tsuda, Y.; Asakoshi, T.; Masitah, H.; Okanishi, T.; Matsui, T.; Eguchi, K. Carbon dioxide methanation over Ni catalysts supported on various metal oxides. J. Catal. 2016, 343, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Zhao, A.; Ying, W.; Zhang, H.; Ma, H.; Fang, D. Ni–Al2O3 catalysts prepared by solution combustion method for syngas methanation. Catal. Comm. 2012, 17, 34–38. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperature. Int. J. Hydrogen Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Song, F.; Zhong, Q.; Yu, Y.; Shi, M.; Wu, Y.; Hu, J.; Song, Y. Obtaining well-dispersed Ni/Al2O3 catalyst for CO2 methanation with a microwave-assisted method. Int. J. Hydrogen Energy 2017, 42, 4174–4183. [Google Scholar] [CrossRef]
- Pradita, T.; Shih, S.J.; Aji, B.B.; Sudibyo. Synthesis of MgO powder from magnesium nitrate using spray pyrolysis. AIP Conf. Proc. 2017, 1823, 020016. [Google Scholar]


| Items | Condition | |
|---|---|---|
| Variable | Reaction Temperature (°C) | 200–450 |
| CH4 and CO2 composition ratio (%) | 65:35, 50:50, 40:60 | |
| GHSV (h−1) | 21,000–50,000 | |
| H2/CO2 mole ratio | 3.5, 4, 4.5, 5 | |
| O2 (cc/min) | 10.5 (4%) | |
| Catalyst | BET(m2/g) | Total Pore Volume (m3/g) | Pore size(Å) | |
|---|---|---|---|---|
| 20 wt% Ni-Mg-Al | Fresh | 180.3 | 0.36 | 81.5 |
| spent | 148.9 | 0.30 | 81.1 | |
| 40 wt% Ni-Mg-Al | Fresh | 152.6 | 0.31 | 82.1 |
| spent | 107.8 | 0.32 | 82.4 |
| Catalyst | Ni Loading (wt%) | Ni Dispersion(%) a | Ni Particle Size (nm) | TOF(s−1) b |
|---|---|---|---|---|
| 20wt% Ni-Mg-Al | 20 | 3.57 | 28.3 | 0.36 |
| 40wt% Ni-Mg-Al | 40 | 3.68 | 27.5 | 0.17 |
| Catalyst | Reaction Temperature (°C) | CO2 Conversion (XCO2) | CH4 Selectivity (SCH4) |
|---|---|---|---|
| 20 wt% Ni-Mg-Al | 350 | 75.0 | 94.2 |
| 400 | 75.6 | 93.0 | |
| 40 wt% Ni-Mg-Al | 350 | 61.2 | 96.4 |
| 400 | 72.2 | 95.3 |
| Element | C | O | Ni | Mg | Al |
|---|---|---|---|---|---|
| Weight (%) | 4.82 | 36.99 | 16.80 | 2.59 | 38.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Kim, Y.; Byun, H.; Cho, W.; Baek, Y. CO2 Methanation of Biogas over 20 wt% Ni-Mg-Al Catalyst: on the Effect of N2, CH4, and O2 on CO2 Conversion Rate. Catalysts 2020, 10, 1201. https://doi.org/10.3390/catal10101201
Han D, Kim Y, Byun H, Cho W, Baek Y. CO2 Methanation of Biogas over 20 wt% Ni-Mg-Al Catalyst: on the Effect of N2, CH4, and O2 on CO2 Conversion Rate. Catalysts. 2020; 10(10):1201. https://doi.org/10.3390/catal10101201
Chicago/Turabian StyleHan, Danbee, Yunji Kim, Hyunseung Byun, Wonjun Cho, and Youngsoon Baek. 2020. "CO2 Methanation of Biogas over 20 wt% Ni-Mg-Al Catalyst: on the Effect of N2, CH4, and O2 on CO2 Conversion Rate" Catalysts 10, no. 10: 1201. https://doi.org/10.3390/catal10101201




