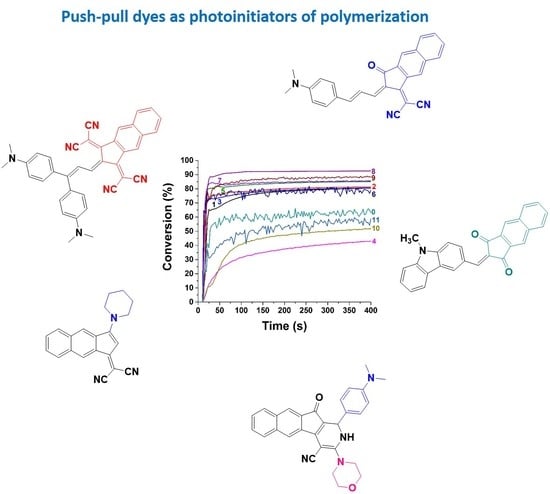
Novel Push–Pull Dyes Derived from 1H-cyclopenta[b]naphthalene-1,3(2H)-dione as Versatile Photoinitiators for Photopolymerization and Their Related Applications: 3D Printing and Fabrication of Photocomposites
Abstract
:1. Introduction
2. Results
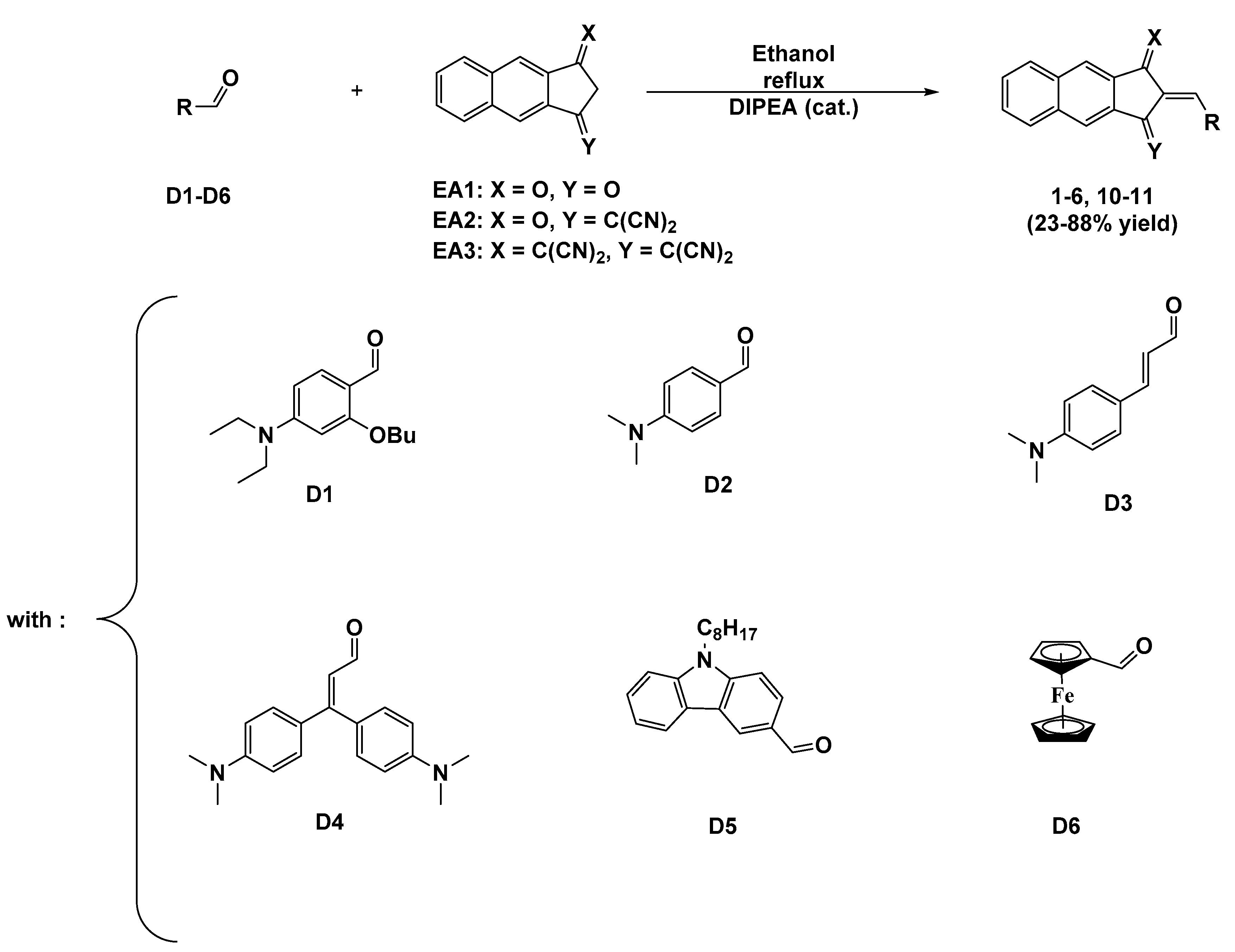
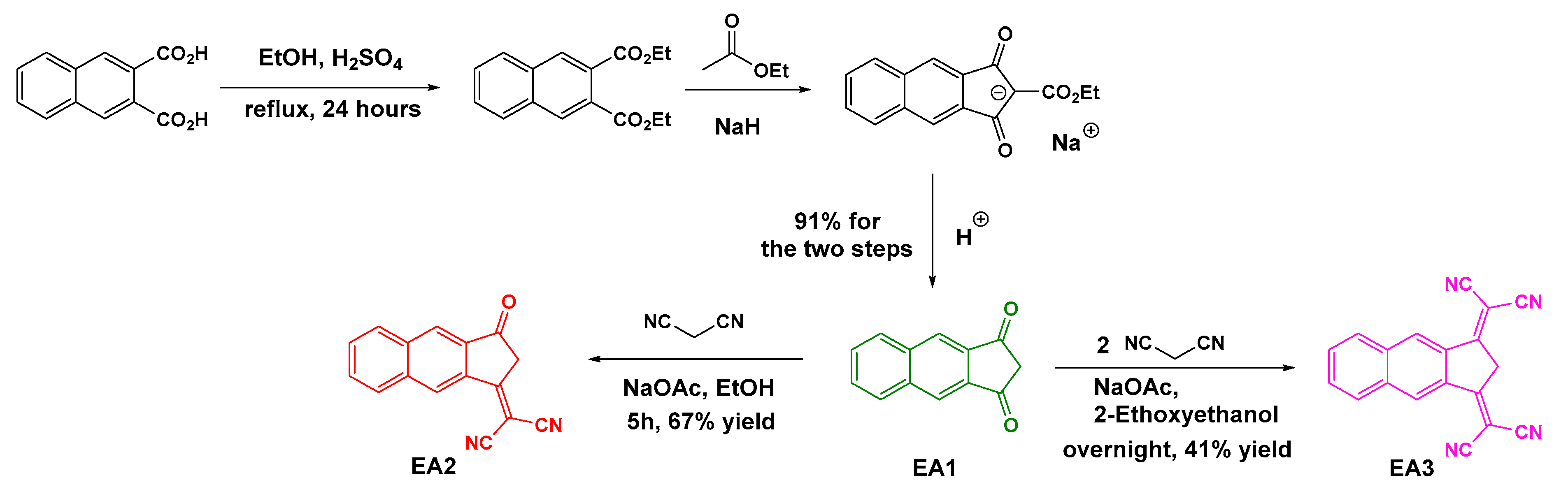
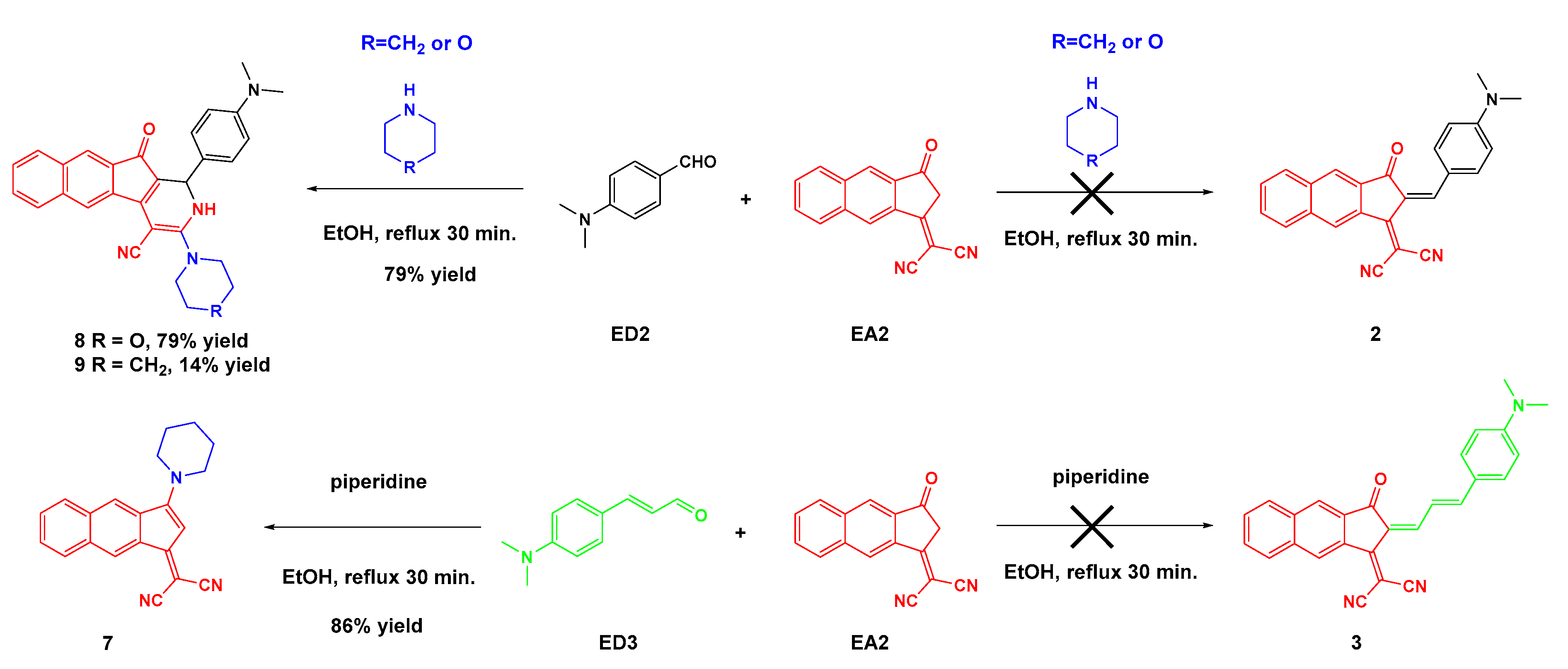
2.1. Synthesis of the Different Dyes
2.2. Photopolymerization Kinetics for Newly Push–Pull Dyes in Three-Component Photoinitiating Systems
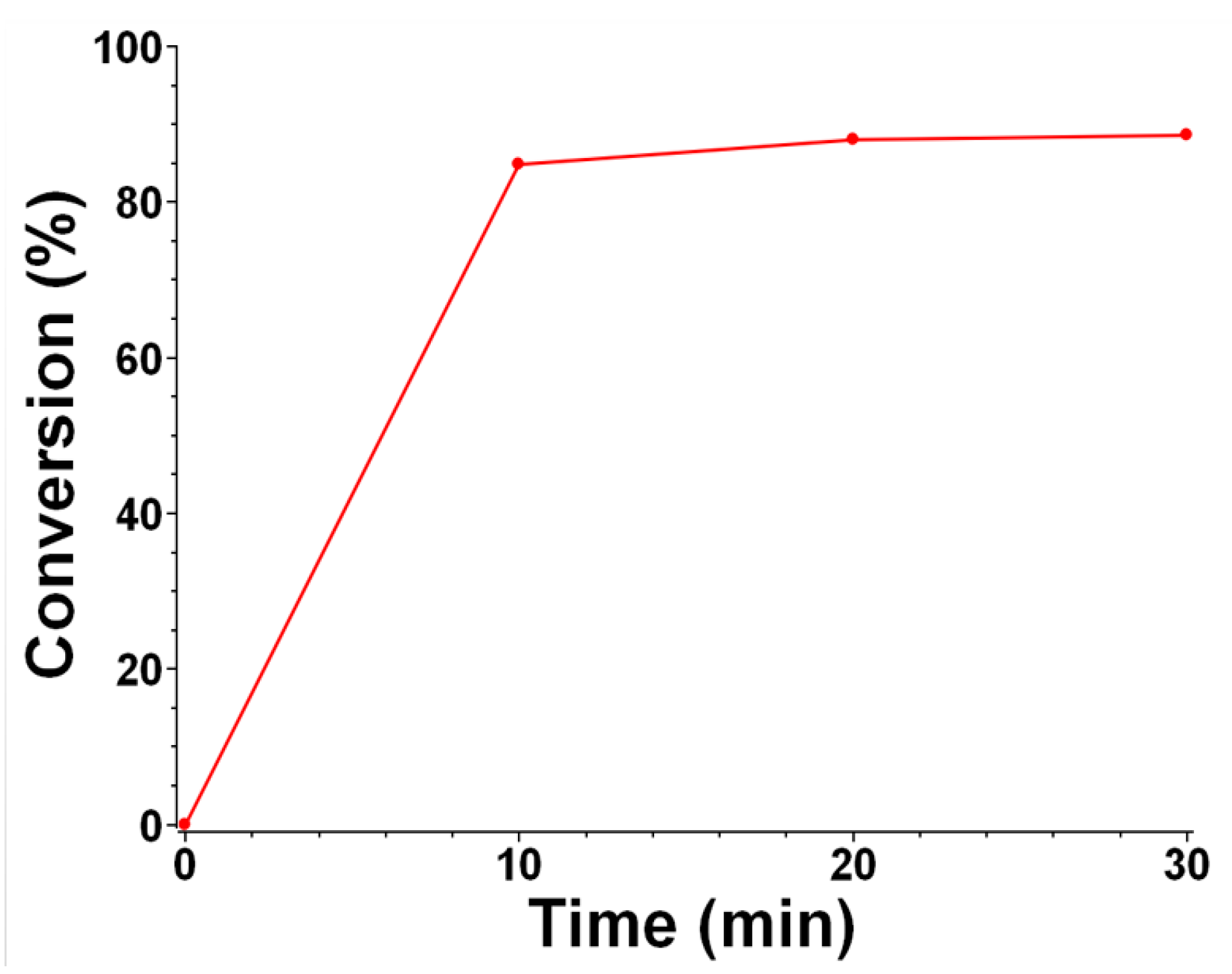
2.3. Photopolymerization Kinetics upon Exposure to Sunlight for Push–Pull Dye 8
3. Discussion
3.1. Light Absorption Properties of the Push–Pull Dyes
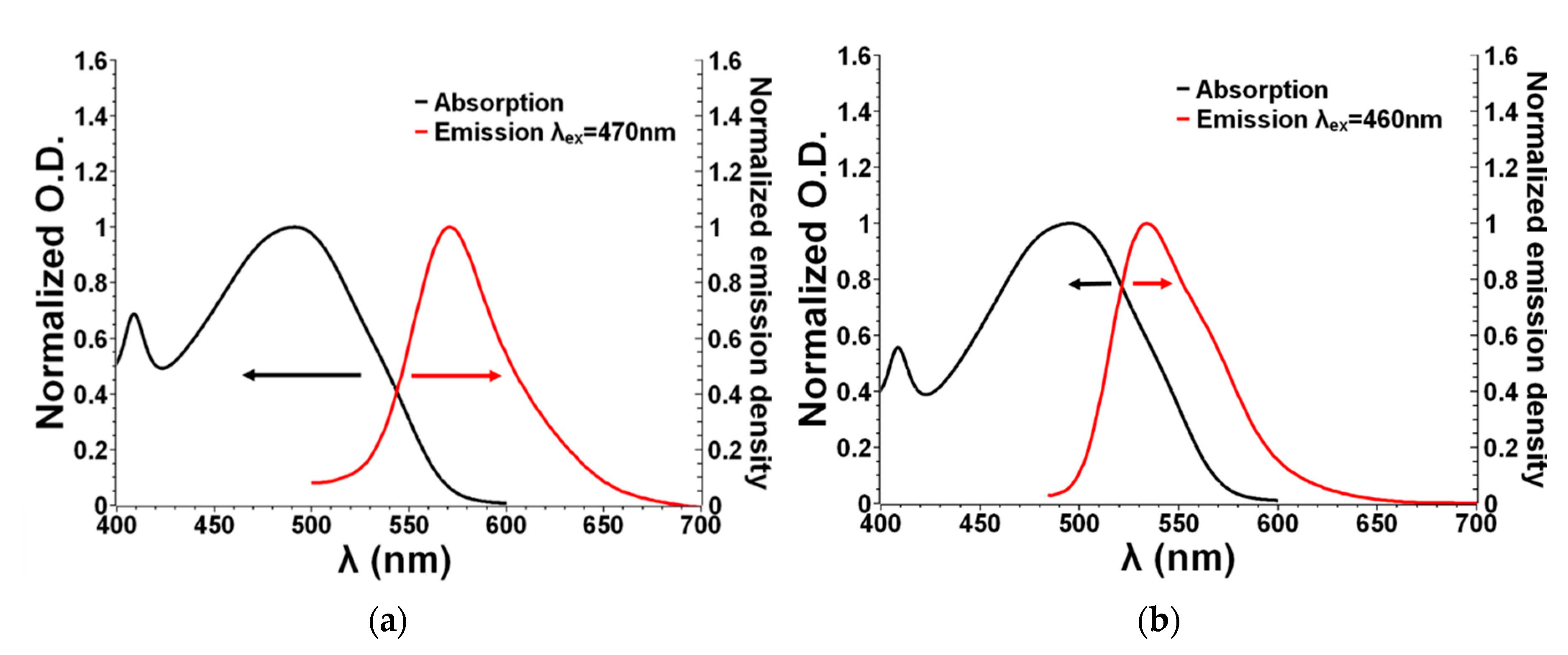
3.2. Optical Properties of Dyes 8 and 9
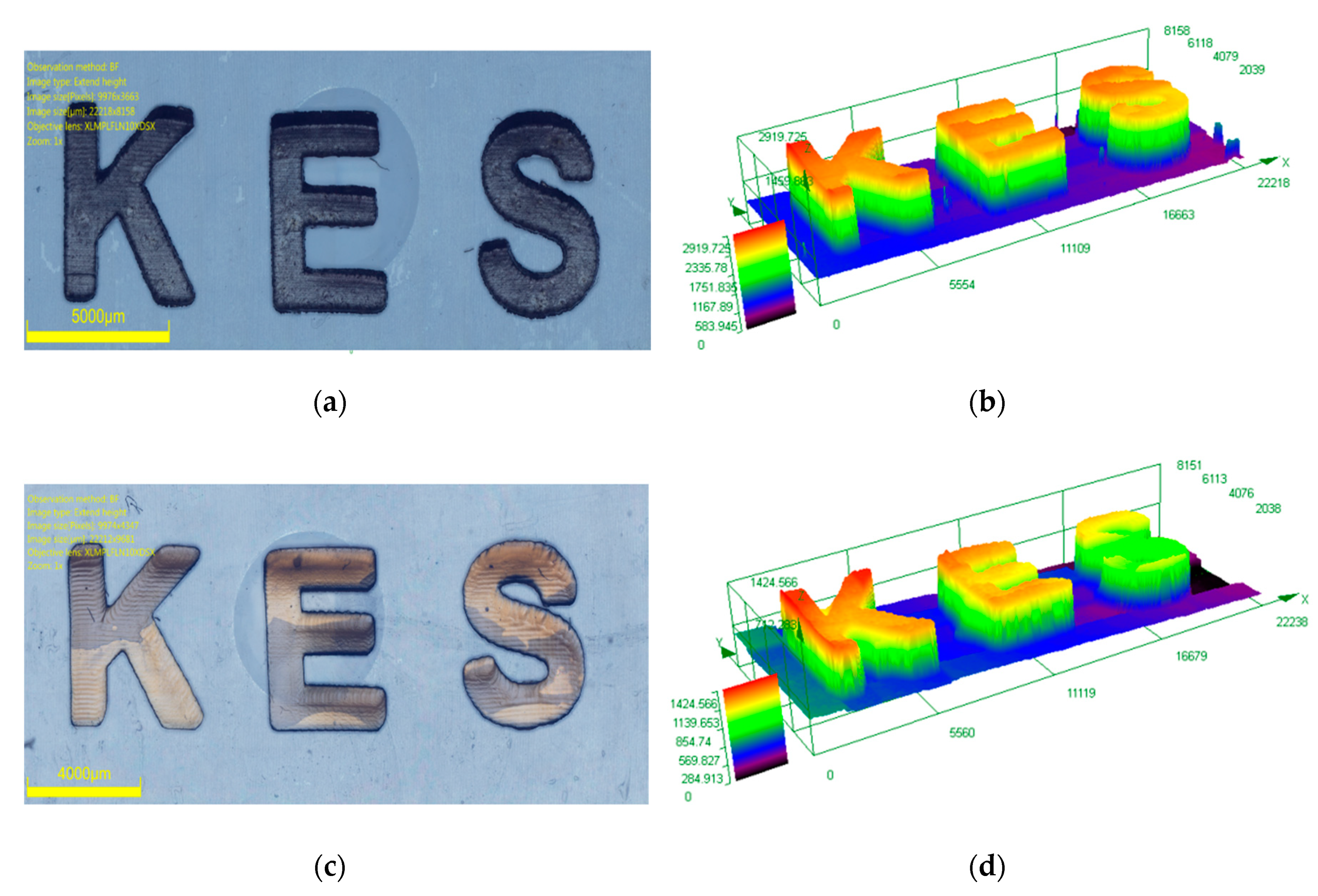
3.3. Laser Writing Experiments with Resins Based on Three-Component Systems and Comprising of Dye 8 or Dye 9 as Photosensitizers
3.4. Potential Chemical Mechanisms of Push–Pull Dyes in Photolysis Reactions
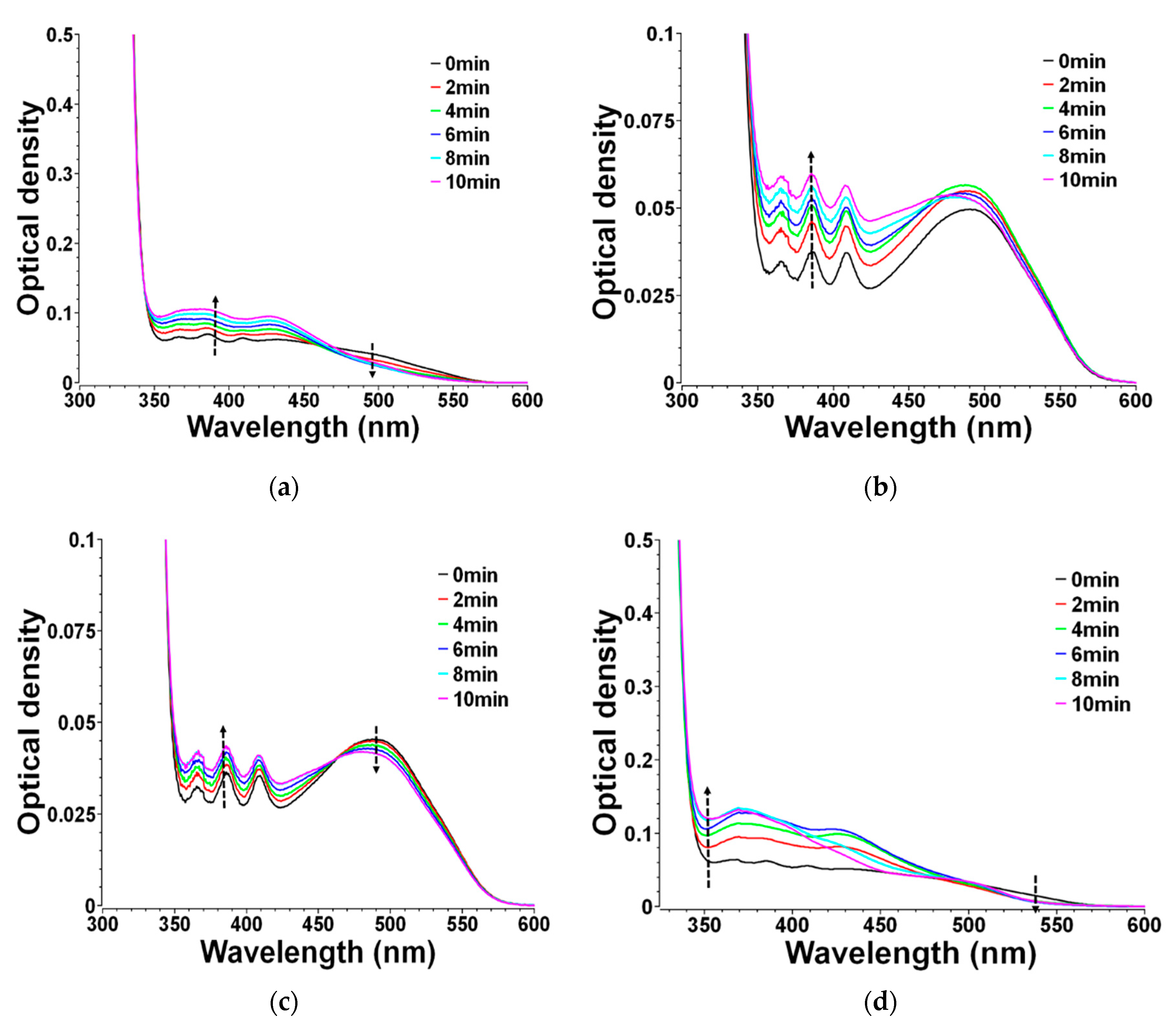
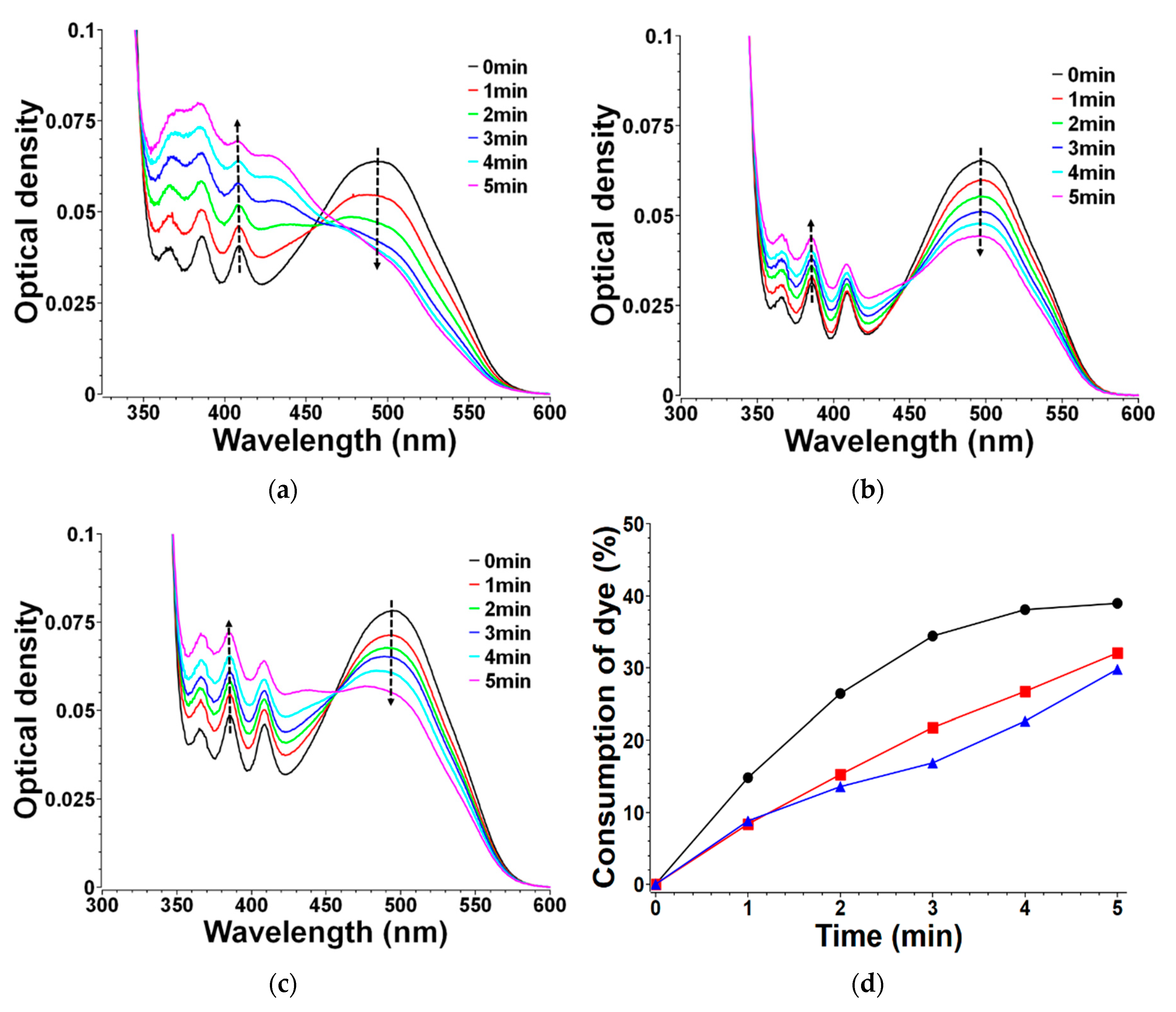
3.5. Mechanistic Investigations in Solutions
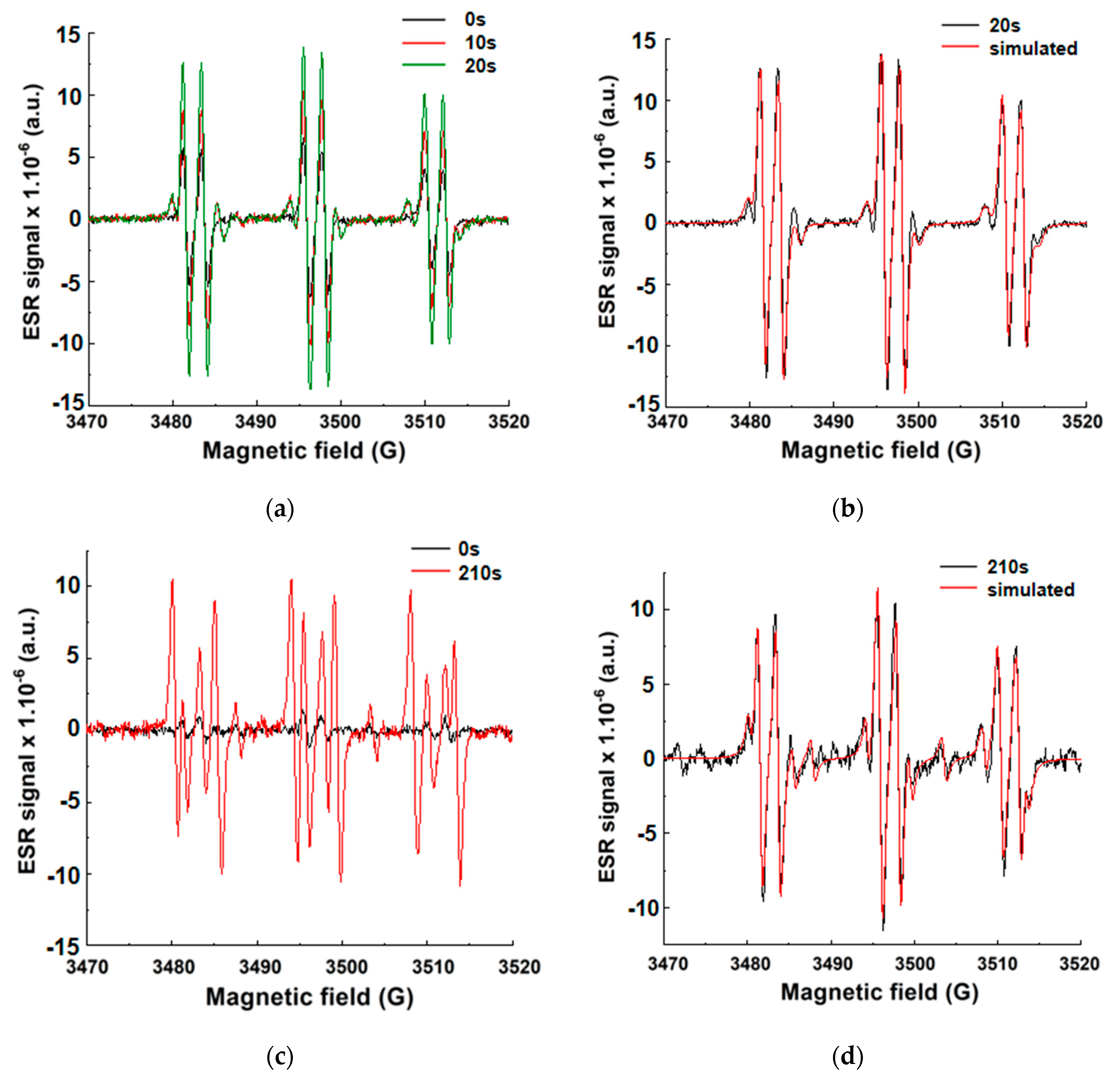
3.6. ESR Spin-Trapping Experiments
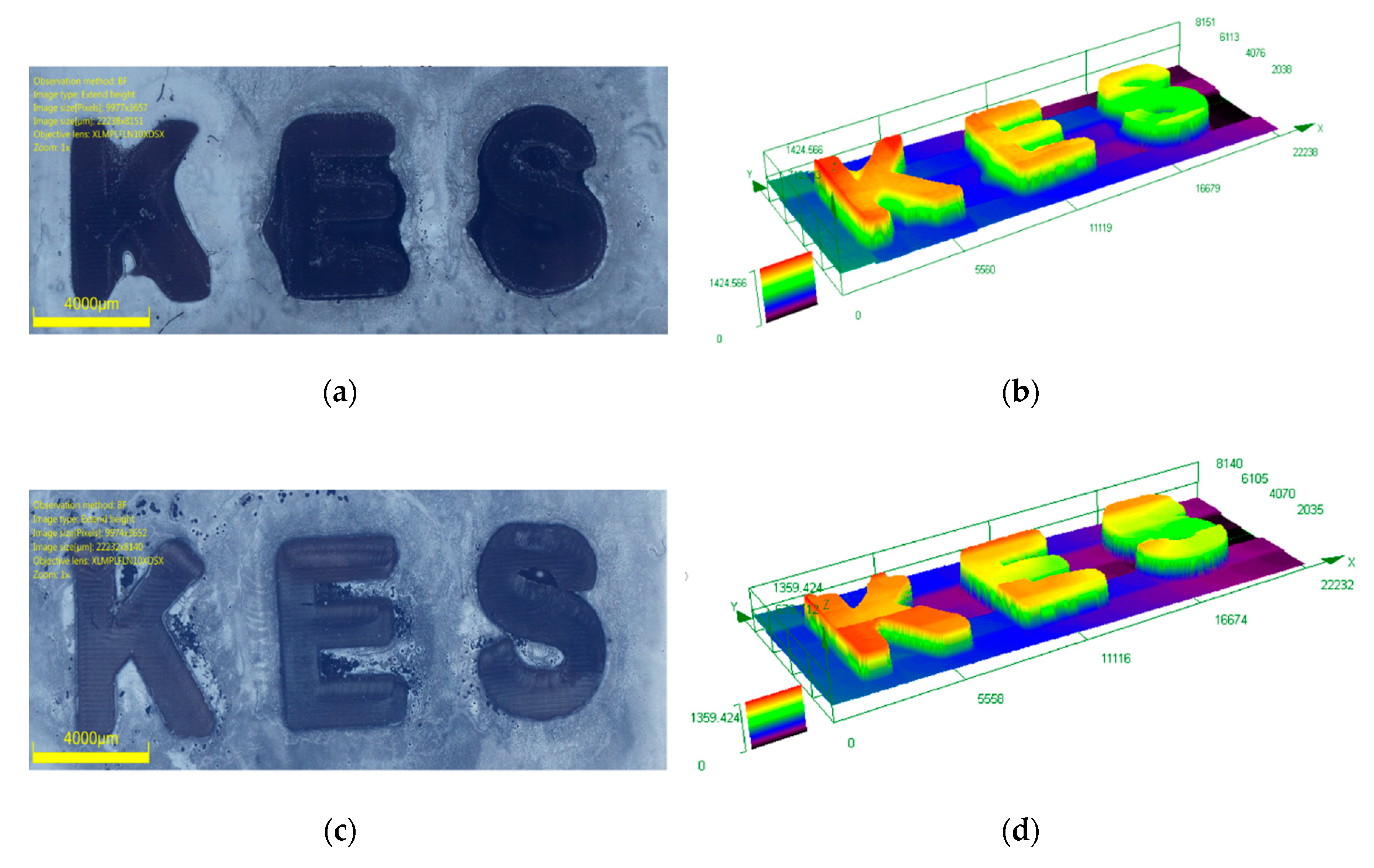
3.7. Laser Writing Experiments for the Photocomposites Prepared by Push–Pull Dye-Based PISs and Silica Fillers
4. Materials and Methods
4.1. Dyes
4.2. Other Materials
4.3. Photopolymerization Experiments
4.4. UV–Visible Absorption, Photolysis and Fluorescent Properties
4.5. Redox Potentials
4.6. Preparation of Photocomposites
4.7. 3D Printing Experiments
4.8. Electron Spin Resonance (ESR) Spin Trapping (ESR-ST)
4.9. Computational Procedure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bures, F. Fundamental aspects of property tuning in push–pull molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef] [Green Version]
- Pigot, C.; Noirbent, G.; Peralta, S.; Duval, S.; Nechab, M.; Gigmes, D.; Dumur, F. Unprecedented Nucleophilic Attack of Piperidine on the Electron Acceptor during the Synthesis of Push-Pull Dyes by a Knoevenagel Reaction. Helv. Chim. Acta 2019, 102, e1900229. [Google Scholar] [CrossRef]
- Guda, R.; Bhaskar, A.; Goodson, T. Ultrafast excited state relaxation dynamics of branched donor-π-acceptor chromophore: Evidence of a charge-delocalized state. J. Phys. Chem. B 2006, 110, 20872–20878. [Google Scholar]
- Kivala, M.; Diederich, F. Acetylene-derived strong organic acceptors for planar and nonplanar push− pull chromophores. Acc. Chem. Res. 2019, 42, 235–248. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Peralta, S.; Duval, S.; Bui, T.T.; Aubert, P.H.; Nechab, M.; Gigmes, D.; Dumur, F. New push-pull dyes based on 2-(3-oxo-2,3-dihydro-1H-cyclopenta[b]naphthalen-1-ylidene) malononitrile: An amine-directed synthesis. Dyes Pigment. 2020, 175, 108182. [Google Scholar] [CrossRef]
- Paek, S.; Lee, J.K.; Ko, J. Synthesis and photovoltaic characteristics of push–pull organic semiconductors containing an electron-rich dithienosilole bridge for solution-processed small-molecule organic solar cells. Sol. Energy Mater. Solar Cells 2014, 120, 209–217. [Google Scholar] [CrossRef]
- Xu, S.J.; Zhou, Z.; Liu, W.; Zhang, Z.; Liu, F.; Yan, H.; Zhu, X. A twisted thieno[3,4-b]thiophene-based electron acceptor featuring a 14-π-electron indenoindene core for high-performance organic photovoltaics. Adv. Mater. 2017, 29, 1704510. [Google Scholar] [CrossRef] [PubMed]
- Turkoglu, G.; Cinar, M.E.; Ozturk, T. Triarylborane-based materials for OLED applications. Molecules 2017, 22, 1522. [Google Scholar] [CrossRef] [PubMed]
- Karak, S.; Liu, F.; Russell, T.P.; Duzhko, V.V. Bulk charge carrier transport in push-pull type organic semiconductor. ACS Appl. Mater. Interfaces 2014, 6, 20904–20912. [Google Scholar] [CrossRef]
- Raimundo, J.M.; Blanchard, P.; Gallego-Planas, N.; Mercier, N.; Ledoux-Rak, I.; Hierle, R.; Roncali, J. Design and synthesis of push-pull chromophores for second-order nonlinear optics derived from rigidifified thiophene-based π-conjugating spacers. J. Org. Chem. 2002, 67, 205–218. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Borbone, F.; Al-Amshany, Z.M.; Tuzi, A.; Barsella, A.; Asiri, A.M.; Roviello, A. Thiazole azo dyes with lateral donor branch: Synthesis, structure and second order NLO properties. Dyes Pigment. 2013, 96, 45–51. [Google Scholar] [CrossRef]
- Cesaretti, A.; Bonaccorso, C.; Elisei, F.; Fortuna, C.G.; Mencaroni, L.; Spalletti, A. Photoinduced intramolecular charge transfer and hyperpolarizability coefficient in push-pull pyridinium salts with increasing strength of the acceptor group. ChemPlusChem 2018, 83, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Motiei, H.; Jafari, A.; Naderali, R. Third-order nonlinear optical properties of organic azo dyes by using strength of nonlinearity parameter and Z-scan technique. Opt. Laser Technol. 2017, 88, 68–74. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Al-Zahrani, F.A.; Afzal, S.M.; Razvi, M.A.N.; Al-Amshany, Z.M.; Bakry, A.H.; Asiri, A.M. Synthesis, linear and nonlinear optical properties of a new dimethine cyanine dye derived from phenothiazine. RSC Adv. 2016, 6, 91546–91556. [Google Scholar] [CrossRef]
- Chaumel, F.; Jiang, H.; Kakkar, A. Sol−gel materials for second-order nonlinear optics. Chem. Mater. 2001, 13, 3389–3395. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kubodera, K.I.; Kurihara, T.; Kaino, T. Nonlinear optical properties of an azo dye attached polymer. Appl. Phys. Lett. 1987, 51, 1–2. [Google Scholar] [CrossRef]
- Gao, S.H.; Xie, M.S.; Wang, H.X.; Niu, H.Y.; Qu, G.R.; Guo, H.M. Highly selective detection of Hg2+ ion by push–pull-type purine nucleoside-based fluorescent sensor. Tetrahedron 2014, 70, 4929–4933. [Google Scholar] [CrossRef]
- Kim, E.; Felouat, A.; Zaborova, E.; Ribierre, J.C.; Wu, J.W.; Senatore, S.; Matthews, C.; Lenne, P.F.; Baffert, C.; Karapetyan, A.; et al. Boron difluoride complexes of hemicurcuminoids as bio-inspired push–pull dyes for bioimaging. Org. Biomol. Chem. 2016, 14, 1311–1324. [Google Scholar] [CrossRef]
- Li, C.; Plamont, M.A.; Aujard, I.; Le Saux, T.; Jullien, L.; Gautier, A. Design and characterization of red fluorogenic push–pull chromophores holding great potential for bioimaging and biosensing. Org. Biomol. Chem. 2016, 14, 9253–9261. [Google Scholar] [CrossRef] [Green Version]
- Noirbent, G.; Dumur, F. Recent advances on nitrofluorene derivatives: Versatile electron acceptors to create dyes absorbing from the visible to the near and far-infrared region. Materials 2018, 11, 2425. [Google Scholar] [CrossRef] [Green Version]
- Blanchard-Desce, M.; Wortmann, R.; Lebus, S.; Lehn, J.M.; Krämer, P. Intramolecular charge transfer in elongated donor-acceptor conjugated polyenes. Chem. Phys. Lett. 1995, 243, 526–532. [Google Scholar] [CrossRef]
- Noirbent, G.; Pigot, C.; Bui, T.-T.; Péralta, S.; Nechab, M.; Gigmes, D.; Dumur, F. Synthesis, optical and electrochemical properties of a series of push-pull dyes based on the 2-(3-cyano-4,5,5-trimethylfuran-2(5H)-ylidene) malononitrile (TCF) acceptor. Dyes Pigment. 2021, 184, 108807. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Push-pull (thio)barbituric acid derivatives in dye photosensitized radical and cationic polymerization reactions under 457/473 nm Laser beams or blue LEDs. Polym. Chem. 2013, 4, 3866–3875. [Google Scholar] [CrossRef]
- Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Organic Electronics: An El Dorado in the quest of new photoCatalysts as photoinitiators of polymerization. Acc. Chem. Res. 2016, 49, 1980–1989. [Google Scholar] [CrossRef] [PubMed]
- Tehfe, M.A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. New push-pull dyes derived from Michler’s ketone for polymerization reactions upon visible lights. Macromolecules 2013, 46, 3761–3770. [Google Scholar] [CrossRef]
- Xiao, P.; Frigoli, M.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Julolidine or fluorenone based push-pull dyes for polymerization upon soft polychromatic visible light or green light. Macromolecules 2014, 47, 106–112. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Blue-to-red light sensitive push-pull structured photoinitiators: Indanedione derivatives for radical and cationic photopolymerization reactions. Macromolecules 2013, 46, 3332–3341. [Google Scholar] [CrossRef]
- Mokbel, H.; Telitel, S.; Dumur, F.; Vidal, L.; Versace, D.-L.; Tehfe, M.-A.; Graff, B.; Toufaily, J.; Fouassier, J.-P.; Gigmes, D.; et al. Photoinitiating systems of polymerization and in-situ incorporation of metal nanoparticles in polymer matrixes upon visible lights: Push-pull malonate and malonitrile based dyes. Polym. Chem. 2013, 4, 5679–5687. [Google Scholar] [CrossRef]
- Sun, K.; Xu, Y.; Dumur, F.; Morlet-Savary, F.; Chen, H.; Dietlin, C.; Graff, B.; Lalevée, J.; Xiao, P. In silico rational design by molecular modeling of new ketones as photoinitiators in three-component photoinitiating systems: Application in 3D printing. Polym. Chem. 2020, 11, 2230–2242. [Google Scholar] [CrossRef]
- Liu, S.; Brunel, D.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. A Monocomponent bifunctional benzophenone-carbazole type II photoinitiator for led photoinitiating systems. Polym. Chem. 2020, 11, 3551–3556. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Sun, K.; Brunel, D.; Gigmes, D.; Morlet-Savary, F.; Zhang, Y.; Liu, S.; Xiao, P.; Dumur, F.; et al. Photoinitiators derived from natural product scaffolds: Monochalcones in three-component photoinitiating systems and their applications in 3D printing. Polym. Chem. 2020, 11, 4647–4659. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Zhang, Y.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Noirbent, G.; Pigot, C.; Brunel, D.; et al. Monocomponent photoinitiators based on benzophenone-carbazole structure for led photoinitiating systems and application on 3D printing. Polymers 2020, 12, 1394. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Noirbent, G.; Brunel, D.; Liu, F.; Gigmes, D.; Sun, K.; Zhang, Y.; Liu, S.; Morlet-Savary, F.; Xiao, P.; et al. Ketone derivatives as photoinitiators for both radical and cationic photopolymerizations under visible LED and application in 3D printing. Eur. Polym. J. 2020, 132, 109737. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Bui, T.T.; Péralta, S.; Gigmes, D.; Nechab, M.; Dumur, F. Push-pull chromophores based on the naphthalene scaffold: Potential candidates for optoelectronic applications. Materials 2019, 12, 1342. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Pigot, C.; Chen, H.; Nechab, M.; Gigmes, D.; Morlet-Savary, F.; Graff, B.; Liu, S.; Xiao, P.; Dumur, F.; et al. Free radical photopolymerization and 3D printing using newly developed dyes: Indane-1, 3-dione and 1H-cyclopentanaphthalene-1, 3-dione derivatives as photoinitiators in three-component systems. Catalysts 2020, 10, 463. [Google Scholar] [CrossRef] [Green Version]
- Pigot, C.; Noirbent, G.; Brunel, D.; Dumur, F. Recent advances on push-pull organic dyes as visible light photoinitiators of polymerization. Eur. Polym. J. 2020, 133, 109797. [Google Scholar] [CrossRef]
- Landmesser, T.; Linden, A.; Hansen, H.J. A Novel Route to 1-Substituted 3-(Dialkylamino)-9-Oxo-9H- Indeno[2,1-c]Pyridine-4-Carbonitriles. Helv. Chim. Acta 2008, 91, 265–284. [Google Scholar] [CrossRef]
- Romanczyk, P.P.; Kurek, S.S. The Reduction Potential of Diphenyliodonium Polymerisation Photoinitiator Is Not −0.2 V vs. SCE. A Computational Study. Electrochim. Acta 2017, 225, 482–485. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Lalevée, J. Photoinitiators for Polymer Synthesis—scope, Reactivity, and Efficiency; John Wiley Sons: Weinheim, Germany, 2012. [Google Scholar]
- Haire, L.D.; Krygsman, P.H.; Janzen, E.G.; Oehler, U.M. Correlation of radical structure with EPR spin adduct parameters: Utility of the proton, carbon-13, and nitrogen-14 hyperfine splitting constants of aminoxyl adducts of PBN-nitronyl-13C for three-parameter scatter plots. J. Org. Chem. 1988, 53, 4535–4542. [Google Scholar] [CrossRef]
- Ohto, N.; Niki, E.; Kamiya, Y. Study of autoxidation by spin trapping. Spin trapping of peroxyl radicals by phenyl N-t-butyl nitrone. J. Chem. Soc. Perkin Trans. 2 1977, 13, 1770–1774. [Google Scholar] [CrossRef]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-savary, F.; Graff, B.; Fouassier, J.P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Morlet-Savary, F.; Fouassier, J.P. Green bulb light source induced epoxy cationic polymerization under air using tris (2, 2′-bipyridine) ruthenium (II) and silyl radicals. Macromolecules 2010, 43, 10191–10195. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet- Savary, F.; Gigmes, D.; Fouassier, J.P. Efficient dual radical/cationic photoinitiator under visible light: A new concept. Polym. Chem. 2011, 2, 1986–1991. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Ding, Z.F.; Liu, F.Y.; Sun, K.; Dietlin, C.; Lalevée, J.; Xiao, P. 3D Printing of Polydiacetylene Photocomposite Materials: Two Wavelengths for Two Orthogonal Chemistries. ACS Appl. Mater. Interf. 2019, 12, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Hijazi, A.; Graff, B.; Fouassier, J.-P.; Rodeghiero, G.; Gualandi, A.; Dumur, F.; Cozzi, P.G.; Lalevée, J. Coumarin derivatives as versatile photoinitiators for 3D printing, polymerization in water and photocomposite synthesis. Polym. Chem. 2019, 10, 872–884. [Google Scholar] [CrossRef]
- Abdallah, M.; Bui, T.-T.; Goubard, F.; Theodosopoulou, D.; Dumur, F.; Hijazi, A.; Fouassier, J.-P.; Lalevée, J. Phenothiazine derivatives as photoredox catalysts for cationic and radical photosensitive resins for 3D printing technology and photocomposite synthesis. Polym. Chem. 2019, 10, 6145–6156. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of fluorescence quenching by electron and H-atom transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Zhang, J.; Dumur, F.; Xiao, P.; Graff, B.; Bardelang, D.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Structure design of naphthalimide derivatives: Toward versatile photoinitiators for near-UV/visible LEDs, 3D printing, and water-soluble photoinitiating systems. Macromolecules 2015, 48, 2054–2063. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Copper complexes in radical photoinitiating systems: Applications to free radical and cationic polymerization upon visible LEDs. Macromolecules 2014, 47, 3837–3844. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Campolo, D.; Poly, J.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Iron complexes as potential photocatalysts for controlled radical photopolymerizations: A tool for modifications and patterning of surfaces. J. Polym. Sci. A Polym. Chem. 2016, 54, 702–713. [Google Scholar] [CrossRef]
- James, B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods; Gaussian Inc.: Wallingford, CT, USA, 1996. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.; Stratmann, R.E.; Burant, J.C.; et al. Gaussian 03, Revision B-2; Gaussian Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
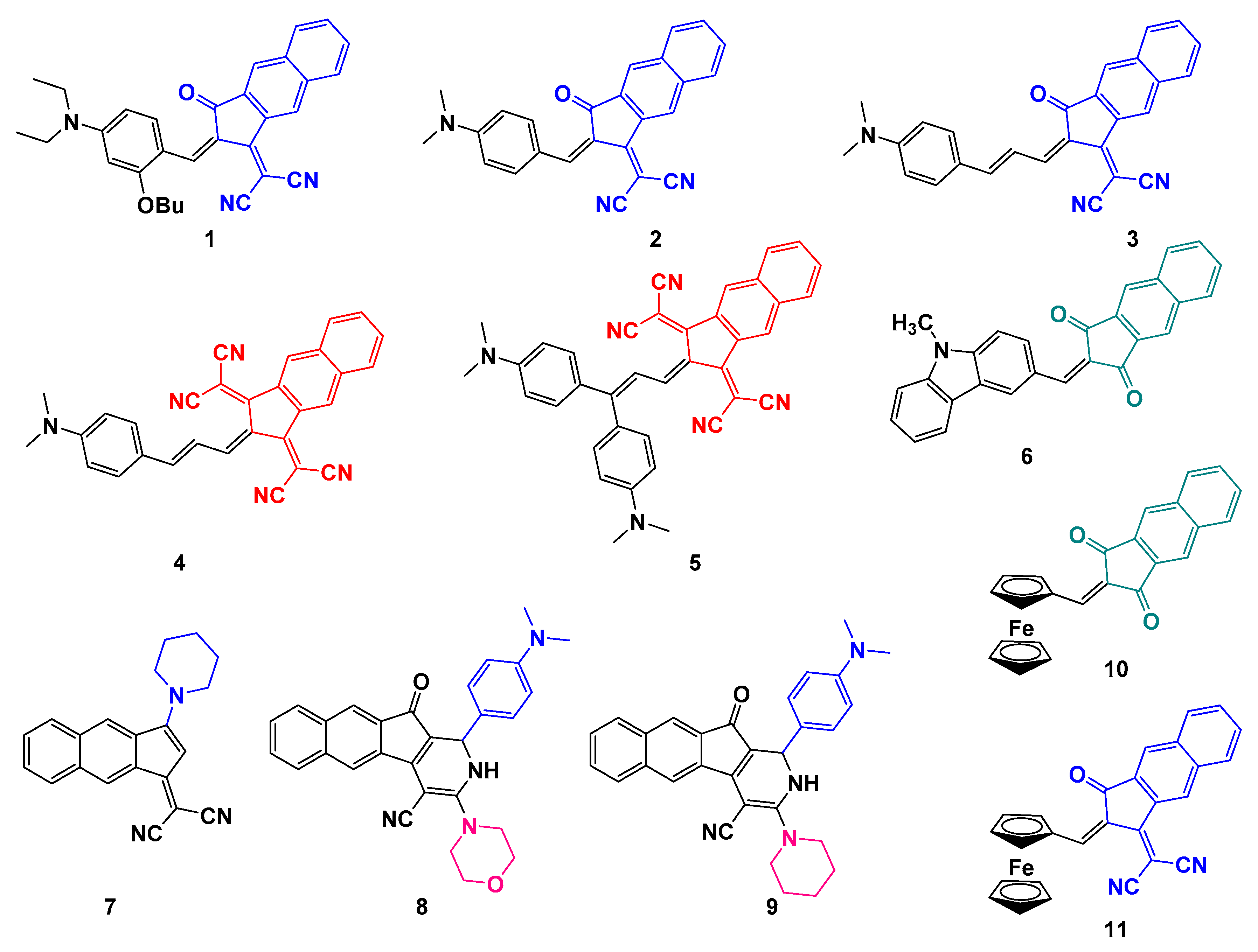
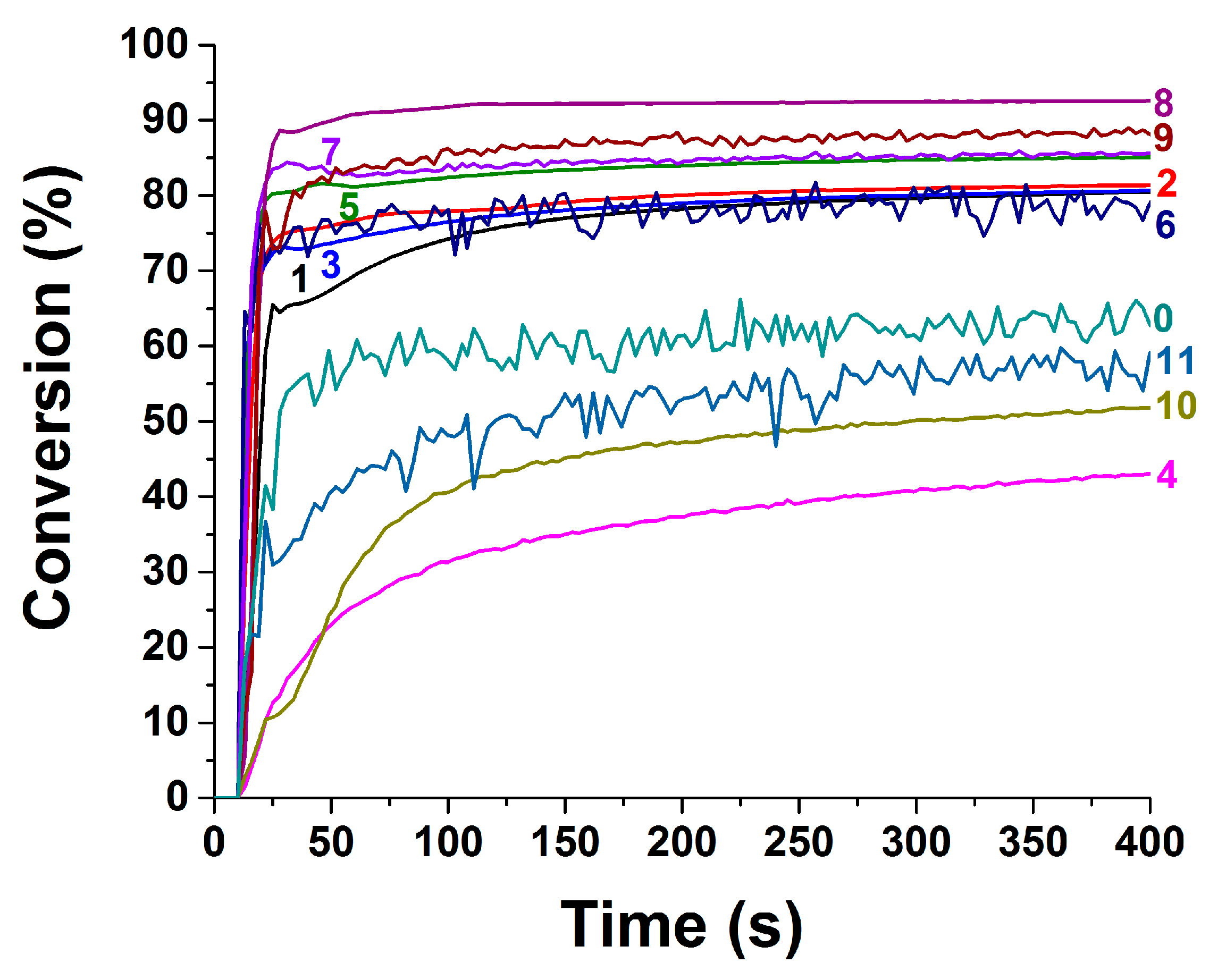

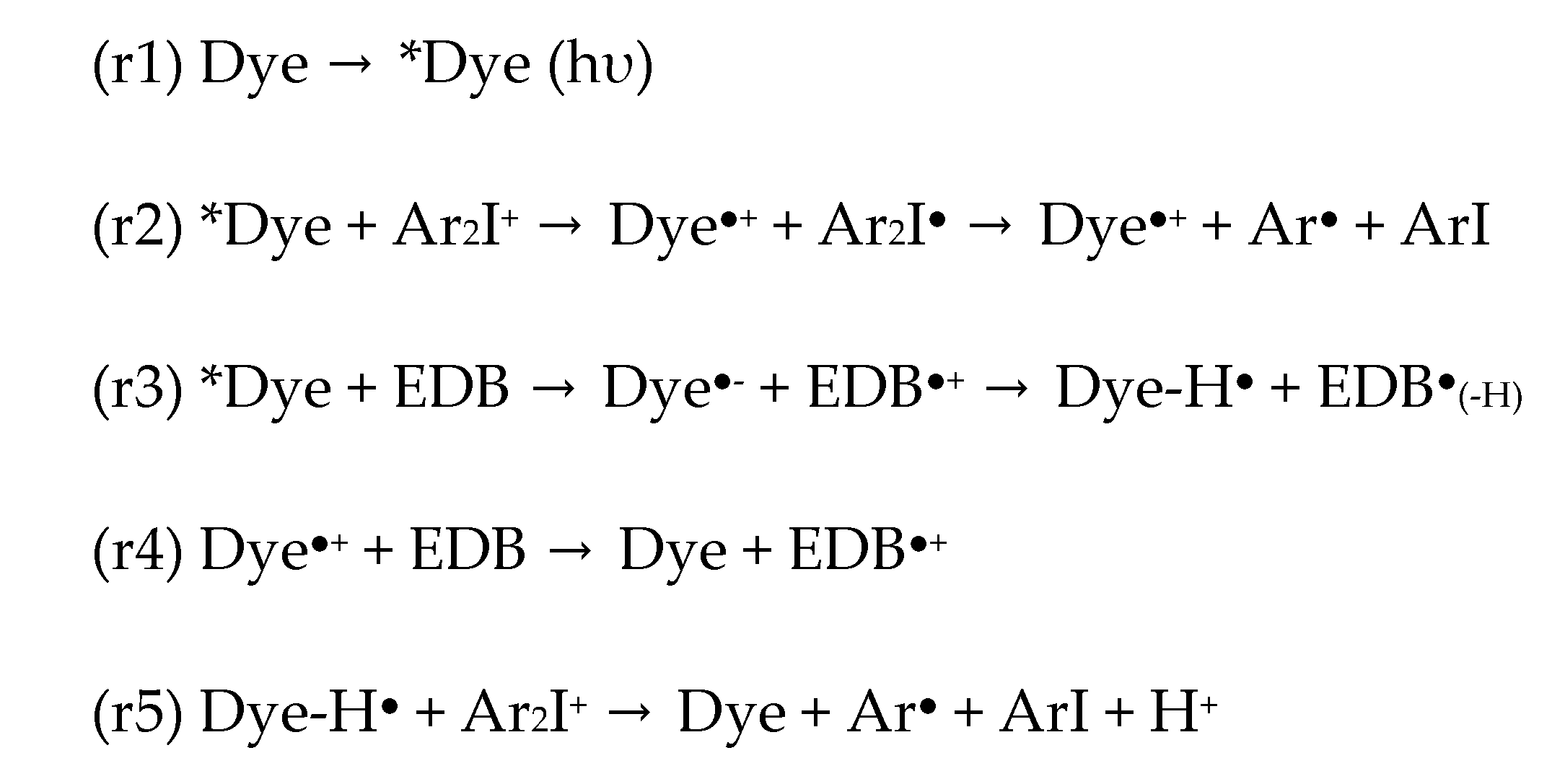

| Dye | 0 (Blank) a | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| FCs | 60% | 78% | 80% | 78% | 43% | 84% |
| [dye] | 0 | 2.31 × 10−3 | 2.93 × 10−3 | 2.74 × 10−3 | 2.44 × 10−3 | 1.93 × 10−3 |
| Dye | 6 | 7 | 8 | 9 | 10 | 11 |
| FCs | 76% | 85% | 91% | 88% | 51% | 56% |
| [dye] | 2.84 × 10−3 | 3.53 × 10−3 | 2.38 × 10−3 | 2.39 × 10−3 | 2.80 × 10−3 | 2.50 × 10−3 |
| λmax (nm) | εmax (M−1 cm−1) | ε@405nm (M−1 cm−1) | |
|---|---|---|---|
| Dye 1 | 601 | 38,580 | 8560 |
| Dye 2 | 582 | 24,872 | 6870 |
| Dye 3 | 661 | 16,330 | 3500 |
| Dye 4 | 537 | 11,600 | 16,090 |
| Dye 5 | 727 | 22,990 | 8320 |
| Dye 6 | 447 | 49,650 | 22,910 |
| Dye 7 | 500 | 14,220 | 3880 |
| Dye 8 | 491 | 5330 | 3340 |
| Dye 9 | 495 | 9763 | 4950 |
| Dye 10 | 591 | 4550 | 8630 |
| Dye 11 | 644 | 5930 | 11,348 |
| ES1 (eV) | Ered (eV) b | Eox (eV) b | ∆GS1Ioda(eV) d | ∆G S1EDB(eV) d | |
|---|---|---|---|---|---|
| Dye 8 | 2.28 | −1.35 | 0.36 | −1.22 | 0.07 |
| Dye 9 | 2.38 | −1.41 | 0.32 | −1.36 | −0.03 |
| ET1 (eV) c | ∆GetT1Iod(eV) d | ∆GetT1EDB(eV) d | Ksv(S1) EDB(M−1) | ϕet(S1) EDB a | |
| Dye 8 | 1.65 | −0.59 | 0.7 | - | - |
| Dye 9 | 1.65 | −0.63 | 0.76 | 129 | 0.937 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.; Liu, S.; Pigot, C.; Brunel, D.; Graff, B.; Nechab, M.; Gigmes, D.; Morlet-Savary, F.; Zhang, Y.; Xiao, P.; et al. Novel Push–Pull Dyes Derived from 1H-cyclopenta[b]naphthalene-1,3(2H)-dione as Versatile Photoinitiators for Photopolymerization and Their Related Applications: 3D Printing and Fabrication of Photocomposites. Catalysts 2020, 10, 1196. https://doi.org/10.3390/catal10101196
Sun K, Liu S, Pigot C, Brunel D, Graff B, Nechab M, Gigmes D, Morlet-Savary F, Zhang Y, Xiao P, et al. Novel Push–Pull Dyes Derived from 1H-cyclopenta[b]naphthalene-1,3(2H)-dione as Versatile Photoinitiators for Photopolymerization and Their Related Applications: 3D Printing and Fabrication of Photocomposites. Catalysts. 2020; 10(10):1196. https://doi.org/10.3390/catal10101196
Chicago/Turabian StyleSun, Ke, Shaohui Liu, Corentin Pigot, Damien Brunel, Bernadette Graff, Malek Nechab, Didier Gigmes, Fabrice Morlet-Savary, Yijun Zhang, Pu Xiao, and et al. 2020. "Novel Push–Pull Dyes Derived from 1H-cyclopenta[b]naphthalene-1,3(2H)-dione as Versatile Photoinitiators for Photopolymerization and Their Related Applications: 3D Printing and Fabrication of Photocomposites" Catalysts 10, no. 10: 1196. https://doi.org/10.3390/catal10101196