Steric Effects of Mesoporous Silica Supported Bimetallic Au-Pt Catalysts on the Selective Aerobic Oxidation of Aromatic Alcohols
Abstract
:1. Introduction
2. Results and Discussions
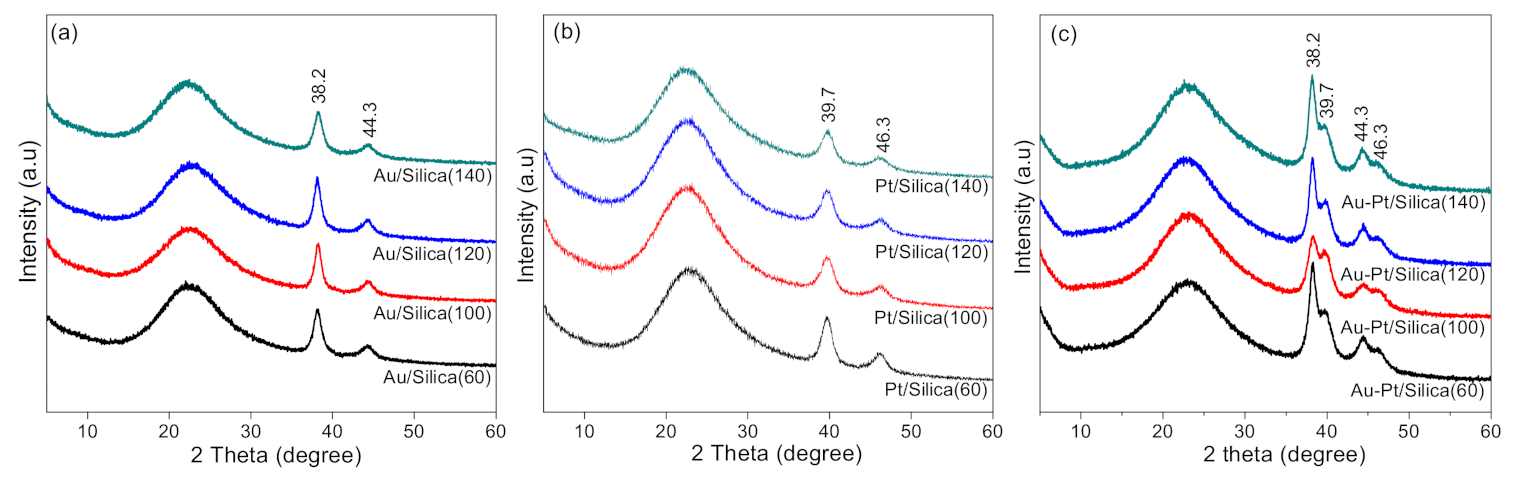
2.1. Crystalline Structures
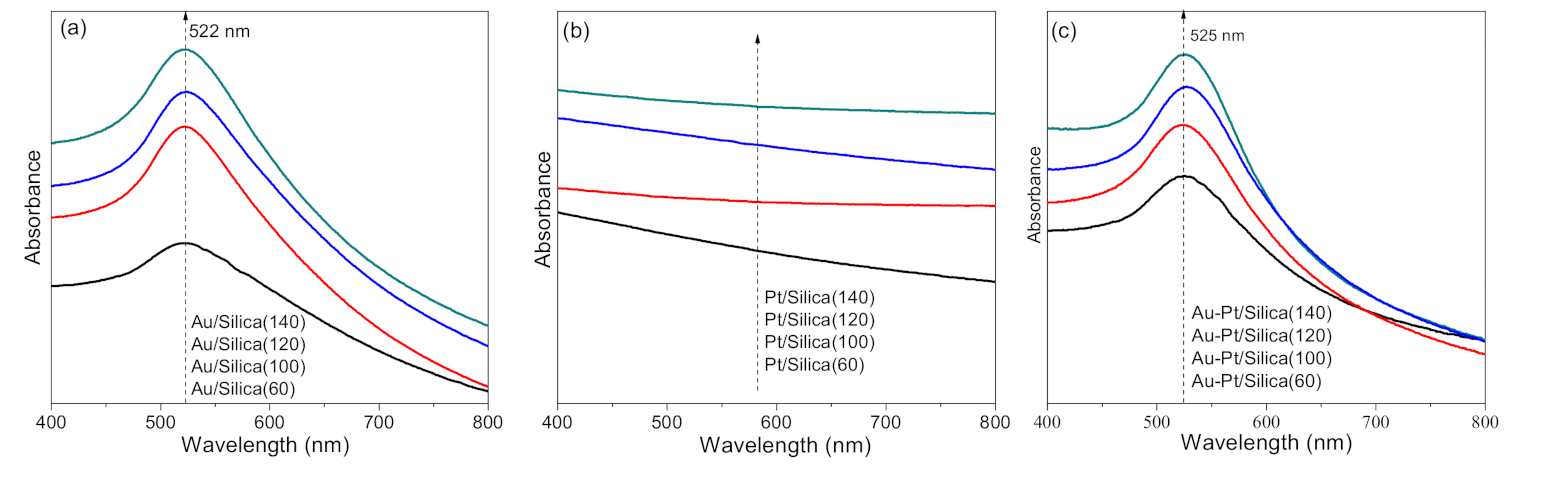
2.2. Optical Properties
2.3. Textural Properties
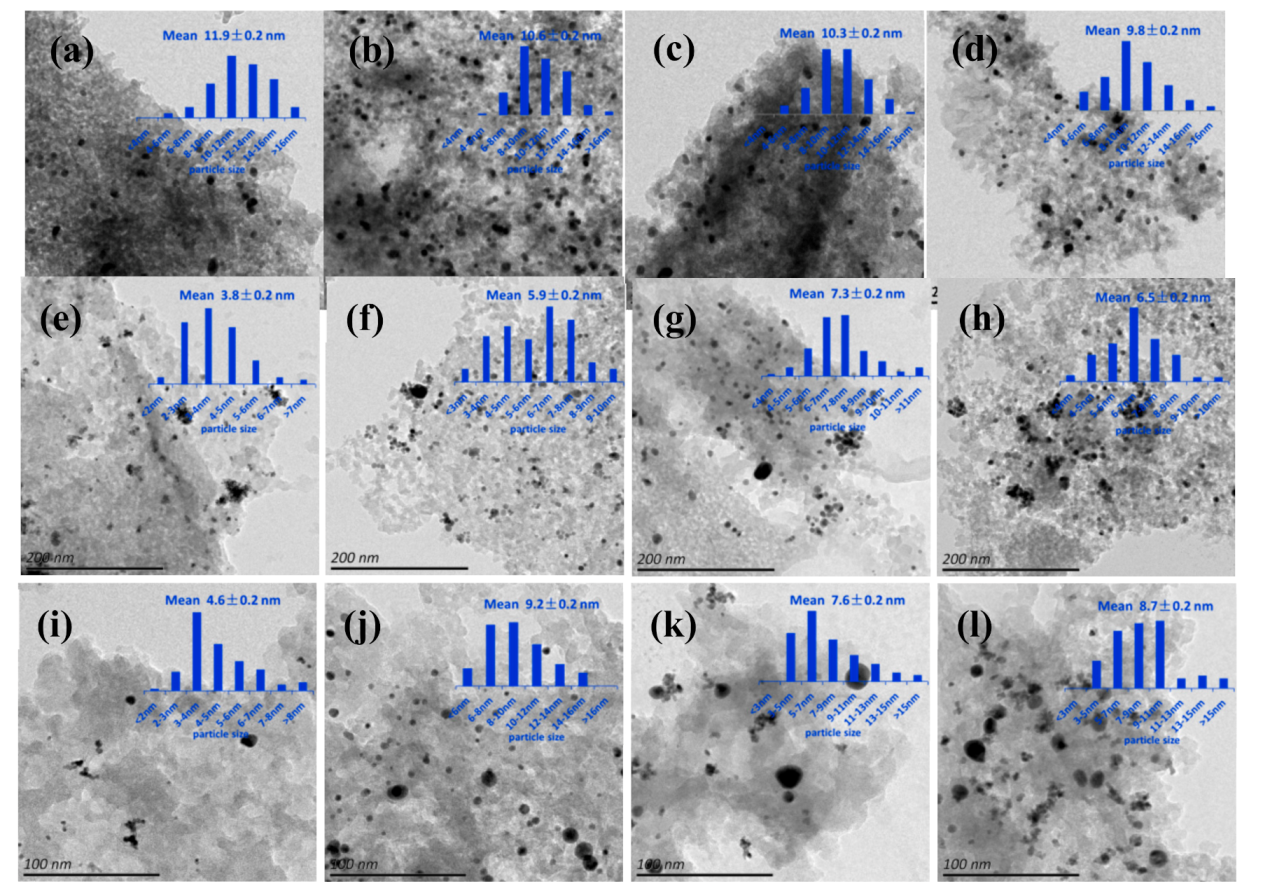
2.4. Morphologies of Catalysts and Particle Size of Metal NPs
2.5. Elemental and XPS Analyses
2.6. Catalytic Performance and Discussions on the Steric Effects
3. Experimental Section
3.1. Raw Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, B.; Wang, L.Y.; Gao, S. Recent advances in aerobic oxidation of alcohols and amines to imines. ACS Catal. 2015, 5, 5851–5876. [Google Scholar] [CrossRef]
- Zhang, X.G.; Wilson, K.; Lee, A.F. Heterogeneously catalyzed hydrothermal processing of C5-C6 sugars. Chem. Rev. 2016, 116, 12328–12368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Liu, B.; Zhang, Q.H.; Deng, W.P.; Wang, Y.; Yang, Y.H. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem. Soc. Rev. 2014, 43, 3480–3524. [Google Scholar] [CrossRef] [PubMed]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.G.; Ke, X.B.; Zhu, H.Y. Zeolite-supported gold nanoparticles for selective photooxidation of aromatic alcohols under visible-light irradiation. Chem. Eur. J. 2012, 18, 8048–8056. [Google Scholar] [CrossRef]
- Yadav, G.D.; Mehta, P.H. Theoretical and experimental analysis of capsule membrane phase transfer catalysis: Selective alkaline hydrolysis ofbenzyl chloride to benzyl alcohol. Catal. Lett. 1993, 21, 391–403. [Google Scholar] [CrossRef]
- Konietzni, F.; Zanthoff, H.W.; Maier, W.F. The role of active oxygen in the AMM-VxSi-catalysed selective oxidation of toluene. J. Catal. 1999, 188, 154–164. [Google Scholar] [CrossRef]
- Martin, A.; Bentrup, U.; Wolf, G.U. The effect of alkali metal promotion on vanadium-containing catalysts in the vapour phase oxidation of methyl aromatics to the corresponding aldehydes. Appl. Catal. A Gen. 2002, 227, 131–142. [Google Scholar] [CrossRef]
- Aellig, C.; Girard, C.; Hermans, I. Aerobic alcohol oxidations mediated by nitric acid. Angew. Chem. Int. Ed. 2011, 50, 12355–12360. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.F.; Zhao, W.N.; Liu, Z.P.; Xu, B.Q. NaOH alone can be a homogeneous catalyst for selective aerobic oxidation of alcohols in water. J. Catal. 2017, 353, 37–43. [Google Scholar] [CrossRef]
- Lavenn, C.; Demessence, A.; Tuel, A. Au25(SPh-pNH2)17 nanoclusters deposited on SBA-15 as catalysts for aerobic benzyl alcohol oxidation. J. Catal. 2015, 322, 130–138. [Google Scholar] [CrossRef]
- Liu, C.H.; Lin, C.Y.; Chen, J.L.; Lu, K.T.; Lee, J.F.; Chen, J.M. SBA-15-supported Pd catalysts: The effect of pretreatment conditions on particle size and its application to benzyl alcohol oxidation. J. Catal. 2017, 350, 21–29. [Google Scholar] [CrossRef]
- Wu, P.P.; Cao, Y.X.; Zhao, L.M.; Wang, Y.; He, Z.K.; Xing, W.; Bai, P.; Mintova, S.; Yan, Z.F. Formation of PdO on Au-Pd bimetallic catalysts and the effect on benzyl alcohol oxidation. J. Catal. 2019, 375, 32–43. [Google Scholar] [CrossRef]
- Tang, Q.H.; Gong, X.N.; Zhao, P.Z.; Chen, Y.T.; Yang, Y.H. Copper-manganese oxide catalysts supported on alumina: Physicochemical features and catalytic performances in the aerobic oxidation of benzyl alcohol. Appl. Catal. A Gen. 2010, 389, 101–107. [Google Scholar] [CrossRef]
- Wang, T.; Yuan, X.; Li, S.R.; Zeng, L.; Gong, J.L. CeO2-modified Au@SBA-15 nanocatalysts for liquid-phase selective oxidation of benzyl alcohol. Nanoscale 2015, 7, 7593–7602. [Google Scholar] [CrossRef]
- Tang, H.L.; Wei, J.K.; Liu, F.; Qiao, B.T.; Pan, X.L.; Li, L.; Liu, J.Y.; Wang, J.H.; Zhang, T. Strong metal-support interactions between gold nanoparticles and nonoxides. J. Am. Chem. Soc. 2016, 138, 56–59. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chen, H.; Xu, C.J.; Sun, Y.M.; Li, S.; Qin, G.W. Control of catalytic activity of nano-au through tailoring the fermi level of support. Small 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Lim, H.M.; Tang, Q.H.; Gao, Y.T.; Sun, T.; Yan, Q.Y.; Yang, Y.H. Solvent-free aerobic oxidation of benzyl alcohol over Pd monometallic and Au-Pd bimetallic catalysts supported on SBA-16 mesoporous molecular sieves. Appl. Catal. A Gen. 2010, 380, 55–65. [Google Scholar] [CrossRef]
- Olmos, C.M.; Chinchilla, L.E.; Villa, A.; Delgado, J.J.; Hungria, A.B.; Blanco, G.; Prati, L.; Calvino, J.J.; Chen, X.W. Size, nanostructure, and composition dependence of bimetallic Au-Pd supported on ceria-zirconia mixed oxide catalysts for selective oxidation of benzyl alcohol. J. Catal. 2019, 375, 44–55. [Google Scholar] [CrossRef]
- Ishida, T.; Murayama, T.; Taketoshi, A.; Haruta, M. Importance of size and contact structure of gold nanoparticles for the genesis of unique catalytic processes. Chem. Rev. 2020, 120, 464–525. [Google Scholar] [CrossRef] [Green Version]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the support in gold-containing nanoparticles as heterogeneous catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Deelen, T.W.; Mejia, C.H.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Yang, S.T.; Yu, C.X.; Yu, L.L.; Miao, S.; Zou, M.M.; Jin, C.Z.; Zhang, D.Z.; Xu, L.Y.; Huang, S. Bridging dealumination and desilication for the synthesis of hierarchical MFI zeolites. Angew. Chem. Int. Ed. 2017, 56, 12553–12556. [Google Scholar] [CrossRef] [PubMed]
- Wilde, N.; Pelz, M.; Gebhardt, S.G.; Glaser, R. Highly efficient nano-sized TS-1 with micro-/mesoporosity from desilication and recrystallization for the epoxidation of biodiesel with H2O2. Green Chem. 2015, 17, 3378–3389. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.R.; Xu, L.; Wu, P. Hierarchical, core-shell meso-ZSM-5@mesoporous aluminosilicate-supported Pt nanoparticles for bifunctional hydrocracking. J. Mater. Chem. A 2014, 2, 15535–15545. [Google Scholar] [CrossRef]
- Wang, N.; Sun, Q.M.; Yu, J.H. Ultrasmall metal nanoparticles confined within crystalline nanoporous materials: A fascinating class of nanocatalysts. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.Y.; Dou, B.J.; Li, J.J.; Cheng, J.; Hu, Q.; Hao, Z.P.; Qiao, S.Z. Catalytic oxidation of benzyl alcohol on Au or Au-Pd nanoparticles confined in mesoporous silica. Appl. Catal. B Environ. 2009, 92, 202–208. [Google Scholar] [CrossRef]
- Li, B.; Xu, Z.X.; Jing, F.L.; Luo, S.Z.; Chu, W. Facile one-pot synthesized ordered mesoporous Mg-SBA-15 supported PtSn catalysts for propane dehydrogenation. Appl. Catal. A Gen. 2017, 533, 17–27. [Google Scholar] [CrossRef]
- Wu, P.P.; Cao, Y.X.; Wang, Y.; Xing, W.; Zhong, Z.Y.; Bai, P.; Yan, Z.F. Ultrastable bimetallic catalyst with tuned surface electronic properties for highly selective oxidation of cyclohexane. Appl. Surf. Sci. 2018, 457, 580–590. [Google Scholar] [CrossRef]
- Zhang, X.G.; Du, A.J.; Zhu, H.Y.; Jia, J.F.; Wang, J.; Ke, X.B. Surface plasmon-enhanced zeolite catalysis under light irradiation and its correlation with molecular polarity of reactants. Chem. Commun. 2014, 50, 13893–13895. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.G.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.A.; Lee, A.F.; Wilson, K. Platinum-catalyzed aqueous-phase hydrogenation of D-glucose to D-sorbitol. ACS Catal. 2016, 6, 7409–7417. [Google Scholar] [CrossRef] [Green Version]
- Hamza, A.; Srinivas, D. Selective oxidation of benzyl alcohol over copper phthalocyanine immobilized on MCM-41. Catal. Lett. 2009, 128, 434–442. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.Z.; Wang, J.Y.; Yang, Q.; Hou, Q.D.; Ju, M.T. Gold nanoparticle-modified TiO2/SBA-15 nanocomposites as active plasmonic photocatalysts for the selective oxidation of aromatic alcohols. RSC Adv. 2016, 6, 70352–70363. [Google Scholar] [CrossRef]
- Escamilla-Perea, L.; Peza-Ledesma, C.L.; Nava, R.; Rivera-Munoz, E.M.; Pawelec, B.; Fierro, J.L.G. CO oxidation at 20 degrees C over Au/SBA-15 catalysts decorated by Fe2O3 nanoparticles. Catal. Commun. 2011, 15, 108–112. [Google Scholar] [CrossRef]
- Wang, G.M.; Yao, R.H.; Xin, H.Y.; Guan, Y.J.; Wu, P.; Li, X.H. At room temperature in water: Efficient hydrogenation of furfural to furfuryl alcohol with a Pt/SiC-C catalyst. RSC Adv. 2018, 8, 37243–37253. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.L.; Su, Y.; Zhang, B.S.; Lee, A.F.; Isaacs, M.A.; Wilson, K.; Li, L.; Ren, Y.G.; Huang, J.H.; Haruta, M.; et al. Classical strong metal-support interactions between gold nanoparticles and titanium dioxide. Sci. Adv. 2017, 3. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Mirescu, A.; Prusse, U. Selective glucose oxidation on gold colloids. Catal. Commun. 2006, 7, 11–17. [Google Scholar] [CrossRef]
- Xu, L.; Peng, H.G.; Zhang, K.; Wu, H.H.; Chen, L.; Liu, Y.M.; Wu, P. Core-shell-structured titanosilicate as a robust catalyst for cyclohexanone ammoximation. ACS Catal. 2013, 3, 103–110. [Google Scholar] [CrossRef]
- Dapsens, P.Y.; Mondelli, C.; Perez-Ramirez, J. Highly selective Lewis acid sites in desilicated MFI zeolites for dihydroxyacetone isomerization to lactic acid. ChemSusChem 2013, 6, 831–839. [Google Scholar] [CrossRef] [PubMed]



| Samples | SBET a (m2 g −1) | Vt b (cm3 g −1) | Dp c (nm) |
|---|---|---|---|
| Au/Silica(140) | 144.4 | 0.702 | 16.2 |
| Au/Silica(120) | 135.8 | 0.647 | 15.8 |
| Au/Silica(100) | 110.8 | 0.587 | 18.4 |
| Au/Silica(60) | 24.3 | 0.085 | -- |
| Pt/Silica(140) | 290.5 | 0.537 | 5.6 |
| Pt/Silica(120) | 192.2 | 0.325 | 5.1 |
| Pt/Silica(100) | 211.7 | 0.278 | 4.2 |
| Pt/Silica(60) | 2.5 | 0.007 | -- |
| Au-Pt/Silica(140) | 328.3 | 0.656 | 7.0 |
| Au-Pt/Silica(120) | 334.2 | 0.738 | 7.0 |
| Au-Pt/Silica(100) | 216.9 | 0.453 | 6.6 |
| Au-Pt/Silica(60) | 14.7 | 0.017 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Shan, L.; Gu, X.; Zhang, X.; Cai, J. Steric Effects of Mesoporous Silica Supported Bimetallic Au-Pt Catalysts on the Selective Aerobic Oxidation of Aromatic Alcohols. Catalysts 2020, 10, 1192. https://doi.org/10.3390/catal10101192
Yan J, Shan L, Gu X, Zhang X, Cai J. Steric Effects of Mesoporous Silica Supported Bimetallic Au-Pt Catalysts on the Selective Aerobic Oxidation of Aromatic Alcohols. Catalysts. 2020; 10(10):1192. https://doi.org/10.3390/catal10101192
Chicago/Turabian StyleYan, Jun, Longlong Shan, Xiaoli Gu, Xingguang Zhang, and Junmeng Cai. 2020. "Steric Effects of Mesoporous Silica Supported Bimetallic Au-Pt Catalysts on the Selective Aerobic Oxidation of Aromatic Alcohols" Catalysts 10, no. 10: 1192. https://doi.org/10.3390/catal10101192
APA StyleYan, J., Shan, L., Gu, X., Zhang, X., & Cai, J. (2020). Steric Effects of Mesoporous Silica Supported Bimetallic Au-Pt Catalysts on the Selective Aerobic Oxidation of Aromatic Alcohols. Catalysts, 10(10), 1192. https://doi.org/10.3390/catal10101192





