1. Introduction
Ever increasing energy demand for rapid industrialization/urbanization, being fulfilled mainly from fossil fuels, is continuously stimulating the alarming and severe threats of environmental pollution and global warming [
1]. It is believed that amongst various global house gases (GHG), increased atmospheric CO
2 gas concentration contributes to the major portion for the respective critical issue [
2,
3,
4]. Industrialization and utilization of fossil fuels are considered as the prime driving factors for excessive CO
2 levels. Therefore, in accordance with the adverse sentiment, it is imperative to adopt and promote an alternative technology that provides an efficient, renewable, and sustainable pathway to counterbalance excessive atmospheric CO
2 level. In this regard, photocatalytic CO
2 conversion (PCC) or photo-reduction to useful/value added chemicals (CO, CH
4, C
2H
6, C
2H
5OH, C
2H
4, CH
3OH, HCOOH, etc.) is one of the most engaging and alluring approaches. PCC is based on the concept of natural photosynthesis, employing a semiconductor material acting as a photocatalyst with a reducing agent (e.g., H
2O/H
2 etc.) for CO
2 conversion to useful chemicals under the light irradiation, a free energy source [
5,
6,
7]. Generally, PCC is considered as a research domain under the umbrella of artificial photosynthesis, targeting normalization of CO
2 level and providing chemicals/fuels in a sustainable manner.
Since the pioneering work of Inoue et al., the photocatalysis field has been researched extensively with respect to materials [
8]. As well-established, titanium dioxide (TiO
2) is counted on as one of the best photocatalytic materials due to its specific characteristics of abundant availability, high stability, better charge lifetime, favorable surface area, non-toxicity, and cost effectiveness [
9]. However, despite certain benefits, TiO
2 also possesses the major drawback of a limited light absorption due to its wide band gap and limited CO
2 surface adsorption. Until now, enormous efforts and researches have been done to achieve an efficient photocatalytic CO
2 conversion on the account of process development, such as materials with broadened light absorption, higher surface area, enhanced CO
2 adsorption, and photocatalytic reactors advancement [
10,
11,
12,
13,
14]. For the case of photocatalytic materials development, certain strategies have been executed such as doping [
15,
16], composite/hybrid/heterojunction formation [
17,
18,
19,
20,
21], nano-architectures [
22,
23,
24], and coupling with noble metals or carbon-based materials [
22,
25,
26]. All such approaches are oriented towards attaining outstanding photocatalytic CO
2 conversion efficiency.
Recently, layered double hydroxide (LDH), an interesting class of layered anionic clays, has captivated the researchers’ interest for their development and application in a variety of research domains such as adsorption [
27], photocatalysis [
28], electrochemistry [
29,
30,
31,
32], and biomedical science [
33]. Generally, LDHs are synthetically produced and rarely occur in nature. The exceptional properties of LDH, i.e., their superb adsorption capacity extended light absorption via compositional variation, cheap synthesis procedure, and unique layered morphology, making them suitable materials for photocatalysis applications [
28]. Until now, a moderate number of researches and studies have been reported employing LDH regarding their synthesis approaches and electronic and physical properties influencing photocatalytic applications [
32,
34]. However, specific to PCC, there exist limited reports employing bare LDH, surface modified LDH, or hybrid LDH based photocatalysts.
In the present review, the recent progress of bare LDH and LDH based photocatalysts employed for photocatalytic CO2 conversion to useful chemicals/fuels are briefly overviewed. The synthesis procedure involved, key reaction conditions, PCC mechanism, and prime parameters promoting the improvement of photocatalytic performance are detailed. In short, the review is a brief overview of LDH based photocatalysts offering excellent performance for PCC.
2. Layered Double Hydroxide (LDH) Structure and Synthesis
LDH are mainly synthetic materials with their structure mimicking natural mineral hydrotalcite (Mg
6Al
2(OH)
16)CO
3. H
2O was discovered in 1842 and synthetically prepared in 1942 [
35]. The detailed structural investigation of the LDH was conducted by Allmann and co-authors in 1969 [
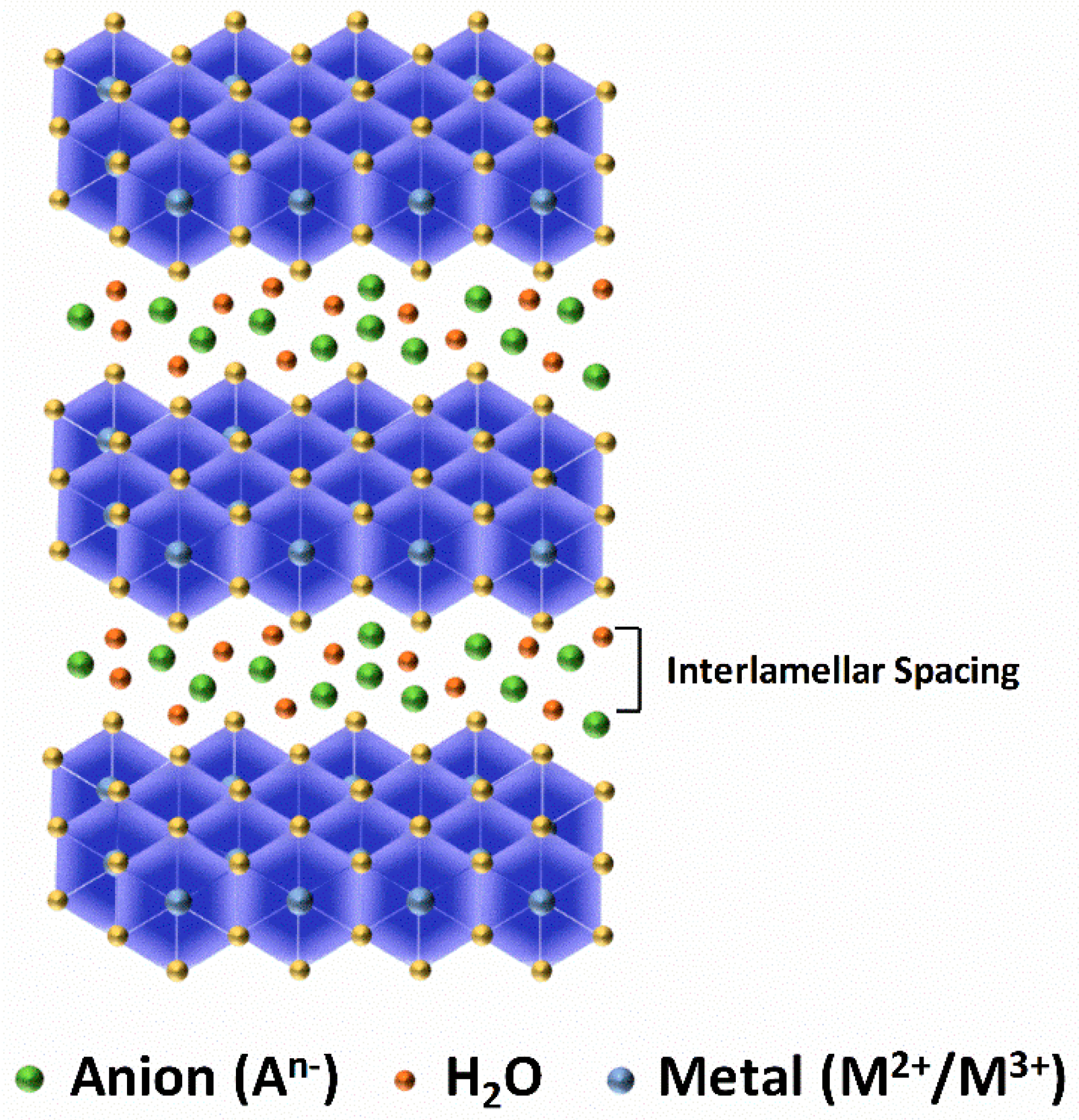
36]. Hence, LDH are composed of layered structure and are also termed hydrotalcite-like compounds comprising brucite like metal hydroxide layers and water molecules balanced by interlayer anions. The commonly accepted formula representing the LDH is
•, where M
2+ (e.g., Mg
2+, Cu
2+, Ni
2+, Zn
2+ etc.) and M
3+ (e.g., Al
3+, Fe
3+, Ga
3+ etc.) represents the divalent and trivalent cations, respectively. A
n− indicates the interlayer gallery ions such as CO
32−, NO
32−, Cl
−, etc. along with water molecules. The illustration of commonly acknowledged and endorsed LDH is shown in
Figure 1.
It is well established that the composition of the LDH, their structure, and certain properties can be controlled and manipulated by the nature of the synthesis procedure. Primarily the synthesis procedure can be classified based on the phase involved, i.e., (1) solid phase and (2) liquid phase synthesis approach. Solid state synthesis approach mainly consists of mechanochemical process employing wet and dry milling methods [
37,
38]. Such approach offers benefit of less pollution in terms of liquids used but again is an expensive method, with a major drawback of structure distortion and nonuniform LDH structure. On the contrary, liquid phase synthesis approach is a commonly adopted and dominant strategy because of its easiness, cost effectiveness, and provision of the prime benefits of complete and uniform LDH structural morphology. The liquid phase synthesis approach is further branched into variety of synthesis methods based on the reaction type [
39,
40,
41]. Some of important and commonly practiced liquid phase synthesis methods can be listed as follows:
- I.
Co-precipitation method;
- II.
Anion exchange method;
- III.
Reconstruction/rehydration method;
- IV.
Solution mixing method.
Amongst the above-mentioned methods, co-precipitation method is the most frequently adopted synthesis approach offering a direct method for the synthesis of LDH. Co-precipitation method practices slow addition of anionic solution to the divalent or trivalent metal cations solution, with addition of alkali or urea, resulting in hydrolysis and finally precipitation of LDH. The addition of base or urea in metal cations solution leads to co-precipitation of metallic salts via condensation of hexa-aqua metal complexes finally resulting in brucite-like metal cations layers with solvated interlamellar anions.
Anion exchange method employs the exchange of the intercalated anions by other targeted anions. In this method, already prepared LDH is subjected to stirring in the targeted anions solution, which are to be intercalated to achieve the required LDH. Moreover, when the co-precipitation method is not feasible, anion exchange method is utilized, e.g., when either metal cations or anions are unstable in alkaline solution or when there is chance of direct reaction between the cations and intercalated anions. Under these specific circumstances, anion exchange method offers viable alternative approach to synthesize the desired LDH by exchanging the intercalated anions with the target anions.
Another interesting synthesis method is the reconstruction method. Like shape memory materials, LDH materials also tend to return to their original structure. Generally, when the LDH materials are calcined at higher temperatures (400–500 °C), the intercalated anions and water molecules are completely erased, and hydroxides are converted to respective metal oxides. These metal oxides are immersed in water or targeted anion solution, and the metal hydroxide and intercalated layers of anions are regenerated, and LDH is reformed. Such process is very fruitful for intercalating the specific targeted anions in the interlayer gallery of the LDH.
Simple solution mixing and then subjecting it to high temperature and pressure conditions such as hydrothermal or solvothermal approaches are another effective mean to fabricate the LDH and/or LDH based composites with other semiconductor materials.
In addition to the abovementioned methods, i.e., hydrothermal [
42], there exist a variety of synthesis methods such as sol–gel [
43], and microemulsion methods [
44], which can be adopted based on the required features and properties of the LDH.
3. Bare and Modified Layered Double Hydroxide (LDH) Photocatalysts
Layered double hydroxide (LDH) is mainly comprised of layered morphology, providing important aspects of (i) broader light absorption (generally in the visible light range) and (ii) better CO
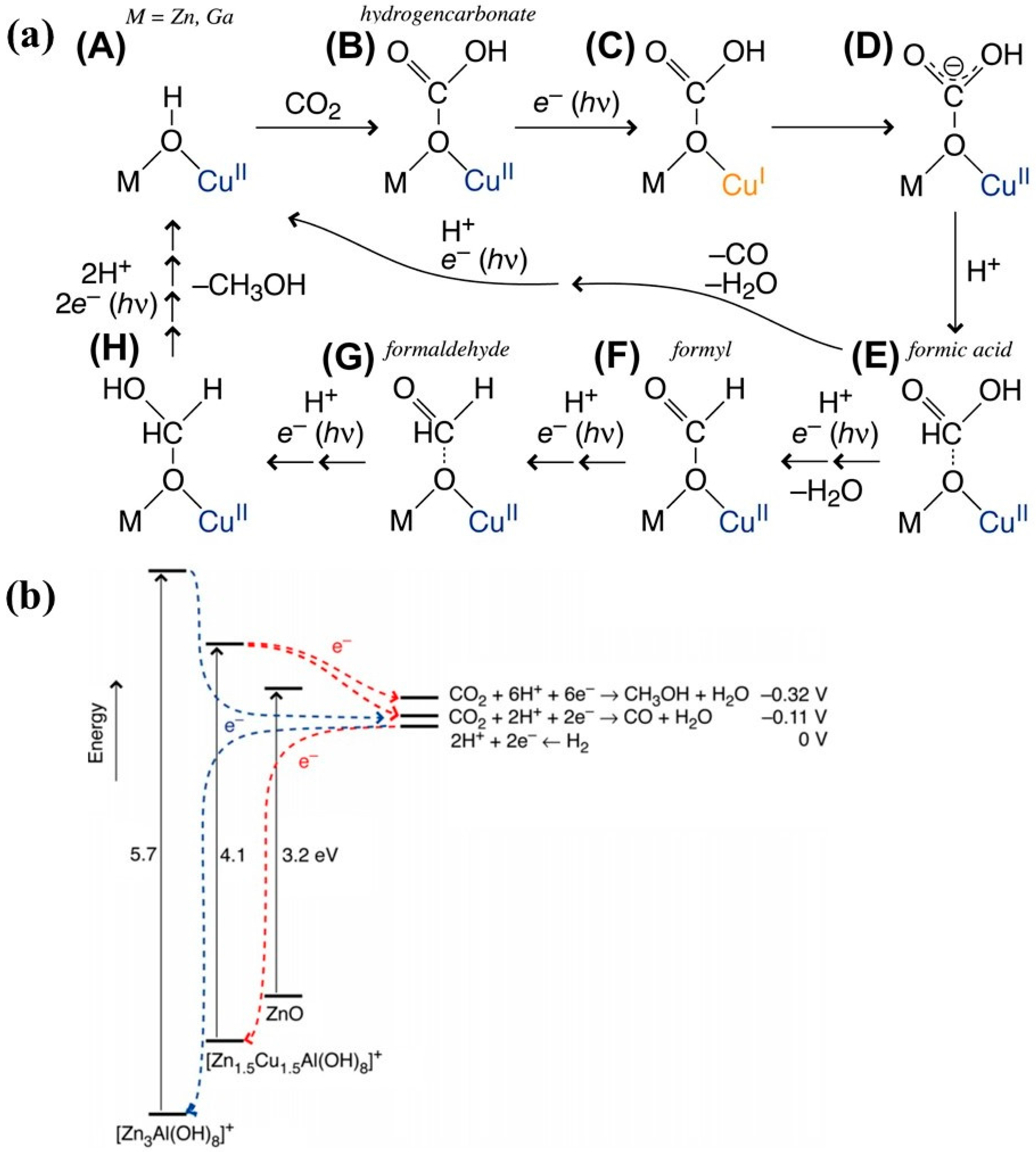
2 sorption capability because of their layered morphology. Moreover, the choice of metal cations within LDH can well tune the photocatalytic activity by influencing its light absorption and sorption attributes. Ahmed et al. [
45] studied the effect of the metal cation for zinc-copper-M (III) LDH, where M stands for aluminum (Al) or gallium (Ga) metal. It was observed that samples composed of ternary metal ions, i.e., ZnCuAl and ZnCuGa LDH, show a red shift in their light absorption as compared to ZnAl and ZnGa LDHs. Hence, the insertion of Cu in both mentioned LDHs extends their absorption towards the visible range, which might be due to the generation of an electronic state induced by Cu ions, thus narrowing down the band gap. Under the UV–Vis photo irradiation and reactant mixture consisting of CO
2 and H
2, the ZnCuAl and ZnCuGa LDH produced mainly CH
3OH as a key product component as compared to ZnAl and ZnGa LDHs, which mainly yielded CO as a key product. Hence, the selectivity of the product mainly from CO switches to CH
3OH with the incorporation of the Cu in the LDH. The role of Cu addition is responsible for increased production of CH
3OH as compared to CO. Cu sites within the LDH might be providing a binding site for CO
2, thus interacting with photogenerated electrons, protons, and probably the Cu
I/Cu
II redox couple. The proposed mechanism is shown in
Figure 2a, where the interlayer hydroxl group linked with Ga/Al and Cu ties with the CO
2 molecule resulting in hydrogen carbonate, which undergoes a series of reduction reactions in presence of protons finally forming the CH
3OH as a major product. Furthermore, the insertion of Cu narrows down the band gap and well, aligning it with the redox level of CO
2/CH
3OH as shown by energy level diagram in
Figure 2b. Such a band gap alignment is favorable for producing CH
3OH under light irradiation.
As investigated in their previous research [
45], the interlayer space within LDH layers acts as an active site for the CO
2 conversion to CH
3OH with higher selectivity when Cu is inserted. Hence, extending their investigation with the modification of the interlayer space of the LDH for enhancing the photocatalytic performance via anionic species between the cationic LDH layers, Ahmed and co-authors [
46] found an increased CH
3OH formation rate. They found, during the synthesis process of Zn
3Ga|CO
3 and Zn
1.5Cu
1.5Ga|CO
3 LDH, the (CO
3)
2− is replaced with [Cu(OH)
4]
2− anions by [CuCl
4]
2− ions hydrolyzed in the alkaline solution. It was observed with the intercalation of the [Cu(OH)
4]
2− anions that the light absorption is shifted towards the lower energy as compared to samples with (CO
3)
2− anions. Moreover, Cu insertion also exhibits the absorption in the visible range due to intrinsic property of Cu ions. The Zn
3Ga|Cu(OH)
4 LDH photocatalyst exhibits 5.9 times higher CH
3OH production as compared to Zn
3Ga|CO
3 and similar to that of Zn
1.5Cu
1.5Ga|CO
3. However, Zn
1.5Cu
1.5Ga|Cu(OH)
4 shows an increase selectivity of CH
3OH up to 88%. The investigation concludes that the modification of the respective LDH photocatalysts with the Cu(OH)
4 anions instead of CO
3 anions leads to increase of interlayer space between the LDH thus resulting in more reactive space. Secondly, the insertion of the Cu ions narrows the bandgap of the resulting LDH photocatalyst with more light absorption and thus better photocatalytic performance.
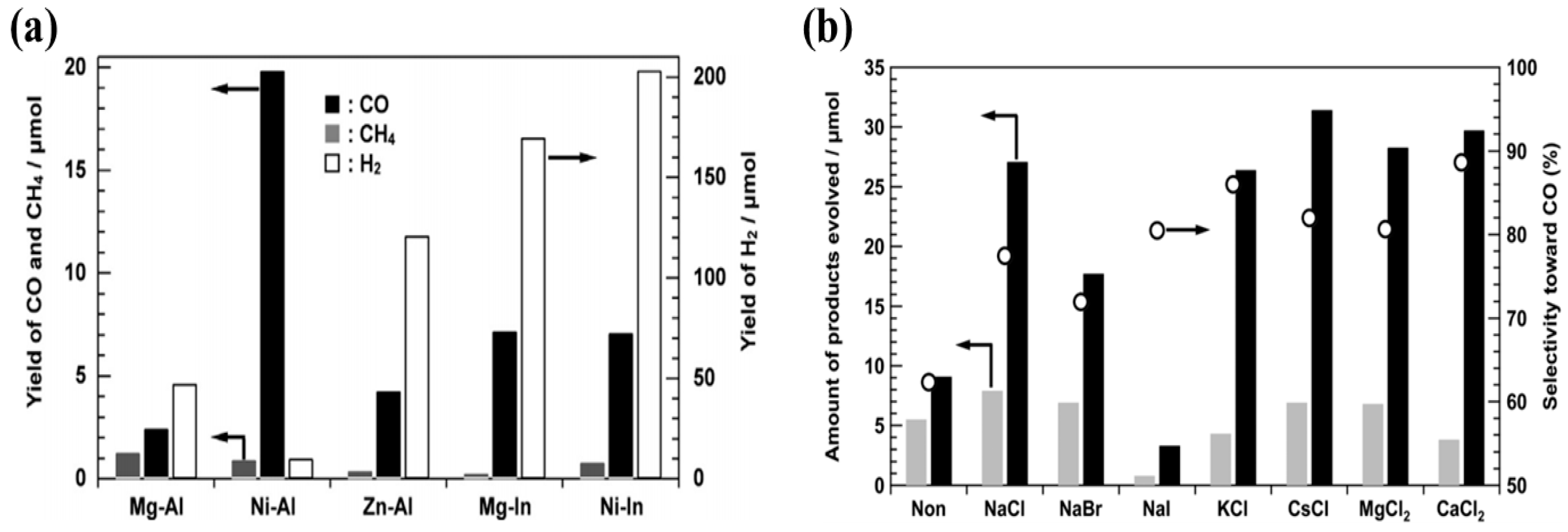
Another report investigates the role of the precursor anions for hydroxide sheets, for various LDH photocatalysts including Ni-Al, Zn-Al, Mg-In, and Ni-In [
47]. The LDH were synthesized by both nitrates (LDH-NO
3−) and chlorides (LDH-Cl
−) and evaluated for their photocatalytic activity in terms of CO
2 conversion to useful chemicals. It was observed that the LDH with Ni/Al ratio of 4 and synthesized using chlorides exhibited the maximum CO
2 conversion to CO. It was noticed that except Ni-Al LDH-Cl
−, all other samples selectively generate H
2; however, Ni-Al LDH-Cl
− mainly yielded CO from CO
2 photo-reduction. The production rate of various products obtained from variety of LDH photocatalysts can be seen in
Figure 3a. The reason for the selective formation of CO for Ni-Al LDH-Cl
− can be attributed to the co-catalytic behavior of the Ni species, which in turn leads to the enhancement of CO formation as the Ni ratio is increased. For the other LDH photocatalysts, H
2 is mainly produced due to competition of H
+ being reduced during the CO
2 photo-reduction, a well-known competitive reaction phenomenon. Furthermore, when the Ni content is decreased within Ni-Al LDH-Cl
−, the CO formation is decreased, and H
2 formation is increased, which endorses Ni species to act as a co-catalyst during the CO
2 photo-reduction reaction. The Ni-Al LDH-NO
3−, on the contrary, showed the production of CH
4 when employed for CO
2 photo-reduction. The authors also found that Cl
− ions in the aqueous solution also act as hole scavengers, thus promoting the selective formation of CO instead of other products.
Continuing the role of Cl
− ions, Iguchi et al. researched the effect of Cl
− ions as a hole scavenger in CO
2 photo-reduction employing Ni-Al LDH [
48]. Various additives were added in the aqueous solution containing suspended LDH photocatalyst, bubbled with CO
2 gas, and irradiated using 400 W high pressure mercury lamp. The additives were NaHCO
3, Na
2CO
3, Na
2SO
4, NaNO
3, and NaCl. It was observed that NaHCO
3 and Na
2CO
3 exhibited a greater selectivity towards H
2 generation due to favorable reduction of H
+ ions generated in water. The Na
2SO
4 and NaNO
3 did not contribute in the formation of either product, H
2 or CO. However, when NaCl was used as an additive, a significant amount of CO was produced with higher selectivity as compared to all other additives. The selectivity along with production rate of H
2 and CO with various inorganic additives can be viewed in
Figure 3b (dark bar represents CO, grey bar H
2 and circle represents selectivity towards CO formation). In order to understand the mechanism involved, DPD (N, N-dimethyl-p-phenylenediamine) test was performed. The DPD test indicated that when the NaCl was added to aqueous solution, the product was consisted of CO, H
2 and HClO under photoirradiation as a reaction amongst CO
2, H
+ and Cl
−. Hence, the formation of HClO as an oxidant product during the photoreaction indicates that Cl
− is expected to be a strong reducing agent and is rapidly oxidized by holes in the solution leading to yield HClO and selective formation of CO.
Another report investigated the role of fluorinated surface of LDH photocatalysts and their role in the CO
2 conversion enhancement [
49]. The authors fabricated fluorinated Mg-Al and Ni-Al LDH photocatalysts and evaluated their photocatalytic performance for the CO
2 conversions in aqueous solution. The fluorination was done by incorporating hexafluoroaluminate (AlF
63−) units within the hydroxide sheets of the LDH. In addition, various samples of LDH were prepared by changing the concentration of fluorine precursor, with the optimized sample providing the maximum yield of CO as a main product of CO
2 photo-reduction. It was observed and confirmed in authors previous works that the Ni-Al LDH is more efficient in selective formation of CO as compared to Mg-Al LDH as Ni species are supposed to act as co-catalyst, thus suppressing the formation of H
2. However, the optimized fluorine content (which was estimated by ratio of fluorine precursor to the aluminum precursor of LDH and was found 7.5) significantly enhances the formation of CO with NaCl as an additive for the hole scavenger. Such an improvement is mainly associated with the increased surface area of the fluorinated LDH, which leads to higher sites for CO
2 adsorption and thus increased CO
2 photo-reduction to CO.
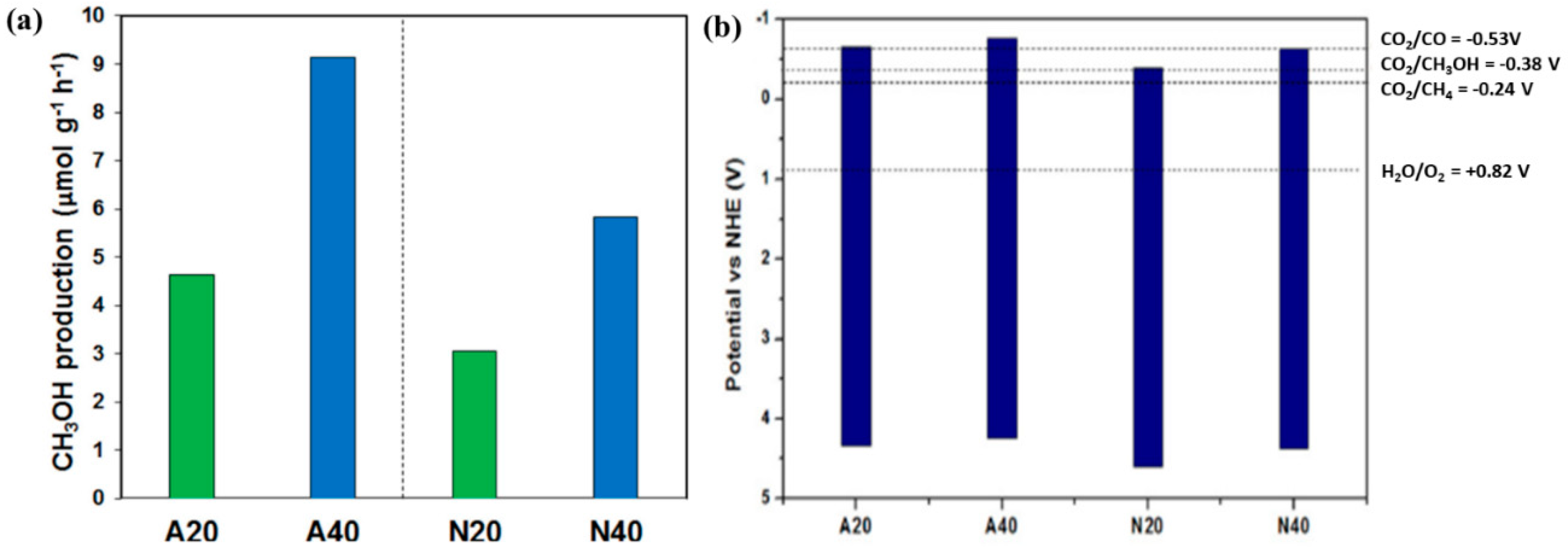
The work done by Flores et al. investigates the role of various Mg sources, organic precursor and a one-step microwave-hydrothermal method on the photocatalytic activity of Mg-Al LDH [
50]. Two different salts of magnesium, i.e., magnesium acetate and magnesium nitrate, were used with organic urea as a source of CO
3− and OH
− within LDH layers. The LDH photocatalysts were then subjected for two different times for microwave radiation for 20 and 40 min and at a temperature of 100 °C. All the samples were employed for photocatalytic CO
2 conversion in gas as well as liquid phase reactions. It was observed that the crystallinity of the synthesized LDH was significantly influenced by the nature of the Mg precursor and microwave irradiation time. The greater microwave irradiation time leads to formation of a larger crystallite size along with decomposition of organic urea resulting in formation of NH
4+, OH
−, H
+, and CO
32− ions, which will readily react with Mg
2+ due to higher dipole moment, thus resulting in better crystallinity. When the prepared LDH photocatalysts were employed in liquid phase for CO
2 conversion, the key product formed was CH
3OH with the highest yield obtained from Mg-Al LDH prepared by respective acetate salt under 40 min microwave radiation. Hence, by increasing the microwave irradiation time, the crystallite size and crystallinity are improved, which leads to decreased impedance, whereas, the decomposition of acetate anions adsorbed on LDH surface in turn facilitated the electron transfer towards the CO
2 sites within LDH. Such decomposition for LDH obtained by nitrates salts resulted in formation of NO
3−, which can occupy the CO
2 active site.
Figure 4a displays the CH
3OH production by various LDH photocatalysts prepared in the reported work. When the optimized LDH photocatalysts were employed for gas phase reactions, the key products obtained were CO and CH
4. Such formation of CO and CH
4 under gas phase is more favorable due to its potential lowering than the CH
3OH formation and deoxygenation of C-species with electrons. The band gap alignment of all the Mg-Al LDH prepared in the respective work is shown in
Figure 4b.
A recent work done by Wang et al. presents synthesis of Co-Al LDH nanosheets for the conversion of very low concentration atmospheric CO
2 and water to CH
4 [
51]. Furthermore, the investigation also focuses on the importance of alkaline OH group and divalent cobalt for efficient CO
2 conversion to CH
4.
Figure 5a,b show the morphology of the prepared Co-Al LDH nanosheets, showing hexagonal sheet like morphology and thickness of 18 nm. The unique attribute was the uniform composition of Co, Al, and O elements. The UV–Vis absorption spectrum for the Co-Al LDH nanosheets suggests the light absorption of the synthesized photocatalyst lies in the visible range with an estimated band gap of 2.1 eV. Thus, the respective photocatalyst can capture the visible range of the light, which can probably lead to improve photocatalytic performance. The synthesized Co-Al LDH nanosheets were found to be active for 55 h of irradiation with atmospheric CO
2 (400 ppm), yielding CH
4 as a main product with rate of 4.3 µmol g
−1 h
−1, which is 13 times higher as compared to standard TiO
2 sample, i.e., P25. The improved CH
4 generation was mainly attributed to the enhanced CO
2 adsorption by surface OH groups and unique effect of divalent cobalt. The divalent characteristic of cobalt was investigated by oxidation of the Co-Al LDH in oxygen atmosphere, and it was observed that the photocatalytic performance was sharply decreased when the LDH was oxidized. Moreover, the LDH photocatalyst exhibited a stable photocatalytic performance over five cycles of repeated reaction as shown in
Figure 5c. The mechanistic view displaying the proposed reduction mechanism for CO
2 conversion upon the surface of Co-Al LDH is shown in
Figure 5d. The CO
2 reduction is proposed to initiate with the chemisorption of CO
2 and OH groups, which can bend linear CO
2 molecule, thus activating them to form carbonate species. These carbonate species in the presence of electrons and protons undergo multistep proton assisted reaction forming CO, which is finally converted to CH
4 and other useful chemicals.
In another work, Tokudome et al. reported synthesis of nanocrystalline Ni-Al LDH (20 nm) by simple homogenous supersaturation process induced via rapid increase of pH form concentrated aqueous salt solutions of precursors [
52]. When Ni-Al LDH was employed for CO
2 conversion in aqueous medium, the CO formation rate (selectivity of 80%) was found 7 times higher as compared to the standard Ni-Al LDH prepared by conventional method. The key for the improved photocatalytic performance was attributed to the metastable surface of the nano LDH due to high degree of supersaturation.
Figure 6a shows the morphology of the nano LDH and standard LDH. Nano LDH mainly consists of 20 nm particle size in agglomerated form whereas the conventional LDH is mainly composed of various size ranges of platelets within a range of 40–200 nm.
Figure 6b shows the photocatalytic CO
2 conversion to CO in aqueous media. It was observed that nano LDH exhibited a significant increase in CO production as compared to standard LDH. This was mainly attributed to the surface affinity of the nano LDH towards the CO
2 adsorption in gaseous phase. Such a specific surface nature is induced mainly due to synthesis process designed in the research work. Furthermore, for the purpose of comparison, various control samples were synthesized in order to investigate the role of morphology and crystallinity of nano LDH for CO
2 conversion. However, it was observed that all those parameters, when improved, exhibited a decrease in photocatalytic performance; hence ultimately, the metastable surface of the nano LDH and its superb affinity towards CO
2 adsorption can only be considered as the key to success of improved photocatalytic performance.
A recently published research work by Gao et al. presented an ultrathin Mg-Al LDH modified with Fe
3O
4 by a simple coprecipitation approach [
53]. It was observed that Fe
3O
4/Mg-Al LDH (FMAL) exhibited significant improvement in the photocatalytic activity, which was due to synergetic effect of efficient separation of electron-hole pairs and reduction of resistance for charges transmission induced by Fe
3O
4 and ultrathin Mg-Al LDH, respectively.
Figure 7a shows the transmission electron microscopy (TEM) image of Fe
3O
4/Mg-Al LDH, which exhibits thin 2D nanosheets of Mg-Al LDH with Fe
3O
4 nanoparticles loaded on nanosheets. The UV–Vis DRS for the Fe
3O
4/Mg-Al LDH is shown in
Figure 7b. The light absorption spectrum covers visible region due to presence of Fe
3O
4 nanoparticles, which possess a narrow band gap and almost act like a conductor. Such conductive behavior leads to inhibition of photogenerated electron-hole recombination, thus contributing to improved photocatalytic performance.
Figure 7c,d show the photocatalytic production of CO and CH
4 by various Fe
3O
4/Mg-Al LDH samples (with varied content of Fe
3O
4). It was observed that the sample FMAL-10 with 10 wt.% of Fe
3O
4, exhibited the highest CO and CH
4 yield, and hence, it was selected as an optimized sample. The key factors as explained above were supposed to be the efficient charge separation by the Fe
3O
4 content and the rapid transformation of the photoexcited charges towards the reactive sites on Fe
3O
4/Mg-Al LDH surface. Further increase of Fe
3O
4 content decreased the light transmission characteristic and hence reduced photocatalytic performance.
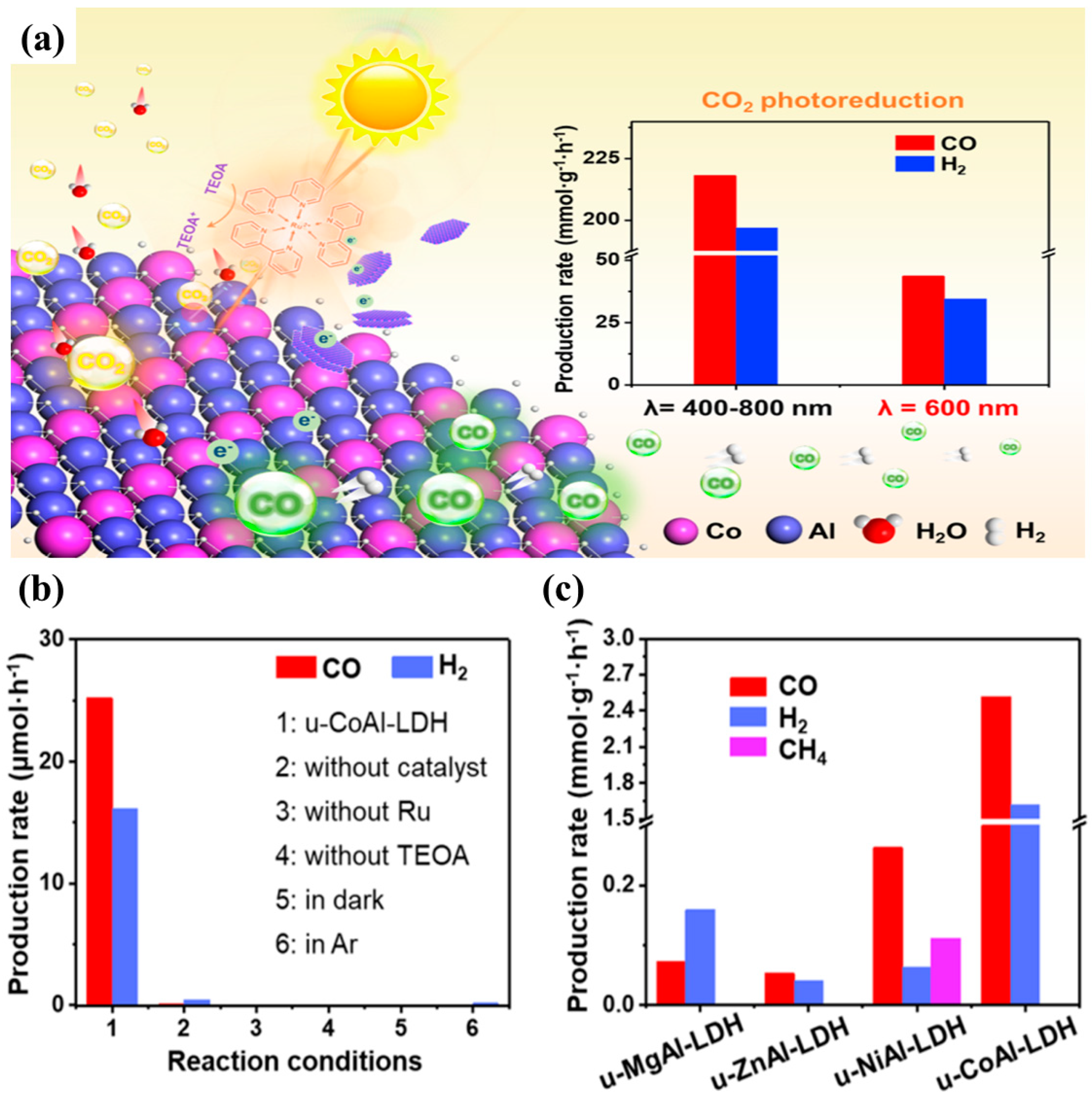
Recently, Bai et al. reported four different types of ultrathin MAl-LDH photocatalysts (u-MAl-LDH, where M stands for Mg
2+, Ni
2+, CO
2+ and Zn
2+) for CO
2 conversion to mainly CO [
54]. The synthesized u-MAl-LDH were tested under visible light irradiation (λ = 400–800 nm) and employed with a [Ru(bpy)
3]Cl
2·6H
2O photosensitizer for photocatalytic CO
2 conversion to CO. Amongst various samples synthesized, the u-CoAl-LDH showed the highest efficiency,
Figure 8a shows the schematic view of proposed photocatalytic CO
2 conversion employing u-CoAl-LDH with production of CO and H
2 when irradiated with visible light (400–800 nm) and light at 600 nm wavelength.
Figure 8b shows that the photocatalytic reactions were not initiated without [Ru(bpy)
3]Cl
2·6H
2O and triethanolamine, thus endorsing the important roles of [Ru(bpy)
3]Cl
2·6H
2O as a photosensitizer and triethanolamine as a hole scavenger for regeneration of LDH photocatalysts valence bands.
Figure 8c illustrates the photocatalytic CO
2 conversion products for all the synthesized u-MAl-LDH photocatalysts. It could be observed that all samples exhibit less amount of CO and H
2 production as than u-CoAl-LDH, which exhibited the highest conversion rate. The u-NiAl-LDH sample also exhibited production of CH
4 due to well-known selectivity of Ni towards CH
4. The key reason for improved photocatalytic performance can be associated to (i) defects on the photocatalyst surface due to its ultrathin nature thus promoting the CO
2 adsorption; (ii) improved light absorption, thus capturing the maximum of visible light region; and (iii) well matched energy levels of u-CoAl-LDH with [Ru(bpy)
3]Cl
2·6H
2O photosensitizer, thus on light irradiation the photogenerated charges are drained towards the LDH surface where they can efficiently react with the adsorbed CO
2.
Another work proposed by Xiong et al. presented the role of trivalent and tetravalent in Zn based LDH photocatalysts employed for photocatalytic CO
2 conversion [
55]. It was observed that choice of trivalent or tetravalent cation in the LDH photocatalyst alters the selectivity of the product. Authors synthesized a variety of ZnM-LDH (where M = Ti
4+, Fe
3+, Co
3+, Ga
3+, Al
3+) and observed that ZnTi-LDH generates CH
4 as main product, whereas ZnFe-LDH and ZnCo-LDH were unable to reduce CO
2 and yield H
2 by water splitting only. Moreover, ZnGa-LDH and ZnAl-LDH yielded CO as main product of CO
2 photo-reduction. By help of in situ diffuse reflection, infrared Fourier transform spectroscopy (DRIFT), and computational calculations, it was revealed that the metal cations with d band level (
ԑd) closer to the fermi level interact favorably with CO
2, leading to its well adsorption, and hence, light irradiation converts the adsorbed CO
2 into carbon containing products, i.e., CH
4 and CO, whereas the metals with d band level far from the fermi level exhibited very less CO
2 adsorption, thus yielding H
2 due to water splitting.
Figure 9a shows the mechanistic view of the synthesized ZnM-LDH photocatalysts and their selectivity to certain products.
Figure 9b shows the photocatalytic product generated by the ZnM-LDH photocatalysts, and
Figure 9c exhibits the respective selectivity.
In addition to the development of LDH photocatalysts, another important and beneficial approach is to use the LDH as a base material to convert it into a highly efficient photocatalyst. Recently, Wang et al. fabricated thin and defective NiO/Al
2O
3 composite from NiAl-LDH [
56]. The NiAl-LDH photocatalyst was transformed to NiO with Ni and oxygen vacancies under oxidation and was investigated at various oxidation temperatures from 200–800 °C. The schematic view of the proposed mechanism for synthesis methodology is exhibited in
Figure 10a. The photocatalytic CO
2 conversion to various products with selectivity can be seen in
Figure 10b,c, respectively. It could be observed, NiO sample synthesized at a temperature of 275 °C, yielded maximum CH
4 under the irradiation of 600 nm wavelength light. Hence, this temperature meant to be an optimum condition for having the best oxygen and nickel vacancies.
The summary of the various bare/pure LDH photocatalysts regarding their research objective, reaction conditions, products yielded, and influential parameters are tabulated in
Table 1.
4. Metals Loaded/Embedded Layered Double Hydroxide (LDH) Photocatalysts
Another effective strategy for the development of efficient LDH photocatalysts is to couple, embed, or dope the metals/non-metals to LDH structures, which will lead to improved photocatalytic performance in terms of CO2 photo-reduction. Until today, a limited amount of investigations has been done with respect to mentioned domain; however, there exists an enormous potential to develop metals embedded or doped LDH, which might result in an enhanced and efficient photocatalytic CO2 conversion to useful chemicals/fuels.
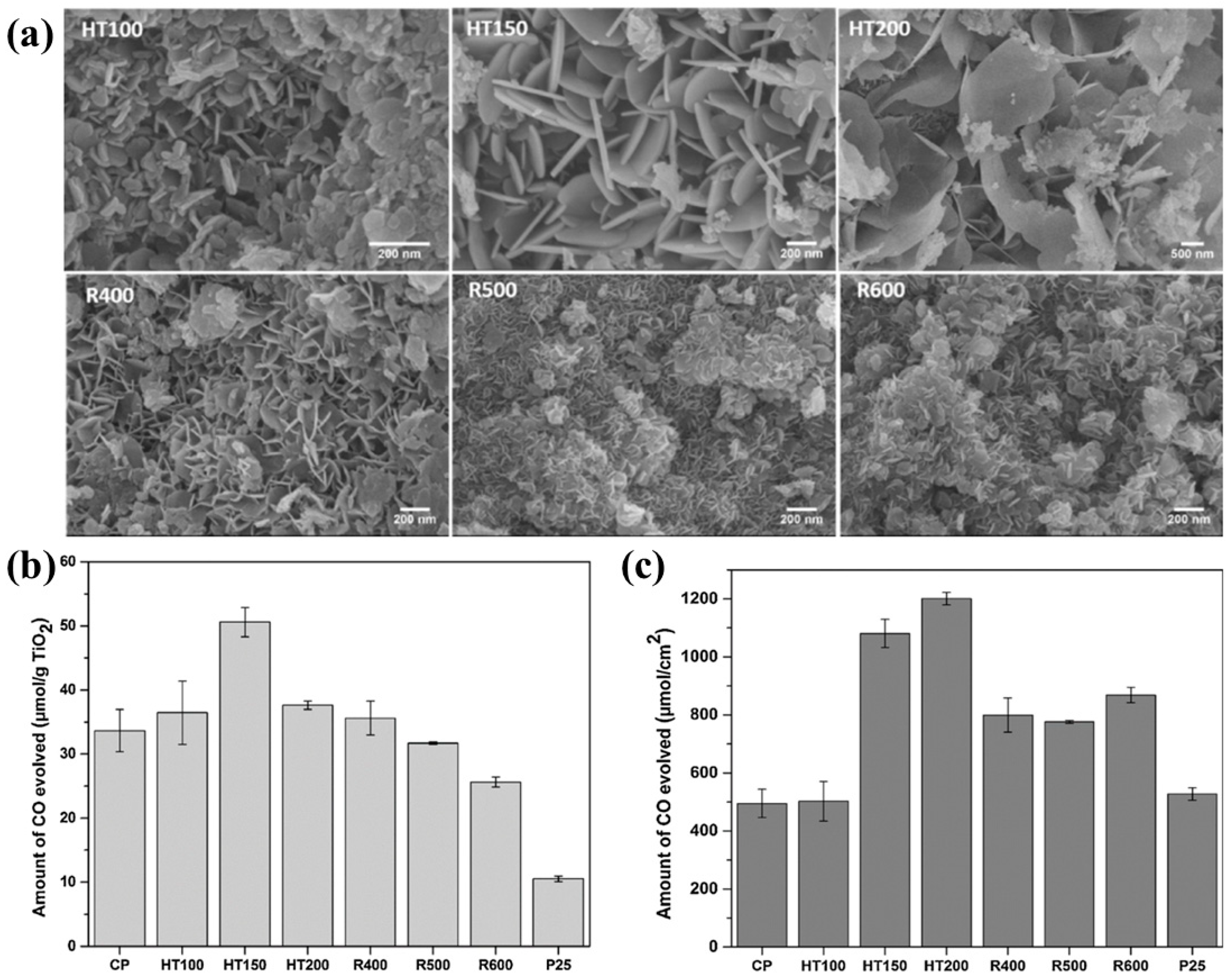
Zhao et al. reported a Ti embedded MgAl-LDH (MgAlTi-LDH) synthesized by three different methodologies and their performance evaluated by photocatalytic CO
2 conversion with water to useful products [
57]. The three different synthesis methodologies include (i) co-precipitation (CP), (ii) co-precipitation and hydrothermal (HT), and (iii) co-precipitation followed by calcination and reconstruction (R). It was observed that the two key factors influencing the photocatalytic activity were crystallinity and surface area of the prepared samples. Moreover, the effect of the temperature treatment was also investigated in accordance with the photocatalytic performance.
Figure 11a shows the surface morphologies for the MgAlTi-LDH samples synthesized by coprecicpitation method followed by HT and R treatment at various temperatures, respectively. It was observed that the CP samples hydrothermally treated at different temperatures go under transformation of their small nanoflakes (50 nm) to large nanoflakes with increased size (500–1000 nm). Such transformation indicates the increase of crystallite size, which was also confirmed by XRD data. On the other hand, calcinated and reconstructed samples (R-MgAlTi LDH) do not exhibit any significant change in the crystallite size with the increase of the temperature. When employed for photocatalytic CO
2 tests, all samples exhibited better CO yield as compared to standard TiO
2-P25.
Figure 11b,c show the photocatalytic CO
2 conversion to CO normalized by catalyst mass and surface area, respectively. It was noticed that HT150 and HT200 samples (hydrothermally treated sample at 150 and 200 °C) exhibit best performance in accordance with both parameters, which can be attributed to the optimum condition of TiO
2 crystallinity, crystallite size, band gap, and surface area of the LDH photocatalyst.
A research work reported by Iguchi et al. presented extension of their previously reported work by modifying the MgAl LDH with Ga
2O
3 and loading Ag nanoparticles on it [
58]. It was observed that by loading 0.25 wt.% of Ag co-catalyst of Ga
2O
3-MgAl LDH, the photocatalytic CO
2 conversion to CO was improved with enhanced selectivity towards CO. The molar concentration of already prepared MgAl LDH was varied in order to optimize the sample with the best performance. It was observed the sample with 95 molar concentration and 0.25 wt.% of loaded Ag co-catalyst, exhibited the best performance, yielding 211.7 µmol/h with 0.19 g of photocatalyst. Such an increased yield was associated to the enhanced CO
2 adsorption by MgAl LDH, improving surface areas and selectivity induced by Ag nanoparticles.
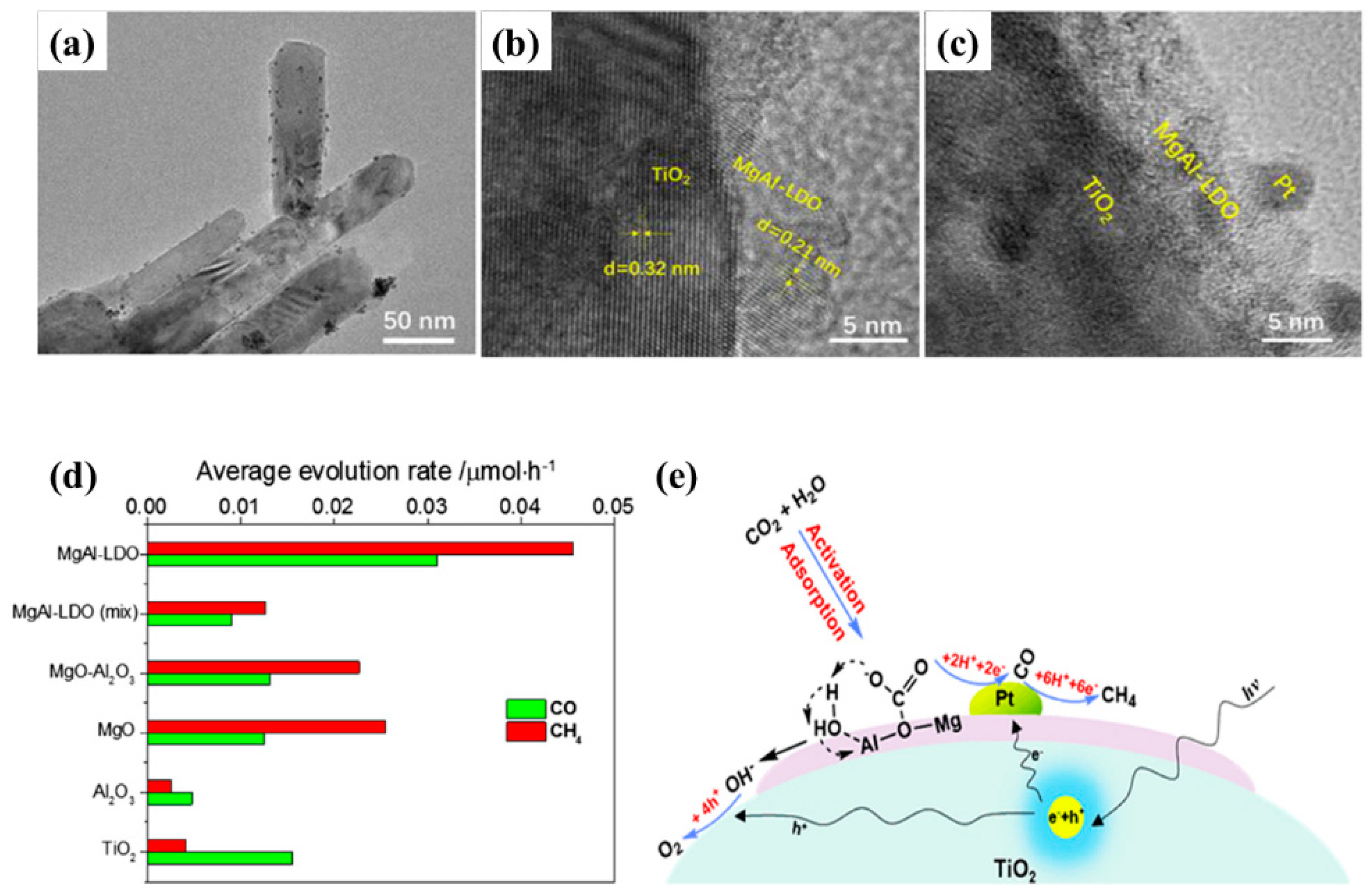
Another research investigated by Chong et al. presents the role of MgAl-LDO as an interlayer in between Pt and TiO
2 for CO
2 photo-reduction to useful chemicals [
59]. It was observed that the insertion of MgAl-LDO layer boosts the photocatalytic CO
2 conversion to CO and CH
4 as compared to reference samples. The authors used rutile TiO
2 rods, as they are more active for water oxidation, thus providing more protons and electrons, which can be utilized for CO
2 photo-reduction. The MgAl-LDH was in situ deposited and then calcined at high temperature to give LDO upon which the Pt (fixed 1 wt.%) was deposited. The sample optimization was performed by varying the loading amount of MgAl-LDH on the TiO
2 surface. It was observed that there exist no significant changes in the sample crystallinity, surface morphology, and surface areas.
Figure 12a–c shows TEM images for the Pt-MgAl-LDO-TiO
2 photocatalyst with clear depiction of the MgAl-LDO interlayer and Pt deposition.
Figure 12d shows the photocatalytic CO and CH
4 evolution rate from CO
2 photo-reduction and it can be noticed that Pt-MgAl-LDO-TiO
2 exhibit the highest production rate as compared to all other samples. The key parameter for promoting and enhancing the photocatalytic CO
2 conversion is synergetic effect of Pt deposition and interlayer of MgAl-LDO. It was proposed that the thin layer of MgAl-LDO optimized by MgAl-LDO deposition promotes the CO
2 adsorption and efficient transfer of photogenerated electrons to Pt where the CO
2 photo-reduction can occur. Moreover, the Pt nanoparticles act as electron sinks, and once they receive the photoexcited electrons, it is difficult for them to go back and recombine. The proposed reaction mechanism is well displayed in
Figure 12e.
Another research conducted by Wang et al. developed series of palladium (Pd) loaded CoAl-LDH (Pd/CoAl-LDH) photocatalysts with varied content of Pd, in combination with a ruthenium-based complex acting as a photosensitizer [
60]. When these photocatalysts employed for photocatalytic CO
2 conversion, the syngas CO/H
2 was the major product, where Pd promotes the production of H
2 and CoAl-LDH yielded CO under visible light irradiation even when extended above 600 nm. It was observed that the ratio of CO/H
2 can be tuned by varying the loading of Pd nanoparticles.
Figure 13a,b show the production rate of CO/H
2 and selectivity with varying the ratio of Pd. It can be noticed that with increasing the Pd amount, the relative amount of H
2 is increased due to reduction of protons. As Pd is a well-known H
2 producing cocatalyst, H
2 is produced by Pd, which captures the photogenerated electrons from the LDH, whereas the photogenerated electrons within the LDH can directly react with the adsorbed CO
2 to convert it into CO. The proposed mechanism of the CO
2 photo-reduction over the prepared photocatalysts is shown in
Figure 13c where triethanolamine (TEOA) assists to regenerate the Ru based photosensitizer.
The summarized overview of up to date developed metal embedded/loaded LDH photocatalysts employed for photocatalytic CO
2 conversion to useful chemicals are presented in
Table 2 in respect to research objective, reaction conditions, products yielded, and important influential parameters contributing to enhanced performance.
5. Composite/Hybrid/Heterojunctioned Layered Double Hydroxide (LDH) Based Photocatalysts
The fabrication of hybrid/heterojunctioned photocatalyst materials has always been an attractive and fascinating domain for the material scientists/researchers. As a well-explored domain, a similar approach is also implicated for the synthesis of hybrid/heterojunctioned photocatalysts employing LDH as one of the components with another established semiconductor material. The LDH support can act as an adsorbent and/or photocatalyst support to capture/convert CO2 into useful products. Hence, the benefits and gains of the hybrid/heterojunctioned materials can be a harvested for CO2 reduction by designing a suitable LDH based hybrid photocatalysts. With plenty of research space, until now, very limited investigations have been done with the respective concept.
A research work reported by Zhao et al. displays hybrid photocatalysts composed of MgAl-LDO coupled with TiO
2 cuboids (MgAl-LDO/TiO
2) [
61]. The photocatalyst was synthesized by the combined approach of hydrothermal and coprecipitation methodologies, and a series of MgAl-LDO/TiO
2 was prepared with varied molar ratio of Mg + Al to Ti, in order to optimize the photocatalyst with best performance. As the MgAl-LDO are reported to be in micrometer size platelets, it was required to synthesize micrometer size TiO
2 which were synthesized in micrometer sized cuboids.
Figure 14a–f shows the morphology of various MgAl-LDO/TiO
2 photocatalysts with varied ratio of Mg + Al. It is obvious that MgAl-LDO platelets were successfully anchored on the TiO
2 cuboids even after calcination and do not change the morphology. Hence, this report is also unique in terms of morphology of the photocatalyst obtained. When employed for CO
2 photo-reduction with water vapors under UV light irradiation, CO was found to be the main product with small amount of CH
4.
Figure 14g shows the CO production rate by photocatalytic CO
2 conversion. It was observed that at a condition of UV light irradiation (4 h) and 50 °C, MgAl-LDO alone had no activity, and also, when coupled with the TiO
2, it did not show any significant photocatalytic activity towards CO
2 conversion to CO. This might be due to weak CO
2 adsorption at lower temperatures by MgAl-LDO. However, at higher temperature, i.e., 150 °C, the 10% Mg-Al LDO/TiO
2 showed a 5 times higher CO production as compared to bare TiO
2 cuboids. Moreover, 10% MgAl-LDO/TiO
2 also exhibited the best performance, hence optimizing the Mg + Al ratio to Ti. Such an enhanced CO production can be attributed to the improved adsorption capacity of MgAl-LDO at higher temperatures. Upon illumination, the photogenerated electron in TiO
2 can easily transfer to the nearby interface of MgAl-LDO and TiO
2, where it can react with adsorbed CO
2 converting it into CO.
Figure 14h shows control tests of 10% MgAl-LDO/TiO
2 under He gas and water vapors as reactants. It was observed that, at a temperature of 50 °C, 10% MgAl-LDO/TiO
2 does not show any CO yield; however, at a temperature of 150 °C, a considerable amount of CO was observed. It was proposed that such CO might be a result of carbonate species on the surface of photocatalyst, which was stable at lower temperature, but at higher temperature, it was converted into CO.
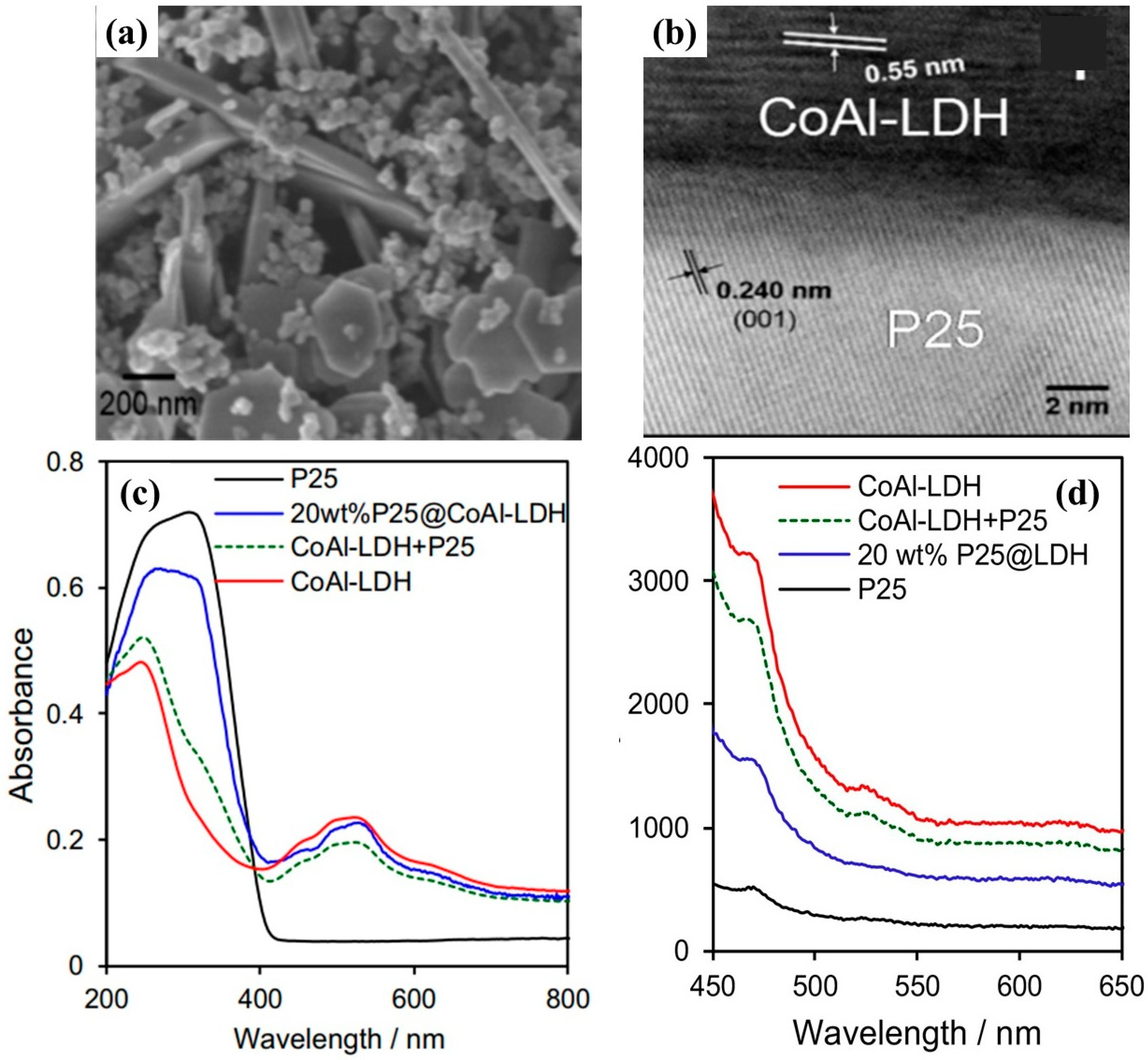
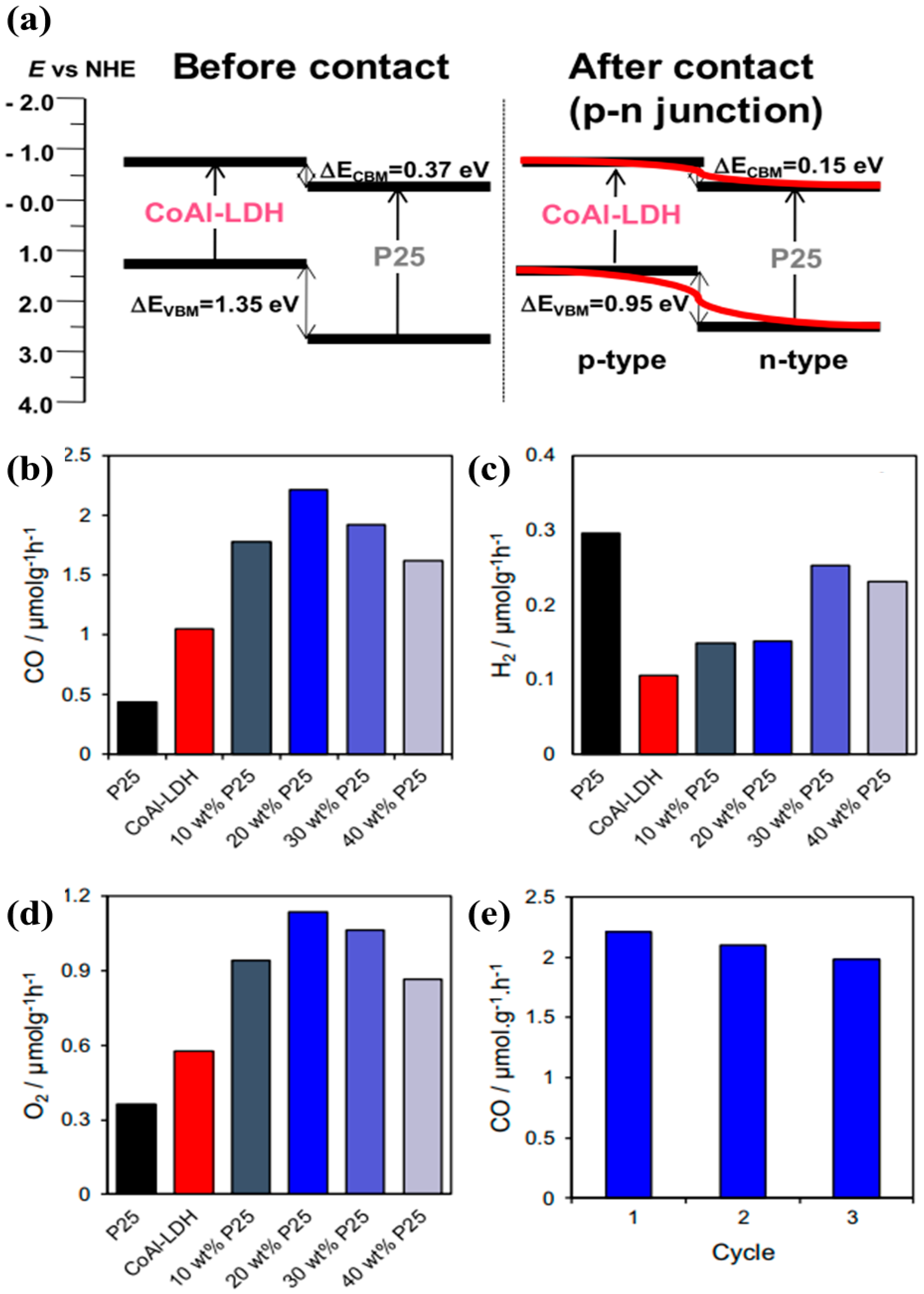
Another work performed by Kumar et al. investigated the heterojunction of CoAl-LDH with P25, a well-known standard TiO
2 [
62]. P25 nanoparticles were encapsulated within microporous CoAl-LDH by a simple one step hydrothermal approach. It was observed that light absorption of the composite CoAl-LDH -P25 was red shifted, thus capturing the solar spectrum which might lead to improved photocatalytic activity. Furthermore, the CO
2 adsorption capacity and matched band alignment also promote the efficient conversion of CO
2 into useful chemicals.
Figure 15a,b shows the morphology of the P25@CoAl-LDH photocatalyst with clear depiction of 2D platelets of CoAl LDH with thickness of approximately 20 nm and irregular nanoparticles of P25 within size range of 20–50 nm.
Figure 15c shows the UV–Vis DRS for all the samples investigated in the research work. P25 showed its characteristic absorption in the UV range with the absorption peak centered around 300 nm, whereas CoAl-LDH exhibited two absorption peaks appearing in the UV and visible range, respectively. The absorption peak appearing around 500 nm was attributed to the d–d transitions of octahedral Co
2+.
Figure 15d shows the steady state photoluminescence (PL) spectroscopy for the samples. The PL peaks for P25@CoAl-LDH were intermediate between pure P25 and CoAl-LDH, thus endorsing the reduced charge recombination within the composite P25@CoAl-LDH photocatalyst. When employed for photocatalytic CO
2 conversion, only three products were formed, i.e., CO, H
2, and O
2, shown in
Figure 16a–d. Pure P25 showed the poorest activity with negligible production; however, CoAl-LDH exhibited certain amount of CO and O
2 production. P25@CoAl-LDH showed a production in between P25 and CoAl-LDH, whereas 20 wt.% P25@CoAl-LDH showed a superior production of CO with negligible amount of CH
4. Such an increased CO production and higher selectivity can be attributed to the well heterojunction formation within P25@CoAl-LDH and was associated to extended light absorption, enhanced CO
2 adsorption, and effective photogenerated charges separation. Furthermore, the P25@CoAl-LDH sample also exhibited a stable performance up to 3 cycles of CO
2 photo-reduction.
Figure 15e shows the band gap alignment and heterojunction formation for the P25@CoAl-LDH photocatalyst. As well established, band gap of P25 is suitable for proton reduction but not suitable for CO
2 photo-reduction. On the contrary, the bandgap of CoAl-LDH with its conduction band edge at −0.75 eV can easily produce CO. Thus, the combination of both P25 and CoAl-LDH leads to formation of p-n junction which is well aligned for the efficient production of CO and photogenerated charge separation, ultimately resulting in enhanced photocatalytic activity for CO
2 conversion into useful product.
A research work executed by Tang et al. demonstrated the synthesis of unique homo-heterojunction of BiOCl nanoplates coupled with ZnCr-LDH by a simple electrostatic interaction method [
63]. As well-known BiOCl generally consists of two directions, that is, {001} and {110} surfaces. Both surfaces form a homojunction within the BiOCl, whereas the ZnCr-LDH can assemble on {001} surface via electrostatic interaction. A built-in field will allow the electrons generated under irradiation to flow from one facet to another facet of BiOCl, from where they can easily flow down to the coupled ZnCr-LDH and react with adsorbed CO
2. Various samples were synthesized with different content of the ZnCr-LDH represented by “Sc” wt.%.
Figure 17a shows the photocatalytic CO
2 conversion for various BiOCl-ZnCr-LDH samples with different contents of ZnCr yielding CH
4 as a main product. The maximum yield was obtained when ZnCr-LDH amount was 10 wt.%. The proposed mechanism for charge transfer within BiOCl homojunction and heterojunction with ZnCr-LDH is shown in
Figure 17b. The key factor indulged in the performance improvement was the efficient charge transfer.
Another research work executed by Yang and coauthors proposed an urchinlike Z-scheme photocatalyst by hierarchical structure of CoZnAl-LDH/RGO/g-C
3N
4 (LDH/RGO/CN) [
64]. The LDH was synthesized by simple hydrothermal method, while for the synthesis of LDH/RGO/CN, the already prepared RGO and CN were added into the ionic precursors of LDH and then subjected for hydrothermal process. For the sake of optimization and achieving of best sample, the weight ratio of CN was varied among the LDH/RGO/CN hybrid photocatalyst. The schematic view of the synthesis procedure designed to achieve LDH/RGO/CN is shown in
Figure 18a. The morphologies of CN and LDH/RGO/CN are shown in
Figure 18b,c, respectively. The CN exhibited a bulky morphology with somewhat lamellar structures, whereas LDH/RGO/CN exhibited a well observable urchin like nanostructure. When employed for photocatalytic CO
2 conversion, CO was observed as the key product, with CH
4 up to 5 h of irradiation; it is shown in
Figure 18d,e. It could be noticed that insertion of RGO between CoZnAl-LDH and CN significantly enhanced the CO yield, specifically for sample LDH/RGO/CN-2 (with 0.1 g of CN in the composite) whose CO yield was 3.4 and 8.5 times higher than LDH/CN and bare CN, respectively. As well established, CO
2 adsorption and photocatalytic activity are two prime contributors for efficient photocatalytic CO
2 conversion. The ternary composite of CoZnAl-LDH shows improved CO
2 adsorption whereas CN is well known for its visible light activity. In addition, the insertion of RGO promotes the efficient charge separation from CoZnAl-LDH to valence band of CN, whereas the holes of CoZnAl-LDH are generated by water oxidation. The proposed schematic based on several analysis techniques is displayed in
Figure 18f.
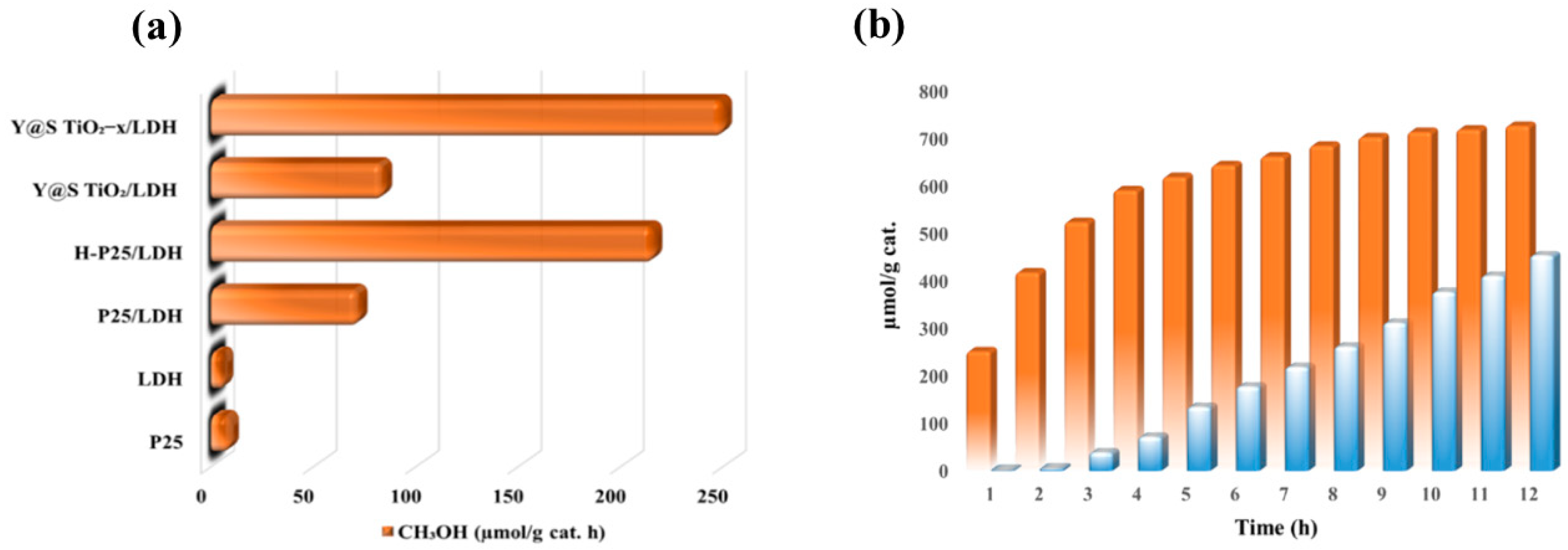
Another excellent work done by Ziarati et al. reported an efficient architecture of 3D yolk@shell TiO
2−x/ CoAl LDH (Y@S TiO
2−x/LDH) for photocatalytic CO
2 conversion to solar fuels [
65]. Such an architecture was synthesized by sequential designing of solvothermal, hydrogen treatment, and hydrothermal steps. When such a 3D architecture was employed for CO
2 photo-reduction, it yielded CH
3OH as a main product for the first 2 h of irradiation; however, the CH
3OH production decreased with the passage of time, and CH
4 yield increased.
Figure 19a shows the CH
3OH production for the 3D architecture photocatalyst along with other reference samples.
Figure 19b exhibits the time dependent photocatalytic CO
2 conversion to solar fuels. Such a conversion of product from CH
3OH to CH
4 is proposed, since, with the passage of time, CH
3OH gets adsorbed on the surface, and photocatalytic is converted into CH
3 radical, which reacts with electron and protons to form CH
4 as the key product. The enhanced performance of the 3D photocatalyst was attributed to the combination of better CO
2 sorption capacity, improved light absorption, and enhanced charge separation at interface of TiO
2−x and LDH.
Another approach represented an LDH based composite photocatalyst with doped strategy. Jo et al. developed a N doped C dots/ CoAl LDH/gC
3N
4 (NCD/LDH/CN, represented as NLC) hybrid photocatalyst with efficient and selective photocatalytic CO
2 conversion to CH
4 [
66]. The N doped carbon dots (NCD) are well researched materials as an alternate to noble metals cocatalysts. In their work, co-authors designed a strategy to employ NCD in replacement of noble metals and found a significant increase and selective production of CH
4. Various NLC photocatalysts were synthesized by varying the contents of LDH to CN as 5, 10, 15, and 20 wt.%, whereas the amount of NCD was kept fixed for all samples, which was 2 wt.%.
Figure 20a shows the SEM image of the NLC sample exhibiting a flower like morphology with clear depiction of LDH and CN.
Figure 20b shows a high resolution TEM image of NLC with clear presence of all three components, i.e., LDH, CN, and NCD in the hybrid sample. When utilized for photocatalytic CO
2 conversion, the key product obtained was CH
4 with small amounts of CO and H
2, as shown in
Figure 20c. It was observed when NLC is formed by 2 wt.% NCD and with LDH to CN ratio of 10, the sample NLC-10 exhibited the maximum production rate as compared to all other samples. The most remarkable selectivity was 99% towards CH
4 for NLC-10 sample. The reusability of the NLC-10 was also evaluated (shown in
Figure 20d), which displayed a durable performance in each cycle, thus representing the stable performance during the prolonged reaction possibly to a good structural stability of the photocatalyst. The prime factors contributing to significantly improved photocatalytic performance of NLC consist of broadened light absorption, optimized surface area, and improved charge separation due to 2D junction in between LDH and CN. Moreover, the product selectivity was attributed to the well aligned band gap with corresponding product redox potential. The proposed mechanism for the photocatalytic CO
2 conversion employing NLC-10 sample is shown in
Figure 20e. Under the visible light irradiation, both LDH and CN photogenerate the charges and electron flowing down to conduction band of LDH whereas holes from LDH towards the valence band of CN where they are regenerated by water oxidation. Due to formation of well-defined 2D–2D heterojunction between LDH and CN, the photogenerated charges are efficiently transferred and react with CO
2 adsorbed on the LDH surface. In addition, NCD also acts as electron sinks for efficient removal of electrons from conduction band of LDH to get reacted with adsorbed CO
2 and converted to CH
4.
Recently Wu and co-authors developed an efficient NiFe-LDH wrapped Cu
2O nanocube (NFC) heterostructure for enhanced photocatalytic CO
2 conversion [
67]. The NFC photocatalysts were synthesized by a simple co-precipitation approach with varied time of aging temperature.
Figure 21a–c shows the SEM images of NiFe-LDH, Cu
2O nanocubes and the NFC heterostructure photocatalyst, respectively. The NiFe-LDH consisted of interconnected layers resulting in a flower like morphology, whereas Cu
2O were in fine nanocubes. The NFC heterostructure shows a smooth structure of nanocubes covered with layers of LDH. The photocatalytic CO
2 conversion for NFC photocatalyst yielded mainly CH
4 as a key product.
Figure 21d shows the CH
4 production rate for various samples with varied aging time, i.e., 1, 2, and 4 h represented by NFC-1, NFC-2, and NFC-4, respectively. The NFC-4 displayed the highest efficiency amongst all the samples. The authors proposed a Z-scheme mechanism for conversion of CO
2 to CH
4, shown in
Figure 21e. It can be assumed that upon light irradiation, the electrons generated in Cu
2O react to the adsorbed CO
2 on NFC surface and are converted to CH
4, while the holes in valence band are filled by the photogenerated electrons from LDH. Thus, the key factors involved for the enhanced efficiency include visible light absorption, efficient charge separation, and improved CO
2 adsorption.
Similarly, Jiang et al. reported Cu
2O loaded ZnCr LDH for CO
2 photo-reduction to useful fuels [
68]. The Cu
2O@ZnCr LDH was synthesized by a ternary CuZnCr LDH via in situ reduction process. A variety of samples were prepared by in situ reduction of Cu
2−xZn
2−2xCr LDH (where
x = 0.05, 0.1, 0.2, and 0.4) resulting in the formation of
xCu
2O@Zn
2−2xCr LDH. The photocatalytic CO
2 conversion employing
xCu
2O@Zn
2−2xCr LDH sample is shown in
Figure 22a,b. The CO
2 conversion to mainly CO was observed from the samples with 0.1Cu
2O@Zn
1.8Cr LDH sample exhibiting the highest yield as compared to its corresponding reference samples (
Figure 22a). The key behind the significantly enhanced activity after Cu loading was its superb electron extraction property from the photocatalyst.
Figure 22c exhibits the CO
2 conversion of various
xCu
2O@Zn
2−2xCr LDH samples, indicating the 0.1Cu
2O@Zn
1.8Cr LDH sample is the most efficient photocatalyst with optimized loading of Cu content. Moreover, the effect of various additives was also investigated for 0.1Cu
2O@Zn
1.8Cr LDH sample and is shown in
Figure 22d. It was observed that Na
2SO
3 and Na
2CO
3 additives suppressed the formation of CO and enhanced the formation of H
2, due to their better hole scavenging properties and occupying the CO
2 adsorption sites.
Figure 22e shows the proposed mechanism of CO
2 photo-reduction for 0.1Cu
2O@Zn
1.8Cr LDH sample. Upon light irradiation, the photogenerated electrons are extracted by Cu
2O nanoparticles where they react with adsorbed CO
2 species to yield CO. Hence the key role of Cu
2O nanoparticles on the surface of 0.1Cu
2O@Zn
1.8Cr LDH sample is an efficient electron separator providing CO
2 active sites.
In another manner, LDH materials can be utilized as based material to synthesize efficient composite/heterojunctioned/hybrid photocatalysts. There are very limited reports regarding the utilization of LDH materials as photocatalyst precursors. To our best knowledge, in our investigation, we found a couple of research works taking advantage of mentioned approach and providing a novel pathway towards efficient photocatalysts development. Ye et al. reported CeO
2−x platelets from monometallic cerium LDH (MCe-LDH) and evaluated their performance for photocatalytic CO
2 conversion [
69]. The synthesis approach consists of two steps including synthesis of MCe-LDH in the first step followed by second step of heat treatment at various temperatures up to 800 °C.
Figure 23a shows the SEM image of a synthesized un-calcined MCe-LDH displaying the quasi-hexagonal platelets.
Figure 23b shows the SEM image of the MCe-LDH calcined at a temperature of 800 °C, thus indicating that thermal treatment does not change significantly the structure except for the increased density of the nanopores.
Figure 23c exhibits the UV–Vis DRS for various samples. It was observed that MCe-LDH exhibits broad hump at 320 nm, whereas when MCe-LDH were calcined, the absorption spectra showed two absorption peaks at 280 and 320 nm, corresponding to the Ce
3+ and Ce
4+, respectively. The inset to UV–Vis DRS shows the absorption ratio for Ce
3+ to Ce
4+ with temperature. The MCe-LDH sample calcined at 800 °C (MCe-LDH-800) shows the maximum ratio, whereas upon increasing the temperature up to 1000 °C, the absorption ratio decreased due to structure distortion.
Figure 23d displays the photocatalytic CO
2 conversion test with the synthesized and reference samples. It was observed that only CO was yielded, and sample calcined at 800 °C yielded the maximum CO production rate, 2.5 times higher than reference CeO
2. Moreover, the optimized sample MCe-LDH-800 shows a stable photocatalytic performance for 4 cycles (8 h of each cycle) of testing. The photocatalytic performance was believed to be enhanced by two key factors of Ce
3+/Ce
4+ redox couple present at the surface of MCe-LDH and enhanced surface areas. The Ce
3+/Ce
4+ redox couple supports in the efficient charge separation, whereas improved surface areas lead to increased active sites for CO
2 adsorption and conversion. The proposed mechanism by authors is shown in
Figure 23e; under light irradiation, Ce
4+ ions and oxygen vacancies will trap the photogenerated electrons. Such oxygen vacancy will promote the CO
2 adsorption which will react with Ce
3+ ions and get converted to CO
2−, which will further react with electron and proton to give CO. On the other hand, Ce
3+ ions after reducing adsorbed CO
2 will be converted to Ce
4+ ions, and thus, the reaction cycle continues.
The summary of the reviewed composite photocatalysts with LDH with respective research aims, reaction condition, solar fuels production, and various contributing parameters to catalytic performance is tabulated in
Table 3.