Recent Advances in Transition Metal Carbide Electrocatalysts for Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Parameters and Mechanism for OER
2.1. Parameters and Measurement Criteria for OER
2.1.1. Overpotential (η)
2.1.2. Tafel Slope
2.1.3. Electrochemically Active Surface Area (ECSA)
2.1.4. Faradaic Efficiency (FE)
2.1.5. Electrochemical Impedance Spectroscopy (EIS)
2.1.6. Stability
2.2. Mechanism for OER
3. Transition Metal Carbide Electrocatalysts for OER
3.1. Synthesis Methods of TMCs
3.2. Nickel Carbide
3.3. Tungsten Carbide
3.4. Iron Carbide
3.5. Molybdenum Carbide
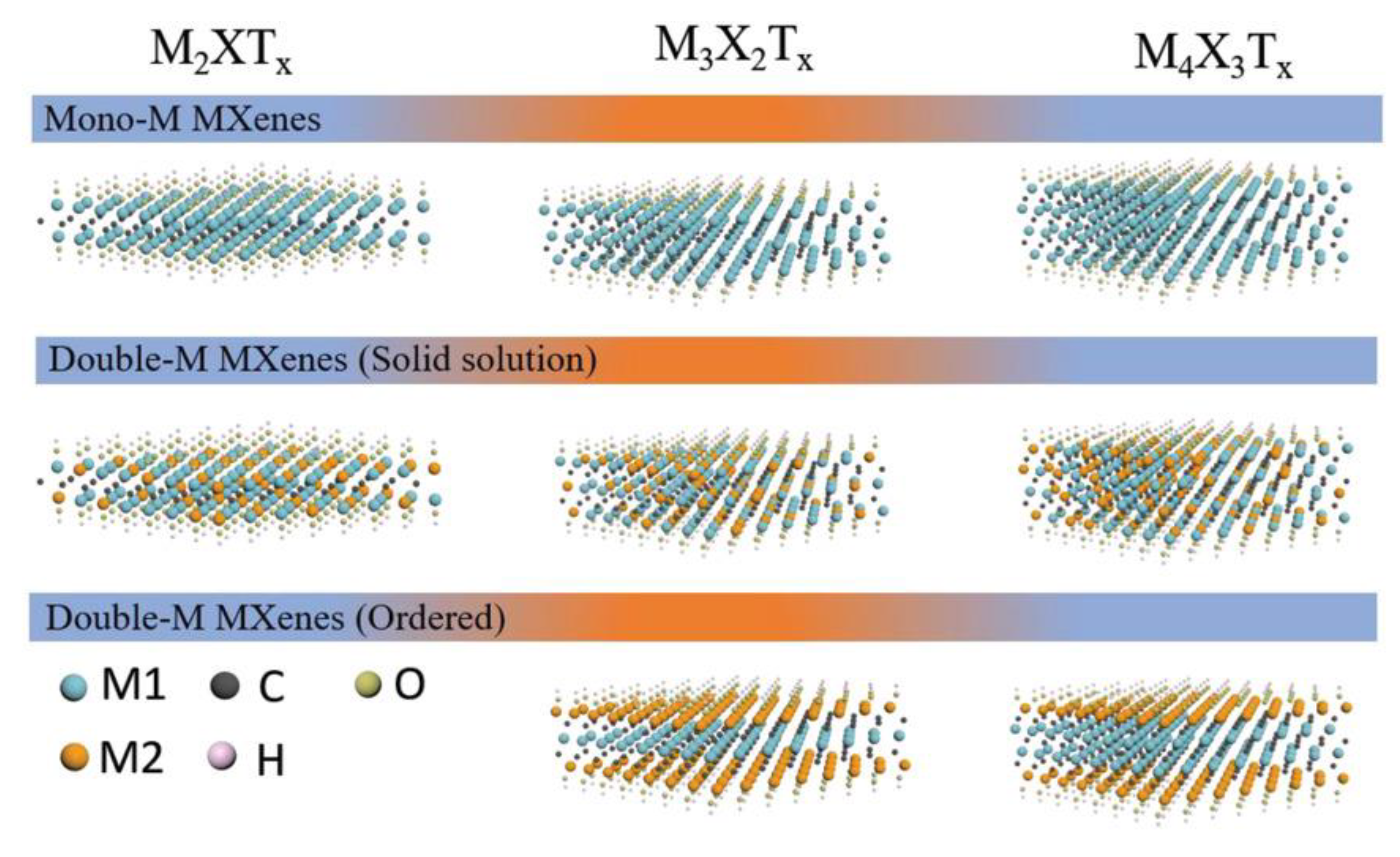
3.6. MXene
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yan, X.D.; Tian, L.H.; He, M.; Chen, X.B. Three-dimensional crystalline/amorphous Co/Co3O4 core/shell nanosheets as efficient electrocatalysts for the hydrogen evolution reaction. Nano Lett. 2015, 15, 6015–6021. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fei, H.L.; Ruan, G.D.; Tour, J.M. Porous cobalt-based thin film as a bifunctional catalyst for hydrogen generation and oxygen generation. Adv. Mater. 2015, 27, 3175–3180. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Jin, K.; Jerng, S.E.; Seo, H.; Kim, D.; Nahm, S.H.; Kim, S.H.; Nam, K.T. Mn5O8 nanoparticles as efficient water oxidation catalysts at neutral ipH. ACS Catal. 2015, 5, 4624–4628. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.S.; Wang, H.F.; Zhang, Q.; Tian, G.L.; Nie, J.Q.; Wei, F. Spatially confined hybridization of nanometer-sized NiFe hydroxides into nitrogen-doped graphene frameworks leading to superior oxygen evolution reactivity. Adv. Mater. 2015, 27, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, R.; Sawant, S.Y.; Nandi, D.K. Hydrogen Evolution Reaction by Atomic Layer-Deposited MoNx on Porous Carbon Substrates: The Effects of Porosity and Annealing on Catalyst Activity and Stability. ChemSusChem 2020, 13, 4159–4168. [Google Scholar] [CrossRef]
- Wu, P.W.; Wu, J.; Si, H.N. 3D Holey-Graphene Architecture Expedites Ion Transport Kinetics to Push the OER Performance. Adv. Energy Mater. 2020, 10, 2001005. [Google Scholar] [CrossRef]
- Chen, B.T.; Morlanes, N.; Adogla, E.; Takanabe, K.; Rodionov, V.O. An efficient and stable hydrophobic molecular cobalt catalyst for water electro-oxidation at neutral pH. ACS Catal. 2016, 6, 4647–4652. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, H.X.; Wang, Z.L.; Meng, F.L.; Zhang, X.B. Integrated three-dimensional carbon paper/carbon tubes/cobalt-sulfide sheets as an efficient electrode for overall water splitting. ACS Nano 2016, 10, 2342–2348. [Google Scholar] [CrossRef]
- Wang, H.; Hsu, Y.Y.; Chen, R.; Chan, T.S.; Chen, H.M.; Liu, B. Ni3+-induced formation of active NiOOH on the spinel Ni–Co oxide surface for efficient oxygen evolution reaction. Adv. Energy Mater. 2015, 5, 5. [Google Scholar] [CrossRef]
- Tian, J.Q.; Liu, Q.; Asiri, A.M.; Sun, X.P. Self-supported nanoporous cobalt phosphide nanowire arrays: An efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Chen, M.X.; Han, Y.Z.; Luo, H.X.; Su, X.J.; Zhang, M.T.; Lin, X.H.; Sun, J.L.; Wang, L.; Deng, L.; et al. Fast and simple preparation of iron-based thin films as highly efficient water-oxidation catalysts in neutral aqueous solution. Angew. Chem. 2015, 127, 4952–4957. [Google Scholar] [CrossRef]
- Chen, P.Z.; Xu, K.; Fang, Z.W.; Tong, Y.; Wu, J.C.; Lu, X.L.; Peng, X.; Ding, H.; Wu, C.Z.; Xie, Y. Metallic Co4N porous nanowire arrays activated by surface oxidation as electrocatalysts for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2015, 54, 14710–14714. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.Q.; Liu, Q.; Cheng, N.Y.; Asiri, A.M.; Sun, X.P. Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from Water. Angew. Chem. Int. Ed. 2014, 53, 9577–9581. [Google Scholar] [CrossRef] [PubMed]
- Bockris, J.O.M. Hydrogen economy in the future. Int. J. Hydrogen Energy 1999, 24, 1–15. [Google Scholar] [CrossRef]
- Chen, Q.; Fu, Y.L.; Jin, J.L.; Zang, W.J.; Liu, X.; Zhang, X.Y.; Huang, W.Z.; Kou, Z.K.; Wang, J.; Zhou, L.; et al. In-situ surface self-reconstruction in ternary transition metal dichalcogenide nanorod arrays enables efficient electrocatalytic oxygen evolution. J. Energy Chem. 2021, 55, 10–16. [Google Scholar] [CrossRef]
- Tang, Y.J.; Liu, C.H.; Huang, W. Bimetallic Carbides-Based Nanocomposite as Superior Electrocatalyst for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 16977–16985. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jensen, J.O.; Zhang, W. Hollow Spheres of Iron Carbide Nanoparticles Encased in Graphitic Layers as Oxygen Reduction Catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef]
- Benchakar, M.; Bilyk, T.; Garnero, C. MXene Supported Cobalt Layered Double Hydroxide Nanocrystals: Facile Synthesis Route for a Synergistic Oxygen Evolution Reaction Electrocatalyst. Adv. Mater. Interfaces 2019, 6, 1901328. [Google Scholar] [CrossRef]
- Munir, A.; Haq, T.U.; Saleem, M.; Qurashi, A.; Hussain, S.Z.; Sher, F.; Hamid, A.U.; Jilani, A.; Hussain, I. Controlled engineering of nickel carbide induced N-enriched carbon nanotubes for hydrogen and oxygen evolution reactions in wide pH range. Electrochim. Acta 2020, 341, 136032. [Google Scholar] [CrossRef]
- Li, G.L.; Zhang, X.B.; Zhang, H. Ultrathin 2D nanosheet based 3D hierarchical hollow polyhedral CoM/C (M = Ni, Cu, Mn) phosphide nanocages as superior electrocatalysts toward oxygen evolution reaction. Chem. Eng. J. 2020, 398, 125467. [Google Scholar] [CrossRef]
- Ren, S.S.; Duan, X.D.; Ge, F.Y. Trimetal-based N-doped carbon nanotubes arrays on Ni foams as self-supported electrodes for hydrogen/oxygen evolution reactions and water splitting. J. Power Sources 2020, 480, 228866. [Google Scholar] [CrossRef]
- Wang, H.P.; Zhu, S.; Deng, J.W.; Zhang, W.C.; Feng, Y.Z.; Ma, J.M. Transition metal carbides in electrocatalytic oxygen evolution reaction. Chin. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suen, N.T.; Hung, S.F.; Quan, Q. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Yu, P.; Wang, F.M.; Shifa, T.A.; Zhan, X.Y.; Lou, X.D.; Xia, F.; He, J. Earth abundant materials beyond transition metal dichalcogenides: A focus on electrocatalyzing hydrogen evolution reaction. Nano Energy 2019, 58, 244–276. [Google Scholar] [CrossRef]
- You, B.; Jiang, N.; Sheng, M.L.; Gul, S.; Yano, J.; Sun, Y.J. High-Performance Overall Water Splitting Electrocatalysts Derived from Cobalt-Based Metal–Organic Frameworks. Chem. Mater. 2015, 27, 7636–7642. [Google Scholar] [CrossRef]
- Fei, H.L.; Dong, J.; Arellano-Jiménez, M.J. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 8668. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, T.; Ye, Y.K.; Dai, W.J.; Zhu, Y.A.; Pan, Y. Stabilizing Oxygen Vacancy in Entropy-Engineered CoFe2O4-Type Catalysts for Co-prosperity of Efficiency and Stability in an Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2020, 12, 32548–32555. [Google Scholar] [CrossRef]
- Frydendal, R.; Paoli, E.A.; Knudsen, B.P.; Wickman, B.; Malacrida, P.; Stephens, I.E.L.; Chorkendorff, I. Benchmarking the Stability of Oxygen Evolution Reaction Catalysts: The Importance of Monitoring Mass Losses. ChemElectroChem 2014, 1, 2075–2081. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhou, J.; Sun, Z. Novel 2D Transition-Metal Carbides: Ultrahigh Performance Electrocatalysts for Overall Water Splitting and Oxygen Reduction. Adv. Funct. Mater. 2020, 2000570. [Google Scholar] [CrossRef]
- Anurupa, M. Cobalt-based heterogeneous catalysts in an electrolyzer system for sustainable energy storage. Dalton Trans. 2020, 49, 11430–11450. [Google Scholar]
- Cheng, W.; Lu, X.F.; Luan, D.; Lou, X.W. NiMn-Based Bimetal-Organic Framework Nanosheets Supported on Multi-Channel Carbon Fibers for Efficient Oxygen Electrocatalysis. Angew. Chem. Int. Ed. 2020, 59, 18234–18239. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Ye, Y.Q.; Wu, C.Y.; Xiao, K.; Liu, Z.Q. Heterostructures Composed of N-Doped Carbon Nanotubes Encapsulating Cobalt and b-Mo2C Nanoparticles as Bifunctional Electrodes for Water Splitting. Angew. Chem. Int. Ed. 2019, 58, 4923–4928. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Qin, R.; Ji, P.; Pu, Z.; Zhu, J.; Lin, C.; Zhao, Y.; Tang, H.; Li, W.; Mu, S. Synergistic Coupling of Ni Nanoparticles with Ni3C Nanosheets for Highly Efficient Overall Water Splitting. Small 2020, 16, 2001642. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Li, Y. Manipulating dehydrogenation kinetics through dual-doping Co3N electrode enables highly efficient hydrazine oxidation assisting self-powered H2 production. Nat. Commun. 2020, 11, 5148–5180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.C.; Zai, S.F.; Zhou, Y.T.; Du, L.; Jiang, Q. Fe3C-Co Nanoparticles Encapsulated in a Hierarchical Structure of N-Doped Carbon as a Multifunctional Electrocatalyst for ORR, OER, and HER. Adv. Funct. Mater. 2019, 29, 1901949. [Google Scholar] [CrossRef]
- Xiu, L.Y.; Wang, Z.Y.; Yu, M.Z.; Wu, X.H.; Qiu, J.S. Aggregation-Resistant 3D MXene-Based Architecture as Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Nano 2018, 12, 8017–8028. [Google Scholar] [CrossRef]
- Du, C.F.; Dinh, K.N.; Liang, Q.; Zheng, Y.; Luo, Y.; Zhang, J.; Yan, Q. Self-Assemble and In Situ Formation of Ni1−xFexPS3 Nanomosaic-Decorated MXene Hybrids for Overall Water Splitting. Adv. Energy Mater. 2018, 8, 1801127. [Google Scholar] [CrossRef]
- Anh, L.T.; Quang, T.N.; Yeseul, H.; Meeree, K.; Hyoyoung, L. Porosity-Engineering of MXene as a Support Material for a Highly Efficient Electrocatalyst toward Overall Water Splitting. ChemSusChem 2020, 13, 945–955. [Google Scholar]
- Li, Z.; Wu, Y. 2D Early Transition Metal Carbides (MXenes) for Catalysis. Small 2019, 15, 15. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Dong, B.; Li, S. Interdiffusion Reaction-Assisted Hybridization of Two-Dimensional Metal–Organic Frameworks and Ti3C2Tx Nanosheets for Electrocatalytic Oxygen Evolution. ACS Nano 2017, 11, 5800–5807. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.H.; Zhong, L.X.; Du, C.F.; Zheng, Y.; Luo, Y.B.; Xu, J.W.; Li, S.Z.; Yan, Q.Y. Mosaic-Structured Cobalt Nickel Thiophosphate Nanosheets Incorporated N-doped Carbon for Efficient and Stable Electrocatalytic Water Splitting. Adv. Funct. Mater. 2018, 28, 1805075. [Google Scholar] [CrossRef]
- Tian, Y.H.; Xu, L.; Qian, J.C.; Bao, J.Y. Fe3C/Fe2O3 heterostructure embedded in N-doped graphene as a bifunctional catalyst for quasi-solid-state zinceair batteries. Carbon 2019, 146, 763–771. [Google Scholar] [CrossRef]
- Zhao, P.P.; Hua, X.; Xu, W.; Luo, W.; Chen, S.L.; Cheng, G.Z. Metal–organic framework-derived hybrid of Fe3C nanorod-encapsulated, N-doped CNTs on porous carbon sheets for highly efficient oxygen reduction and water oxidation. Catal. Sci. Technol. 2016, 6, 6365–6371. [Google Scholar] [CrossRef]
- Fan, H.S.; Yu, H.; Zhang, Y.F.; Zheng, Y.; Dai, Z.F.; Li, B.; Zong, Y. Fe-Doped Ni3C Nanodots in N-Doped Carbon Nanosheets for Efficient Hydrogen-Evolution and Oxygen-Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2017, 56, 12566–12570. [Google Scholar] [CrossRef]
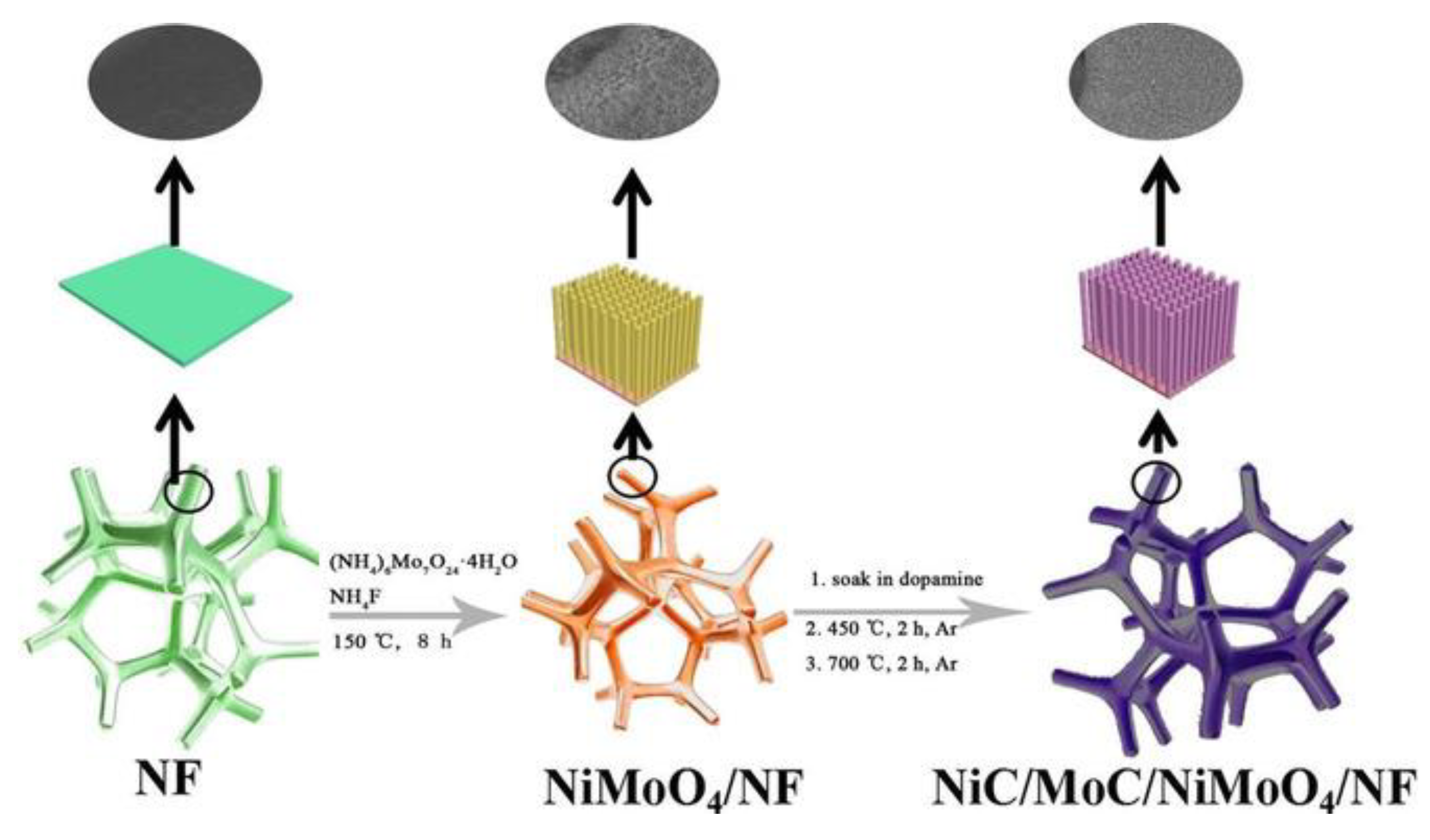
- Geng, S.; Yang, W.W.; Yu, Y.S. Fabrication of NiC/MoC/NiMoO4 Heterostructured Nanorod Arrays as Stable Bifunctional Electrocatalysts for Efficient Overall Water Splitting. Chem. Asian J. 2019, 14, 1013–1020. [Google Scholar] [CrossRef]
- He, P.; Yu, X.Y.; Lou, X.W.D. Carbon-Incorporated Nickel-Cobalt Mixed Metal Phosphide Nanoboxes with Enhanced Electrocatalytic Activity for Oxygen Evolution. Angew. Chem. Int. Ed. 2017, 56, 3897–3900. [Google Scholar] [CrossRef]
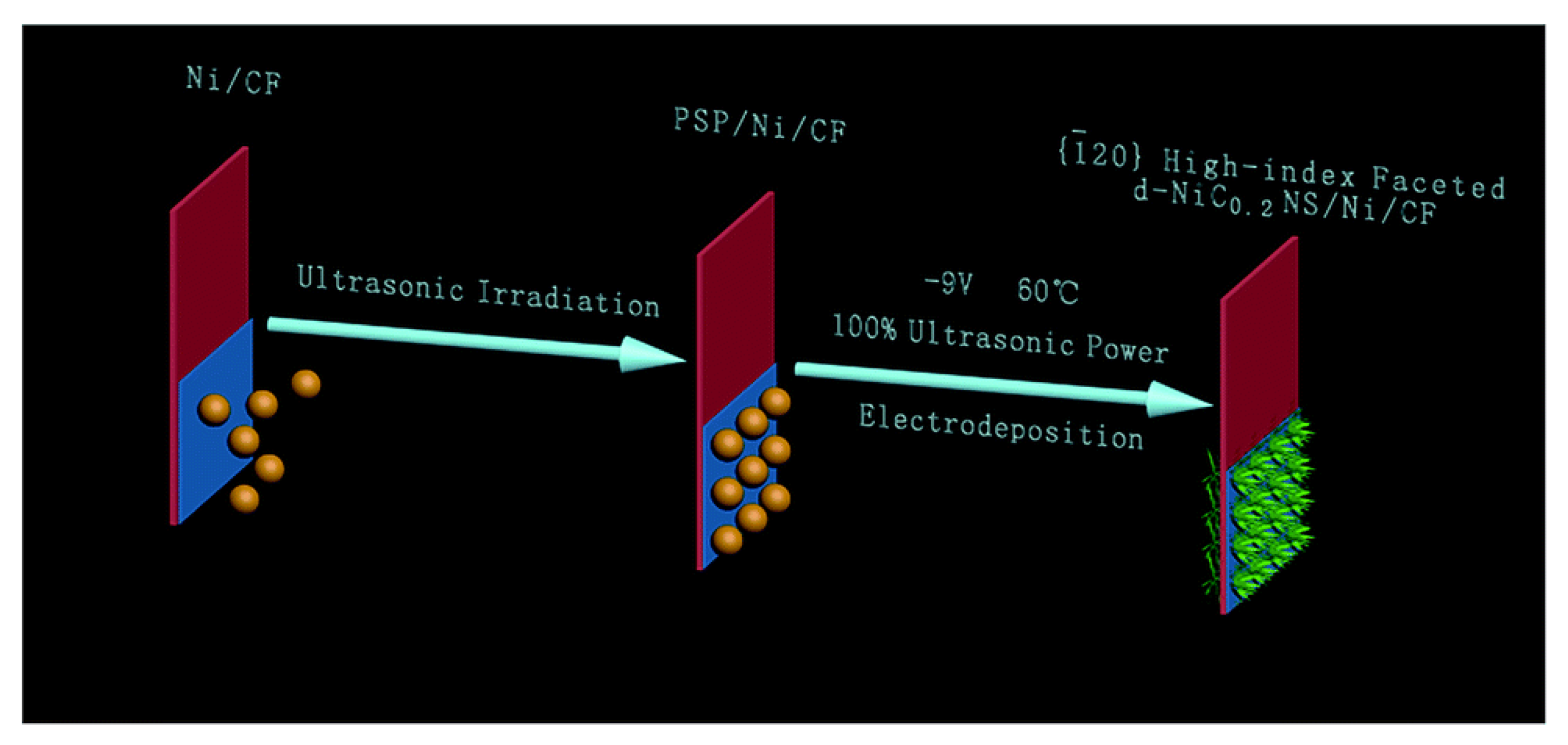
- Yang, H.; Luo, S.; Li, X. Controllable orientation-dependent crystal growth of high-index faceted dendritic NiC0.2 nanosheets as high-performance bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2016, 4, 18499–18508. [Google Scholar] [CrossRef]
- Joy, J.; Sekar, A.; Vijayaraghavan, S.; Kumar, P.; Pillai, V.K.; Alwarappan, S. Nickel-Incorporated, Nitrogen-Doped Graphene Nanoribbons as Efficient Electrocatalysts for Oxygen Evolution Reaction. J. Electrochem. Soc. 2018, 165, H141–H146. [Google Scholar] [CrossRef]
- Regmi, Y.N.; Wan, C.; Duffee, K.D.; Leonard, B.M. Nanocrystalline Mo2C as a Bifunctional Water Splitting Electrocatalyst. ChemCatChem 2015, 7, 3911–3915. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Cao, Y.J.; Sun, C.; Zou, G.F.; Huang, J.W.; Kuai, X.X.; Zhao, J.Q.; Gao, L.J. Strongly Coupled Molybdenum Carbide on Carbon Sheets as a Bifunctional Electrocatalyst for Overall Water Splitting. ChemSusChem 2017, 10, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Santra, S.; Nanda, K.K. In Situ Fabrication of a Nickel/Molybdenum Carbide-Anchored N-Doped Graphene/CNT Hybrid: An Efficient (Pre)catalyst for OER and HER. ACS Appl. Mater. Interfaces 2018, 10, 35025–35038. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.M.A.; Hee, L.M.; Sung, L.J. Boron- and Nitrogen-Codoped Molybdenum Carbide Nanoparticles Imbedded in a BCN Network as a Bifunctional Electrocatalyst for Hydrogen and Oxygen Evolution Reactions. ACS Catal. 2018, 8, 8296–8305. [Google Scholar]
- Zhao, Y.X.; Bowers, C.W.; Spain, I.L. Graphitic nature of chemical vapor-deposited carbon filaments grown on silicon surfaces from acetylene. Carbon 1988, 26, 291–293. [Google Scholar] [CrossRef]
- Fadil, N.A.; Saravanna, G.; Ramesh, G.V. Synthesis and electrocatalytic performance of atomically ordered nickel carbide (Ni3C) nanoparticles. Chem. Commun. 2014, 50, 6451–6453. [Google Scholar] [CrossRef]
- Wan, C.; Leonard, B.M. Iron-Doped Molybdenum Carbide Catalyst with High Activity and Stability for the Hydrogen Evolution Reaction. Chem. Mater. 2015, 27, 4281–4288. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, G.F.; Zheng, Y.T.; Luo, W.H.; Jin, C.; Wang, R.; Wang, Z.; Zheng, W.J. Controlled synthesis of Ni3C/nitrogen-doped carbon nanoflakes for efficient oxygen evolution. Electrochim. Acta 2019, 320. [Google Scholar] [CrossRef]
- Kang, J.; Duan, X.G.; Wang, C.; Sun, H.Q.; Tan, X.Y.; Tade, M.O.; Wang, S.B. Nitrogen-doped bamboo-like carbon nanotubes with Ni encapsulation for persulfate activation to remove emerging contaminants with excellent catalytic stability. Chem. Eng. J. 2018, 332, 398–408. [Google Scholar] [CrossRef]
- Qin, Q.; Hao, J.; Zheng, W.J. Ni/Ni3C Core/Shell Hierarchical Nanospheres with Enhanced Electrocatalytic Activity for Water Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 17827–17834. [Google Scholar] [CrossRef]
- Zi, Y.Y.; Yu, D.; Min, R.G. A one-dimensional porous carbon-supported Ni/Mo2C dual catalyst for efficient water splitting. Chem. Sci. 2017, 8, 968–973. [Google Scholar]
- Rostami, S.; Pour, A.N.; Salimi, A.; Abolghasempour, A. Hydrogen adsorption in metal-organic frameworks (MOFs): Effects of adsorbent architecture. Int. J. Hydrogen Energy 2018, 43, 7072–7080. [Google Scholar] [CrossRef]
- Lee, J.Y.; Farha, O.K.; Roberts, J. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2019, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Liu, Y.Y. Fluorescent Aromatic Tag-Functionalized MOFs for Highly Selective Sensing of Metal Ions and Small Organic Molecules. Inorg. Chem. 2016, 55, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.X.; Wang, M.H.; Liu, G. Mixed-metal MOF-derived Co-doped Ni3C/Ni NPs embedded in carbon matrix as an efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 24572–24579. [Google Scholar] [CrossRef]
- Levy, R.B.; Boudart, M. Platinum-Like Behavior of Tungsten Carbide in Surface Catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef]
- Glibin, V.; Svirko, R.; Bashtan-Kandybovich, R. Synthesis, surface chemistry and electrocatalytic properties of oxygen-modified tungsten carbide in relation to the electrochemical hydrogen oxidation. Surf. Sci. 2010, 604, 500–507. [Google Scholar] [CrossRef]
- Palanker, V.S.; Gajyev, R. On adsorption and electro-oxidation of some compounds on tungsten carbide; their effect on hydrogen electro-oxidation. Electrochim. Acta 1977, 22, 133–136. [Google Scholar] [CrossRef]
- Zhou, X.S.; Qiu, Y.J. Tungsten carbide nanofibers prepared by electrospinning with high electrocatalytic activity for oxygen reduction. Int. J. Hydrogen Energy 2011, 36, 7398–7404. [Google Scholar] [CrossRef]
- Han, N.; Yang, K.R.; Lu, Z. Nitrogen-doped tungsten carbide nanoarray as an efficient bifunctional electrocatalyst for water splitting in acid. Nat. Commun. 2018, 9, 924. [Google Scholar] [CrossRef] [Green Version]
- Bukola, S.; Merzougui, B.; Akinpelu, A.; Zeama, M. Cobalt and Nitrogen Co-Doped Tungsten Carbide Catalyst for Oxygen Reduction and Hydrogen Evolution Reactions. Electrochim. Acta 2016, 190, 1113–1123. [Google Scholar] [CrossRef]
- Kim, D.W.; Li, O.L.; Pootawang, P. Solution plasma synthesis process of tungsten carbide on N-doped carbon nanocomposite with enhanced catalytic ORR activity and durability. RSC Adv. 2014, 4, 16813–16819. [Google Scholar] [CrossRef]
- Zhao, T.; Gao, J.K.; Wu, J.; He, P.P.; Li, Y.W.; Yao, J.M. Highly Active Cobalt/Tungsten Carbide@N-Doped Porous Carbon Nanomaterials Derived from Metal-Organic Frameworks as Bifunctional Catalysts for Overall Water Splitting. Energy Technol. 2019, 7, 1800969. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.H.; Shi, F.; Zhan, J.Y.; Tu, J.P.; Fan, H.J. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G. Carbide and Nitride Overlayers on Early Transition Metal Surfaces: Preparation, Characterization, and Reactivities. Chem. Rev. 1996, 96, 1477–1498. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Pu, Z.; Liu, X. Multifunctional Mo–N/C@MoS2 Electrocatalysts for HER, OER, ORR, and Zn–Air Batteries. Adv. Funct. Mater. 2017, 27, 1702300. [Google Scholar] [CrossRef]
- Qian, Y.H.; Hu, Z.G.; Ge, X.M.; Yang, S.L.; Peng, Y.W.; Kang, Z.X.; Liu, Z.L.; Lee, J.Y.; Zhao, D. A metal-free ORR/OER bifunctional electrocatalyst derived from metal-organic frameworks for rechargeable Zn-Air batteries. Carbon 2017, 111, 641–650. [Google Scholar] [CrossRef]
- Jiang, H.; Gu, J.X.; Zheng, X.S.; Liu, M.; Qiu, X.Q. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ. Sci. 2019, 12, 322–333. [Google Scholar] [CrossRef]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Schalkwijk, W.V. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst Approaches and Challenges for Automotive Fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.P.; Fowler, M.; Chen, Z.W. Electrically Rechargeable Zinc–Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xu, D.; Xu, J.J.; Zhang, X.B. Oxygen electrocatalysts in metal–air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef] [PubMed]
- Defilippi, C.; Shinde, D.V.; Dang, Z.; Manna, L.; Hardacre, C.; Greer, A.J.; D’Agostino, C.; Giordano, C. HfN Nanoparticles: An Unexplored Catalyst for the Electrocatalytic Oxygen Evolution Reaction. Angew. Chem. 2019, 131, 15610–15616. [Google Scholar] [CrossRef]
- Song, J.Y.; Ren, Y.R.; Li, J.S.; Huang, X.B.; Cheng, F.Y.; Tang, Y.G.; Wang, H.Y. Coreshell Co/CoNx@C nanoparticles enfolded by Co-N doped carbon nanosheets as a highly efficient electrocatalyst for oxygen reduction reaction. Carbon 2018, 138, 300–308. [Google Scholar] [CrossRef]
- Wu, Y.X.; Wang, T.H.; Zhang, Y.D.; Xin, S.; He, X.J.; Zhang, D.W.; Shui, J.L. Electrocatalytic performances of g-C3N4-LaNiO3 composite as bi-functional catalysts for lithium-oxygen batteries. Sci. Rep. 2016, 6, 24314. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Han, Q.; Zhao, Y.; Wang, L.X.; Li, Y.; Qu, L.T. Sulfur-doped graphitic carbon nitride decorated with graphene quantum dots for an efficient metal-free electrocatalyst. J. Mater. Chem. 2015, 3, 1841–1846. [Google Scholar] [CrossRef]
- Wang, Q.C.; Lei, Y.P.; Chen, Z.Y. Fe/Fe3C@C nanoparticles encapsulated in N-doped graphene–CNTs framework as an efficient bifunctional oxygen electrocatalyst for robust rechargeable Zn–air batteries. J. Mater. Chem. A 2017, 6, 516–526. [Google Scholar] [CrossRef]
- Wu, N.; Lei, Y.P.; Wang, Q.C.; Wang, B.; Han, C.; Wang, Y.D. Facile synthesis of FeCo@NC core–shell nanospheres supported on graphene as an efficient bifunctional oxygen electrocatalyst. Nano Res. 2017, 10, 2332–2343. [Google Scholar] [CrossRef]
- Su, C.Y.; Cheng, H.; Li, W.; Liu, Z.Q.; Li, N.; Hou, Z.; Bai, F.Q.; Zhang, H.X.; Ma, T.Y. Atomic Modulation of FeCo-Nitrogen-Carbon Bifunctional Oxygen Electrodes for Rechargeable and Flexible All-Solid-State Zinc-Air Battery. Adv. Energy Mater. 2017, 7, 1602420. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.B.; Dai, L.M.; Yao, J.N. Scalable Fabrication of Nanoporous Carbon Fiber Films as Bifunctional Catalytic Electrodes for Flexible Zn-Air Batteries. Adv. Mater. 2016, 28, 3000–3006. [Google Scholar] [CrossRef]
- Cui, Z.M.; Li, Y.T.; Fu, G.T. Robust Fe3Mo3C Supported IrMn Clusters as Highly Efficient Bifunctional Air Electrode for Metal–Air Battery. Adv. Mater. 2017, 29, 1702385. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.F.; Wang, C.H.; Sasaki, K. Highly active and durable nanostructured molybdenum carbide electrocatalysts for hydrogen production. Energy Environ. Sci. 2013, 6, 943–951. [Google Scholar] [CrossRef]
- Liao, L.; Wang, S.N.; Xiao, J.J. A nanoporous molybdenum carbide nanowire as an electrocatalyst for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.B.; Xia, B.Y.; Yu, L. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 2015, 6, 6512. [Google Scholar] [CrossRef] [PubMed]
- Regmi, Y.N.; Waetzig, G.R.; Duffee, K.D.; Schmuecker, S.M.; Thode, J.M.; Leonard, B.M. Carbides of group IVA, VA and VIA transition metals as alternative HER and ORR catalysts and support materials. J. Mater. Chem. A 2015, 3, 10085–10091. [Google Scholar] [CrossRef]
- Zhu, J.; Holmen, A.; Chen, D. Carbon Nanomaterials in Catalysis: Proton Affinity, Chemical and Electronic Properties, and their Catalytic Consequences. ChemCatChem 2013, 5, 378–401. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, C.L.; Chen, L.C. Functionalized Carbon Nanomaterial Supported Palladium Nano-Catalysts for Electrocatalytic Glucose Oxidation Reaction. Electrochim. Acta 2015, 152, 408–416. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, Z. Design Principles for Dual-Element-Doped Carbon Nanomaterials as Efficient Bifunctional Catalysts for Oxygen Reduction and Evolution Reactions. ACS Catal. 2016, 6, 1553–1558. [Google Scholar] [CrossRef]
- Podyacheva, O.Y.; Ismagilov, Z.R. Nitrogen-doped carbon nanomaterials: To the mechanism of growth, electrical conductivity and application in catalysis. Catal. Today 2015, 249, 12–22. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.; Song, D.; Oh, S.; Chang, K.J.; Cho, E. Promotion of electrochemical oxygen evolution reaction by chemical coupling of cobalt to molybdenum carbide. Appl. Catal. B Environ. 2018, 227, 340–348. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, Q.; Zeng, C. Cobalt/molybdenum carbide@N-doped carbon as a bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. J. Mater. Chem. A 2017, 5, 16929–16935. [Google Scholar] [CrossRef]
- Dutta, S.; Indra, A.; Han, H.S. An Intriguing Pea-Like Nanostructure of Cobalt Phosphide on Molybdenum Carbide Incorporated Nitrogen-Doped Carbon Nanosheets for Efficient Electrochemical Water Splitting. ChemSusChem 2018, 11, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, X.; Huang, L. Cobalt doped beta-molybdenum carbide nanoparticles encapsulated within nitrogen-doped carbon for oxygen evolution. Chem. Commun. 2019, 55, 9995–9998. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, L.B.; Liu, Z.B.; Chen, L.; Guo, J.K.; Kang, N.; Xiu, L.M.; Cheng, H.M.; Ren, W.C. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zha, X.H.; Chen, F.Y. A two-dimensional zirconium carbide by selective etching of Al3C3 from nanolaminated Zr3Al3C5. Angew. Chem. Int. Ed. 2016, 128, 5092–5097. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Xie, X.; Ren, C.E.; Makaryan, T.; Anasori, B.; Wang, G.; Gogotsi, Y. Hollow MXene Spheres and 3D Macroporous MXene Frameworks for Na-Ion Storage. Adv. Mater. 2017, 29, 1702410. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Wang, Z.Y.; Yu, M.Z.; Xiu, L.Y.; Qiu, J.S. Stabilizing the MXenes by Carbon Nanoplating for Developing Hierarchical Nanohybrids with Efficient Lithium Storage and Hydrogen Evolution Capability. Adv. Mater. 2017, 29, 1607017. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Ran, J.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Phosphorus-Doped Graphitic Carbon Nitrides Grown In Situ on Carbon-Fiber Paper: Flexible and Reversible Oxygen Electrodes. Angew. Chem. Int. Ed. 2015, 54, 4646–4650. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.Y.; Cao, J.L.; Jaroniec, M.; Qiao, S.Z. Interacting Carbon Nitride and Titanium Carbide Nanosheets for High-Performance Oxygen Evolution. Angew. Chem. Int. Ed. 2016, 55, 1138–1142. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Metal-Organic Framework Composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Zhao, S.L.; Wang, Y.; Dong, J.C.; He, C.T.; Yin, H.J.; An, P.F.; Zhao, K.; Zhang, X.F.; Gao, C.; Zhang, L.J.; et al. Ultrathin Metal–Organic Framework Nanosheets for Electrocatalytic Oxygen Evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.H.; Jing, Y. MXene-Based Nanocomposites: Surface Modified MXene-Based Nanocomposites for Electrochemical Energy Conversion and Storage. Small 2019, 15, 1970133. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Milligan, C.; Ma, T.; Zhou, L.; Cui, Y.; Qi, Z.; Libretto, N.; Xu, B.; Luo, J.; et al. Two-dimensional transition metal carbides as supports for tuning the chemistry of catalytic nanoparticles. Nat. Commun. 2018, 9, 5258. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Geng, D.C.; Zhao, X.X.; Chen, Z.X.; Sun, W.W.; Fu, W.; Chen, J.Y.; Liu, W.; Zhou, W.; Loh, K.P. Direct Synthesis of Large-Area 2D Mo2C on In Situ Grown Graphene. Adv. Mater. 2017, 29, 1700072. [Google Scholar] [CrossRef]
- Chaitoglou, S.; Giannakopoulou, T.; Speliotis, T.; Vavouliotis, A.; Trapalis, C.; Dimoulas, A. Mo2C/graphene heterostructures: Low temperature chemical vapor deposition on liquid bimetallic Sn-Cu and hydrogen evolution reaction electrocatalytic properties. Nanotechnology 2018, 30, 125401. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Xiong, Y. In Situ X-ray Absorption Spectroscopy of a Synergistic Co-Mn Oxide Catalyst for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2019, 146, 1463–1466. [Google Scholar] [CrossRef]
- Massel, F.; Ahmadi, S.; Hahlin, M.; Liu, Y.S.; Guo, J.H.; Edvinsson, T.; Rensmo, H.; Duda, L.C. Transition metal doping effects in Co-phosphate catalysts for water splitting studied with XAS. J. Electron Spectrosc. Relat. Phenom. 2018, 224, 3–7. [Google Scholar] [CrossRef]
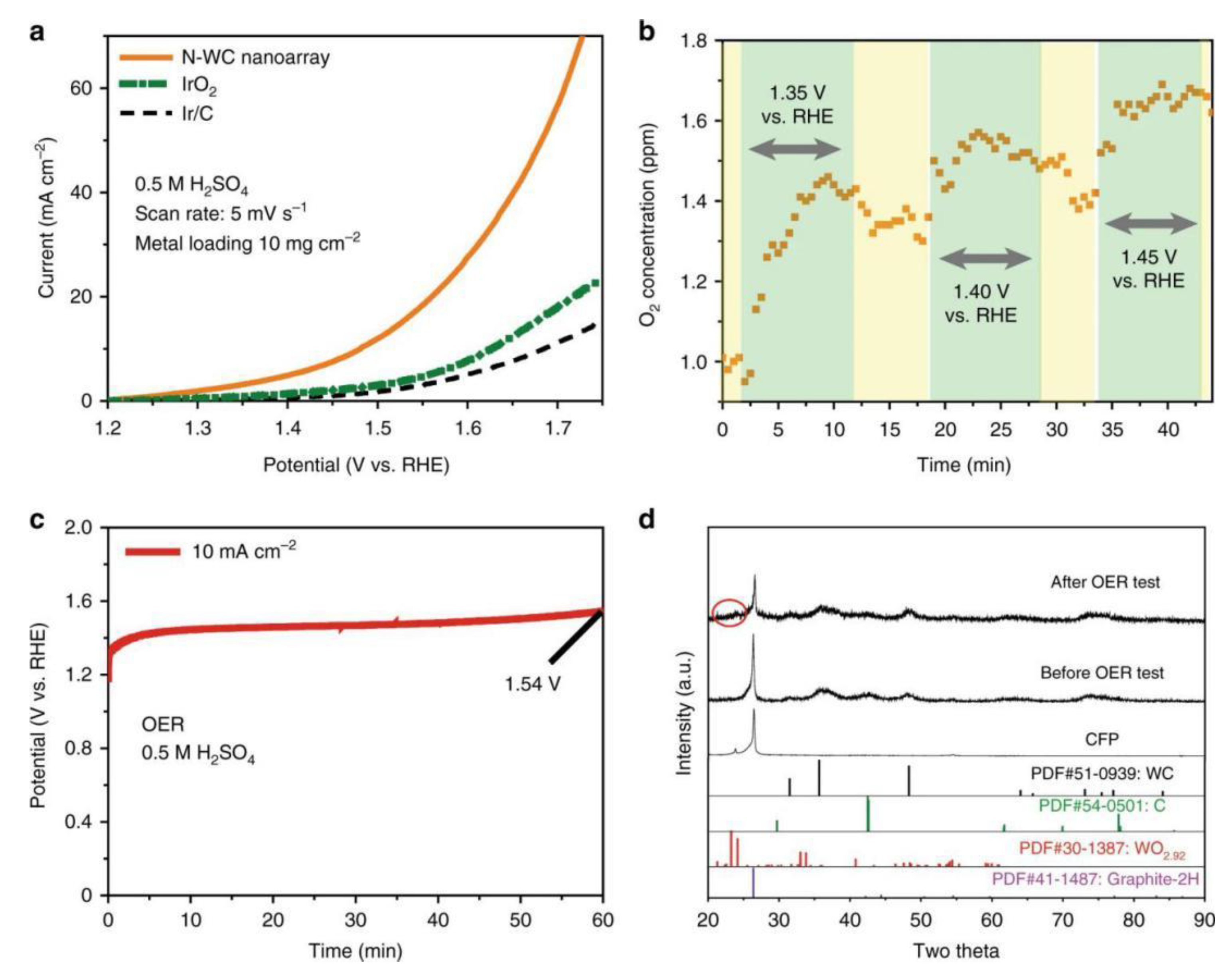
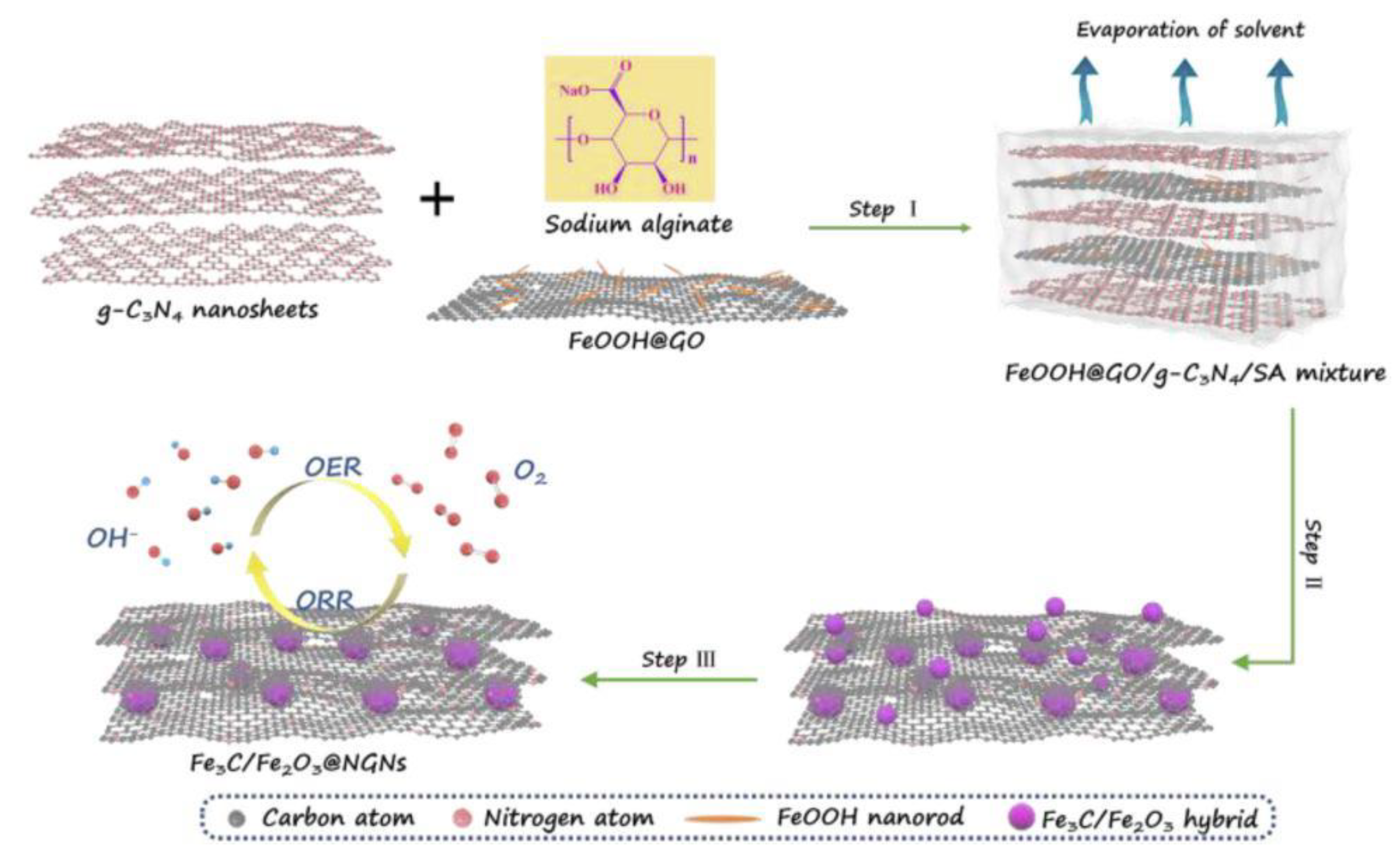
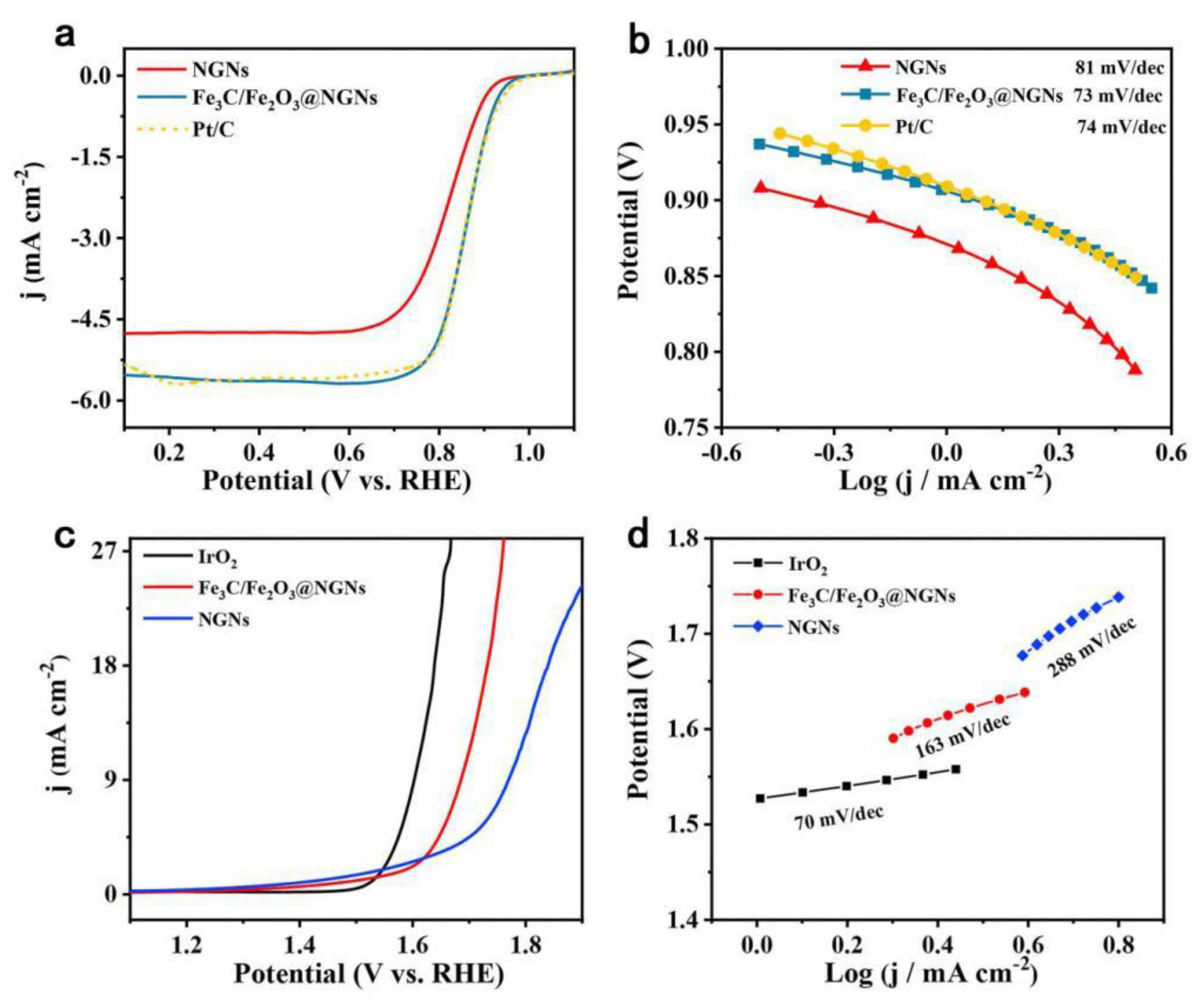
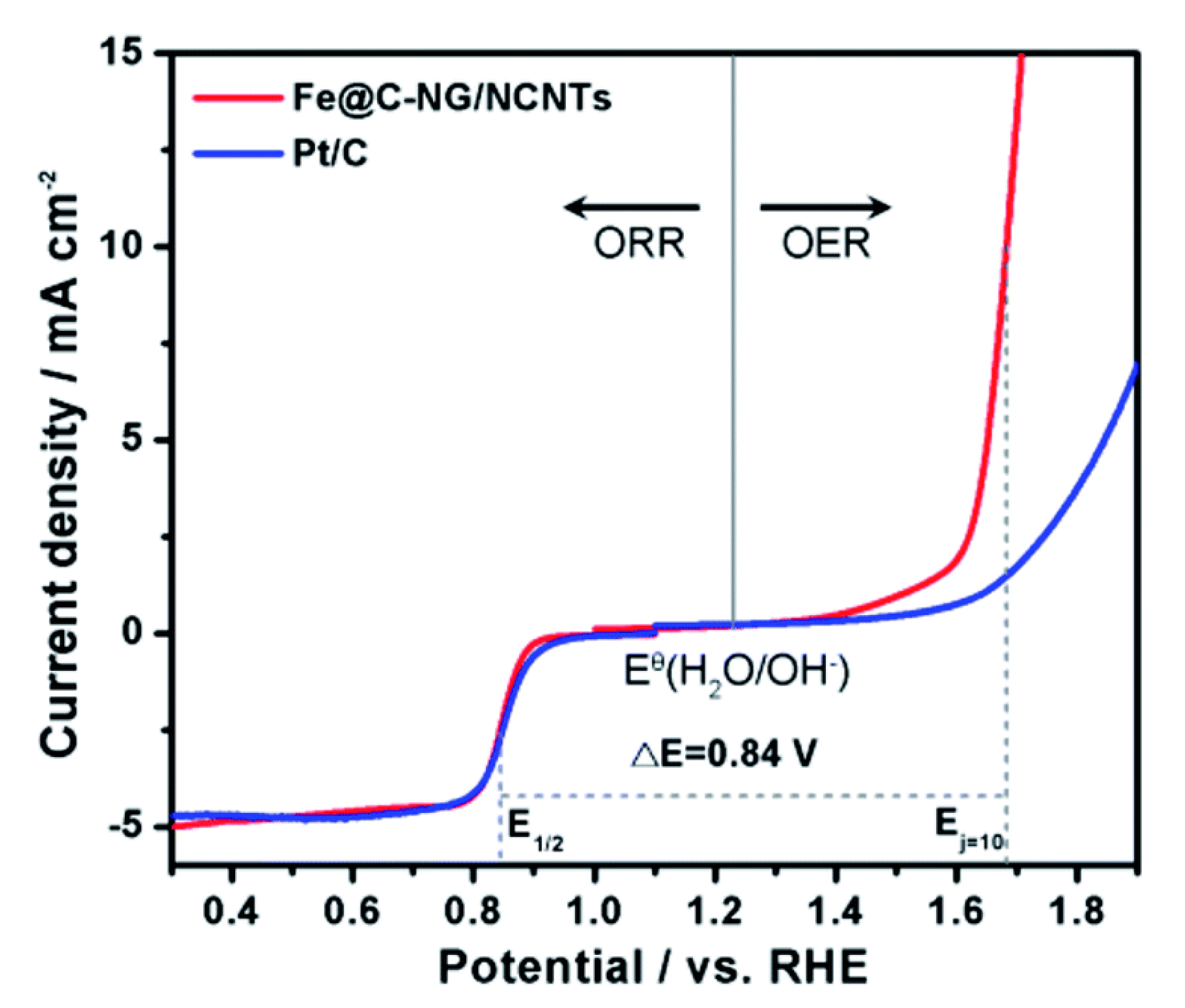

| Catalysts | Overpotential (mV) for OER at a Specific Current Density | Synthesis Strategies | Reference |
|---|---|---|---|
| MXene | |||
| Co-P/3D Ti3C2 MXene | 298 @ 10 mA·cm−2 | Constructing aggregation-resistant 3D Mxene architecture via a capillary-forced assembling strategy (template free). | [37] |
| Ni0.7Fe0.3PS3 @ Ti3C2Tx | 282 @ 10 mA·cm−2 | Decorating nickel-based bimetal phosphorus trisulfide nanomosaic on the surface of MXene nanosheets (template free). | [38] |
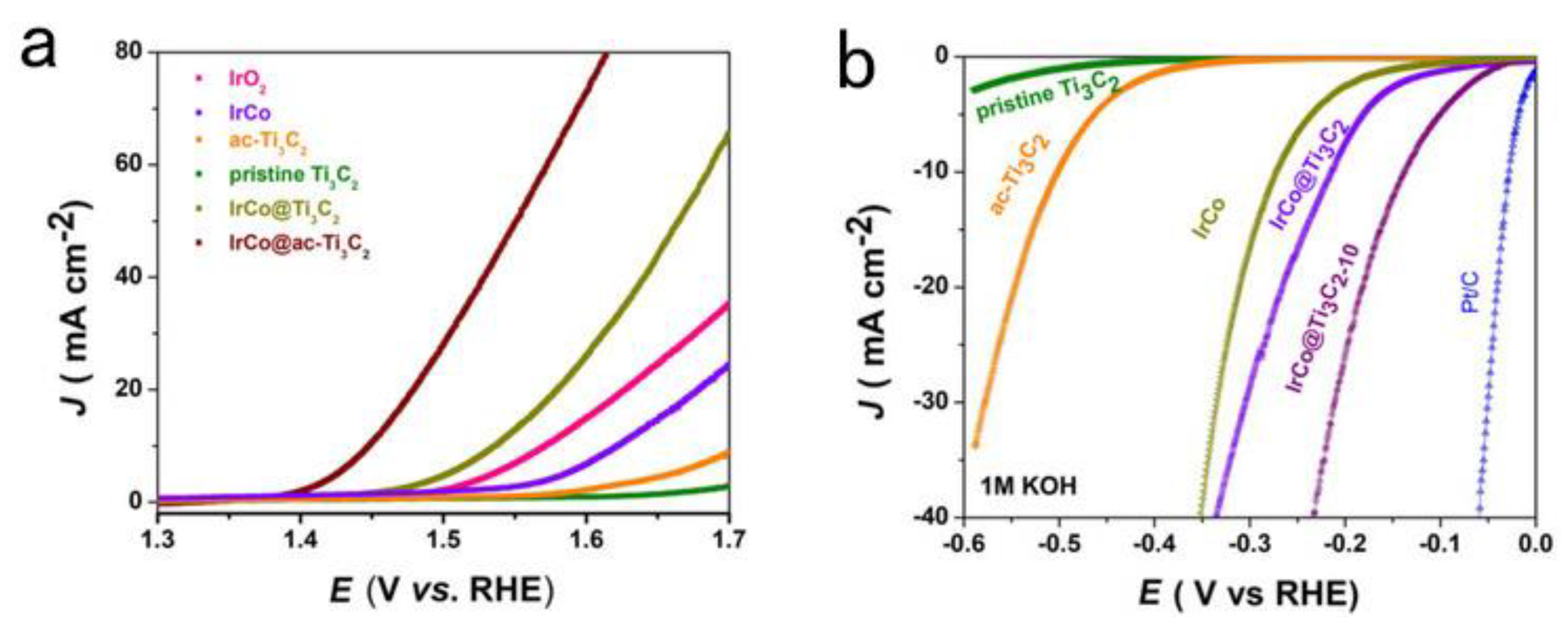
| IrCo @ ac-Ti3C2 | 220 @ 10 mA·cm−2 | Incorporating IrCo into basal-plane-porous titanium carbide MXene (template free). | [39] |
| TCCN | 420 @ 10 mA·cm−2 | Free-standing flexible films with hierarchically porous structure was constructed from 2D graphitic carbon nitride and titanium carbide nanosheets (template free). | [40] |
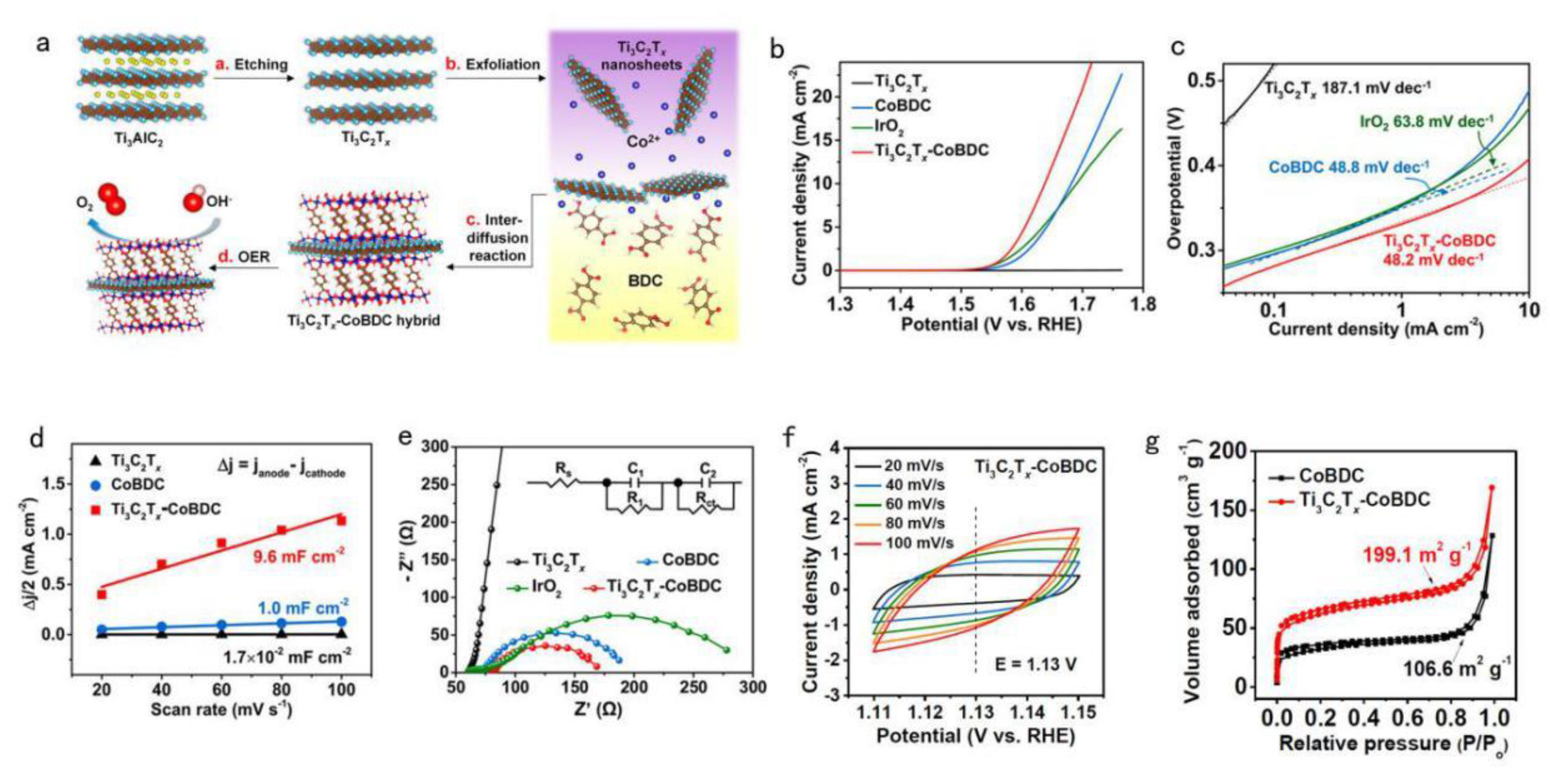
| Ti3C2TX−CoBDC | 410 @ 10 mA·cm−2 | Hybridizing 2D cobalt 1,4-benzenedicarboxylate (CoBDC) with Ti3C2T x nanosheets via an interdiffusion reaction-assisted process (template free). | [41] |
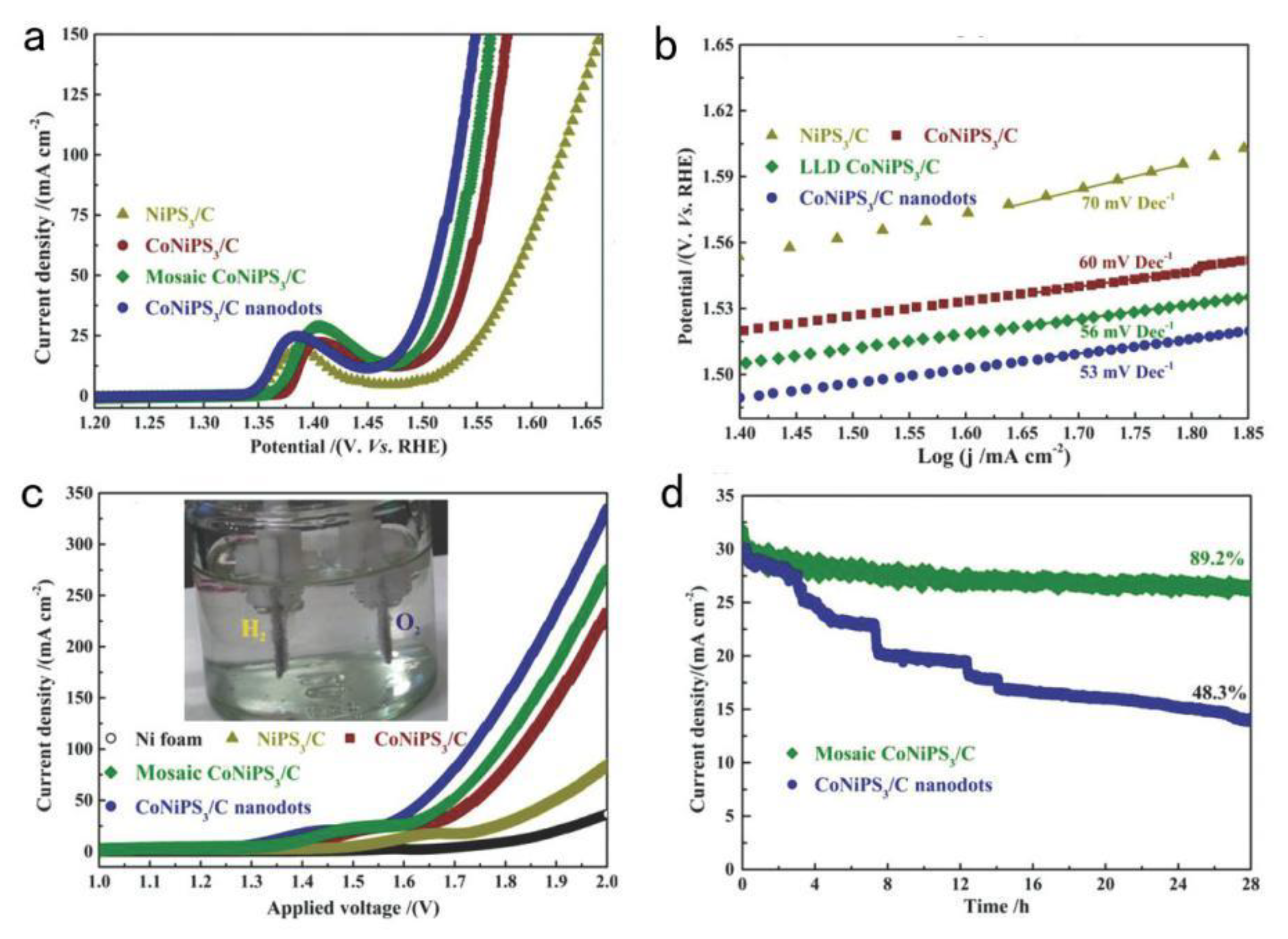
| CoNiPS3/C | 262 @ 30 mA·cm−2 | Simultaneous phosphorization and sulfurization processes and Co-Ni Prussian-blue analogues (PBA) as templating precursor (template assisted). | [42] |
| Fe3C(Cementite) | |||
| Fe3C/Fe2O3 @ NGNs | - | Assembling graphene oxide with graphitic carbon nitride and FeOOH nanorods, nanostructure of carbon layers coated iron species was constructed (template free). | [43] |
| Fe @ C-NG/NCNTs | 450 @ 10 mA·cm−2 | Annealing a Fe-based MOF (MIL-88B) loaded with melamine at 800 °C in N2 to get a hybrid structure (template assisted). | [44] |
| NiC | |||
| Fe-Ni3C-2% | 275 @ 10 mA·cm−2 | Carburizing treatment is carried out in argon/H2 atmosphere and then dope heteroatom of Fe into Ni3C nanodots via a co-precipitation method (template assisted). | [45] |
| NiC/MoC/NiMoO4 | 280 @ 10 mA·cm−2 | After preparing NiMoO4/NF nanorod arrays, NiMoO4 nanorods react with dopamine at a high annealing temperature to form 3D NiC/MoC/NiMoO4 (template assisted). | [46] |
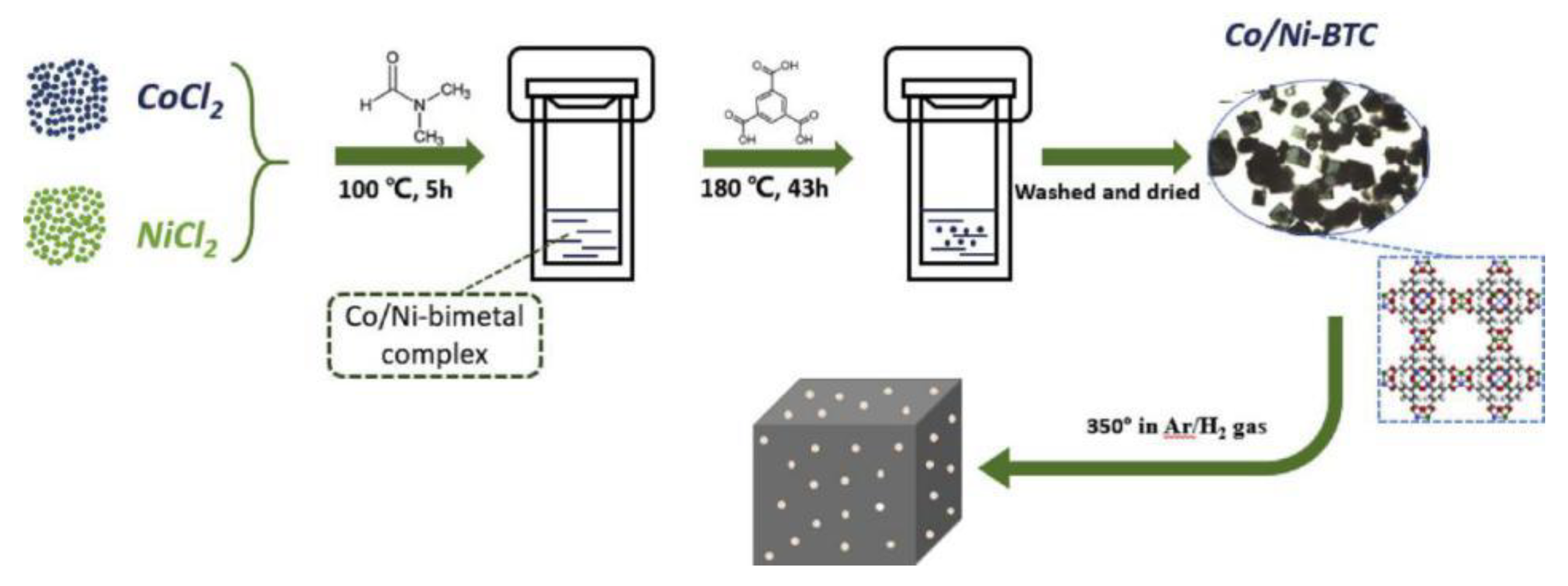
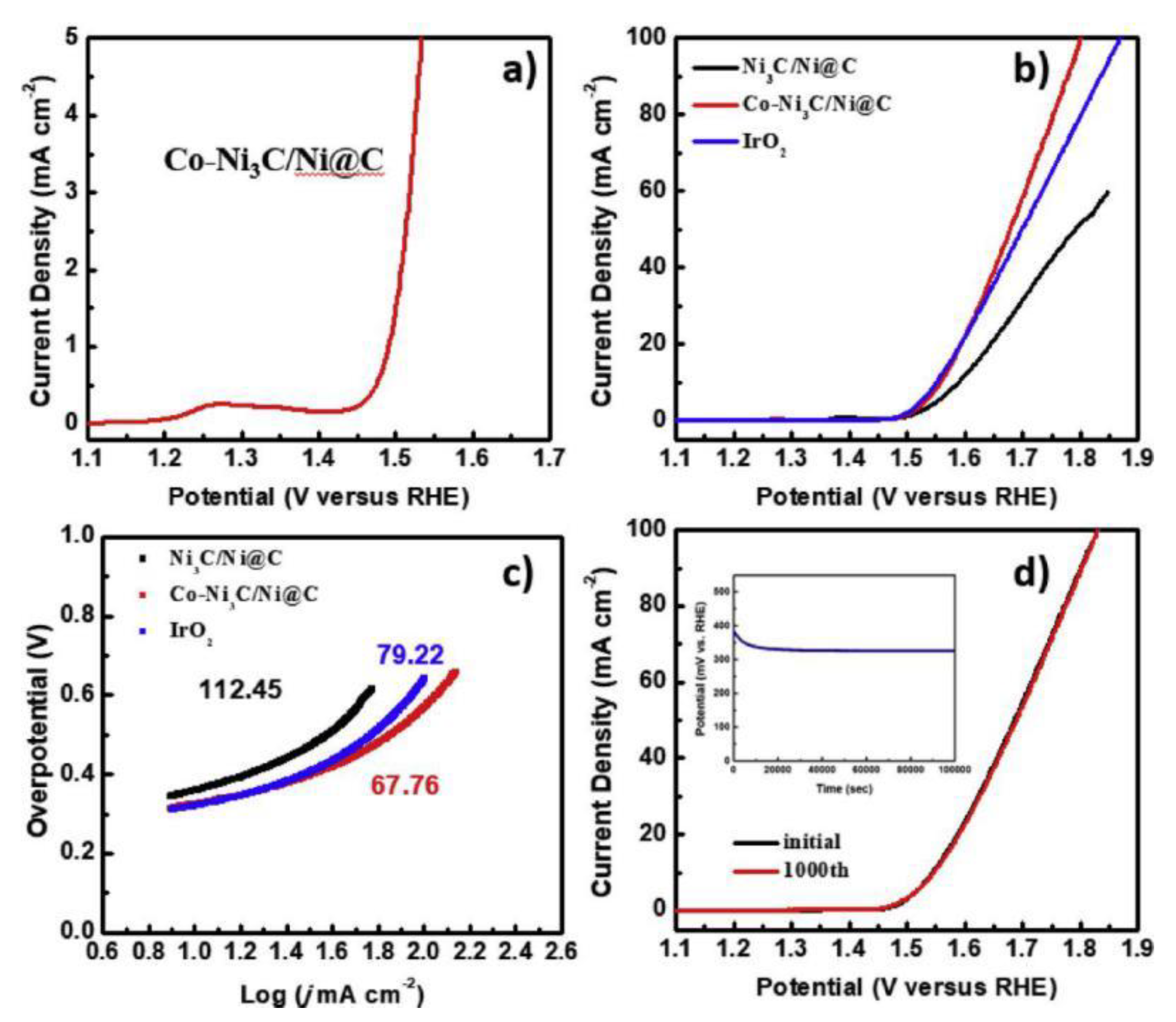
| Co-Ni3C/Ni @ C | 325 @ 10 mA·cm−2 | Co-Ni3C/Ni @ C is prepared via pyrolysis of the bimetallic MOF (Co/Ni-BTC) (template assisted). | [47] |
| d-NiC0.2NS/Ni/CF | 228 @ 10 mA·cm−2 | Nanosheets are prepared on nickel-coated copper foil by mild electrodeposition with high index polyhedral dendritic hexagonal NiCx (template free). | [48] |
| Ni/NGNRs | 380 @ 10 mA·cm−2 | Mix MWCNTs together with MERCK via a solvothermal process and then collect the resultant mixture for the post treatment of further heating under argon atmosphere (template free). | [49] |
| Mo2C | |||
| β-Mo2C | 267 @ 5 mA·cm−2 | Reduction of MoO3 with decolorizing carbon (Mo2C-DC) and multiwalled carbon nanotubes (Mo2C-MW) at 950 °C resulted in phase pure Mo2C (template free). | [50] |
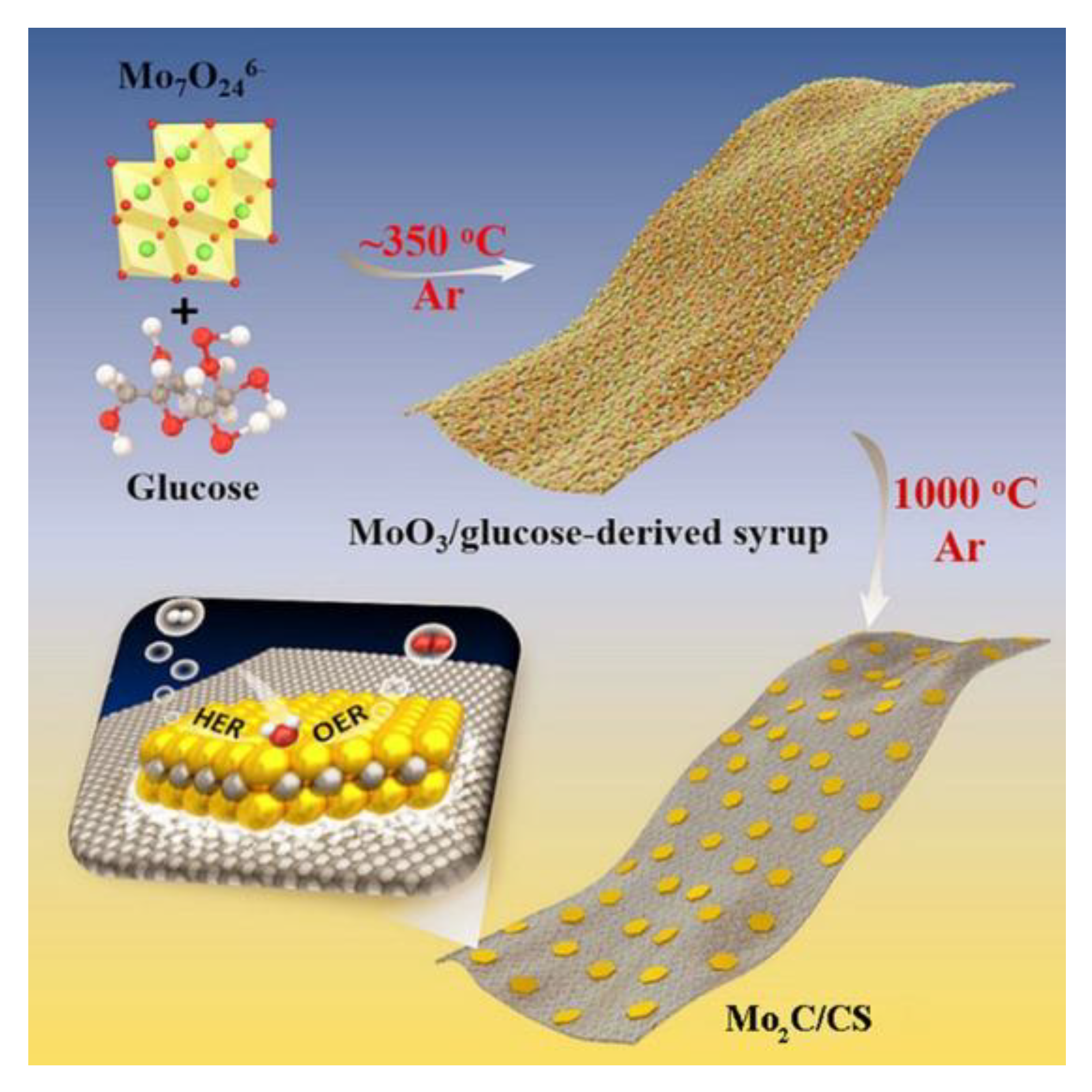
| Mo2C @ CS | 320 @ 10 mA·cm−2 | Mo2C @ CS is synthesized through a one-pot process with ammonium molybdate and glucose as the Mo and C source (template free). | [51] |
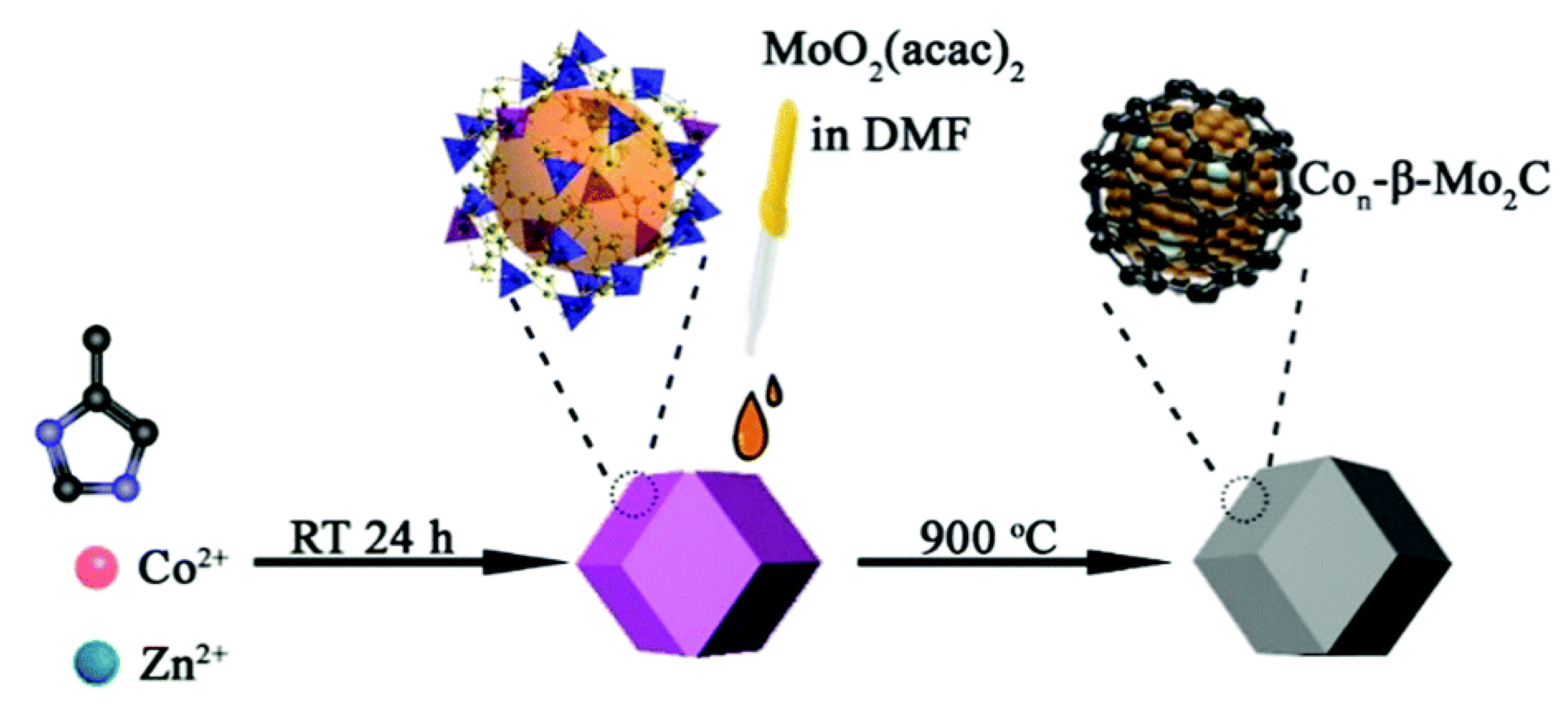
| Ni-Mo2C @ NC | 328 @ 10 mA·cm−2 | NiMoO4·xH2O nanobelts serve as a single source for the synthesis of both Ni and MoxC nanoparticles, while melamine simultaneously serves as the precursor and dopant for the formation of Ni-Mo2C @ NC (template assisted). | [52] |
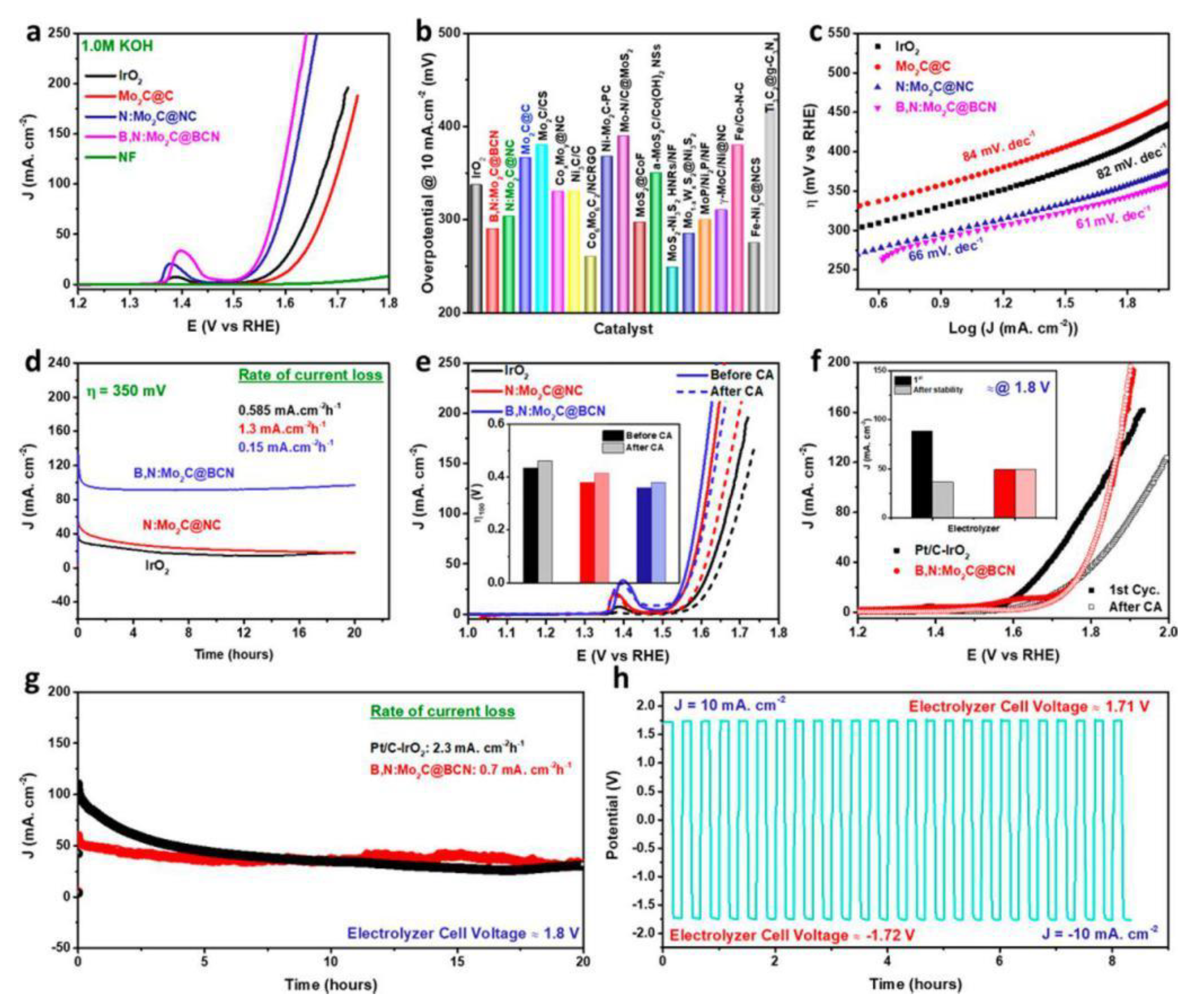
| B,N:Mo2C @ BCN | 360 @ 100 mA·cm−2 | Obtaining hybrid structure via an eco-friendly organometallic complex of Mo imidazole and boric acid (template assisted). | [53] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, Q.; Zhang, B.; Tian, L.; Li, K.; Zhang, X. Recent Advances in Transition Metal Carbide Electrocatalysts for Oxygen Evolution Reaction. Catalysts 2020, 10, 1164. https://doi.org/10.3390/catal10101164
Wang Y, Wu Q, Zhang B, Tian L, Li K, Zhang X. Recent Advances in Transition Metal Carbide Electrocatalysts for Oxygen Evolution Reaction. Catalysts. 2020; 10(10):1164. https://doi.org/10.3390/catal10101164
Chicago/Turabian StyleWang, Yuanfei, Qimeng Wu, Bicheng Zhang, Lei Tian, Kexun Li, and Xueli Zhang. 2020. "Recent Advances in Transition Metal Carbide Electrocatalysts for Oxygen Evolution Reaction" Catalysts 10, no. 10: 1164. https://doi.org/10.3390/catal10101164
APA StyleWang, Y., Wu, Q., Zhang, B., Tian, L., Li, K., & Zhang, X. (2020). Recent Advances in Transition Metal Carbide Electrocatalysts for Oxygen Evolution Reaction. Catalysts, 10(10), 1164. https://doi.org/10.3390/catal10101164









