Rendering Visible-Light Photocatalytic Activity to Undoped ZnO via Intrinsic Defects Engineering
Abstract
:1. Introduction
2. Results and Discussions
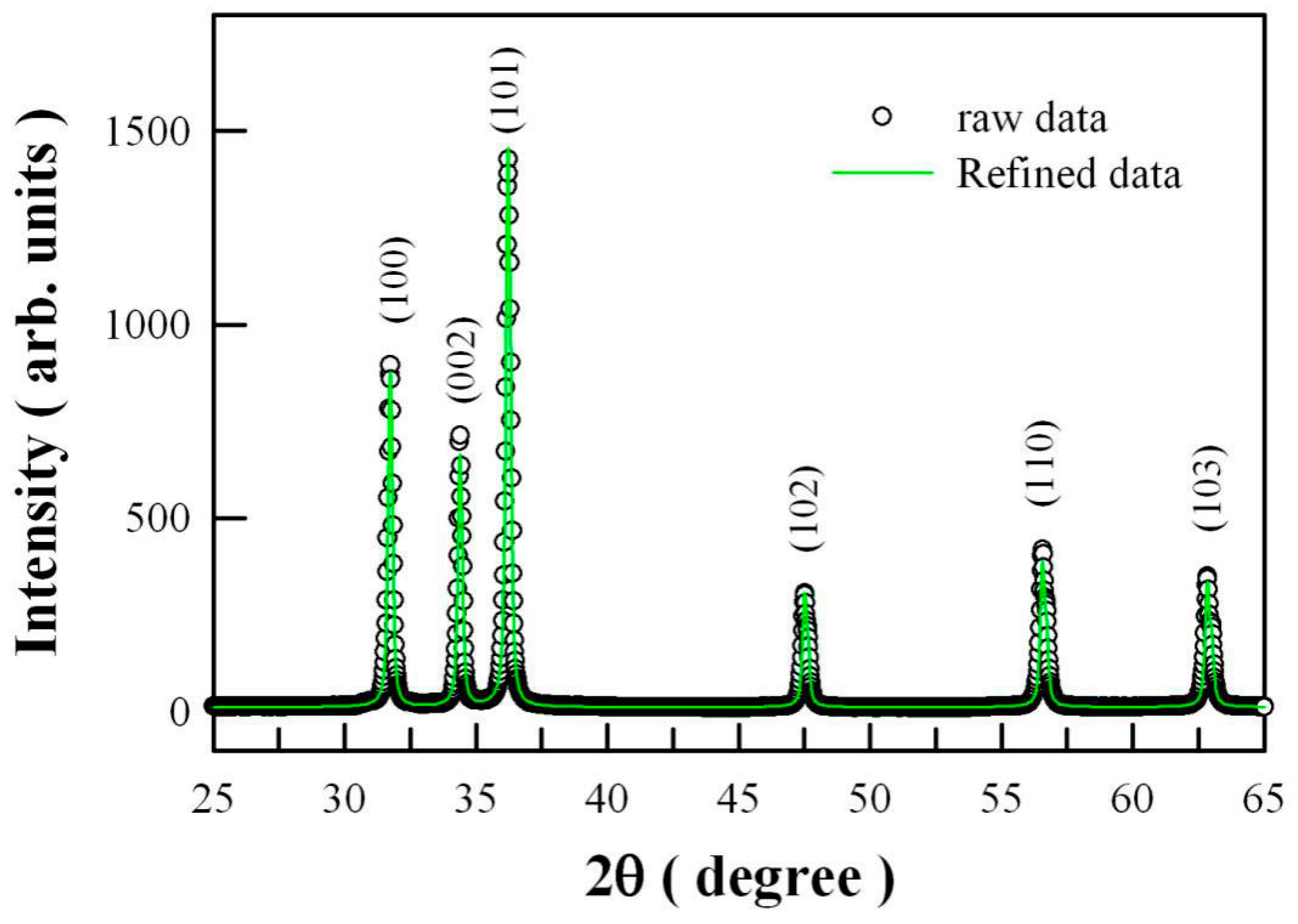
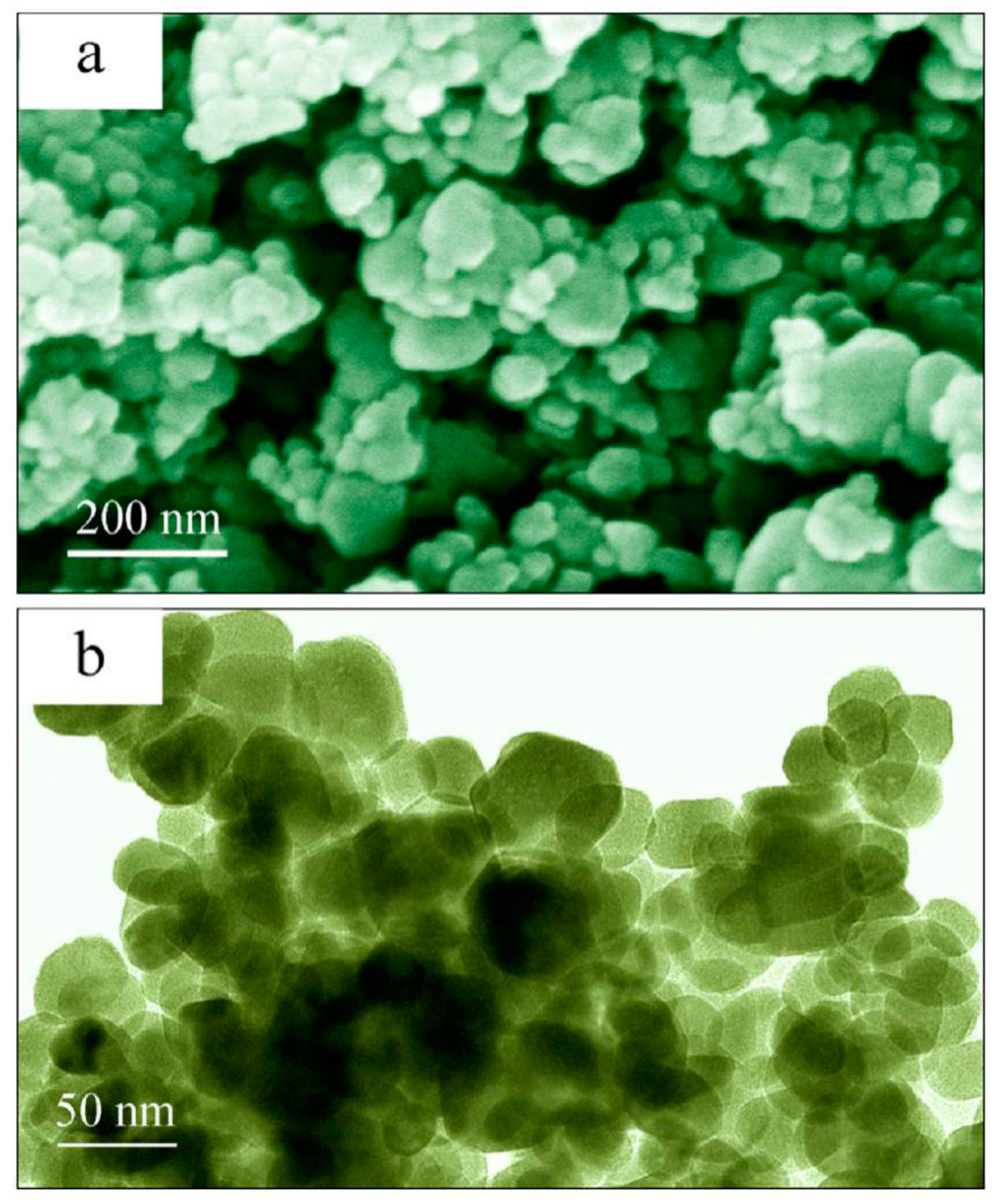
2.1. Morphology and Crystal Structure of ZnO Nanocrystals
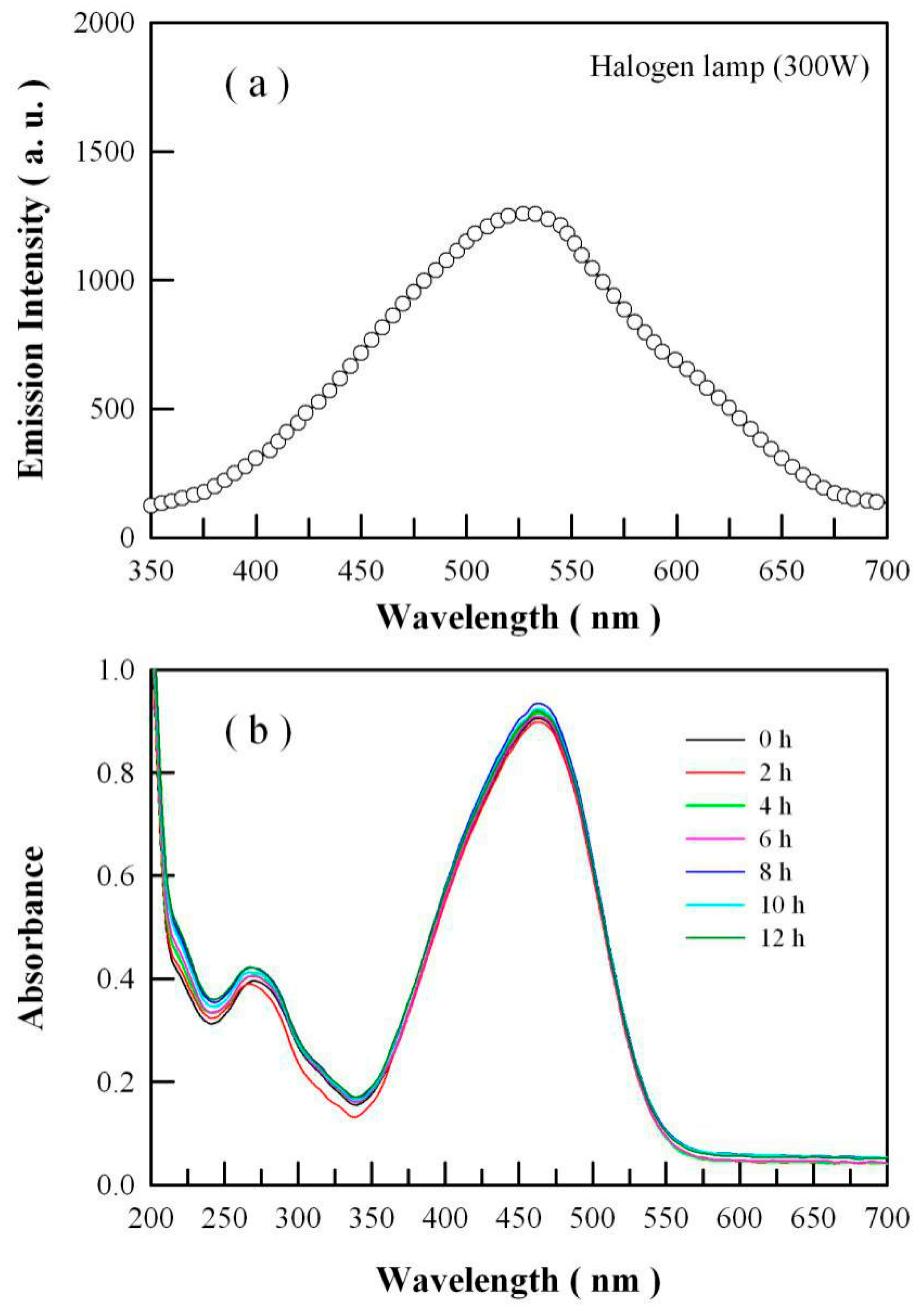
2.2. Absorption and Photoluminescence (PL) Spectra of ZnO Nanocrystals
2.3. PL Spectrum of the Halogen Lamp
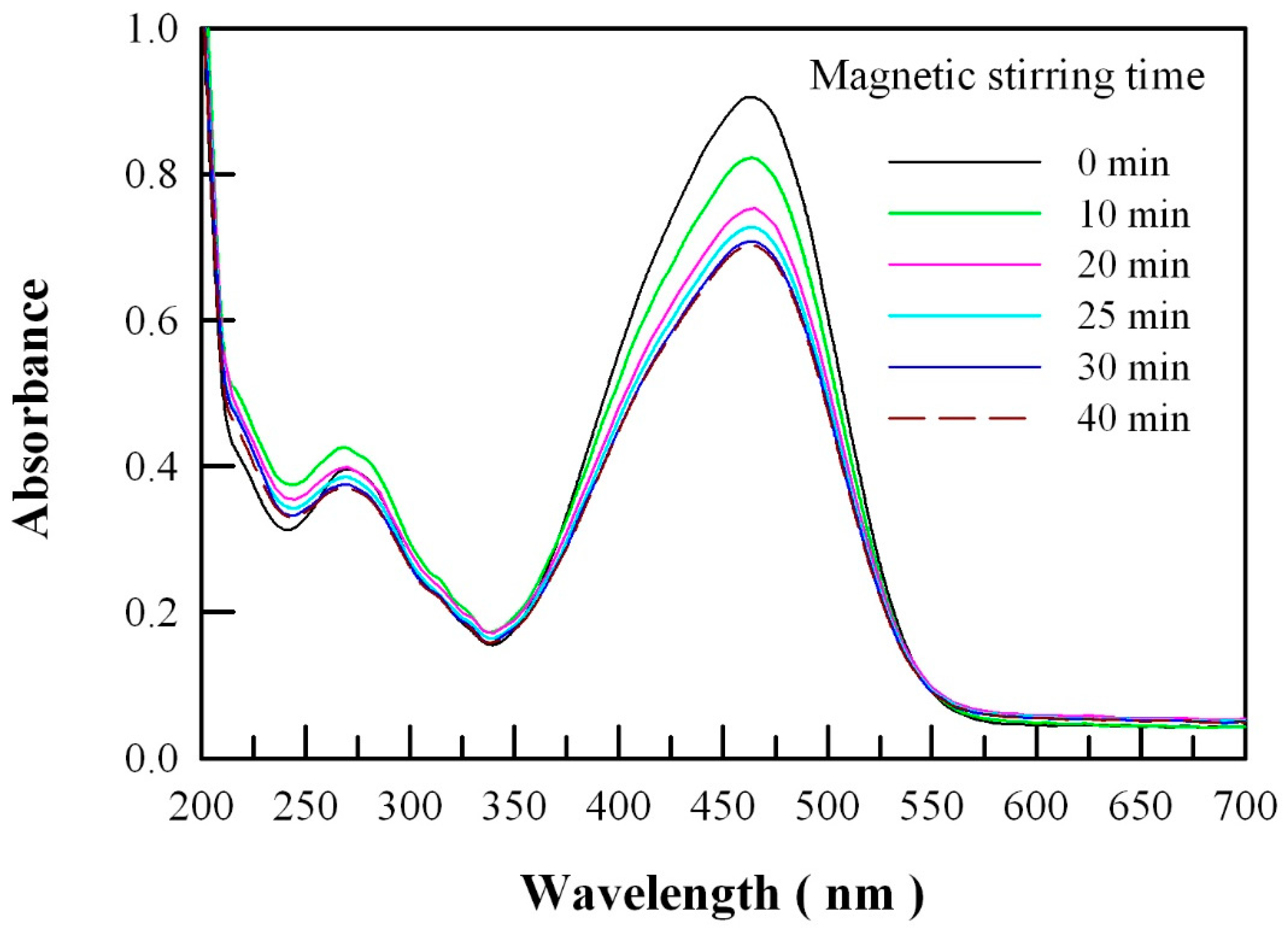
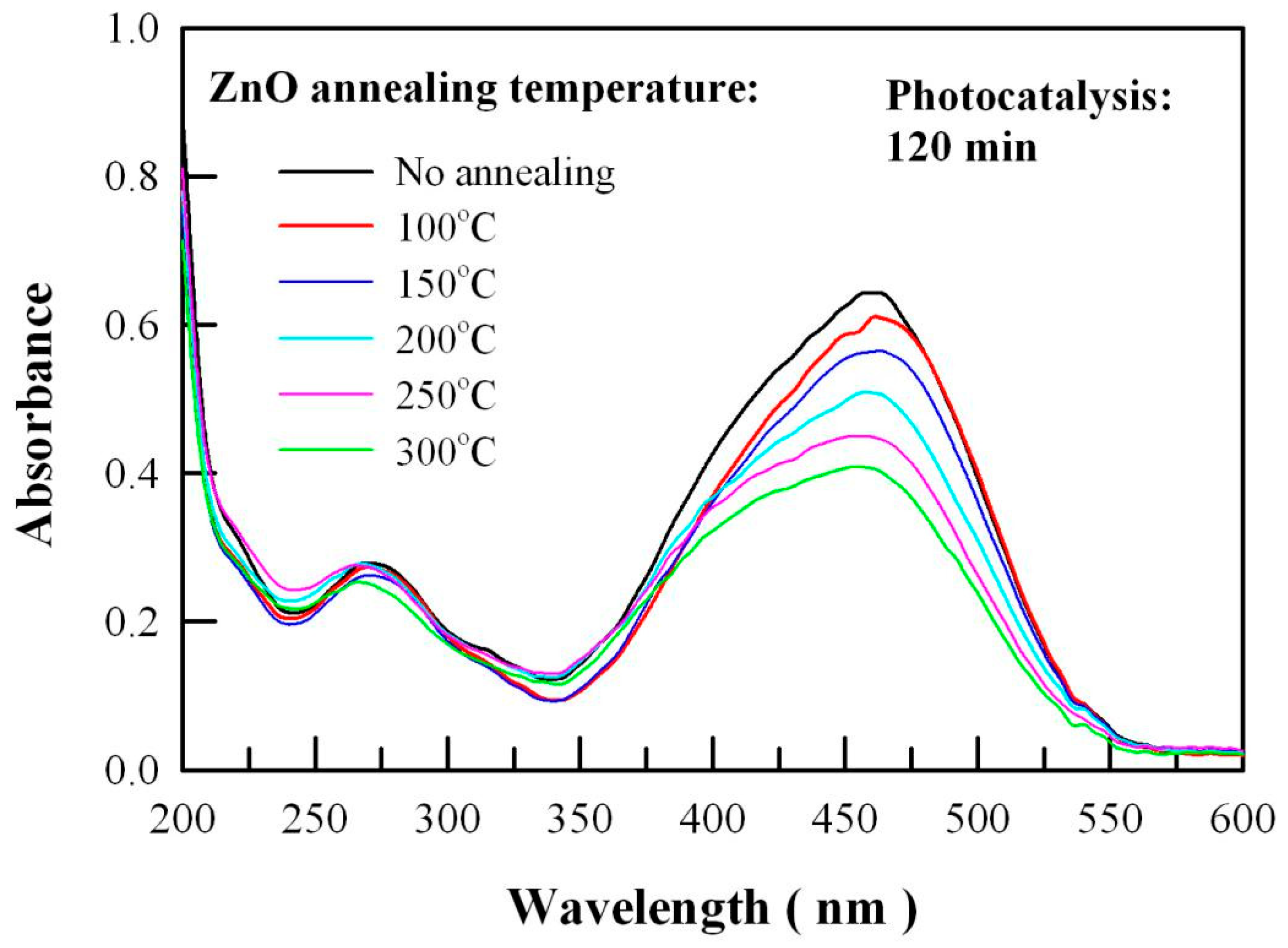
2.4. Photocatalytic Activity of Undoped ZnO Nanocrystals
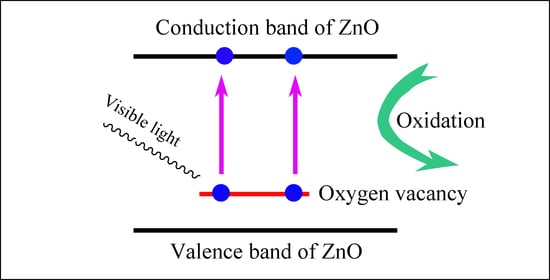
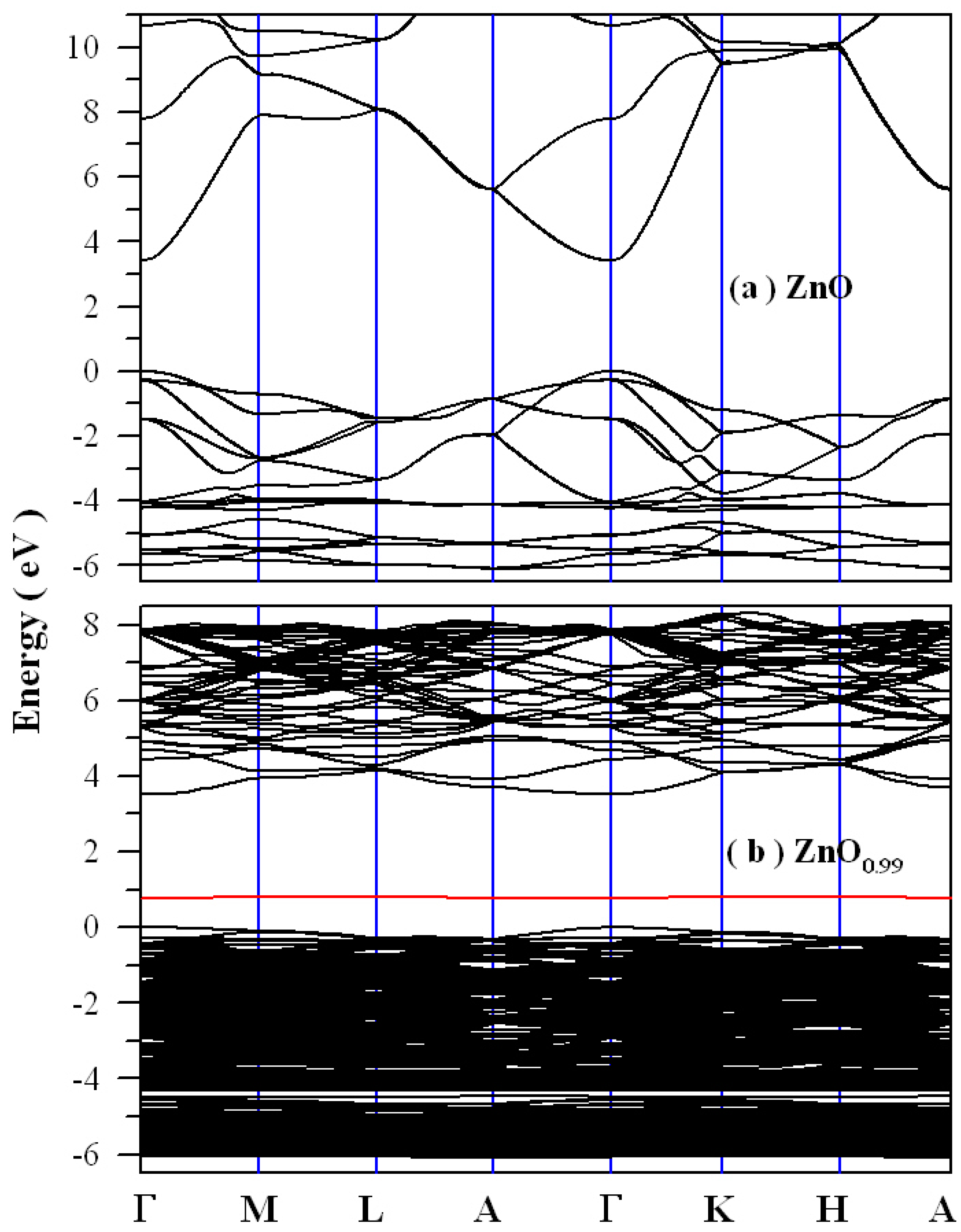
2.5. Electronic Structures of Oxygen Deficient ZnO
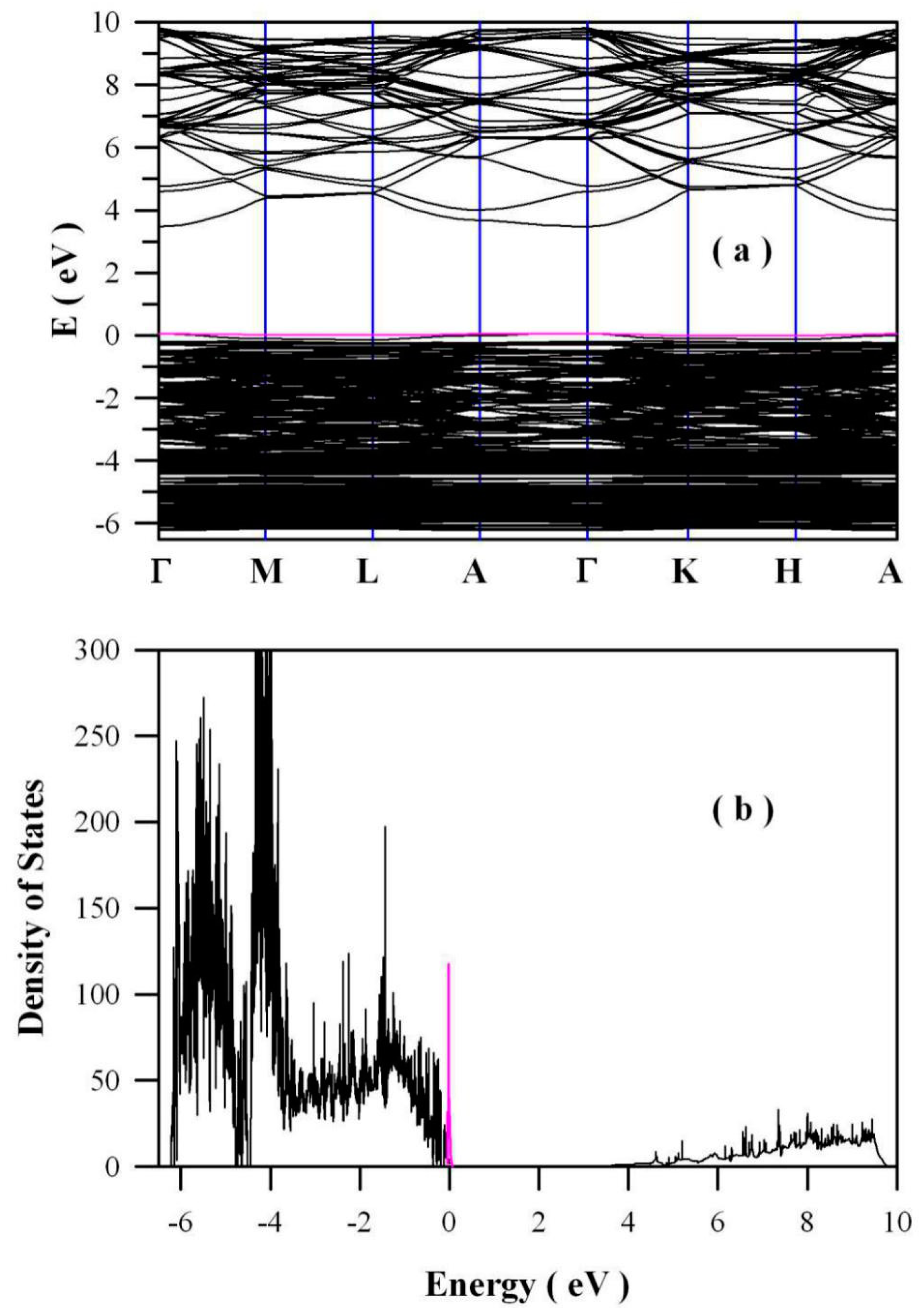
2.6. Electronic Structures of Zinc Deficient ZnO
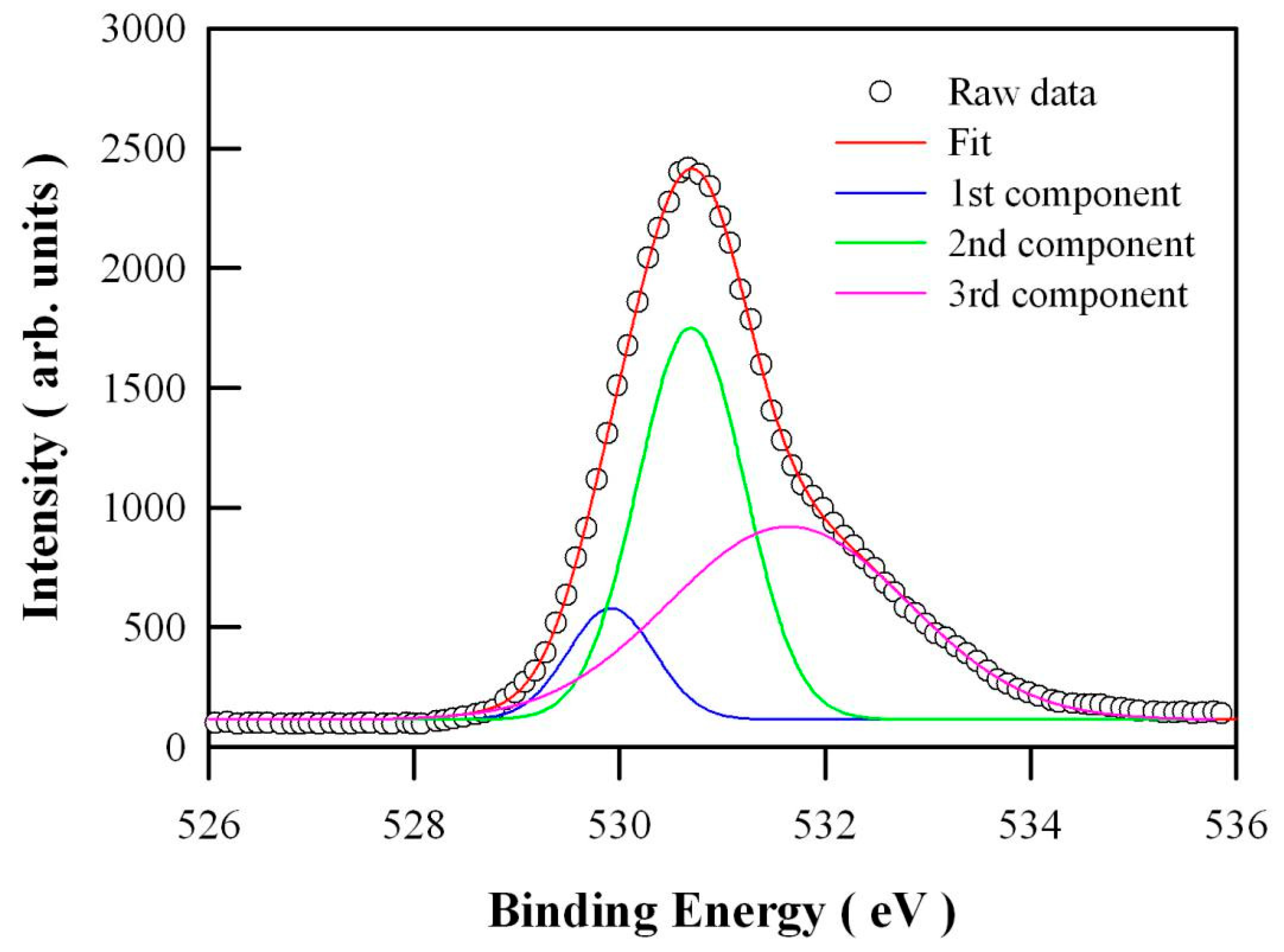
2.7. X-ray Photoelectron Spectroscopic (XPS) Spectrum of O1s in Undoped ZnO Nanocrystals
2.8. Visible-Light Photocatalytic Activity of Thermally-Annealed ZnO Nanocrystals
3. Materials, Characterizations and Calculation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rehman, S.; Ullah, R.; Butt, A.M.; Gohar, N.D. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, J.; Zhu, Y. Luminescence and photocatalytic activity of ZnO nanocrystals: Correlation between structure and property. Inorg. Chem. 2007, 46, 6675–6682. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Ma, Q.L.; Zhai, B.G. Core–shell Zn/ZnO structures with improved photocatalytic properties synthesized by aqueous solution method. Funct. Mater. Lett. 2013, 6, 1350058. [Google Scholar] [CrossRef]
- Ma, Q.L.; Xiong, R.; Zhai, B.G.; Huang, Y.M. Core–shelled Zn/ZnO microspheres synthesized by ultrasonic irradiation for photocatalytic applications. Micro Nano Lett. 2013, 8, 491–495. [Google Scholar] [CrossRef]
- Ma, Q.L.; Xiong, R.; Zhai, B.G.; Huang, Y.M. Ultrasonic synthesis of fern–like ZnO nanoleaves and their enhanced photocatalytic activity. Appl. Surf. Sci. 2015, 324, 842–848. [Google Scholar] [CrossRef]
- Ibrahim, A.; Kumar, R.; Umar, A.; Kim, S.H.; Bumajdad, A.; Ansari, Z.A.; Baskoutas, S. Cauliflower–shaped ZnO nanomaterials for electrochemical sensing and photocatalytic applications. Electrochim. Acta 2016, 222, 463–472. [Google Scholar] [CrossRef]
- Pawar, R.C.; Choi, D.H.; Lee, J.S.; Lee, C.S. Formation of polar surfaces in microstructured ZnO by doping with Cu and applications in photocatalysis using visible light. Mater. Chem. Phys. 2015, 151, 167–180. [Google Scholar] [CrossRef]
- Ullah, R.; Dutta, J. Photocatalytic degradation of organic dyes with manganese–doped ZnO nanoparticles. J. Hazard. Mater. 2008, 156, 194–200. [Google Scholar] [CrossRef]
- He, R.; Hocking, R.K.; Tsuzuki, T. Co–doped ZnO nanopowders: Location of cobalt and reduction in photocatalytic activity. Mater. Chem. Phys. 2012, 132, 1035–1040. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Y.; Wang, D.; Wang, L.; Xie, T.; Jiang, T. A high performance cobalt–doped ZnO visible light photocatalyst and its photogenerated charge transfer properties. Nano. Res. 2011, 4, 1144–1152. [Google Scholar] [CrossRef]
- Wang, R.; Xin, J.H.; Yang, Y.; Liu, H.; Xu, L.; Hu, J. The characteristics and photocatalytic activities of silver doped ZnO nanocrystallites. Appl. Surf. Sci. 2004, 227, 312–317. [Google Scholar] [CrossRef]
- Zhai, B.G.; Yang, L.; Ma, Q.L.; Huang, Y.M. Visible light driven photocatalytic activity of Fe–doped ZnO nanocrystals. Funct. Mater. Lett. 2017, 10, 1750002. [Google Scholar] [CrossRef]
- Uum, Y.R.; Han, B.S.; Lee, H.M.; Hong, S.M.; Kim, G.M.; Rhee, C.K. Magnetic and photocatalytic effect of Fe–doped nano–rod ZnO synthesized by the hydrolysis of metal powders. Phys. Status Solidi A 2007, 4, 4408–4411. [Google Scholar] [CrossRef]
- Abed, C.; Bouzidi, C.; Elhouichet, H.; Gelloz, B.; Ferida, M. Mg doping induced high structural quality of sol–gel ZnO nanocrystals: Application in photocatalysis. Appl. Surf. Sci. 2015, 349, 855–863. [Google Scholar] [CrossRef]
- SKhayyat, A.; Abaker, M.; Umar, A.; Alkattan, M.O.; Alharbi, N.D.; Baskoutas, S. Synthesis and characterizations of Cd–doped ZnO multipods for environmental remediation application. J. Nanosci. Nanotechnol. 2012, 12, 8453–8458. [Google Scholar] [CrossRef]
- Huang, Y.M.; Ma, Q.L.; Zhai, B.G. A simple method to grow one–dimensional ZnO nanostructures in air. Mater. Lett. 2013, 93, 266–268. [Google Scholar] [CrossRef]
- Huang, Y.M.; Ma, Q.L.; Zhai, B.G. Controlled morphology of ZnO nanostructures by adjusting the zinc foil heating temperature in an air–filled box furnace. Mater. Chem. Phys. 2014, 147, 788–795. [Google Scholar] [CrossRef]
- Ischenko, V.; Polarz, S.; Grote, D.; Stavarache, V.; Fink, K.; Driess, M. Zinc oxide nanoparticles with defects. Adv. Funct. Mater. 2003, 15, 1945–1954. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, M.S.; Yahia, I.S.; Knoff, W.; Story, T. Oxygen–defected ZnO: Facial Synthesis and high photocatalytic performance under visible light. Optik 2018, 158, 1123–1130. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Dong, Y.; Chen, R.; Xiang, L.; Komarneni, S. Defect–rich ZnO nanosheets of high surface area as an efficient visible–light photocatalyst. Appl. Catal. B Environ. 2016, 192, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.L.; Huang, Y.M. Improved photovoltaic performance of dye sensitized solar cell by decorating TiO2 photoanode with Li–doped ZnO nanorods. Mater. Lett. 2015, 148, 171–173. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zhai, B.G.; Huang, Y.M. Sol–gel derived ZnO/porous silicon composites for tunable photoluminescence. J. Sol-Gel Sci. Technol. 2012, 64, 110–116. [Google Scholar] [CrossRef]
- Zhai, B.G.; Ma, Q.L.; Yang, L.; Huang, Y.M. Synthesis of morphology–tunable ZnO nanostructures via the composite hydroxide mediated approach for photocatalytic applications. Mater. Res. Express 2016, 3, 105045. [Google Scholar] [CrossRef]
- Pei, Z.; Ding, L.; Hu, J.; Weng, S.; Zheng, Z.; Huang, M.; Liu, P. Defect and its dominance in ZnO films: A new insight into the role of defect over photocatalytic activity. Appl. Catal. B Environ. 2013, 142–143, 736–743. [Google Scholar] [CrossRef]
- Liu, F.Z.; Guo, M.Y.; Leung, Y.H. Influence of native defects on photocatalytic activity of ZnO. AIP Conf. Proc. 2013, 1566, 75. [Google Scholar]
- Fang, J.; Fan, H.; Ma, Y.; Wang, Z.; Chang, Q. Surface defects control for ZnO nanorods synthesized by quenching and their anti–recombination in photocatalysis. Appl. Surf. Sci. 2015, 332, 47–54. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Chen, Q.; Ren, B.; Guan, R.; Cao, X.; Yang, X.; Duan, R. Interface–defect–mediated photocatalysis of mesocrystalline ZnO assembly synthesized in–situ via a template–free hydrothermal approach. Appl. Surf. Sci. 2017, 412, 517–528. [Google Scholar] [CrossRef]
- Huang, Y.M.; Zhai, B.G.; Zhou, F.F. Effects of photo–irradiation on the optical properties and electronic structures of an azo–containing bent–core liquid crystal. Mol. Cryst. Liq. Cryst. 2009, 510, 34–42. [Google Scholar] [CrossRef]
- Janotti, A.; van de Walle, C.G. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- McCluskey, M.D.; Jokela, S.J. Defects in ZnO. J. Appl. Phys. 2009, 106, 071101. [Google Scholar] [CrossRef] [Green Version]
- Kappers, L.A.; Gilliam, O.R.; Evans, S.M.; Halliburton, L.E.; Giles, N.C. EPR and optical study of oxygen and zinc vacancies in electron–irradiated ZnO. Nucl. Instrum. Methods Phys. Res. B 2008, 266, 2953. [Google Scholar] [CrossRef]
- Zhai, B.G.; Yang, L.; Zhou, F.F.; Shi, J.S.; Huang, Y.M. Strong photo–oxidative capability of ZnWO4 nanoplates with highly exposed {0–11} facets. Catalysts 2019, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.M.; Li, M.Y.; Yang, L.; Zhai, B.G. Eu2+ and Eu3+ doubly doped ZnWO4 nanoplates with superior photocatalytic performance for dye degradation. Nanomaterials 2018, 8, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Kayaci, F.; Vempati, S.; Donmez, I.; Biyikli, N.; Uyar, T. Role of zinc interstitials and oxygen vacancies of ZnO in photocatalysis: A bottom–up approach to control defect density. Nanoscale 2014, 6, 10224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bora, T.; Sathe, P.; Laxman, K.; Dobretsov, S.; Dutta, J. Defect engineered visible light active ZnO nanorods for photocatalytic treatment of water. Catal. Today 2017, 284, 11–18. [Google Scholar] [CrossRef]
- Baruah, S.; Rafique, R.F.; Dutta, J. Visible light photocatalysis by tailoring crystal defects in zinc oxide nanostructures. Nano 2008, 3, 399–407. [Google Scholar] [CrossRef]
- Zhai, B.G.; Huang, Y.M. Origin of thermal–annealing induced orange emissions from solution–grown ZnO nanocrystals. Mater. Res. Innov. 2015, 19, S7-45–S7-50. [Google Scholar] [CrossRef]
- Zhai, B.G.; Xu, H.; Zhuo, F.; Huang, Y.M. Annealing temperature dependent photoluminescence and afterglow of undoped CaAl2O4. J. Alloys Compd. 2020, 821, 153563. [Google Scholar] [CrossRef]
- Zhai, B.G.; Yang, L.; Huang, Y.M. Intrinsic defect engineering in Eu3+ doped ZnWO4 for annealing temperature tunable photoluminescence. Nanomaterials 2019, 9, 99. [Google Scholar] [CrossRef] [Green Version]
- Tran, F.; Blaha, P. Accurate band gaps of semiconductors and insulators with a semilocal exchange–correlation potential. Phys. Rev. Lett. 2009, 102, 226401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koller, D.; Tran, F.; Blaha, P. Merits and limits of the modified Becke–Johnson exchange potential. Phys. Rev. B 2011, 83, 195134. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-l.; Zhai, B.-g.; Huang, Y.M. Rendering Visible-Light Photocatalytic Activity to Undoped ZnO via Intrinsic Defects Engineering. Catalysts 2020, 10, 1163. https://doi.org/10.3390/catal10101163
Chen L-l, Zhai B-g, Huang YM. Rendering Visible-Light Photocatalytic Activity to Undoped ZnO via Intrinsic Defects Engineering. Catalysts. 2020; 10(10):1163. https://doi.org/10.3390/catal10101163
Chicago/Turabian StyleChen, Lan-li, Bao-gai Zhai, and Yuan Ming Huang. 2020. "Rendering Visible-Light Photocatalytic Activity to Undoped ZnO via Intrinsic Defects Engineering" Catalysts 10, no. 10: 1163. https://doi.org/10.3390/catal10101163