Abstract
Sb-containing catalysts (SbZrOx (SbZr), SbCeOx (SbCe), SbCeZrOx (SbCeZr)) were prepared by citric acid method and investigated for the selective catalytic reduction (SCR) of NOx with NH3 (NH3-SCR). SbCeZr outperformed SbZr and SbCe and exhibited the highest activity with 80% NO conversion in the temperature window of 202–422 °C. Meanwhile, it also had good thermal stability and resistance against H2O and SO2. Various characterization methods, such as XRD, XPS, H2-TPR, NH3-TPD, and in situ diffuse reflectance infrared Fourier transform (DRIFT), were applied to understand their different behavior in NOx removal. The presence of Sb in the metal oxides led to the difference in acid distribution and redox property, which closely related with the NH3 adsorption and NO oxidation. Brønsted acid and Lewis acid were evenly distributed on SbCe, while Brønsted acid dominated on SbCeZr. Compared with Brønsted acid, Lewis acid was slightly active in NH3-SCR. The competition between NH3 adsorption and NO oxidation was dependent on SbOx and metal oxides, which were found on SbCe while not on SbCeZr.
1. Introduction
Nitrogen oxide (NOx), one of the main environmental contaminants emitted from stationary sources and mobile sources, causes photochemical smog, acid rain, and the depletion of the ozone layer [,]. Several technologies have been proposed for NOx removal, such as direct decomposition of NOx, NOx storage reduction (NSR), and the selective catalytic reduction (SCR) process with ammonia (NH3-SCR) or hydrocarbons (HC-SCR). The NH3-SCR process has been proven to be the efficient method to satisfy the restrict regulations of NOx emissions []. In general, the NH3-SCR reaction is a competitive process between NH3 oxidation and NOx reduction, which are closely affected by reaction temperature. The enhanced oxidation activity at low temperature promotes NOx selective reduction, while it also takes a risk for the depletion of NH3 at high temperature leading to the decrease in NO reduction. Meanwhile, the reaction thermal shock and exhaust components (H2O and SO2) put forward strict requirements on the design of NH3-SCR catalysts.
CeO2 possesses unique oxygen storage-release capability by the formation of Ce3+/Ce4+ redox couples [], but the maximum NO conversion on CeO2 was about 30% in the whole temperature range. Compared with mono-metal oxides, dual metal oxides have been more active in NO abatement. The presence of Ti, W, Mo, Mn, and Nb can efficiently broaden the temperature window, especially the introduction of W. Nearly 100% NOx conversion could be achieved on CeWOx in a wide temperature range (250–425 °C) under a rather high gas hourly space velocity (GHSV) (500,000 h−1). The effects of SO2 on the SCR over CeO2 was strongly dependent on the SO2 content and reaction conditions. An appropriate level for sulfated pretreatment can promote SCR, and the presence of excess SO2 shows obvious negative effects. Li studied that SO2 was preferentially bonded with CeO2 as sulfate species leading to a significant decrease in reducibility and the loss of surface active oxygen groups []. Suitable sulfation promoted NH3 adsorption and favored the formation of oxygen vacancies which facilitated NH3-SCR []. Hong’s research indicated that the optimal condition for sulfation treatment was 800 ppm SO2 1 h at 500 °C []. Meanwhile, the thermal stability of the sulfated Ce-based catalyst needs to be further improved [].
Sb compounds are generally classified into the valences of +3 and +5, in which the oxidation state of +5 is more stable. The Sb-containing oxides are Sb2O3, Sb2O5, and mixed oxide Sb2O4 that is composed of Sb3+ and Sb5+ []. Sb has been widely used as a promoter for NH3-SCR catalysts [,]. The surface acid and the redox capability are generally considered to be two necessary factors for NH3-SCR catalysts [,,]. The addition of Sb can improve the dispersion of active species [] and increase acid sites and the redox property [], which are due to the strong interaction between SbOx and other metal oxides [,]. Besides the promotion in activity, the resistance of K2O poisoning [] and SO2 are also found [] in Sb-containing catalysts. The role of Sb as a promoter reflects in minimizing the binding affinity between the catalytic surface and the ammonium (bi)sulfates species, which is beneficial for the low-temperature SO2 resistance [].
The substitution of Ce4+ by Zr4+ helps create structural defect, accelerating oxygen diffusion and the formation of surface oxygen species [], which can consequently promote the redox property [,]. CeZrOx mixed oxide with remarkable thermal stability shows an enormous advantage in NH3-SCR [,]. In this study, Sb-containing metal oxides (SbZr, SbCe, SbCeZr) were prepared by citric acid method and investigated for NOx removal by NH3-SCR. The SbCeZr exhibited higher catalytic activity than SbCe and SbZr catalysts, especially in the presence of H2O and SO2. The correlation between physicochemical properties and SCR performance was explored by various characterization techniques, and the mechanism was also studied using in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFT).
2. Results and Discussion
2.1. Catalyst Activity
2.1.1. NH3-SCR Performance
NOx conversions over the catalysts as a function of temperature are illustrated in Figure 1a. Pristine SbOx and SbZr showed negligible catalytic activity with NOx conversion below 10% in the whole temperature range. Even for CeO2, the maximum NOx conversion was only 29% at 360 °C. The addition of Sb promoted the NOx conversion over Ce-containing catalysts, and SbCeZr exhibited better NH3-SCR performance than SbCe. The temperature for 50% conversion (T50) was 175 °C over SbCeZr, which was lower than that of SbCe (223 °C). The operation temperature window (NOx conversion over 80%) was 202–422 °C over SbCeZr, while it was 261–407 °C over SbCe.
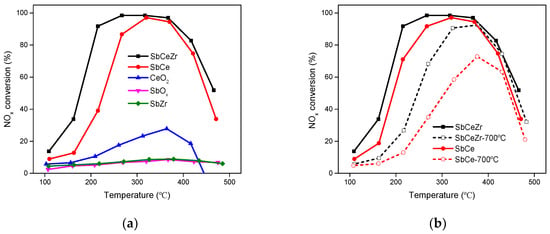
Figure 1.
(a) NOx conversions and (b) thermal stability over the catalysts (reaction conditions: 500 ppm NO, 500 ppm NH3, 5% O2, with Ar balance).
The apparent activation energy of the NH3-SCR reaction is an important factor to evaluate the role and efficiency of the catalyst in the reaction []. By calculating the reaction rate constant of the NH3-SCR reaction over SbCeZr and SbCe, the apparent activation energy of the catalysts were obtained. The reaction activation energy over the SbCeZr catalyst (33.3 kJ/mol) was lower than that of the SbCe catalyst (42.1 kJ/mol), which further confirmed the excellent NH3-SCR reaction performance of SbCeZr.
In order to investigate the thermal stability of SbCeZr and SbCe, the catalysts were aged at 700 °C for 4 h in the air before testing. As shown in Figure 1b, aging had a side effect on catalytic activity, which led to the loss in activity and shrink from the operation temperature window. The presence of Zr improved the thermal resistance of thermal shock. T50 of aged SbCeZr turned out to be 247 °C and the operation temperature window narrowed to 300–413 °C, resulting from the sintering. The maximum NOx conversion over aged SbCe was only 73% at 373 °C, i.e., aging led to the significant deactivation.
2.1.2. Effects of H2O and SO2
SO2 and H2O are unavoidable in the feed gas, so the effect on the catalytic activities over SbCeZr and SbCe were investigated. As shown in Figure 2a, the NH3-SCR performance of SbCeZr was slightly affected by SO2 or H2O at temperatures below 220 °C. However, the presence of H2O significantly reduced the catalytic activity at temperatures above 420 °C, which could be attributed to the competitive adsorption between H2O and NH3 [,]. In the presence of O2 and SO2, the deposited sulfates on the catalysts led to the decrease of the specific surface area (SSA) [] and blocked the active sites at low temperature []. With increased temperature, the decomposition of sulfates led to the restoration of active sites []. The presence of H2O facilitated SO2 oxidation at low temperature and inhibited sulfate decomposition at high temperature. Therefore, the presence of H2O and SO2 had a more detrimental effect than H2O or SO2.
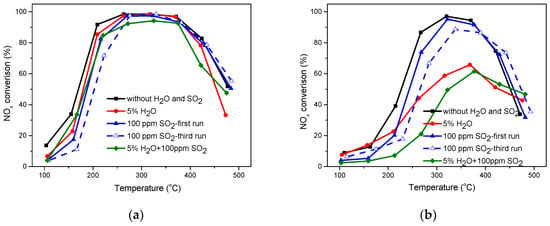
Figure 2.
Effect of H2O and SO2 on the NH3-SCR performance over (a) the SbCeZr catalyst and (b) the SbCe catalyst (reaction conditions: 500 ppm NO, 500 ppm NH3, 5% O2, 5% H2O, 100 ppm SO2, with Ar balance).
The presence of SO2 on SbCe had an influence similar to that on SbCeZr. Unfortunately, the presence of H2O had a detrimental effect on NH3-SCR performance over SbCe. The light-off temperature (T50) shifted to 284 °C, and the maximum NOx conversion was only 66% at 370 °C. When both H2O and SO2 were introduced simultaneously, the synergy effect made the NH3-SCR performance drop even more. T50 shifted to high temperature and the maximum NOx conversion also decreased accordingly. The decrease of NH3-SCR performance was not only related to the formation of ammonium salt on the catalyst surface but also connected with the fact that the active phase was sulfated and then formed stable sulfate species [].
Furthermore, repeated temperature ramps (three times) containing SO2 over the SbCeZr catalyst and the SbCe catalyst were also conducted. For SbCeZr, the second time was consistent with the third time, and slightly less active than the first time below 250 °C. It suggests that SbCeZr had a better performance of resistance to SO2. For SbCe, during the three test ramps, the T80 kept shifting to higher temperatures, which narrowed the operation window.
2.2. Catalyst Characterization
2.2.1. XRD
The XRD patterns of the fresh and aging catalysts are shown in Figure 3. The XRD patterns of CeO2 and ZrO2 showed sharp diffraction peaks corresponding to CeO2 with a cubic fluorite structure (JCPDS 34-0394, 2θ = 28.6°, 47.5°, and 56.4°) and tetrahedral ZrO2 (JCPDS 50-1089, 2θ = 30.1°, 50.4°, and 60.0°). The typical peaks at 19.8°, 25.7°, and 29.0° over SbOx were observed and assigned to Sb2O4 (JCPDS 11-0694). Compared with the XRD pattern of CeO2, the diffraction peaks of CeZr shifted to high angles, suggesting that Zr was incorporated into the CeO2 crystal lattice to form a CeZrO4 solid solution.
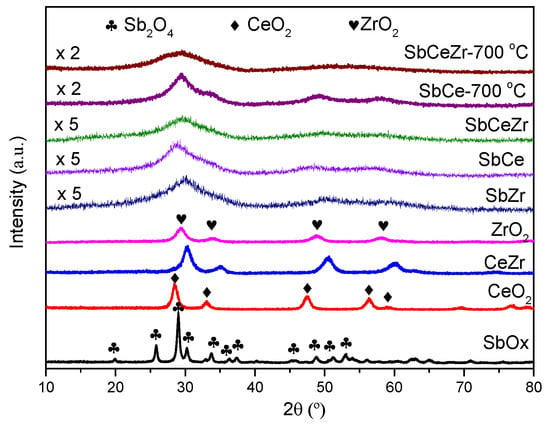
Figure 3.
XRD patterns of the catalysts.
With the introduction of Sb, the diffraction peaks were broadened and weakened, which led to the absence of obvious peaks. No diffraction peaks related to SbOx were detected, and a completely amorphous structure was formed on the Sb-containing catalysts. Compared with the fresh samples, the diffraction peaks of aged catalysts (aging at 700 °C in air for 4 h) intensified, indicating the increase in the grain size.
According to Table 1, the specific surface area (SSA) of SbCeZr (73 m2/g) was slightly higher than that of SbCe (68 m2/g). Combining the value of specific surface area (Table 1) with activity showed that specific surface area was not the decisive factor in catalyst activity. It thus suggested that other factors were affecting activity. The loss of SSA was found after aging, especially on SbCe. The SSA of SbCe-700 °C was only 9 m2/g, which was only 13% of fresh SbCe. However, 57% SSA of SbCeZr was lost after aging. Combining the results of XRD and SSA showed that SbCeZr possessed the higher thermal stability as compared to SbCe.

Table 1.
Brunauer–Emmett–Teller (BET) specific surface area (SSA), bulk phase, and surface atom concentration of the catalysts.
2.2.2. XPS
Figure 4 presented the XPS spectra of Sb 3d, Ce 3d, and O 1s for Sb-containing catalysts, and the surface atom concentration and chemical state are summarized in Table 1. For the SbCeZr catalyst, the bulk phase mole ratio of Sb/(Ce + Zr) was 0.57, slightly lower than the surface atom concentration ratio 0.66. However, as for the SbCe catalyst and SbZr catalyst, the bulk phase mole ratio was much lower than the surface concentration. These results indicated that more Sb existed on the surface of CeO2 and ZrO2 over SbCe and SbZr, while more Sb was incorporated into the CeZrOx.
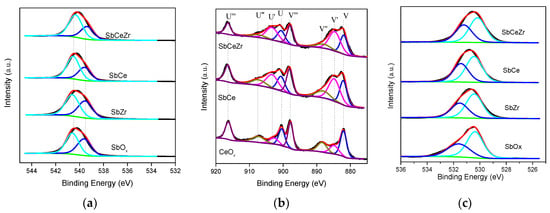
Figure 4.
XPS spectra for (a) Sb3d, (b) Ce3d, and (c) O1s over the catalysts.
Figure 4a showed the Sb 3d3/2 spectra of Sb-containing catalysts and SbOx. Because of the O 1s and Sb 3d5/2 photoemission line overlapping around 530.0 eV, the Sb3d3/2 spectra at higher binding energy were used. This peak could be separated into two contributions—the Gaussian line centered at 540.4 eV corresponds to antimony with an oxidation state of Sb5+, while the peak at 539.6 eV corresponds to Sb3+ [,,]. As shown in Table 1, the proportion of Sb5+ increase followed the order of SbOx < SbZr < SbCe < SbCeZr. The ratio of Sb5+ over SbOx was 50.3%, meaning an equal proportion of Sb5+ and Sb3+, i.e., Sb2O4 was the main species in SbOx, which was in line with the XRD results. It was obvious that the concentration of Sb5+ over the SbCeZr was significantly improved to 64.3%.
Figure 4b presents the Ce 3d spectra of the catalysts. The Ce 3d peaks could be deconvoluted into four pairs of spin-orbit doublets. Four main peaks in 3d5/2 at 881.9, 884.9, 887.8, and 898.0 eV were labeled as V, V′, V″, and V′″, respectively. The four main peaks in 3d3/2 at 900.8, 903.0, 907.4, and 916.2 eV were labeled as U, U′, U″, and U′″, respectively. The peaks labeled as V’ and U’ corresponded to Ce3+ and the other peaks labeled as V, V″, V′″, U, U″, and U′″ corresponded to Ce4+ [,]. From the Ce 3d XPS spectra, it could be concluded that the Ce3+ and Ce4+ species coexisted in all the samples, and the ratios of Ce3+/(Ce4+ + Ce3+) of all the catalysts are listed in Table 1. The Ce3+/(Ce4+ + Ce3+) ratio over SbCeZr (42.1%) was higher than that over SbCe (32.9%). The presence of Sb5+ led to the increase of the Ce3+ concentration due to the redox reaction Sb3+ + Ce4+ ↔ Sb5+ + Ce3+. The transformation of Ce4+ to Ce3+ created the charge imbalance and oxygen vacancy, facilitating the adsorption of oxygen species or activation of reactants in NH3-SCR [].
The O 1s spectra of all the catalysts are displayed in Figure 4c. The spectra were deconvoluted into two peaks. The peak at 530.2 eV was attributed to lattice oxygen (Oβ), and the other one at around 531.2 eV was denoted to surface chemisorbed oxygen (Oα). Oα species usually correlated with oxygen defects or surface oxygen, such as O22− or O2− [,,]. The relative ratios of Oα/(Oα + Oβ) by peak deconvolution over the samples were 47.8%, 40.6%, and 33.4% over SbCeZr, SbCe, and SbZr, respectively, suggesting more surface chemisorbed oxygen formed over SbCeZr.
2.2.3. H2-TPR
The redox property of catalysts plays an important role in the NH3-SCR reaction [,]. Figure 5 shows the H2-TPR profiles of the catalysts. CeO2 showed two reduction peaks centered at 474 and 781 °C, which were attributed to the reduction of surface oxygen and bulk oxygen of CeO2, respectively [,]. SbOx also showed two reduction peaks centered at 616 and 701 °C, which could be ascribed to the reduction of Sb5+ to Sb3+ and Sb3+ to Sb0, respectively []. The reduction profiles of SbZr were similar to those of SbOx with the reduction temperature shifting to lower temperature.
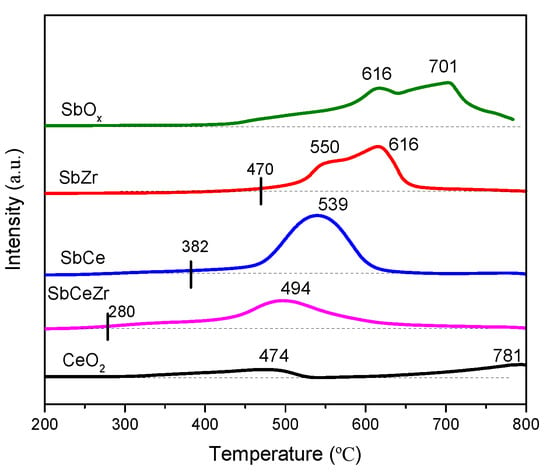
Figure 5.
H2-TPR profiles of the catalysts.
The reduction behavior of SbCe and SbCeZr was different from that of CeO2 and SbOx. A broad peak was observed on SbCe and SbCeZr centered at 539 °C and 494 °C, respectively, which could be attributed to the combination of reduction peaks of SbOx and CeO2 []. The broad reduction peak of SbCeZr shifted to lower Tonset (the onset reduction temperature of active oxygen) compared to the other catalysts (SbCe and SbZr). The lower reduction temperature of SbCeZr indicated the increase in redox and the generation of easily reducible species.
H2-TPR is usually used as a descriptor for the relative strength of the interaction among active components. The similar reduction profiles of SbOx and SbZr suggested the rather weak interaction between SbOx and ZrO2. However, a strong interaction was found on SbCe due to the co-reduction of SbOx and CeO2, which also led to the higher reduction temperature. By contrast, with the help of ZrO2, the strong interaction between SbOx and CeO2 was weakened, resulting in the lower onset reduction temperature of active oxygen.
2.2.4. NO-TPD
NO-TPD experiments were performed to investigate the adsorption and oxidation properties of NOx on different catalysts. NO-TPD profiles and the calculated NOx adsorption capacity are presented in Figure 6 and Table 2, respectively. The NO desorption peak below 100 °C was attributed to physical adsorbed NO, and the one at about 260 °C was attributed to chemical adsorbed NO []. Since the strength of NO bonding to the ceria surface depends on the reduction degree of support (unsaturated sites) [], it could be concluded that the Ce3+ content on the SbCeZr surface was much higher than that of SbCe, which was consistent with the XPS result.
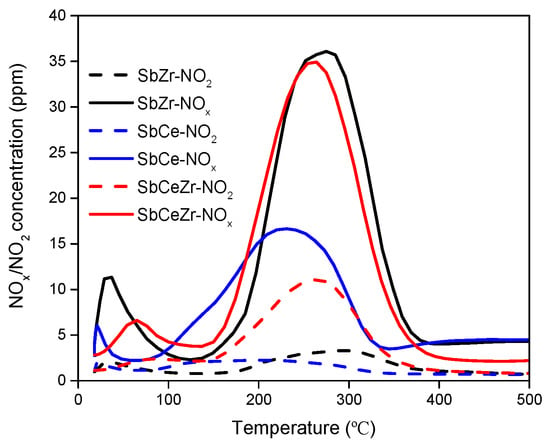
Figure 6.
NO-TPD profiles of the catalysts.

Table 2.
H2 consumption, NH3 desorption amount, and strong acid and NOx adsorption capability of the catalysts.
NO2 came from the oxidation of adsorbed NO by active oxygen on the catalyst surface. The desorption temperature and peak area correlated with the adsorption strength and oxidation ability of NO. The ability of the catalyst for oxidizing NO to NO2 plays an important role in improving the catalytic activities since it can promote the occurrence of the “fast SCR” reaction [,]. Considering the temperature range of the NH3-SCR reaction, the desorbed peaks in the temperature range of 150–400 °C were mainly discussed.
The main NOx desorption peak of SbCe was at 245 °C, slightly lower than those of SbCeZr (258 °C) and SbZr (273 °C). The calculated NOx amount was in the order of the following sequence: SbCeZr > SbZr > SbCe. There were no obvious differences in the NOx desorption amount between SbCeZr and SbZr, but the amount of NOx desorption on SbCeZr was almost 2 times higher than that of SbCe. It is worth mentioning that 31.7% NO converted to NO2 on SbCeZr, while only 13.5% NO did so on SbCe. With the help of NO2, the “fast SCR” occurred and facilitated NOx removal.
2.2.5. NH3-TPD
Surface acidity of the catalysts plays a key role in the adsorption and activation of NH3 [,]. NH3-TPD experiments were conducted to characterize the acid amount and strength according to peak area and desorption temperature, and the profiles are shown in Figure 7. Four broad peaks could be observed in the NH3-TPD profiles in the temperature range of 100–450 °C. NH3 desorption peaks at low temperature (<200 °C) were associated with NH3 desorption from weak acid sites derived from coordinatively unsaturated Cen+ or Zrn+ sites [,], and the peaks in the temperature range of 200–300 °C were assigned to the dissociation of NH4+ on Brønsted acid sites associated with surface hydroxyl groups. The peaks at temperatures higher than 300 °C were denoted as NH3 desorption from strong Lewis acid [,,]. The original weak Lewis acidity of CeO2 and ZrO2 was also increased by SbOx loading, which could be attributed to the electron withdrawing of SbOx species in proximity of Cen+/Zrn+ Lewis acid sites. The NH3 species desorbed from acid sites (>200 °C) played a decisive role in the NH3-SCR reaction [,].
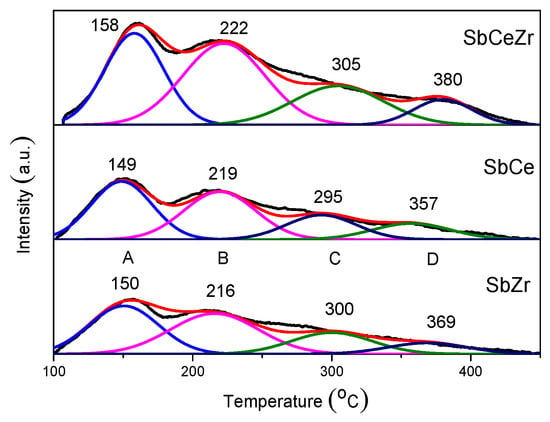
Figure 7.
NH3-TPD profiles of the catalysts.
The nature of the active sites could be assessed based on the quantity of sites and their intrinsic activity []. Quantitative analysis was employed to compare the amount of acid sites, and the ratios of different species based on the deconvoluted results are also shown in Table 2. The amount of acid sites had the following sequence: SbCeZr > SbCe > SbZr. The large total acid amount was found over SbCeZr (1.46 mL/g), which was nearly 2 times as much as that on SbZr (0.78 mL/g). It suggested that the interaction among the catalyst components facilitated the increase in acid amount.
2.3. In Situ DRIFT
2.3.1. Adsorption of NH3
The adsorption and activation of NH3 play an important role in the NH3-SCR reaction []. The adsorption of NH3 was investigated by in situ DRIFT shown in Figure 8. The bands at 3166, 3241, and 3357 cm−1 were assigned to N-H stretching vibration modes of the coordinated NH3 [,]. The bands at 1198 and 1599 cm−1 were attributed to coordinated NH3 linked to Lewis acid sites (NH3-L) [,], and the bands at 1440 and 1689 cm−1 were assigned to asymmetric and symmetric bending vibrations of NH4+ bounded to Brønsted acid sites (NH4+-B) [,,]. The band at 1258 cm−1 was assigned to the deformation vibration of NH3 bonded to Lewis acid [].
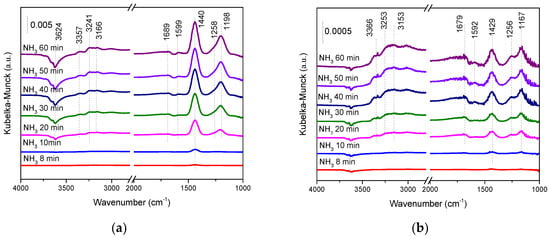
Figure 8.
The in situ diffuse reflectance infrared Fourier transform (DRIFT) of NH3 adsorption over (a) the SbCeZr and (b) the SbCe catalysts at 200 °C.
NH4+-B species (1440 cm−1) appeared after exposing in NH3 for 8 min over SbCeZr as shown in Figure 8a, and the adsorbed band turned intensive with the consumption of surface hydroxyl groups (3624 cm−1). However, the appearance of NH3-L species (1198 cm−1) lagged behind. Unlike SbCeZr, NH4+-B species (1429, 1679 cm−1) and NH3-L species (1167, 1256 cm−1) were observed synchronously over SbCe (Figure 8b). Compared with SbCe, an obvious blue shift of NH3 adsorption on acid sites was found over SbCeZr, presenting the strong NH3 adsorption on SbCeZr. After exposure in NH3 for 60 min, the difference in the distribution of NH4+-B and NH3-L species was found. It should be recalled that a great discrepancy was noticed for the bands of NH4+-B and NH3-L species in two samples. By calculating the band intensity of NH3 adsorption, a high proportion of NH4+-B species was found on SbCeZr, while the proportions of NH4+-B and NH3-L species on SbCe were similar. The presence of abundant Brønsted and Lewis acid sites on the SbCeZr catalyst should be responsible for the improvement in SCR activities over a wide temperature range [].
2.3.2. Adsorption of NH3 Followed by Introduction of NO + O2
The spectra of introducing NO + O2 into the catalysts after NH3 adsorption as a function of time were recorded and are shown in Figure 9. The decrease of NH4+-B and NH3-L species on SbCeZr with the introduction of NO + O2 presented the reaction between NOx and adsorbed NH3 species (Figure 9a). The intensities of the bands ascribed to NH3-L and NH4+-B species were reduced after NH3 adsorption, showing that these surface species were able to react with the mixture of NO and O2 at 200 °C. A deeper insight into the reduction of band intensity with the reaction time over the catalysts was essential. The further investigation on the consumption rate of NH3-L and NH4+-B species showed the slightly faster disappearance of NH3-L species than NH4+-B species in the reaction. Extending the exposure time led to the appearance of nitrate species, such as bridged nitrate (1605 and 1247 cm−1) [], bidentate nitrate (1579, 1559, and 1516 cm−1) [], and monodentate nitrate (1541 cm−1) []. The recovery of the negative broad band of surface hydroxyl groups (3441 cm−1) was attributed to the formation of water, which further confirmed the occurrence of the reaction.
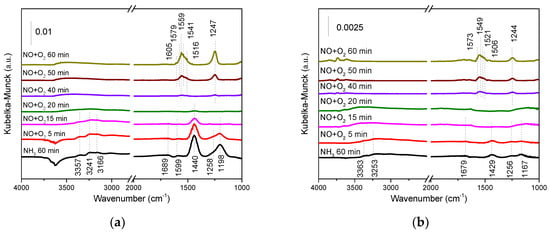
Figure 9.
The in situ DRIFT of NO + O2 reacting with preadsorbed NH3 species over (a) the SbCeZr and (b) the SbCe catalysts at 200 °C.
As shown in Figure 9b, the phenomenon that occurred on SbCe was similar to that on SbCeZr, i.e., the appearance of nitrate species at the expense of the disappearance of adsorbed NH3. The disappearance of NH3-L species confirmed that NH3-L species could be involved in the reaction, and NH3-L species were slightly active compared with NH4+-B species. The adsorbed NH3 on the catalyst surface reacted with NO/NO2 in the gas phase, following the Eley-Rideal (E-R) mechanism.
2.3.3. Co-Adsorption of NO + O2
The adsorption of NO + O2 was investigated by in situ DRIFT. As shown in Figure 10a, bridged nitrate (1245, 1606 cm−1), bidentate nitrate (1579, 1559, 1519 cm−1), and monodentate nitrate (1539 cm−1) were formed on SbCeZr after exposure to NO + O2 for 30 min. The NH3 adsorption rate was much faster than the NOx− generation rate. With the exposure time prolonged, the bands of nitrate intensified accordingly. Bridged nitrate seemed dominant in all the kinds of nitrates. In the case of SbCe (Figure 10b), rather weak bands of nitrates were observed after the exposure to the same feed gas for the same time, and the adsorption intensity of bridged nitrate was rather weaker than that of the monodentate and bidentate nitrates. It could be seen that Sb interacted with different metals (Ce or CeZr), resulting in the difference in the NO3- species distribution on the catalyst surface.
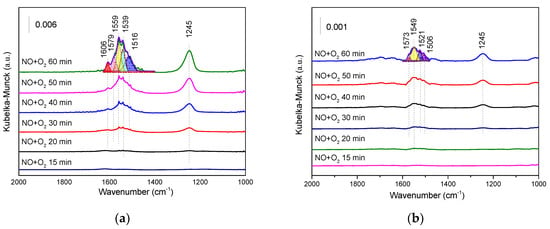
Figure 10.
The in situ DRIFT of NO + O2 co-adsorption over (a) the SbCeZr and (b) the SbCe catalysts at 200 °C.
2.3.4. Adsorption of NO + O2 Followed by Introduction of NH3
Compared with the results of NO + O2 reacting with preadsorbed NH3 (Figure 9), the effects of adding NH3 to preadsorbed NO + O2 (Figure 11) on activity were not so obvious. It is worth mentioning that a new band linked to NH4+-B species (1440 cm−1) gradually formed over SbCeZr as the exposure time increased. However, the bands linked to NH3-L (1599 and 1198 cm−1) were not detected. Therefore, the formed nitrate species had detrimental effects on NH3 adsorption on Lewis sites, but negligent effects on NH3 adsorption on Brønsted acid sites.
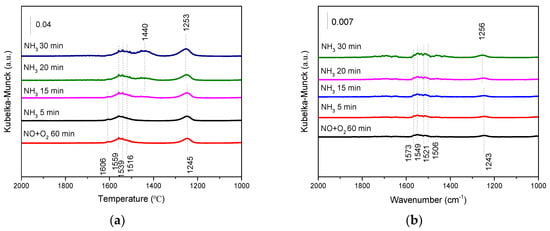
Figure 11.
The in situ DRIFT of NH3 reacting with preadsorbed NO + O2 over (a) the SbCeZr and (b) the SbCe catalysts at 200 °C.
On the contrary, there were no bands related to NH3 adsorption over SbCe even after prolonging the exposure time up to 30 min. The phenomena illustrated that the formed nitrate species significantly inhibited NH3 adsorption. Combined with the results of Figure 11, this indicated that NH3 adsorption and NOx oxidation could be competitive on SbCe, but not on SbCeZr.
2.4. Discussion
NOx conversions over CeO2 and SbOx were very low, but it increased significantly when they were combined together. Compared with CeO2, the reason for the improvement of catalytic activity over SbCeOx was due to alleviating nitrate adsorption and leaving more active sites for NH3 adsorption and activation []. Considering the competitive adsorption between NH3 and nitrate species, we prepared SbCeZr and investigated it for NH3-SCR performance. SbCeZr outperformed SbCe not only in activity and stability but also in SO2 and water tolerance. SbCeZr exhibited high activity with NOx conversion over 80% in the temperature range of 202–422 °C. The introduction of H2O and SO2 had little influence on the activity of SbCeZr, but an adverse effect on the catalytic activity of SbCe was found.
The presence of Zr influenced the distribution of Sb on the surface or bulk. According to ICP-AES and XPS results (Table 1), the surface amount of Sb on SbCe was much higher than that on SbCeZr, displaying the enrichment of Sb on the SbCe surface. However, H2-TPR indicated the strong interaction between SbOx and CeO2. Unlike SbCe, the presence of Zr made the distribution of Sb, Ce, and Zr uniform and weakened the interaction between SbOx and CeO2. The weakened interaction also brought about abundant Sb5+ species on the catalyst surface (Table 1) and the lower onset reduction temperature of active oxygen (Figure 5). Furthermore, the generation of NO2 during NO-TPD (Figure 6) also illustrated the enhancement in reducibility, which facilitated the “fast SCR” reaction during NH3-SCR [].
The adsorption of NH3 on the catalyst surface was considered as the initial step of the NH3-SCR reaction [], so NH3 adsorption played an important role during NOx removal. Compared with SbCe, NH3 adsorption was enhanced on SbCeZr, which was attributed to the increase of Brønsted and Lewis acid amounts (Figure 7). The acid distribution on the catalysts was different. The amount of Brønsted acid was higher than the amount of Lewis acid on SbCeZr, whereas the amount of Brønsted acid was similar to that of Lewis acid on SbCe. Meanwhile, in situ DRIFT experiments of NH3 adsorption also confirmed the strong adsorption of NH3 and the faster adsorption rate on SbCeZr (Figure 8). The reaction rate of NOx with NH3 adsorbed on Lewis acid was slightly faster than that with NH4+ adsorbed on Brønsted acid (Figure 9) (E-R mechanism).
It is worth mentioning that the formed nitrates had negligible effects on the adsorption of NH3 over SbCeZr; however, detrimental impacts were found over SbCe. It meant that NO oxidation and NH3 adsorption were both around Cen+ sites, so there was competition between them. Amorphous ZrO2 possessed abundant hydroxyl [], which showed obvious acidity properties. Then, the strong withdrawing effects of SbOx further increased the acid amount and strength of ZrO2, which was confirmed by the high NH3 desorption amount (Figure 7). Therefore, the separation of active sites alleviated the competition effects between NO oxidation and NH3 adsorption, thus promoting NOx removal.
Given the above discussion, it was shown that the strong acid and weak reducibility of SbZr made it less active in NH3-SCR. However, a strengthened interaction was found between SbOx and CeO2, favoring NOx removal. Unfortunately, the competition between NO oxidation and NH3 adsorption on the same active sites still needed to be alleviated to improve its thermostability and activity in the presence of H2O and SO2. In the presence of ZrO2, NH3 adsorption and NO oxidation on different active sites widened the operation window.
3. Materials and Methods
3.1. Catalyst Preparation
The SbCe, SbCeZr, and SbZr catalysts, where the molar ratio of Sb/Ce and Zr/Ce was 1.0, were prepared by the citric acid method. First, Sb(CH3COO)3 was dissolved in a solution of citric acid, then Zr(NO3)4 and Ce(NO3)4 were added. The molar ratio of citric acid to total metal components (the sum of Ce, Zr, and Sb) was 1.5. Then, the resultant mixture was stirred at 80 °C in a water bath until forming a gel. The gel was dried at 110 °C for 12 h to form a porous, foamlike solid. Finally, the precursor was calcined at 500 °C for 3 h in a muffle furnace (SX2, Shanghai Pudong Rong-Feng Scientific Instrument Co. Ltd., Shanghai, China). After calcining, the sample was crushed and sieved to 40–60 mesh prior to evaluation. CeO2, SbOx, and ZrO2 were also prepared by the same method.
3.2. Catalytic Activity Testing
The catalytic activities of the catalysts for the selective catalytic reduction of NOx with NH3 in excess oxygen were conducted at atmospheric pressure in a fixed-bed continuous flow quartz reactor. In the evaluation, a 200 mg portion of catalyst (40–60 mesh) was applied, and the feed gas contained 500 ppm NO, 500 ppm NH3, 5% O2, 5% H2O (when used), 100 ppm SO2 (when used), and Ar balance. The total flow was 300 mL/min and the gas hourly space velocity (GHSV) was about 150,000 h−1. A Thermo Fisher NO-NOx-chemiluminescence analyzer (Waltham, MA, USA) was used to detect the concentration of NO and NO2 before and after the reaction. The catalyst bed was heated at 2 °C/min, held for 45 min every 50 °C, and then the concentration of NOx was measured. NOx conversion was calculated using the following equation:
where [NOx]in and [NOx]out represented the inlet and outlet concentrations of NOx, respectively.
3.3. Catalyst Characterization
The N2 adsorption–desorption isotherms were measured on a Quantachrome NOVA1200 analyzer (Norcross, GA, USA) at −196 °C to obtain the surface area of the catalysts. Before measurement, the catalyst was degassed at 180 °C until it reached a stable vacuum of approximately 5 mTorr. After calculating the desorption data using the Brunauer–Emmett–Teller (BET) method, the specific surface area was obtained. The X-ray diffraction (XRD) patterns were carried out on a Bruker/D8 diffractometer (Karlsruhe, Germany) with Cu Kα radiation (λ = 0.154 nm). The X-ray photoelectron spectroscopy (XPS) spectra were obtained using a Thermo Scientific ESCALAB 250 spectrometer analyzer (Waltham, MA, USA), using a monochromatized Al-Kα source (1486.6 eV), and the binding energy of adventitious carbon (284.6 eV) was taken as a reference.
The temperature-programmed reduction of H2 (H2-TPR) and the temperature-programmed desorption of NH3 (NH3-TPD) were performed on a PX200 apparatus (Tianjin Pengxiang Technology Co. Ltd., Tianjin, China) with a thermal conductivity detector (TCD). For the H2-TPR experiment, a 100 mg portion of catalyst was pretreated in N2 at 300 °C for 30 min, then cooled down to 30 °C. Finally, the catalyst was heated from 30 °C to 800 °C at a rate of 10 °C/min in 5% H2/Ar (40 mL/min). For the NH3-TPD, a 100 mg portion of catalyst was pretreated at 500 °C under a flow of Ar (50 mL/min) for 1 h, and the pretreated catalyst was exposed to a flow of 10% NH3/Ar (40 mL/min) for 1 h at 100 °C. Then, the sample was flushed by Ar (50 mL/min) for 1 h and heated from 100 °C to 500 °C at 10 °C/min.
The temperature-programmed desorption of NO (NO-TPD) experiments were conducted on custom-made equipment with a NOx analyzer. Before testing, a 200 mg portion of catalyst was pretreated at 500 °C under a flow of Ar (300 mL/min) for 1 h; after cooling to room temperature, the pretreated catalyst adsorbed NO with a flow of 500 ppm NO/Ar (300 mL/min) for 1 h. The catalyst was flushed by Ar (300 mL/min) for 1 h and then heated from room temperature to 500 °C at 10 °C/min.
In situ DRIFT spectra were collected using an FTIR spectrometer (Thermo Nicolet 6700, Waltham, MA, USA) with an MCT detector cooled by liquid nitrogen. Before each experiment, the catalyst was pretreated at 300 °C for 1 h in Ar (50 mL/min). After the temperature cooling to 200 °C, 1000 ppm NH3/Ar was introduced into the IR cell and the background was subtracted from the sample spectra. After adsorption saturation, 1000 ppm NO + 5% O2/Ar was introduced to replace NH3 at 200 °C. The experiment of NO + O2 adsorption and reaction with NH3 was carried out following a similar process. First, the catalyst was pretreated at 300 °C for 1 h in Ar (50 mL/min), then 1000 ppm NO + 5% O2/Ar adsorption was carried out at 200 °C, and finally 1000 ppm NH3/Ar was introduced to replace NO + O2.
4. Conclusions
Sb-containing metal oxides (SbZr, SbCe, SbCeZr) were prepared by citric acid method and investigated for NH3-SCR. SbCeZr exhibited the higher performance in NH3-SCR than SbCe and SbZr, and NOx could be removed with 80% conversion in the temperature range of 202–422 °C. SbCeZr owned excellent activity even in the presence of water and SO2, and the thermostability was improved compared with SbCe. Hence, SbCeZr seemed to be a good candidate for the practical application in deNOx. The presence of Sb in the metal oxides led to the difference in acid distribution and redox property, which was closely related to the NH3 adsorption and NO oxidation. Brønsted acid and Lewis acid distributed evenly on SbCe, while Brønsted acid dominated on SbCeZr. Although high amounts of acid were on SbZr, poor redox led to its negligent performance in NH3-SCR. The enhancement in redox ability promoted the generation of nitrates on SbCe. However, the competition between NO oxidation and NH3 adsorption on the same active sites limited its application. In the presence of ZrO2, the interaction between Sb and Ce was weakened, which further increased the acid amount and redox property. The form of “dual active sites” favored the NH3 adsorption and nitrate generation.
Author Contributions
Conceptualization, Q.X. and L.W.; methodology, Q.X., D.L. and C.W.; validation, L.W. and Y.G. (Yun Guo); formal analysis, Q.X., L.W., Q.K. and M.N.H.; investigation, D.L., Q.K. and M.N.H.; resources, W.Z., Y.G. (Yun Guo) and Y.G. (Yanglong Guo); data curation, Q.X., Q.K. and L.W.; writing—original draft preparation, Q.X. and L.W.; writing—review and editing, Q.X., L.W. and W.Z.; project administration, Y.G. (Yanglong Guo); funding acquisition, Y.G. (Yun Guo) and Y.G. (Yanglong Guo). All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported financially by the National Key Research and Development Program of China (2016YFC0204300) and the National Natural Science Foundation of China (21976057 and 21922602), as well as the “Shanghai Science and Technology Innovation Plan” Program (19DZ1208000), and Fundamental Research Funds for the Central Universities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Irfan, M.F.; Goo, J.H.; Kim, S.D. Co3O4 based catalysts for NO oxidation and NOx reduction in fast SCR process. Appl. Catal. B Environ. 2008, 78, 267–274. [Google Scholar] [CrossRef]
- Fu, M.; Li, C.; Lu, P.; Qu, L.; Zhang, M.; Zhou, Y.; Yu, M.; Fang, Y. A review on selective catalytic reduction of NOx by supported catalysts at 100–300 °C—Catalysts, mechanism, kinetics. Catal. Sci. Technol. 2014, 4, 14–25. [Google Scholar] [CrossRef]
- Xu, Q.; Fang, Z.L.; Chen, Y.Y.; Guo, Y.L.; Guo, Y.; Wang, L.; Wang, Y.S.; Zhang, J.S.; Zhan, W.C. Titania–Samarium–Manganese Composite Oxide for the Low-Temperature Selective Catalytic Reduction of NO with NH3. Environ. Sci. Technol. 2020, 54, 2530–2538. [Google Scholar] [CrossRef]
- Zhan, W.C.; Guo, Y.; Gong, X.Q.; Guo, Y.L.; Wang, Y.Q.; Lu, G.Z. Current status and perspectives of rare earth catalytic materials and catalysis. Chin. J. Catal. 2014, 35, 1238–1250. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, D.; Li, B.; Wang, C.; Li, J.; Crittenden, J.; Hao, J. Impacts of Pb and SO2 Poisoning on CeO2−WO3/TiO2−SiO2 SCR Catalyst. Environ. Sci. Technol. 2017, 51, 11943–11949. [Google Scholar] [CrossRef]
- Xu, T.; Wu, X.; Gao, Y.; Lin, Q.; Hu, J.; Wang, D. Comparative study on sulfur poisoning of V2O5-Sb2O3/TiO2 and V2O5-WO3/TiO2 monolithic catalysts for low-temperature NH3-SCR. Catal. Commun. 2017, 93, 33–36. [Google Scholar] [CrossRef]
- Kwon, D.W.; Hong, S.C. Enhancement of performance and sulfur resistance of ceria-doped V/Sb/Ti by sulfation for selective catalytic reduction of NOx with ammonia. RSC Adv. 2016, 6, 1169–1181. [Google Scholar] [CrossRef]
- Xu, L.; Wang, C.; Chang, H.; Wu, Q.; Zhang, T.; Li, J. New Insight into SO2 Poisoning and Regeneration of CeO2–WO3/TiO2 and V2O5–WO3/TiO2 Catalysts for Low-Temperature NH3–SCR. Environ. Sci. Technol. 2018, 52, 7064–7071. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, K.; Feng, Z.; Ying, P.; Li, C. Studies on the SbOx species of SbOx/SiO2 catalysts for methane-selective oxidation to formaldehyde. Appl. Catal. A Gen. 2006, 305, 110–119. [Google Scholar] [CrossRef]
- Qu, R.; Gao, X.; Cen, K.; Li, J. Relationship between structure and performance of a novel cerium-niobium binary oxide catalyst for selective catalytic reduction of NO with NH3. Appl. Catal. B Environ. 2013, 142, 290–297. [Google Scholar] [CrossRef]
- Topsøe, N.Y. Mechanism of the selective catalytic reduction of nitric oxide by ammonia elucidated by in situ on-line Fourier transform infrared spectroscopy. Science 1994, 265, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Qu, R.; Su, W.; Li, J. A novel Ce–Ta mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2015, 176, 338–346. [Google Scholar] [CrossRef]
- Guo, R.; Sun, X.; Liu, J.; Pan, W.; Li, M.; Sun, P.; Liu, S. Enhancement of the NH3-SCR catalytic activity of MnTiOx catalyst by the introduction of Sb. Appl. Catal. A Gen. 2018, 558, 1–8. [Google Scholar] [CrossRef]
- Shi, R.H.; Lin, X.Y.; Zheng, Z.G.; Feng, R.; Liu, Y.M.; Ni, L.F.; Yuan, B.H. Selective catalytic reduction of NOx with NH3 over Sb modified CeZrOx catalyst. React. Kinet. Mech. Cat. 2018, 124, 217–227. [Google Scholar] [CrossRef]
- Liu, J.; Li, G.; Zhang, Y.; Liu, X.; Wang, Y.; Li, Y. Novel Ce-W-Sb mixed oxide catalyst for selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2017, 401, 7–16. [Google Scholar] [CrossRef]
- Danh, H.T.; Kumar, P.A.; Jeong, Y.E.; Ha, H.P. Enhanced NH3-SCR activity of Sb-V/CeO2–TiO2 catalyst at low temperatures by synthesis modification. Res. Chem. Intermed. 2016, 42, 155–169. [Google Scholar] [CrossRef]
- Du, X.; Gao, X.; Fu, Y.; Gao, F.; Luo, Z.; Cen, K. The co-effect of Sb and Nb on the SCR performance of the V2O5/TiO2 catalyst. J. Colloid Interface Sci. 2012, 368, 406–412. [Google Scholar] [CrossRef]
- Phil, H.H.; Reddy, M.P.; Kumar, P.A.; Ju, L.K.; Hyo, J.S. SO2 resistant antimony promoted V2O5/TiO2 catalyst for NH3-SCR of NOx at low temperatures. Appl. Catal. B Environ. 2008, 78, 301–308. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Kwon, D.W.; Lee, K.Y.; Ha, H.P. SO32−/SO42− functionalization-tailorable catalytic surface features of Sb-promoted Cu3V2O8 on TiO2 for selective catalytic reduction of NOX with NH3. Appl. Catal. A Gen. 2019, 570, 355–366. [Google Scholar] [CrossRef]
- Ding, Y.Q.; Wu, Q.Q.; Li, B.; Guo, Y.L.; Guo, Y.; Wang, Y.S.; Wang, L.; Zhan, W.C. Superior catalytic activity of a Pd catalyst in methane combustion by fine-tuning the phase of ceria-zirconia support. Appl. Catal. B Environ. 2020, 266, 118631. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, H.; Li, D.; Qin, Y.; Xie, Y.; Wang, S. ChemInform Abstract: WO3/CeO2—ZrO2, a Promising Catalyst for Selective Catalytic Reduction (SCR) of NOx with NH3 in Diesel Exhaust. Chem. Commun. 2008, 12, 1470–1472. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Wang, Y.; Wang, F.; Liu, T. The effect of Ce–Zr on NH3 -SCR activity over MnOx(0.6)/Ce0.5 Zr0.5 O2 at low temperature. Chem. Eng. J. 2014, 236, 171–180. [Google Scholar] [CrossRef]
- Ning, P.; Song, Z.; Li, H.; Zhang, Q.; Liu, X.; Zhang, J.; Tang, X.; Huang, Z. Selective catalytic reduction of NO with NH3 over CeO2–ZrO2–WO3 catalysts prepared by different methods. Appl. Surf. Sci. 2014, 236, 171–180. [Google Scholar] [CrossRef]
- Ding, S.; Liu, F.; Shi, X.; Liu, K.; Lian, Z.; Xie, L.; He, H. Significant Promotion Effect of Mo Additive on a Novel Ce-Zr Mixed Oxide Catalyst for the Selective Catalytic Reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2015, 7, 9497–9506. [Google Scholar] [CrossRef]
- Hu, H.; Zha, K.; Li, H.; Shi, L.; Zhang, D. In situ DRIFTs investigation of the reaction mechanism over MnOx-MOy/Ce0.75Zr0.25O2 (M = Fe, Co, Ni, Cu) for the selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2016, 387, 921–928. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.; Li, J. Structure–activity relationship of VOx/CeO2 nanorod for NO removal with ammonia. Appl. Catal. B Environ. 2014, 144, 538–546. [Google Scholar] [CrossRef]
- Peng, Y.; Li, J.; Huang, X.; Li, X.; Su, W.; Sun, X.; Wang, D.; Hao, J. Deactivation mechanism of potassium on the V2O5/CeO2 catalysts for SCR reaction: Acidity, reducibility and adsorbed-NOx. Environ. Sci. Technol. 2014, 48, 4515–4520. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Gao, Y.; Yao, X.; Ge, C.; Gao, F.; Deng, Y.; Tang, C.; Dong, L. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2015, 165, 589–598. [Google Scholar] [CrossRef]
- Xu, H.; Sun, M.; Liu, S.; Li, Y.; Wang, J.; Chen, Y. Effect of the calcination temperature of cerium–zirconium mixed oxides on the structure and catalytic performance of WO3/CeZrO2 monolithic catalyst for selective catalytic reduction of NOx with NH3. RSC Adv. 2017, 7, 24177–24187. [Google Scholar] [CrossRef]
- Chen, Z.; Si, Z.; Cao, L.; Wu, X.; Ran, R.; Weng, D. Decomposition behavior of ammonium nitrate on ceria catalysts and its role in the NH3-SCR reaction. Catal. Sci. Technol. 2017, 7, 2531–2541. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, Z.; Liu, Z. Combined effect of H2O and SO2 on V2O5/AC catalysts for NO reduction with ammonia at lower temperatures. Appl. Catal. B Environ. 2002, 39, 361–368. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, R.; Shi, X.; Pan, W.; Liu, J.; Sun, X.; Liu, S.; Liu, X.; Qin, H. The enhanced performance of Sb-modified Cu/TiO2 catalyst for selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2019, 475, 334–341. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Z.P.; Brosnahan, J.T.; Zhang, S.; Guo, Y.L.; Guo, Y.; Wang, L.; Wang, Y.S.; Zhan, W.C. Ru/CeO2 Catalyst with Optimized CeO2 Support Morphology and Surface Facets for Propane Combustion. Environ. Sci. Technol. 2019, 53, 5349–5358. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Chen, L.; Yuan, F.; Li, R.; Zhang, T.; Bakhtiar, S.H.; Leng, X.; Niu, X.; Zhu, Y. Synergistic effect between copper and cerium on the performance of Cux-Ce0.5-x-Zr0.5 (x = 0.1–0.5) oxides catalysts for selective catalytic reduction of NO with ammonia. Appl. Catal. B Environ. 2017, 210, 223–234. [Google Scholar] [CrossRef]
- Guan, B.; Lin, H.; Zhu, L.; Huang, Z. Selective Catalytic Reduction of NOx with NH3 over Mn, Ce Substitution Ti0.9V0.1O2−δ Nanocomposites Catalysts Prepared by Self-Propagating High-Temperature Synthesis Method. J. Phys. Chem. C 2011, 115, 12850–12863. [Google Scholar] [CrossRef]
- Carja, G.; Kameshima, Y.; Okada, K.; Madhusoodana, C.D. Mn–Ce/ZSM5 as a new superior catalyst for NO reduction with NH3. Appl. Catal. B Environ. 2007, 73, 60–64. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Q.; Qiu, C.; Lin, T.; Gong, M.; Chen, Y. Tungsten modified MnOx–CeO2/ZrO2 monolith catalysts for selective catalytic reduction of NOx with ammonia. Chem. Eng. Sci. 2012, 76, 120–128. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, Y.; Zhang, S.; Zhu, T.; Zhu, J.; Wang, J. Selective catalytic reduction of NOx with NH3 over Mn-Ce mixed oxide catalyst at low temperatures. Catal. Today 2013, 216, 76–81. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, W.; Liu, J.; Zheng, H.; Zhao, Z.; Xu, C.; Wei, Y.; Hensen, E. Simultaneous NOx and Particulate Matter Removal from Diesel Exhaust by Hierarchical Fe-Doped Ce–Zr Oxide. ACS Catal. 2017, 7, 3883–3892. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Zeng, H.; Xu, Q. A novel Ce-Sb binary oxide catalyst for the selective catalytic reduction of NOx with NH3. Catal. Sci. Technol. 2016, 6, 8063–8071. [Google Scholar] [CrossRef]
- Adamowska, M.; Krzton, A.; Najbar, M.; Costa, P.D. DRIFT study of the interaction of NO and O2 with the surface of Ce0.62Zr0.38O2 as deNOx catalyst. Catal. Today 2008, 137, 288–291. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, Y.; Wang, L.; Lu, G. A highly effective catalyst of Sm-Mn mixed oxide for the selective catalytic reduction of NOx with ammonia: Effect of the calcination temperature. J. Mol. Catal. A Chem. 2016, 420, 272–281. [Google Scholar] [CrossRef]
- Shao, C.; Liu, X.; Meng, D.; Xu, Q.; Guo, Y.; Guo, Y.; Zhan, W.; Wang, L.; Lu, G. Catalytic performance of Co–Fe mixed oxide for NH3-SCR reaction and the promotional role of cobalt. RSC Adv. 2016, 6, 66169–66179. [Google Scholar] [CrossRef]
- Liu, F.; Shan, W.; Lian, Z.; Xie, L.; Yang, W.; He, H. Novel MnWOx catalyst with remarkable performance for low temperature NH3-SCR of NOx. Catal. Sci. Technol. 2013, 3, 2699–2707. [Google Scholar] [CrossRef]
- Chen, L.; Si, Z.; Wu, X.; Weng, D. DRIFT Study of CuO–CeO2–TiO2 Mixed Oxides for NOx Reduction with NH3 at Low Temperatures. ACS Appl. Mater. Interfaces 2014, 6, 8134–8145. [Google Scholar] [CrossRef] [PubMed]
- Onfroy, T.; Clet, G.; Houalla, M. Correlations between Acidity, Surface Structure, and Catalytic Activity of Niobium Oxide Supported on Zirconia. J. Phys. Chem. B 2005, 109, 14588–14594. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wu, X.; Si, Z.; Weng, D.; Ma, J.; Xu, T. Impacts of niobia loading on active sites and surface acidity in NbOx/CeO2–ZrO2 NH3–SCR catalysts. Appl. Catal. B Environ. 2015, 179, 380–394. [Google Scholar] [CrossRef]
- Zuo, J.; Chen, Z.; Wang, F.; Yu, Y.; Wang, L.; Li, X. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Novel Mn–Zr Mixed Oxide Catalysts. Ind. Eng. Chem. Res. 2014, 53, 2647–2655. [Google Scholar] [CrossRef]
- Roy, S.; Hegde, M.S.; Madras, G. Catalysis for NOx abatement. Appl. Eng. 2009, 86, 2283–2297. [Google Scholar] [CrossRef]
- Lee, K.J.; Kumar, P.A.; Maqbool, M.S.; Rao, K.N.; Song, K.H.; Ha, H.P. Ceria added Sb-V2O5/TiO2 catalysts for low temperature NH3 SCR: Physico-chemical properties and catalytic activity. Appl. Catal. B Environ. 2013, 142–143, 705–717. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Ma, K.; Zou, W.; Cao, Y.; Xiong, Y.; Tang, C.; Dong, L. Ultra-low loading of copper modified TiO2/CeO2 catalysts for low-temperature selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2017, 207, 366–375. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, S.; Qiu, T.; Shen, S. A novel catalyst of CeO2/Al2O3 for selective catalytic reduction of NO by NH3. Catal. Commun. 2009, 11, 20–23. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Liu, S.; Zheng, C.; Gao, X.; Nova, I.; Tronconi, E. Improvement in activity and alkali resistance of a novel V-Ce(SO4)2/Ti catalyst for selective catalytic reduction of NO with NH3. Appl. Catal. B Environ. 2017, 206, 449–460. [Google Scholar] [CrossRef]
- Nam, K.B.; Kwon, D.W.; Hong, S.C. DRIFT study on promotion effects of tungsten-modified Mn/Ce/Ti catalysts for the SCR reaction at low-temperature. Appl. Catal. A Gen. 2017, 542, 55–62. [Google Scholar] [CrossRef]
- Kumar, P.A.; Jeong, Y.E.; Ha, H.P. Low temperature NH3 -SCR activity enhancement of antimony promoted vanadia-ceria catalyst. Catal. Today 2017, 293–294, 61–72. [Google Scholar] [CrossRef]
- Yang, N.; Guo, R.; Pan, W.; Chen, Q.; Wang, Q.; Lu, C. The promotion effect of Sb on the Na resistance of Mn/TiO2 catalyst for selective catalytic reduction of NO with NH3. Fuel 2016, 169, 87–92. [Google Scholar] [CrossRef]
- Matrails, H.K.; Ciardelli, M.; Ruwet, M.; Grange, P. Vanadia Catalysts Supported on Mixed TiO2-Al2O3 Supports: Effect of Composition on the Structure and Acidity. J. Catal. 1995, 157, 368–379. [Google Scholar] [CrossRef]
- Amiridis, M.D.; Duevel, R.V.; Wachs, I.E. The effect of metal oxide additives on the activity of V2O5/TiO2 catalysts for the selective catalytic reduction of nitric oxide by ammonia. Appl. Catal. B Environ. 1999, 20, 111–122. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, T.; Weng, X.; Wang, Y.; Wu, Z.; Wang, H. DRIFT Studies on the Selectivity Promotion Mechanism of Ca-Modified Ce-Mn/TiO2 Catalysts for Low-Temperature NO Reduction with NH3. J. Phys. Chem. C 2012, 116, 16582–16592. [Google Scholar] [CrossRef]
- Jiang, B.; Li, Z.; Lee, S. Mechanism study of the promotional effect of O2 on low-temperature SCR reaction on Fe–Mn/TiO2 by DRIFT. Chem. Eng. J. 2013, 225, 52–58. [Google Scholar] [CrossRef]
- Ma, Z.; Xu, R.; Yang, C.; Wei, W.; Li, W.; Sun, Y. Preparation and Surface Properties of Different Zirconia Polymorphs. Acta Phys.-Chim. Sin. 2004, 20, 1221–1225. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).