Abstract
The Fe/(SZr) and S(Fe/Zr) sulfated iron-based catalysts, prepared by impregnation methods through changing the loading order of Fe2O3 and SO42− on ZrO2, were investigated on selective catalytic reduction (SCR) of NOx by ammonia. It was studied that the existent forms of Fe2O3 and SO42− on the surface of catalysts were affected by the loading order. The Fe/(SZr) catalyst surface had isolated Fe2O3 and SO42− species and followed both the L-H mechanism and the E-R mechanism, whereas the S(Fe/Zr) catalyst contained SO42− specie and sulfate only and mainly followed the E-R pathway. These factors affected the redox ability and NH3 adsorption, which might be key to the SCR reaction.
1. Introduction
Nitrogen oxides usually come from stationary sources, such as coal-fired power plants, industrial boilers, and mobile sources, like diesel engines and marine engines. They have caused lots of harmful problems to the ecosystem health. For example, the acid rain would dissolve toxic metals into the water, resulting in reducing species. The photochemical smog could harm plants and reduce atmospheric visibility. Besides, nitrogen oxides made people face existential threats. The resulting photochemical fumes could irritate the eyes, invade the lungs, and even cause death. Therefore, it is important to remove nitrogen oxides. Currently, selective catalytic reduction of NOx by ammonia (NH3-SCR) is an efficient technology to abate NOx in the flue gas from stationary sources [1]. The core of this method is catalysts. Typically, the V2O5-WO3/TiO2 is the most commonly used catalysts [2]. However, there are still some shortcomings of vanadium-based catalysts that need to be addressed, including the narrow temperature window, the low N2 selectivity at high temperatures, losing SCR activity by SO2 poisoned, and the secondary pollution to the environment. Due to these disadvantages, the development of other metals for vanadium and the modification of the support have been investigated over the past several decades. Iron-based catalysts have received considerable attention for its well NH3-SCR activity and N2 selectivity, such as α-Fe2O3 [3], γ-Fe2O3 [3], MgFe2O4 [4], Fe2O3/SiO2 [5], Fe2O3/TiO2 [6], Fe2O3/TiO2-pillared clays [1], Fe-ZSM5 [7], and Fe-MOR [8]. Besides iron-based catalysts, other sulfated catalysts were reported to be active for SCR reaction. Grange et al. [9,10] reported that V2O5-WO3 catalyst supported on TiO2-SO42− extended high activity up to 450 °C, and for sulfate, increased the acid sites and improved the interaction between V2O5 and WO3. Ke et al. [11] reported that cobalt sulfate exhibited high NOx conversion and well resistance to H2O and SO2 poisoning, because the existence of sulfate reduced the number of bulk Co3O4 and cooperated with cobalt oxides to improve the activity of SCR reaction. Gu et al. [12] reported that sulfated CeO2 possessed high NOx conversion and N2 selectivity in the temperature range of 200–570 °C, and suggested that sulfate enhanced the active oxygen species and NH3 adsorption on surface, which were responsible for the higher reactivity of sulfated CeO2.
The iron-based oxide and sulfate catalysts for SCR of NOx by NH3 have been investigated in recent years. However, few studies have been performed on the preparation process about sulfating. In this study, a series of sulfated iron-based catalysts were prepared through different sulfate-loaded order and characterized by XRD, BET, XPS, Raman, and NH3-TPD. The high activities of sulfated iron-base catalysts were also explained on the basis of the characterization results.
2. Results
2.1. SCR Catalytic Activity and Influence of SO2
The activity curves of the sulfated iron-based catalysts are shown in Figure 1a. The Fe/Zr catalyst exhibited the lowest activity in the temperature range of 150–500 °C, and its NOx conversion reached the highest 46.6% at 350 °C and the NOx conversion decreased sharply to negative value in the temperature range of 400–500 °C, which was attributed to the NH3 oxidation to NOx at the high temperature. Notably, the activities of sulfated iron-based catalysts improved remarkably in the temperature range of 250–500 °C compared with Fe/Zr catalyst. The NOx conversion of Fe/(SZr) catalyst reached above 90% in 300–450 °C, amazingly, the NOx conversions increased to 98%, but which decreased to 50% at 500 °C. The temperature window of NOx conversion of Fe/(SZr) catalysts became narrow and moved to high temperature. The NOx conversion of Fe/(SZr) catalyst was reached 90% at 400–450 °C. The SCR reaction of the catalysts mainly follows the Eley−Rideal (E-R) mechanism and/or Langmuir–Hinshelwood mechanism (L-H) [13,14]. It is the critical reaction step to adsorb NH3 to form activated ammonia species on the catalyst and then react with NO or adsorbed oxynitride species to generate N2 and H2O. Therefore, the acidic sites are key for the SCR reaction. The sulfated iron-based catalysts were prepared by adding SO42−, which could remarkably increase the acidic sites. Therefore, the activity improves obviously with the sulfated iron-based catalyst.
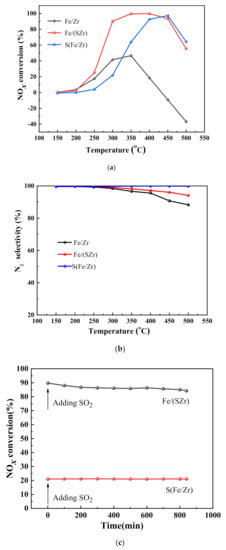
Figure 1.
(a)NH3–selective catalytic reduction (SCR) activity, (b) N2 selectivity, and (c) influence of SO2 on NOx conversion of Fe/Zr, Fe/(SZr), and S(Fe/Zr) for the NOx reduction with NH3.
The N2 selectivity is one of the important indicators for the activities of NOx selective catalytic reduction by NH3. The N2 selectivity of Fe/Zr, Fe/(SZr), and S(Fe/Zr) is showed in Figure 1b. S(Fe/Zr) showed excellent N2 selectivity in 150–500 °C. The N2 selectivity of Fe/Zr catalyst decreased from 250 °C, and the N2 selectivity of Fe/Zr catalyst dropped greatly at 500 °C to 88%. The slight decrease in N2 selectivity in the high temperature range might be due to the higher ability of oxidization of NH3 to NO [15], which resulted in less production of N2. Besides, according to He et al. [16], the intermediate specie -HNO would combine with -NH to produce N2O after the oxidization of NH3, which led to less N2 selectivity.
As shown in Figure 1c, this work investigated the effect of SO2 on the activity of Fe/(SZr) and S(Fe/Zr) catalysts. In the figure, the activity of the S(Fe/Zr) catalyst remained stable for 840 min in the presence of SO2, but the NOx conversion rate was relatively low. For the Fe/(SZr) catalyst, the de-NOx reactivity slightly decreased and the NOx conversion rate kept stable between 770 and 840 min when there was SO2. This demonstrated that the Fe/(SZr) catalyst had superior SO2 tolerance and exhibited higher activity. Typically, it was recognized that the Langmuir–Hinshelwood (L-H) mechanism or/and Eley–Rideal (E-R) mechanism were followed by catalysts during the reaction [17]. Meanwhile, it could be assumed that there was competitive adsorption between SO2 and NO, in which SO2 was easier to adsorb than the other. Therefore, the Langmuir–Hinshelwood (L-H) mechanism was suppressed, but the Eley–Rideal (E-R) mechanism still existed on the Fe/(SZr) catalyst. It was the reason that Fe/(SZr) had stronger SO2 resistance. These would be analyzed in more detail in Discussion section.
2.2. XRD
XRD patterns of the ZrO2, α-Fe2O3, Fe/Zr, Fe/(SZr), and S(Fe/Zr) catalysts are shown in the Figure 2. Both the ZrO2 and α-Fe2O3 showed obvious characteristic peaks. However, the patterns of Fe/Zr, Fe/(SZr), and S(Fe/Zr) exhibited only one phase, which corresponded to the cubic crystal structure of ZrO2. In other words, the X-ray diffraction peaks of α-Fe2O3 or Fe2(SO4)3 did not appear in the patterns, which demonstrated that Fe2O3 and SO42− dispersed well on the surface of ZrO2. According to references [15,18], the shape of peaks would be changed or new peak would appear if Fe2O3 or/and SO42− entered into the crystal ZrO2. These proved that the sulfating process did not affect the crystal form of Fe/Zr, Fe/(SZr), and S(Fe/Zr) catalysts. In fact, the change of crystal structure and high dispersion had significant influences on the catalytic performance [15], which might affect the existent forms of active species and pathways followed on catalysts.
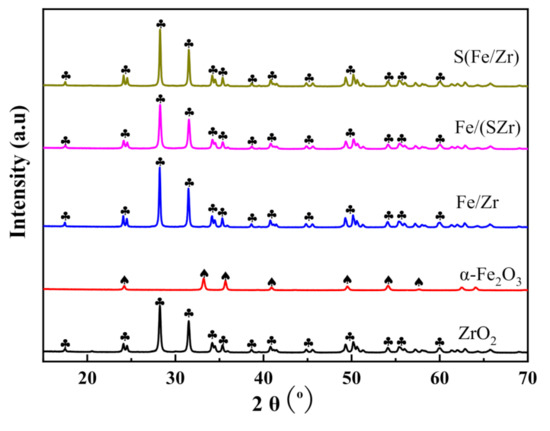
Figure 2.
XRD patterns of different iron-based catalysts.
2.3. BET and ICP
It is well known that the surface area is crucial to catalytic activity because large surface area could facilitate to well dispersion. However, the surface areas of sulfated iron-based catalysts were so low (Table 1) that the distinction among them could be neglected. Meanwhile, integra mass concentrations of Fe, S, and Zr measured by the ICP equipment are also summarized in Table 1. The integra mass concentrations of Fe, S, and Zr were close to their calculated values.

Table 1.
Brunauer-Emmett-Teller (BET) surface area and integral mass concentration of various catalysts by Inductive Coupled Plasma (ICP) results.
2.4. Raman
To investigate the species on the surface of sulfated iron-based catalysts, the Raman spectra were collected at room temperature. Figure 3 showed the Raman spectra of different sulfated iron-based catalysts and pure Fe2O3. The Fe/(SZr), S(Fe/Zr), and ZrO2 catalysts all showed similar peak shapes, but the strength was different in the wavenumber range of 0–650 cm−1, which meant that the sulfate and Fe2O3 species on catalyst surfaces did not have effects on ZrO2. Besides, several bands in the range of 800–1500 cm−1 were detected, in which the peak centered at 1278 cm−1 on Fe/(SZr) was assigned the fingerprint peak of Fe2O3 [19]. There was no characteristic peak in the range of 800–1500 cm−1 on ZrO2. Only one peak appeared at 992 cm−1 on the surface of S(Fe/Zr) catalyst, which was assigned to the S=O bands of isolated sulfate. For Fe/(SZr) catalyst, three peaks located at 992, 1082, and 1278 cm−1 could be detected, respectively. Among them, the band at 1082 cm−1 was induced by the S=O bond of the polynuclear sulfate type [10]. These demonstrated that species such as Fe2O3, isolated SO42−, and sulfate existed on Fe/(SZr) catalyst. In short, it could be concluded that the S(Fe/Zr) catalyst surface had isolated SO42− and sulfate because of the different sulfation preparation processes, which led to different species on the surface of catalysts. These affected the mechanisms followed by catalysts.
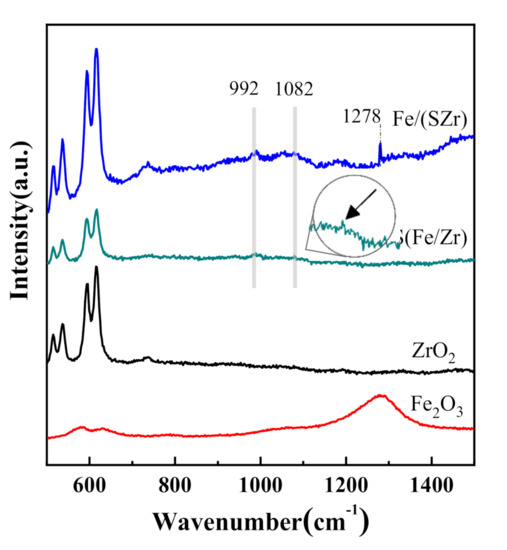
Figure 3.
Raman patterns of different iron-based catalysts.
2.5. XPS
X-ray photoelectron spectroscopy (XPS) was used to characterize chemical states and surface atomic concentrations of Fe, O, S, and Zr elements on Fe/(SZr) and S(Fe/Zr) catalysts. Peaks were fitted by Gaussian–Lorentz curves [20]. These results are shown in Figure 4. Meanwhile, the surface atomic concentrations of Fe, S, and Zr are summarized in Table 2.
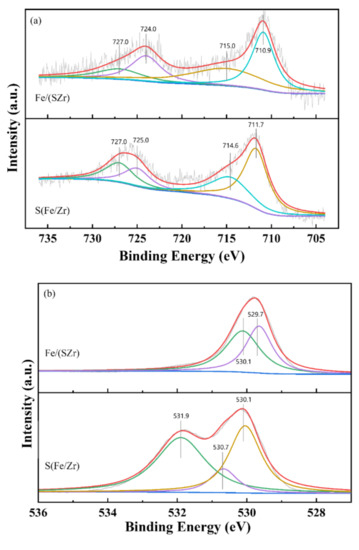
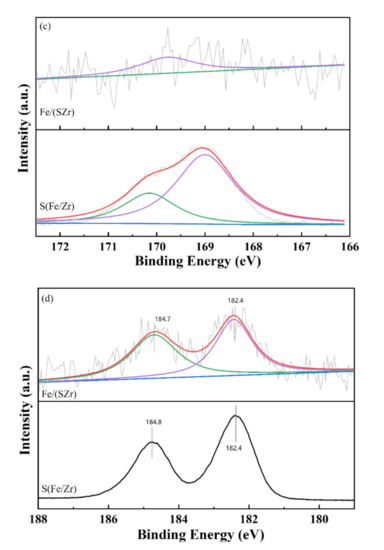
Figure 4.
XPS results of (a) Fe 2p, (b) O 1s, (c) S 2p, and (d) Zr 3d in Fe/(SZr) and S (Fe/Zr) catalysts.

Table 2.
Surface atomic concentration of various catalysts by XPS results.
The curves of Fe2p are displayed in the Figure 4a. The peak located at 727.0 eV was corresponded to the Fe2p1/2 of Fe3+, and peaks referred to the Fe2p3/2 of Fe3+ were at the banding energy of 710.9 and 711.7 eV. Besides, the banding energy at 724.0 and 725.0 eV were corresponded to the Fe2p1/2 of Fe2+. According to the previous studies [18], the peaks at 715.0 and 714.6 eV were identified as the fingerprint peak influenced by SO42−. The ratio of Fe3+/(Fe2+ + Fe3+) on Fe/(SZr) was 69.6%, which was larger than that of S(Fe/Zr) (58.1%). It was known that the catalyst surface containing more Fe3+ species would exhibit better performances during the redox reaction because the transformation between Fe2+ and Fe3+ could irritate the redox circle, which agreed with the results of SCR catalytic activity. Besides, these results also indicated that the main specie on the surface of Fe/(SZr) was Fe2O3, corresponding to the Raman results.
Additionally, Figure 4b showed the XPS results of O 1s. The profiles of O 1s exhibited large differences between Fe/(SZr) and S(Fe/Zr) catalysts. For the Fe/(SZr) catalyst, the two peaks appeared at 530.1 and 529.7 eV. The banding energy at 530.1 eV was attributed to the surface-chemisorbed labile oxygen (denoted as ), and the 529.7 eV peak was corresponded to the lattice oxygen (denoted as ) [21]. However, three peaks located at 531.9, 530.7, and 530.1 eV could be observed on the S(Fe/Zr) catalyst, which demonstrated that there were three forms of oxygen on this catalyst. In addition to the surface-chemisorbed oxygen and the lattice oxygen, the O22− contained in the SO42− species led to the appearance of the peak located at 531.9 eV, which was in accordance with our previous research [2]. Typically, the surface-chemisorbed labile oxygen played important roles in the SCR reaction. More the content of , the higher catalytic activity of catalysts because the surface oxygen (Oα) could promote the activation of NH2− formed by NH3 due to the more efficient mobility of electrons than lattice oxygen [21]. Therefore, the Fe/(SZr) catalyst had superior redox activity. For Fe/(SZr) catalyst, the content of /( + ) was 53.5%, which was higher than that of S(Fe/Zr) (22.7%). This meant that Fe/(SZr) had better redox ability in the SCR reaction, which was in accordance with the results of SCR catalytic activity and H2–TPR.
The XPS results of S 2p are shown in Figure 4c. For Fe/(SZr), the peak representing S was unconspicuous. However, two obvious peaks could be detected on S(Fe/Zr) catalyst. The banding energy at 170.1 and 169.0 eV was referred to the S6+ in SO42− [22]. These results manifested that the main species on the surface of Fe/(SZr) was Fe2O3. Although the isolated SO42− species existed on the Fe/(SZr) catalyst, the surface atomic concentration was extremely low (only 0.3%) (Table 1). It was because XPS detected the surface atomic concentration and only a small amount of SO42− existed on the surface, although SO42− was the main species on the surface of S(Fe/Zr). These results corresponded with the O 1s results.
The XPS spectra of Zr 3d are presented in Figure 4d. Each catalyst was fitted with two characteristic peaks. For the Fe/(SZr) catalyst, the banding energy located at 184.8 eV was corresponded with the Zr 3d3/2. Another peak at 182.4 eV was referred to the Zr4+ species. The positions of peaks were similar, which certified that the existence of Zr species was stable and not affected by the sulfating process.
The ICP results demonstrated that the integral mass concentrations of Fe, S, and Zr were all extremely similar for sulfated iron-based catalysts. Combined with the XPS results of concentrations of virous elements, it could be concluded that the preparation method of Fe/(SZr) and S(Fe/Zr) merely affected the surface atomic concentrations, rather than the integral atomic concentrations.
2.6. H2–TPR
H2–TPR technique was used to investigate the interaction between Fe2O3 and SO42−. The H2–TPR curves of all the catalysts are shown in Figure 5. The profile of Fe/Zr showed three peaks centered at 329, 417, and 540 °C. The peaks at 329 and 522 °C could be attributed to the reduction of the surface Fe2O3 (Fe2O3–Fe3O4–Fe) [23], and another peak at 417 °C could be assigned to the peak produced by the machine. However, for the other three catalysts, the intensity of this peak was too slight too be observed. The pattern of SZr showed only one peak centered at 585 °C, which corresponded to the reduction of SO42− [24]. The H2–TPR curves of Fe/(SZr) and S(Fe/Zr) showed only one peak, which centered at 403 and 486 °C, respectively. Their reduction peak temperature (Tred) were all higher than that of Fe/Zr catalyst and lower than that of SZr catalyst, which were referred to the overlapping of the stepwise reduction peaks of Fe2O3 and SO42−. According to reference [25], lower the Tred is, the stronger is the redox ability of the catalyst. Among Fe/(SZr) and S(Fe/Zr) catalysts, the redox abilities were as following: Fe/(SZr) > S(Fe/Zr). By combining the Raman results, it was concluded that the surface species were different because of the different sulfated order. In other words, the existent forms of Fe2O3 and SO42− on the surface of ZrO2 resulted in the different interaction of Fe2O3 and SO42−, which impacted the redox ability.
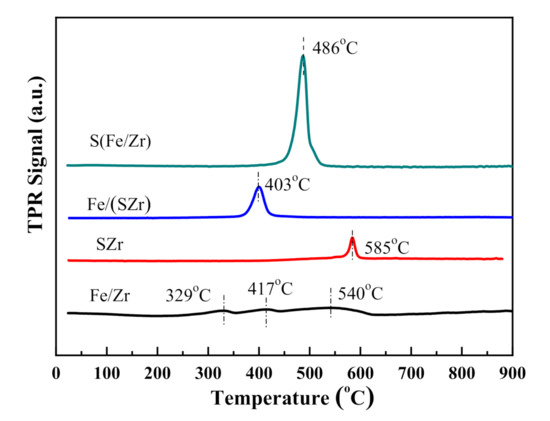
Figure 5.
H2–temperature-programmed reduction (H2-TPR) patterns of different iron-based catalysts.
2.7. NH3–TPD
The NH3–TPD patterns of Fe/(SZr) and S(Fe/Zr) catalysts are shown in Figure 6. One wide NH3 desorption peak of these three catalysts was displayed in the temperature range of 30–500 °C in the figure. The amounts of NH3 desorption were different greatly among these two catalysts, and the sequence was following: Fe/(SZr) (38.1 μmol·g−1) > S(Fe/Zr)(6.3 μmol·g−1). The NH3 species desorbed in high temperature range on Fe/(SZr) catalyst reduced a little, but the NH3 species desorbed in low and medium temperature range increased more greatly than that on the surface of S(Fe/Zr) catalyst, which might be one of the important reason for that Fe/(SZr) catalyst exhibited the higher SCR activity in low and medium temperature range than other catalysts.
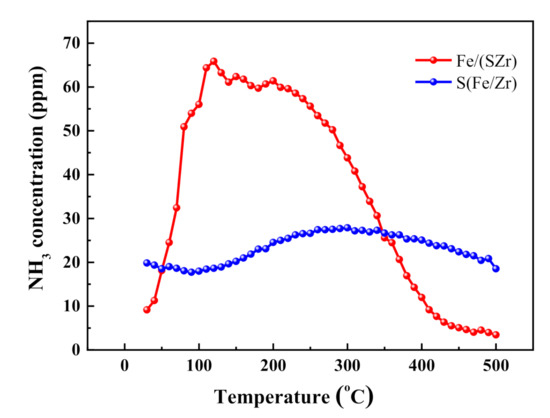
Figure 6.
NH3–TPD profiles of sulfated iron-based catalysts. Reaction conditions: 0.05% NH3, N2 balanced, 300 mL/min, and 0.5 g.
2.8. In Situ DRIFTS Studying of the NH3-Adsorption on Different Catalysts
The in situ DRIFTS spectra of Fe/(SZr) and S(Fe/Zr) catalysts, which were adsorbed and saturated with NH3 at 30 °C and then purged with N2, is displayed in Figure 7. Several absorbing vibration peaks at 3202, 3043, 2840, 1820, 1685, 1600, 1440, and 1257 cm−1 appeared over both Fe/(SZr) and S(Fe/Zr) catalysts. Among them, the absorbing vibration peaks at 3202, 3043, and 2840 cm−1 were assigned to the N-H bond. For Fe/(SZr) catalyst, the absorbing vibration peaks at 1820 and 1257 cm−1 were detected. The absorbing vibration peaks at 1440 and 1257 cm−1 of S(Fe/Zr) catalysts were observed. The peaks at 1685 and 1440 cm−1 were attributed to the symmetric and asymmetric absorbing vibration adsorbed to Brønsted acid sites [26], while the peaks at 1600 and 1257 cm−1 were ascribed to absorbing vibration adsorbed on Lewis acid sites [27,28]. Compared with S(Fe/Zr) catalyst, the peak intensities of absorbed NH3 species bounded to both Brønsted and Lewis acidic sites on the Fe/(SZr) catalyst surface were stronger in the figure. These mainly related to various forms of species on the Fe/(SZr) catalyst, such as isolated Fe2O3, SO42−, and sulfate species. Fe2O3 could provide Lewis acid sites, whereas SO42− supplied Brønsted acid sites, which could adsorb more NH3 species and were in accordance with the results of NH3–TPD.
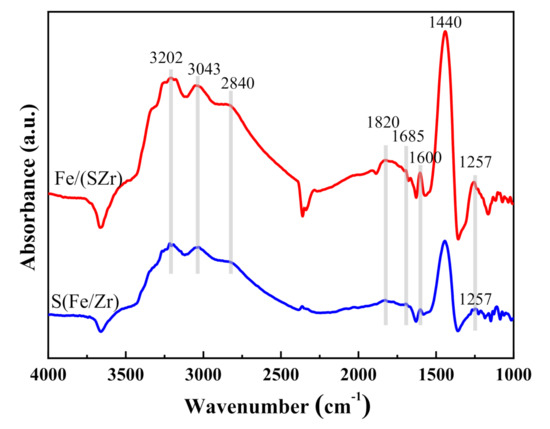
Figure 7.
In situ diffuse reflectance infrared Fourier transform spectra (DRIFTS) of Fe/(SZr) and S(Fe/Zr).
2.9. Transient Reaction Studies over Different Catalysts
2.9.1. Transient Reaction Studies over the Fe/(SZr) Catalyst
In Figure 8a, after the sample exposed to NO + O2 reached saturation and was purged with N2 at 250 °C, the nitrate species were stabilized on the surface of Fe/(SZr) catalyst (1525, 1442, 1380, and 1324 cm−1) [29,30]. After NH3 was introduced into the reaction cell, the absorbed NH3 species bounded to different acid sites on catalysts surface were observed (such as the peak at 1608 and 1300 cm−1, which could be attributed to the symmetric and asymmetric stretching vibrational mode of NH3 species on Lewis surface acid sites [31,32,33]). The peak at 1442 cm−1 could be assigned to the symmetric stretching vibration of NH4+ species bound to Brønsted acid sites [14].) After NH3 was introduced sustainably for 60 min, the adsorbed peaks did not change on the surface of catalysts. These suggested that the nitrate species adsorbed on catalyst surface were active in the SCR reaction, which could react with NH3 existed in air quickly for the redox reaction. As shown in Figure 8b, the stable absorbing peaks attributed to NH3 species were formed over Fe/(SZr) catalyst after introduction of NH3 and purging at 250 °C. (The peaks located at 1608 and 1300 cm−1 can be assigned to the symmetric and asymmetric stretching vibrational peaks of NH3 species on Lewis surface acid sites [21,31,33]. The peak at 1430 cm−1 can be attributed to the symmetric stretching vibration of NH4+ species bounded to Brønsted acid sites [34,35].) With the introduction of NO + O2 for 1 min, the NH3 species on catalyst surface became weaker, and the peak at 1631 cm−1 related to stretching vibration of NO2 appeared [36,37]. That was to say that the NH3 and oxynitride species can coexist on the surface of catalysts. After introducing NO + O2 for approximately 3 min, the NH3 species almost disappeared and the peaks of stretching vibration of NO2 enhanced. Meanwhile, some steady nitrate species (1324 cm−1) formed on catalyst surface, indicating that NH3 species adsorbed on the surface of catalysts were active species in the reaction. Overall, both adsorbed nitrate and NH3 species were active species to proceed SCR reaction.
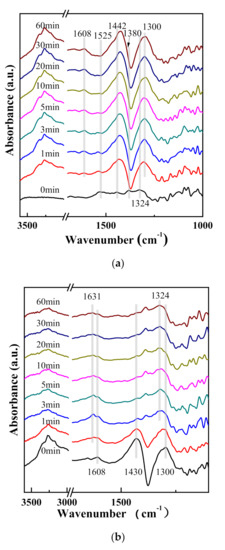
Figure 8.
In situ DRIFT spectra of (a) NH3 reacted with preadsorbed NO + O2 and (b) NO + O2 reacted with preadsorbed NH3 on Fe/(SZr).
2.9.2. Transient Reaction Studies over the S (Fe/Zr) Catalyst
When the S (Fe/Zr) catalyst was saturated with NO + O2 and purged with N2 at 250 °C, a small amount of nitrate species was formed on the surface (1592 and 1334 cm−1). Several absorbing peaks, which were assigned to weakly adsorbed NH3 species, appeared immediately on catalyst surface after introducing NH3. (The peaks at 1608 and 1324 cm−1 were ascribed to the symmetric and asymmetric stretching vibrational peaks of NH3 species bounded to Lewis surface acid sites [38]. The absorbing peak at 1429 cm−1 was due to the symmetric stretching vibration of NH4+ species on Brønsted acid sites [39].) As shown in Figure 9b, steady peaks representing absorbed NH4+ species located at 1431 and 1321 cm−1 appeared after saturation with NH3 and purging at 250 °C, which could be attributed to symmetric stretching vibration of NH4+ species bounded to Brønsted acid sites [40]. The amount of NH4+ species decreased on the catalyst surface after introduction of NO + O2 for 1 min, and then the stretching vibration of H2O species appeared (1625 cm−1) [41]. The stretching vibration peaks of H2O enhanced, and the NH4+ species still existed at 3 min. After the introduction of NO + O2 for 5 min, the stretching vibration of H2O species became weaker. Meanwhile, the NO2 (1637 cm−1) and nitrate species (1540 cm−1) appeared [42,43,44]. After introducing NO + O2 for 10 min, the NO2 stretching vibration peaks almost disappeared, but NH4+ species remained adsorbed on Brønsted acid sites (1431 and 1321 cm−1) [44]. The NH4+ stretching vibration peaks still existed at 60 min, suggesting that NH4+ species adsorbed on the catalyst surface were extremely stable and only a fraction of them participated in the reaction. Therefore, although the NH3 species on the surface of catalysts could participate in the reaction, the steady NH4+ species affected the catalytic activity of SCR reaction. It could be deduced that the SCR reaction on S(Fe/Zr) catalyst surface also followed the E-R pathway: First, NH3 adsorbed on SO42− existed on the catalyst surface to form the NH4+ species, which could react with NOx conducting the oxidation reaction, and further generated N2 and H2O.
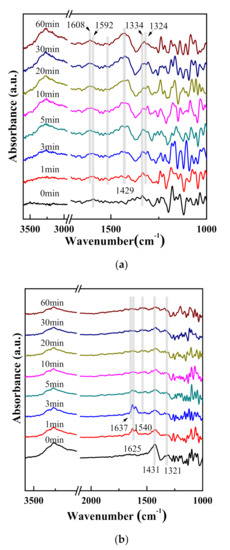
Figure 9.
In situ DRIFT spectra of (a) NH3 reacted with preadsorbed NO + O2 and (b) NO + O2 reacted with preadsorbed NH3 on S (Fe/Zr).
In conclusion, considering the reaction mechanisms of Fe/(SZr) and S(Fe/Zr) catalysts, it could be inferred that Fe3+ and SO42− afforded crucial active sites of sulfated iron-based catalysts, and the active species on sulfated iron-based catalyst surface were Fe2O3, SO42−, and Fe2(SO4)3.
3. Discussion
It could be concluded from the results of transient studies that the NH3-SCR reaction on Fe/(SZr) catalyst followed Langmuir–Hinshelwood (L-H) and Eley–Rideal (E-R) mechanisms. It was not only that the NH3 species adsorbed on the surface could react with gaseous NO + O2 but also, at the same time, NOx on the catalyst surface could form large amount of highly active NO2 species, which could react with adsorbed NH3 for SCR reaction. The E-R mechanism was executed on S(Fe/Zr) surface in the reaction, which meant that the gaseous NH3 absorbed first on the surface of catalysts, and then reacted with NOx in air for redox reaction. Besides, the adsorbed NH4+ on catalyst surface was very stabilized and only partly activated to participate in the reaction over the S(Fe/Zr) catalyst.
Combined results of Raman and XPS spectra showed that there were different forms of species on Fe/(SZr) and S(Fe/Zr) catalysts. The existent forms of Fe2O3 and SO42− on catalysts influenced the mutually coordinated impact on these two catalysts, which determined large differences in the redox ability and adsorption performance of catalysts, and also affected the nitrogen oxide and NH3 adsorption species participating in the reaction on the catalyst surface. There were more Fe3+ and surface-chemisorbed labile oxygen on the surface of Fe/(SZr) catalyst, which facilitated the redox cycle and led to better performance during the reaction. The differences caused the SCR reaction on the surface of Fe/(SZr) and S(Fe/Zr) catalysts to follow different mechanisms, which resulted in the different SCR reaction path on the catalyst surface, and the SCR reaction activity of the catalyst were influenced. Not only the sulfate species but also isolated Fe2O3 and SO42− ions existed on the Fe/(SZr) catalyst. The isolated Fe2O3 on the catalyst was beneficial to the adsorption of sulfate and more NH3 species on the surface, which promoted the operation of L-H mechanism and the NOx removal efficiency over Fe/(SZr) catalyst at middle-low temperature. This might be the main reason for the higher catalytic activity of Fe/(SZr) catalyst. For S(Fe/Zr) catalyst, only the SO42− and sulfate were existed on the surface. The Fe3+ was covered by isolated SO42− due to the different orders of loading on S(Fe/Zr) catalyst. The Fe3+ ion of the catalyst could not participate well in the redox reaction, so the activity of the catalyst was affected, which explained the reason that the low-temperature activity of the S(Fe/Zr) catalyst was lower than that of the (FeS)/Zr catalyst. In addition, according to the catalytic activity results of the effect of SO2 on the activity of catalysts at different sulfated positions, the Fe/(SZr) catalyst had poor stability against SO2 poisoning. The Fe/(SZr) catalyst had Fe2O3 species that could promote the activity at medium and low temperature. Meanwhile, Fe2O3 easily combined with SO2 to generate ferrous sulfate or ferric sulfate, occupying the active center, thus leading to the SO2 poisoning of the Fe/(SZr) catalyst. Therefore, it could be concluded that the different forms of Fe2O3 and SO42− affected the catalytic activity and caused different mechanisms followed on catalysts. Besides, different species on the surface of catalysts influenced the property of resistance to SO2 poisoning.
4. Experimental
4.1. Catalyst Preparation
All the catalysts were synthesized through the method of impregnation. The specific experimental steps were as follows: First, the weighed ZrO2 power was dissolved in distilled water, and some quantities of Fe(NO3)3·9H2O and (NH4)2SO4 were added subsequently. Then, the solution was heated to 70 °C. Meanwhile, it was kept stirring till the paste was formed. After that, the paste was dried overnight at 120 °C and calcined under air at 500 °C for 4 h. Finally, several iron-based catalysts with different molar ratios were obtained, signed as FexSyZr (x = 3, y = 0; x = 0, y = 5; x is the wt.% of Fe2O3 and y is the wt.% of SO42−). In this paper, Fe3Zr and S5Zr were abbreviated as FeZr and SZr, respectively.
Some SZr power was dissolved and a certain amount of Fe(NO3)3·9H2O was added. Meanwhile, some FeZr catalyst was dissolved and some quantities of (NH4)2SO4 were added. These two solutions were heated to 70 °C. Similarly, these solutions were kept stirred until they formed paste and then were dried overnight at 120 °C. Afterwards, they were calcined under air at 500 °C for 4 h. Finally, Fe/(SZr) and S/(FeZr) catalysts were obtained.
4.2. Catalytic Activity Measurement
Measurements of catalytic activity were performed in a fixed-bed quartz reactor with an inner diameter of 9 mm. Precisely, 0.5 g of 40–60 mesh catalysts were used in the reactor. Specific experimental mixture gas conditions were as follows: 500 ppm NO, 500 ppm NH3, 3 vol.% O2, and N2 were used as the balance gas. The total flow rate was 300 mL/min, whereas corresponding small space-time velocity of the gas was approximately 47,000 h−1. The Fourier-transform infrared spectrometer (FT-IR) gas analyzer (Gasmet Dx-4000) was used to measure NOx, N2O, and NH3 concentrations in both the inlet and outlet. When the catalytic reaction reached a stable state of half an hour at each temperature, the activity data were collected.
4.3. Catalyst Characterization
X-ray diffraction (XRD) was carried out on a D/MAX-RB system with Cu Ka radiation. The diffraction patterns were recorded in the range of 2u from 10° to 90° in steps of 0.018 and 1 s/step.
The Quantachrome Autosorb AS-1 System was used to measure the BET specific surface area, pore size, and pore volume through N2 adsorption at 77 K.
X-ray photoelectron spectroscopy (XPS) was used to observe chemical valences of catalysts, atomic concentration, and surface species. These experiments were conducted on ESCALab 220i-XL electron spectrometer with radiations of 300 W Mg Ka.
The results of Raman spectra were obtained by using the Raman microscope (InVia reflection, Renishaw), which was equipped with a deep depletion thermoelectric cooled charge-coupled device (CCD) array detector and an advanced Leica microscope (long working distance objective lens 50×).
H2-temperature-programmed reduction (TPR) curves were recorded through the chemisorption analyzer (Micromeritics ChemiSorb 2720, Micromeritics Norcross, America) using 40 mg of samples. First, samples were pretreated in N2 at 300 °C for 1 h and then cooled down to the room temperature. Subsequently, using 10% H2/Ar reducing gas with a flow rate of 50 mL/min, the catalyst was reduced from room temperature to 1000 °C with a temperature gradient of 10 °C/min. The consumed H2 was calculated by integrating the corresponding TCD signal strength.
NH3 temperature-programmed desorption (TPD) was performed in a fixed-bed quartz reactor. First, each sample was degassed under the atmosphere of N2 at 500 °C for 1 h. Second, 500 ppm NH3 was adsorbed for 1 h after cooling to room temperature. Third, the desorption was carried out in N2 at the temperature of the last-step response experiment until no NH3 was detected. Finally, temperature-programmed desorption (TPD stage) was performed at 10 °C/min up to 500 °C. During these experiments, typically, the sample mass of 0.5 g and a gas flow rate of 300 mL/min were needed.
The Nicolet NEXUS 870 FT-IR (Nicolet Madison, America) spectrometer was used to characterize the in situ DRIFTS spectra. Before the experiments, each sample was pretreated at 300 °C for 1 h in N2 with the flow rate of 100 cm3/min.
5. Conclusions
The Fe/(SZr) and S(Fe/Zr) sulfated iron-based catalysts synthesized by impregnation methods were investigated in this research. The activities of sulfated iron-based catalysts improved remarkably compared with Fe/Zr catalyst in the temperature range of 250–500 °C. The NOx conversion and N2 selectivity exhibited large distinction between Fe/(SZr) and S(Fe/Zr) sulfated iron-based catalysts. The study results revealed that the existence of sulfate, isolated Fe2O3, and SO42− species on Fe/(SZr) catalyst supplied more acid sites, which could adsorb more NH3 species and reacted with gaseous NO + O2. Besides, the reaction between highly active NO2 species and NOx on the catalyst surface was beneficial to the catalytic activity. Thus, the SCR reaction on Fe/(SZr) catalyst surface followed both the L-H mechanism and the E-R mechanism. For S(Fe/Zr) catalyst, the SO42− and sulfate were existed on the surface. The Fe3+ was covered by the independent SO42−, so the Fe3+ of the catalyst could not participate in the redox reaction well, which affected the progress of the SCR reaction on the catalyst. The absorbed NH4+ reacted with NOx in air and made the S(Fe/Zr) catalyst mainly follow the E-R pathway. In other words, different loading positions of sulfate caused distinctive active components on Fe/(SZr) and S(Fe/Zr) surface, leading to the difference of reaction mechanisms and affected the redox ability and NH3 adsorption. Therefore, the catalytic activity was affected.
Author Contributions
Writing—original draft preparation, B.F.; writing—review and editing, Z.Z. and Q.L.; supervision, C.L. All authors have read and agree to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (2016YFC0205302 and 2016YFC0205300), the National Youth Natural Science Foundation of China (No. 21507100), and the Science and Technology Project of Hebei Province (NO.206Z3702G).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Long, R.Q.; Yang, R.T. The promoting role of rare earth oxides on Fe-exchanged TiO2-pillared clay for selective catalytic reduction of nitric oxide by ammonia. Appl. Catal. B 2000, 27, 87–95. [Google Scholar] [CrossRef]
- Guo, M.; Liu, Q.; Zhao, P.; Han, J.; Li, X.; Ha, Y.; Fu, Z.; Song, C.; Ji, N.; Liu, C.; et al. Promotional effect of SO2 on Cr2O3 catalysts for the marine NH3-SCR reaction. Chem. Eng. J. 2019, 361, 830–838. [Google Scholar] [CrossRef]
- Liu, C.; Yang, S.; Ma, L.; Peng, Y.; Hamidreza, A.; Chang, H.; Li, J. Comparison on the performance of α-Fe2O3 and γ-Fe2O3 for selective catalytic reduction of nitrogen oxides with ammonia. Catal. Lett. 2013, 143, 697–704. [Google Scholar] [CrossRef]
- Ramis, G.; Yi, L.; Busca, G.; Turco, M.; Kotur, E.; Willey, R.J. Adsorption, activation, and oxidation of ammonia over SCR catalysts. J. Catal. 1995, 157, 523–535. [Google Scholar] [CrossRef]
- Fabrizioli, P.; Bürgi, T.; Baiker, A. Environmental catalysis on iron oxide–Silica Aerogels: Selective oxidation of NH3 and reduction of NO by NH3. J. Catal. 2002, 206, 143–154. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Selective catalytic oxidation of ammonia to nitrogen over Fe2O3-TiO2 prepared with a sol-gel method. J. Catal. 2002, 207, 158–165. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Characterization of Fe-ZSM-5 catalyst for selective catalytic reduction of nitric oxide by ammonia. J. Catal. 2000, 194, 80–90. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Selective catalytic oxidation (SCO) of ammonia to nitrogen over Fe-exchanged zeolites. J. Catal. 2001, 201, 145–152. [Google Scholar] [CrossRef]
- Jung, S.M.; Grange, P. Investigation of the promotional effect of V2O5 on the SCR reaction and its mechanism on hybrid catalyst with V2O5 and TiO2-SO42− catalysts. Appl. Catal. B 2002, 36, 207–215. [Google Scholar] [CrossRef]
- Jung, S.M.; Grange, P. Characterization and reactivity of V2O5-WO3 supported on TiO2-SO42− catalyst for the SCR reaction. Appl. Catal. B 2001, 32, 123–131. [Google Scholar] [CrossRef]
- Ke, R.; Li, J.; Liang, X.; Hao, J. Novel promoting effect of SO2 on the selective catalytic reduction of NOx by ammonia over Co3O4 catalyst. Catal. Commun. 2007, 8, 2096–2099. [Google Scholar] [CrossRef]
- Gu, T.; Liu, Y.; Weng, X.; Wang, H.; Wu, Z. The enhanced performance of ceria with surface sulfation for selective catalytic reduction of NO by NH3. Catal. Commun. 2010, 12, 310–313. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Wang, H.; Ning, P.; Zhang, Y.; Ma, Y.; Wang, J.; Wang, L.; Zhang, Q. Highly efficient WO3-FeOx catalysts synthesized using a novel solvent-free method for NH3-SCR. J. Hazard. Mater. 2020, 388, 121812. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Asakura, K.; He, H.; Shan, W.; Shi, X.; Zhang, C. Influence of sulfation on iron titanate catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B 2011, 103, 369–377. [Google Scholar] [CrossRef]
- Zhang, L.; He, H. Mechanism of selective catalytic oxidation of ammonia to nitrogen over Ag/Al2O3. J. Catal. 2009, 268, 18–25. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Y.; Li, J. Activity enhancement of sulphated Fe2O3 supported on TiO2–ZrO2 for the selective catalytic reduction of NO by NH3. Appl. Surf. Sci. 2020, 528, 146695. [Google Scholar] [CrossRef]
- Zhu, N.; Shan, W.; Lian, Z.; Zhang, Y.; Liu, K.; He, H. A superior Fe-V-Ti catalyst with high activity and SO2 resistance for the selective catalytic reduction of NOx with NH3. J. Hazard. Mater. 2020, 382, 120970. [Google Scholar] [CrossRef]
- Tan, W.; Wang, J.; Li, L.; Liu, A.; Song, G.; Guo, K.; Luo, Y.; Liu, F.; Gao, F.; Dong, L. Gas phase sulfation of ceria-zirconia solid solutions for generating highly efficient and SO2 resistant NH3-SCR catalysts for NO removal. J. Hazard. Mater. 2020, 388, 121729. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, X.; Li, Y.; Zhang, T.; Yuan, F.; Niu, X.; Zhu, Y. Effect of W on the acidity and redox performance of the Cu0.02Fe0.2WaTiOx (a = 0.01, 0.02, 0.03) catalysts for NH3-SCR of NO. Appl. Catal. B 2019, 248, 226–238. [Google Scholar] [CrossRef]
- Chen, J.P.; Yang, R.T. Selective Catalytic Reduction of NO with NH3 on SO−24/TiO2 Superacid Catalyst. J. Catal. 1993, 139, 277–288. [Google Scholar] [CrossRef]
- Apostolescu, N.; Geiger, B.; Hizbullah, K.; Jan, M.T.; Kureti, S.; Reichert, D.; Schott, F.; Weisweiler, W. Selective catalytic reduction of nitrogen oxides by ammonia on iron oxide catalysts. Appl. Catal. B 2006, 62, 104–114. [Google Scholar] [CrossRef]
- Ma, L.; Li, J.; Ke, R.; Fu, L. Catalytic performance, characterization, and mechanism study of Fe2(SO4)3/TiO2 catalyst for selective catalytic reduction of NOx by Ammonia. J. Phys. Chem. C 2011, 115, 7603–7612. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Wang, J.; Xu, C.; Duan, A.; Jiang, G.; Yang, Q. The highly active catalysts of nanometric CeO2-supported cobalt oxides for soot combustion. Appl. Catal. B 2008, 84, 185–195. [Google Scholar] [CrossRef]
- Yang, J.; Ren, S.; Zhou, Y.; Su, Z.; Yao, L.; Cao, J.; Jiang, L.; Hu, G.; Kong, M.; Yang, J.; et al. In situ IR comparative study on N2O formation pathways over different valence states manganese oxides catalysts during NH3–SCR of NO. Chem. Eng. J. 2020, 397, 125446. [Google Scholar] [CrossRef]
- Zhan, S.; Zhang, H.; Zhang, Y.; Shi, Q.; Li, Y.; Li, X. Efficient NH3-SCR removal of NOx with highly ordered mesoporous WO3(χ)-CeO2 at low temperatures. Appl. Catal. B 2017, 203, 199–209. [Google Scholar] [CrossRef]
- Wang, S.; Fan, C.; Zhao, Z.; Liu, Q.; Xu, G.; Wu, M.; Chen, J.; Li, J. A facile and controllable in situ sulfation strategy for CuCeZr catalyst for NH3-SCR. Appl. Catal. A 2020, 597, 117554. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, J.; Ning, P.; Song, Z.; Liu, X.; Wang, L.; Wang, J.; Wang, H.; Long, K. In situ DRIFTS investigation of NH3-SCR reaction over CeO2/zirconium phosphate catalyst. Appl. Surf. Sci. 2018, 435, 1037–1045. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Z.; Liu, L.; Chen, Y.; Zhang, Z.; Wang, X. In situ DRIFTS investigation on the SCR of NO with NH3 over V2O5 catalyst supported by activated semi-coke. Appl. Surf. Sci. 2014, 313, 660–669. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zhao, Q.; Ke, J.; Xiao, H.; Lv, X.; Liu, S.; Tadé, M.; Wang, S. Mechanistic investigation of the enhanced NH3-SCR on cobalt-decorated Ce-Ti mixed oxide: In Situ FTIR analysis for structure-activity correlation. Appl. Catal. B 2017, 200, 297–308. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Liu, S.; Jia, W. The effect of SO2 on NH3-SCO and SCR properties over Cu/SCR catalyst. Appl. Surf. Sci. 2020, 507, 145153. [Google Scholar] [CrossRef]
- Song, L.; Yue, H.R.; Ma, K.; Tian, W.; Liu, W.Z.; Liu, C.J.; Tang, S.Y.; Liang, B. Mechanistic aspects of highly efficient FeaSbTiOx catalysts for the NH3-SCR reaction: Insight into the synergistic effect of Fe and S species. Ind. Eng. Chem. Res. 2020, 59, 8164–8173. [Google Scholar] [CrossRef]
- Shu, Y.; Aikebaier, T.; Quan, X.; Chen, S.; Yu, H. Selective catalytic reaction of NOx with NH3 over Ce–Fe/TiO2-loaded wire-mesh honeycomb: Resistance to SO2 poisoning. Appl. Catal. B 2014, 150–151, 630–635. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.; Cavataio, G.; McCabe, R.W.; Fu, L.; Li, J. In situ DRIFTS and temperature-programmed technology study on NH3-SCR of NOx over Cu-SSZ-13 and Cu-SAPO-34 catalysts. Appl. Catal. B 2014, 156–157, 428–437. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Reaction mechanism of selective catalytic reduction of NO with NH3 over Fe-ZSM-5 catalyst. J. Catal. 2002, 207, 224–231. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Li, Y.; Su, H.; Ma, L. WO3 promoted Mn–Zr mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2016, 283, 1044–1050. [Google Scholar] [CrossRef]
- Yan, Q.; Gao, Y.; Li, Y.; Vasiliades, M.A.; Chen, S.; Zhang, C.; Gui, R.; Wang, Q.; Zhu, T.; Efstathiou, A.M. Promotional effect of Ce doping in Cu4Al1Ox–LDO catalyst for low-T practical NH3-SCR: Steady-state and transient kinetics studies. Appl. Catal. B 2019, 255, 117749. [Google Scholar] [CrossRef]
- France, L.J.; Yang, Q.; Li, W.; Chen, Z.; Guang, J.; Guo, D.; Wang, L.; Li, X. Ceria modified FeMnOx—Enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl. Catal. B 2017, 206, 203–215. [Google Scholar] [CrossRef]
- Shan, W.; Liu, F.; He, H.; Shi, X.; Zhang, C. A superior Ce-W-Ti mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B 2012, 115–116, 100–106. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Guo, R.-T.; Guan, Z.-Z.; Shi, X.; Pan, W.-G.; Fu, Z.-G.; Qin, H.; Liu, X.-Y. The promotion effect of Cr additive on CeZr2Ox catalyst for the low-temperature selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2019, 485, 133–140. [Google Scholar] [CrossRef]
- Wang, A.; Guo, Y.; Gao, F.; Peden, C.H.F. Ambient-Temperature NO oxidation over amorphous CrOx-ZrO2 mixed oxide catalysts: Significant promoting effect of ZrO2. Appl. Catal. B 2017, 202, 706–714. [Google Scholar] [CrossRef]
- Liu, H.; Fan, Z.; Sun, C.; Yu, S.; Feng, S.; Chen, W.; Chen, D.; Tang, C.; Gao, F.; Dong, L. Improved activity and significant SO2 tolerance of samarium modified CeO2-TiO2 catalyst for NO selective catalytic reduction with NH3. Appl. Catal. B 2019, 244, 671–683. [Google Scholar] [CrossRef]
- Gong, P.; Xie, J.; Fang, D.; He, F.; Li, F.; Qi, K. Enhancement of the NH3-SCR property of Ce-Zr-Ti by surface and structure modification with P. Appl. Surf. Sci. 2020, 505, 144641. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).