Doping of Graphitic Carbon Nitride with Non-Metal Elements and Its Applications in Photocatalysis
Abstract
:1. Introduction
2. Oxygen-Doped g-C3N4
3. Nitrogen-Doped g-C3N4
| Precursor | Synthetic Method | C/N Atomic Ratio, Doped (Pristine) | Photocatalytic Process | Conditions of the Process | Efficiency Doped/Pristine | References |
|---|---|---|---|---|---|---|
| Melamine, N-N dimethylformamide (DMF) | Hydrothermal treatment | 0.407 (0.540) by EDS, 0.457 (0.575) by Organic Elemental Analysis (OEA) | H2 evolution | 300W Xe lamp, filter λ > 400 nm), co-catalysts Pt nanoparticles, triethanolamine (TEOA) as a hole quencher | 128.5 h−1/ 58.6 h−1, | [57] |
| Melamine, hydrazine hydrate | Thermal condensation | 0.67 (0.73) by elemental analysis | H2 evolution | 300W Xe lamp, filter λ > 400 nm, TEOA (10 vol.%, Pt co-catalyst (3 wt.%) | 44.28 μmol·h−1/ 7.86 μmol⋅h−1 | [49] |
| dicyandiamide | Secondary calcination | 57.57 (56.88) at.% by EDS | methylene blue degradation | 300W Xe lamp, filter λ > 420 nm | 0.02355 min−1/ 0.00829 min−1 | [50] |
| Dicyandiamide, DMF | Thermal copolymerization | 0.67 (0.76) by XPS | Tetracycline (TC) degradation | 300W Xe lamp, filter λ > 420 nm | 76.78 (52.21)% of TC was degraded in 60 min | [52] |
| Urea, DMF | Thermal copolymerization | 0.74 (0.59) at.% by organic elemental analysis | H2 evolution | 300W Xe lamp, filter λ > 400 nm, TEOA (10 vol.%), Pt co-catalyst (3 wt.%) | 5268 μmol g−1·h−1/ 3579 μmol g−1·h−1/ | [51] |
| Urea, monoethanolamine | Amine functionalization of g-C3N4 | — | CO2 reduction | 300W Xe lamp, gas phase reaction | CH4: 0.34 μmol·h−1·g−1/trace; CH3OH: 0.26 μmol·h−1·g−1/ 0.26 μmol·h−1·g−1 | [58] |
| Melamine, NH4Cl | Thermal polymerization | N content (at.%) doped 47.32 doped; 45.59 pristine | RhB degradation | 300W Xe lamp, 420 nm cutoff filter, | 0.01954 min−1/ 0.00391 min−1 | [59] |
| Melamine, NH4Cl | Second-calcination approach | 1.14 (1.63) by XPS analysis | H2 evolution | 420-nm LED, lactic acid (10 vol%) solution, (Pt co-catalyst (1 wt%) | 15.5 mmol·h−1/ 7.5 mmol·h−1 | [65] |
| Melamine, NH4Cl | Precursor formation by hydrothermal method; thermal polymerization in NH3, N2, Ar, air | 0.55-0.59 (0.69) by elemental analysis | CO2 reduction | 300 W Xe-lamp, CoCl2, 2,2-bipyridine, TEOA and methyl cyanide | 103.6 μmol·g−1·h−1/ 6.1 μmol·g−1·h−1 | [62] |
| Citric acid, urea | Thermal polymerization | without change | H2 evolution | 300W Xe lamp, 420 nm cutoff filter, Pt nanoparticles (3 wt.%), triethanolamine as a hole quencher | 64 μmol·h−1/15 μmol·h−1 | [63] |
| Dicyandiamide, citric acid, urea | Thermal polymerization | — | Indomethacine degradation | 350 W xenon lamp with 420 nm cutoff filter; 500 W mercury lamp; 350 W xenon lamp with a 290 nm cut-off filter | Photocatalytic activity was 1.1 (UV light irradiation), 1.8 (simulated sunlight), and 13.6 (visible light irradiation) times higher than that of pristine g-C3N4 | [66] |
| Melamine, urea | Hydrothermal treatment | C:N mass ratio doped 0.53/0.73 by OEA; C:N atomic ratio 0.47/0.72 by quantitative XPS | H2 evolution | 300W Xe lamp, 420 nm cutoff filter, Pt nanoparticles (1 wt.%), 20% vol. lactic acid | 3579 μmol·h−1·g−1/ 147 μmol·h−1·g−1 | [64] |
| Urea, N2 | Thermal polymerization | doped 0.71/0.73 by XPS | Bisphenol A oxidation; Cr(VI) reduction | 300W Xe lamp, 420 nm cutoff filter | Complete degradation of BPA 60 min/90 min; Photoreduction of Cr(VI) over 120 min: 10%/60% | [34] |
4. Carbon-Doped g-C3N4
5. Sulfur-Doped g-C3N4
6. P-Doped g-C3N4
| Precursor | Synthetic Method | C/Doping Element Atomic Ratio, Doped (Pristine) | Photocatalytic Process | Conditions of the Process | Efficiency Doped/Pristine | References |
|---|---|---|---|---|---|---|
| g-C3N4, P powder | Thermal modification | P 2p signal at around 133.7 eV | H2 evolution | 300 W Xe lamp (λ > 300 nm), filter λ > 420 nm, 10% vol% TEOA, Pt co-catalyst (3 wt.%) | λ > 300 nm 261.2 µmol·g−1·h−1/81.6 µmol·g−1·h−1 λ > 420 nm 171.6 µmol·g−1·h−1/81.6 µmol·g−1·h−1 | [109] |
| Urea, phosphonitrilic chloride | Thermal condensation | 4.4 atomic% by EDS 5.72 atomic% by XPS | H2O2 generation | Visible light irradiation (420 nm ≤ λ ≤ 700 nm) | 1968 μmol·g−1·h−1/ 68 μmol·g−1·h−1 | [108] |
| NH4SCN, NH4PF6 | Thermal condensation | — | RhB destruction | 300 W Xe lamp, filter λ > 420 nm | 0.09856 min−1/ 0.03679 min−1 | [110] |
| g-C3N4, phosphorene | Mechanically mixing | 1.8 wt.% | H2 evolution | 300 W Xe lamp, filter λ > 400 nm, lactic acid (88 vol%) | 571 µmol·g−1·h−1/ 43 μmol·g−1·h−1 | [117] |
| g-C3N4, sodium hypophosphite | Thermal treatment method | 13.52 wt.% | RhB destruction | 300 W Xe lamp, λ: 420–780 nm, | 0.0525 min−1/ 0.0126min−1 | [115] |
| Urea, adenosine phosphate | Thermal condensation followed by thermal exfoliation method | 2.17 atomic% | H2 evolution | 300 W Xe lamp, filters λ: 400, 420, 435, 450, 550 nm; 10% vol% TEOA, Pt co-catalyst (3 wt.%) | 9523.7 µmol·g−1·h−1/ 458 μmol·g−1·h−1 | [118] |
| Urea, NH4H2PO2 | Thermal condensation | — | H2 evolution | 300 W Xe lamp, filter λ 400 nm, 20% vol% TEOA, Pt co-catalyst (1 wt.%) | 5.7 times that of pristine | [119] |
| Dicyandiamide, NH4Cl, (NH4)2HPO4 | Thermal condensation | 1.53 wt% by EDS | H2 evolution | 300 W Xe lamp, filter λ 420 nm, 10% vol% TEOA, Pt co-catalyst (3 wt.%) | 33.2 µmol·g−1·h−1/ 10.7 μmol·g−1·h−1 | [126] |
7. Vacancy-Doped g-C3N4
8. Photocatalytic Applications of Non-Metal Elements Doped g-C3N4
8.1. Photocatalytic CO2 Reduction
8.2. H2-Evolution
8.3. Degradation of Dyes and Organic Pollutants
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Kroke, E. Novel group 14 nitrides. Coord. Chem. Rev. 2004, 248, 493–532. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Hasija, V.; Raizada, P.; Sudhaik, A.; Sharma, K.; Kumar, A.; Singh, P.; Jonnalagadda, S.B.; Thakur, V.K. Recent advances in noble metal free doped graphitic carbon nitride based nanohybrids for photocatalysis of organic contaminants in water: A review. Appl. Mater. Today 2019, 15, 494–524. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Shen, Y.; Liu, S.; Zhang, Y. Molecular engineering of polymeric carbon nitride: Advancing applications from photocatalysis to biosensing and more. Chem. Soc. Rev. 2018, 47, 2298–2321. [Google Scholar] [CrossRef]
- Wang, A.; Wang, C.; Fu, L.; Wong-Ng, W.; Lan, Y. Recent Advances of Graphitic Carbon Nitride-Based Structures and Applications in Catalyst, Sensing, Imaging, and LEDs. Nano-Micro Lett. 2017, 9, 47. [Google Scholar] [CrossRef]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B Environ. 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503, (1–31). [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, D.; Zhu, M.Y.; Shi, T.Y.; Li, H.N.; Wang, K. An effective strategy for fabricating highly dispersed nanoparticles on O-C3N4 with enhanced electrocatalytic activity and stability. J. Alloy. Compd. 2018, 741, 1203–1211. [Google Scholar] [CrossRef]
- Yousefi, M.; Villar-Rodil, S.; Paredes, J.I.; Moshfegh, A.Z. Oxidized graphitic carbon nitride nanosheets as an effective adsorbent for organic dyes and tetracycline for water remediation. J. Alloy. Compd. 2019, 809, 11. [Google Scholar] [CrossRef]
- Zhu, W.R.; Hao, N.; Lu, J.W.; Dai, Z.; Qian, J.; Yang, X.D.; Wang, K. Highly active metal-free peroxidase mimics based on oxygen-doped carbon nitride by promoting electron transfer capacity. Chem. Commun. 2020, 56, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Chubenko, E.B.; Baglov, A.V.; Leonenya, M.S.; Yablonskii, G.P.; Borisenko, V.E. Structure of Photoluminescence Spectra of Oxygen-Doped Graphitic Carbon Nitride. J. Appl. Spectrosc. 2020, 87, 9–14. [Google Scholar] [CrossRef]
- Denisov, N.M.; Chubenko, E.B.; Bondarenko, V.P.; Borisenko, V.E. Synthesis of Oxygen-Doped Graphitic Carbon Nitride from Thiourea. Tech. Phys. Lett. 2019, 45, 108–110. [Google Scholar] [CrossRef]
- Gao, Y.W.; Zhu, Y.; Lyu, L.; Zeng, Q.Y.; Xing, X.C.; Hu, C. Electronic Structure Modulation of Graphitic Carbon Nitride by Oxygen Doping for Enhanced Catalytic Degradation of Organic Pollutants through Peroxymonosulfate Activation. Environ. Sci. Technol. 2018, 52, 14371–14380. [Google Scholar] [CrossRef]
- Huang, J.; Nie, G.; Ding, Y.B. Metal-Free Enhanced Photocatalytic Activation of Dioxygen by g-C3N4 Doped with Abundant Oxygen-Containing Functional Groups for Selective N-Deethylation of Rhodamine B. Catalysts 2020, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Pan, S.G.; Shi, L.L.; Yu, A.P.; Wang, X.; Fu, Y.S. Hollow porous prismatic graphitic carbon nitride with nitrogen vacancies and oxygen doping: A high-performance visible light-driven catalyst for nitrogen fixation. Nanoscale 2020, 12, 1833–1841. [Google Scholar] [CrossRef]
- Li, F.; Han, M.E.; Jin, Y.; Zhang, L.L.; Li, T.; Gao, Y.W.; Hu, C. Internal electric field construction on dual oxygen group-doped carbon nitride for enhanced photodegradation of pollutants under visible light irradiation. Appl. Catal. B Environ. 2019, 256, 10. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, H.W.; Cui, W.; Dong, F.; Zhang, Y.H. Band structure engineering and efficient charge transport in oxygen substituted g-C3N4 for superior photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 230, 115–124. [Google Scholar] [CrossRef]
- Li, Q.; Wang, S.C.; Sun, Z.X.; Tang, Q.J.; Liu, Y.Q.; Wang, L.Z.; Wang, H.Q.; Wu, Z.B. Enhanced CH4 selectivity in CO2 photocatalytic reduction over carbon quantum dots decorated and oxygen doping g-C3N4. Nano Res. 2019, 12, 2749–2759. [Google Scholar] [CrossRef]
- Wei, F.Y.; Liu, Y.; Zhao, H.; Ren, X.N.; Liu, J.; Hasan, T.; Chen, L.H.; Li, Y.; Su, B.L. Oxygen self-doped g-C3N4 with tunable electronic band structure for unprecedentedly enhanced photocatalytic performance. Nanoscale 2018, 10, 4515–4522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Yao, J.H.; Qiu, P.X.; Xu, C.M.; Jiang, F.; Wang, X. Facile surfactant assistant synthesis of porous oxygen-doped graphitic carbon nitride nanosheets with enhanced visible light photocatalytic activity. Mater. Res. Bull. 2017, 91, 42–48. [Google Scholar] [CrossRef]
- Putri, L.K.; Ng, B.J.; Er, C.C.; Ong, W.J.; Chang, W.S.; Mohamed, A.R.; Chai, S.P. Insights on the impact of doping levels in oxygen-doped gC(3)N(4) and its effects on photocatalytic activity. Appl. Surf. Sci. 2020, 504. [Google Scholar] [CrossRef]
- Sun, S.D.; Li, J.; Cui, J.; Gou, X.F.; Yang, Q.; Liang, S.H.; Yang, Z.M.; Zhang, J.M. Constructing oxygen-doped g-C3N4 nanosheets with an enlarged conductive band edge for enhanced visible-light-driven hydrogen evolution. Inorg. Chem. Front. 2018, 5, 1721–1727. [Google Scholar] [CrossRef]
- Chen, Y.L.; Liu, X.Q.; Hou, L.; Guo, X.R.; Fu, R.W.; Sun, J.M. Construction of covalent bonding oxygen-doped carbon nitride/graphitic carbon nitride Z-scheme heterojunction for enhanced visible-light-driven H2 evolution. Chem. Eng. J. 2020, 383. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Wang, W.; Chen, Q.W.; Pu, Y.Y.; He, H.; Zhuang, W.M.; He, J.Q.; Huang, L.M. A hierarchical carbon nitride tube with oxygen doping and carbon defects promotes solar-to-hydrogen conversion. J. Mater. Chem. A 2020, 8, 3160–3167. [Google Scholar] [CrossRef]
- Yuan, X.J.; Xie, R.L.; Zhang, Q.; Sun, L.; Long, X.J.; Xia, D.S. Oxygen functionalized graphitic carbon nitride as an efficient metal-free ozonation catalyst for atrazine removal: Performance and mechanism. Sep. Purif. Technol. 2019, 211, 823–831. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Liu, X.; Liu, C.B.; Wang, L.L.; Xia, Y.C.; Zhang, S.Q.; Luo, S.L.; Pei, Y. Scalable one-step production of porous oxygen-doped g-C3N4 nanorods with effective electron separation for excellent visible-light photocatalytic activity. Appl. Catal. B Environ. 2018, 224, 1–9. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.Y.; Li, Y.; Shuai, D.M. Visible-light-driven photocatalytic disinfection of human adenovirus by a novel heterostructure of oxygen-doped graphitic carbon nitride and hydrothermal carbonation carbon. Appl. Catal. B Environ. 2019, 248, 11–21. [Google Scholar] [CrossRef]
- Wang, Y.X.; Wang, H.; Chen, F.Y.; Cao, F.; Zhao, X.H.; Meng, S.G.; Cui, Y.J. Facile synthesis of oxygen doped carbon nitride hollow microsphere for photocatalysis. Appl. Catal. B Environ. 2017, 206, 417–425. [Google Scholar] [CrossRef]
- Mei, R.G.; Ma, L.; An, L.A.; Wang, F.; Xi, J.J.; Sun, H.Y.; Luo, Z.K.; Wu, Q.X. Layered Spongy-like O-Doped g-C3N4: An Efficient Non-Metal Oxygen Reduction Catalyst for Alkaline Fuel Cells. J. Electrochem. Soc. 2017, 164, F354–F363. [Google Scholar] [CrossRef]
- Wang, C.; Fan, H.Q.; Ren, X.H.; Ma, J.W.; Fang, J.W.; Wang, W.J. Hydrothermally Induced Oxygen Doping of Graphitic Carbon Nitride with a Highly Ordered Architecture and Enhanced Photocatalytic Activity. Chemsuschem 2018, 11, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, Y.H.; Hu, S.Z.; Pei, Y.B.; Ma, W.T.; Fan, Z.P. Hydrothermal Synthesis of Band Gap-Tunable Oxygen-Doped g-C3N4 with Outstanding “Two-Channel” Photocatalytic H2O2 Production Ability Assisted by Dissolution-Precipitation Process. Nano 2019, 14. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.J.; Zhao, Y.; Song, H.; Wang, H. Facile synthesis of oxygen doped mesoporous graphitic carbon nitride with high photocatalytic degradation efficiency under simulated solar irradiation. In Colloids and Surfaces a-Physicochemical and Engineering Aspects; Elsevier: Amsterdam, The Netherlands, 2019; Volume 580. [Google Scholar] [CrossRef]
- Zhang, J.W.; Gong, S.; Mahmood, N.; Pan, L.; Zhang, X.W.; Zou, J.J. Oxygen-doped nanoporous carbon nitride via water-based homogeneous supramolecular assembly for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 221, 9–16. [Google Scholar] [CrossRef]
- Zhu, K.; Ouyang, J.; Liu, J.M.; Zhu, Y.X.; Zeng, Q.; Cui, Y.J. Preparation and Photocatalytic Hydrogen Evolution from Water of Oxygen Doped Carbon Nitride Nanosheets. Chin. J. Inorg. Chem. 2019, 35, 1005–1012. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Z.H.; Gao, Y.W.; Hu, C. General synthesis of carbon and oxygen dual-doped graphitic carbon nitride via copolymerization for non-photochemical oxidation of organic pollutant. J. Hazard. Mater. 2020, 394. [Google Scholar] [CrossRef]
- Tang, R.; Ding, R.L.; Xie, X.C. Preparation of oxygen-doped graphitic carbon nitride and its visible-light photocatalytic performance on bisphenol A degradation. Water Sci. Technol. 2018, 78, 1023–1033. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.X.; Rao, L.; Wang, P.F.; Guo, Y.; Guo, X.; Zhang, L.X. Porous oxygen-doped carbon nitride: Supramolecular preassembly technology and photocatalytic degradation of organic pollutants under low-intensity light irradiation. Environ. Sci. Pollut. Res. 2019, 26, 15710–15723. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Gu, P.C.; Ma, R.; Wen, T.; Zhao, G.X.; Li, L.; Ai, Y.J.; Hu, C.; Wang, X.K. Enhanced photodegradation of toxic organic pollutants using dual-oxygen-doped porous g-C3N4: Mechanism exploration from both experimental and DFT studies. Appl. Catal. B Environ. 2019, 248, 1–10. [Google Scholar] [CrossRef]
- Song, P.; Liang, S.H.; Cui, J.; Ren, D.; Duan, R.Y.; Yang, Q.; Sun, S.D. Purposefully designing novel hydroxylated and carbonylated melamine towards the synthesis of targeted porous oxygen-doped g-C3N4 nanosheets for highly enhanced photocatalytic hydrogen production. Catal. Sci. Technol. 2019, 9, 5150–5159. [Google Scholar] [CrossRef]
- Fu, J.W.; Zhu, B.C.; Jiang, C.J.; Cheng, B.; You, W.; Yu, J.G. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Wang, J.J.; Gillan, E.G. Rapid, facile synthesis of nitrogen-rich carbon nitride powders. J. Mater. Chem. 2002, 12, 2463–2469. [Google Scholar] [CrossRef]
- Miller, D.R.; Swenson, D.C.; Gillan, E.G. Synthesis and structure of 2,5,8-triazido-s-heptazine: An energetic and luminescent precursor to nitrogen-rich carbon nitrides. J. Am. Chem. Soc. 2004, 126, 5372–5373. [Google Scholar] [CrossRef]
- Miller, D.R.; Holst, J.R.; Gillan, E.G. Nitrogen-rich carbon nitride network materials via the thermal decomposition of 2,5,8-triazido-s-heptazine. Inorg. Chem. 2007, 46, 2767–2774. [Google Scholar] [CrossRef]
- Huynh, M.H.V.; Hiskey, M.A.; Archuleta, J.G.; Roemer, E.L.; Gilardi, R. 3,6-di(azido)-1,2,4,5-tetrazine: A precursor for the preparation of carbon nanospheres and nitrogen-rich carbon nitrides. Angew. Chem. Int. Edit. 2004, 43, 5658–5661. [Google Scholar] [CrossRef]
- Gillan, E.G. Synthesis of nitrogen-rich carbon nitride networks from an energetic molecular azide precursor. Chem. Mat. 2000, 12, 3906–3912. [Google Scholar] [CrossRef]
- Fang, J.W.; Fan, H.Q.; Li, M.M.; Long, C.B. Nitrogen self-doped graphitic carbon nitride as efficient visible light photocatalyst for hydrogen evolution. J. Mater. Chem. A 2015, 3, 13819–13826. [Google Scholar] [CrossRef]
- Xu, Q.X.; Xu, G.Q.; Yu, Q.B.; Yang, K.; Li, H.Q. Nitrogen self-doped high specific surface area graphite carbon nitride for photocatalytic degradating of methylene blue. J. Nanopart. Res. 2019, 21, 13. [Google Scholar] [CrossRef]
- Guo, F.; Wang, L.J.; Sun, H.R.; Li, M.Y.; Shi, W.L. High-efficiency photocatalytic water splitting by a N-doped porous g-C3N4 nanosheet polymer photocatalyst derived from urea and N,N-dimethylformamide. Inorg. Chem. Front. 2020, 7, 1770–1779. [Google Scholar] [CrossRef]
- Jiang, L.B.; Yuan, X.Z.; Zeng, G.M.; Liang, J.; Wu, Z.B.; Yu, H.B.; Mo, D.; Wang, H.; Xiao, Z.H.; Zhou, C.Y. Nitrogen self-doped g-C3N4 nanosheets with tunable band structures for enhanced photocatalytic tetracycline degradation. J. Colloid Interface Sci. 2019, 536, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Li, Q.; Zhang, S.; Chen, Z.S.; Wang, W.X.; Zhao, G.X.; Zhuang, L.; Hu, B.W.; Wang, X.K. Visible-light-driven N-2-g-C3N4 as a highly stable and efficient photocatalyst for bisphenol A and Cr(VI) removal in binary systems. Catal. Today 2019, 335, 110–116. [Google Scholar] [CrossRef]
- Dong, S.S.; Liu, C.; Chen, Y.G. Boosting exciton dissociation and molecular oxygen activation by in-plane grafting nitrogen-doped carbon nanosheets to graphitic carbon nitride for enhanced photocatalytic performance. J. Colloid Interface Sci. 2019, 553, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.L.; Liu, Y.N.; Li, C.Y.; Zou, X.H.; Huang, Y.D.; Wang, Y.G. Precursor-reforming protocol to synthesis of porous N-doped g-C3N4 for highly improved photocatalytic water treatments. Mater. Lett. 2020, 264, 4. [Google Scholar] [CrossRef]
- Che, H.N.; Che, G.B.; Zhou, P.J.; Liu, C.B.; Dong, H.J.; Li, C.X.; Song, N.; Li, C.M. Nitrogen doped carbon ribbons modified g-C3N4 for markedly enhanced photocatalytic H2-production in visible to near-infrared region. Chem. Eng. J. 2020, 382, 9. [Google Scholar] [CrossRef]
- Hao, Q.G.; Song, Y.H.; Ji, H.Y.; Mo, Z.; She, X.J.; Deng, J.J.; Muhmood, T.; Wu, X.Y.; Yuan, S.Q.; Xu, H.; et al. Surface N modified 2D g-C3N4 nanosheets derived from DMF for photocatalytic H2 evolution. Appl. Surf. Sci. 2018, 459, 845–852. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, J.G.; Cao, S.W.; Cui, C.; Cheng, B. Efficient photocatalytic reduction of CO2 by amine-functionalized g-C3N4. Appl. Surf. Sci. 2015, 358, 350–355. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, C.J.; Li, P.R.; Chen, B.H.; Zhang, S.S.; Dong, X.P.; Xi, F.N.; Liu, J.Y. Nitrogen-rich graphitic carbon nitride: Controllable nanosheet-like morphology, enhanced visible light absorption and superior photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 257–264. [Google Scholar] [CrossRef]
- Gao, B.R.; Wang, J.; Dou, M.M.; Huang, X.; Yu, X.X. Novel nitrogen-rich g-C3N4 with adjustable energy band by introducing triazole ring for cefotaxime removal. Sep. Purif. Technol. 2020, 241, 9. [Google Scholar] [CrossRef]
- Wu, X.H.; Gao, D.D.; Wang, P.; Yu, H.G.; Yu, J.G. NH4Cl-induced low-temperature formation of nitrogen-rich g-C3N4 nanosheets with improved photocatalytic hydrogen evolution. Carbon 2019, 153, 757–766. [Google Scholar] [CrossRef]
- Mo, Z.; Zhu, X.W.; Jiang, Z.F.; Song, Y.H.; Liu, D.B.; Li, H.P.; Yang, X.F.; She, Y.B.; Lei, Y.C.; Yuan, S.Q.; et al. Porous nitrogen-rich g-C3N4 nanotubes for efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. 2019, 256, 7. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, L.X.; Huang, W.M.; Kong, Q.L.; Fan, X.Q.; Wang, M.; Shi, J.L. N-doped graphitic carbon-incorporated g-C3N4 for remarkably enhanced photocatalytic H2 evolution under visible light. Carbon 2016, 99, 111–117. [Google Scholar] [CrossRef]
- Tian, N.; Zhang, Y.H.; Li, X.W.; Xiao, K.; Du, X.; Dong, F.; Waterhouse, G.I.N.; Zhang, T.R.; Huang, H.W. Precursor-reforming protocol to 3D mesoporous g-C3N4 established by ultrathin self-doped nanosheets for superior hydrogen evolution. Nano Energy 2017, 38, 72–81. [Google Scholar] [CrossRef]
- Wu, M.; Gong, Y.S.; Nie, T.; Zhang, J.; Wang, R.; Wang, H.W.; He, B.B. Template-free synthesis of nanocage-like g-C3N4 with high surface area and nitrogen defects for enhanced photocatalytic H2 activity. J. Mater. Chem. A 2019, 7, 5324–5332. [Google Scholar] [CrossRef]
- Wang, F.L.; Chen, P.; Feng, Y.P.; Xie, Z.J.; Liu, Y.; Su, Y.H.; Zhang, Q.X.; Wang, Y.F.; Yao, K.; Lv, W.Y.; et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ. 2017, 207, 103–113. [Google Scholar] [CrossRef]
- Asadzadeh-Khaneghah, S.; Habibi-Yangjeh, A.; Nakata, K. Decoration of carbon dots over hydrogen peroxide treated graphitic carbon nitride: Exceptional photocatalytic performance in removal of different contaminants under visible light. J. Photochem. Photobiol. A Chem. 2019, 374, 161–172. [Google Scholar] [CrossRef]
- Deng, P.H.; Li, H.Y.; Wang, Z.D.; Hou, Y. Enhanced photocatalytic hydrogen evolution by carbon-doped carbon nitride synthesized via the assistance of cellulose. Appl. Surf. Sci. 2020, 504. [Google Scholar] [CrossRef]
- Duan, Y.; Deng, L.; Shi, Z.; Liu, X.; Zeng, H.X.; Zhang, H.J.; Crittenden, J. Efficient sulfadiazine degradation via in-situ epitaxial grow of Graphitic Carbon Nitride (g-C3N4) on carbon dots heterostructures under visible light irradiation: Synthesis, mechanisms and toxicity evaluation. J. Colloid Interface Sci. 2020, 561, 696–707. [Google Scholar] [CrossRef]
- Jeong, T.; Piao, H.; Park, S.; Yang, J.H.; Choi, G.; Wu, Q.; Kang, H.; Woo, H.J.; Jung, S.J.; Kim, H.; et al. Atomic and electronic structures of graphene-decorated graphitic carbon nitride (g-C3N4) as a metal-free photocatalyst under visible-light. Appl. Catal. B Environ. 2019, 256, 7. [Google Scholar] [CrossRef]
- Cao, J.S.; Fan, H.Q.; Wang, C.; Ma, J.W.; Dong, G.Z.; Zhang, M.C. Facile synthesis of carbon self-doped g-C3N4 for enhanced photocatalytic hydrogen evolution. Ceram. Int. 2020, 46, 7888–7895. [Google Scholar] [CrossRef]
- Chatzoudis, A.; Giannopoulos, V.; Hollmann, F.; Smonou, I. Surface-Doped Graphitic Carbon Nitride Catalyzed Photooxidation of Olefins and Dienes: Chemical Evidence for Electron Transfer and Singlet Oxygen Mechanisms. Catalysts 2019, 9, 639. [Google Scholar] [CrossRef] [Green Version]
- Ge, F.Y.; Xu, Y.G.; Zhou, Y.H.; Tian, D.; Huang, S.Q.; Xie, M.; Xu, H.; Li, H.M. Surface amorphous carbon doping of carbon nitride for efficient acceleration of electron transfer to boost photocatalytic activities. Appl. Surf. Sci. 2020, 507. [Google Scholar] [CrossRef]
- Li, Z.H.; Huang, D.L.; Zhou, C.Y.; Xue, W.J.; Lei, L.; Deng, R.; Yang, Y.; Chen, S.; Wang, W.J.; Wang, Z.W. Metal-free carbon nitride with boosting photo-redox ability realized by the controlled carbon dopants. Chem. Eng. J. 2020, 382. [Google Scholar] [CrossRef]
- Liu, H.P.; Liang, J.; Fu, S.; Li, L.; Cui, J.H.; Gao, P.H.; Zhao, F.Y.; Zhou, J.G. N doped carbon quantum dots modified defect-rich g-C3N4 for enhanced photocatalytic combined pollutions degradation and hydrogen evolution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 13. [Google Scholar] [CrossRef]
- Long, D.; Chen, W.L.; Rao, X.; Zheng, S.H.; Zhang, Y.P. Synergetic effect of C-60/g-C3N4 nanowire composites for enhanced photocatalytic H2 evolution under visible light irradiation. ChemCatChem 2020, 12, 2022–2031. [Google Scholar] [CrossRef]
- Meng, L.R.; Yin, W.H.; Wang, S.S.; Wu, X.G.; Hou, J.H.; Yin, W.Q.; Feng, K.; Ok, Y.S.; Wang, X.Z. Photocatalytic behavior of biochar-modified carbon nitride with enriched visible-light reactivity. Chemosphere 2020, 239. [Google Scholar] [CrossRef]
- Wang, X.F.; Cheng, J.J.; Yu, H.G.; Yu, J.G. A facile hydrothermal synthesis of carbon dots modified g-C3N4 for enhanced photocatalytic H2-evolution performance. Dalton Trans. 2017, 46, 6417–6424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Cai, H.Y.; Qian, F.F.; Li, Y.M.; Yu, J.Q.; Yang, X.L.; Bao, M.T.; Li, X.M. Facile one-step synthesis of onion-like carbon modified ultrathin g-C3N4 2D nanosheets with enhanced visible-light photocatalytic performance. J. Colloid Interface Sci. 2019, 533, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Li, D.; Fang, Z.Y.; Chen, R.J.; Luo, B.F.; Shi, W.D. Controlling carbon self-doping site of g-C3N4 for highly enhanced visible-light-driven hydrogen evolution. Appl. Catal. B Environ. 2019, 254, 128–134. [Google Scholar] [CrossRef]
- Li, X.L.; Bai, J.F.; Li, J.Q.; Li, C.; Zhong, X.Y.; Deng, S.P. The effect of n-pi* electronic transitions on the N-2 photofixation ability of carbon self-doped honeycomb-like g-C3N4 prepared via microwave treatment. RSC Adv. 2020, 10, 7019–7025. [Google Scholar] [CrossRef] [Green Version]
- Ran, M.X.; Li, J.R.; Cui, W.; Li, Y.H.; Li, P.D.; Dong, F. Efficient and stable photocatalytic NO removal on C self-doped g-C3N4: Electronic structure and reaction mechanism. Catal. Sci. Technol. 2018, 8, 3387–3394. [Google Scholar] [CrossRef]
- Xu, Q.L.; Jiang, C.J.; Cheng, B.; Yu, J.G. Enhanced visible-light photocatalytic H2-generation activity of carbon/g-C3N4 nanocomposites prepared by two-step thermal treatment. Dalton Trans. 2017, 46, 10611–10619. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, J.; Wang, Z.P.; Zhu, Y.F. Enhanced visible-light photocatalytic degradation and disinfection performance of oxidized nanoporous g-C3N4 via decoration with graphene oxide quantum dots. Chin. J. Catal. 2020, 41, 474–484. [Google Scholar] [CrossRef]
- Bao, N.; Hu, X.D.; Zhang, Q.Z.; Miao, X.H.; Jie, X.Y.; Zhou, S. Synthesis of porous carbon-doped g-C3N4 nanosheets with enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2017, 403, 682–690. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, T.T.; Yu, X.; Wu, Q.L.; Zhu, Q.H.; Zhang, L.Z.; Li, J.H.; Fang, W.P.; Yi, X.D. Gradual carbon doping of graphitic carbon nitride towards metal-free visible light photocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 15310–15319. [Google Scholar] [CrossRef]
- Luo, L.; Ma, J.N.; Zhu, H.X.; Tang, J.W. Embedded carbon in a carbon nitride hollow sphere for enhanced charge separation and photocatalytic water splitting. Nanoscale 2020, 12, 7339–7346. [Google Scholar] [CrossRef] [Green Version]
- Li, J.X.; Ma, W.Q.; Chen, J.J.; An, N.; Zhao, Y.; Wang, D.J.; Mao, Z.Y. Carbon vacancies improved photocatalytic hydrogen generation of g-C3N4 photocatalyst via magnesium vapor etching. Int. J. Hydrogen Energy 2020, 45, 13939–13946. [Google Scholar] [CrossRef]
- Ma, L.; Hu, S.Z.; Li, P.; Wang, Q.; Ma, H.F.; Li, W. In situ synthesis of sulfur doped carbon nitride with enhanced photocatalytic performance using DBD plasma treatment under H2S atmosphere. J. Phys. Chem. Solids 2018, 118, 166–171. [Google Scholar] [CrossRef]
- Lv, H.Q.; Huang, Y.; Koodali, R.T.; Liu, G.M.; Zeng, Y.B.; Meng, Q.G.; Yuan, M.Z. Synthesis of Sulfur-Doped 2D Graphitic Carbon Nitride Nanosheets for Efficient Photocatalytic Degradation of Phenol and Hydrogen Evolution. ACS Appl. Mater. Interfaces 2020, 12, 12656–12667. [Google Scholar] [CrossRef]
- Wang, W.J.; Zeng, Z.T.; Zeng, G.M.; Zhang, C.; Xiao, R.; Zhou, C.Y.; Xiong, W.P.; Yang, Y.; Lei, L.; Liu, Y.; et al. Sulfur doped carbon quantum dots loaded hollow tubular g-C3N4 as novel photocatalyst for destruction of Escherichia coli and tetracycline degradation under visible light. Chem. Eng. J. 2019, 378, 11. [Google Scholar] [CrossRef]
- Joseph, S.; Abraham, S.; Priyanka, R.N.; Abraham, T.; Suresh, A.; Mathew, B. In situ S-doped ultrathin gC(3)N(4) nanosheets coupled with mixed-dimensional (3D/1D) nanostructures of silver vanadates for enhanced photocatalytic degradation of organic pollutants. New J. Chem. 2019, 43, 10618–10630. [Google Scholar] [CrossRef]
- Sakthivel, A.; Chandrasekaran, A.; Jayakumar, S.; Manickam, P.; Alwarappan, S. Sulphur Doped Graphitic Carbon Nitride as an Efficient Electrochemical Platform for the Detection of Acetaminophen. J. Electrochem. Soc. 2019, 166, B1461–B1469. [Google Scholar] [CrossRef]
- Fang, Y.X.; Li, X.C.; Wang, Y.; Giordano, C.; Wang, X.C. Gradient sulfur doping along polymeric carbon nitride films as visible light photoanodes for the enhanced water oxidation. Appl. Catal. B Environ. 2020, 268, 6. [Google Scholar] [CrossRef]
- Fan, Q.J.; Liu, J.J.; Yu, Y.C.; Zuo, S.L.; Li, B.S. A simple fabrication for sulfur doped graphitic carbon nitride porous rods with excellent photocatalytic activity degrading RhB dye. Appl. Surf. Sci. 2017, 391, 360–368. [Google Scholar] [CrossRef]
- Lin, Y.R.; Dizon, G.V.C.; Yamada, K.; Liu, C.Y.; Venault, A.; Lin, H.Y.; Yoshida, M.; Hu, C.C. Sulfur-doped g-C3N4 nanosheets for photocatalysis: Z-scheme water splitting and decreased biofouling. J. Colloid Interface Sci. 2020, 567, 202–212. [Google Scholar] [CrossRef]
- Lu, C.H.; Zhang, P.; Jiang, S.J.; Wu, X.; Song, S.Q.; Zhu, M.S.; Lou, Z.Z.; Li, Z.; Liu, F.; Liu, Y.H.; et al. Photocatalytic reduction elimination of UO22+ pollutant under visible light with metal-free sulfur doped g-C3N4 photocatalyst. Appl. Catal. B Environ. 2017, 200, 378–385. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.S.; Cheng, B.; Ho, W.K.; Yu, J.G. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B Environ. 2015, 176, 44–52. [Google Scholar] [CrossRef]
- Praus, P.; Smykalova, A.; Foniok, K.; Velisek, P.; Cvejn, D.; Zadny, J.; Storch, J. Post-Synthetic Derivatization of Graphitic Carbon Nitride with Methanesulfonyl Chloride: Synthesis, Characterization and Photocatalysis. Nanomaterials 2020, 10, 193. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.F.; Lv, W.H.; Bai, J.R.; Zhou, Y.; Wen, Y.P.; He, Q.T.; Tang, J.H.; Wang, L.B.; Zhou, Q.F. Sulfur-doped porous graphitic carbon nitride heterojunction hybrids for enhanced photocatalytic H2 evolution. J. Mater. Sci. 2019, 54, 4811–4820. [Google Scholar] [CrossRef]
- Ke, L.; Li, P.F.; Wu, X.; Jiang, S.J.; Luo, M.B.; Liu, Y.H.; Le, Z.G.; Sun, C.Z.; Song, S.Q. Graphene-like sulfur-doped g-C3N4 for photocatalytic reduction elimination of UO22+ under visible Light. Appl. Catal. B Environ. 2017, 205, 319–326. [Google Scholar] [CrossRef]
- Raziq, F.; Humayun, M.; Ali, A.; Wang, T.T.; Khan, A.; Fu, Q.Y.; Luo, W.; Zeng, H.P.; Zheng, Z.P.; Khan, B.; et al. Synthesis of S-Doped porous g-C3N4 by using ionic liquids and subsequently coupled with Au-TiO2 for exceptional cocatalyst-free visible-light catalytic activities. Appl. Catal. B Environ. 2018, 237, 1082–1090. [Google Scholar] [CrossRef]
- Li, Y.F.; Wang, S.; Chang, W.; Zhang, L.H.; Wu, Z.S.; Song, S.Y.; Xing, Y. Preparation and enhanced photocatalytic performance of sulfur doped terminal-methylated g-C3N4 nanosheets with extended visible-light response. J. Mater. Chem. A 2019, 7, 20640–20648. [Google Scholar] [CrossRef]
- Cui, Y.J.; Li, M.; Wang, H.; Yang, C.F.; Meng, S.G.; Chen, F.Y. In-situ synthesis of sulfur doped carbon nitride microsphere for outstanding visible light photocatalytic Cr (VI) reduction. Sep. Purif. Technol. 2018, 199, 251–259. [Google Scholar] [CrossRef]
- Mohammad, A.; Khan, M.E.; Cho, M.H. Sulfur-doped-graphitic-carbon nitride (S-g-C3N4) for low cost electrochemical sensing of hydrazine. J. Alloy. Compd. 2020, 816, 10. [Google Scholar] [CrossRef]
- Guo, H.; Shu, Z.; Chen, D.H.; Tan, Y.G.; Zhou, J.; Meng, F.Y.; Li, T.T. One-step synthesis of S-doped g-C3N4 nanosheets for improved visible-light photocatalytic hydrogen evolution. Chem. Phys. 2020, 533, 7. [Google Scholar] [CrossRef]
- Xie, L.L.; Dai, Y.R.; Zhou, Y.J.; Chang, X.; Yin, L.F. Sulfur (VI) modified graphite carbon nitride nanosheets with chrysanthemum-like structure and enhanced photocatalytic activity. Chem. Phys. Lett. 2018, 693, 1–7. [Google Scholar] [CrossRef]
- Cao, J.J.; Wang, H.; Zhao, Y.J.; Liu, Y.; Wu, Q.Y.; Huang, H.; Shao, M.W.; Kang, Z.H. Phosphorus-doped porous carbon nitride for efficient sole production of hydrogen peroxide via photocatalytic water splitting with a two-channel pathway. J. Mater. Chem. A 2020, 8, 3701–3707. [Google Scholar] [CrossRef]
- Zhu, Y.K.; Li, J.Z.; Cao, J.M.; Lv, C.X.; Huang, G.Q.; Zhang, G.L.; Xu, Y.; Zhang, S.C.; Meng, P.P.; Zhan, T.R.; et al. Phosphorus-doped polymeric carbon nitride nanosheets for enhanced photocatalytic hydrogen production. APL Mater. 2020, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Chai, B.; Yan, J.T.; Wang, C.L.; Ren, Z.D.; Zhu, Y.C. Enhanced visible light photocatalytic degradation of Rhodamine B over phosphorus doped graphitic carbon nitride. Appl. Surf. Sci. 2017, 391, 376–383. [Google Scholar] [CrossRef]
- Chegeni, M.; Dehghan, N. Preparation of Phosphorus Doped Graphitic Carbon Nitride Using a Simple Method and Its Application for Removing Methylene Blue. Phys. Chem. Res. 2020, 8, 31–44. [Google Scholar] [CrossRef]
- Huang, J.X.; Li, D.G.; Li, R.B.; Zhang, Q.X.; Chen, T.S.; Liu, H.J.; Liu, Y.; Lv, W.Y.; Liu, G.G. An efficient metal-free phosphorus and oxygen co-doped g-C3N4 photocatalyst with enhanced visible light photocatalytic activity for the degradation of fluoroquinolone antibiotics. Chem. Eng. J. 2019, 374, 242–253. [Google Scholar] [CrossRef]
- Huang, J.X.; Li, D.G.; Liu, Y.; Li, R.B.; Chen, P.; Liu, H.J.; Lv, W.Y.; Liu, G.G.; Feng, Y.P. Ultrathin Ag2WO4-coated P-doped g-C3N4 nanosheets with remarkable photocatalytic performance for indomethacin degradation. J. Hazard. Mater. 2020, 392, 13. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, T.; Partheeban, T.; Vivekanantha, M.; Prabu, N.; Kundu, M.; Premkumar, S.; Umapathy, S.; Vinu, A.; Sasidharan, M. Design of P-Doped Mesoporous Carbon Nitrides as High-Performance Anode Materials for Li-Ion Battery. ACS Appl. Mater. Interfaces 2020, 12, 24007–24018. [Google Scholar] [CrossRef]
- Li, J.J.; Tian, C.; Zhao, H.; Mei, J.; Zhang, J.; Yang, S.J. Controllable fabrication of a red phosphorus modified g-C3N4 photocatalyst with strong interfacial binding for the efficient removal of organic pollutants. J. Alloy. Compd. 2019, 810. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis. In Principal Component Analysis; Springer: New York, NY, USA, 1986; pp. 115–128. [Google Scholar] [CrossRef]
- Ran, J.R.; Guo, W.W.; Wang, H.L.; Zhu, B.C.; Yu, J.G.; Qiao, S.Z. Metal-Free 2D/2D Phosphorene/g-C3N4 Van der Waals Heterojunction for Highly Enhanced Visible-Light Photocatalytic H2 Production. Adv. Mater. 2018, 30, 6. [Google Scholar] [CrossRef]
- Su, C.Y.; Zhou, Y.Z.; Zhang, L.L.; Yu, X.H.; Gao, S.; Sun, X.J.; Cheng, C.; Liu, Q.Q.; Yang, J. Enhanced n →pi* electron transition of porous P-doped g-C3N4 nanosheets for improved photocatalytic H2 evolution performance. Ceram. Int. 2020, 46, 8444–8451. [Google Scholar] [CrossRef]
- Sun, Y.J.; He, J.Y.; Zhang, D.; Wang, X.J.; Zhao, J.; Liu, R.H.; Li, F.T. Simultaneous construction of dual-site phosphorus modified g-C3N4 and its synergistic mechanism for enhanced visible-light photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 517, 8. [Google Scholar] [CrossRef]
- Wang, P.Y.; Guo, C.S.; Hou, S.; Zhao, X.; Wu, L.L.; Pei, Y.Y.; Zhang, Y.; Gao, J.F.; Xu, J. Template-free synthesis of bubble-like phosphorus-doped carbon nitride with enhanced visible-light photocatalytic activity. J. Alloy. Compd. 2018, 769, 503–511. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Xie, C.; Cui, H.D.; Wang, Q.Y.; Shu, Z.; Zhou, J.; Li, T.T. Scalable one-pot synthesis of phosphorus-doped g-C3N4 nanosheets for enhanced visible-light photocatalytic hydrogen evolution. Diam. Relat. Mat. 2020, 104, 8. [Google Scholar] [CrossRef]
- Deng, Y.C.; Tang, L.; Zeng, G.M.; Zhu, Z.J.; Yan, M.; Zhou, Y.Y.; Wang, J.J.; Liu, Y.N. Insight into highly efficient simultaneous photocatalytic removal of Cr (VI) and 2,4-diclorophenol under visible light irradiation by phosphorus doped porous ultrathin g-C3N4 nanosheets from aqueous media: Performance and reaction mechanism. Appl. Catal. B Environ. 2017, 203, 343–354. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Ren, T.Z.; Yuan, Z.Y. Mesoporous Phosphorus-Doped g-C3N4 Nanostructured Flowers with Superior Photocatalytic Hydrogen Evolution Performance. ACS Appl. Mater. Interfaces 2015, 7, 16850–16856. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, Z.X.; Li, Y.X.; Peng, S.Q.; Liu, B. The nonmetal modulation of composition and morphology of g-C3N4-based photocatalysts. Appl. Catal. B Environ. 2020, 269, 21. [Google Scholar] [CrossRef]
- Guo, S.E.; Tang, Y.Q.; Xie, Y.; Tian, C.G.; Feng, Q.M.; Zhou, W.; Jiang, B.J. P-doped tubular g-C3N4 with surface carbon defects: Universal synthesis and enhanced visible-light photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 218, 664–671. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, L.X.; Liu, J.J.; Fan, X.Q.; Wang, B.Z.; Wang, M.; Ren, W.C.; Wang, J.; Li, M.L.; Shi, J.L. Brand new P-doped g-C3N4: Enhanced photocatalytic activity for H2 evolution and Rhodamine B degradation under visible light. J. Mater. Chem. A 2015, 3, 3862–3867. [Google Scholar] [CrossRef]
- Li, X.H.; Zhang, J.; Zhou, F.; Zhang, H.L.; Bai, J.; Wang, Y.J.; Wang, H.Y. Preparation of N-vacancy-doped g-C3N4 with outstanding photocatalytic H2O2 production ability by dielectric barrier discharge plasma treatment. Chin. J. Catal. 2018, 39, 1090–1098. [Google Scholar] [CrossRef]
- Li, H.L.; Jin, C.; Wang, Z.Y.; Liu, Y.Y.; Wang, P.; Zheng, Z.K.; Whangbo, M.H.; Kou, L.Z.; Li, Y.J.; Dai, Y.; et al. Effect of the intra- and inter-triazine N-vacancies on the photocatalytic hydrogen evolution of graphitic carbon nitride. Chem. Eng. J. 2019, 369, 263–271. [Google Scholar] [CrossRef]
- Peng, G.M.; Wu, J.W.; Wang, M.Z.; Niklas, J.; Zhou, H.; Liu, C. Nitrogen-Defective Polymeric Carbon Nitride Nanolayer Enabled Efficient Electrocatalytic Nitrogen Reduction with High Faradaic Efficiency. Nano Lett. 2020, 20, 2879–2885. [Google Scholar] [CrossRef]
- Li, X.X.; Tan, T.N.; Zhang, J.; Zhang, N.; He, J.W.; Han, W.M.; Wang, Y.D.; Pan, M. Nitrogen Deficient Graphitic Carbon Nitride as Anodes for Lithium-ion Batteries. J. Wuhan Univ. Technol. Mat. Sci. Edit. 2020, 35, 263–271. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Y.X.; Huang, Z.H.; Zhang, J.Y.; Jia, X.F.; Wang, X.S.; Ye, J.H. Two types of cooperative nitrogen vacancies in polymeric carbon nitride for efficient solar-driven H2O2 evolution. Appl. Catal. B Environ. 2020, 265, 7. [Google Scholar] [CrossRef]
- Wang, J.; Gao, B.; Dou, M.M.; Huang, X.; Ma, Z.K. A porous g-C3N4 nanosheets containing nitrogen defects for enhanced photocatalytic removal meropenem: Mechanism, degradation pathway and DFT calculation. Environ. Res. 2020, 184, 10. [Google Scholar] [CrossRef]
- Wang, Y.X.; Rao, L.; Wang, P.F.; Guo, Y.; Shi, Z.Y.; Guo, X.; Zhang, L.X. Synthesis of nitrogen vacancies g-C3N4 with increased crystallinity under the controlling of oxalyl dihydrazide: Visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2020, 505, 10. [Google Scholar] [CrossRef]
- Yu, W.W.; Shan, X.; Zhao, Z.K. Unique nitrogen-deficient carbon nitride homojunction prepared by a facile inserting-removing strategy as an efficient photocatalyst for visible light-driven hydrogen evolution. Appl. Catal. B Environ. 2020, 269, 9. [Google Scholar] [CrossRef]
- Li, X.X.; Zhang, K.L.; Zhou, M.; Yang, K.; Yang, S.; Ma, X.H.; Yu, C.L.; Xie, Y.; Huang, W.Y.; Fan, Q.Z. A Novel Approach to Synthesize Nitrogen-Deficient g-C3N4 for the Enhanced Photocatalytic Contaminant Degradation and Electrocatalytic Hydrogen Evolution. Nano 2020, 15, 13. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.X.; Chu, D.L.; Jia, G.R.; Yao, M.G.; Liu, B.B. Significantly narrowed bandgap and enhanced charge separation in porous, nitrogen-vacancy red g-C3N4 for visible light photocatalytic H2 production. Appl. Surf. Sci. 2020, 504, 8. [Google Scholar] [CrossRef]
- Huang, J.J.; Du, J.M.; Du, H.W.; Xu, G.S.; Yuan, Y.P. Control of Nitrogen Vacancy in g-C3N4 by Heat Treatment in an Ammonia Atmosphere for Enhanced Photocatalytic Hydrogen Generation. Acta Phys. Chim. Sin. 2020, 36, 8. [Google Scholar] [CrossRef]
- Li, W.S.; Guo, Z.; Jiang, L.T.; Zhong, L.; Li, G.N.; Zhang, J.J.; Fan, K.; Gonzalez-Cortes, S.; Jin, K.J.; Xu, C.J.; et al. Facile in situ reductive synthesis of both nitrogen deficient and protonated g-C3N4 nanosheets for the synergistic enhancement of visible-light H2 evolution. Chem. Sci. 2020, 11, 2716–2728. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Di, J.; Ding, P.H.; Zhao, J.Z.; Gu, K.Z.; Chen, X.L.; Yan, C.; Yin, S.; Xia, J.X.; Li, H.M. Ultrathin g-C3N4 with enriched surface carbon vacancies enables highly efficient photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2019, 553, 530–539. [Google Scholar] [CrossRef]
- Li, Y.H.; Gu, M.L.; Shi, T.; Cui, W.; Zhang, X.M.; Dong, F.; Cheng, J.S.; Fan, J.J.; Lv, K.L. Carbon vacancy in C3N4 nanotube: Electronic structure, photocatalysis mechanism and highly enhanced activity. Appl. Catal. B Environ. 2020, 262, 11. [Google Scholar] [CrossRef]
- Li, S.N.; Dong, G.H.; Hailili, R.; Yang, L.P.; Li, Y.X.; Wang, F.; Zeng, Y.B.; Wang, C.Y. Effective photocatalytic H2O2 production under visible light irradiation at g-C3N4 modulated by carbon vacancies. Appl. Catal. B Environ. 2016, 190, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Barrio, J.; Volokh, M.; Shalom, M. Polymeric carbon nitrides and related metal-free materials for energy and environmental applications. J. Mater. Chem. A 2020, 8, 11075–11116. [Google Scholar] [CrossRef]
- Liu, X.L.; Ma, R.; Zhuang, L.; Hu, B.W.; Chen, J.R.; Liu, X.Y.; Wang, X.K. Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants. Crit. Rev. Environ. Sci. Technol. 2020, 40. [Google Scholar] [CrossRef]
- Mun, S.J.; Park, S.J. Graphitic Carbon Nitride Materials for Photocatalytic Hydrogen Production via Water Splitting: A Short Review. Catalysts 2019, 9, 805. [Google Scholar] [CrossRef] [Green Version]
- Talapaneni, S.N.; Singh, G.; Kim, I.Y.; AlBahily, K.; Al-Muhtaseb, A.H.; Karakoti, A.S.; Tavakkoli, E.; Vinu, A. Nanostructured Carbon Nitrides for CO2 Capture and Conversion. Adv. Mater. 2020, 32, 21. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Shen, S.H. Progress and Prospects of Non-Metal Doped Graphitic Carbon Nitride for Improved Photocatalytic Performances. Acta Phys. Chim. Sin. 2020, 36, 14. [Google Scholar] [CrossRef]
- Zhu, A.H.; Qiu, B.C.; Du, M.M.; Ji, J.H.; Nasir, M.; Xing, M.Y.; Zhang, J.L. Dopant-Induced Edge and Basal Plane Catalytic Sites on Ultrathin C3N4 Nanosheets for Photocatalytic Water Reduction. Acs Sustain. Chem. Eng. 2020, 8, 7497–7502. [Google Scholar] [CrossRef]
- Wanninayake, N.; Ai, Q.; Zhou, R.; Hoque, M.A.; Herrell, S.; Guzman, M.I.; Risko, C.; Kim, D.Y. Understanding the effect of host structure of nitrogen doped ultrananocrystalline diamond electrode on electrochemical carbon dioxide reduction. Carbon 2020, 157, 408–419. [Google Scholar] [CrossRef]
- Zhao, H.L.; Liu, L.J.; Andino, J.M.; Li, Y. Bicrystalline TiO2 with controllable anatase-brookite phase content for enhanced CO2 photoreduction to fuels. J. Mater. Chem. A 2013, 1, 8209–8216. [Google Scholar] [CrossRef] [Green Version]
- Hoque, M.A.; Guzman, M.I. Photocatalytic Activity: Experimental Features to Report in Heterogeneous Photocatalysis. Materials 2018, 11, 1990. [Google Scholar] [CrossRef] [Green Version]
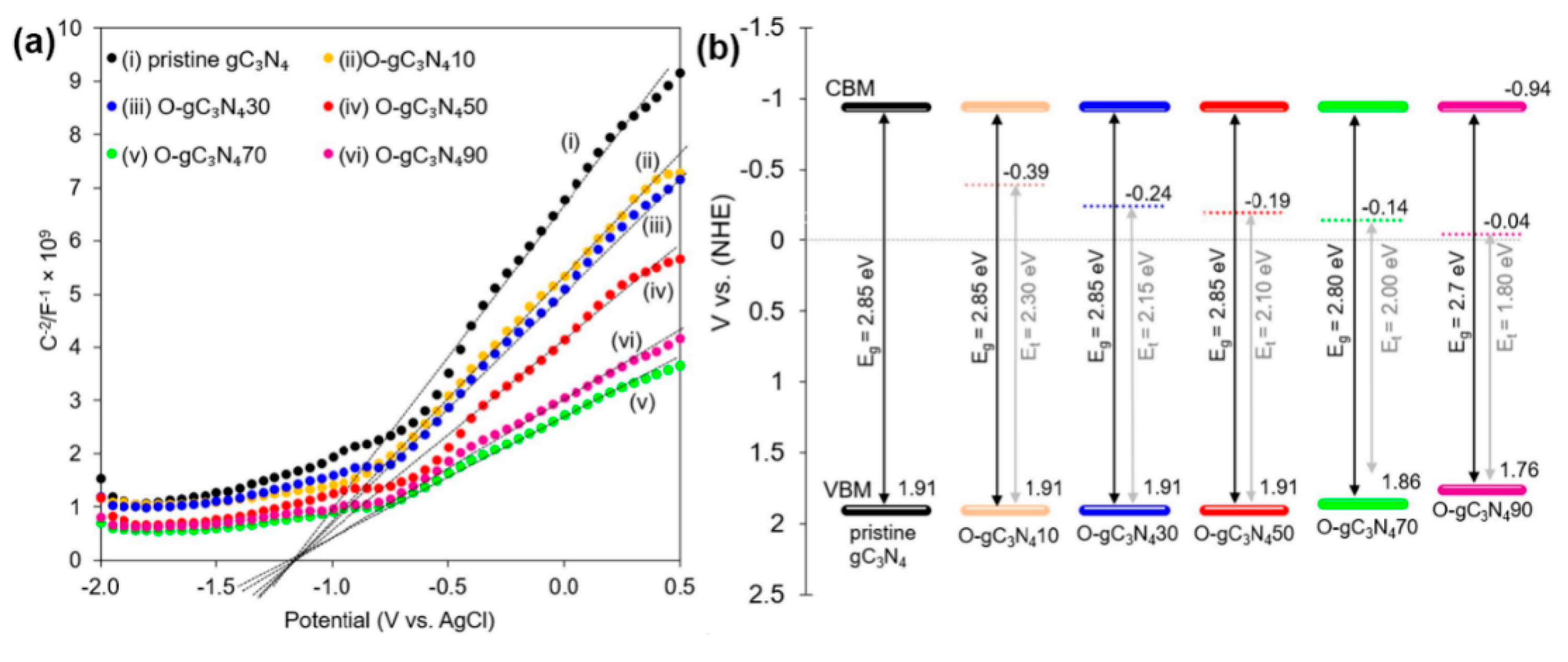
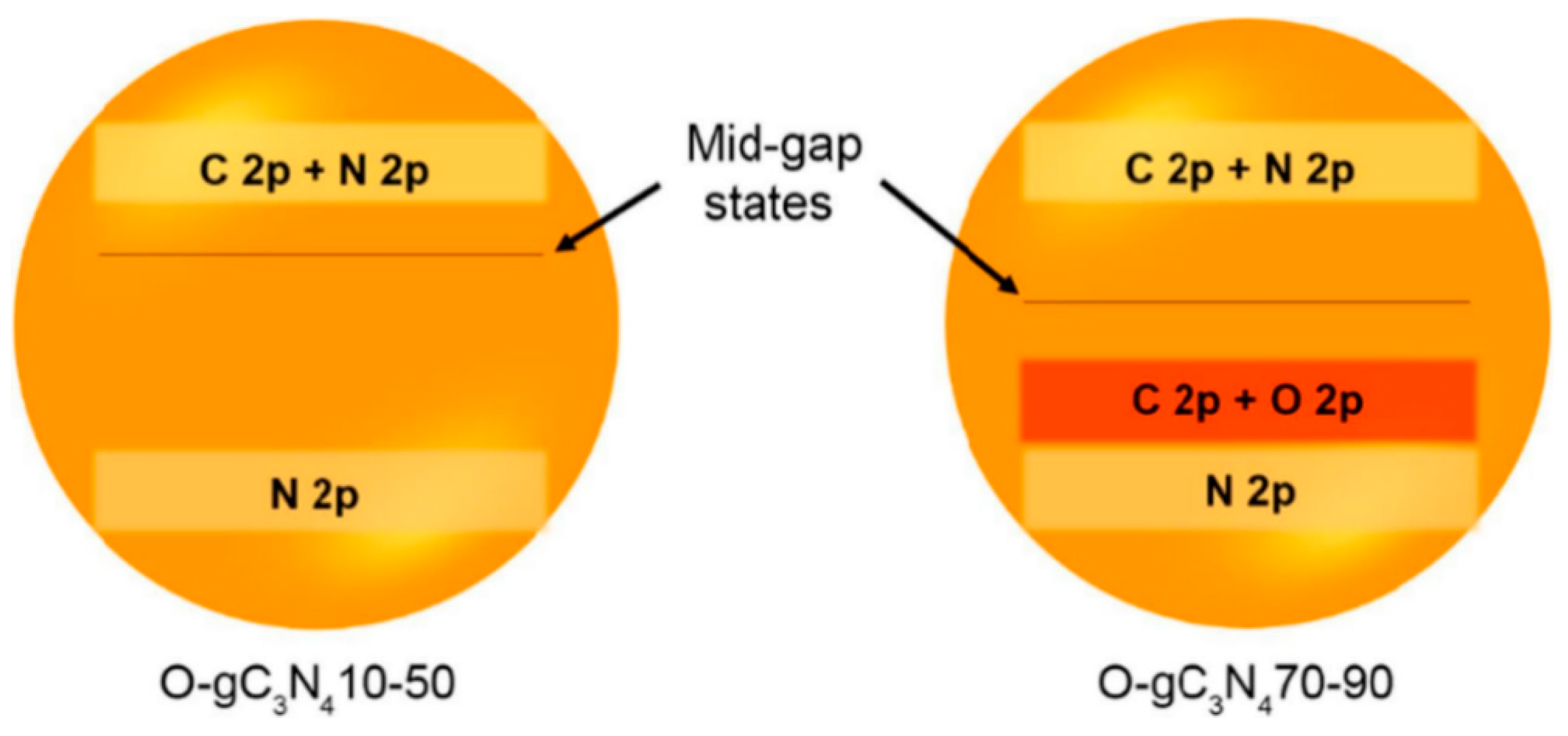
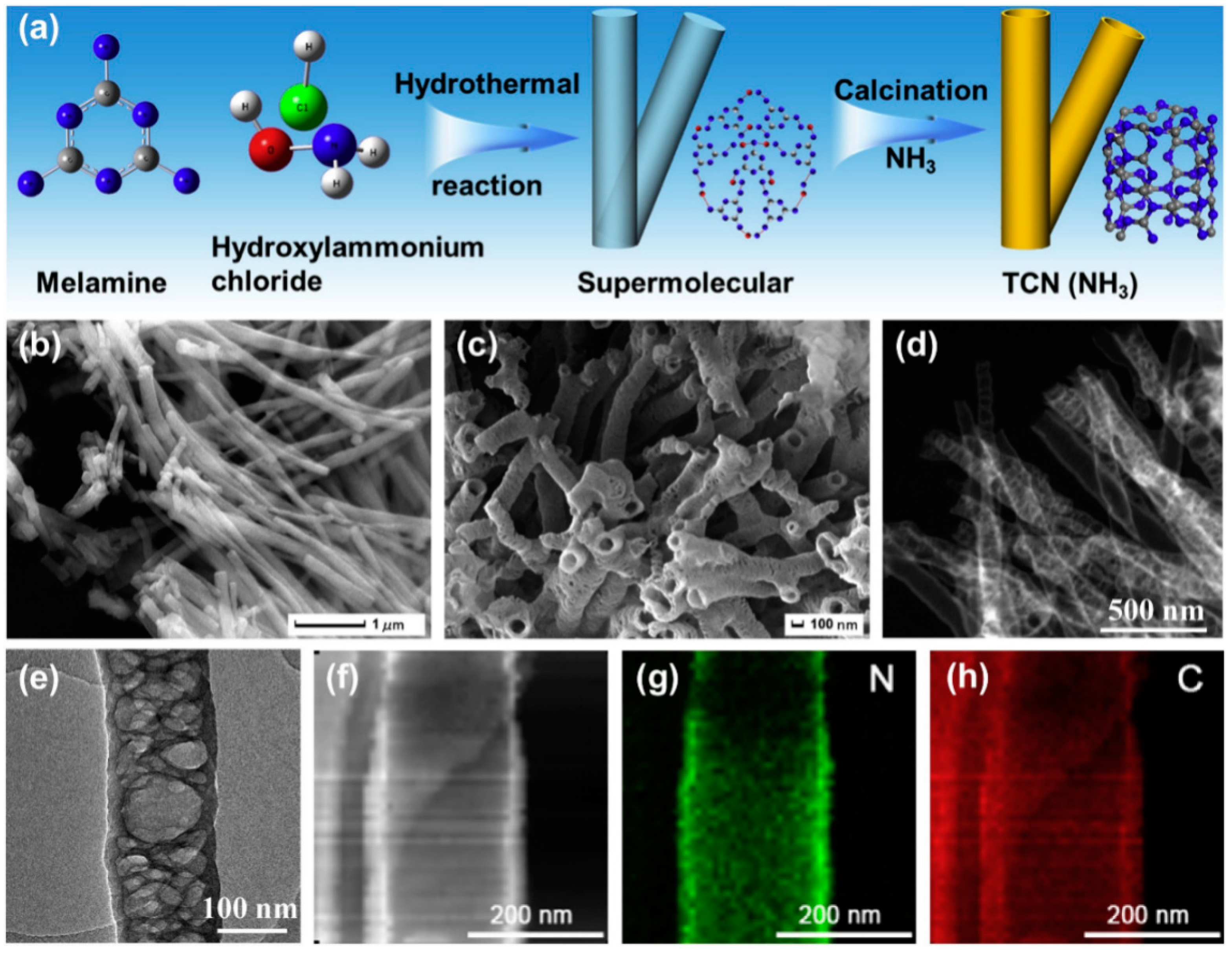

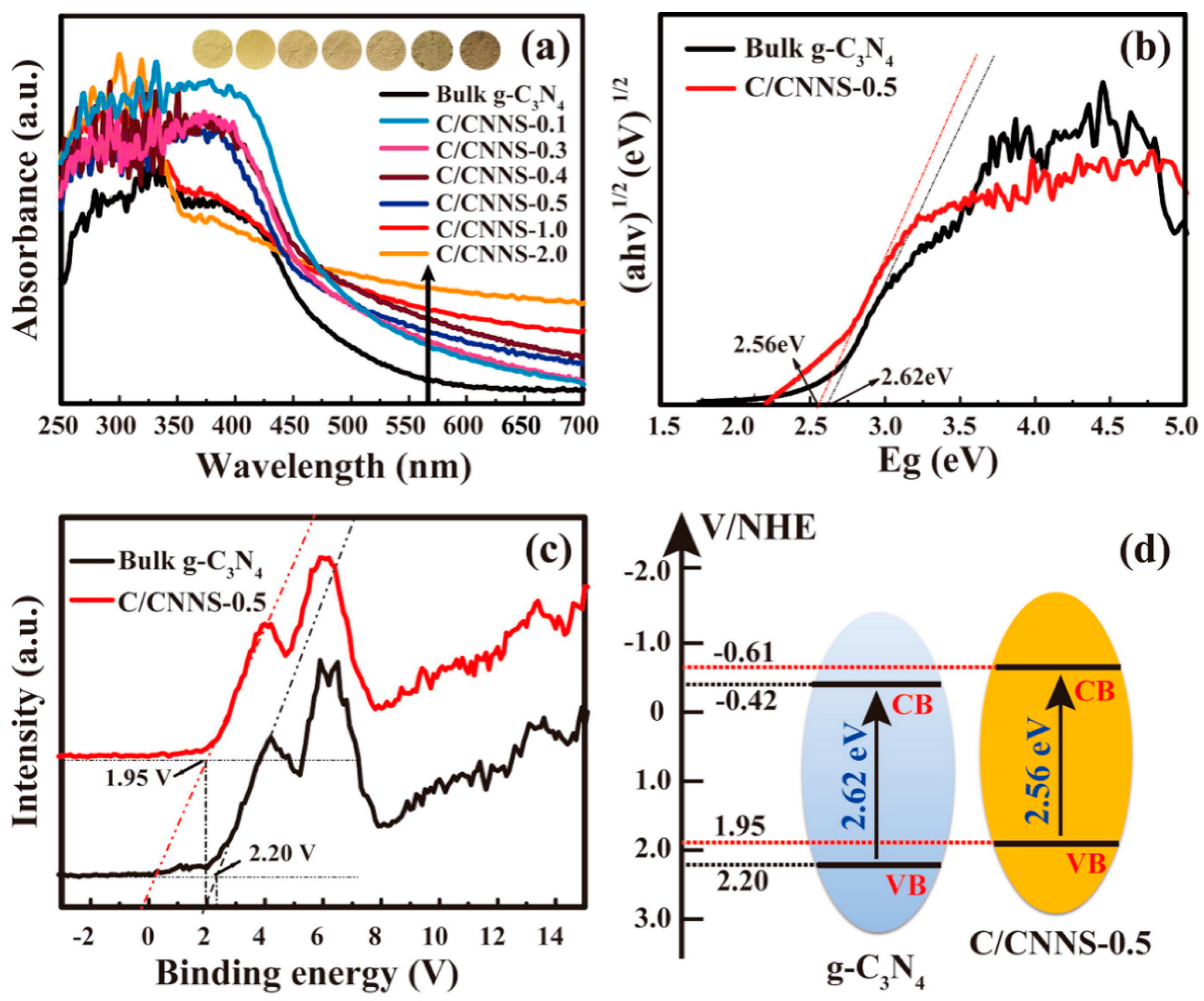
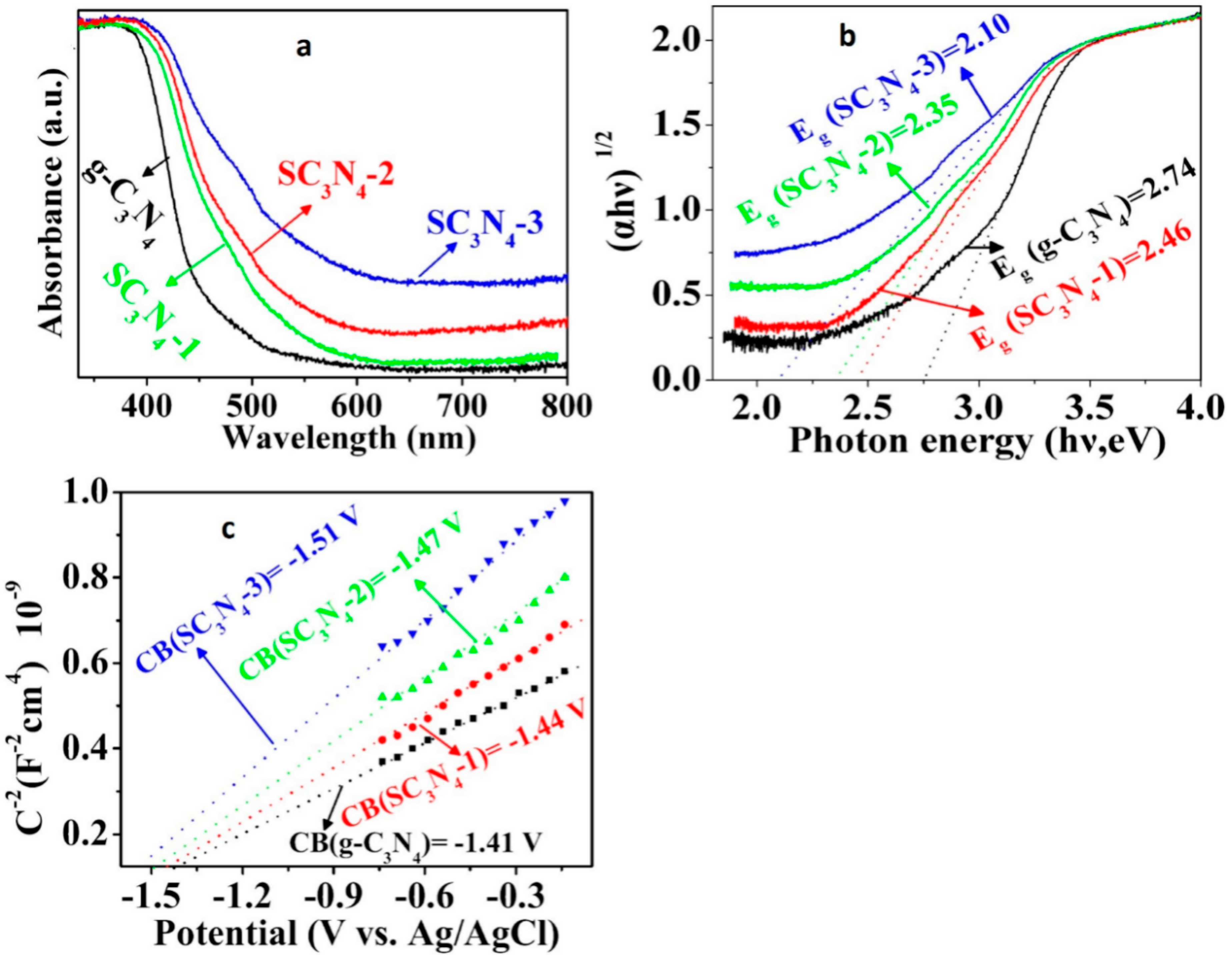

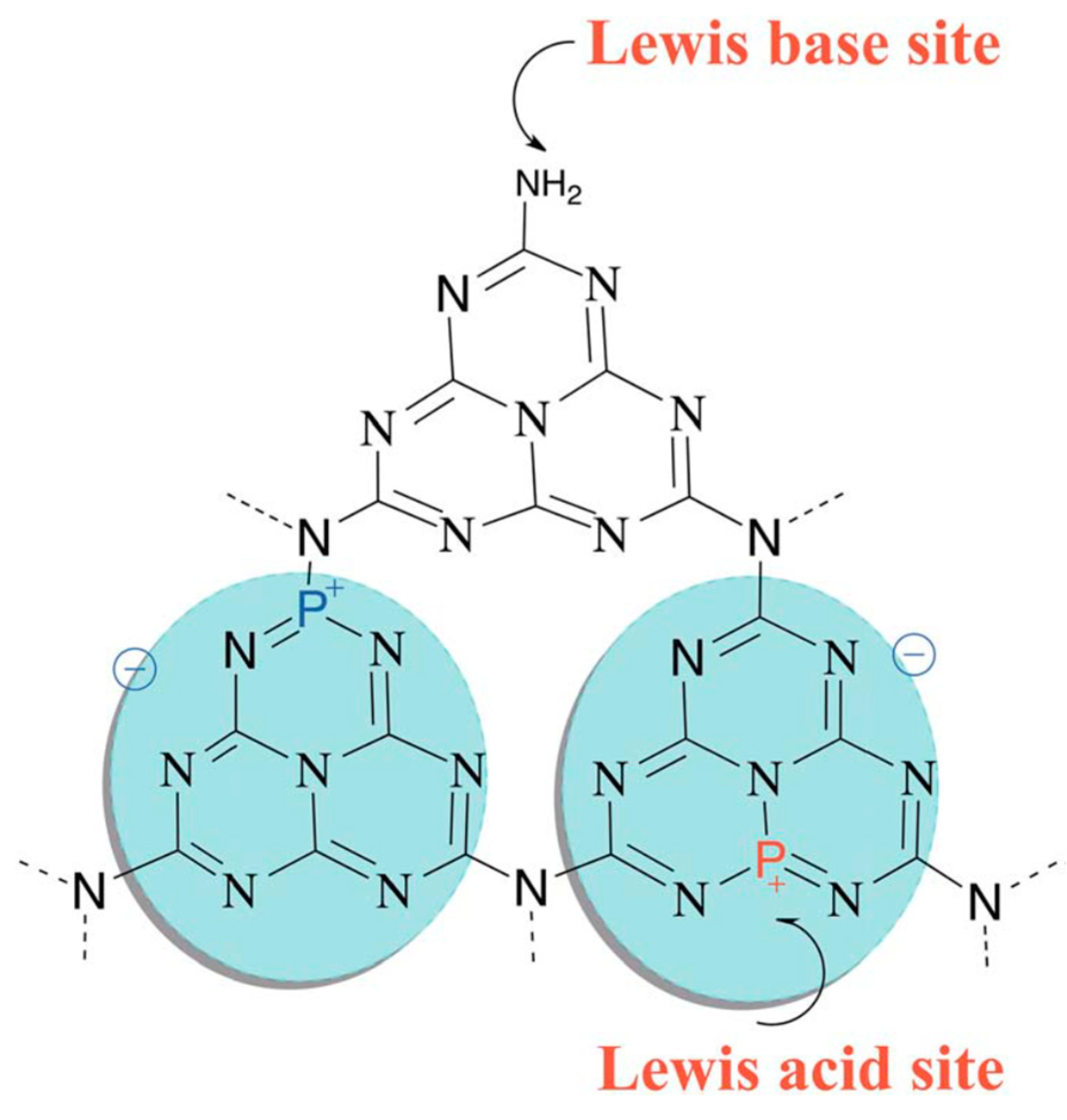
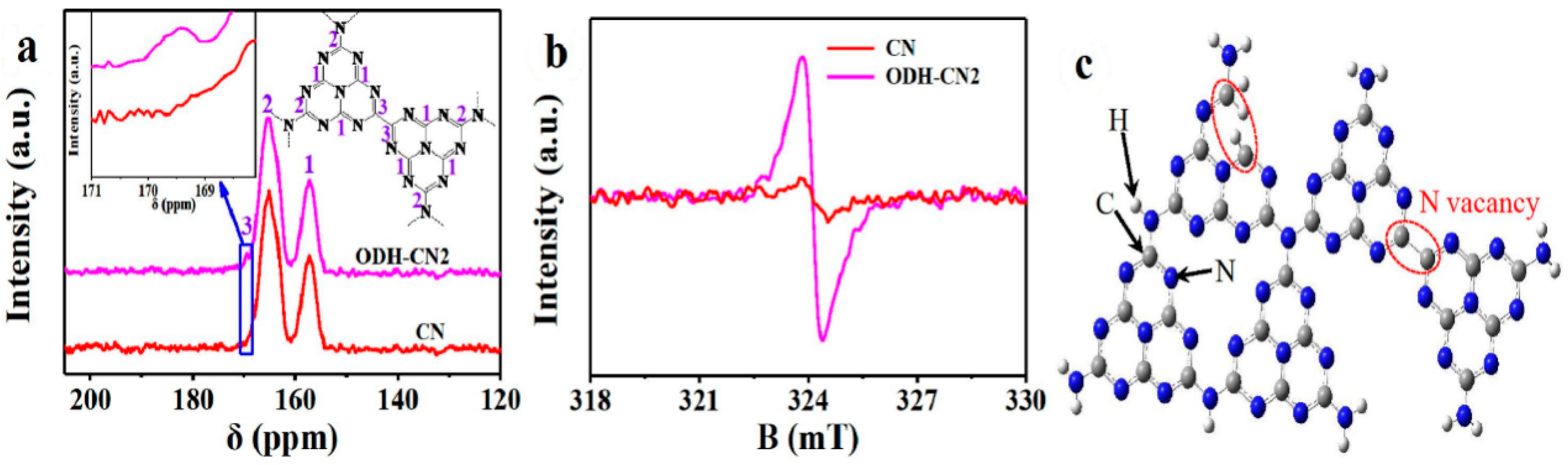
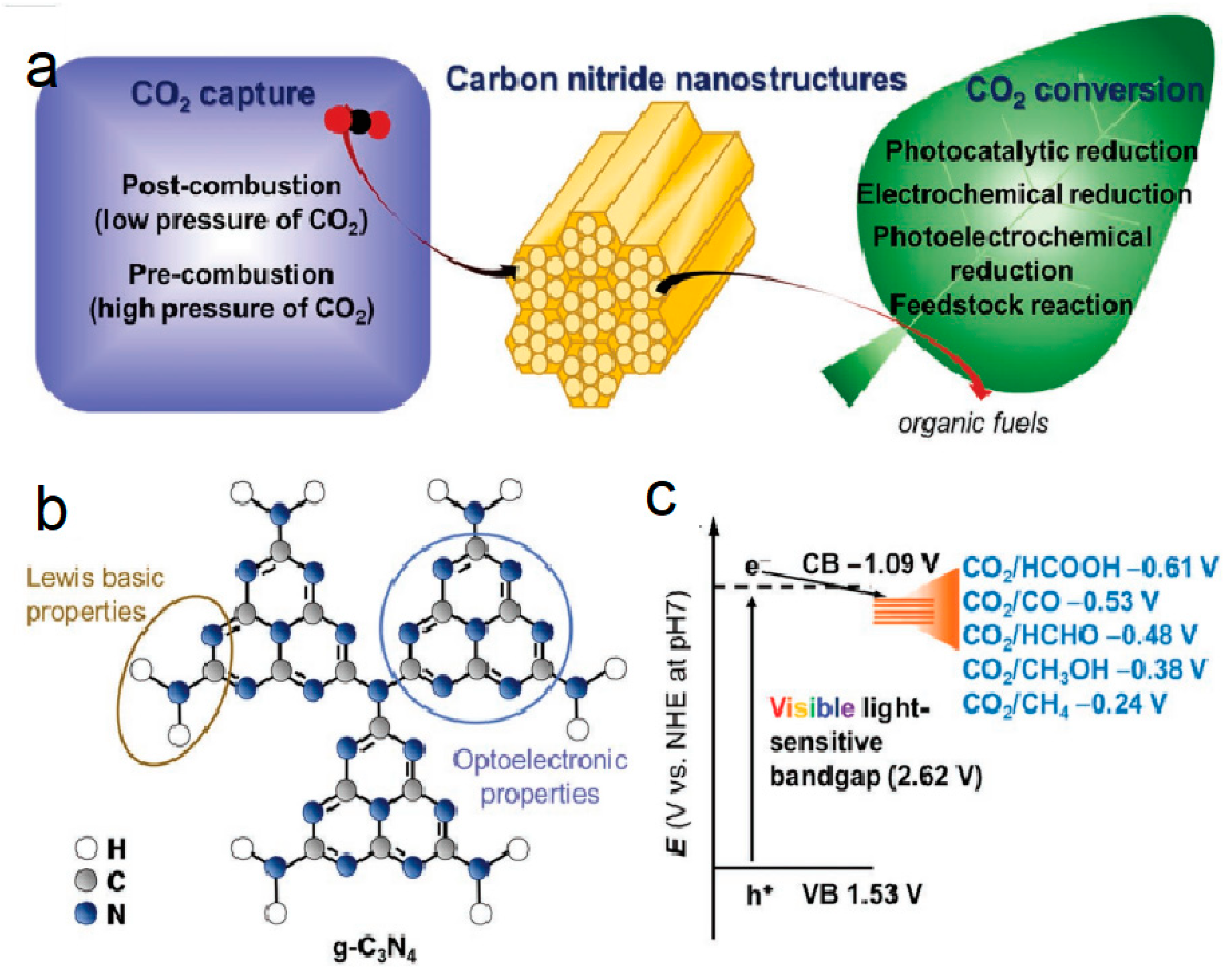
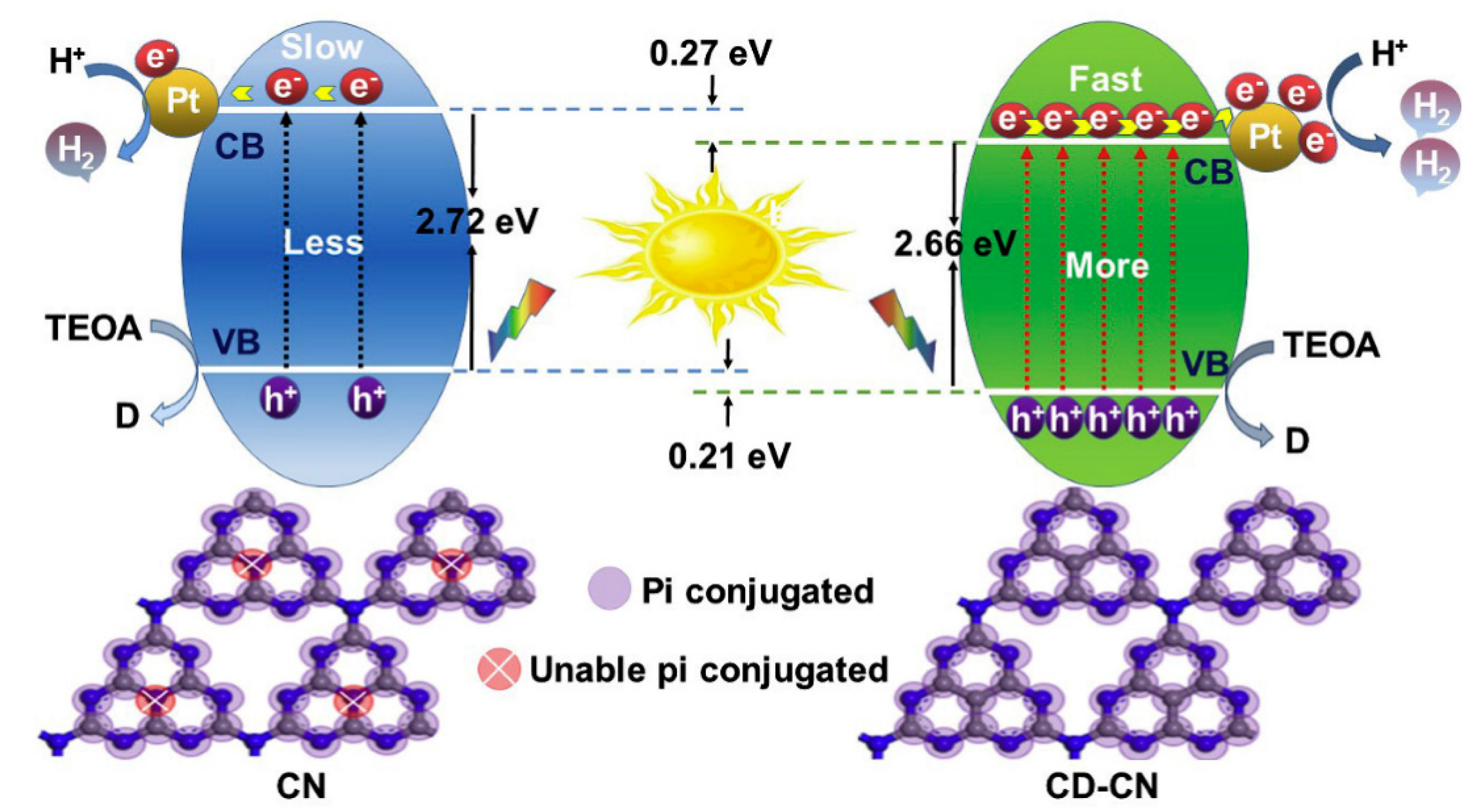
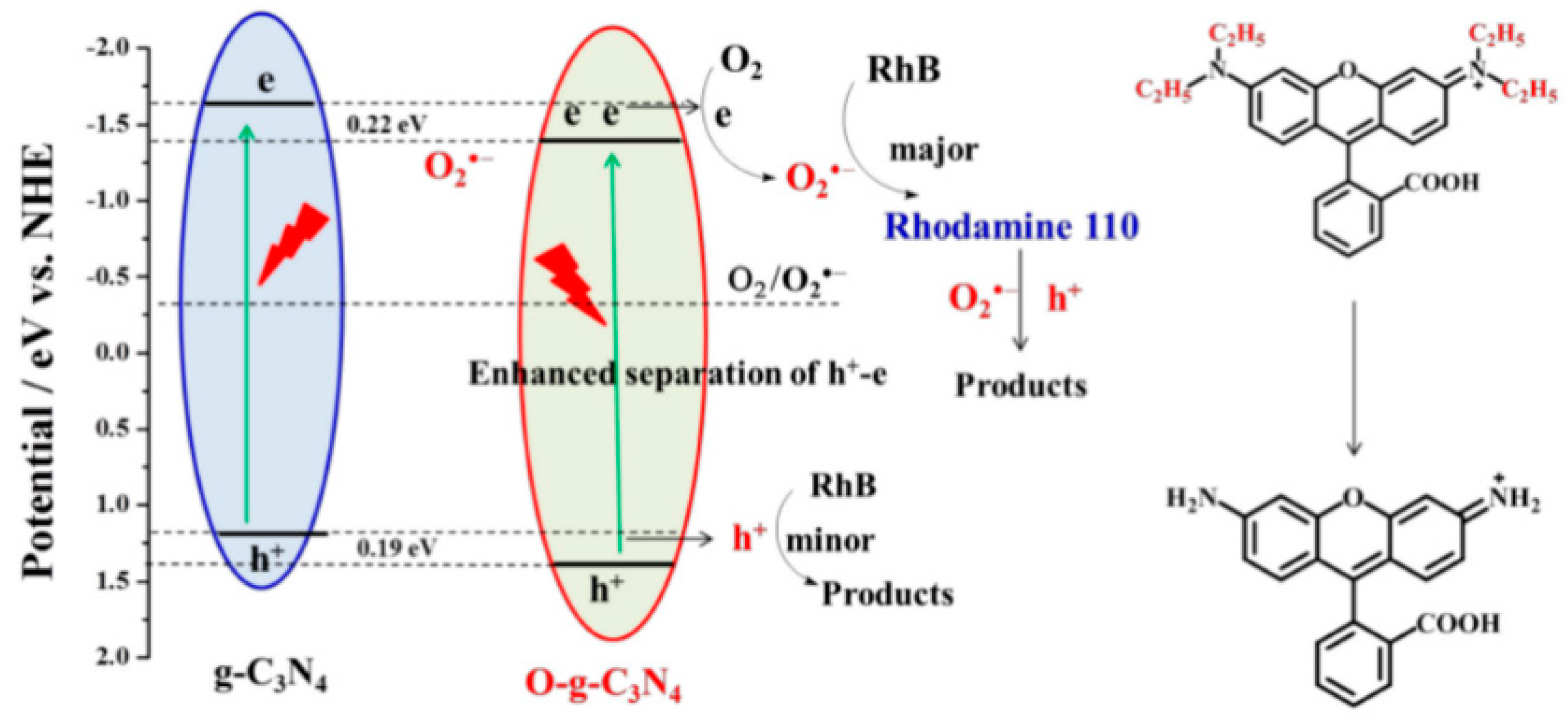
| Precursor | Synthetic Method | C/O Ratio, Doped (Pristine) | Photocatalytic Process | Conditions of the Process | Efficiency Doped/Pristine | References |
|---|---|---|---|---|---|---|
| Urea | H2O2 hydrothermal treatment, 120 °C | Surface O at.% in O-doped g-C3N4 3.23–6.59 by XPS | H2 evolution | 500 W Xe lamp, simulate solar irradiation; 30 vol% triethanolamine (TEOA) | 408.4 μmol·g−1/ 317.9 μmol·g−1 | [24] |
| Melamine | PMS hydrothermal treatment, 60 °C | 3.16%/2.73% by Energy-Dispersive X-Ray Spectroscopy (EDS) 1.8%/2.8% by XPS | Rhodamine B (RhB) destruction | 500 W halogen lamp, filter λ > 420 nm | 0.079 min−1/0.0032 min−1 | [17] |
| Urea | H2O2 hydrothermal treatment, 120 °C | — | RhB, Methyl orange (MO) destruction | 500 W Xe lamp, simulated solar irradiation | RhB:0.1074 h−1/0.0170 h−1 MO: 0.2287 h−1/0.0095 h−1 | [41] |
| Urea, ammonium acetate | two-step thermal treatment | 4.3/8.8 by elemental analysis (EA) | bisphenol A (BPA), phenol (Ph), 2-chlorphenol (2-Ph), diphenhydramine destruction (DP) | Light-emitting diode (LED) lamp, λ = 420–780 nm | Total organic carbon (TOC) removal rate: BPA 72.79%/9.27%; Ph 67.3%, 2-Ph 61.5%, DP 55.0% | [19] |
| Melamine, ethanol | Thermal polymerization | Atom.% (O) 3.25/- by XPS | H2 evolution | 350 W Xe lamp, filter λ > 420 nm, 10% vol% TEOA, Pt co-catalyst (1 wt.%) | 64.30 μmol·h−1/ 3.6 μmol·h−1 | [42] |
| 1,3,5-Trichloro-triazine, dicyandiami-de | Solvothermal method, 200 °C | C:N:O 1.08:1:0.23/ 0.69:1:0.03 by XPS | H2 evolution; RhB destruction | Visible light, λ > 420, Pt co-catalyst (0.3 wt.%) | H2 evolution: 3174 μmol·h−1 g −1/846 μmol·h−1·g −1 RhB degradation: 0.249 min−1/0.007min−1 | [22] |
| Melamine | Thermal polycondensation | 12.5/trace by XPS | CO2 reduction | 350 W Xe lamp, filter λ > 420 nm | CH3OH production 0.88 µmol·g−1·h−1/ 0.17 µmol·g−1·h−1 | [43] |
| Dicyandia-midine | Hydrothermal followed by calcination | O content (wt.%) 5.23/0.17 by EA | N2 fixation | 500 W Xenon lamp filter λ > 420 nm, 10 vol.% methanol as sacrificial agents | 118.8 mg·l−1·h−1·gcat−1/ 5.86 mg·l−1·h−1·gcat−1 | [18] |
| Precursor | Synthetic Method | C/N Doped (Pristine) | Photocatalytic Process | Conditions of the Process | Efficiency Doped/Pristine | References |
|---|---|---|---|---|---|---|
| Dicyanamide, dimethylformamide | Thermal copolymerization | C/N mass ratio 0.61(0.59) by elemental analysis | H2 evolution | 300W Xe lamp, filter λ > 400 nm), Pt co-catalyst (1 wt.%), triethanolamine (TEOA) as a hole quencher | 35.5 μmol/ 6.78 μmol in 8 h | [71] |
| Melamine, cellulose | thermal treatment | C/N mass ratio 33.39 (30.12) by elemental analysis | H2 evolution | 300W Xe lamp, filter λ > 420 nm), Pt (3%), TEOA (10 vol%) | 1024 μmol·g−1·h−1/ 59.6 μmol·g−1·h−1 | [68] |
| Melamine, Urea, phenylmalonic acid | Precursor copolymerization on the surface of g-C3N4 | C/N at. Ratio 68.9 (43.0) C, % 33.52 (33.45) by elemental analysis | bisphenol A (BPA) destruction H2 evolution | 300W Xe lamp, filter λ > 420 nm, Pt (3%), TEOA (10 vol%) | BPA destruction: 0.0507 min−1/0.0038 min−1; H2 evolution: 31 μmol·h−1/10 μmol·h−1 | [73] |
| Cyanuric acid, ethylene glycol, melamine | microwave treatment of supramolecular aggregates | C/N at. Ratio 0.688 (0.669) C, %: 39.98 (39.51) by elemental analysis | N2 photofixation | 250W high-pressure Na lamp (400 < l < 800 nm) | 5.3 mg·L−1·gcat−1/ 0.48 mg·L−1·gcat−1 | [88] |
| Urea, C60 nanorods | liquid-liquid interfacial precipitation method | - | H2 evolution | 500 W Xe lamp, filter λ > 420 nm, Pt (3%), TEOA (17 vol%) | 8.7 μmol·h−1/ 1.85 μmol·h−1 | [76] |
| Agar-melamine gel | one-step thermal condensation method | C/N at. Ratio 0.69 (0.67) C, %: 34.9 (35.33) by elemental analysis C/N at. Ratio 0.90 (0.87) by XPS | RhB, Phenol, BPA, Phe destruction | 300 W Xe lamp, filter λ > 420 nm | RhB destruction 0.042 min−1/ 0.016 min−1 BPA destruction 0.145 min−1/ 0.113min−1 | [79] |
| Urea, sacharose | Thermal polymerization | C/N at. Ratio 0.58 (0.57) C, %: 34.83 (34.81) by XPS analysis | NO removal | Xe lamp, filter λ > 420 nm | NO removal ratio 56.77%/ 50.89% | [82] |
| Melamine, carbon dots (CD) | combining g-C3N4 treated with H2O2 and CD | C wt.%: 46.77(39.78) by EDX | MB, RhB, fuchsine, Phe destruction; Cr(VI) photoreduction | 50 W LED lamp, visible light irradiation | RhB destruction 0.0675 min−1/ 0.0019 min−1 | [67] |
| Precursor | Synthetic Method | Content of Sulfur | Photocatalytic Process | Conditions of the Process | Efficiency Doped/Pristine | References |
|---|---|---|---|---|---|---|
| Melamine, sulfur | Thermal polycondensation, 520 °C | - | O2 evolution; scheme H2O splitting; bactericidal activity | 400 W halide lamp (λmax = 360 nm), 150 W Xe lamp, filter λ > 400 nm [Co(bpy)3]SO4 as electron mediator, Ru/SrTiO3:Rh as cocatalyst | O2 evolution: 40.3 μmol·h−1/- Z-scheme H2O splitting: 29.3 μmol·h−1/-; 70% of bacteria were killed | [96] |
| Melamine, (NH4)2SO4 | Upgraded gas templating method | — | H2 evolution | 350 W Xe lamp, filter λ > 420 nm, 10% vol% TEOA, Pt co-catalyst (1 wt.%) | 572 μmol·h−1·g−1/ 78 μmol·h−1·g−1 | [106] |
| Urea, thioacetamide | One-pot copolymerization | 0.1 at.% S 0.2 by XPS | Procion Red MX-5B degradation H2 evolution | 500 W Xe lamp, monochromic light provided by using a 420 ± 15, 450 ± 15, 475 ± 15 and 520 ± 15 nm band pass filter TEOA, Pt co-catalyst (1 wt.%) | Dye degradation 0.072 min−1/0.024 min−1 H2 evolution 3.17 mmol·g−1·h−1/ 0.84 mmol·g−1·h−1 | [103] |
| Thiourea | Thermal polycondensation followed by thermal oxidative etching | S content: 0.45 by OEA, 1.58 by XPS | Phenol degradation; H2 evolution | 300 W Xe lamp, 5% vol% TEOA, Pt co-catalyst (1 wt.%) | 75%/100% of Phenol was decomposed; H2 evolution: 127.4 μmol·h−1/ 0.5 μmol·h−1 | [90] |
| Thiourea, mesyl chloride | Post-synthetic derivatization of g-C3N4 | - | Acid Orange 7 dye degradation | UVA tube lamp, λmax = 368 nm | 0.113 min−1/0.022 min−1 | [99] |
| 1,3,5-trichlorotriazine, Melamine, Trithiocyanuric acid | Solvothermal condensation process, 180 °C | - | Cr(VI) reduction | Irradiation with λ > 420 nm | 1.85 min−1/0.03 min−1 | [104] |
| Melamine, Trithiocyanuric acid | Thermal polycondensation of the supramolecular complex | S (wt%): 0.63 | RhB degradation | 500 W Xe lamp, filter λ > 420 nm | 0.0167 min−1/0.0013 min−1 | [95] |
| Urea, benzyl disulfide | Thermal polycondensation, 520 °C | - | Reduction elimination of UO22+ | 350 W Xe lamp, λ ≥ 420 nm | 0.16 min−1/0.07 min−1 | [101] |
| Urea, thiourea | Thermal polycondensation, 550 °C | - | H2 evolution | 300 W Xe lamp, filter λ > 420 nm, 10% vol% TEOA | 95.3 μmol·h−1/ 36.4 μmol·h−1 | [100] |
| Melamine, sulfuric acid | Sulfuring and sonicating bulk g-C3N4 | - | 4-nitrophenol degradation | 500 W Xe lamp, filter λ > 400 nm | 3.47 × 10−2 min−1/ 7.04 × 10−4 min−1 | [107] |
| Thiourea | Thermal polycondensation, 520 °C | S atomic% 0.05 by EA | CO2 reduction | 300 W Xe lamp, Pt co-catalyst (1 wt.%) | CH3OH formation: 1.12 μmol·g−1/0.81 μmol·g−1 | [98] |
| Precursor | Synthetic Method | C/N Element Atomic Ratio, Doped (Pristine) | Photocatalytic Process | Conditions of the Process | Efficiency Doped/Pristine | References |
|---|---|---|---|---|---|---|
| Urea, oxalyl dihydrazide (ODH) | Thermal copolymerization | 0.74 (0.65) by element analysis; 0.67 (0.61) by XPS 1.87 (1.09) by EDX | Tetracycline hydrochloride (TC-HCl) and sulfamethoxazole (SMZ) destruction; H2 evolution | 300 W Xe lamp, filter λ > 420 nm, Pt co-catalyst (1wt.%), TEOA | SMZ destruction: 0.0203min−1/ 0.0066 min−1; H2 evolution: 5833.1 μmol·h−1·g−1/ 1458.2 μmol·g−1 | [133] |
| Urea, dicyandiamide | Post-thermal treatment of g-C3N4 | — | H2 evolution | Visible light irradiation | 6.5 μmol·g−1/ 2.1 μmol·g−1 | [137] |
| Melamine | Polymerization in atmosphere of: CCl4; H2; Ar | 0.61 (0.65) by elemental analysis | H2 evolution | 300W Xe lamp, filter λ > 420 nm), Pt (3%), TEOA (10 vol%) | 0.079 min−1/ 0.0032 min−1 | [128] |
| Urea | KOH-assisted calcination treatment | 1.45 (1.51) by organic elemental analysis 1.32 (1.64) by XPS | H2O2 production | simulated sunlight lamp 20 vol% ethanol | 152.6 μmol·h−1/ 10.2 μmol·h−1 | [131] |
| Dicyandiamide, NH4Cl, 3-amino-1,2,4-triazol | Thermal polymerization with post treatment in N2 | 1.260 (1.489) by element analysis | H2 evolution | 300W Xe lamp, filter λ > 400 nm), Pt (3%), TEOA (10 vol%) | 3882.5 μmol·h−1·g−1/ 85.0 μmol·h−1·g−1 | [134] |
| Urea, Mg powder | magnesium vapor etching | 0.51 (0.78) by EDX 0.92 (1.14) by XPS | H2 evolution | 300W Xe lamp, filter λ > 400 nm), Pt (3%), TEOA (10 vol%) | 450 μmol·h−1·g−1/ 225 μmol·h−1·g−1 | [88] |
| Dicyandiamide | two-step calcination | 0.81 (0.85) by XPS | N2 fixation | 300W Xe lamp | NH4+ formation: 54 mmol·L−1/ 24 mmol·L−1 | [139] |
| Urea, melamine | precursor preprocessing and thermolysis in N2 | — | NO oxidation | LED lamp (λ ≥ 448 nm) | the NO oxidation in 30 min of irradiation 47.7%/22% | [140] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starukh, H.; Praus, P. Doping of Graphitic Carbon Nitride with Non-Metal Elements and Its Applications in Photocatalysis. Catalysts 2020, 10, 1119. https://doi.org/10.3390/catal10101119
Starukh H, Praus P. Doping of Graphitic Carbon Nitride with Non-Metal Elements and Its Applications in Photocatalysis. Catalysts. 2020; 10(10):1119. https://doi.org/10.3390/catal10101119
Chicago/Turabian StyleStarukh, Halyna, and Petr Praus. 2020. "Doping of Graphitic Carbon Nitride with Non-Metal Elements and Its Applications in Photocatalysis" Catalysts 10, no. 10: 1119. https://doi.org/10.3390/catal10101119
APA StyleStarukh, H., & Praus, P. (2020). Doping of Graphitic Carbon Nitride with Non-Metal Elements and Its Applications in Photocatalysis. Catalysts, 10(10), 1119. https://doi.org/10.3390/catal10101119






