Metal Chalcogenides Based Heterojunctions and Novel Nanostructures for Photocatalytic Hydrogen Evolution
Abstract
1. Introduction
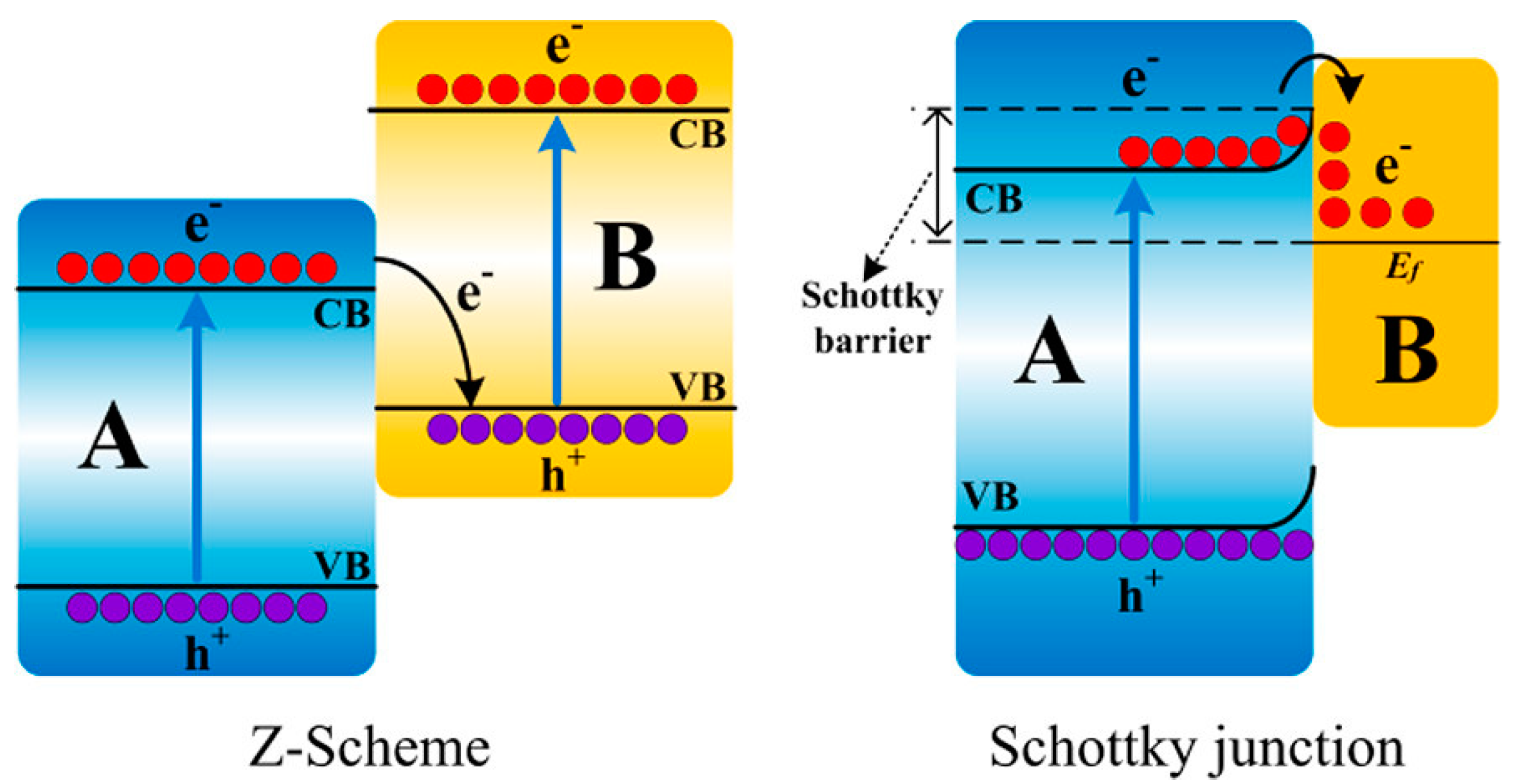
2. Heterojunctions for Enhanced Charge Carriers Separation and Light Absorption
2.1. Metal Chalcogenides Based Binary Heterojunctions
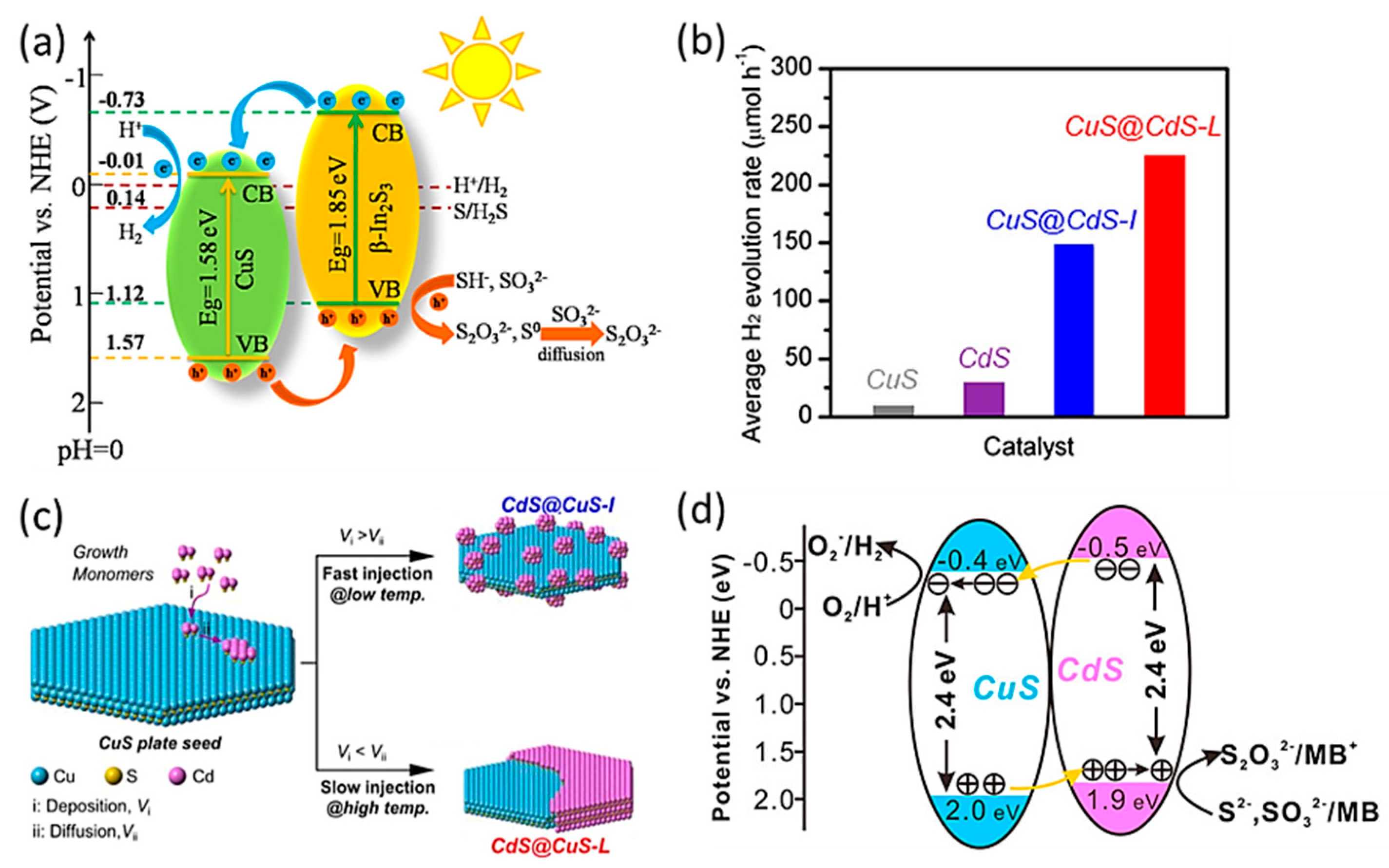
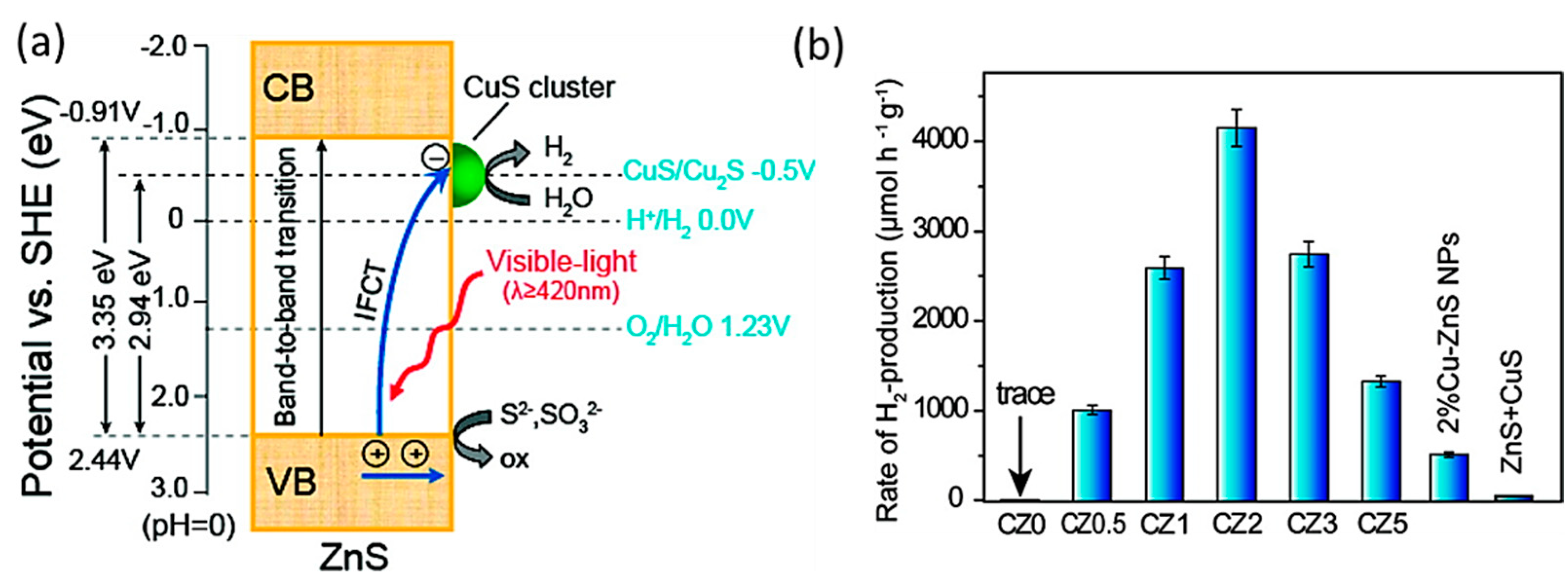
2.1.1. CuS-Based Binary Heterojunctions
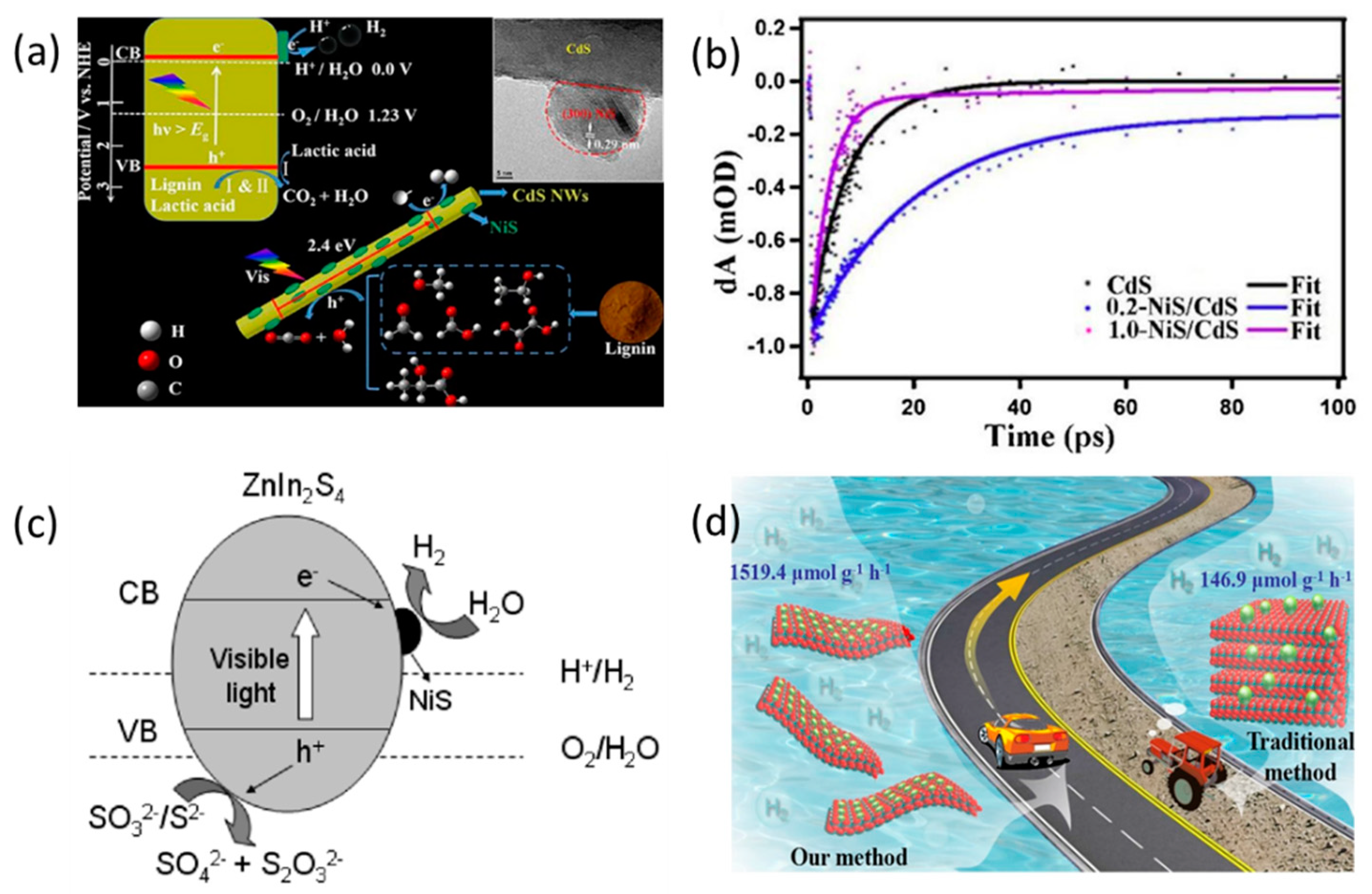
2.1.2. NiS-Based Binary Heterojunctions
2.2. Metal Chalcogenides Based Polynary Heterojunctions
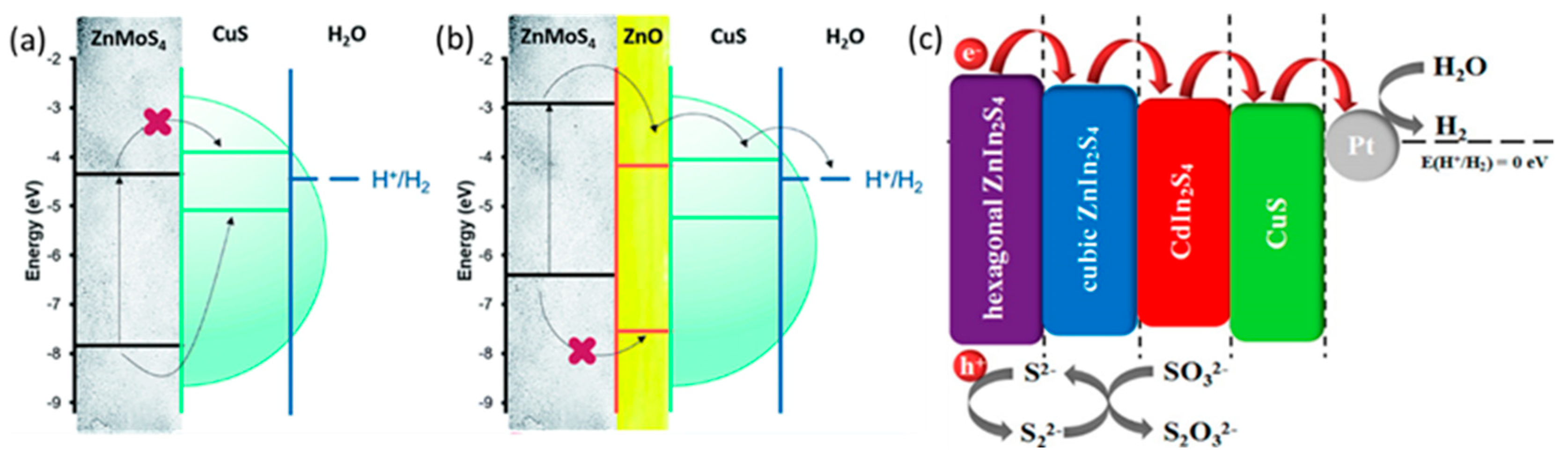
2.2.1. CuS Based Polynary Heterojunctions
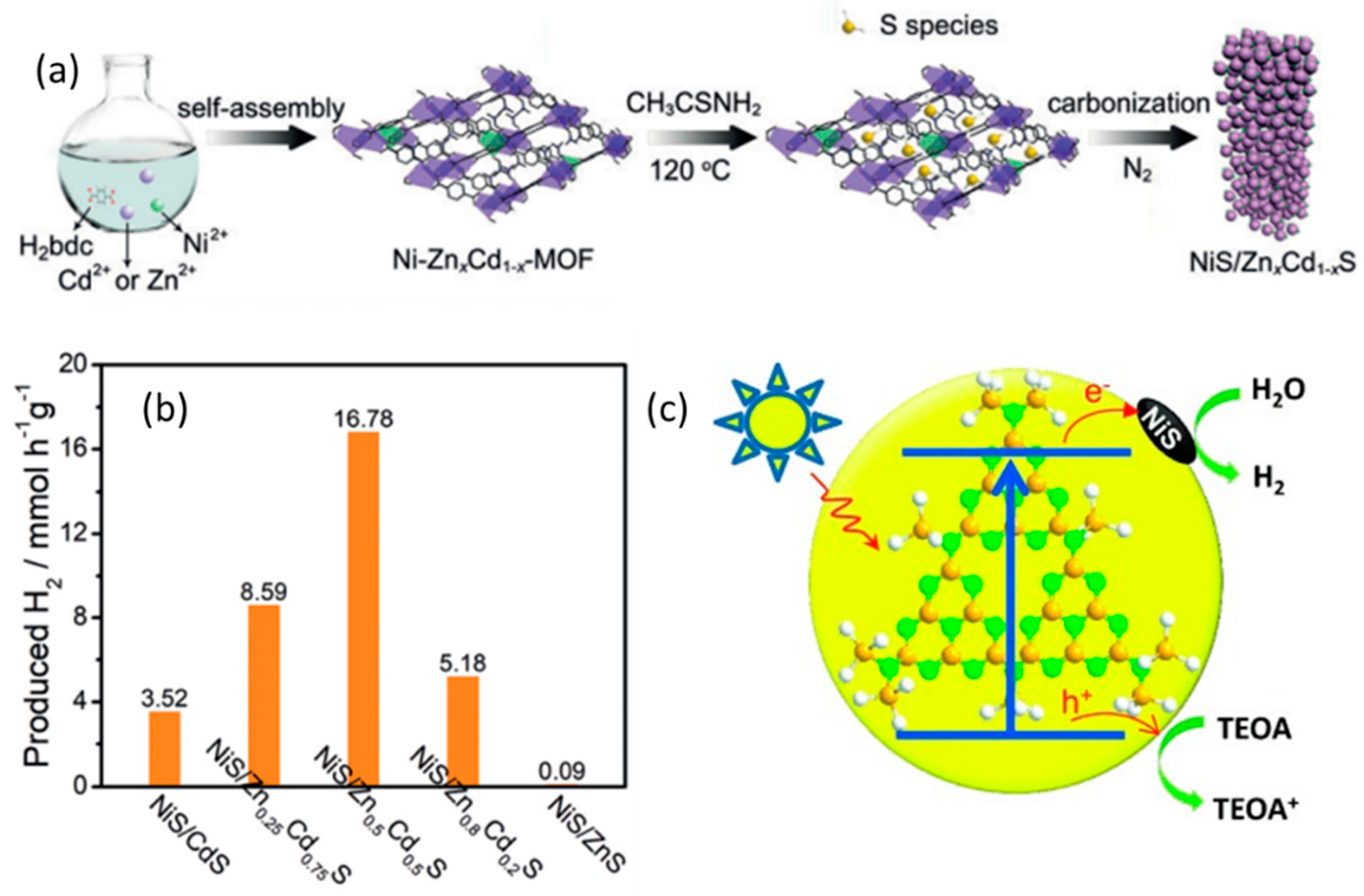
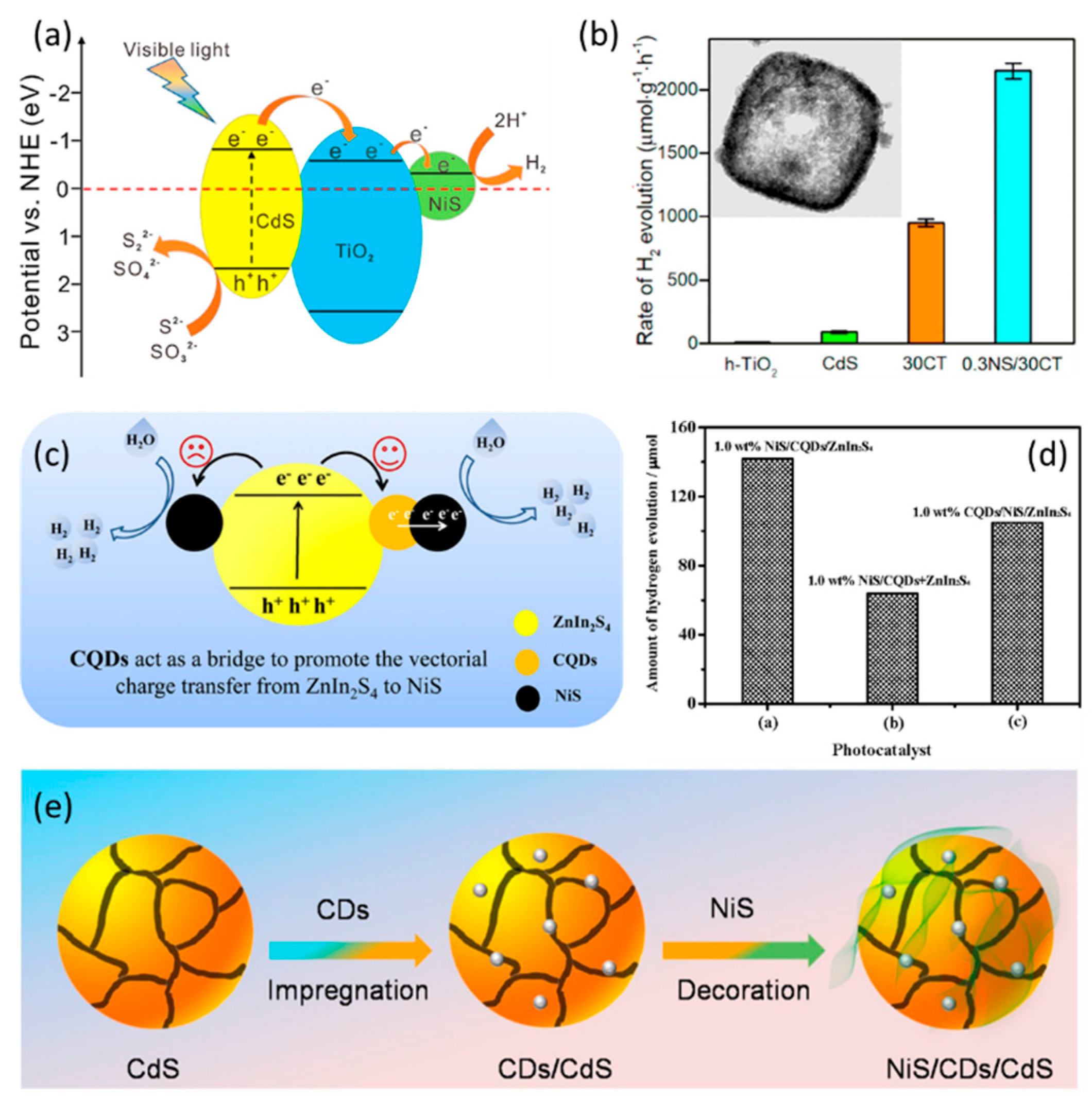
2.2.2. NiS-Based Polynary Heterojunctions
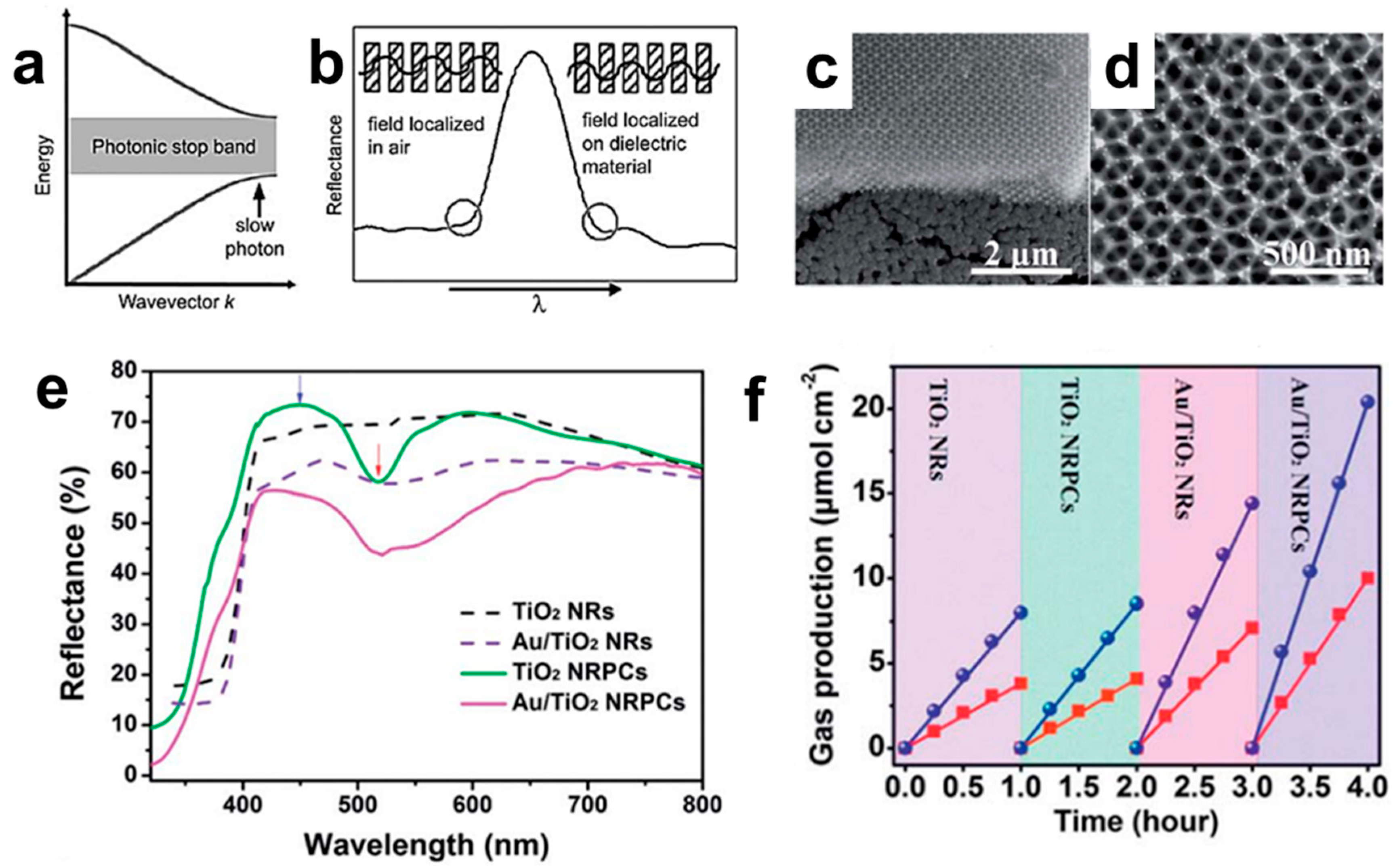
3. 3D Nanostructure an Accelerator of the Photochemistry
4. Imogolite Clay Nanotubes (INT)
4.1. A Promising Tunable Nanoreactor
4.2. Applications in (Photo)Catalysis
4.2.1. Catalysis and Fenton Reaction
4.2.2. Photocatalysis
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A.; Remhof, A.; Borgschulte, A.; Friedrichs, O. Hydrogen: The future energy carrier. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3329–3342. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S. Toward cost-effective solar energy use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Anusuyadevi, P.R.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef] [PubMed]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Zhang, P.; Lou, X.W. Design of Heterostructured Hollow Photocatalysts for Solar-to-Chemical Energy Conversion. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of Interfaces in Two-Dimensional Photocatalyst for Water Splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Selinsky, R.S.; Ding, Q.; Faber, M.S.; Wright, J.C.; Jin, S. Quantum dot nanoscale heterostructures for solar energy conversion. Chem. Soc. Rev. 2013, 42, 2963–2985. [Google Scholar] [CrossRef]
- Gao, X.; Li, J.; Du, R.; Zhou, J.; Huang, M.-Y.; Liu, R.; Li, J.; Xie, Z.; Wu, L.-Z.; Liu, Z.; et al. Direct Synthesis of Graphdiyne Nanowalls on Arbitrary Substrates and Its Application for Photoelectrochemical Water Splitting Cell. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Li, J.; Gao, X.; Liu, B.; Feng, Q.; Li, X.-B.; Huang, M.-Y.; Liu, Z.; Zhang, J.; Tung, C.-H.; Wu, L.-Z. Graphdiyne: A Metal-Free Material as Hole Transfer Layer to Fabricate Quantum Dot-Sensitized Photocathodes for Hydrogen Production. J. Am. Chem. Soc. 2016, 138, 3954–3957. [Google Scholar] [CrossRef] [PubMed]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Yuan, Y.-P.; Ruan, L.-W.; Barber, J.; Loo, S.C.J.; Xue, C. Hetero-nanostructured suspended photocatalysts for solar-to-fuel conversion. Energy Environ. Sci. 2014, 7, 3934–3951. [Google Scholar] [CrossRef]
- Li, J.-X.; Li, Z.-J.; Ye, C.; Li, X.-B.; Zhan, F.; Fan, X.-B.; Li, J.; Chen, B.; Tao, Y.; Tung, C.-H.; et al. Visible light-induced photochemical oxygen evolution from water by 3,4,9,10-perylenetetracarboxylic dianhydride nanorods as an n-type organic semiconductor. Catal. Sci. Technol. 2016, 6, 672–676. [Google Scholar] [CrossRef]
- Zhong, M.; Hisatomi, T.; Kuang, Y.; Zhao, J.; Liu, M.; Iwase, A.; Jia, Q.; Nishiyama, H.; Minegishi, T.; Nakabayashi, M.; et al. Surface Modification of CoOx Loaded BiVO4 Photoanodes with Ultrathin p-Type NiO Layers for Improved Solar Water Oxidation. J. Am. Chem. Soc. 2015, 137, 5053–5060. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, L. Metal sulphide semiconductors for photocatalytic hydrogen production. Catal. Sci. Technol. 2013, 3, 1672–1690. [Google Scholar] [CrossRef]
- Nie, L.; Zhang, Q. Recent progress in crystalline metal chalcogenides as efficient photocatalysts for organic pollutant degradation. Inorg. Chem. Front. 2017, 4, 1953–1962. [Google Scholar] [CrossRef]
- Borgarello, E.; Kalyanasundaram, K.; Grätzel, M.; Pelizzetti, E. Visible Light-Induced Generation of Hydrogen from H2S in CdS Dispersions; Hole Transfer Catalysis by RuO2. Helv. Chim. Acta 1982, 65, 243–248. [Google Scholar] [CrossRef]
- Gesesse, G.D.; Li, C.; Paineau, E.; Habibi, Y.; Remita, H.; Colbeau-Justin, C.; Ghazzal, M.N. Enhanced Photogenerated Charge Carriers and Photocatalytic Activity of Biotemplated Mesoporous TiO2 Films with a Chiral Nematic Structure. Chem. Mater. 2019, 31, 4851–4863. [Google Scholar] [CrossRef]
- Gesesse, G.D.; le Neel, T.; Cui, Z.; Bachelier, G.; Remita, H.; Colbeau-Justin, C.; Ghazzal, M.N. Plasmonic core–shell nanostructure as an optical photoactive nanolens for enhanced light harvesting and hydrogen production. Nanoscale 2018, 10, 20140–20146. [Google Scholar] [CrossRef]
- Li, J.; Gao, X.; Li, Z.; Wang, J.-H.; Zhu, L.; Yin, C.; Wang, Y.; Li, X.-B.; Liu, Z.; Zhang, J.; et al. Superhydrophilic Graphdiyne Accelerates Interfacial Mass/Electron Transportation to Boost Electrocatalytic and Photoelectrocatalytic Water Oxidation Activity. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, X.; Jiang, X.; Li, X.-B.; Liu, Z.; Zhang, J.; Tung, C.-H.; Wu, L.-Z. Graphdiyne: A Promising Catalyst–Support to Stabilize Cobalt Nanoparticles for Oxygen Evolution. ACS Catal. 2017, 7, 5209–5213. [Google Scholar] [CrossRef]
- Prakash, A.; Dan, M.; Yu, S.; Wei, S.; Li, Y.; Wang, F.; Zhou, Y. In2S3/CuS nanosheet composite: An excellent visible light photocatalyst for H2 production from H2S. Sol. Energy Mater. Sol. Cells 2018, 180, 205–212. [Google Scholar] [CrossRef]
- Li, N.; Fu, W.; Chen, C.; Liu, M.; Xue, F.; Shen, Q.; Zhou, J. Controlling the Core–Shell Structure of CuS@CdS Heterojunction via Seeded Growth with Tunable Photocatalytic Activity. ACS Sustain. Chem. Eng. 2018, 6, 15867–15875. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J.; Zhang, Y.; Li, Q.; Gong, J.R. Visible Light Photocatalytic H2-Production Activity of CuS/ZnS Porous Nanosheets Based on Photoinduced Interfacial Charge Transfer. Nano Lett. 2011, 11, 4774–4779. [Google Scholar] [CrossRef]
- Huang, J.; Shi, Z.; Dong, X. Nickel sulfide modified TiO2 nanotubes with highly efficient photocatalytic H2 evolution activity. J. Energy Chem. 2016, 25, 136–140. [Google Scholar] [CrossRef]
- Qiao, P.; Wu, J.; Li, H.; Xu, Y.; Sun, B.; Ren, L.; Pan, K.; Wang, L.; Zhou, W. Improved charge separation of NiS nanoparticles modified defect-engineered black TiO2 hollow nanotubes for boosting solar-driven photocatalytic H2 evolution. Nanotechnology 2019, 30, 125703. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Naghadeh, S.B.; Zhang, J.Z.; Fang, P. Visible light driven hydrogen evolution by photocatalytic reforming of lignin and lactic acid using one-dimensional NiS/CdS nanostructures. Appl. Catal. B 2018, 227, 229–239. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Y.; Zhao, J.; Li, Z. Preparation of NiS/ZnIn2S4 as a superior photocatalyst for hydrogen evolution under visible light irradiation. Beilstein J. Nanotechnol. 2013, 4, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liang, S.; Wu, L.; Wang, X. Ultrasmall NiS decorated HNb3O8 nanosheeets as highly efficient photocatalyst for H2 evolution reaction. Catal. Today 2019, 330, 195–202. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.; Liu, J.; Shi, W.; Yang, G.; Wang, G.C.; Cheng, P. An Efficient, Visible-Light-Driven, Hydrogen Evolution Catalyst NiS/ZnxCd1−xS Nanocrystal Derived from a Metal-Organic Framework. Angew. Chem. Int. Ed. Engl. 2018, 57, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Wang, Y.; Wang, Y.; Zhang, W.; Xu, R. Noble-metal-free NiS/C3N4 for efficient photocatalytic hydrogen evolution from water. ChemSusChem 2013, 6, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.Y.; Wu, H.; Lim, Y.-F.; Ho, G.W. Facilitating the charge transfer of ZnMoS4/CuS p–n heterojunctions through ZnO intercalation for efficient photocatalytic hydrogen generation. J. Mater. Chem. A 2018, 6, 11416–11423. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Zhang, W.; Li, Y.; Song, Q.; Dong, L. Fabricate Globular Flower-like CuS/CdIn2S4/ZnIn2S4 with High Visible Light Response via Microwave-assisted One–step Method and Its Multipathway Photoelectron Migration Properties for Hydrogen Evolution and Pollutant Degradation. ACS Sustain. Chem. Eng. 2016, 4, 6680–6688. [Google Scholar] [CrossRef]
- Li, N.; Huang, H.; Bibi, R.; Shen, Q.; Ngulube, R.; Zhou, J.; Liu, M. Noble-metal-free MOF derived hollow CdS/TiO2 decorated with NiS cocatalyst for efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2019, 476, 378–386. [Google Scholar] [CrossRef]
- Wang, B.; Ding, Y.; Deng, Z.; Li, Z. Rational design of ternary NiS/CQDs/ZnIn2S4 nanocomposites as efficient noble-metal-free photocatalyst for hydrogen evolution under visible light. Chin. J. Catal. 2019, 40, 335–342. [Google Scholar] [CrossRef]
- Wei, R.-B.; Huang, Z.-L.; Gu, G.-H.; Wang, Z.; Zeng, L.; Chen, Y.; Liu, Z.-Q. Dual-cocatalysts decorated rimous CdS spheres advancing highly-efficient visible-light photocatalytic hydrogen production. Appl. Catal. B 2018, 231, 101–107. [Google Scholar] [CrossRef]
- Yang, X.; Cao, C.; Erickson, L.; Hohn, K.; Maghirang, R.; Klabunde, K. Photo-catalytic degradation of Rhodamine B on C-, S-, N-, and Fe-doped TiO2 under visible-light irradiation. Appl. Catal. B 2009, 91, 657–662. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- In, S.; Orlov, A.; Berg, R.; García, F.; Pedrosa-Jimenez, S.; Tikhov, M.S.; Wright, D.S.; Lambert, R.M. Effective Visible Light-Activated B-Doped and B,N-Codoped TiO2 Photocatalysts. J. Am. Chem. Soc. 2007, 129, 13790–13791. [Google Scholar] [CrossRef] [PubMed]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Band gap narrowing of titanium dioxide by sulfur doping. Appl. Phys. Lett. 2002, 81, 454–456. [Google Scholar] [CrossRef]
- Chatterjee, D.; Mahata, A. Visible light induced photodegradation of organic pollutants on dye adsorbed TiO2 surface. J. Photochem. Photobiol. A 2002, 153, 199–204. [Google Scholar] [CrossRef]
- Abe, R.; Hara, K.; Sayama, K.; Domen, K.; Arakawa, H. Steady hydrogen evolution from water on Eosin Y-fixed TiO2 photocatalyst using a silane-coupling reagent under visible light irradiation. J. Photochem. Photobiol. A 2000, 137, 63–69. [Google Scholar] [CrossRef]
- Nehme, A.S.; Haydous, F.; Halaoui, L. Amplification in Light Energy Conversion at Q-CdTe Sensitized TiO2 Photonic Crystal, Photoelectrochemical Stability in Se2– Electrolyte, and Size-Dependent Type II Q-CdTe/CdSe Formation. J. Phys. Chem. C 2016, 120, 4766–4778. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Chaoui, N.; Trzpit, M.; Ghazzal, N.; Robert, D.; Weber, J.V. UV–vis versus visible degradation of Acid Orange II in a coupled CdS/TiO2 semiconductors suspension. J. Photochem. Photobiol. A 2006, 183, 218–224. [Google Scholar] [CrossRef]
- Park, H.; Choi, W.; Hoffmann, M.R. Effects of the preparation method of the ternary CdS/TiO2/Pt hybrid photocatalysts on visible light-induced hydrogen production. J. Mater. Chem. 2008, 18, 2379–2385. [Google Scholar] [CrossRef]
- Chen, J.I.L.; von Freymann, G.; Choi, S.Y.; Kitaev, V.; Ozin, G.A. Slow photons in the fast lane in chemistry. J. Mater. Chem. 2008, 18, 369–373. [Google Scholar] [CrossRef]
- Yablonovitch, E. Inhibited Spontaneous Emission in Solid-State Physics and Electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef]
- Deparis, O.; Vigneron, J.P. Modeling the photonic response of biological nanostructures using the concept of stratified medium: The case of a natural three-dimensional photonic crystal. Mater. Sci. Eng. B 2010, 169, 12–15. [Google Scholar] [CrossRef]
- Vukusic, P.; Sambles, J.R. Photonic structures in biology. Nature 2003, 424, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Ghazzal, M.N.; Deparis, O.; Errachid, A.; Kebaili, H.; Simonis, P.; Eloy, P.; Vigneron, J.P.; de Coninck, J.; Gaigneaux, E.M. Porosity control and surface sensitivity of titania/silica mesoporous multilayer coatings: Applications to optical Bragg resonance tuning and molecular sensing. J. Mater. Chem. 2012, 22, 25302–25310. [Google Scholar] [CrossRef]
- Ghazzal, M.N.; Deparis, O.; de Coninck, J.; Gaigneaux, E.M. Tailored refractive index of inorganic mesoporous mixed-oxide Bragg stacks with bio-inspired hygrochromic optical properties. J. Mater. Chem. C 2013, 1, 6202–6209. [Google Scholar] [CrossRef]
- Aguirre, C.I.; Reguera, E.; Stein, A. Tunable Colors in Opals and Inverse Opal Photonic Crystals. Adv. Funct. Mater. 2010, 20, 2565–2578. [Google Scholar] [CrossRef]
- Mekis, A.; Chen, J.C.; Kurland, I.; Fan, S.; Villeneuve, P.R.; Joannopoulos, J.D. High Transmission through Sharp Bends in Photonic Crystal Waveguides. Phys. Rev. Lett. 1996, 77, 3787–3790. [Google Scholar] [CrossRef]
- Ghazzal, M.N.; Joseph, M.; Kebaili, H.; de Coninck, J.; Gaigneaux, E.M. Tuning the selectivity and sensitivity of mesoporous dielectric multilayers by modifiying the hydrophobic–hydrophilic balance of the silica layer. J. Mater. Chem. 2012, 22, 22526–22532. [Google Scholar] [CrossRef]
- Puzzo, D.P.; Scotognella, F.; Zavelani-Rossi, M.; Sebastian, M.; Lough, A.J.; Manners, I.; Lanzani, G.; Tubino, R.; Ozin, G.A. Distributed Feedback Lasing from a Composite Poly(phenylene vinylene)−Nanoparticle One-Dimensional Photonic Crystal. Nano Lett. 2009, 9, 4273–4278. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Lee, S.-T.; Yang, S.; Kang, Z. Coupling surface plasmon resonance of gold nanoparticles with slow-photon-effect of TiO2 photonic crystals for synergistically enhanced photoelectrochemical water splitting. Energy Environ. Sci. 2014, 7, 1409–1419. [Google Scholar] [CrossRef]
- Park, H.G.; Jung, Y. Carbon nanofluidics of rapid water transport for energy applications. Chem. Soc. Rev. 2014, 43, 565–576. [Google Scholar] [CrossRef]
- Miners, S.A.; Rance, G.A.; Khlobystov, A.N. Chemical reactions confined within carbon nanotubes. Chem. Soc. Rev. 2016, 45, 4727–4746. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.; Melchionna, M. Enter the Tubes: Carbon Nanotube Endohedral Catalysis. Catalysts 2019, 9, 128. [Google Scholar] [CrossRef]
- Huang, H.; Tu, S.; Zeng, C.; Zhang, T.; Reshak, A.H.; Zhang, Y. Macroscopic Polarization Enhancement Promoting Photo- and Piezoelectric-Induced Charge Separation and Molecular Oxygen Activation. Angew. Chem. Int. Ed. 2017, 56, 11860–11864. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, H.; Guo, L.; Zhang, Y.; Ma, T. The Role of Polarization in Photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 10061–10073. [Google Scholar] [CrossRef] [PubMed]
- Paineau, E. Imogolite Nanotubes: A Flexible Nanoplatform with Multipurpose Applications. Appl. Sci. 2018, 8, 1921. [Google Scholar] [CrossRef]
- Paineau, E.; Launois, P. Nanomaterials from Imogolite: Structure, Properties, and Functional Materials. In Nanomaterials from Clay Minerals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 257–284. [Google Scholar]
- Yoshinaga, N.; Aomine, S. Imogolite in some ando soils. Soil Sci. Plant Nutr. 1962, 8, 22–29. [Google Scholar] [CrossRef]
- Farmer, V.; Fraser, A.; Tait, J.M. Synthesis of Imogolite: A Tubular Aluminium Silicate Polymer. J. Chem. Soc. Chem. Commun. 1977, 12, 462–463. [Google Scholar] [CrossRef]
- Guimaraes, L.; Enyashin, A.N.; Frenzel, J.; Heine, T.; Duarte, H.A.; Seifert, G. Imogolite Nanotubes: Stability, Electronic, and Mechanical Properties. ACS Nano 2007, 1, 362–368. [Google Scholar] [CrossRef]
- Teobaldi, G.; Beglitis, N.S.; Fisher, A.J.; Zerbetto, F.; Hofer, A.A. Hydroxyl vacancies in single-walled aluminosilicate and aluminogermanate nanotubes. J. Phys. Condens. Matter 2009, 21. [Google Scholar] [CrossRef]
- Maillet, P.; Levard, C.; Spalla, O.; Masion, A.; Rose, J.; Thill, A. Growth kinetic of single and double-walled aluminogermanate imogolite-like nanotubes: An experimental and modeling approach. Phys. Chem. Chem. Phys. 2011, 13, 2682–2689. [Google Scholar] [CrossRef]
- Yucelen, G.I.; Kang, D.-Y.; Schmidt-Krey, I.; Beckham, H.W.; Nair, S. A generalized kinetic model for the formation and growth of single-walled metal oxide nanotubes. Chem. Eng. Sci. 2013, 90, 200–212. [Google Scholar] [CrossRef]
- Monet, G.; Amara, M.S.; Rouzière, S.; Paineau, E.; Chai, Z.; Elliott, J.D.; Poli, E.; Liu, L.-M.; Teobaldi, G.; Launois, P. Structural resolution of inorganic nanotubes with complex stoichiometry. Nat. Commun. 2018, 9, 2033. [Google Scholar] [CrossRef] [PubMed]
- Yucelen, G.I.; Kang, D.-Y.; Guerrero-Ferreira, R.C.; Wright, E.R.; Beckham, H.W.; Nair, S. Shaping Single-Walled Metal Oxide Nanotubes from Precursors of Controlled Curvature. Nano Lett. 2012, 12, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Thill, A.; Guiose, B.; Bacia-Verloop, M.; Geertsen, V.; Belloni, L. How the Diameter and Structure of (OH)3Al2O3SixGe1–xOH Imogolite Nanotubes Are Controlled by an Adhesion versus Curvature Competition. J. Phys. Chem. C 2012, 116, 26841–26849. [Google Scholar] [CrossRef]
- Amara, M.-S.; Paineau, E.; Bacia-Verloop, M.; Krapf, M.-E.M.; Davidson, P.; Belloni, L.; Levard, C.; Rose, J.; Launois, P.; Thill, A. Single-step formation of micron long (OH)3Al2O3Ge(OH) imogolite-like nanotubes. Chem. Commun. 2013, 49, 11284–11286. [Google Scholar] [CrossRef]
- Amara, M.S.; Paineau, E.; Rouzière, S.; Guiose, B.; Krapf, M.-E.M.; Taché, O.; Launois, P.; Thill, A. Hybrid, Tunable-Diameter, Metal Oxide Nanotubes for Trapping of Organic Molecules. Chem. Mater. 2015, 27, 1488–1494. [Google Scholar] [CrossRef]
- Chemmi, A.; Brendle, J.; Marichal, C.; Lebeau, B. Key Steps Influencing the Formation of Aluminosilicate Nanotubes by the Fluoride Route. Clays Clay Miner. 2015, 63, 132–143. [Google Scholar] [CrossRef]
- Picot, P.; Gobeaux, F.; Coradin, T.; Thill, A. Dual internal functionalization of imogolite nanotubes as evidenced by optical properties of Nile red. Appl. Clay Sci. 2019, 178, 105133. [Google Scholar] [CrossRef]
- Arancibia-Miranda, N.; Escudey, M. Imogolite-Like Family. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2016; Volume 7, pp. 458–483. [Google Scholar]
- Farmer, V.; Adams, M.; Fraser, A.; Palmieri, F. Synthetic imogolite: Properties synthesis and possible applications. Clay Miner. 1983, 18, 459–472. [Google Scholar] [CrossRef]
- Van Nordstrand, R.A. Hydrocarbons Hydroprocessing with Imogolite Catalyst. U.S. Patent 4394253, 19 July 1983. [Google Scholar]
- Imamura, S.; Hayashi, Y.; Kajiwara, K.; Hoshino, H.; Kaito, C. Imogolite: A possible new type of shape-selective catalyst. Ind. Eng. Chem. Res. 1993, 32, 600–603. [Google Scholar] [CrossRef]
- Ookawa, M.; Onishi, Y.; Fukukawa, S.-I.; Matsumoto, K.-I.; Watanabe, M.; Yamaguchi, T.; Suzuki, M.J. Catalytic Property of Synthetic Imogolite. Clay Sci. Soc. Jpn. 2006, 45, 184–187. (In Japanese) [Google Scholar]
- Olson, N.; Deshpande, N.; Gunduz, S.; Ozkan, U.S.; Brunelli, N.A. Utilizing imogolite nanotubes as a tunable catalytic material for the selective isomerization of glucose to fructose. Catal. Today 2019, 323, 69–75. [Google Scholar] [CrossRef]
- Shafia, E.; Esposito, S.; Manzoli, M.; Chiesa, M.; Tiberto, P.; Barrera, G.; Menard, G.; Allia, P.; Freyria, F.S.; Garrone, E.; et al. Al/Fe isomorphic substitution versus Fe2O3 clusters formation in Fe-doped aluminosilicate nanotubes (imogolite). J. Nanopart. Res. 2015, 17, 336. [Google Scholar] [CrossRef]
- Ookawa, M.; Inoue, Y.; Watanabe, M.; Suzuki, M.; Yamaguchi, T. Synthesis and Characterization of Fe Containing Imogolite. Clay Sci. 2006, 12, 280–284. [Google Scholar]
- Ookawa, M.; Takata, Y.; Suzuki, M.; Inukai, K.; Maekawa, T.; Yamaguchi, T. Oxidation of aromatic hydrocarbons with H2O2 catalyzed by a nano-scale tubular aluminosilicate, Fe-containing imogolite. Res. Chem. Intermed. 2008, 34, 679–685. [Google Scholar] [CrossRef]
- Shafia, E.; Esposito, S.; Armandi, M.; Bahadori, E.; Garrone, E.; Bonelli, B. Reactivity of bare and Fe-doped alumino-silicate nanotubes (imogolite) with H2O2 and the azo-dye Acid Orange 7. Catal. Today 2016, 277, 89–96. [Google Scholar] [CrossRef]
- Imamura, S.; Kokubu, T.; Yamashita, T.; Okamoto, Y.; Kajiwara, K.; Kanai, H. Shape-selective copper-loaded Imogolite catalyst. J. Catal. 1996, 160, 137–139. [Google Scholar] [CrossRef]
- Qi, X.; Yoon, H.; Lee, S.-H.; Yoon, J.; Kim, S.-J. Surface-modified imogolite by 3-APS-OsO4 complex: Synthesis, characterization and its application in the dihydroxylation of olefins. J. Ind. Eng. Chem. 2008, 14, 136–141. [Google Scholar] [CrossRef]
- Katsumata, K.-I.; Hou, X.; Sakai, M.; Nakajima, A.; Fujishima, A.; Matsushita, N.; MacKenzie, K.J.D.; Okada, K. Visible-light-driven photodegradation of acetaldehyde gas catalyzed by aluminosilicate nanotubes and Cu(II)-grafted TiO2 composites. Appl. Catal. B Environ. 2013, 138, 243–252. [Google Scholar] [CrossRef]
- Bahadori, E.; Vaiano, V.; Esposito, S.; Armandi, M.; Sannino, D.; Bonelli, B. Photo-activated degradation of tartrazine by H2O2 as catalyzed by both bare and Fe-doped methyl-imogolite nanotubes. Catal. Today 2018, 304, 199–207. [Google Scholar] [CrossRef]
- Gustafsson, J.P. The surface chemistry of imogolite. Clays Clay Miner. 2001, 49, 73–80. [Google Scholar] [CrossRef]
- Poli, E.; Elliott, J.D.; Hine, N.D.M.; Mostofi, A.A.; Teobaldi, G. Large-scale density functional theory simulation of inorganic nanotubes: A case study on Imogolite nanotubes. Mater. Res. Innov. 2015, 19, S272–S282. [Google Scholar] [CrossRef]
- Poli, E.; Elliott, J.D.; Ratcliff, L.E.; Andrinopoulos, L.; Dziedzic, J.; Hine, N.D.M.; Mostofi, A.A.; Skylaris, C.-K.; Haynes, P.D.; Teobaldi, G. The potential of imogolite nanotubes as (co-)photocatalysts: A linear-scaling density functional theory study. J. Phys. Condens. Matter 2016, 28, 074003. [Google Scholar] [CrossRef]
- Elliott, J.D.; Poli, E.; Scivetti, I.; Ratcliff, L.E.; Andrinopoulos, L.; Dziedzic, J.; Hine, N.D.M.; Mostofi, A.A.; Skylaris, C.-K.; Haynes, P.D.; et al. Chemically Selective Alternatives to Photoferroelectrics for Polarization-Enhanced Photocatalysis: The Untapped Potential of Hybrid Inorganic Nanotubes. Adv. Sci. 2017, 4, 1600153. [Google Scholar] [CrossRef]
- Poli, E.; Elliott, J.D.; Chulkov, S.K.; Watkins, M.B.; Teobaldi, G. The role of cation-vacancies for the electronic and optical properties of aluminosilicate imogolite nanotubes: A non-local, linear-response TDDFT study. Front. Chem. 2019, 7, 210. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalysis for Hydrogen Production and CO2 Reduction: The Case of Copper-Catalysts. ChemCatChem 2019, 11, 368–382. [Google Scholar] [CrossRef]



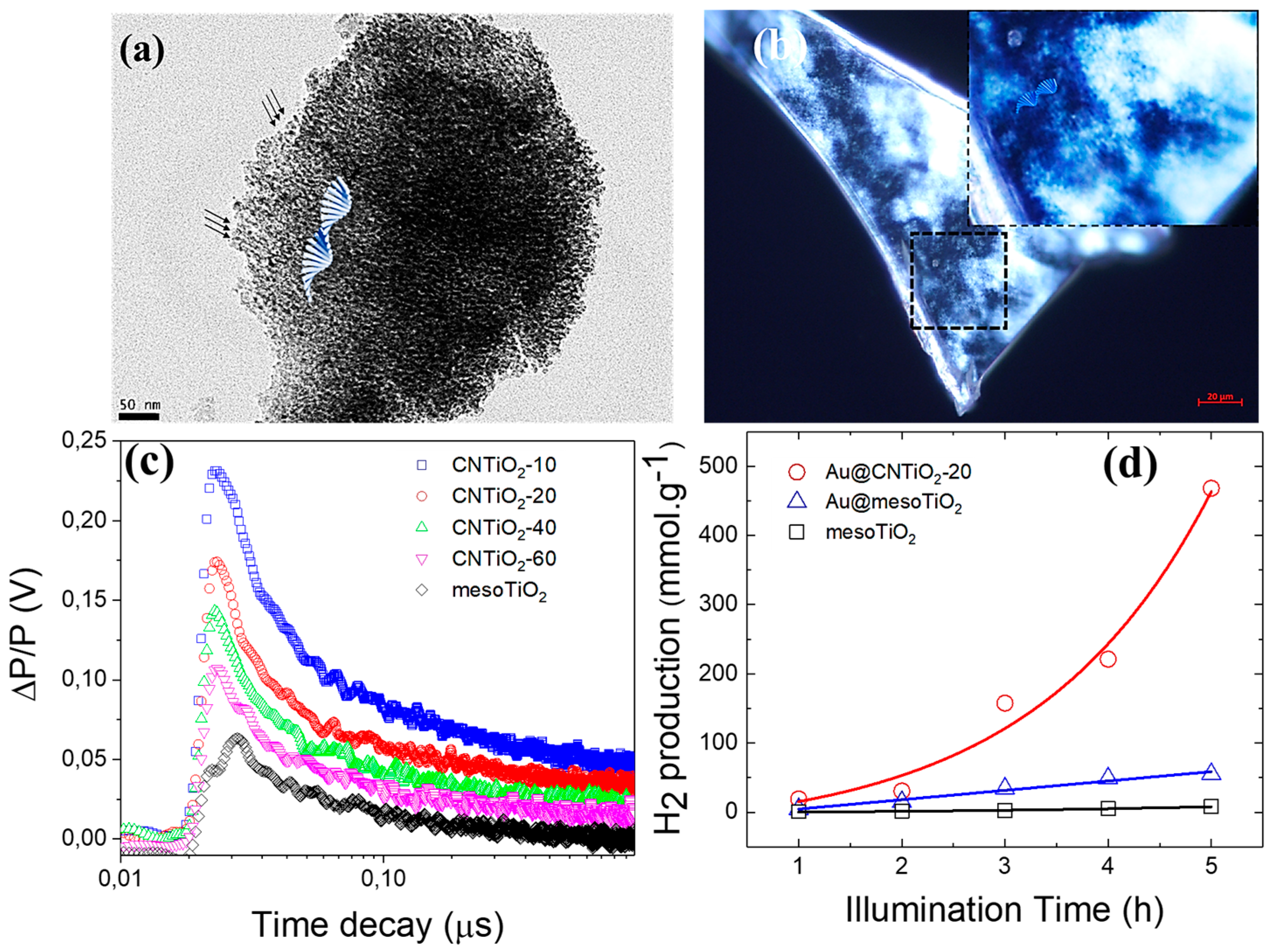
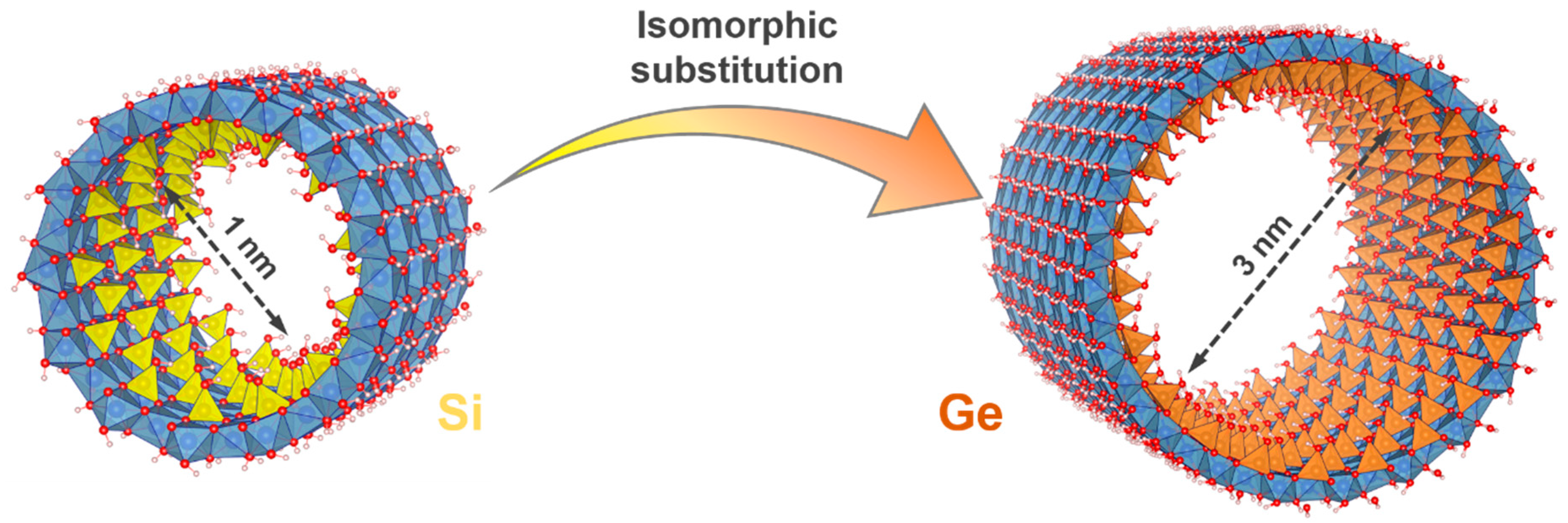

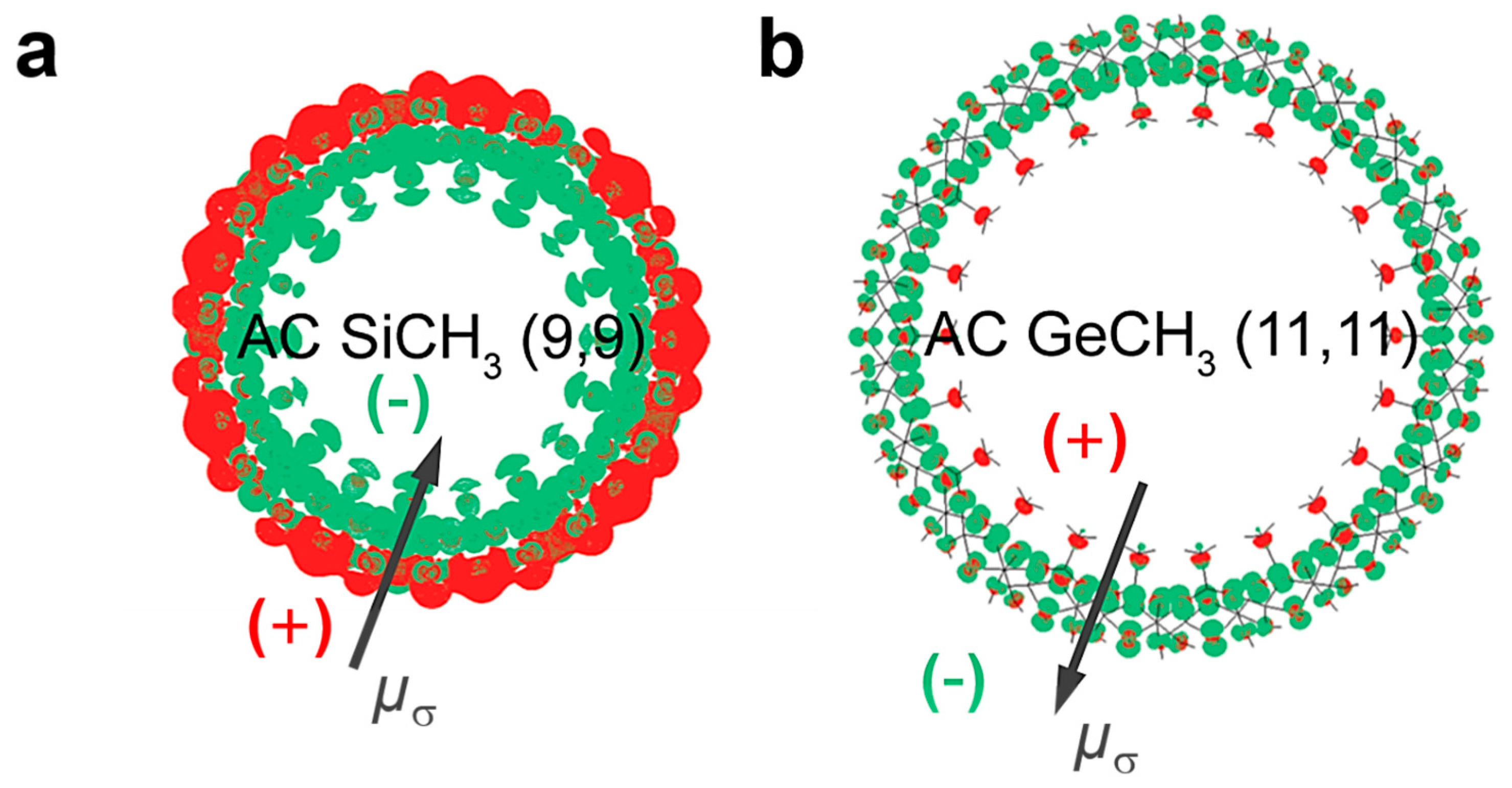
| Photocatalysts | Sacrificial Agent | HER Rate | Stability | Ref |
|---|---|---|---|---|
| In2S3/CuS | Na2S/Na2SO3 along with 3M H2S | 14,950 μmol g−1 h−1 | 13 h | [25] |
| CuS@CdS | Na2S/Na2SO3 | 11,140 μmol g−1 h−1 | 100 h | [26] |
| CuS/ZnS | Na2S/Na2SO3 | 4147 μmol g−1 h−1 | No data | [27] |
| NiS/TNTs | methanol | 7486 μmol g−1 h−1 | No data | [28] |
| NiS/NBTNs | methanol | 3170 μmol g−1 h−1 | 14 h | [29] |
| NiS/CdS | lignin and lactic acid | 1512.4 μmol g−1 h−1 | 15 h | [30] |
| NiS/ZnIn2S4 | Na2S/Na2SO3 | 2094 μmol g−1 h−1 | 15 h | [31] |
| NiS/HNb3O8 | Triethanolamine (TEOA) | 1519.4 μmol g−1 h−1 | 28 h | [32] |
| NiS/ZnxCd1−xS | Na2S/Na2SO3 | 16780 μmol g−1 h−1 | 20 h | [33] |
| NiS/C3N4 | TEOA | 482 μmol g−1 h−1 | 24 h | [34] |
| ZnMoS4/ZnO/CuS | Na2S/Na2SO3 | 38,220 μmol g−1 h−1 | 8 h | [35] |
| CuS/CdIn2S4/ZnIn2S4 | Na2S/Na2SO3 | 358.4 μmol g−1 h−1 | No data | [36] |
| NiS/CdS/TiO2 | Na2S/Na2SO3 | 2149 μmol g−1 h−1 | 16 h | [37] |
| CQDs/NiS/ZnIn2S4 | TEOA | 600 μmol g−1 h−1 | 15 h | [38] |
| NiS/CDs/CdS | Na2S/Na2SO3 | 1444.5 μmol g−1 h−1 | 15 h | [39] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Jiménez-Calvo, P.; Paineau, E.; Ghazzal, M.N. Metal Chalcogenides Based Heterojunctions and Novel Nanostructures for Photocatalytic Hydrogen Evolution. Catalysts 2020, 10, 89. https://doi.org/10.3390/catal10010089
Li J, Jiménez-Calvo P, Paineau E, Ghazzal MN. Metal Chalcogenides Based Heterojunctions and Novel Nanostructures for Photocatalytic Hydrogen Evolution. Catalysts. 2020; 10(1):89. https://doi.org/10.3390/catal10010089
Chicago/Turabian StyleLi, Jian, Pablo Jiménez-Calvo, Erwan Paineau, and Mohamed Nawfal Ghazzal. 2020. "Metal Chalcogenides Based Heterojunctions and Novel Nanostructures for Photocatalytic Hydrogen Evolution" Catalysts 10, no. 1: 89. https://doi.org/10.3390/catal10010089
APA StyleLi, J., Jiménez-Calvo, P., Paineau, E., & Ghazzal, M. N. (2020). Metal Chalcogenides Based Heterojunctions and Novel Nanostructures for Photocatalytic Hydrogen Evolution. Catalysts, 10(1), 89. https://doi.org/10.3390/catal10010089







