Abstract
Alkali earth metal molybdates (MMoO4, M = Mg, Ca, Sr, and Ba) were investigated as catalysts for the selective oxidation of methanol to formaldehyde in the search for more stable alternatives to the current industrial iron molybdate catalyst. The catalysts were prepared by either sol-gel synthesis or co-precipitation with both stoichiometric ratio (Mo:M = 1.0) and 10 mol% to 20 mol% excess Mo (Mo:M = 1.1 to 1.2). The catalysts were characterized by X-ray diffraction (XRD), nitrogen physisorption, Raman spectroscopy, temperature programmed desorption of CO2 (CO2-TPD), and inductively coupled plasma (ICP). The catalytic performance of the catalysts was measured in a lab-scale, packed bed reactor setup by continuous operation for up to 100 h on stream at 400 °C. Initial selectivities towards formaldehyde of above 97% were achieved for all samples with excess molybdenum oxide at MeOH conversions between 5% and 75%. Dimethyl ether (DME) and dimethoxymethane (DMM) were the main byproducts, but CO (0.1%–2.1%) and CO2 (0.1%–0.4%) were also detected. It was found that excess molybdenum oxide evaporated from all the catalysts under operating conditions within 10 to 100 h on stream. No molybdenum evaporation past the point of stoichiometry was detected.
1. Introduction
Formaldehyde is a major industrial chemical used in the production of urea-, phenol-, melamine, and polyacetal resins, accounting for 70% of the consumption, but with other important products as well [1]. Formaldehyde is commercially available as formalin, which is an aqueous solution of formaldehyde (typically 37 wt.%), as formaldehyde is very reactive and, in pure form, polymerizes to the solid paraformaldehyde. In 2017, the annual production was 50 million metric tons of formalin [2] and it is expected to grow to 60 million tons per year in the late 2020s [3], with a growth rate of 5.76% per year of the market value from 2018 to 2022 [4].
The synthesis of formaldehyde was first reported by Butlerov in 1859, and industrial production started in Germany in 1889. There are two processes applied industrially today—the silver process, which was patented in 1910, and the Formox process using a metal oxide catalyst, which was first patented in 1921 [5]. Today, around two-thirds of the global capacity is based on the Formox technology [6].
In the Formox process, an iron molybdate catalyst with excess molybdenum oxide (MoO3/Fe2(MoO4)3) has been used since 1952 [7], giving overall plant yields of 90%–95% depending, among other things, on the degree of catalyst deactivation [3]. The main cause of deactivation for the industrial catalyst is the volatilization of molybdenum species [8,9,10], which leads to a catalyst life time of only 12–18 months [7]. The active phase has been shown to be a thin amorphous surface layer of MoOx [11,12,13,14,15,16], where the role of the crystalline MoO3 is only to replenish the MoOx surface upon evaporation [11]. Thus, it is of major interest to either increase the stability of the iron molybdate or find alternative catalysts with similar activity and selectivity, but better stability and less volatile components. In the literature, much investigation has gone into various vanadium-based systems such as vanadium phosphates (90%–96% selectivity) [17,18], iron vanadates (90% selectivity) [3,19,20,21,22,23,24,25,26], supported V2O5 [27,28,29,30,31,32], and other vanadium-based catalysts [33,34]. The vanadium-based catalysts mostly show good activity, lower selectivity than iron molybdate, and the vanadium is also subject to volatilization [22,35]. On the other hand only few publications looking into alternative molybdate catalysts are found in the literature [3,36,37,38], and have mostly been done by Popov et al. [36,37,38] during the 1970s and 1980s and, more recently, by the Andersson group at Lund University (Sweden) [3,20]. Unfortunately, details on the stability of the systems are often not provided, even though this is the main motivation for finding an alternative catalyst system for the industry. The iron molybdate catalysts are deliberately sintered during the synthesis to decrease the activity of the catalyst for better temperature control; hence the activity is not an issue for the industry, though this is often the focus in the scientific literature. The CaMoO4 system was tested by Popov et al. [38] for methanol oxidation, achieving 96% selectivity to formaldehyde, and by Said and Goda [39] for the anaerobic dehydrogenation to anhydrous formaldehyde, where a yield of 98% was reported. Also, SrMoO4 and BaMoO4 have been tested by Popov et al. [36], but the selectivities to CH2O, CO, and CO2 were not clearly presented.
In this study, an investigation of alkali earth metal molybdates as catalysts for the selective oxidation of methanol to formaldehyde was conducted with respect to selectivity, activity, and, importantly, stability for a period of up to 100 h under conditions of accelerated deactivation at 400 °C. This is correlated to the catalyst composition and structure determined by nitrogen physisorption using BET theory, X-ray diffraction (XRD), Raman spectroscopy, inductively coupled plasma (ICP), and surface basicity by temperature programmed desorption of CO2 (CO2-TPD). The potential of using the alkali earth metal molybdates for industrial production of formaldehyde is discussed.
2. Results
2.1. Characterization
2.1.1. XRD, BET, and ICP
All the catalyst samples were characterized by X-ray powder diffraction both as fresh and spent samples, see Figure 1.
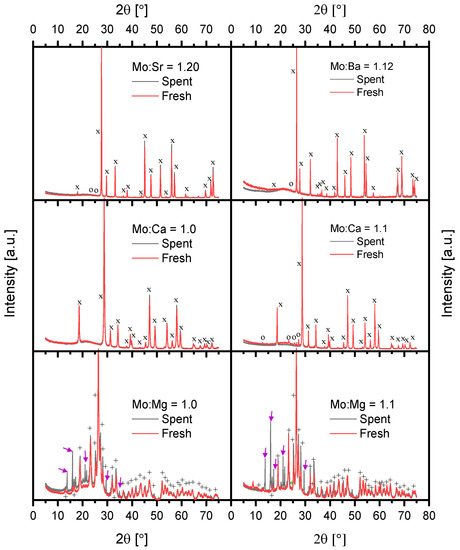
Figure 1.
Normalized X-ray powder diffractograms for all catalyst samples. Symbols mark the peaks for the different phases. x = MMoO4 (M = Ca, Sr, Ba) with a scheelite-CaWO4 tetragonal structure, + = MgMoO4 with a CoMoO4 monoclinic structure, o = MoO3 with orthorhombic structure, * = Mo3O9·H2O from the ICSD database [40] (collection codes: 20418, 194078, 239418, 193795, 35076, 420492). Arrows: Reflections unaccounted for.
The diffractograms of CaMoO4, SrMoO4, and BaMoO4 were very similar as they all had the scheelite-CaWO4 structure. The peaks shifted towards lower diffraction angles owing to the increasing size of the tetragonal unit cell when going down the alkali earth group. MgMoO4 displayed several peaks in comparison, because the unit cell of MgMoO4 is monoclinic. The phase compositions from Rietveld analysis as well as the crystallite sizes for the fresh catalysts are shown in Table 1.

Table 1.
Overview of the stoichiometric ratios between molybdenum and the alkali earth metal (M = Mg, Ca, Sr, or Ba), the specific surface area (SSA) measured by BET, and the phase composition and crystallite size of the stoichiometric molybdate determined by XRD on fresh samples.
All the fresh samples consisted of a stoichiometric alkali earth metal molybdate phase and, for the cases where Mo was in excess, one additional molybdenum oxide phase (see Table 1). The Mo3O9·H2O phase was believed to come from solvation of MoO3 by water in the air and is expected to dehydrate under operating conditions. It should be noted that all the molybdenum phases have Mo in the +6 oxidation state. The crystallite size increased down through the group and, in addition, samples with excess Mo had larger crystal sizes compared with the stoichiometric samples (see Table 1), indicating that molybdenum promotes sintering. These observations from XRD were in agreement with the trends for the specific surface area determined by BET, as the specific surface area decreased down through the group, and the samples with excess Mo had lower specific surface areas (see Table 1). For information on the effect of calcination temperature, see Tables S1–S5 and Figures S1–S4 in the Supplementary Material (SM). For the spent Mo:Ba = 1.12, Mo:Sr = 1.20, and Mo:Ca = 1.1 samples, it was observed that the peaks from excess Mo (MoO3 and BaMo3O10) had disappeared after the activity tests and no new peaks appeared (Figure 1). Thus, the catalysts were phase pure molybdates after the reaction. There were no differences between the spent Mo:Ca = 1.1 and spent Mo:Ca = 1.0 samples. The Mo:Mg = 1.0 and Mo:Mg = 1.1 samples showed the formation of new peaks after the reaction, but also the disappearance of the excess MoO3. The new peaks were mostly visible for the Mo:Mg = 1.1 catalyst. The new peaks were likely a partially reduced Mo phase, as it was most visible in the Mo:Mg = 1.1 catalyst. The HighScore+ and software in combination with the ICDD PDF-4+ database and the Topas 4 software were used in an attempt to index the additional peaks, however, no reasonable match could be identified.
2.1.2. Raman Spectroscopy
All samples were investigated with Raman spectroscopy both as fresh samples and after the catalytic tests.
The spectrum for the fresh Mo:Mg = 1.1 (Figure 2a) showed clear bands from crystalline MoO3 as peaks at 994, 818, and 666 cm−1, but the double peak at 282 and 290 cm−1 were also visible, which corresponds to the peaks described for MoO3 by various authors [41,42,43] (ID: R100217). A very small peak has also been reported for MoO3 at 471 cm−1 [42], which was also seen for the fresh Mo:Mg = 1.1. The peak at 740 cm−1 could indicate the presence of MoO2 [44,45], which was not seen by XRD. If comparing the spectra to the literature on MgMoO4, the general shape of the Mo:Mg = 1.1 spent and Mo:Mg = 1.0 spent samples were in good agreement with these spectra [41,42]. The peaks were reported at 116, 153, 178, 204, 277, 335, 349, 371, 384, 856, 910, 958, and 970 cm−1, with small “shoulders” at 302, 425, and 876 cm−1 [41,42]. This leaves one clear, non-explained peak at 551 cm−1, which was especially well defined for the spent Mo:Mg = 1.1 sample. This may be the same non-identified phase observed with XRD. From the spectra, it was observed that the free MoO3 in the Mo:Mg = 1.1 catalyst evaporated during the 100 h on stream, leaving only the MgMoO4 phase. The spectrum for Mo:Mg = 1.0 fresh was not obtainable because of fluorescence.
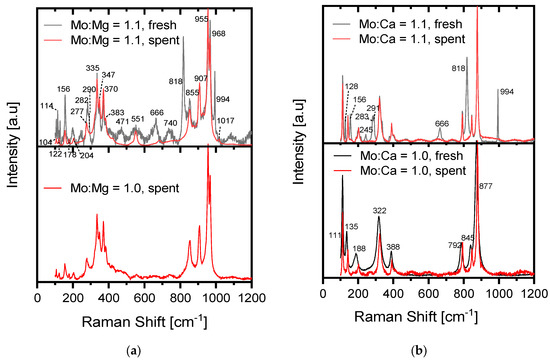
Figure 2.
Raman spectra measured for (a) magnesium molybdate and (b) calcium molybdate catalysts before and after 100 h on stream at 400 °C.
Also, the Mo:Ca = 1.1 sample was synthesized to have 10 mol% excess of Mo, which, from the XRD, was found as crystalline MoO3. For the fresh catalyst, a MoO3 phase was also found by Raman spectroscopy (Figure 2b), as there were distinguished peaks at 994, 818, and 666 cm−1, and the double peak at 291 and 283 cm−1 [41,42,43] (ID: R100217). However, the spent Mo:Ca = 1.1 catalyst no longer displayed the peaks attributed to MoO3. The spent Mo:Ca = 1.1 catalyst exhibited the same peaks as the Mo:Ca = 1.0 catalyst did (both fresh and spent), showing that all excess MoO3 evaporated from the Mo:Ca = 1.1 sample, and that no free MoO3 was present in the Mo:Ca = 1.0 sample (neither fresh or spent). Furthermore, the Mo:Ca = 1.0 catalyst did not show any change in the peaks after the reaction, only smaller variations in the relative magnitude of the peaks. The peaks could be assigned to the CaMoO4 phase, which has all of the peaks at 877, 845, 792, 388, 322 [43] (ID:R100180, R050355) [46,47], 188 cm−1 [47] (192 cm−1 [46]), and 111 cm−1 [43] (ID: R050355) [46,47]. However, the position of the last peak at 135 cm−1 differed from the value of 145 cm−1 reported in the literature [43] (ID:R100180, R050355) [46,47]. There was no evidence of the molybdenum evaporating from the CaMoO4 phase under reaction conditions by Raman, as there were no signs of CaO, Ca(OH)2, or CaCO3 in the spectra (peaks at 155, 282, 680, 713, and 1080 cm−1) [48].
For the Mo:Sr = 1.20 sample (Figure 3a), the excess of Mo was again found as MoO3 from the peaks at 994, 818, 666, and 290 cm−1 [41,42,43] (ID: R100217), in agreement with the XRD results for the fresh catalyst. After 8 h on stream, the sample no longer exhibited the peaks at 994, 818, 666, 290, 240, 154, and 127 cm−1. Thus, the MoO3 had evaporated already after 8 h on stream, which was also observed for an iron molybdate catalyst [49]. The peaks at 885, 842, 793, 380, 366, 326, 231, and 181 cm−1 were attributed to the vibrational modes of the MoO42− in SrMoO4 [46,47]. Additionally, SrMoO4 was reported to have external optical modes at 111, 137, and 157 cm−1 [46,47], thus accounting for all the observed bands in the Raman spectrum. Comparing the spectrum of the spent strontium molybdate catalyst with the spectrum of the CaMoO4 (Mo:Ca = 1.0) catalyst, the similarity was striking. The same applies for comparing with the spent barium molybdate catalyst (Figure 3b). This is also in agreement with peaks for BaMoO4 in the literature at 891, 838, 792, 360, 346, 325, 189, 143, 136, and 107 cm−1. This shows that in all three cases, the spent samples consisted of the stoichiometric molybdates, as these all have a scheelite structure and exhibit bands from the same symmetry operations. For the fresh barium molybdate catalyst, it was found that it did not contain the excess Mo in the form of MoO3, in agreement with XRD; however, it did show extra bands compared with the spent catalyst. The spent catalyst only showed the BaMoO4 bands [46,50] (890, 837, 790, 359, 346, 324, 185, 132, and 107 cm−1, all reported with small variations) after only 8 h on stream. The additional peaks in the fresh barium molybdate catalyst were likely attributed to the BaMo3O10 phase found by XRD, however, a reference Raman spectrum was not found.
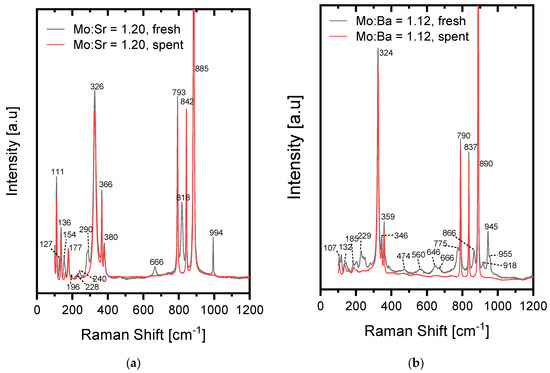
Figure 3.
Raman spectra measured for (a) strontium molybdate and (b) barium molybdate catalysts before and after 8 h on stream at 400 °C.
2.2. Catalytic Activity
The activity and selectivity of the catalysts were evaluated at 250 °C, 300 °C, 350 °C, and 400 °C in a lab-scale, packed bed reactor. The results are shown as the corrected selectivity versus the corrected conversion in Figure 4. Furthermore, the samples were compared to a molybdenum trioxide/iron molybdate industrial reference catalyst.
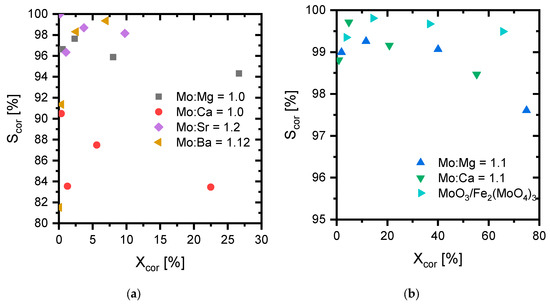
Figure 4.
Reversible byproducts corrected selectivity towards formaldehyde vs. the corrected conversion obtained by measurements at 250 °C, 300 °C, 350 °C, and 400 °C, in a flow of 150 NmL/min with MeOH/O2/N2 = 5/10/85 molar ratio with 25 mg of 150–250 µm catalyst diluted in 150 mg SiC. (a) catalysts with low maximum conversion; (b) catalysts with high maximum conversion.
The mass based activity decreased down the group (i.e., with increasing molar mass) for the samples with excess Mo, which was in correspondence with the tendency of the specific surface areas shown in Table 1. It should be noted that the MgMoO4 and CaMoO4 samples with Mo:M = 1.1 behaved almost identically. Both the selectivity and activity of the stoichiometric samples were significantly lower, even though the surface areas were significantly higher. Thus, the corrected selectivity, for all catalysts with an excess molybdenum oxide phase detected by XRD, was above 97%. However, they do not have quite as high selectivity as the industrial reference for which the selectivity was above 99.3%. The individual selectivities and activity are visualized in Figure 5 and Figure 6, and full details are shown in Tables S6 and S7 and Figure S5 in the SM. See also Tables S8 and S13 and Figures S6 and S8 for information on the calcination temperature and preparation method.

Figure 5.
Selectivities and conversions for the alkali earth metal molybdates at 250 °C, 300 °C, 350 °C, and 400 °C in a flow of 150 NmL/min with MeOH/O2/N2 = 5/10/85 molar ratio with 25 mg of 150–250 µm catalyst diluted in 150 mg SiC.
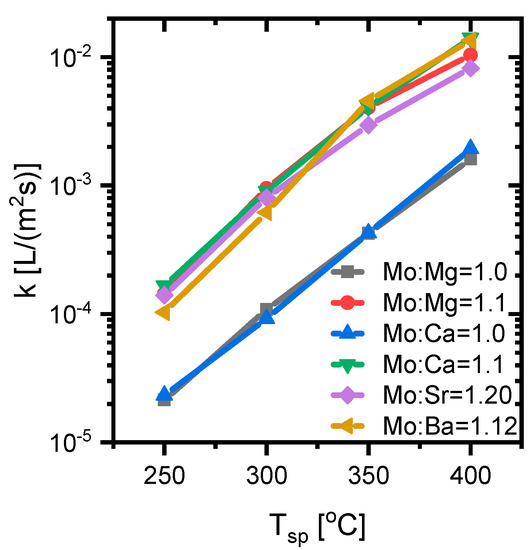
Figure 6.
Non-corrected, surface area based rate constants for the alkali earth metal molybdates at 250 °C, 300 °C, 350 °C, and 400 °C, in a flow of 150 NmL/min with MeOH/O2/N2 = 5/10/85 molar ratio with 25 mg of 150–250 µm catalyst diluted in 150 mg SiC.
The selectivity towards DMM was significant for most samples, except for the BaMoO4 catalyst and the MgMoO4 catalyst with Mo:Mg = 1.0. The presence of the over-oxidation products (CO and CO2) indicates the presence of sites with stronger basic character [51]. All the samples also formed some DME, which was generally recognized to stem from strong acidic sites [51]. All the samples with excess Mo had a surface area normalized, non-corrected activity on the order of 1 × 10−2 L/(m2·s) at 400 °C, which indicates that the excess Mo was important for the activity for all the samples. This may be explained by the excess MoO3 being needed to form a surface monolayer acting as the most active phase in the catalysts, in agreement with previous studies [11,12,13,14,15,16].
The stability of the samples was investigated with time on stream for up to 100 h at 400 °C, see Figure 7, which may be considered as the maximum temperature in the hot spot region in industrial reactors under normal operation. This was done in order to investigate accelerated deactivation corresponding to a longer time on stream at a lower temperature in the industrial reactor.
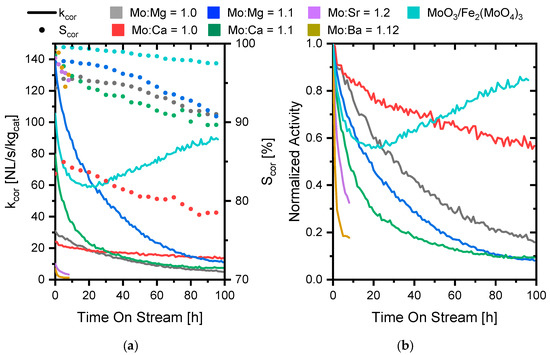
Figure 7.
(a) Activity and reversible byproduct corrected selectivity towards formaldehyde (b) normalized activity (normalized activity = kcor(t)/kcor(t = 0)) vs. time on stream at 400 °C, in a flow of 150 NmL/min with MeOH/O2/N2 = 5/10/85 molar ratio with 25 mg of 150–250 µm catalyst diluted in 150 mg SiC.
The selectivity and activity of all the samples (including the industrial reference) decreased with time on stream, but the industrial reference reactivated after about 20 h on stream. The reactivation was observed previously by Raun et al. [49], who found that the reactivation started when the excess crystalline MoO3 had evaporated from the catalyst. For the stoichiometric Ca- and MgMoO4, an initial increase in the selectivity was also observed within the first 10 h (Figure 7a), which was believed to stem from a restructuring of the Mo on the surface, as the surface of mixed metal oxide catalysts was reported to consist of only a single metal oxide under reaction conditions [52].
The stability of the catalysts with Mo in excess decreased down through the group, so the BaMoO4 lost 80% of the activity within 8 h, whereas it took 37 h and 55 h for the CaMoO4 and MgMoO4, respectively, to lose 80% activity. After 100 h on stream, the activity of the catalysts with excess Mo came close to the activity of the stoichiometric molybdates and, in the case of MgMoO4, the selectivities of the two samples were also identical after 100 h. This indicates that the catalysts became very similar once the excess molybdenum evaporated. CaMoO4 with excess MoO3 even became less active than the stoichiometric sample, though the selectivity did not fall to the same level. Comparing the surface area based activity of the samples after 100 h (with an assumption of no change in surface area to be able to make the comparison, as the sample size was too small to measure the change in surface area by BET), the rate constants became very close, being 1.1 × 10−3 L/(m2·s) and 1.3 × 10−3 L/(m2·s) for the Mo:Ca = 1.0 and 1.1, respectively. For MgMoO4, the same comparison showed 2.6 × 10−4 L/(m2·s) and 8.3 × 10−4 L/(m2·s) for the Mo:Mg = 1.0 and 1.1 samples, respectively, which is not as close as for the CaMoO4 samples. The reason for this difference is perhaps the assumption of no change in the surface area, and it is likely that the two samples approach each other with further time on stream.
3. Discussion
The Raman spectra showed that excess molybdenum oxide evaporated from all samples under reaction conditions of 400 °C, 150 NmL/min of 5% MeOH, 10% O2 and 85% N2, and 25 mg catalyst, yielding only the stoichiometric alkali earth metal molybdates. This was shown to happen within 8 h for the strontium and barium molybdates. The stoichiometric molybdates, however, did not undergo significant structural or chemical changes during the time on stream, as the Raman spectra before and after reaction were essentially identical. This is different from the industrial MoO3/Fe2(MoO4)3 catalysts, where the Mo has been shown by Raun et al. [49] to evaporate from the ferric molybdate phase, first forming ferrous molybdate and then iron oxide: Fe2(MoO4)3 →FeMoO4 →Fe2O3.
The corrected formaldehyde selectively measured initially was on a similar level, between 94% and 99% for all samples, when the conversion was large enough to measure selectivity accurately (Figure 4, when above 2% conversion approximately), except for the stoichiometric CaMoO4 sample. Also, with time on stream, the selectivity of this sample was significantly lower than the selectivities of the other samples. The low selectivity of the stoichiometric CaMoO4 could stem from small variations in the surface Mo distribution leading to Mo deficient zones, which is detrimental to the formaldehyde selectivity, as shown by Popov et al. [38]. Increased selectivity towards over-oxidation products (MF, CO, and CO2) owing to increased basicity/nucleophilicity has also been reported after the addition of K2O to a V2O5/Al2O3 catalyst by Wachs et al. [29], from the difference in desorption products of methanol on SiO2, Al2O3, F/Al2O3, MgO, TiO2, ZrO2, ThO2, and Fe2O3 by Busca et al. [53], and proposed as a way of probing a catalyst surface by Tatibouet [51]. This was supported by the 3.5–9 times higher surface basicity (µmol CO2/g) for this sample as measured by CO2-TPD (see Figure S9 and Table S14 in the SM for more details). The SrMoO4 and BaMoO4 samples lost their selectivity at the same rate as the Mo:Ca = 1.1 sample, for the first 8 h on stream. This may point towards the MoO3 evaporation happening at the same rate, and thus the excess MoO3 in the calcium sample may mostly be gone after 8 h on stream, as seen with Raman spectroscopy for the strontium and barium samples.
The activity measurements showed that the activity and selectivity of the samples with and without initial excess of molybdenum became almost equal after 100 h on stream. Combined with the Raman and XRD measurements, this points towards having essentially the same catalyst in the reactor after operation independent of the initial Mo:M ratio.
From the literature [49,54], it seemed the MoO3 evaporation from the industrial catalyst pellets (ring shaped cylinders with outer diameter = 4.55 mm, hole diameter = 1.70 mm, and length = 4.00 mm) was a diffusion limited process, as it took much longer time for the excess MoO3 to disappear from pellets than the sieve fraction used here (150–250 µm), under similar conditions. Additionally, a reactor model indicated that reducing MoO3 evaporation only for the catalyst layer near the inlet might to a large extent solve the problem of increasing pressure drop [55]. In this study, the CaMoO4, MgMoO4, and Mo:Mg = 1.1 samples initially showed a smaller relative decrease of the activity than the industrial reference (Figure 7), and much less MoO3 per catalyst mass evaporated from them according to Raman spectroscopy. The selectivity of the CaMoO4 sample was probably too low to be used industrially. However, the MgMoO4 samples had high enough selectivity to be of interest as inlet layer catalyst, in front of the regular industrial catalyst, to partly convert the feed. In this way, the methanol concentration would be decreased and the problem of MoO3 evaporation from the iron molybdate catalyst would be less severe, as it would not experience as high methanol concentrations as normal. This could increase the longevity of the industrial type catalyst, but further studies are needed to confirm this.
4. Materials and Methods
4.1. Catalyst Synthesis
Two different methods, sol-gel and co-precipitation, were used to synthesize the catalysts in order to achieve sufficiently high phase purity, surface area, and catalytic activity of all the alkali earth metal molybdates. For the synthesis, Mg(NO3)2·6H2O (Alfa Aesar, 98%), Ca(NO3)2·4H2O (Sigma-Aldrich, puriss. P.a., ACS reagent, 99%–103%), citric acid C6H8O7·H2O (Sigma-Aldrich, 99.0%), (NH4)6Mo7O24·4H2O (Sigma-Aldrich, puriss. p.a., ACS reagent, 99.0% (T)), Sr(NO3)2 (Fluka, puriss. P.a. ACS; 99.0% (KT)), Ba(NO3)2 (Alfa Aesar, ACS, 99+%), and 25 wt.% aqueous NH3 (VWR AnalaR NORMAPUR) were used. Water was demineralized in all cases.
4.1.1. Sol-Gel
Samples with stoichiometric and 10 mol% excess of Mo were prepared for MgMoO4 and CaMoO4 using a citric acid sol-gel method. Mg(NO3)2·6H2O (5.16 g or 5.56 g) or Ca(NO3)2·4H2O (8.82 g or 9.45 g) was dissolved in 100 mL of H2O together with a 1/1 molar amount of C6H8O7·H2O and a 1/0.143 or 1/0.157 molar amount of (NH4)6Mo7O24·4H2O (giving Mo:M ratios of 1.0 and 1.1) (see Table S15 in the SM for full details). The water was evaporated in a rotary evaporator until the liquid became highly viscous and greenish. The samples were then slowly dried in an oven at 80 °C, where they swelled to multiple times the original liquid volume. The samples were calcined in static air in a muffle furnace at 500 °C for 4 h with a heating rate of 5 °C/min.
4.1.2. Co-Precipitation
For SrMoO4 and BaMoO4, a co-precipitation procedure was used to synthesize phase pure materials. Sr(NO3)2 (4.27 g) or Ba(NO3)2 (4.41 g) was dissolved in 100 mL of H2O together with a 1/1 molar amount of C6H8O7·H2O. Then, 10/7 molar amounts of (NH4)6Mo7O24·4H2O (giving Mo:M = 10 in the solution, see Table S16 in the SM for more details) were dissolved separately in 130 mL of H2O. The nitrate/citric acid solution was heated to 45 °C on a magnetic stirrer. The molybdate solution was added slowly under stirring and, finally, the pH was adjusted to 7.0 with 25 wt.% aqueous NH3. The solution was aged for 10 min at 45 °C under stirring, filtered hot, and washed with 600 mL of H2O. The samples were dried at 110 °C and subsequently calcined at 500 °C for 4 h with a heating rate of 5 °C/min.
4.2. Catalytic Activity and Selectivity
The samples were tested in a lab-scale, packed bed reactor setup described in detail elsewhere [49,56,57]. For the tests, 25 mg of sample in a 150–250 µm sieve fraction was diluted with 150 mg of SiC (150–355 µm sieve fraction). The bed was held in place by two quartz wool plugs. The flow rate was 150 NmL/min with 5 vol.% MeOH and 10 vol.% O2 in N2. The MeOH was fed by bubbling the gas mixture through liquid methanol kept at 5 °C. The temperature of the catalyst bed was controlled by a Siemens PLC running a PID loop with a LabVIEW application as the interface. A thermocouple touching the top of the catalyst bed was used to control the temperature. The effluent was analyzed by a ThermoFisher Trace Ultra GC equipped with both TCD and FID, quantifying MeOH, DME, DMM, MF, CO, and CO2. The catalyst temperature was ramped stepwise with activity measurements performed at 250 °C, 300 °C, 350 °C, and 400 °C (one GC analysis at each temperature, after 15 min of stabilization with 40 min in total at each temperature), after which the conditions were kept steady for up to 100 h (SrMoO4 and BaMoO4 were only kept for 8 h).
The selectivities and conversion were calculated based on the GC measurements on the effluent according to Equations (1)–(4).
where yi is the gas mole fraction of the ith product, and vi is the number of carbons in the ith product. Xcor and Scor are the reversible byproduct corrected conversion and selectivity towards formaldehyde. Here, DME was treated like two MeOH, DMM like two MeOH and one CH2O, and MF as one MeOH and one CO, as they would be converted back to these species at high MeOH conversion. This made the comparison between catalysts easier as the reversible byproduct corrected selectivity was mostly independent of temperature and conversion. This is further illustrated in Figure S10 in the SM. The reaction rate was assumed to follow (pseudo-)first order kinetics, as reported in the literature [58,59,60,61,62], and the rate constant was calculated from the design equation for a PFR reactor as in Equation (5) [63].
where V0 is the volumetric feed flow at reaction conditions, w is the catalyst mass, and X is the conversion. For the calculation of kcor (corrected rate constant), Xcor was used instead.
4.3. Characterization
4.3.1. X-Ray Powder Diffraction
The fresh samples were investigated with a PanAlytical X’Pert Pro instrument in Bragg-Brentano Geometry in reflectance mode using a Cu Kα radiation source (λ = 1.541 Å) at ambient conditions. The scan range was 5–70° with a step size of 0.017°. The Rietveld analysis was performed using the Topas Software. Spent samples were investigated using a PanAlytical Empyrean instrument using a Cu Kα radiation source (λ = 1.541 Å) mounted with a Ni beta filter, a pair of 0.04 radian soller slits, a beam stop, and a capillary spinner. The scan range was 5–75° with a step size of 0.14°. The samples were measured in open capillaries at ambient conditions. Relevant fresh samples were measured for comparison.
4.3.2. Surface Area Measurement
The surface areas of the samples were measured using a Quantachrome NOVATouch instrument and analyzed using the BET method. The samples were degassed at 350 °C for 4 h under vacuum before the nitrogen physisorption at 77 K with up to six points in the p/p0 range of 0.05–0.3, depending on the R2-value of the linear regression.
4.3.3. Elemental Analysis
The samples prepared by precipitation were subjected to inductively coupled plasma optical emission spectrometry (ICP-OES), to determine the Mo to alkali earth metal ratio. The samples were melted with K2S2O7 and dissolved with HCl. Calibration curves including a blank were made before measurement. The apparatus was a Perkin Elmer model Optima 3000, Agilent 720 ICP, or a similar instrument. Samples prepared by the sol-gel method were assumed to have the nominal composition.
4.3.4. Raman Spectroscopy
Raman spectroscopy was performed on all the samples as prepared (fresh) and after the performance tests (spent). The Raman spectra were recorded with a Horiba LabRam microscope at ambient conditions. For the measurement, a HeNe laser (red, λ = 633 nm) was used as a monochromatic light source, however, owing to large fluorescence, the Mo:Ca = 1.0 samples (both fresh and spent) were measured with an argon laser (blue, λ = 488 nm). The Mo:Mg = 1.0 fresh fluoresced so much that a Raman spectrum was not obtainable, neither by changing excitation wavelength or by initial dehydration. The raw data were baseline subtracted in OriginPro software using the Peak Analyzer. The spectra were normalized with respect to peak height before plotting.
4.3.5. CO2-TPD
Temperature programmed desorption of CO2 (CO2-TPD) was performed in the setup used for the activity test by a modification connecting a Pfeiffer Vacuum OMNIStarTM MS before the GC. For the measurements, 200 mg of sample was used, which was mounted between two quartz wool plugs. The samples were dried in a mixture of 10% O2 and 90% N2 to ensure no change in the surface. Full details on the procedure are given in the SM.
5. Conclusions
Alkali earth metal molybdates have successfully been synthesized by sol-gel synthesis and co-precipitation, as confirmed by XRD and Raman spectroscopy. The specific surface areas of the stoichiometric molybdates were higher than for the respective molybdates with excess MoO3, indicating that molybdenum promotes sintering. It was further observed that the surface area decreased down through group 2 (Mg > Ca > Sr > Ba). The most active catalyst (on mass basis) was the MgMoO4 catalyst with 10 mol% Mo in excess, which achieved over 75% conversion at 400 °C and more than 97% reversible byproduct corrected selectivity towards formaldehyde. All the catalysts with excess MoO3 had similar surface area based activity at 400 °C on the order of 1 × 10−2 L/(m2·s). Among the samples with excess MoO3, the MgMoO4 showed the highest stability through 100 h on stream, while the most stable catalyst tested was the stoichiometric CaMoO4 catalyst. However, this catalyst only had a corrected selectivity towards formaldehyde of 85%. The surface area based activity of the CaMoO4 catalysts with Mo:Ca = 1.0 and 1.1 catalysts was very similar after 100 h on stream, indicating that the catalyst with initial excess of Mo approached the stoichiometric samples, as excess Mo was lost by volatilization. This was substantiated by Raman measurements on fresh and spent samples. As the selectivity of the industrial reference was significantly better than even the Mo:Mg = 1.1 sample, and also with time on stream, the industrial applicability of these catalysts seemed limited. The Mo:Mg = 1.1 may be applicable as the inlet layer catalyst to increase the longevity of the current industrial catalyst, but further studies would be needed to confirm this.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/1/82/s1: Motivation for choice of different synthesis methods for the different alkali earth metal molybdates, XRD and BET analysis of additional samples by variation in calcination temperature and non-phase pure samples not reported in the main manuscript, full results of activity and selectivity measurements, and full details of sample preparation.
Author Contributions
Conceptualization, U.V.M., M.T., A.D.J., and M.H.; Formal analysis, J.T. and L.F.L.; Funding acquisition, A.D.J. and M.H.; Investigation, J.T.; Methodology, J.T., L.F.L., P.B., A.D.J., and M.H.; Visualization, J.T.; Writing—Original draft, J.T.; Writing—Review & editing, L.F.L., P.B., U.V.M., M.T., A.D.J., and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Independent Research Fund Denmark, grant number DFF-4184-00336.
Acknowledgments
Help by Caroline Piper Hem (XRD), Aino Nielsen (Raman), and other technical staff at Haldor Topsøe A/S with the characterization is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IHS Markit Formaldehyde. Available online: https://www.ihs.com/products/formaldehyde-chemical-economics-handbook.html (accessed on 28 September 2017).
- Andersson, L.-O. Informally Speaking A Formaldehyde Magazine from Johnson Mathey; Johnson Mathey: Hässleholm, Sweden, December 2017; p. 2. [Google Scholar]
- Andersson, A.; Holmberg, J.; Häggblad, R. Process Improvements in Methanol Oxidation to Formaldehyde: Application and Catalyst Development. Top. Catal. 2016, 59, 1589–1599. [Google Scholar] [CrossRef]
- Cision Global Formaldehyde Market 2018–2022. Available online: https://www.prnewswire.com/news-releases/global-formaldehyde-market-2018-2022-300633054.html (accessed on 25 April 2019).
- Franz, A.W.; Kronemayer, H.; Pfeiffer, D.; Pilz, R.D.; Reuss, G.; Disteldorf, W.; Gamer, A.O.; Hilt, A. Formaldehyde. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 1–34. [Google Scholar]
- Andersson, L.-O. Informally Speaking A Formaldehyde Magazine from Johnson Mathey; Johnson Mathey: Teeside, United Kingdom, February 2019; pp. 12–13. [Google Scholar]
- Gerberich, H.R.; Seaman, G.C. Formaldehyde. In Kirk-Othmer Encyclopedia of Chemical Technology; Elsevier Science: Amsterdam, The Netherlands, 2013; pp. 24–26. [Google Scholar]
- Andersson, A.; Hernelind, M.; Augustsson, O. A study of the ageing and deactivation phenomena occurring during operation of an iron molybdate catalyst in formaldehyde production. Catal. Today 2006, 112, 40–44. [Google Scholar] [CrossRef]
- Ivanov, K.I.; Dimitrov, D.Y. Deactivation of an industrial iron-molybdate catalyst for methanol oxidation. Catal. Today 2010, 154, 250–255. [Google Scholar] [CrossRef]
- Popov, B.I.; Bibin, V.N.; Boreskov, G.K. Study of an Iron-Molybdenum oxide catalyst for the oxidation of methanol to formaldehyde. IV. Entrainment of Molybdenum from the catalyst, the main reason for the decrease in its activity during use. Kinet. Katal. 1976, 17, 371–377. [Google Scholar]
- Routray, K.; Zhou, W.; Kiely, C.J.; Grünert, W.; Wachs, I.E. Origin of the synergistic interaction between MoO3 and iron molybdate for the selective oxidation of methanol to formaldehyde. J. Catal. 2010, 275, 84–98. [Google Scholar] [CrossRef]
- Söderhjelm, E.; House, M.P.; Cruise, N.; Holmberg, J.; Bowker, M.; Bovin, J.-O.; Andersson, A. On the Synergy Effect in MoO3–Fe2(MoO4)3 Catalysts for Methanol Oxidation to Formaldehyde. Top. Catal. 2008, 50, 145–155. [Google Scholar] [CrossRef]
- Bowker, M.; Brookes, C.; Carley, A.F.; House, M.P.; Kosif, M.; Sankar, G.; Wawata, I.; Wells, P.P.; Yaseneva, P. Evolution of active catalysts for the selective oxidative dehydrogenation of methanol on Fe2O3 surface doped with Mo oxide. Phys. Chem. Chem. Phys. 2013, 15, 12056. [Google Scholar] [CrossRef]
- House, M.P.; Shannon, M.D.; Bowker, M. Surface segregation in iron molybdate catalysts. Catal. Lett. 2008, 122, 210–213. [Google Scholar] [CrossRef]
- Brookes, C.; Wells, P.P.; Cibin, G.; Dimitratos, N.; Jones, W.; Morgan, D.J.; Bowker, M. Molybdenum Oxide on Fe2O3 Core–Shell Catalysts: Probing the Nature of the Structural Motifs Responsible for Methanol Oxidation Catalysis. ACS Catal. 2014, 4, 243–250. [Google Scholar] [CrossRef]
- Burcham, L.J.; Briand, L.E.; Wachs, I.E. Quantification of Active Sites for the Determination of Methanol Oxidation Turn-over Frequencies Using Methanol Chemisorption and in Situ Infrared Techniques. 1. Supported Metal Oxide Catalysts. Langmuir 2001, 17, 6164–6174. [Google Scholar] [CrossRef]
- Behera, G.C.; Parida, K. Selective gas phase oxidation of methanol to formaldehyde over aluminum promoted vanadium phosphate. Chem. Eng. J. 2012, 180, 270–276. [Google Scholar] [CrossRef]
- Behera, G.C.; Parida, K.; Dummer, N.F.; Whiting, G.; Sahu, N.; Carley, A.F.; Conte, M.; Hutchings, G.J.; Bartley, J.K. Tungstate promoted vanadium phosphate catalysts for the gas phase oxidation of methanol to formaldehyde. Catal. Sci. Technol. 2013, 3, 1558–1564. [Google Scholar] [CrossRef]
- Routray, K.; Zhou, W.; Kiely, C.J.; Wachs, I.E. Catalysis science of methanol oxidation over iron vanadate catalysts: Nature of the Catalytic Active sites. ACS Catal. 2011, 1, 54–66. [Google Scholar] [CrossRef]
- Massa, M.; Häggblad, R.; Hansen, S.; Andersson, A. Oxidation of methanol to formaldehyde on cation vacant Fe-V-Mo-oxide. Appl. Catal. A Gen. 2011, 408, 63–72. [Google Scholar] [CrossRef]
- Häggblad, R.; Hansen, S.; Wallenberg, L.R.; Andersson, A. Stability and performance of vacant Fe3−x−yVx□yO4 spinel phase catalysts in methanol oxidation. J. Catal. 2010, 276, 24–37. [Google Scholar] [CrossRef]
- Massa, M.; Häggblad, R.; Andersson, A. Cation Vacant Fe3−x−yVx□yO4 Spinel-Type Catalysts for the Oxidation of Methanol to Formaldehyde. Top. Catal. 2011, 54, 685–697. [Google Scholar] [CrossRef]
- Häggblad, R.; Wagner, J.B.; Hansen, S.; Andersson, A. Oxidation of methanol to formaldehyde over a series of Fe1−xAlx-V-oxide catalysts. J. Catal. 2008, 258, 345–355. [Google Scholar] [CrossRef]
- Routray, K.; Briand, L.E.; Wachs, I.E. Is there a relationship between the M{double bond, long}O bond length (strength) of bulk mixed metal oxides and their catalytic activity? J. Catal. 2008, 256, 145–153. [Google Scholar] [CrossRef]
- Klissurski, D.; Abadzhieva, N.; Kassabov, S.; Stefanov, P.; Kovacheva, D.; Uzunov, I. Selective oxidation of methanol to formaldehyde on iron vanadate catalyst. Comptes Rendus L’Academie Bulg. Sci. 2009, 62, 1073–1078. [Google Scholar]
- Heller, P.; Wells, P.P.; Gianolio, D.; Bowker, M. VOx/Fe2O3 Shell-Core Catalysts for the Selective Oxidation of Methanol to Formaldehyde. Top. Catal. 2018, 61, 357–364. [Google Scholar] [CrossRef]
- Burcham, L.J.; Wachs, I.E. The origin of the support effect in supported metal oxide catalysts: In situ infrared and kinetic studies during methanol oxidation. Catal. Today 1999, 49, 467–484. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Popova, G.Y.; Chesalov, Y.A.; Saraev, A.A.; Andrushkevich, T.V.; Bukhtiyarov, V.I. Active component of supported vanadium catalysts in the selective oxidation of methanol. Kinet. Catal. 2016, 57, 82–94. [Google Scholar] [CrossRef]
- Wang, X.; Wachs, I.E. Designing the activity/selectivity of surface acidic, basic and redox active sites in the supported K2O-V2O5/Al2O3 catalytic system. Catal. Today 2004, 96, 211–222. [Google Scholar] [CrossRef]
- Kim, T.; Wachs, I.E. CH3OH oxidation over well-defined supported V2O5/Al2O3 catalysts: Influence of vanadium oxide loading and surface vanadium-oxygen functionalities. J. Catal. 2008, 255, 197–205. [Google Scholar] [CrossRef]
- Isaguliants, G.V.; Belomestnykh, I.P. Selective oxidation of methanol to formaldehyde over V–Mg–O catalysts. Catal. Today 2005, 100, 441–445. [Google Scholar] [CrossRef]
- Vining, W.C.; Strunk, J.; Bell, A.T. Investigation of the structure and activity of VOx/CeO2/SiO2 catalysts for methanol oxidation to formaldehyde. J. Catal. 2012, 285, 160–167. [Google Scholar] [CrossRef]
- Yang, Y.; Du, G.; Lim, S.; Haller, G.L. Radius of curvature effect of V-MCM-41 probed by methanol oxidation. J. Catal. 2005, 234, 318–327. [Google Scholar] [CrossRef]
- Chen, S.; Ma, X. The role of oxygen species in the selective oxidation of methanol to dimethoxymethane over VOx/TS-1 catalyst. J. Ind. Eng. Chem. 2017, 45, 296–300. [Google Scholar] [CrossRef]
- Bronkema, J.L.; Leo, D.C.; Bell, A.T. Mechanistic Studies of Methanol Oxidation to Formaldehyde on Isolated Vanadate Sites Supported on High Surface Area Anatase. J. Phys. Chem. C 2007, 111, 14530–14540. [Google Scholar] [CrossRef]
- Popov, B.I.; Shkuratova, L.N.; Orlova, L.B. Effect of excess molybdenum trioxide on the activity and selectivity of some molybdates in methanol oxidation. React. Kinet. Catal. Lett. 1976, 4, 323–328. [Google Scholar] [CrossRef]
- Popov, B.I.; Bibin, V.N. Catalytic properties of bismuth molybdate and its constituent oxides in methanol oxidation. React. Kinet. Catal. Lett. 1975, 3, 337–341. [Google Scholar] [CrossRef]
- Popov, B.I.; Shkuratova, L.N.; Maksimov, Y.V.; Gustov, V.V. Catalytic properties and radiothermoluminescence of calcium molybdate with MoO3 additives. React. Kinet. Catal. Lett. 1982, 20, 293–297. [Google Scholar] [CrossRef]
- Said, A.E.A.A.; Goda, M.N. Superior catalytic performance of CaMoO4 catalyst in direct dehydrogenation of methanol into anhydrous formaldehyde. Chem. Phys. Lett. 2018, 703, 44–51. [Google Scholar] [CrossRef]
- Karlsruhe, F. Inorganic Crystal Structure Database ICSD. Available online: https://icsd.fiz-karlsruhe.de/search/basic.xhtml;jsessionid=83E66C26C80F40E7E12A0DAEC8C8C6AD (accessed on 14 August 2019).
- Chang, S.C.; Leugers, M.A.; Bare, S.R. Surface chemistry of magnesium oxide-supported molybdenum oxide: An in situ Raman spectroscopic study. J. Phys. Chem. 1992, 96, 10358–10365. [Google Scholar] [CrossRef]
- Vrieland, G.E.; Murchison, C.B. Anaerobic oxidation of butane to butadiene over magnesium molybdate catalysts. I. Magnesia supported catalysts. Appl. Catal. A Gen. 1996, 134, 101–121. [Google Scholar] [CrossRef]
- RRUFF project. Available online: http://rruff.info/ (accessed on 14 August 2019).
- Zhang, Q.; Li, X.; Ma, Q.; Zhang, Q.; Bai, H.; Yi, W.; Liu, J.; Han, J.; Xi, G. A metallic molybdenum dioxide with high stability for surface enhanced Raman spectroscopy. Nat. Commun. 2017, 8, 14903. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, H.; Li, J.; Yue, X.; Han, Y.; Shen, P.K.; Cui, Y. Porous MoO2 Nanosheets as Non-noble Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Mater. 2016, 28, 3785–3790. [Google Scholar] [CrossRef]
- Liegeois-Duyckaerts, M.; Tarte, P. Vibrational studies of molybdates, tungstates and related compounds-I. New infrared data and assignments for the scheelite-type compounds XIIMoO4 and XIIWO4. Spectrochim. Acta Part A Mol. Spectrosc. 1972, 28, 2029–2036. [Google Scholar] [CrossRef]
- Porto, S.P.S.; Scott, J.F. Raman spectra of CaWo4, SrWo4, CaMoo4, and SrMoo4. Phys. Rev. 1967, 157, 716–719. [Google Scholar] [CrossRef]
- Schmid, T.; Dariz, P. Shedding light onto the spectra of lime: Raman and luminescence bands of CaO, Ca(OH)2 and CaCO3. J. Raman Spectrosc. 2014, 46, 141–146. [Google Scholar] [CrossRef]
- Raun, K.V.; Lundegaard, L.F.; Chevallier, J.; Beato, P.; Appel, C.C.; Nielsen, K.; Thorhauge, M.; Jensen, A.D.; Høj, M. Deactivation behavior of an iron-molybdate catalyst during selective oxidation of methanol to formaldehyde. Catal. Sci. Technol. 2018, 8, 4626–4637. [Google Scholar] [CrossRef]
- Marques, A.P.A.; Picon, F.C.; Melo, D.M.A.; Pizani, P.S.; Leite, E.R.; Varela, J.A.; Longo, E. Effect of the order and disorder of BaMoO4 powders in photoluminescent properties. J. Fluoresc. 2008, 18, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Tatibouët, J.M. Methanol oxidation as a catalytic surface probe. Appl. Catal. A Gen. 1997, 148, 213–252. [Google Scholar] [CrossRef]
- Wachs, I.E.; Routray, K. Catalysis science of bulk mixed oxides. ACS Catal. 2012, 2, 1235–1246. [Google Scholar] [CrossRef]
- Busca, G.; Lamotte, J.; Lavalley, J.-C.; Lorenzelli, V.; Erba, C. FT-IR study of the adsorption and transformation of formaldehyde on oxide surfaces. J. Am. Chem. Soc. 1987, 109, 5197. [Google Scholar] [CrossRef]
- Raun, K.V.; Johannessen, J.; McCormack, K.; Appel, C.C.; Baier, S.; Thorhauge, M.; Høj, M.; Jensen, A.D. Modeling of the molybdenum loss in iron molybdate catalyst pellets for selective oxidation of methanol to formaldehyde. Chem. Eng. J. 2019, 361, 1285–1295. [Google Scholar] [CrossRef]
- Raun, K.V.; Thorhauge, M.; Høj, M.; Jensen, A.D. Modeling of molybdenum transport and pressure drop increase in fixed bed reactors used for selective oxidation of methanol to formaldehyde using iron molybdate catalysts. Chem. Eng. Sci. 2019, 202, 347–356. [Google Scholar] [CrossRef]
- Høj, M.; Kessler, T.; Beato, P.; Jensen, A.D.; Grunwaldt, J.D. Structure, activity and kinetics of supported molybdenum oxide and mixed molybdenum-vanadium oxide catalysts prepared by flame spray pyrolysis for propane OHD. Appl. Catal. A Gen. 2014, 472, 29–38. [Google Scholar] [CrossRef]
- Høj, M.; Jensen, A.D.; Grunwaldt, J.-D. Structure of alumina supported vanadia catalysts for oxidative dehydrogenation of propane prepared by flame spray pyrolysis. Appl. Catal. A Gen. 2013, 451, 207–215. [Google Scholar] [CrossRef]
- Cheshkova, K.T.; Bibin, V.N.; Popov, B.I. Kinetics of oxidation of methanol with air on a chromium-molybdenum oxide catalyst supported on porous α-Al2O3. React. Kinet. Catal. Lett. 1976, 4, 307–313. [Google Scholar] [CrossRef]
- Bhattacharyya, S.K.; Janakiram, K.; Ganguly, N.D. Kinetics of the Vapor-Phase Oxidation of Methyl Alcohol on Vanadium Pentoxide Catalyst. J. Catal. 1967, 18, 128–136. [Google Scholar] [CrossRef]
- Deshmukh, S.A.R.K.; Van Sint Annaland, M.; Kuipers, J.A.M. Kinetics of the partial oxidation of methanol over a Fe-Mo catalyst. Appl. Catal. A Gen. 2005, 289, 240–255. [Google Scholar] [CrossRef]
- Mann, R.S.; Dosi, M.K. Kinetics of the Vapor-Phase Oxidation of Methyl Alcohol on Vanadium Pentoxide-Molybdenum Trioxide Catalyst. J. Catal. 1973, 28, 282–288. [Google Scholar] [CrossRef]
- Mann, R.S.; Hahn, K.W. Kinetics of vapor-phase oxidation of methyl alcohol on Manganese Dioxide-Molybdenum Trioxide Catalyst. J. Catal. 1969, 15, 329–341. [Google Scholar] [CrossRef]
- Fogler, H.S. Elements of Chemical Reaction Engineering, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2016. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).