Interfacial Biocatalytic Performance of Nanofiber-Supported β-Galactosidase for Production of Galacto-Oligosaccharides
Abstract
1. Introduction
2. Results and Discussion
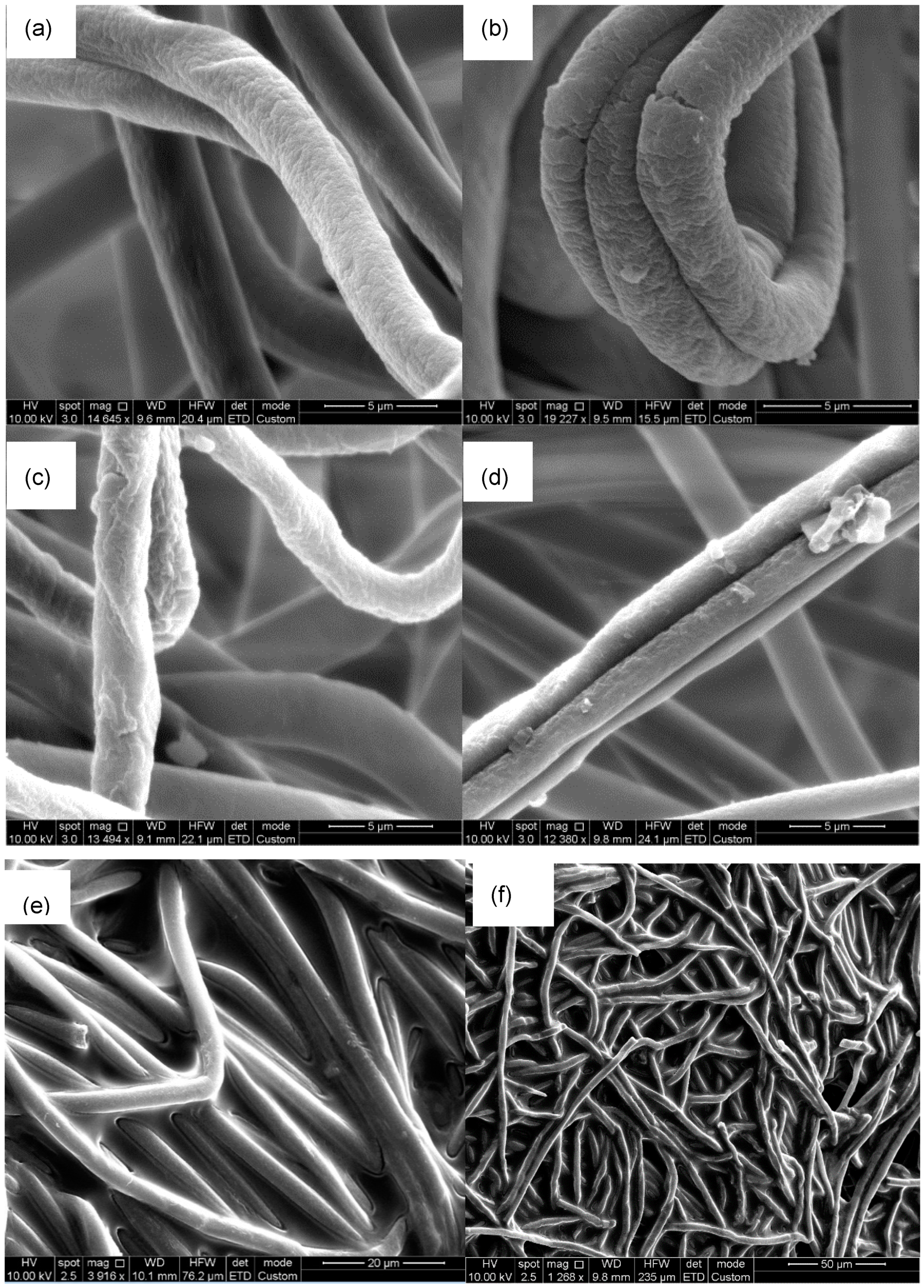
2.1. Textural Properties of Polymer Nanofibers
2.2. Physical Characterization of Nanofibers/β-Galactosidase Assembly
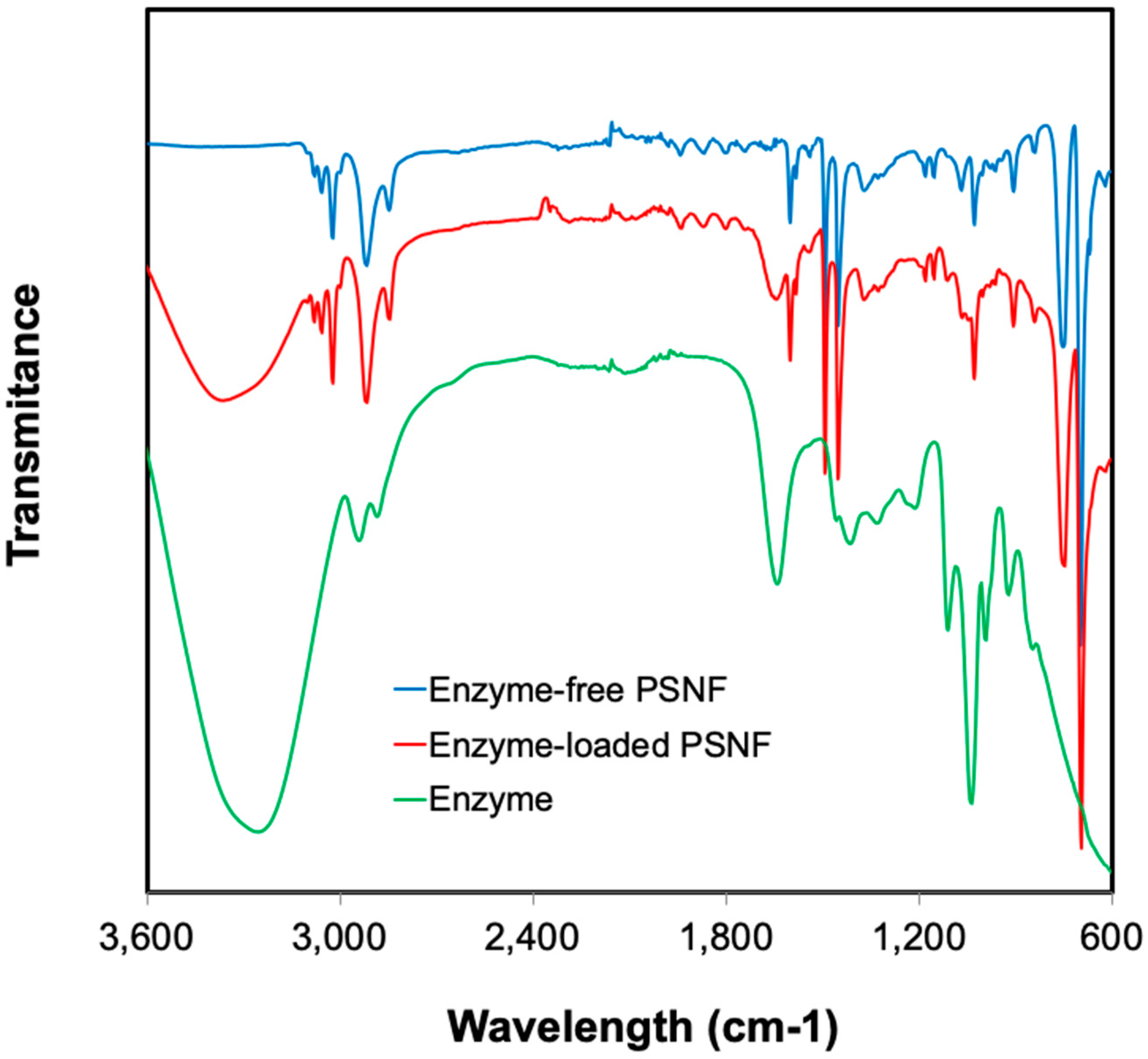
2.3. Chemical Characterization of β-Galactosidase/Nanofibers
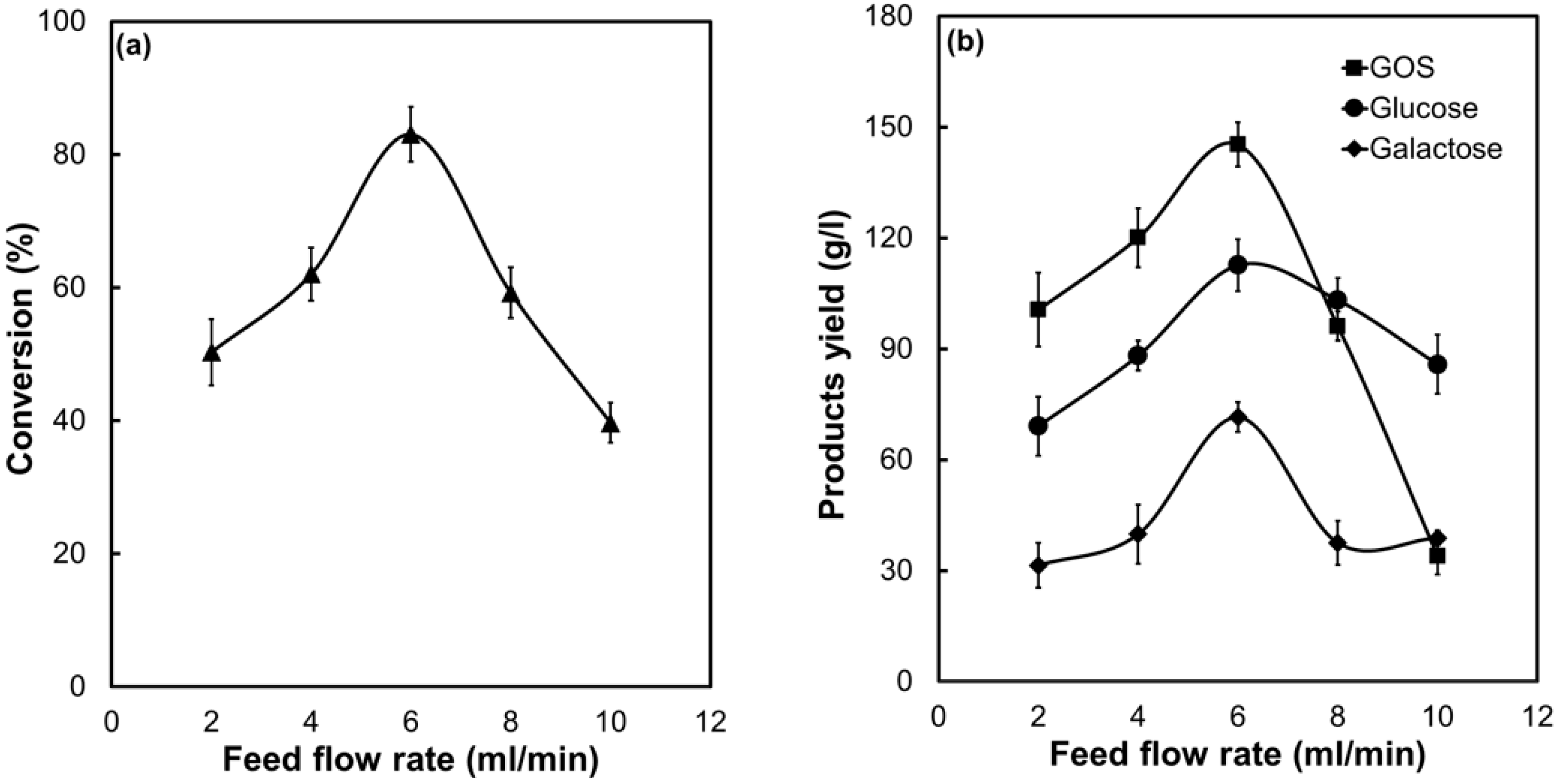
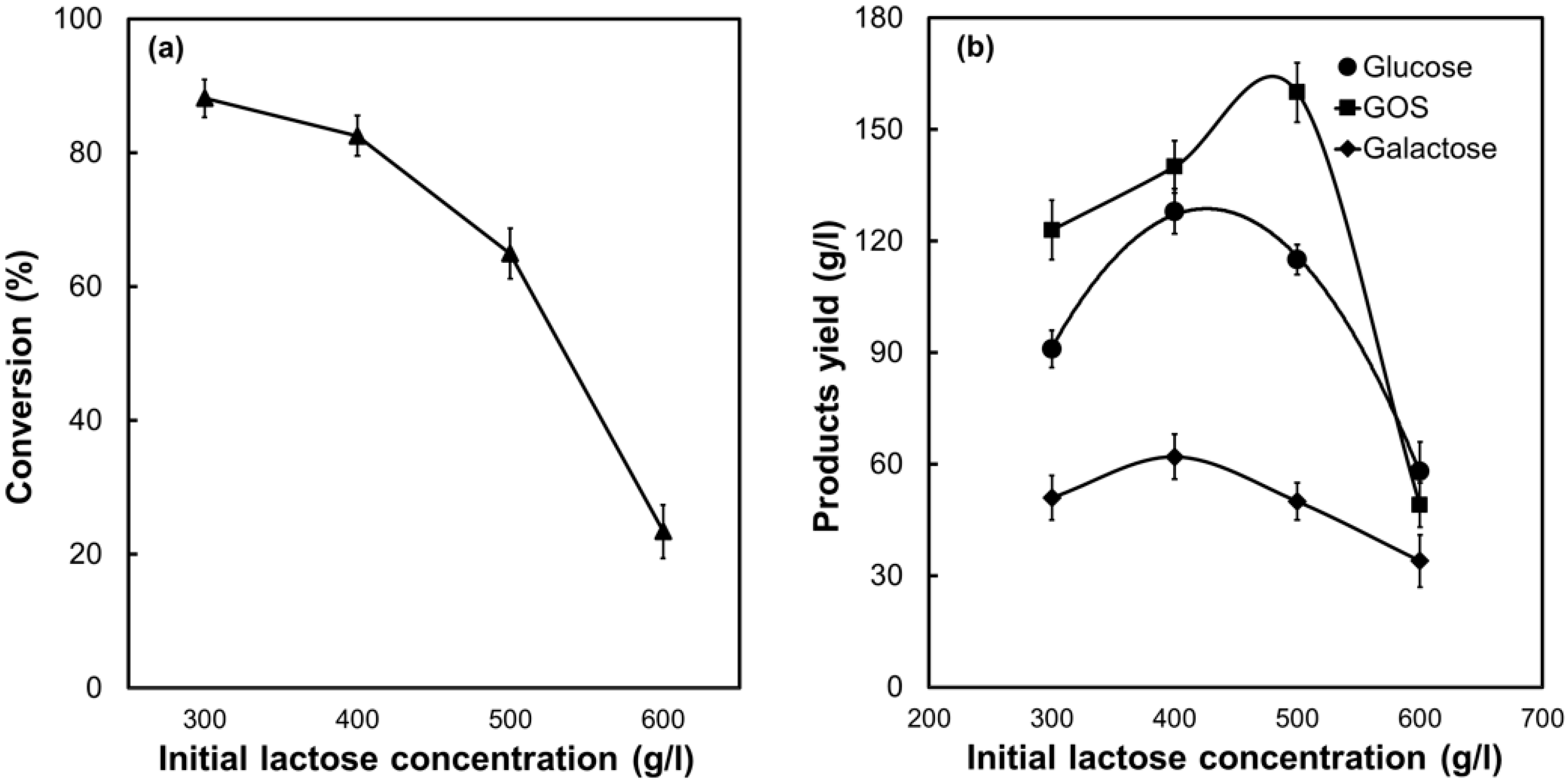
2.4. Biocatalytic Performance of PSNF-β-Galactosidase
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Polymer Nanofibers
3.3. Nanofibers Modification and β-Galactosidase Immobilization
3.4. Characterization of Nanobiocatalyst
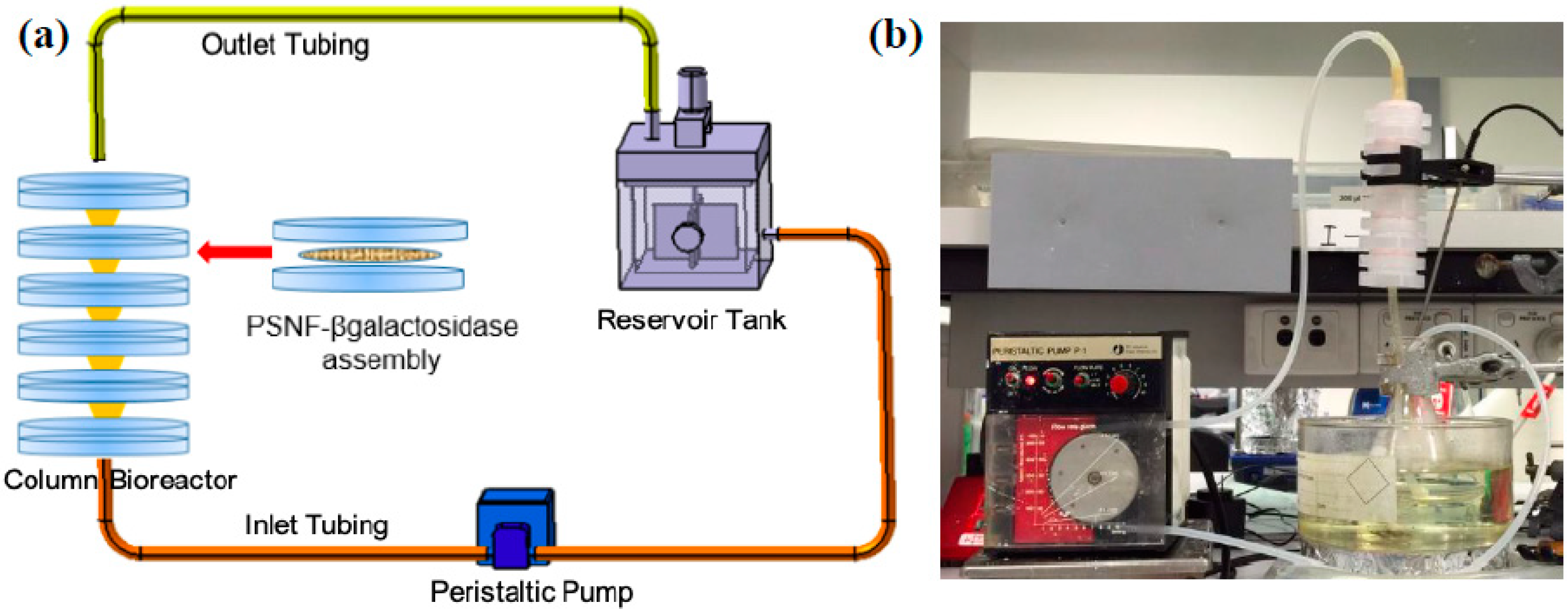
3.5. Experimental Set-Up of a Recirculating Column Reactor
3.6. Chemical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aissaoui, N.; Landoulsi, J.; Bergaoui, L.; Boujday, S.; Lambert, J.-F. Catalytic activity and thermostability of enzymes immobilized on silanized surface: Influence of the crosslinking agent. Enzym. Microb. Technol. 2013, 52, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Zhao, H.; Zhang, S.; Tian, C.; Zhang, D.; Du, P.; Liu, C.; Cao, H.; Li, H. High catalytic activity of immobilized laccase on core–shell magnetic nanoparticles by dopamine self-polymerization. J. Mol. Catal. B Enzym. 2015, 112, 15–24. [Google Scholar] [CrossRef]
- He, T.; Tian, Y.-L.; Qi, L.; Zhang, J.; Zhang, Z.-Q. Improved performance of α-amylase immobilized on poly (glycidyl methacrylate-co-ethylenedimethacrylate) beads. Int. J. Biol. Macromol. 2014, 65, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Secundo, F. Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef]
- Ba, O.M.; Hindie, M.; Marmey, P.; Gallet, O.; Anselme, K.; Ponche, A.; Duncan, A.C. Protein covalent immobilization via its scarce thiol versus abundant amine groups: Effect on orientation, cell binding domain exposure and conformational lability. Colloids Surf. B Biointerfaces 2015, 134, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Zhang, Y.; Zhu, J.; An, R.; Li, B.; Mu, L.; Ying, H.; Wu, J.; Zhou, J.; Chen, Y.; et al. Influences of geometrical topography and surface chemistry on the stable immobilization of adenosine deaminase on mesoporous TiO2. Chem. Eng. Sci. 2016, 139, 142–151. [Google Scholar] [CrossRef]
- Misson, M.; Dai, S.; Jin, B.; Chen, B.H.; Zhang, H. Manipulation of nanofiber-based β-galactosidase nanoenvironment for enhancement of galacto-oligosaccharide production. J. Biotechnol. 2016, 222, 56–64. [Google Scholar] [CrossRef]
- Husain, Q.; Ansari, S.A.; Alam, F.; Azam, A. Immobilization of Aspergillus oryzae β galactosidase on zinc oxide nanoparticles via simple adsorption mechanism. Int. J. Biol. Macromol. 2011, 49, 37–43. [Google Scholar] [CrossRef]
- Du Plessis, D.M.; Botes, M.; Dicks, L.M.T.; Cloete, T.E. Immobilization of commercial hydrolytic enzymes on poly (acrylonitrile) nanofibers for anti-biofilm activity. J. Chem. Technol. Biotechnol. 2012, 88, 585–593. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, R. Reversible His-tagged enzyme immobilization on functionalized carbon nanotubes as nanoscale biocatalyst. Methods Mol. Biol. 2011, 743, 95–106. [Google Scholar]
- Johnson, P.A.; Park, H.J.; Driscoll, A.J. Enzyme nanoparticle fabrication: Magnetic nanoparticle synthesis and enzyme immobilization. In Enzyme Stabilization and Immobilization Methods in Molecular Biology; Minteer, S.D., Ed.; Hanna Publisher: Shallowater, TX, USA, 2011; Volume 679, pp. 183–191. [Google Scholar]
- Tran, D.T.; Chen, C.L.; Chang, J.S. Immobilization of Burkholderia sp. lipase on a ferric silica nanocomposite for biodiesel production. J. Biotechnol. 2012, 158, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-X.; Li, Y.-F.; Zhao, G.-H.; Yang, L.-Q.; Wang, J.-Z.; Shan, X.; Yan, X. Preparation and characterization of graphite nanosheets decorated with Fe3O4 nanoparticles used in the immobilization of glucoamylase. Carbon 2012, 50, 2976–2986. [Google Scholar] [CrossRef]
- Misson, M.; Zhang, H.; Jin, B. Nanobiocatalyst advancements and bioprocessing applications. J. R. Soc. Interface 2015, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Je, H.H.; Noh, S.; Hong, S.-G.; Ju, Y.; Kim, J.; Hwang, D.S. Cellulose nanofibers for magnetically-separable and highly loaded enzyme immobilization. Chem. Eng. J. 2017, 323, 425–433. [Google Scholar] [CrossRef]
- Canbolat, M.F.; Savas, H.B.; Gultekin, F. Enzymatic behavior of laccase following interaction with γ-CD and immobilization into PCL nanofibers. Anal. Biochem. 2017, 528, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chaganti, S.R.; Prakasham, R.S. Polyaniline nanofiber as a novel immobilization matrix for the anti-leukemia enzyme l-asparaginase. J. Mol. Catal. B Enzym. 2012, 74, 132–137. [Google Scholar] [CrossRef]
- Maryšková, M.; Ardao, I.; García-González, C.A.; Martinová, L.; Rotková, J.; Ševců, A. Polyamide 6/chitosan nanofibers as support for the immobilization of Trametes versicolor laccase for the elimination of endocrine disrupting chemicals. Enzym. Microb. Technol. 2016, 89, 31–38. [Google Scholar] [CrossRef]
- Dorraki, N.; Safa, N.N.; Jahanfar, M.; Ghomi, H.; Ranaei-Siadat, S.-O. Surface modification of chitosan/PEO nanofibers by air dielectric barrier discharge plasma for acetylcholinesterase immobilization. Appl. Surf. Sci. 2015, 349, 940–947. [Google Scholar] [CrossRef]
- Silva, T.R.; Rodrigues, D.P.; Rocha, J.M.S.; Gil, M.H.; Pinto, S.C.S.; Lopes-da-Silva, J.A.; Guiomar, A.J. Immobilization of trypsin onto poly(ethylene terephthalate)/poly(lactic acid) nonwoven nanofiber mats. Biochem. Eng. J. 2015, 104, 48–56. [Google Scholar] [CrossRef]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Knystautas, E.J.; Verma, M.; Surampalli, R.Y. Degradation of chlortetracycline using immobilized laccase on Polyacrylonitrile-biochar composite nanofibrous membrane. Sci. Total Environ. 2017, 605–606, 315–321. [Google Scholar] [CrossRef]
- Misson, M.; Jin, B.; Chen, B.; Zhang, H. Enhancing enzyme stability and metabolic functional ability of β-galactosidase through functionalized polymer nanofiber immobilization. Bioprocess Biosyst. Eng. 2015, 38, 1915–1923. [Google Scholar] [CrossRef]
- Misson, M.; Jin, B.; Zhang, H. Recirculating spiral bioreactor for galactooligosaccharide production using polymer nanofiber-β-galactosidase assembly. Ind. Eng. Chem. Res. 2017, 56, 12479–12487. [Google Scholar] [CrossRef]
- Ganzle, M.G. Enzymatic synthesis of galacto-oligosaccharides and other lactose derivatives (hetero-oligosaccharides) from lactose. Int. Dairy J. 2012, 22, 116–122. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef]
- An, H.; Jin, B.; Dai, S. Fabricating polystyrene fiber-dehydrogenase assemble as a functional biocatalyst. Enzym. Microb. Technol. 2015, 68, 15–22. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Eisl, I.; Nidetzky, B. Advanced characterization of immobilized enzymes as heterogeneous biocatalysts. Catal. Today 2016, 259, 66–80. [Google Scholar] [CrossRef]
- Mahmoudifard, M.; Soudi, S.; Soleimani, M.; Hosseinzadeh, S.; Esmaeili, E.; Vossoughi, M. Efficient protein immobilization on polyethersolfone electrospun nanofibrous membrane via covalent binding for biosensing applications. Mater. Sci. Eng. C 2016, 58, 586–594. [Google Scholar] [CrossRef]
- Pinto, S.C.; Rodrigues, A.R.; Saraiva, J.A.; Lopes-da-Silva, J.A. Catalytic activity of trypsin entrapped in electrospun poly (ϵ-caprolactone) nanofibers. Enzym. Microb. Technol. 2015, 79–80, 8–18. [Google Scholar] [CrossRef]
- Liang, C.Y.; Krimm, S. Infrared spectra of high polymers. VI. Polystyrene. J. Polym. Sci. 1958, 27, 241–254. [Google Scholar] [CrossRef]
- Verma, M.L.; Barrow, C.J.; Kennedy, J.F.; Puri, M. Immobilization of beta-d-galactosidase from Kluyveromyces lactis on functionalized silicon dioxide nanoparticles: Characterization and lactose hydrolysis. Int. J. Biol. Macromol. 2012, 50, 432–437. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Bordbar, A.K.; Zare, D.; Davoodi, D.; Noruzi, M.; Barkhi, M.; Tabatabaei, M. Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem. Eng. J. 2011, 171, 669–673. [Google Scholar] [CrossRef]
- Shen, Q.; Yang, R.; Hua, X.; Ye, F.; Wang, H.; Zhao, W.; Wang, K. Enzymatic synthesis and identification of oligosaccharides obtained by transgalactosylation of lactose in the presence of fructose using beta-galactosidase from Kluyveromyces lactis. Food Chem. 2012, 135, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Pant, H.; Jain, R.; Khare, S.K. Galacto-oligosaccharide synthesis by immobilized Aspergillus oryzae b-galactosidase. Food Chem. 2006, 97, 426–430. [Google Scholar] [CrossRef]
- Jovanovic-Malinovska, R.; Fernandes, P.; Winkelhausen, E.; Fonseca, L. Galacto-oligosaccharides synthesis from lactose and whey by beta-galactosidase immobilized in PVA. Appl. Biochem. Biotechnol. 2012, 168, 1197–1211. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Hernández, O.; Ruiz-Matute, A.I.; Olano, A.; Moreno, J.F.; LuzSanz, M. Comparison of fractionation techniques to obtain prebiotic galactooligosaccharides. Int. Dairy J. 2009, 19, 531–536. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Lee, C. Comparative study of changes in reaction profile and microbial community structure in two anaerobic repeated-batch reactors started up with different seed sludges. Bioresour. Technol. 2013, 129, 495–505. [Google Scholar] [CrossRef]
- Machado, J.; Coutinho, J.; Maceso, E. Solid–liquid equilibrium of a-lactose in ethanol/water. Fluid Phase Equilib. 2000, 173, 121–134. [Google Scholar] [CrossRef]
- Zheng, P.; Yu, H.; Sun, Z.; Ni, Y.; Zhang, W.; Fan, Y.; Xu, Y. Production of galacto-oligosaccharides by immobilized recombinant beta-galactosidase from Aspergillus candidus. Biotechnol. J. 2006, 1, 1464–1470. [Google Scholar] [CrossRef]
- Ren, H.; Fei, J.; Shi, X.; Zhao, T.; Cheng, H.; Zhao, N.; Chen, Y.; Ying, H. Continuous ultrafiltration membrane reactor coupled with nanofiltration for the enzymatic synthesis and purification of galactosyl-oligosaccharides. Sep. Purif. Technol. 2015, 144, 70–79. [Google Scholar] [CrossRef]
- Benjamins, E.; Boxem, L.; KleinJan-Noeverman, J.; Broekhuis, T.A. Assessment of repetitive batch-wise synthesis of galacto-oligosaccharides from lactose slurry using immobilised β-galactosidase from Bacillus circulans. Int. Dairy J. 2014, 38, 160–168. [Google Scholar] [CrossRef]
- Urrutia, P.; Mateo, C.; Guisan, J.M.; Wilson, L.; Illanes, A. Immobilization of Bacillus circulans β-galactosidase and its application in the synthesis of galacto-oligosaccharides under repeated-batch operation. Biochem. Eng. J. 2013, 77, 41–48. [Google Scholar] [CrossRef]
- Rodriguez-Colinas, B.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.O.; Plou, F.J. Galacto-oligosaccharide synthesis from lactose solution or skim milk using the β-galactosidase from Bacillus circulans. J. Agric. Food Chem. 2012, 60, 6391–6398. [Google Scholar] [CrossRef]
- Neri, D.F.M.; Balcao, V.M.; Costa, R.S.; Rocha, I.C.A.P.; Ferreira, E.M.F.C.; Torres, D.P.M.; Rodrigues, L.R.M.; Carvalho, L.B., Jr.; Teixeira, J.A. Galacto-oligosaccharides production during lactose hydrolysis by free Aspergillus oryzae b-galactosidase and immobilized on magnetic polysiloxane-polyvinyl alcohol. Food Chem. 2009, 115, 92–99. [Google Scholar] [CrossRef]

| Flow Rate (mL/min) | Capacity of Handling Flow Rate Cycle (h−1) | Retention Time (h) |
|---|---|---|
| 2 | 20 | 0.050 |
| 4 | 40 | 0.025 |
| 6 | 60 | 0.017 |
| 8 | 80 | 0.013 |
| 10 | 100 | 0.010 |
| Source of β-Galactosidase | Reactor | Support Material | Lactose | Conversion | GOS | Reference |
|---|---|---|---|---|---|---|
| - | - | - | g/L | % | % | - |
| Kluyveromyces lactis | Disc reactor | Polystyrene nanofibers | 300 | 88 | 41 | This work |
| 400 | 83 | 35 | This work | |||
| Kluyveromyces lactis | Spiral reactor | Polystyrene nanofibers | 300 | 86 | 31 | [38] |
| 400 | 86 | 40 | [38] | |||
| Kluyveromyces lactis | Batch process | Polystyrene nanofibers | 300 | 38 | 20 | [41] |
| 400 | 40 | 28 | [41] | |||
| Kluyveromyces lactis | Continuous ultrafiltration membrane reactor | Membrane | 300 | 76 | 80 | [42] |
| Bacillus circulans | Repetitive batch-wise | Eupergit C250L | 550 | - | 64 | [43] |
| Bacillus circulans | Repeated batch | Glyoxyl agarose | 500 | 60 | 39 | [44] |
| Bacillus circulans | Batch process | - | 400 | 50 | 41 | [45] |
| Aspergillus oryzae | Batch process | Polysiloxane-polyvinyl alcohol | 500 | 55 | 26 | [46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misson, M.; Jin, B.; Dai, S.; Zhang, H. Interfacial Biocatalytic Performance of Nanofiber-Supported β-Galactosidase for Production of Galacto-Oligosaccharides. Catalysts 2020, 10, 81. https://doi.org/10.3390/catal10010081
Misson M, Jin B, Dai S, Zhang H. Interfacial Biocatalytic Performance of Nanofiber-Supported β-Galactosidase for Production of Galacto-Oligosaccharides. Catalysts. 2020; 10(1):81. https://doi.org/10.3390/catal10010081
Chicago/Turabian StyleMisson, Mailin, Bo Jin, Sheng Dai, and Hu Zhang. 2020. "Interfacial Biocatalytic Performance of Nanofiber-Supported β-Galactosidase for Production of Galacto-Oligosaccharides" Catalysts 10, no. 1: 81. https://doi.org/10.3390/catal10010081
APA StyleMisson, M., Jin, B., Dai, S., & Zhang, H. (2020). Interfacial Biocatalytic Performance of Nanofiber-Supported β-Galactosidase for Production of Galacto-Oligosaccharides. Catalysts, 10(1), 81. https://doi.org/10.3390/catal10010081









