Rhodium-Catalyzed Aqueous Biphasic Olefin Hydroformylation Promoted by Amphiphilic Cyclodextrins
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Remarks
3.2. General Procedure for Rhodium-Catalyzed Hydroformylation Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Hedges, A.R. Industrial applications of cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Tilloy, S.; Binkowski-Machut, C.; Menuel, S.; Bricout, H.; Monflier, E. Phosphane-Based Cyclodextrins as Mass Transfer Agents and Ligands for Aqueous Organometallic Catalysis. Molecules 2012, 17, 13062–13072. [Google Scholar] [CrossRef] [PubMed]
- Hapiot, F.; Bricout, H.; Tilloy, S.; Monflier, E. Functionalized Cyclodextrins as First and Second Coordination Sphere Ligands for Aqueous Organometallic Catalysis. Eur. J. Org. Chem. 2012, 2012, 1571–1578. [Google Scholar] [CrossRef]
- Tilloy, S.; Bricout, H.; Menuel, S.; Hapiot, F.; Monflier, E. Cyclodextrins Modified by Metal-Coordinating Groups for Aqueous Organometallic Catalysis: What Remains to be Done? Curr. Organocatal. 2016, 3, 24–31. [Google Scholar] [CrossRef]
- Reetz, M.T.; Waldvogel, S.R. β-Cyclodextrin-Modified Diphosphanes as Ligands for Supramolecular Rhodium Catalysts. Angew. Chem. Int. Ed. 1997, 36, 865–867. [Google Scholar] [CrossRef]
- Armspach, D.; Matt, D. Metal-capped α-cyclodextrins: The crowning of the oligosaccharide torus with precious metals. Chem. Commun. 1999, 12, 1073–1074. [Google Scholar] [CrossRef]
- Guitet, M.; Marcelo, F.; de Beaumais, S.A.; Zhang, Y.M.; Jimenez-Barbero, J.; Tilloy, S.; Monflier, E.; Menand, M.; Sollogoub, M. Diametrically Opposed Carbenes on an α-Cyclodextrin: Synthesis, Characterization of Organometallic Complexes and Suzuki-Miyaura Coupling in Ethanol and in Water. Eur. J. Org. Chem. 2013, 18, 3691–3699. [Google Scholar] [CrossRef]
- Dindulkar, S.D.; Jeong, D.; Kim, H.; Jung, S. Functionalized beta-cyclodextrin as supramolecular ligand and their Pd(OAc)(2) complex: Highly efficient and reusable catalyst for Mizoroki-Heck cross-coupling reactions in aqueous medium. Carbohydr. Res. 2016, 430, 85–94. [Google Scholar] [CrossRef]
- Khan, R.I.; Pitchumani, K. A pyridinium modified beta-cyclodextrin: An ionic supramolecular ligand for palladium acetate in C-C coupling reactions in water. Green Chem. 2016, 18, 5518–5528. [Google Scholar] [CrossRef]
- Khan, R.I.; Pitchumani, K. Water-Soluble Palladium Complex of N’-(pyridin-2-yl)propane-1,3-diamine modified beta-Cyclodextrin: An efficient Catalyst for Transfer Hydrogenation of Carbonyl Compounds. ACS Sustain. Chem. Eng. 2018, 6, 16130–16138. [Google Scholar] [CrossRef]
- Sak, H.; Mawick, M.; Krause, N. Sustainable Gold Catalysis in Water Using Cyclodextrin-tagged NHC-Gold Complexes. ChemCatChem. 2019, 11, 5821–5829. [Google Scholar] [CrossRef]
- Bricout, H.; Hapiot, F.; Ponchel, A.; Tilloy, S.; Monflier, E. Chemically Modified Cyclodextrins: An Attractive Class of Supramolecular Hosts for the Development of Aqueous Biphasic Catalytic Processes. Sustainability 2009, 1, 924–945. [Google Scholar] [CrossRef]
- Hapiot, F.; Menuel, S.; Bricout, H.; Tilloy, S.; Monflier, E. Recent developments in cyclodextrin-mediated aqueous biphasic hydroformylation and Tsuji–Trost reactions. Appl. Organomet. Chem. 2015, 29, 580–587. [Google Scholar] [CrossRef]
- Hapiot, F.; Leclercq, L.; Azaroual, N.; Fourmentin, S.; Tilloy, S.; Monflier, E. Rhodium-catalyzed hydroformylation promoted by modified cyclodextrins: Current scope and future developments. Curr. Org. Synth. 2008, 5, 162–172. [Google Scholar] [CrossRef]
- Matsinha, L.C.; Siangwata, S.; Smith, G.S.; Makhubela, B.C.E. Aqueous biphasic hydroformylation of olefins: From classical phosphine-containing systems to emerging strategies based on water-soluble nonphosphine ligands. Catal. Rev. 2019, 61, 111–133. [Google Scholar] [CrossRef]
- Hapiot, F.; Bricout, H.; Tilloy, S.; Monflier, E. Hydroformylation in Aqueous Biphasic Media Assisted by Molecular Receptors. In Hydroformylation for Organic Synthesis; Taddei, M., Mann, A., Eds.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2013; Volume 342, pp. 49–78. [Google Scholar]
- Hapiot, F.; Leclercq, L.; Azaroual, N.; Fourmentin, S.; Tilloy, S.; Monflier, E. Rhodium-Catalyzed Hydroformylation Promoted by Modified Cyclodextrins: Current Scope and Future Developments. Adv. Org. Synth. 2013, 14, 36–63. [Google Scholar]
- Bricout, H.; Hapiot, F.; Ponchel, A.; Tilloy, S.; Monflier, E. Cyclodextrins as Mass Transfer Additives in Aqueous Organometallic Catalysis. Curr. Org. Chem. 2010, 14, 1296–1307. [Google Scholar] [CrossRef]
- Monflier, E.; Fremy, G.; Castanet, Y.; Mortreux, A. Molecular Recognition between Chemically Modified β-Cyclodextrin and Dec-1-ene: New Prospects for Biphasic Hydroformylation of Water-Insoluble Olefins. Angew. Chem. Int. Ed. 1995, 34, 2269–2271. [Google Scholar] [CrossRef]
- Sieffert, N.; Wipff, G. Importance of Interfacial Adsorption in the Biphasic Hydroformylation of Higher Olefins Promoted by Cyclodextrins: A Molecular Dynamics Study at the Decene/Water Interface. Chem. Eur. J. 2007, 13, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Sieffert, N.; Wipff, G. Adsorption at the Liquid−Liquid Interface in the Biphasic Rhodium Catalyzed Hydroformylation of Olefins Promoted by Cyclodextrins: A Molecular Dynamics Study. J. Phys. Chem. B 2006, 110, 4125–4134. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, L.; Bricout, H.; Tilloy, S.; Monflier, E. Biphasic aqueous organometallic catalysis promoted by cyclodextrins: Can surface tension measurements explain the efficiency of chemically modified cyclodextrins? J. Colloid Interface Sci. 2007, 307, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Monflier, E.; Tilloy, S.; Fremy, G.; Castanet, Y.; Mortreux, A. A further breakthrough in biphasic, rhodium-catalyzed hydroformylation: The use of per(2,6-di-O-methyl)-beta-cyclodextrin as inverse phase transfer catalyst. Tetrahedron Lett. 1995, 36, 9481–9484. [Google Scholar] [CrossRef]
- Fu, H.; Li, M.; Chen, H.; Li, X. Higher olefin hydroformylation in organic/aqueous biphasic system accelerated by double long-chain cationic surfactants. J. Mol. Catal. A Chem. 2006, 259, 156–160. [Google Scholar] [CrossRef]
- Desset, S.L.; Reader, S.W.; Cole-Hamilton, D.J. Aqueous-biphasic hydroformylation of alkenes promoted by “weak” surfactants. Green Chem. 2009, 11, 630–637. [Google Scholar] [CrossRef]
- Pogrzeba, T.; Müller, D.; Hamerla, T.; Esche, E.; Paul, N.; Wozny, G.; Schomäcker, R. Rhodium-Catalyzed Hydroformylation of Long-Chain Olefins in Aqueous Multiphase Systems in a Continuously Operated Miniplant. Ind. Eng. Chem. Res. 2015, 54, 11953–11960. [Google Scholar] [CrossRef]
- Illner, M.; Müller, D.; Esche, E.; Pogrzeba, T.; Schmidt, M.; Schomäcker, R.; Wozny, G.; Repke, J.-U. Hydroformylation in Microemulsions: Proof of Concept in a Miniplant. Ind. Eng. Chem. Res. 2016, 55, 8616–8626. [Google Scholar] [CrossRef]
- Bibouche, B.; Peral, D.; Stehl, D.; Söderholm, V.; Schomäcker, R.; von Klitzing, R.; Vogt, D. Multiphasic aqueous hydroformylation of 1-alkenes with micelle-like polymer particles as phase transfer agents. RSC Adv. 2018, 8, 23332–23338. [Google Scholar] [CrossRef]
- Cocq, A.; Rousseau, C.; Bricout, H.; Oliva, E.; Bonnet, V.; Djedaini-Pilard, F.; Monflier, E.; Tilloy, S. Oleic Acid Based Cyclodextrins for the Development of New Hydrosoluble Amphiphilic Compounds. Eur. J. Org. Chem. 2019, 1236–1241. [Google Scholar] [CrossRef]
- Cocq, A.; Rousseau, C.; Bricout, H.; Oliva, E.; Bonnet, V.; Djedaïni-Pilard, F.; Monflier, E.; Tilloy, S. Highly Water-Soluble Amphiphilic Cyclodextrins Bearing Branched and Cyclic Oleic Grafts. Eur. J. Org. Chem. 2019, 4863–4868. [Google Scholar] [CrossRef]
- Mathivet, T.; Meliet, C.; Castanet, Y.; Mortreux, A.; Caron, L.; Tilloy, S.; Monflier, E. Rhodium catalyzed hydroformylation of water insoluble olefins in the presence of chemically modified beta-cyclodextrins: Evidence for ligand-cyclodextrin interactions and effect of various parameters on the activity and the aldehydes selectivity. J. Mol. Catal. A Chem. 2001, 176, 105–116. [Google Scholar] [CrossRef]
- Gärtner, B.; Cornils, H.; Springer, P. Lappe. DE Patent 3235030, 22 September 1982. [Google Scholar]
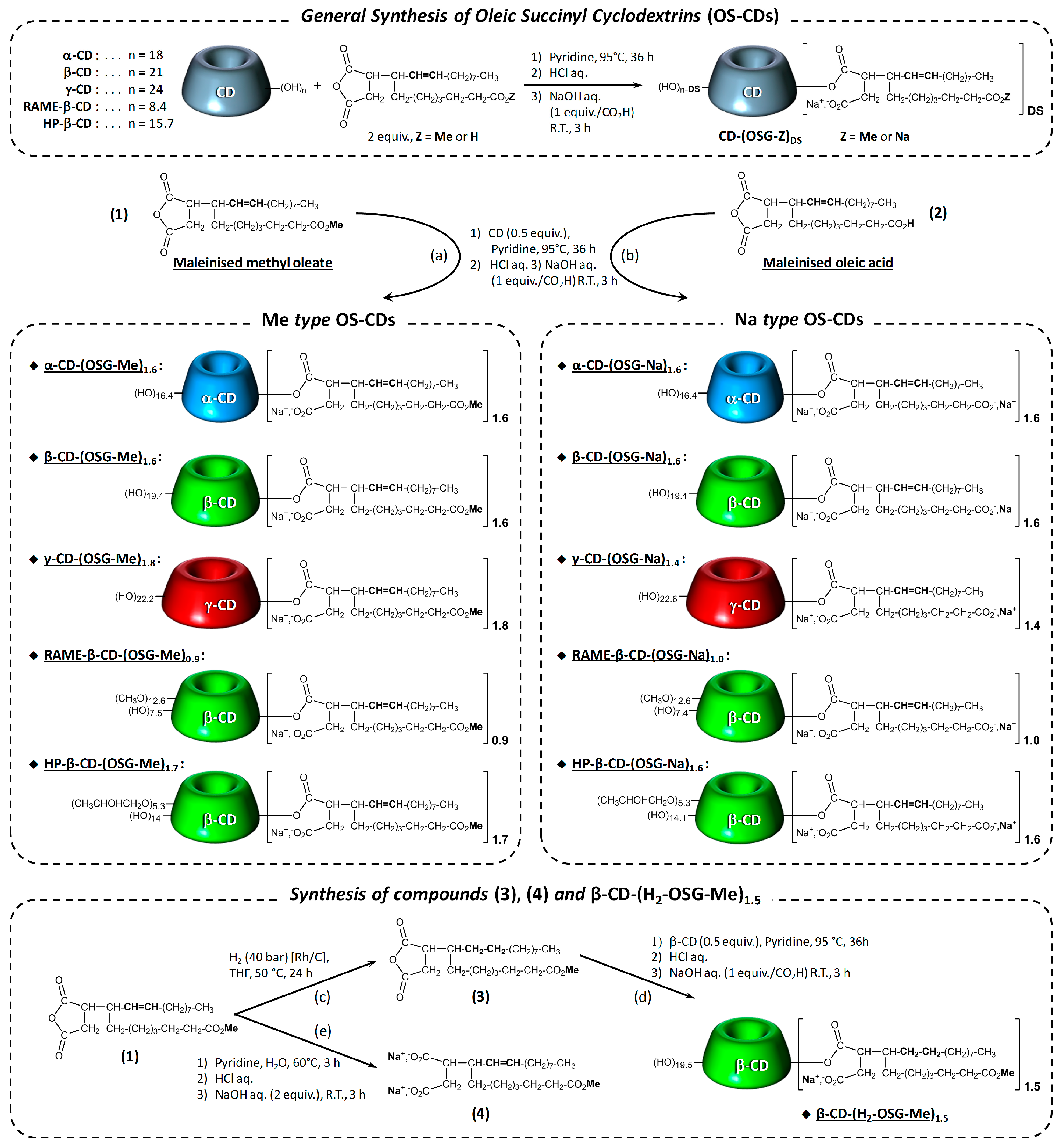
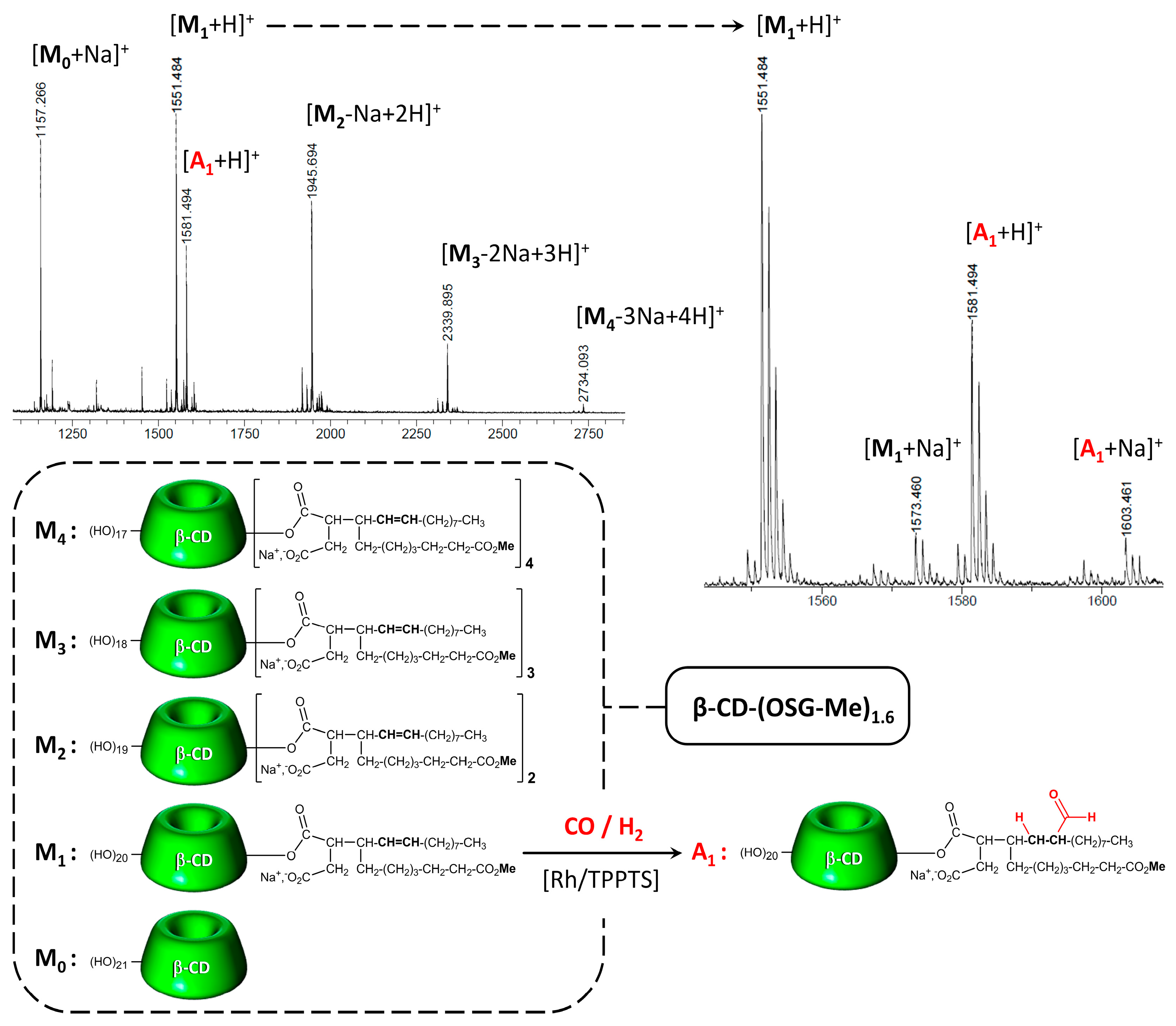
| Entry | OS-CD | Solubility at 20 °C (mM) | CAC at 20 °C (µM) | Surface Tension 1 (mN/m) |
|---|---|---|---|---|
| 1 | α-CD-(OSG–Me)1.6 | 52 | 15 | 33 |
| 2 | α-CD-(OSG–Na)1.6 | 121 | 206 | 36 |
| 3 | β-CD-(OSG–Me)1.6 | 28 | 11 | 39 |
| 4 | β-CD-(OSG–Na)1.6 | 158 | 94 | 38 |
| 5 | γ-CD-(OSG–Me)1.8 | 43 | 8 | 30 |
| 6 | γ-CD-(OSG–Na)1.4 | 176 | 190 | 39 |
| 8 | RAME-β-CD-(OSG–Me)0.9 | 247 | 24 | 32 |
| 9 | RAME-β-CD-(OSG–Na)1.0 | 288 | 18 | 35 |
| 10 | HP-β-CD-(OSG–Me)1.7 | 146 | 46 | 36 |
| 11 | HP-β-CD-(OSG–Na)1.6 | 178 | 54 | 52 |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Substrate | Promotor | Promotor (/Rh) | Time (h) | Conversion 2 (%) | Selectivity 3 (%) | l/b |
| 1 | C10H20 | (-) | (-) | 6 | 3 | 65 | 2.7 |
| 2 | C10H20 | β-CD-(OSG–Me)1.6 | 1 | 4 | 100 | 61 | 2.3 |
| 3 | C10H20 | β-CD-(OSG–Me)1.6 | 1 | 2 | 93 | 59 | 2.4 |
| 4 | C10H20 | β-CD-(OSG–Me)1.6 | 1 | 0.5 | 85 | 58 | 2.6 |
| 5 | C10H20 | β-CD | 1 | 6 | 6 | 80 | 2.6 |
| 6 | C10H20 | RAME-β-CD | 1 | 6 | 21 | 90 | 2.1 |
| 7 | C10H20 | (4) | 1.6 | 6 | 20 | 53 | 2.7 |
| 8 | C10H20 | β-CD + (4) | 1 + 1.6 | 6 | 45 | 53 | 2.5 |
| 9 | C16H32 | (-) | (-) | 6 | 1 | 62 | 2.7 |
| 10 | C16H32 | β-CD-(OSG–Me)1.6 | 1 | 6 | 65 | 52 | 2.8 |
| 11 | C16H32 | β-CD | 1 | 6 | 3 | 78 | 2.4 |
| 12 | C16H32 | RAME-β-CD | 1 | 6 | 9 | 90 | 2.1 |
| 13 | C16H32 | (4) | 1.6 | 6 | 10 | 53 | 2.7 |
| 14 | C16H32 | β-CD + (4) | 1 + 1.6 | 6 | 23 | 53 | 2.5 |
| 15 | C16H32 | β-CD-(H2–OSG–Me)1.6 | 1 | 6 | 68 | 58 | 2.6 |
| 16 | C16H32 | β-CD-(OSG–Me)1.6 4 | 1 | 6 | 63 | 50 | 2.8 |
| 17 | C16H32 | β-CD-(OSG–Me)1.6 5 | 1 | 6 | 67 | 51 | 2.8 |
 | |||||
|---|---|---|---|---|---|
| Entry | Promotor | Eq/Rh | Conversion 2 (%) | Selectivity 3 (%) | l/b |
| 1 | α-CD-(OSG–Me)1.6 | 1 | 57 | 61 | 2.5 |
| 2 | β-CD-(OSG–Me)1.6 | 1 | 65 | 52 | 2.8 |
| 3 | γ-CD-(OSG–Me)1.6 | 1 | 39 | 51 | 3.0 |
| 4 | RAME-β-CD-(OSG–Me)0.9 | 1 | 94 | 53 | 2.5 |
| 5 | HP-β-CD-(OSG–Me)1.7 | 1 | 66 | 55 | 2.5 |
| 6 | α-CD-(OSG–Na)1.6 | 1 | 15 | 62 | 2.9 |
| 7 | β-CD-(OSG–Na)1.6 | 1 | 22 | 58 | 1.8 |
| 8 | γ-CD-(OSG–Na)1.4 | 1 | 9 | 65 | 2.9 |
| 9 | RAME-β-CD-(OSG–Na)1.0 | 1 | 31 | 62 | 2.6 |
| 10 | HP-β-CD-(OSG–Na)1.6 | 1 | 32 | 66 | 2.6 |
| 11 | RAME-β-CD | 1 | 9 | 90 | 2.1 |
| 12 | RAME-β-CD | 10 | 18 | 90 | 2.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocq, A.; Bricout, H.; Djedaïni-Pilard, F.; Tilloy, S.; Monflier, E. Rhodium-Catalyzed Aqueous Biphasic Olefin Hydroformylation Promoted by Amphiphilic Cyclodextrins. Catalysts 2020, 10, 56. https://doi.org/10.3390/catal10010056
Cocq A, Bricout H, Djedaïni-Pilard F, Tilloy S, Monflier E. Rhodium-Catalyzed Aqueous Biphasic Olefin Hydroformylation Promoted by Amphiphilic Cyclodextrins. Catalysts. 2020; 10(1):56. https://doi.org/10.3390/catal10010056
Chicago/Turabian StyleCocq, Aurélien, Hervé Bricout, Florence Djedaïni-Pilard, Sébastien Tilloy, and Eric Monflier. 2020. "Rhodium-Catalyzed Aqueous Biphasic Olefin Hydroformylation Promoted by Amphiphilic Cyclodextrins" Catalysts 10, no. 1: 56. https://doi.org/10.3390/catal10010056
APA StyleCocq, A., Bricout, H., Djedaïni-Pilard, F., Tilloy, S., & Monflier, E. (2020). Rhodium-Catalyzed Aqueous Biphasic Olefin Hydroformylation Promoted by Amphiphilic Cyclodextrins. Catalysts, 10(1), 56. https://doi.org/10.3390/catal10010056






