Characterization and Comparative Performance of TiO2 Photocatalysts on 6-Mercaptopurine Degradation by Solar Heterogeneous Photocatalysis
Abstract
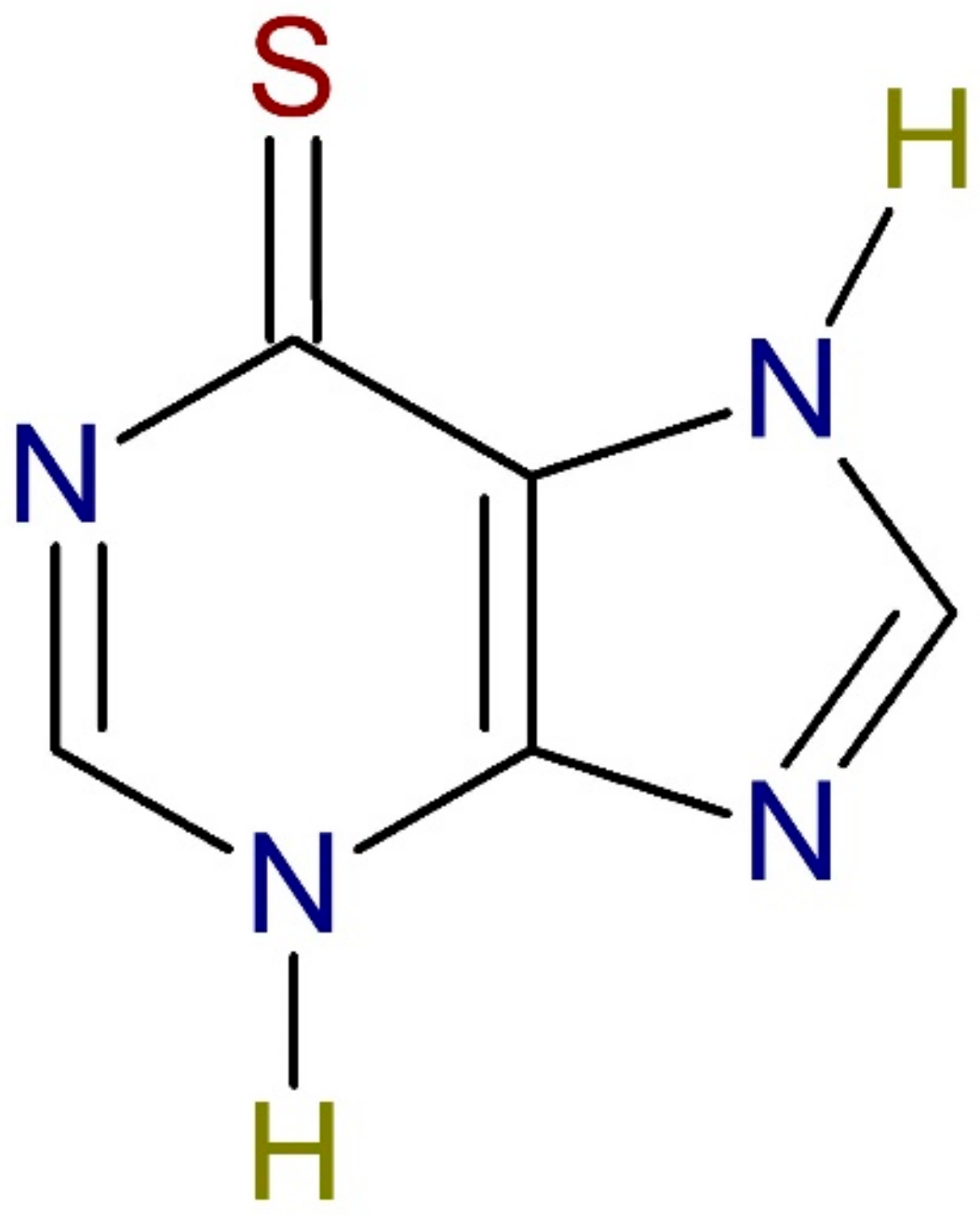
1. Introduction
2. Results
2.1. Structural Analysis of Photocatalysts
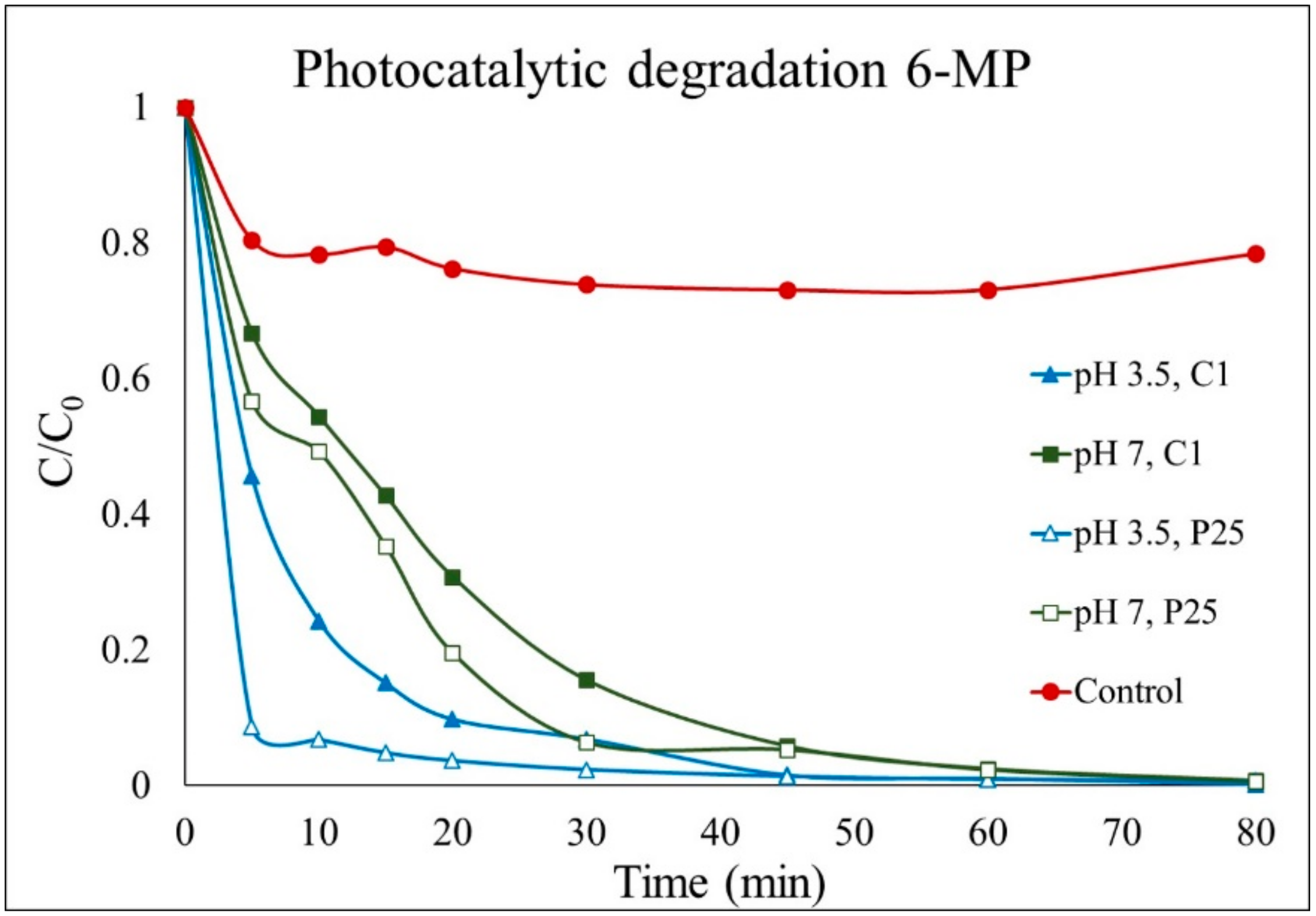
2.2. Comparison of Semiconductors Performance in 6-MP Degradation
3. Materials and Methods
3.1. Chemical Reagents
3.2. TiO2 Photocatalysts Characterization
3.3. Comparison of Semiconductor Performance in 6-MP Degradation
3.3.1. Photocatalytic Reactors
3.3.2. Experimentation
3.3.3. Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, P.; Yang, X.; Wei, G.; Zhang, W.; Shan, L. A novel advanced oxidation process to degrade organic pollutants in wastewater: Microwave-activated persulfate oxidation. J. Environ. Sci. 2009, 21, 1175–1180. [Google Scholar] [CrossRef]
- Fasnabi, P.A. Studies on Advanced Oxidation Processes for the Removal of Acetamiprid from Wastewater. Ph.D. Thesis, Cochin University of Science and Technology, Kerala, India, 2015. [Google Scholar]
- Schmiegelow, K.; Bretton-Meyer, U. 6-mercaptopurine dosage and pharmacokinetics influence the degree of bone marrow toxicity following high-dose methotrexate in children with acute lymphoblastic leukemia. Leukemia 2001, 15, 74–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lennard, L.; Rees, C.A.; Lilleyman, J.S.; Maddocks, J.L. Childhood leukaemia: A relationship between intracellular 6-mercaptopurine metabolites and neutropenia. Br. J. Clin. Pharmacol. 1983, 16, 359–363. [Google Scholar] [CrossRef]
- Schmiegelow, K.; Nielsen, S.N.; Frandsen, T.L.; Nersting, J. Mercaptopurine/Methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: Clinical facts and fiction. J. Pediatr. Hematol. Oncol. 2014, 36, 503–517. [Google Scholar] [CrossRef]
- Medscape. Available online: https://reference.medscape.com/drug/purinethol-purixan-mercaptopurine-342094 (accessed on 15 November 2018).
- Drugs.com. Available online: https://www.drugs.com/dosage/mercaptopurine.html (accessed on 16 November 2018).
- Qin, X.; Jing, L.; Tian, G.; Qu, Y.; Feng, Y. Enhanced photocatalytic activity for degrading Rhodamine B solution of commercial Degussa P25 TiO2 and its mechanisms. J. Hazard. Mater. 2009, 172, 1168–1174. [Google Scholar] [CrossRef]
- Sousa, M.A.; Gonçalves, C.; Vilar, V.J.; Boaventura, R.A.; Alpendurada, M.F. Suspended TiO2-assisted photocatalytic degradation of emerging contaminants in a municipal WWTP effluent using a solar pilot plant with CPCs. Chem. Eng. J. 2012, 198, 301–309. [Google Scholar] [CrossRef]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Menglin, L.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible-light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Constantin, L.A.; Nitoi, I.; Cristea, I.; Oancea, P. Kinetics of 5-Fluorouracil Degradation by Heterogenous TiO2 Photocatalysis. Rev. Chim. 2016, 67, 1447–1450. [Google Scholar]
- Tizaoui, C.; Mezughi, K.; Bickley, R. Heterogeneous photocatalytic removal of the herbicide clopyralid and its comparison with UV/H2O2 and ozone oxidation techniques. Desalination 2011, 273, 197–204. [Google Scholar] [CrossRef]
- Morones, M.M.; Pantoja, J.C.; Proal, J.B.; Cháirez, I.; Gurrola, J.N.; Ávila, M. Uso de un reactor de placa plana (TiO2/vidrio) para la degradación de 2,5-diclorofenol por fotocatálisis solar. Rev. Int. Contam. Ambie. 2017, 33, 605–616. [Google Scholar] [CrossRef]
- Núñez-Núñez, C.M.; Chairez-Hernández, I.; García-Roig, M.; García-Prieto, J.C.; Melgoza-Alemán, R.M.; Proal-Nájera, J.P. UV-C/H2O2 heterogeneous photocatalytic inactivation of coliforms in municipal wastewater in a TiO2/SiO2 fixed bed reactor: A kinetic and statistical approach. React. Kinet. Mech. Catal 2018, 125, 1159–1177. [Google Scholar] [CrossRef]
- Pantoja-Espinoza, J.C.; Proal-Nájera, J.B.; García-Roig, M.; Cháirez-Hernández, I.; Osorio-Revilla, G.I. Eficiencias comparativas de inactivación de bacterias coliformes en efluentes municipales por fotólisis (UV) y por fotocatálisis (UV/TiO2/SiO2). Caso: Depuradora de aguas de Salamanca, España. Rev. Mex. Ing. Quím. 2015, 14, 119–135. [Google Scholar]
- Tayade, R.J.; Surolia, P.K.; Kulkarni, R.G.; Jasra, R.V. Photocatalytic degradation of dyes and organic contaminants in water using nanocrystalline anatase and rutile TiO2. Sci. Technol. Adv. Mater. 2007, 8, 455–462. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tsai, H.L.; Huang, C.L. Phase transformation of precipitated TiO2 nanoparticles. Mater. Sci. Eng. A 2003, 344, 209–214. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase Characterization of TiO2 Powder by XRD and TEM. Kasetsart J. Nat. Sci. 2008, 42, 357–361. [Google Scholar]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray Diffraction, 1st ed.; Dover Publications: NewYork, NY, USA; EUA: Brussels, Belgium, 1990; pp. 41–67. [Google Scholar]
- Velasco-Arias, D. Obtención De Nanoestructuras Hechas a Base De Bismuto. Cerovalente, Bi2O3, Bi¬2Mo3O12 y Bi2Mo2O9. Ph.D. Thesis, Faculty of Chemistry, Universidad Nacional Autónoma de México, Mexico, Mexico, 2013. [Google Scholar]
- Sheng, Y.; Liang, L.; Xu, Y.; Wu, D.; Sun, Y. Low temperature deposition of the high-performance anatase-titania optical films via a modified sol–gel route. Opt. Mater. 2008, 30, 1310–1315. [Google Scholar] [CrossRef]
- Sochacka, J.; Pawelczar, B. Characterization of 6-mercaptopurine binding site on human α1-acid glycoprotein (Orosomucoid) using molecular docking. Acta Pol. Pharm. 2012, 69, 161–166. [Google Scholar] [PubMed]
- Lin, A.P.; Peng, J.D.; Zhou, M.; Zhang, J. Resonance light scattering determination of 6-mercaptopurine coupled with HPLC technique. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 154, 1–7. [Google Scholar] [CrossRef]
- Horn, J. Review article: Understanding the pharmacodynamic and pharmacokinetic differences between proton pump inhibitors – focus on pKa and metabolism. Aliment. Pharmacol. Ther. Symp. Ser. 2006, 2, 340–350. [Google Scholar] [CrossRef]
- Newton, D.W. Drug incompatibility chemistry. Am. J. Health-Syst. Pharm. 2009, 66, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Avdeef, A. Absorption and Drug Development: Solubility, Permeability, and Change State, 2nd ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 22–24. [Google Scholar] [CrossRef]
- Carbajo, J. Aplicación De La Fotocatálisis Solar a La Degradación De Contaminantes Orgánicos En Fase Acuosa Con Catalizadores Nanoestructurados De TiO2. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, España, 2013. [Google Scholar]
- Yan, M.; Chen, F.; Zhang, J.; Anpo, M. Preparation of Controllable Crystalline Titania and Study on the Photocatalytic Properties. J. Phys. Chem. B 2005, 109, 8673–8678. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sangchaya, W.; Sikonga, L.; Kooptarnonda, K. Comparison of photocatalytic reaction of commercial P25 and synthetic TiO2-AgCl nanoparticles. Procedia Eng. 2012, 32, 590–596. [Google Scholar] [CrossRef]
- Santiago, D.E.; Doña-Rodríguez, J.M.; Araña, J.; Fernández-Rodríguez, C.; González-Díaz, O.; Pérez-Peña, J.; Silva, A.M.T. Optimization of the degradation of imazalil by photocatalysis: Comparison between commercial and lab-made photocatalysts. Appl. Catal. B-Environ. 2013, 138, 391–400. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Gatto, S.; Pirola, C.; Naldoni, A.; Di Michele, A.; Cerrato, G.; Crocellà, V.; Capucci, V. Photocatalytic degradation of acetone, acetaldehyde and toluene in gas-phase: Comparison between nano and micro-sized TiO2. Appl. Catal. B-Environ. 2014, 146, 123–130. [Google Scholar] [CrossRef]
- Hossein, M.; Vosooghian, H. Photocatalytic degradation of some organic sulfides as environmental pollutants using titanium dioxide suspension. J. Photochem. Photobiol. A Chem. 2005, 174, 45–52. [Google Scholar] [CrossRef]
- Stintzing, A. Solar Photocatalytic Treatment of Textile Wastewater at a Pilot Plant in Menzel Temime/Tunisia. Bachelor’s Thesis, Institut für Thermische Verfahrenstechnik der Technischen Universität Clausthal, Clausthal, Germany, 2003. [Google Scholar]
- Hemmens, V.J.; Moore, D.E. Photo-oxidation of 6-Mercaptopurine in Aqueous Solution. J. Chem. Soc. Perkin Trans. 1984, 2, 209–211. [Google Scholar] [CrossRef]
- Kuhn, H.; Fösterling, H. Kinetics of Chemical Reactions. In Principles of Physical Chemistry; Wiley: West Sussex, UK, 2000; pp. 677–682. [Google Scholar]
- Lente, G. Facts and alternative facts in chemical kinetics: Remarks about the kinetic use of activities, termolecular processes, and linearization techniques. Curr. Opin. Chem. Eng. 2018, 21, 76–83. [Google Scholar] [CrossRef]
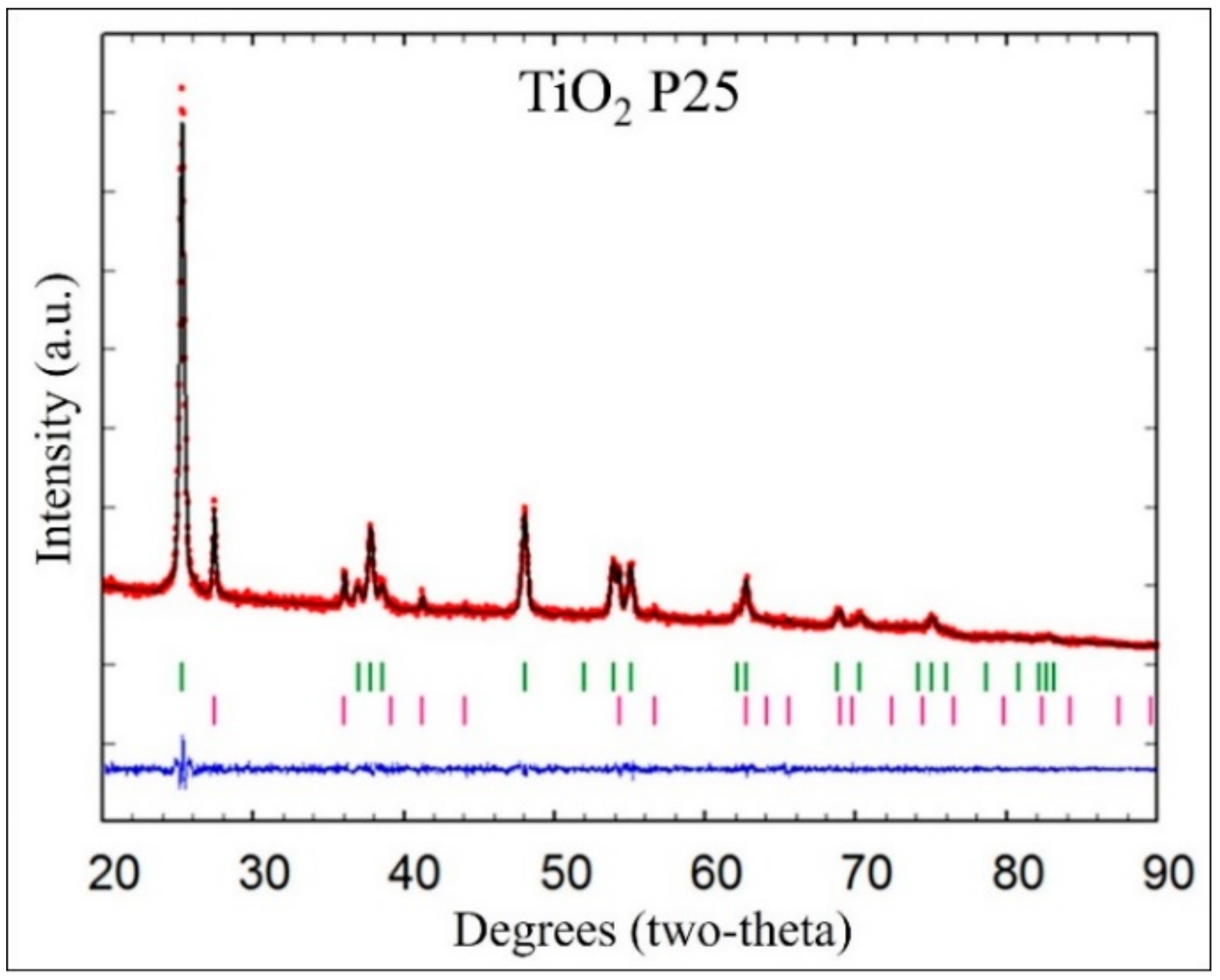
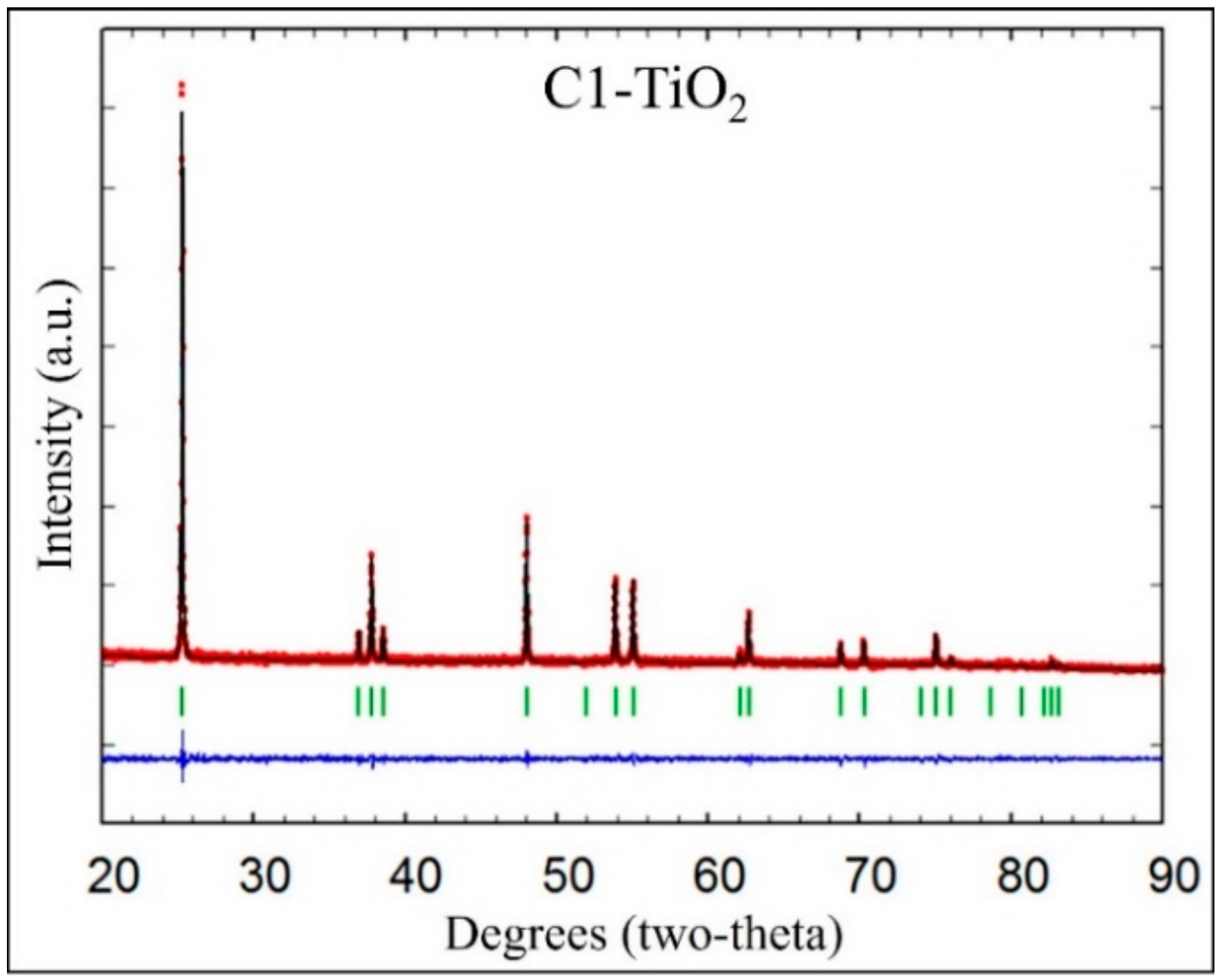
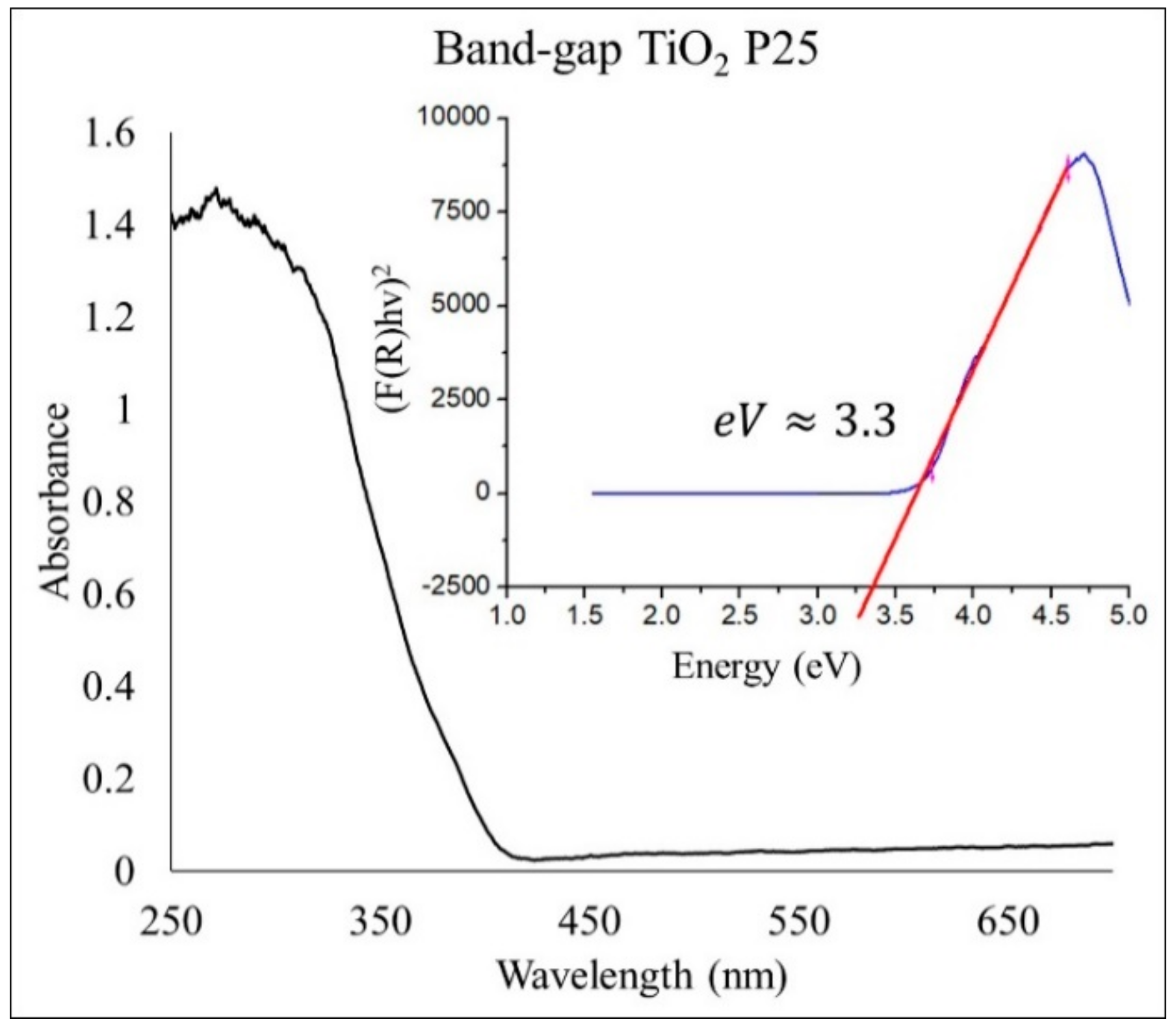
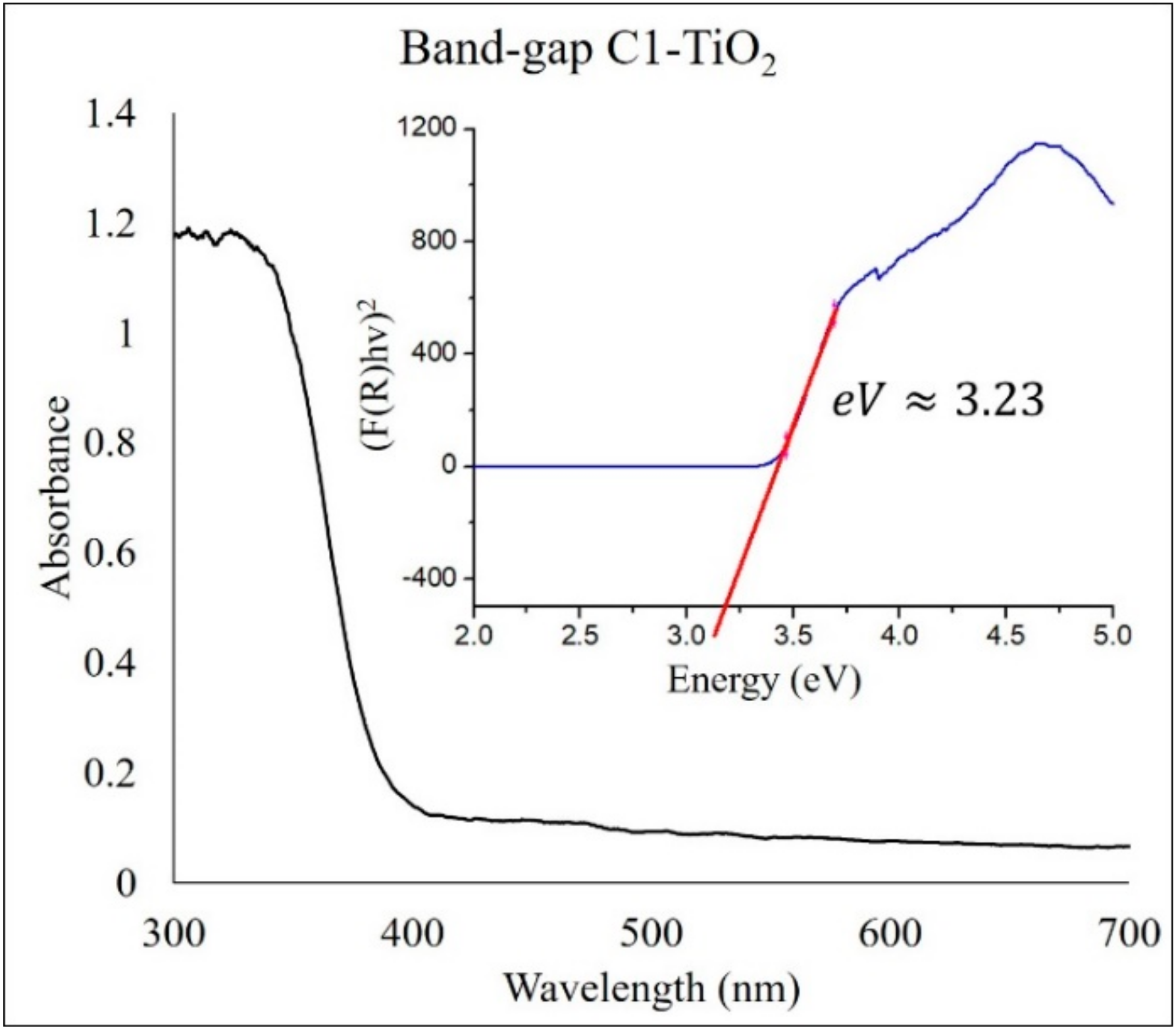

| Photocatalyst | Crystalline Phase | Space Group | Phases Percentage | Crystalline Size (nm) | Crystalline Form |
|---|---|---|---|---|---|
| TiO2 P25 | Tetragonal (anatase) | I 41/a m d [141] | 85.27 | 20.97 | Spherical |
| Tetragonal (rutile) | P42/m n m [136] | 14.73 | 33.96 | Spherical | |
| C1-TiO2 | Tetragonal (anatase) | I 41/a m d [141] | 100 | 80.71 | Spherical |
| Reactor | 1 Phc | 2 Exp. (H2O2 3 mM) | 3 ħυ (W/m2) | λ = 324 nm 4 (%Deg) |
|---|---|---|---|---|
| 1/10 m2 | P25 | pH 3.5 | 904.85 | 99.27 |
| pH 7.0 | 909.84 | 99.38 | ||
| C1 | pH 3.5 | 925.67 | 99.79 | |
| pH 7.0 | 865.80 | 99.27 | ||
| 1 m2 | P25 | pH 3.5 | 773.32 | 99.09 |
| C1 | pH 3.5 | 551.60 | 98.55 |
| Reactor | 1 Phc | pH | 2 Kop (min−1) | 3 Ʈ (min) |
|---|---|---|---|---|
| 1/10 m2 | P25 | 3.5 | 0.2812 | 2.4652 |
| 7.0 | 0.0838 | 8.2747 | ||
| C1 | 3.5 | 0.1351 | 5.1321 | |
| 7.0 | 0.0643 | 10.7785 | ||
| 1 m2 | P25 | 3.5 | 0.3361 | 2.0623 |
| C1 | 3.5 | 0.3158 | 2.1949 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Burciaga, L.A.; Núñez-Núñez, C.M.; Morones-Esquivel, M.M.; Avila-Santos, M.; Lemus-Santana, A.; Proal-Nájera, J.B. Characterization and Comparative Performance of TiO2 Photocatalysts on 6-Mercaptopurine Degradation by Solar Heterogeneous Photocatalysis. Catalysts 2020, 10, 118. https://doi.org/10.3390/catal10010118
González-Burciaga LA, Núñez-Núñez CM, Morones-Esquivel MM, Avila-Santos M, Lemus-Santana A, Proal-Nájera JB. Characterization and Comparative Performance of TiO2 Photocatalysts on 6-Mercaptopurine Degradation by Solar Heterogeneous Photocatalysis. Catalysts. 2020; 10(1):118. https://doi.org/10.3390/catal10010118
Chicago/Turabian StyleGonzález-Burciaga, Luis A., Cynthia M. Núñez-Núñez, Miriam M. Morones-Esquivel, Manuel Avila-Santos, Adela Lemus-Santana, and José B. Proal-Nájera. 2020. "Characterization and Comparative Performance of TiO2 Photocatalysts on 6-Mercaptopurine Degradation by Solar Heterogeneous Photocatalysis" Catalysts 10, no. 1: 118. https://doi.org/10.3390/catal10010118
APA StyleGonzález-Burciaga, L. A., Núñez-Núñez, C. M., Morones-Esquivel, M. M., Avila-Santos, M., Lemus-Santana, A., & Proal-Nájera, J. B. (2020). Characterization and Comparative Performance of TiO2 Photocatalysts on 6-Mercaptopurine Degradation by Solar Heterogeneous Photocatalysis. Catalysts, 10(1), 118. https://doi.org/10.3390/catal10010118





