Photodegradation of Gas Phase Benzene by SnO2 Nanoparticles by Direct Hole Oxidation Mechanism
Abstract
:1. Introduction
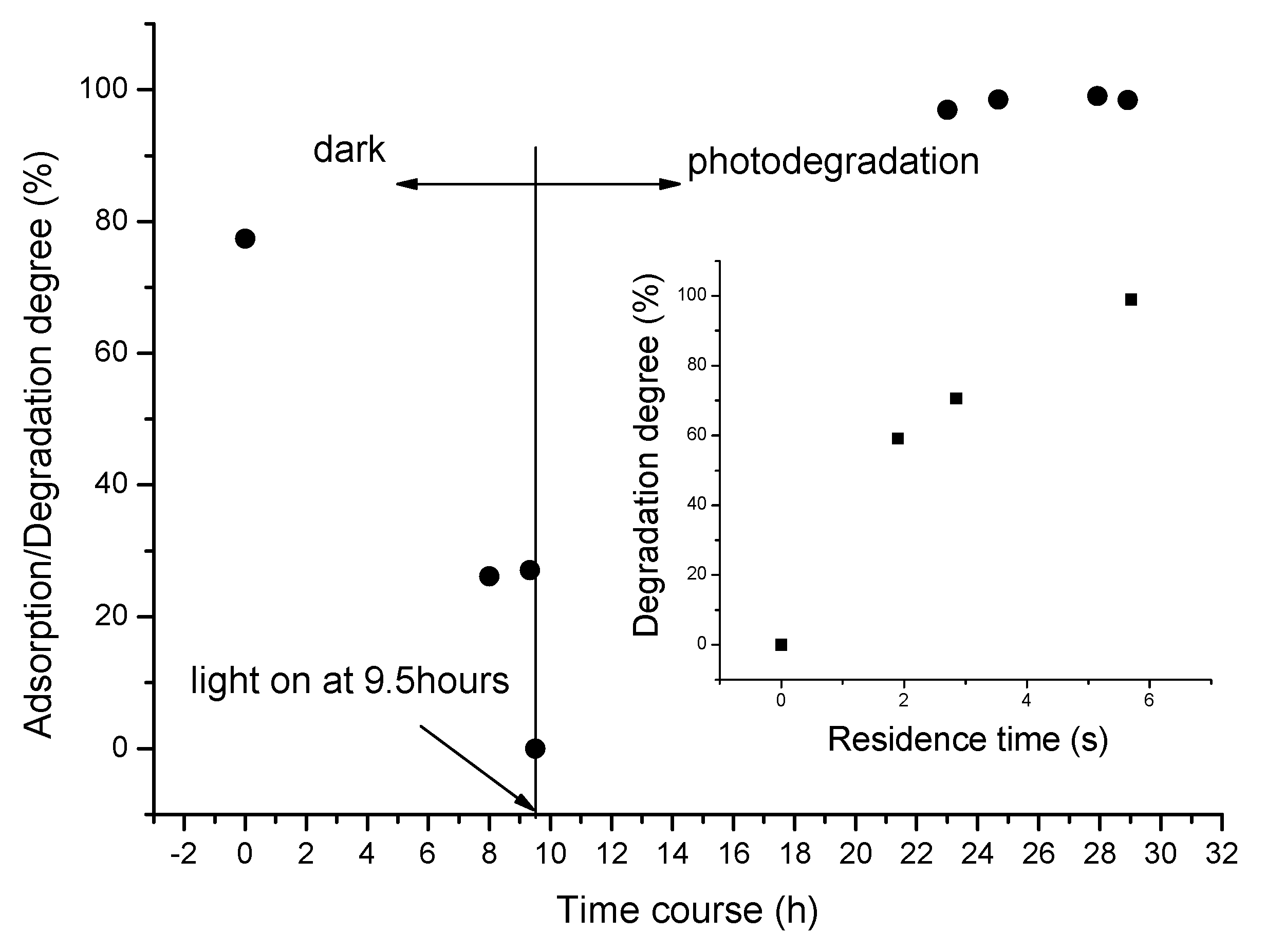
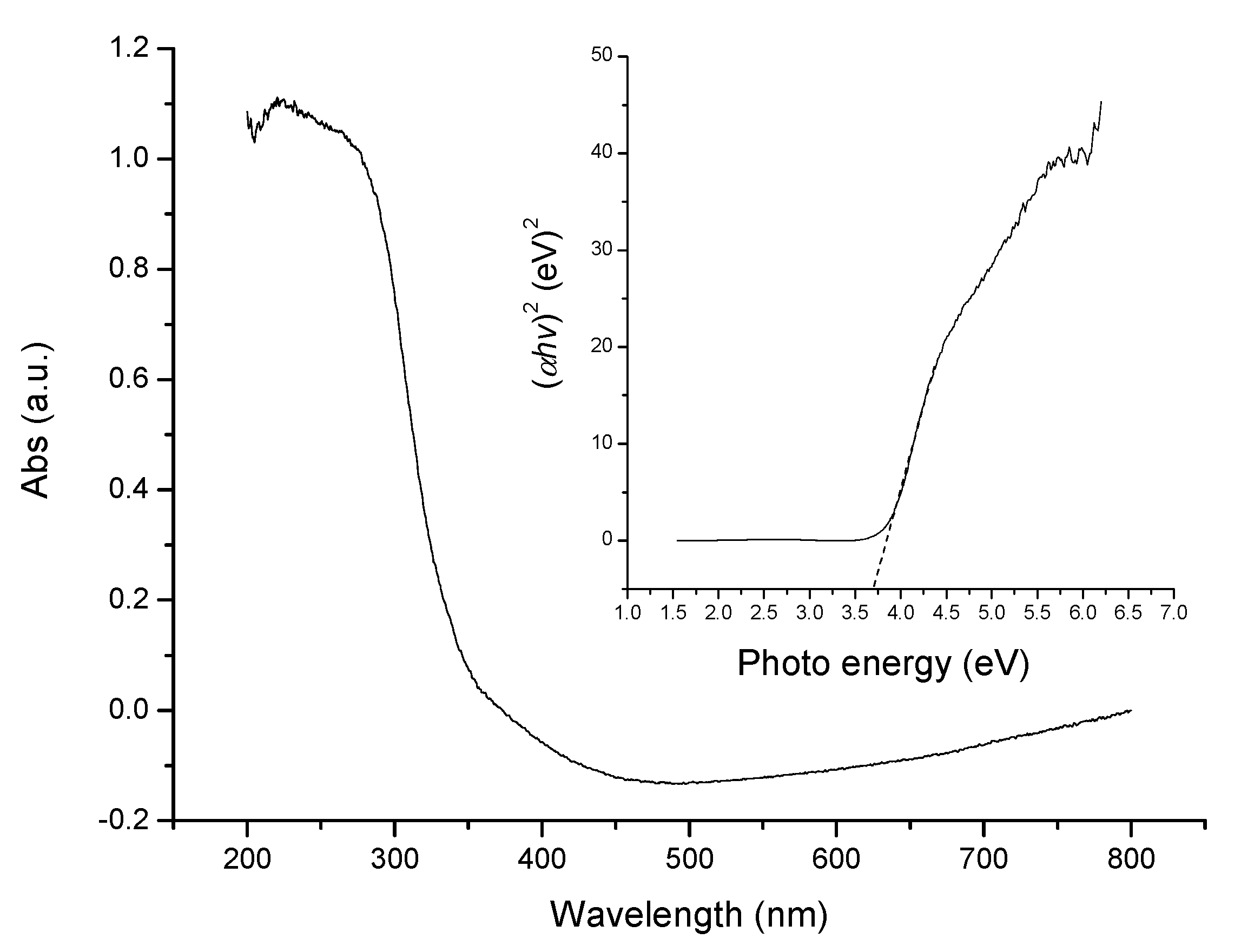
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of SnO2 Nanoparticles
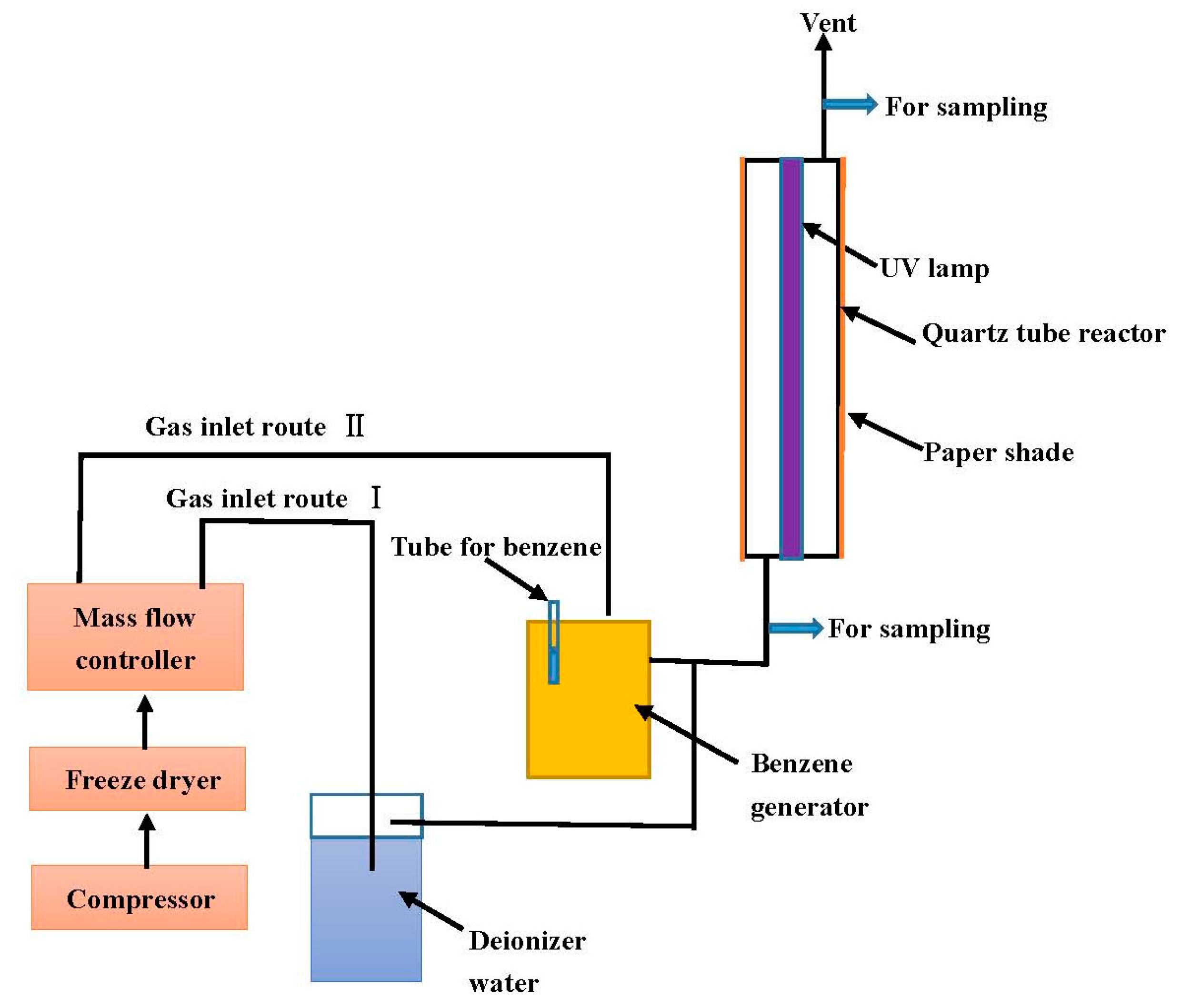
3.2. Experimental Apparatus and Methods for Photodegradation Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brillasa, E.; Mur, E.; Sauleda, R.; Sanchez, L.; Peral, J.; Domenech, X.; Casado, J. Aniline mineralization by AOP’s: Anodic oxidation, photocatalysis, electro-Fenton and photoelectro-Fenton processes. Appl. Catal. B Environ. 1998, 16, 16–31. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Kohl, P.A.; Frank, S.N.; Bard, A.J. Semiconductor electrodes. XI. Behavior of n- and p-type single crystal semiconductors covered with thin n-TiO2 films. J. Electrochem. Soc. 1977, 124, 225–229. [Google Scholar] [CrossRef]
- Xie, H.Y.; Zhu, L.P.; Wang, L.L.; Chen, S.W.; Yang, D.D.; Yang, L.J.; Gao, G.L.; Yuan, H. Photodegradation of benzene by TiO2 nanoparticles prepared by flame CVD process. Particuology 2011, 9, 75–79. [Google Scholar] [CrossRef]
- Alberici, R.M.; Jardim, W.E. Photocatalytic destruction of VOCs in the gas-phase using titanium dioxide. Appl. Catal. B Environ. 1997, 14, 55–68. [Google Scholar] [CrossRef]
- Rao, T.N.; Fujishima, A. Recent advances in electrochemistry of diamond. Diam. Relat. Mater. 2000, 9, 384–389. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Bedja, I.; Kamat, P.V. Nanostructured semiconductor films for photocatalysis. Photoelectrochemical behavior of SnO2/TiO2 composite systems and its role in photocatalytic degradation of a textile azo dye. Chem. Mater. 1996, 8, 2180–2187. [Google Scholar] [CrossRef]
- Chiang, K.; Amal, R.; Tran, T. Photocatalytic oxidation of cyanide: Kinetic and mechanistic studies. J. Mol. Catal. A Chem. 2003, 193, 285–297. [Google Scholar] [CrossRef]
- Park, D.R.; Zhang, J.L.; Ikeue, K.; Yamashita, H.; Anpo, M. Photocatalytic oxidation of ethylene to CO2 and H2O on ultrafine powdered TiO2 photocatalysts in the presence of O2 and H2O. J. Catal. 1999, 185, 114–119. [Google Scholar] [CrossRef]
- Minero, C.; Mariella, G.; Maurino, V.; Pelizzetti, E. Photocatalytic transformation of organic compounds in the presence of inorganic anions. 1. Hydroxyl-mediated and direct electron-transfer reactions of phenol on a titanium dioxide-fluoride system. Langmuir 2000, 16, 2632–2641. [Google Scholar] [CrossRef]
- Minero, C.; Mariella, G.; Maurino, V.; Vione, D.; Pelizzetti, E. Photocatalytic transformation of organic compounds in the presence of inorganic ions. 2. Competitive reactions of phenol and alcohols on a titanium dioxide-fluoride system. Langmuir 2000, 16, 8964–8972. [Google Scholar] [CrossRef]
- Palominos, R.; Freer, J.; Mondaca, M.A.; Mansilla, H.D. Evidence for hole participation during the photocatalytic oxidation of the antibiotic flumequine. J. Photochem. Photobiol. A Chem. 2008, 193, 139–145. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C.; Yu, J.Z.; Xu, J.H. Photodegradation of formaldehyde by photocatalyst TiO2: Effects on the presences of NO, SO2 and VOCs. Appl. Catal. B Environ. 2004, 54, 41–50. [Google Scholar] [CrossRef]
- Chang, C.P.; Chen, J.N.; Lu, M.C. Heterogeneous photocatalytic oxidation of acetone for air purification by near UV-irradiated titanium dioxide. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2003, 38, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ollis, D.F. Heterogeneous photocatalytic oxidation of trichloroethylene and toluene mixtures in air: Kinetic promotion and inhibition, time-dependent catalyst activity. J. Catal. 1996, 163, 1–11. [Google Scholar] [CrossRef]
- Kim, S.B.; Hong, S.C. Kinetic study for photocatalytic degradation of volatile organic compounds in air using thin film TiO2 photocatalyst. Appl. Catal. B Environ. 2002, 35, 305–315. [Google Scholar] [CrossRef]
- Obee, T.N.; Hay, S.O. Effects of moisture and temperature on the photooxidation of ethylene on Titania, Environ. Sci. Technol. 1997, 31, 2034–2038. [Google Scholar] [CrossRef]
- Miller, R.; Fox, R. Treatment of organic contaminanants in air by photocatalytic oxidation-a commercialization perspective. Trace Met. Environ. 1993, 3, 573–578. [Google Scholar]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Levy, B.; Liu, W.; Gilbert, S. Directed photocurrents in nanostructured TiO2/SnO2 heterojunction diodes. J. Phys. Chem. B 1997, 101, 1810–1816. [Google Scholar] [CrossRef]
- Shang, J.; Yao, W.Q.; Zhu, Y.F.; Wu, N. Structure and photocatalytic performances of glass/SnO2/TiO2 interface composite film. Appl Catal. A 2004, 257, 25–32. [Google Scholar] [CrossRef]
- Solis-Casadors, D.; Vigueras-Santiago, E.; Hernandez-Lopez, S.; Camacho-Lopez, A. Characterization and Photocatalytic Performance of Tin Oxide. Ind. Eng. Chem. Res. 2009, 48, 1249–1252. [Google Scholar] [CrossRef]
- Yuan, Y.; Gong, M.; Li, C.; Yan, P. Theoretical and experimental study on transverse mode competition in a partial-coiled multimode fiber laser. Laser Phys. 2008, 18, 52–57. [Google Scholar] [CrossRef]
- Einaga, H.; Futamura, S.; Ibusuki, T. Photocatalytic decomposition of benzene over TiO2 in a humidified airstream. Phys. Chem. Chem. Phys. 1999, 1, 4903–4908. [Google Scholar] [CrossRef]
- Einaga, H.; Futamura, S.; Ibusuki, T. Heterogeneous photocatalytic oxidation of benzene, toluene, cyclohexene and cyclohexane in humidified air: Comparison of decomposition behavior on photoirradiated TiO2 catalyst. Appl Catal. B Environ. 2002, 38, 215–225. [Google Scholar] [CrossRef]
- Zhao, W.R.; Yang, Y.N.; Dai, J.S.; Liu, F.F.; Wang, Y. VUV photolysis of naphthalene in indoor air: Intermediates, pathways, and health risk. Chemosphere 2013, 91, 1002–1008. [Google Scholar] [CrossRef]
- Xie, H.Y.; Gao, G.L.; Tian, Z.; Bing, N.C.; Wang, L.J. Synthesis of TiO2 nanoparticles by propane/air turbulent flame CVD process. Particuology 2009, 7, 204–210. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis and physical properties of the semiconducting electrode WO2. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Nasr, C.; Kamat, P.V.; Hotchandani, S. Photoelectrochemistry of composite semiconductor thin films. Photosensitization of the SnO2/TiO2 coupled system with a ruthenium polypyridyl complex. J. Phys. Chem. B 1998, 102, 10047–10056. [Google Scholar] [CrossRef]
- Xie, H.Y.; Zhang, Y.N.; Xu, Q.L. Study of photocatalytic degradation of methanol in air over TiO2 nanofilm. Proc. China Assoc. Sci. Technol. 2009, 5, 41. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Sun, Z.; Zhang, L.; Xie, H. Photodegradation of Gas Phase Benzene by SnO2 Nanoparticles by Direct Hole Oxidation Mechanism. Catalysts 2020, 10, 117. https://doi.org/10.3390/catal10010117
Chen S, Sun Z, Zhang L, Xie H. Photodegradation of Gas Phase Benzene by SnO2 Nanoparticles by Direct Hole Oxidation Mechanism. Catalysts. 2020; 10(1):117. https://doi.org/10.3390/catal10010117
Chicago/Turabian StyleChen, Shi, Zhiguo Sun, Li Zhang, and Hongyong Xie. 2020. "Photodegradation of Gas Phase Benzene by SnO2 Nanoparticles by Direct Hole Oxidation Mechanism" Catalysts 10, no. 1: 117. https://doi.org/10.3390/catal10010117
APA StyleChen, S., Sun, Z., Zhang, L., & Xie, H. (2020). Photodegradation of Gas Phase Benzene by SnO2 Nanoparticles by Direct Hole Oxidation Mechanism. Catalysts, 10(1), 117. https://doi.org/10.3390/catal10010117




