Differentially Expressed MicroRNAs in Meningiomas Grades I and II Suggest Shared Biomarkers with Malignant Tumors
Abstract
:1. Introduction
2. Results
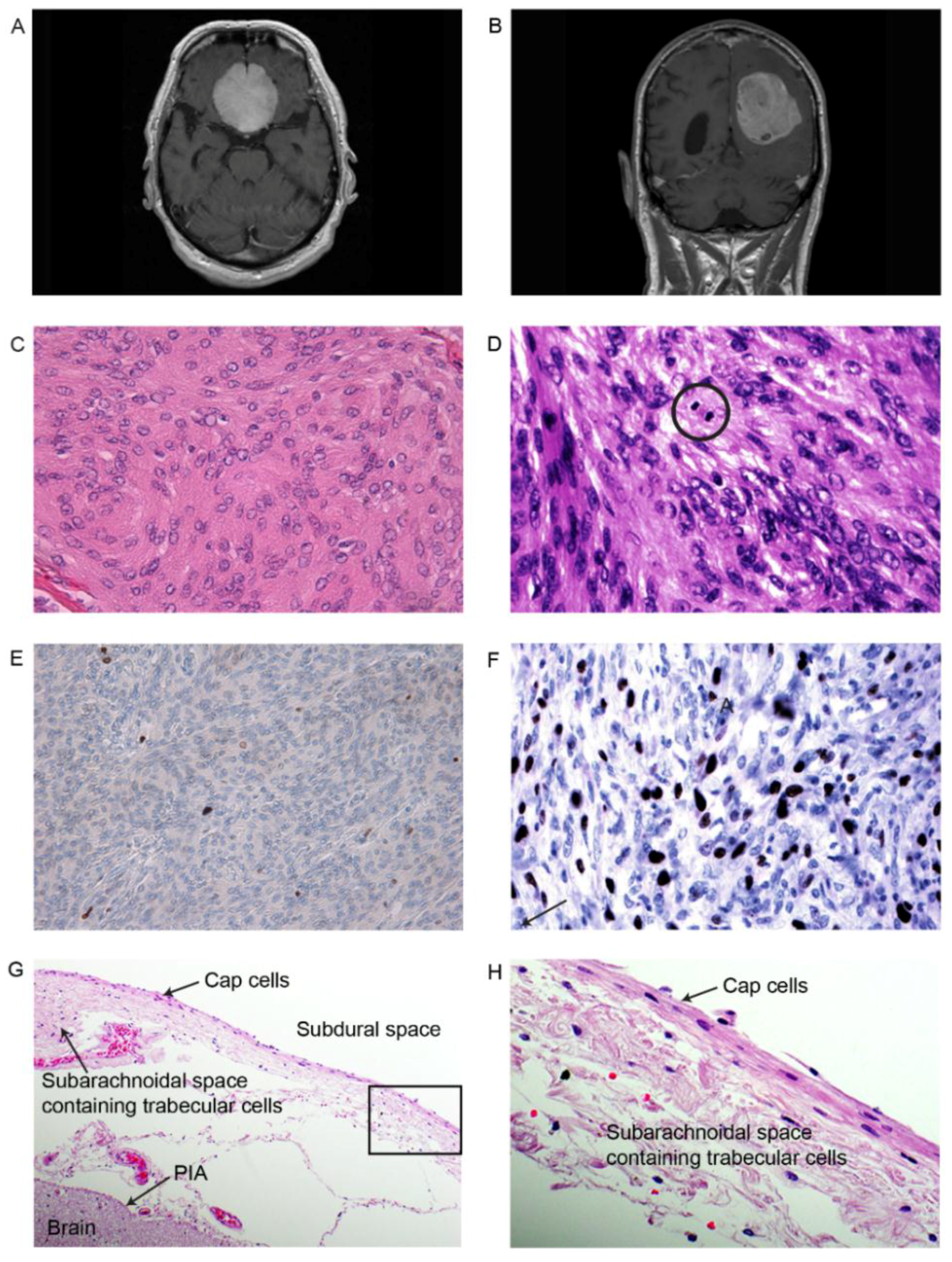
2.1. Pathological and Histological Classifications
2.2. MiRNA Expression Profile from SOLiD Deep Sequencing
2.3. RT-qPCR Re-Evaluation of Differentially Expressed miRNAs in Meningioma Versus Normal
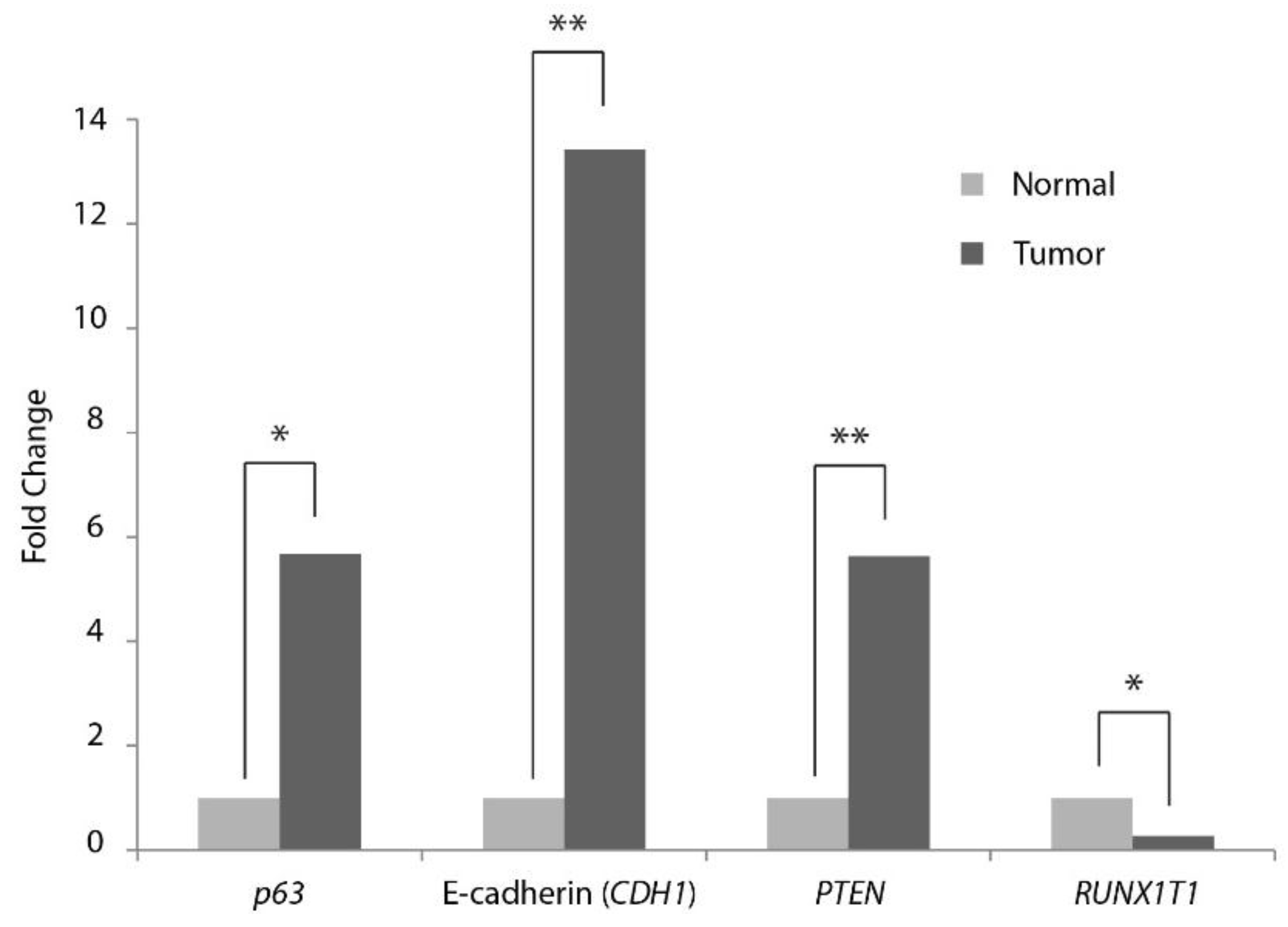
2.4. RT-qPCR Evaluation of Expression of Selected mRNA Targets in Meningiomas
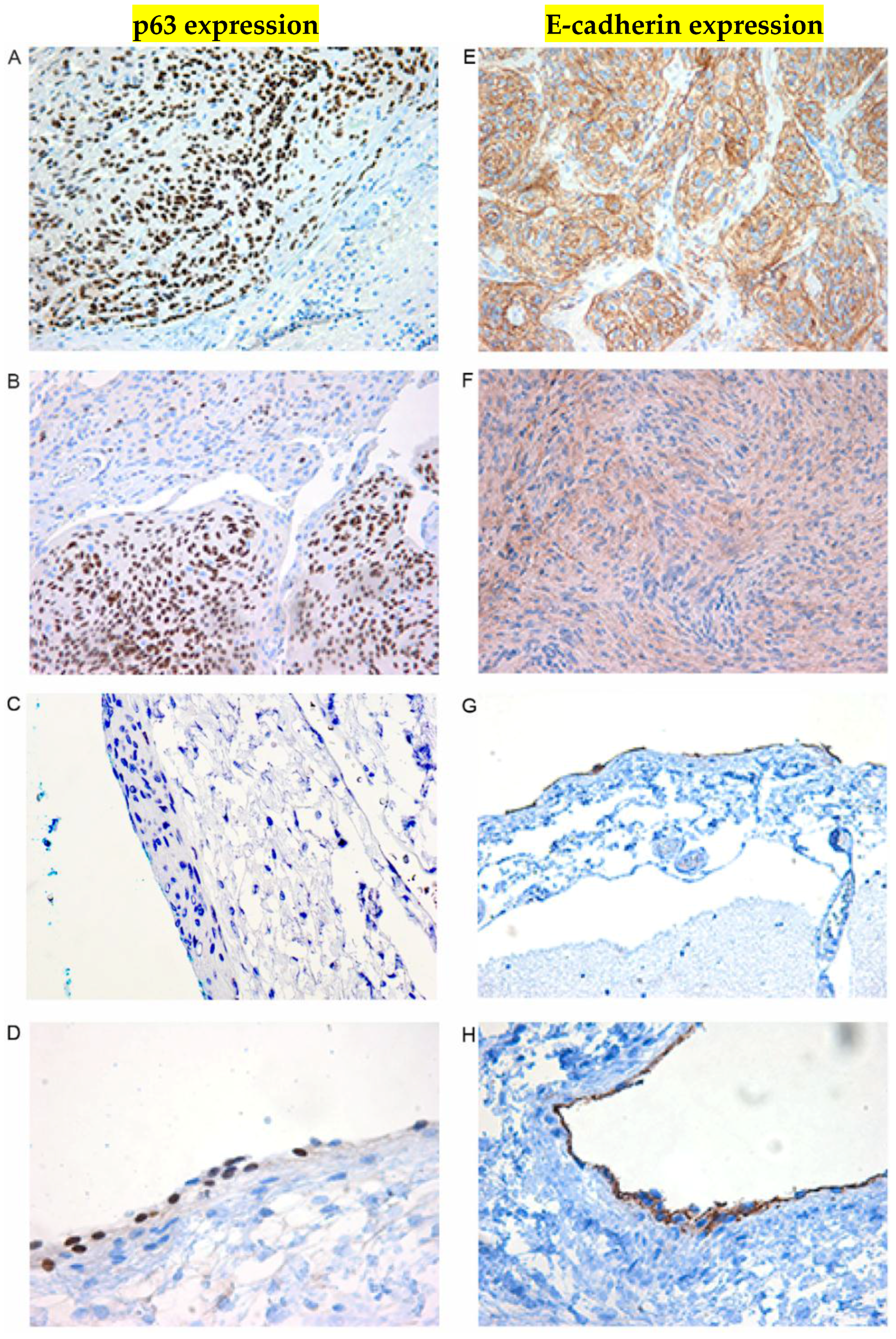
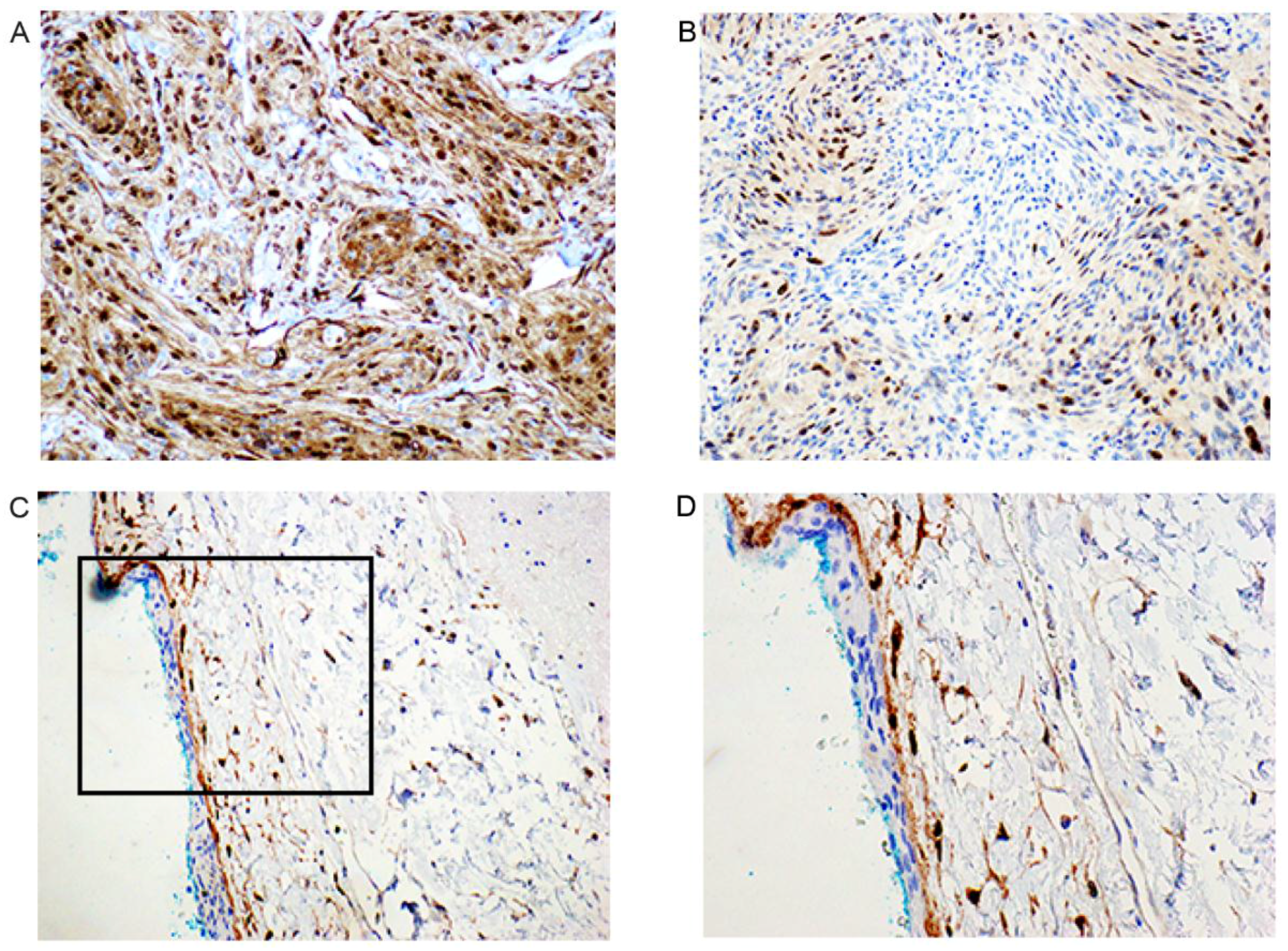
2.5. Immunohistochemical Examination
3. Discussion
3.1. Differentially Expressed Micro RNAs
3.2. Differentially Expressed mRNA Selected Putative Targets
4. Materials and Methods
4.1. Ethical Considerations
4.2. Patient Samples
4.3. Immunohistochemistry (IHC)
4.4. Total RNA Extraction
4.5. SOLiD Sequencing
4.6. Sequence Data Analysis
4.7. Validation of miRNA Expression in Meningioma and Normal Dura by Real-Time Quantitative PCR (RT-qPCR)
4.8. Validation of Selected Possible mRNA Targets Expression in Benign Meningiomas and Normal Dura Biopsies (N and NN) by RT-qPCR
5. Conclusions
Supplementary Material
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marosi, C.; Hassler, M.; Roessler, K.; Reni, M.; Sant, M.; Mazza, E.; Vecht, C. Meningioma. Crit. Rev. Oncol. Hematol. 2008, 67, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2006–2010. Neuro-oncology 2013, 15, ii1–ii56. [Google Scholar] [CrossRef] [PubMed]
- Mawrin, C.; Perry, A. Pathological classification and molecular genetics of meningiomas. J. Neurooncol. 2010, 99, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Marino-Enriquez, A.; Fletcher, C.D.M. Shouldn’t we care about the biology of benign tumours? Nat. Rev. Cancer 2014, 14, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Shi, L.; Gao, F.; Russin, J.; Zeng, L.; He, S.; Chen, T.C.; Giannotta, S.L.; Weisenberger, D.J.; Zada, G.; et al. Genomic and transcriptomic analysis revealing an oncogenic functional module in meningiomas. Neirosurg. Focus 2013, 35, E3. [Google Scholar] [CrossRef] [PubMed]
- Serna, E.; Morales, J.M.; Mara, M.; Gonzalez-Darder, J.; San Miguel, T.; Gil-Benso, R.; Lopez-Gines, C.; Cerda-Nicolas, M.; Monleon, D. Gene expression profiles of metabolic aggressiveness and tumor recurrence in benign meningioma. PLoS ONE 2013, 8, e67291. [Google Scholar] [CrossRef] [PubMed]
- Abedalthagafi, M.S.; Merrill, P.H.; Bi, W.L.; Jones, R.T.; Listewnik, M.L.; Ramkissoon, S.H.; Thornes, A.R.; Dunn, I.F.; Beroukhim, R.; Alexander, B.M.; et al. Angiomatous meningiomas have a distinct genetic profile with multiple chromosomal polysomies including polysomy of chromosome 5. Oncotarget 2014, 5, 10596–10606. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martin, M.; Lassaketta, L.; Isla, A.; de Campos, J.M.; Pinto, G.R.; Burbano, R.R.; Castresana, J.S.; Melendez, B.; Rey, J.A. Global expression profile in low grade meningiomas and schwannomas shows upregulation of PDGFD, CDH1 and SLIT2 compared to their healthy tissue. Oncol. Rep. 2014, 32, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J. Germline and somatic mutations in meningiomas. Cancer Genet. 2015, 208, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.E.; Erson-Omay, E.Z.; Serin, A.; Yin, J.; Cotney, J.; Ozduman, K.; Avşar, T.; Li, J.; Murray, P.B.; Henegariu, O.; et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013, 339, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Nickel, A.; Stadler, S.C. Role of epigenetic mechanisms in epithelial-to-mesenchymal transition of breast cancer cells. Transl. Res. 2015, 165, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Cech, T.R.; Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Murnyák, B.; Bognár, L.; Klekner, Á.; Hortobágyi, T. Epigenetics of Meningiomas. Biomed. Res. Int. 2015, 2015, 532451. [Google Scholar] [CrossRef] [PubMed]
- Venza, M.; Visalli, M.; Beninati, C.; Catalano, T.; Biondo, C.; Teti, D.; Venza, I. Involvement of epimutations in meningioma. Br. Tumor Path. 2015, 32, 163–168. [Google Scholar] [CrossRef] [PubMed]
- miRBase: The microRNA Database Homepage. Available online: http://www.mirbase.org (accessed on 19 February 2016).
- Ohtsuka, M.; Ling, H.; Doki, Y.; Mori, M.; Calin, G.A. MicroRNA Processing and Human Cancer. J. Clin. Med. 2015, 4, 1651–1667. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer. 2015, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell. Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Wilczynska, A.; Bushell, M. The complexity of miRNA-mediated repression. Cell. Death Differ. 2015, 22, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Lieberman, J. Dysregulation of microRNA biogenesis and gene silencing in cancer. Sci. Signal. 2015, 17, re3. [Google Scholar]
- Takasaki, S. Roles of microRNAs in cancers and development. Methods Mol. Biol. 2015, 1218, 375–413. [Google Scholar] [PubMed]
- Pua, H.H.; Ansel, K.M. MicroRNA regulation of allergic inflammation and asthma. Curr. Opin. Immunol. 2015, 36, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Koturbash, I.; Tolleson, W.H.; Guo, L.; Yu, D.; Chen, S.; Hong, H.; Mattes, W.; Ning, B. microRNAs as pharmacogenomic biomarkers for drug efficacy and drug safety assessment. Biomark. Med. 2015, 9, 1153–1176. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, G.; Hayashi, K.; Xi, Y.; Kudo, K.; Uchida, K.; Takasaki, K.; Yamamoto, M.; Ju, J. Non-coding microRNAs hsa-let-7g and hsa-miR-181b are associated with chemoresponse to S-1 in colon cancer. Cancer Genom. Proteom. 2006, 3, 317–324. [Google Scholar]
- Fornari, F.; Gramantieri, L.; Giovannini, C.; Veronese, A.; Ferracin, M.; Sabbioni, S.; Calin, G.A.; Grazi, G.L.; Croce, C.M.; Tavolari, S.; et al. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009, 69, 5761–5767. [Google Scholar] [CrossRef] [PubMed]
- Trompeter, H.I.; Abbad, H.; Iwaniuk, K.M.; Hafner, M.; Renwick, N.; Tuschl, T.; Schira, J.; Müller, H.W.; Wernet, P. MicroRNAs MiR-17, MiR-20a, and MiR-106b act in concert to modulate E2F activity on cell cycle arrest during neuronal lineage differentiation of USSC. PLoS ONE 2011, 6, e16138. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, T.; Kozaki, K.; Uesugi, A.; Furuta, M.; Hirasawa, A.; Imoto, I.; Susumu, N.; Aoki, D.; Inazawa, J. miR-152 is a tumor suppressor microRNA that is silenced by DNA hypermethylation in endometrial cancer. Cancer Res. 2011, 71, 6450–6462. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xie, H.; Mori, M.A.; Alexander, R.; Yuan, B.; Hattangadi, S.M.; Liu, Q.; Kahn, C.R.; Lodish, H.F. Mir193b-365 is essential for brown fat differentiation. Nat. Cell. Biol. 2011, 13, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Feilotter, H.E.; Paré, G.C.; Zhang, X.; Pemberton, J.G.; Garady, C.; Lai, D.; Yang, X.; Tron, V.A. MicroRNA-193b represses cell proliferation and regulates cyclin D1 in melanoma. Am. J. Pathol. 2010, 176, 2520–2529. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Heo, M.J.; Lee, C.G.; Yang, Y.M.; Kim, S.G. Increase of miR-199a-5p by protoporphyrin IX, a photocatalyzer, directly inhibits E2F3, sensitizing mesenchymal tumor cells to anti-cancer agents. Oncotarget 2015, 6, 3918–3931. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, X.; Liu, X.; Xiao, S.; Zhang, Y.; Xiang, T.; Shen, X.; Wang, G.; Sheng, B. miR-21 promotes human nucleus pulposus cell proliferation through PTEN/AKT signaling. Int. J. Mol. Sci. 2014, 15, 4007–4018. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, A.; Kozaki, K.; Tsuruta, T.; Furuta, M.; Morita, K.; Imoto, I.; Omura, K.; Inazawa, J. The tumor suppressive microRNA miR-218 targets the mTOR component Rictor and inhibits AKT phosphorylation in oral cancer. Cancer Res. 2011, 71, 5765–5778. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Birks, D.K.; Balakrishnan, I.; Alimova, I.; Harris, P.S.; Patel, P.R.; Handler, M.H.; Dubuc, A.; Taylor, M.D.; Foreman, N.K.; et al. MicroRNA 218 acts as a tumor suppressor by targeting multiple cancer phenotype-associated genes in medulloblastoma. J. Biol. Chem. 2013, 288, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Dong, Y.; Wu, C.W.; Zhao, Z.; Ng, S.S.; Chan, F.K.; Sung, J.J.; Yu, J. MicroRNA-218 inhibits cell cycle progression and promotes apoptosis in colon cancer by downregulating BMI1 polycomb ring finger oncogene. Mol. Med. 2013, 18, 1491–1498. [Google Scholar] [PubMed]
- Palumbo, T.; Faucz, F.R.; Azevedo, M.; Xekouki, P.; Iliopoulos, D.; Stratakis, C.A. Functional screen analysis reveals miR-26b and miR-128 as central regulators of pituitary somatomammotrophic tumor growth through activation of the PTEN-AKT pathway. Oncogene 2013, 32, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, L.; Luo, J.; Gao, J.; Guo, J.; Xie, X. MiR-34a inhibits proliferation and migration of breast cancer through down-regulation of Bcl-2 and SIRT1. Clin. Exp. Med. 2013, 13, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Fu, G.; Cui, S.; Zhao, S.; Bernaudo, S.; Bai, Y.; Ding, Y.; Zhang, Y.; Yang, B.B.; Peng, C. MicroRNA 376c enhances ovarian cancer cell survival by targeting activin receptor-like kinase 7: Implications for Chemoresistance. J. Cell. Sci. 2011, 124, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, S.; He, J.; Liu, X.; Qu, Y.; Yan, W.; Fan, J.; Li, R.; Xi, H.; Fu, W.; et al. MicroRNA-451 regulates stemness of side population cells via PI3K/Akt/mTOR signaling pathway in multiple myeloma. Oncotarget 2015, 6, 14993–15007. [Google Scholar] [CrossRef] [PubMed]
- Chikh, A.; Matin, R.N.; Senatore, V.; Hufbauer, M.; Lavery, D.; Raimondi, C.; Ostano, P.; Mello-Grand, M.; Ghimenti, C.; Bahta, A.; et al. iASPP/p63 autoregulatory feedback loop is required for the homeostasis of stratified epithelia. EMBO J. 2011, 30, 4261–4273. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, Z.; Tan, X.; Zhou, F.; Tan, F.; Gao, Y.; Sun, N.; Xu, X.; Shao, K.; He, J. MicroRNA-99a/100 promotes apoptosis by targeting mTOR in human esophageal squamous cell carcinoma. Med. Oncol. 2013, 30, 411. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.G.; Luo, X.; Wu, S.; Jian, B. MiR-99a inhibits cell proliferation and tumorigenesis through targeting mTOR in human anaplastic thyroid cancer. Asian Pac. J. Cancer Prev. 2015, 16, 4937–4944. [Google Scholar] [CrossRef] [PubMed]
- Peltier, H.J.; Latham, G.J. Normalization of microRNA expression levels in quantitative RT-PCR assays: Identification of Suitable Reference RNA Targets in Normal and Cancerous Human Solid Tissues. RNA 2008, 14, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Saydam, O.; Shen, Y.; Würdinger, T.; Senol, O.; Boke, E.; James, M.F.; Tannous, B.A.; Stemmer-Rachamimov, A.O.; Yi, M.; Stephens, R.M.; et al. Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/beta-catenin signaling pathway. Mol. Cell. Biol. 2009, 29, 5923–5940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, F.; Zhou, G.; Wang, S.; Shi, Y.; Peng, Y.; Shao, N.; Guan, W.; Qu, H.; Zhang, Y.; Wang, Q.; et al. A microRNA expression signature predicts meningioma recurrence. Int. J. Cancer 2012, 132, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, E.; Perander, M.; Fiskaa, T.; Johansen, S.D. Performance Comparison and Data Analysis Strategies for MicroRNA Profiling in Cancer Research. In Next Generation Sequencing in Cancer Research; Wei, W., Hani, C., Eds.; Springer International Publishing AG: Basel, Switzerland, 2015; Volume 2, pp. 239–265. [Google Scholar]
- Knutsen, E.; Fiskaa, T.; Ursvik, A.; Jørgensen, T.E.; Perander, M.; Lund, E.; Seternes, O.M.; Johansen, S.D.; Andreassen, M. Performance comparison of digital microRNA profiling technologies applied on human breast cancer cell lines. PLoS ONE 2013, 8, e75813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.H.; Pfeffer, S.R.; Sims, M.; Yue, J.; Wang, Y.; Linga, V.G.; Paulus, E.; Davidoff, A.M.; Pfeffer, L.M. The oncogenic microRNA-21 inhibits the tumor suppressive activity of FBXO11 to promote tumorigenesis. J. Biol. Chem. 2015, 290, 6037–6046. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Kim, Y.J.; Mueller, S.C.; Backes, C.; Werner, T.V.; Galata, V.; Sartorius, E.; Bohle, R.M.; Keller, A.; Meese, E. Posttranscriptional deregulation of signaling pathways in meningioma subtypes by differential expression of miRNAs. Neuro-oncology 2015, 17, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, P.; Li, A.; Jiang, W.; Wang, H.; Wang, J.; Xie, K. Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J. Exp. Clin. Cancer Res. 2012, 22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, C.; Zhang, W.; Chen, Z.; Ma, L. Down regulation of miR-143 is related with tumor size, lymph node metastasis and HPV16 infection in cervical squamous cancer. Diagn. Pathol. 2014, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, Y.; Ti, H.; Zhao, J.; Wang, Y.; Li, T.; Zhang, B. Down-regulation of miR-145 and miR-143 might be associated with DNA methyltransferase 3B overexpression and worse prognosis in endometrioid carcinomas. Hum. Pathol. 2013, 44, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kong, F.; Wu, K.; Song, K.; He, J.; Sun, W. miR-193b directly targets STMN1 and uPA genes and suppresses tumor growth and metastasis in pancreatic cancer. Mol. Med. Rep. 2014, 10, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, C.; Bertero, T.; Xu, N.; Bourget-Ponzio, I.; Lebrigand, K.; Fourre, S.; Popa, A.; Cardot-Leccia, N.; Meneguzzi, G.; Sonkoly, E.; et al. miR-193b/365a cluster controls progression of epidermal squamous cell carcinoma. Carcinogenesis 2014, 35, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Fan, M.; Zhang, X. microRNA-218 inhibits prostate cancer cell growth and promotes apoptosis by repressing TPD52 expression. Biochem. Biophys. Res. Comm. 2015, 456, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, R.; Goto, Y.; Sakamoto, S.; Chiyomaru, T.; Enokida, H.; Kojima, S.; Kinoshita, T.; Yamamoto, N.; Nakagawa, M.; Naya, Y.; et al. Tumor-suppressive microRNA-218 inhibits cancer cell migration and invasion via targeting of LASP1 in prostate cancer. Cancer Sci. 2014, 105, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Peng, L.; Chao, C.; Fu, B.; Wang, G.; Wang, Y.; Zhu, X. miR-451 inhibits invasion and proliferation of bladder cancer by regulating EMT. Int. J. Clin. Exp. Pathol. 2014, 7, 7653–7662. [Google Scholar] [PubMed]
- Huang, J.Y.; Zhang, K.; Chen, D.Q.; Chen, J.; Feng, B.; Song, H.; Chen, Y.; Zhu, Z.; Lu, L.; De, W.; et al. MicroRNA-451: Epithelial-mesenchymal transition inhibitor and prognostic biomarker of hepatocelluar carcinoma. Oncotarget 2015, 6, 18613–18630. [Google Scholar] [CrossRef] [PubMed]
- Misso, G.; Di Martino, M.T.; de Rosa, G.; Farooqi, A.A.; Lombardi, A.; Campani, V.; Zarone, M.R.; Gullà, A.; Tagliaferri, P.; Tassone, P.; et al. Mir-34: A new weapon against cancer? Mol. Ther. Nucleic Acids 2014, 3, e194. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Jain, D.; Roy, S.; Mehta, V.S. Correlation of p63 protein expression with histological grade of meningiomas: An Immunohistochemical Study. Int. J. Surg. Pathol. 2012, 20, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Beetham, H.; Black, M.A.; Priya, R.; Telford, B.J.; Guest, J.; Wiggins, G.A.; Godwin, T.D.; Yap, A.S.; Guilford, P.J. E-cadherin loss alters cytoskeletal organization and adhesion in non-malignant breast cells but is insufficient to induce an epithelial-mesenchymal transition. BMC Cancer 2014, 14, 552. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Simeone, P.; Latorre, D.; Cascione, F.; Leporatti, S.; Trerotola, M.; Giudetti, A.M.; Capobianco, L.; Lunetti, P.; Rizzello, A.; et al. Proteomics analysis of E-cadherin knockdown in epithelial breast cancer cells. J. Biotechnol. 2014, 202, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhang, W.; Jiang, L.Y.; Qin, W.X.; Wang, X. Reduced E-Cadherin expression is a prognostic biomarker of non-small cell lung cancer: A meta-analysis based on 2395 subjects. Int. J. Clin. Exp. Med. 2014, 7, 4352–4356. [Google Scholar] [PubMed]
- Shargh, S.A.; Sakizli, M.; Khalaj, V.; Movafagh, A.; Yazdi, H.; Hagigatjou, E.; Sayad, A.; Mansouri, N.; Mortazavi-Tabatabaei, S.A.; Khorram Khorshid, H.R. Downregulation of E-cadherin expression in breast cancer by promoter hypermethylation and its relation with progression and prognosis of tumor. Med. Oncol. 2014, 31, 250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Wang, G.; Wang, Y.; Jin, H.; Yang, S.; Liu, C. The potential involvement of E-cadherin and beta-catenins in meningioma. PLoS ONE 2010, 5, e11231. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, K.; Zhou, L.; Birchmeier, W. E-Cadherin in human brain tumours: Loss of Immunoreactivity in malignant meningiomas. Virchows. Arch. 1998, 432, 163–167. [Google Scholar] [CrossRef] [PubMed]
- El-Habr, E.A.; Levidou, G.; Trigka, E.A.; Sakalidou, J.; Piperi, C.; Chatziandreou, I.; Spyropoulou, A.; Soldatos, R.; Tomara, G.; Petraki, K.; et al. Complex interactions between the components of the PI3K/AKT/mTOR pathway, and with components of MAPK, JAK/STAT and Notch-1 pathways, indicate their involvement in meningioma development. Virchows. Arch. 2014, 465, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.F.; Wu, Z.P.; Chen, Y.; Zhu, Q.S.; Hamidi, S.; Navab, R. MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS ONE 2014, 9, e103698. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.T.; Klausen, C.; Leung, P.C. E-cadherin inhibits tumor cell growth by suppressing PI3K/Akt signaling via β-catenin-Egr1-mediated PTEN expression. Oncogene 2011, 30, 2753–2766. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak-Celińska, A.; Paluszczak, J.; Szalata, M.; Barciszewska, A.M.; Nowak, S.; Baer-Dubowska, W. DNA methylation analysis of benign and atypical meningiomas: Correlation between RUNX3 Methylation and WHO Grade. J. Cancer Res. Clin. Oncol. 2015, 141, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Bae, S.C.; Chuang, L.S. The RUNX family: Developmental regulators in cancer. Nat. Rev. Cancer 2015, 15, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Jirawatnotai, S.; Sharma, S.; Michowski, W.; Suktitipat, B.; Geng, Y.; Quackenbush, J.; Elias, J.E.; Gygi, S.P.; Wang, Y.E.; Sicinski, P. The cyclin D1-CDK4 oncogenic interactome enables identification of potential novel oncogenes and clinical prognosis. Cell Cycle 2014, 13, 2889–2900. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, L.; Lv, W.; Dong, C.; Wang, Y.; Zhang, J. Overexpression of cyclin D1 in meningioma is associated with malignancy grade and causes abnormalities in apoptosis, invasion and cell cycle progression. Med. Oncol. 2015, 32, 439. [Google Scholar] [CrossRef] [PubMed]
| Patient No. | Sample Type | Histology Grade/Subclass * | Gender | Age | Notes |
|---|---|---|---|---|---|
| 1 | T + N | IA | M | 71 | + Deep sequencing |
| 2 | T + N | II | M | 74 | + Deep sequencing |
| 3 | T | II | M | 60 | |
| 4 | T | IA + D | F | 70 | |
| 5 | T | IB | F | 68 | |
| 6 | T | II | M | 75 | |
| 7 | T | IA | M | 69 | |
| 8 | T | IC | F | 55 | |
| 9 | T + N *** | IC | F | 42 | |
| 10 | T + N | IA | M | 68 | |
| 11 | T | IC | F | 62 | |
| 12 | T | IC | M | 47 | |
| 13 | T + N | IA | F | 35 | |
| 14 | T *** | IC | F | 43 | |
| 15 | T + N | IC | M | 59 | |
| 16 | NN | Normal | M | 80 | + Deep sequencing |
| 17 (cadaver) | NN-a | Arachnoid | M | 55 | |
| 18 (cadaver) | NN-a | Arachnoid | M | 59 | |
| 19 (cadaver) | NN-a | Arachnoid | M | 73 | |
| 20 (cadaver) | NN-a | Arachnoid | M | 49 | |
| 21 (cadaver) | NN-a | Arachnoid | M | 54 | |
| 22 | NN | Normal | M | 64 | |
| 23 | NN ** | Normal | M | 76 | Only Deep sequencing |
| miRNA | Change Related to | * Fold Change | Selected 10 Putative Target mRNA for Analysis by RT-qPCR |
|---|---|---|---|
| let-miR-7g | II/N | +10.48 | Cyclin D, E2F, p53 [24] |
| miR-1221 | II/N | −81.56 | Cyclin G1 [25] |
| miR-17 | II/N | −10.34 | Cyclin D1, E2F1, PTEN [26] |
| miR-130a | II/N | +6.32 | |
| miR-143 | IA/N | −5.17 | |
| miR-148b | II/N | −6.02 | |
| miR-152 | II/N | +6.31 | E2F3, RICTOR [27] |
| miR-193b | IA/N | −5.76 | RUNX1T1 (Cyclin D-related) [28], (Cyclin D1) [29] |
| miR-199a-5p | IA/N | −6.20 | E2F3 [30] |
| miR-21 | IA/II: | −5.45 | PTEN [31] |
| miR-218 | II/N | +8.55 | RICTOR [32,33], mTOR [32], regulating p53 [34] |
| IA/N | +7.59 | ||
| miR-26b | IA/II | +8.86 | Activation of PTEN–Akt pathway [35] |
| miR-34a | IA/II | −8.51 | Sirt1 [36] |
| miR-342-3p | IA/N | +7.52 | |
| miR-376c | II/N | +8.14 | ALK7 [37] |
| IA/II | −7.92 | ||
| miR-424 | II/N | +6.33 | |
| miR-451 | IA/N | −10.07 | PI3K/Akt/mTOR signaling pathway [38] |
| IA/II | −6.90 | ||
| miR-574-3p | IA/II | +13.12 | P63 [39] |
| miR-99a | IA/II | −6.09 | mTOR [40,41] |
| II/N | +5.42 |
| miRNA | Fold Change | p-Value |
|---|---|---|
| let-7g | 1.132 | 0.444 |
| miR-130a | 2.254 | 0.004 |
| miR-143 | −3.867 | 0.001 |
| miR-148b | 1.522 | 0.041 |
| miR-152 | 2.218 | 0.000 |
| miR-17 | −2.089 | 0.000 |
| miR-193b | −4.063 | 0.007 |
| miR-199a-5p | −1.548 | 0.325 |
| miR-21 | −3.710 | 0.003 |
| miR-218 | 4.473 | 0.003 |
| miR-26b | 1.156 | 0.377 |
| miR-342-3p | 1.146 | 0.496 |
| miR-34a | 3.133 | 0.002 |
| miR-376c | 1.372 | 0.395 |
| miR-424 | 1.806 | 0.103 |
| miR-451 | −18.452 | 0.000 |
| miR-574-3p | −1.286 | 0.212 |
| miR-99a | 1.110 | 0.760 |
| mRNA | Fold Change | p-Value |
|---|---|---|
| p63 | 5.7 | 0.044 |
| E-Cadherin (CDH1) | 13.4 | 0.000 |
| PTEN | 5.6 | 0.000 |
| RUNX1T1 (Cyclin D related) | −3.7 | 0.021 |
| RICTOR | 1.5 | 0.298 |
| p53 | 1.5 | 0.213 |
| Patient NO. | Sample Type | Histology | P 63 (Nucleus) | E-Cad (Membrane) | Cyclin D1 (Nucleus) |
|---|---|---|---|---|---|
| 1 | T | IA | ++ | ++(+) | ++ |
| 2 | T | II | + | ++ | ++(+) |
| 3 | T | II | + | ++(+) | + |
| 4 | T | IA+IB | ++ | ++(+) | + |
| 5 | T | IB | - | + | ++(+) |
| 6 | T | II | +++ | +++ | +++ |
| 7 | T | IA | + | ++(+) | +++ |
| 8 | T | IC | + | ++ | ++ |
| 9 | T | IC | ++ | ++ | ++ |
| 10 | T | IA+II | + | +++ | +++ |
| 11 | T | IC | ++ | ++ | +++ |
| 12 | T | IC | ++ | ++ | ++(+) |
| 13 | T | IA | ++(+) | +++ | + |
| 14 | T | IC | + | +++ | ++(+) |
| 15 | T | IC | ++ | ++ | ++(+) |
| 17 | Arachnoidea Autopsy | Normal (NN-a) | + | + | + |
| 18 | Arachnoidea Autopsy | Normal (NN-a) | - | + | Negative in cap cells. Positive in some of the trabecular cells in the subarachnoidal space |
| 19 | Arachnoidea Autopsy | Normal (NN-a) | - | + | As previous |
| 20 | Arachnoidea Autopsy | Normal (NN-a) | - | + | As previous |
| 21 | Arachnoidea Autopsy | Normal (NN-a) | - | + | - |
| 22 | Dura | Normal NN | - | + | Positive in single cells, not well oriented |
| For Proliferation | n Code | Expression Relative to Controls | Prognosis | Anti-Proliferation/Anti-Malignancy | n Code | Expression Relative to Controls | nn Prognosis |
|---|---|---|---|---|---|---|---|
| miR-143 1st | TS | Under |  | miR-21 | ONC | Under |  |
| miR-193b 1st | TS | Under |  | miR-34a | TS | Over |  |
| miR-451 | TS | Under |  | miR-218 | TS | Over |  |
| RUNX1T1 1st | TS | Under |  | PTEN | TS | Over |  |
| P63; also positive by IHC | Pro-Growth | Over |  | E-cadherin (CDH1); also positive by IHC | TS | Over |  |
| Cyclin D1 | ONC | Over; by IHC |  |
 Bad prognostic sign,
Bad prognostic sign,  Good prognostic sign.
Good prognostic sign.© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gewely, M.R.; Andreassen, M.; Walquist, M.; Ursvik, A.; Knutsen, E.; Nystad, M.; Coucheron, D.H.; Myrmel, K.S.; Hennig, R.; Johansen, S.D. Differentially Expressed MicroRNAs in Meningiomas Grades I and II Suggest Shared Biomarkers with Malignant Tumors. Cancers 2016, 8, 31. https://doi.org/10.3390/cancers8030031
El-Gewely MR, Andreassen M, Walquist M, Ursvik A, Knutsen E, Nystad M, Coucheron DH, Myrmel KS, Hennig R, Johansen SD. Differentially Expressed MicroRNAs in Meningiomas Grades I and II Suggest Shared Biomarkers with Malignant Tumors. Cancers. 2016; 8(3):31. https://doi.org/10.3390/cancers8030031
Chicago/Turabian StyleEl-Gewely, Mohamed Raafat, Morten Andreassen, Mari Walquist, Anita Ursvik, Erik Knutsen, Mona Nystad, Dag H. Coucheron, Kristin Smistad Myrmel, Rune Hennig, and Steinar D. Johansen. 2016. "Differentially Expressed MicroRNAs in Meningiomas Grades I and II Suggest Shared Biomarkers with Malignant Tumors" Cancers 8, no. 3: 31. https://doi.org/10.3390/cancers8030031
APA StyleEl-Gewely, M. R., Andreassen, M., Walquist, M., Ursvik, A., Knutsen, E., Nystad, M., Coucheron, D. H., Myrmel, K. S., Hennig, R., & Johansen, S. D. (2016). Differentially Expressed MicroRNAs in Meningiomas Grades I and II Suggest Shared Biomarkers with Malignant Tumors. Cancers, 8(3), 31. https://doi.org/10.3390/cancers8030031








