Biomarkers for Basal-like Breast Cancer
Abstract
:1. Introduction
2. Biomarker Panels for Basal-like Breast Cancer
2.1. Current Examples: Immunohistochemical Definitions of Basal-like Breast Cancer
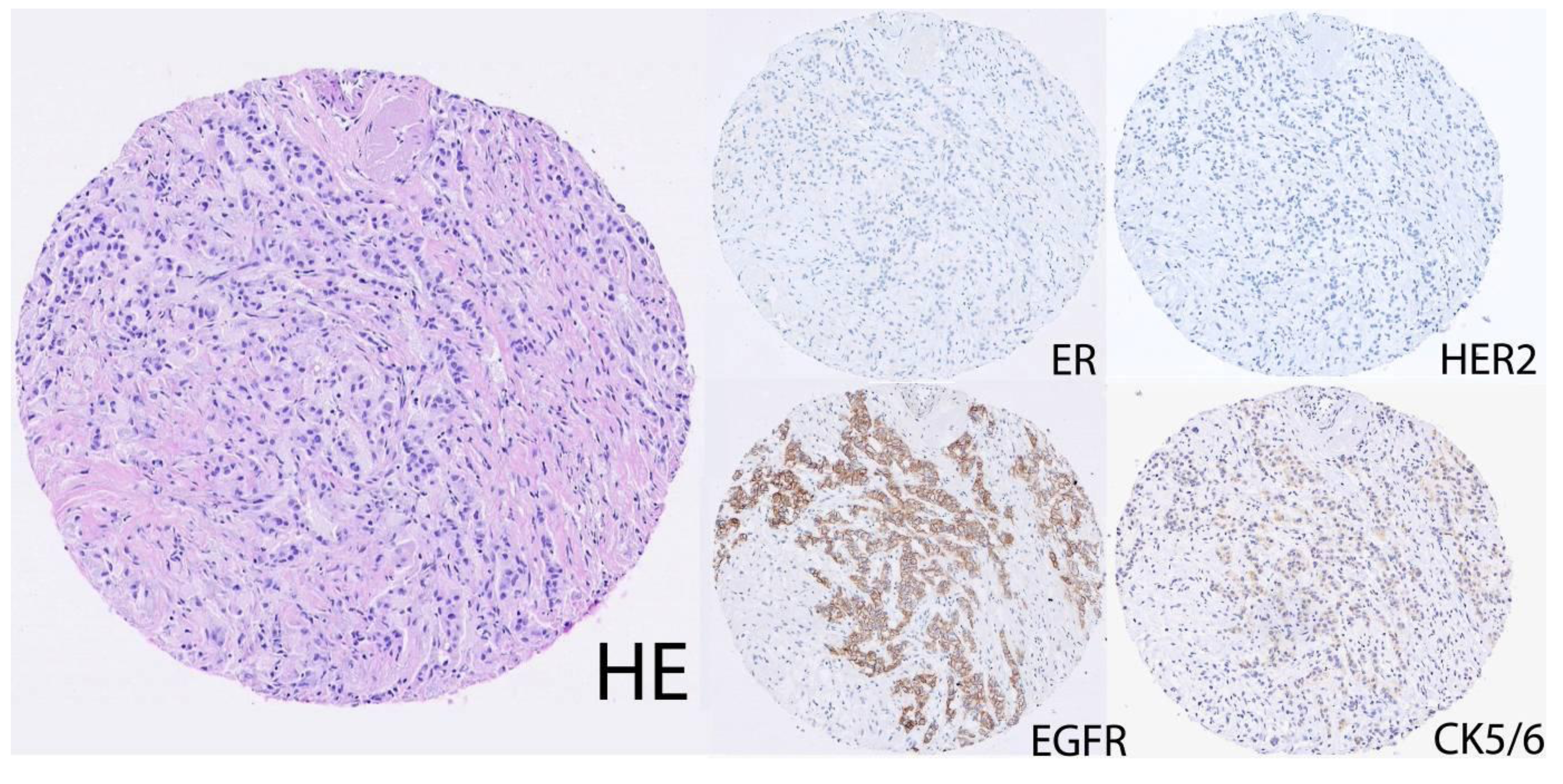
2.2. Lack of a Consensus
3. Biomarkers Associated with a Basal-like Breast Cancer Phenotype
3.1. Structural
3.2. Extracellular Interactions & Signal Transduction
3.3. Transcription, Cell Cycle Regulation and DNA Damage Repair
3.4. Biomarkers of Miscellaneous Function
| Biomarker | Experiment Format | Basal-like Definition | Frequency Among Basal-like (%) | Frequency Among Non-basal-like (%) | Literature References |
|---|---|---|---|---|---|
| Vimentin | TMA | Combined | 21/27 (78) | 30/194 (16) | [57] |
| Fascin | TMA | Combined | 14/26 (54) | 43/198 (22) | [59] |
| Nestin | Whole sections | TNP | 12/21 (57) | 12/129 (9) | [61] |
| Whole sections | TNP | 14/16 (88) | 0/32 (0) | [62] | |
| TMA | Combined | 15/22 (68) | 3/143 (2) | [63] | |
| Moesin* | TMA | Combined | 23/28 (82) | 14/64 (22) | [65] |
| Claudin 1 | TMA | Combined | 11/18 (61) | ND | [68] |
| Claudin 4 | TMA | Combined | 34/38 (90) | 42/66 (64) | [69] |
| Caveolin 1 | TMA | Combined | 17/53 (32) | 25/314 (8) | [71] |
| TMA | Combined | 11/53 (21) | 10/435 (2) | [72] | |
| Whole sections | Combined | 21/30 (70) | 1/202 (0) | [73] | |
| Caveolin 2* | TMA | Combined | 10/50 (20) | 5/270 (2) | [71] |
| TMA | Combined | 11/28 (39) | 1/173 (0) | [74] | |
| Osteopontin* | Whole sections | TNP | ND | ND | [37] |
| Laminin | TMA | Combined | 11/26 (42) | 28/193 (15) | [57] |
| β4 Integrin | Whole | TNP | 15/27 (56) | 18/71 (25) | [86] |
| NGFR** | TMA | Combined | 10/33 (30) | 1/190 (0) | [87] |
| CD109 | Whole sections | TNP | 18/30 (60) | 0/53 (0) | [88] |
| P-cadherin | TMA | Combined | 10/12 (83) | 34/128 (27) | [4] |
| Whole sections | Combined | 6/8 (75) | 13/68 (19) | [90] | |
| CD44 (high) | TMA | Combined | 20/23 (87) | 61/141 (43) | [93] |
| OATP2 | TMA | Basal CK | 23/161 (14) | 20/394 (5) | [10] |
| CD280* | TMA | Combined | 6/28 (21) | 2/175 (3) | [94] |
| TMA | Combined | 9/66 (14) | 11/302 (4) | [94] | |
| CD146 | TMA | TNP | 25/76 (33) | 13/425 (0) | [97] |
| EGFR* | TMA | GEP | 41/93 (44) | 41/521 (8) | [5] |
| Whole sections | TNP | 163/284 (57) | ND | [101] | |
| c-Kit | TMA | Basal CK | 32/102 (31) | 67/605 (11) | [5] |
| VEGF | Whole sections | Basal CK | 15/54 (28) | 4/46 (9) | [105] |
| Sox2 | TMA | Combined | 13/30 (43) | 16/147 (11) | [119] |
| FOXC1 | TMA | Combined | ND | ND | [120,121] |
| FOXC2 | TMA | NS | 8/18 (44) | 4/99 (4) | [122] |
| E2F-5 | Whole sections | TNP | 14/27 (52) | 5/30 (17) | [123] |
| Whole sections | Combined | 14/25 (56) | 5/32 (16) | [123] | |
| YB-1 | TMA | TNP | 27/37 (73) | ND | [103] |
| p-JNK | Whole sections | Combined | 16/25 (64) | 59/134 (44) | [126] |
| p63 | TMA | Combined | 6/11 (56) | 24/137 (18) | [4] |
| Whole sections | Basal CK | 13/19 (68) | 3/83 (4) | [127] | |
| p53 | Whole sections | Basal CK | 7/19 (37) | 28/83 (34) | [127] |
| TMA | TNP | 13/32 (41) | 44/103 (43) | [128] | |
| Whole sections | Basal CK | 32/95 (34) | 27/151 (18) | [130] | |
| Whole sections | Basal CK | 25/49 (51) | 100/278 (36) | [169] | |
| p16 (strong) | Whole sections | GEP | 22/33 (69) | 10/86 (12) | [140] |
| Cyclin E* | Whole sections | Basal CK | 41/92 (45) | 22/150 (15) | [130] |
| Ki67 | TMA | Combined | 6/11 (55) | 27/125 (22) | [4] |
| Whole sections | Basal CK | 15/19 (79) | 32/83 (39) | [127] | |
| Whole sections | Basal CK | 39/49 (80) | 81/278 (29) | [169] | |
| IMP3* | Whole sections | TNP | 25/32 (78) | 20/106 (19) | [162] |
| ALDH1 | Combined | Combined | 9/23 (39) | 24/160 (15) | [164] |
| AQP1* | TMA | TNP | 10/45 (22) | 1/157 (0) | [165] |
| PPH3* | Whole sections | Combined | 19/21 (90) | 65/219 (30) | [168] |
| P-glycoprotein | Whole sections | Basal CK | 29/49 (59) | 85/278 (31) | [169] |
| CAIX* | Whole sections | Combined | 16/62 (26) | 43/394 (11) | [40] |
| FABP7** | TMA | Basal CK | 43/155 (28) | 40/393 (10) | [10] |
| Whole sections | Combined | 10/11 (91) | 14/77 (18) | [171] | |
| αB-crystallin*,** | TMA | Combined | 18/40 (45) | 17/288 (6) | [172] |
| Whole sections | Combined | 26/32 (81) | 0/21 (0) | [173] |
4. What Is Next in Basal-like Breast Cancer Biomarker Research?
5. Conclusions
Acknowledgements
References
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- van't Veer, L.J.; Paik, S.; Hayes, D.F. Gene Expression Profiling of Breast Cancer: A New Tumor Marker. J. Clin. Oncol. 2005, 23, 1631–1635. [Google Scholar] [CrossRef]
- Matos, I.; Dufloth, R.; Alvarenga, M.; Zeferino, L.; Schmitt, F. p63, cytokeratin 5, and P-cadherin: three molecular markers to distinguish basal phenotype in breast carcinomas. Virchows Arch. 2005, 447, 688–694. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowan, D.; Dressler, L.; et al. Immunohistochemical and Clinical Characterization of the Basal-Like Subtype of Invasive Breast Carcinoma. Clin. Cancer Res. 2004, 10, 5367–5374. [Google Scholar] [CrossRef]
- van de Rijn, M.; Perou, C.M.; Tibshirani, R.; Haas, P.; Kallioniemi, O.; Kononen, J.; Torhorst, J.; Sauter, G.; Zuber, M.; Kochli, O.R.; et al. Expression of Cytokeratins 17 and 5 Identifies a Group of Breast Carcinomas with Poor Clinical Outcome. Am. J. Pathol. 2002, 161, 1991–1996. [Google Scholar] [CrossRef]
- Bertucci, F.; Finetti, P.; Cervera, N.; Charafe-Jauffret, E.; Buttarelli, M.; Jacquemier, J.; Chaffanet, M.; Maraninchi, D.; Viens, P.; Birnbaum, D. How different are luminal A and basal breast cancers? Int. J. Cancer 2009, 124, 1338–1348. [Google Scholar] [CrossRef]
- Linn, S.C.; van't Veer, L.J. Clinical relevance of the triple-negative breast cancer concept: Genetic basis and clinical utility of the concept. Eur. J. Cancer 2009, 45, 11–26. [Google Scholar] [CrossRef]
- Rakha, E.; El-Sayed, M.; Reis-Filho, J.; Ellis, I. Patho-biological aspects of basal-like breast cancer. Breast Cancer Res. Treat. 2009, 113, 411–422. [Google Scholar] [CrossRef]
- Zhang, H.; Rakha, E.; Ball, G.; Spiteri, I.; Aleskandarany, M.; Paish, E.; Powe, D.; Macmillan, R.; Caldas, C.; Ellis, I.; et al. The proteins FABP7 and OATP2 are associated with the basal phenotype and patient outcome in human breast cancer. Breast Cancer Res. Treat. 2009, 121, 41–51. [Google Scholar]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, Breast Cancer Subtypes, and Survival in the Carolina Breast Cancer Study. J Am. Med. Assoc. 2006, 295, 2492–2502. [Google Scholar] [CrossRef]
- Cheang, M.C.U.; Voduc, D.; Bajdik, C.; Leung, S.; McKinney, S.; Chia, S.K.; Perou, C.M.; Nielsen, T.O. Basal-Like Breast Cancer Defined by Five Biomarkers Has Superior Prognostic Value than Triple-Negative Phenotype. Clin. Cancer Res. 2008, 14, 1368–1376. [Google Scholar] [CrossRef]
- El-Rehim, D.M.A.; Pinder, S.E.; Paish, C.E.; Bell, J.; Blamey, R.W.; Robertson, J.F.R.; Nicholson, R.I.; Ellis, I.O. Expression of luminal and basal cytokeratins in human breast carcinoma. J. Pathol. 2004, 203, 661–671. [Google Scholar] [CrossRef]
- Reis-Filho, J.S.; Milanezi, F.; Steele, D.; Savage, K.; Simpson, P.T.; Nesland, J.M.; Pereira, E.M.; Lakhani, S.R.; Schmitt, F.C. Metaplastic breast carcinomas are basal-like tumours. Histopathology 2006, 49, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Lae, M.; Freneaux, P.; Sastre-Garau, X.; Chouchane, O.; Sigal-Zafrani, B.; Vincent-Salomon, A. Secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to the basal-like carcinoma spectrum. Mod. Pathol. 2008, 22, 291–298. [Google Scholar]
- Bertucci, F.; Finetti, P.; Cervera, N.; Charafe-Jauffret, E.; Mamessier, E.; Adelaide, J.; Debono, S.; Houvenaeghel, G.; Maraninchi, D.; Viens, P.; et al. Gene Expression Profiling Shows Medullary Breast Cancer Is a Subgroup of Basal Breast Cancers. Cancer Res. 2006, 66, 4636–4644. [Google Scholar] [CrossRef]
- Azoulay, S.; Lae, M.; Freneaux, P.; Merle, S.; Al Ghuzlan, A.; Chnecker, C.; Rosty, C.; Klijanienko, J.; Sigal-Zafrani, B.; Salmon, R.; et al. KIT is highly expressed in adenoid cystic carcinoma of the breast, a basal-like carcinoma associated with a favorable outcome. Mod. Pathol. 2005, 18, 1623–1631. [Google Scholar]
- Weigelt, B.; Mackay, A.; A'Hern, R.; Natrajan, R.; Tan, D.S.P.; Dowsett, M.; Ashworth, A.; Reis-Filho, J.S. Breast cancer molecular profiling with single sample predictors: a retrospective analysis. Lancet Oncol. 2010, 11, 339–349. [Google Scholar] [CrossRef]
- Sorlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. 2003, 100, 8418–8423. [Google Scholar] [CrossRef]
- Stingl, J.; Caldas, C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat. Rev. Cancer 2007, 7, 791–799. [Google Scholar] [CrossRef]
- Lim, E.; Vaillant, F.; Wu, D.; Forrest, N.C.; Pal, B.; Hart, A.H.; Asselin-Labat, M.-L.; Gyorki, D.E.; Ward, T.; Partanen, A.; et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat. Med. 2009, 15, 907–913. [Google Scholar] [CrossRef]
- Bocker, W.; Moll, R.; Poremba, C.; Holland, R.; van Diest, P.J.; Dervan, P.; Burger, H.; Wai, D.; Ina Diallo, R.; Brandt, B.; et al. Common Adult Stem Cells in the Human Breast Give Rise to Glandular and Myoepithelial Cell Lineages: A New Cell Biological Concept. Lab. Invest. 2002, 82, 737–746. [Google Scholar]
- Gusterson, B. Do 'basal-like' breast cancers really exist? Nat. Rev. Cancer. 2009, 9, 128–134. [Google Scholar] [CrossRef]
- Gusterson, B.; Ross, D.; Heath, V.; Stein, T. Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res. 2005, 7, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, N.; Srivastava, S.; LaBaer, J. Applications of protein microarrays for biomarker discovery. Proteomics 2008, 2, 1444–1459. [Google Scholar]
- Fadare, O.; Tavassoli, F.A. Clinical and pathologic aspects of basal-like breast cancers. Nat. Clin. Prac. Oncol. 2008, 5, 149–159. [Google Scholar] [CrossRef]
- Ross, J.S.; Linette, G.P.; Stec, J.; Clark, E.; Ayers, M.; Leschly, N.; Symmans, W.F.; Hortobagyi, G.N.; Pusztai, L. Breast cancer biomarkers and molecular medicine: part II. Expert Rev. Mol. Diagn. 2004, 4, 169–188. [Google Scholar] [CrossRef]
- Tainsky, M.A. Genomic and proteomic biomarkers for cancer: A multitude of opportunities. Biochim. Biophys. Acta Rev. Cancer 2009, 1796, 176–193. [Google Scholar] [CrossRef]
- Ward, T.H.; Cummings, J.; Dean, E.; Greystoke, A.; Hou, J.M.; Backen, A.; Ranson, M.; Dive, C. Biomarkers of apoptosis. Br. J. Cancer 2008, 99, 841–846. [Google Scholar] [CrossRef]
- Mohammadizadeh, F.; Naimi, A.; Rajabi, P.; Ghasemibasir, H.; Eftekhari, A. Expression of basal and luminal cytokeratins in breast cancer and their correlation with clinicopathological prognostic variables. Indian J. Med. Sci. 2009, 63, 152–162. [Google Scholar] [CrossRef]
- Camp, R.L.; Neumeister, V.; Rimm, D.L. A Decade of Tissue Microarrays: Progress in the Discovery and Validation of Cancer Biomarkers. J. Clin. Oncol. 2008, 26, 5630–5637. [Google Scholar] [CrossRef]
- Tang, P.; Skinner, K.A.; Hicks, D.G. Molecular Classification of Breast Carcinomas by Immunohistochemical Analysis: Are We Ready? Diagn. Mol. Pathol. 2009, 18, 125–132. [Google Scholar] [CrossRef]
- Simon, R. Advances in clinical trial designs for predictive biomarker discovery and validation. Curr. Breast Cancer Rep. 2009, 1, 216–221. [Google Scholar] [CrossRef]
- Simon, R.M.; Paik, S.; Hayes, D.F. Use of Archived Specimens in Evaluation of Prognostic and Predictive Biomarkers. J. Natl. Cancer Inst. 2009, jp335. [Google Scholar]
- Nielsen, T.O.; Jewell, S.D.; Thor, A.D.; Gao, D.; Perou, C.M.; Broadwater, G.; Harris, L.N.; Hayes, D.F.; Berry, D.A.; Ellis, M.J. Intrinsic subtype and response to paclitaxel in CALGB 9344 tissue microarrays. In Presented at the ASCO 2009 Breast Cancer Symposium, San Francisco, CA, USA, October 2009.
- Bertucci, F.; Finetti, P.; Cervera, N.; Esterni, B.; Hermitte, F.; Viens, P.; Birnbaum, D. How basal are triple-negative breast cancers? Int. J. Cancer 2008, 123, 236–240. [Google Scholar] [CrossRef]
- Wang, X.; Chao, L.; Ma, G.; Chen, L.; Tian, B.; Zang, Y.; Sun, J. Increased expression of osteopontin in patients with triple-negative breast cancer. Eur. J. Clin. Invest. 2008, 38, 438–446. [Google Scholar] [CrossRef]
- Kim, J.M.; Hwang, T.Y.; Kang, S.H.; Lee, S.J.; Bae, Y.K. Prognostic Significance of Basal Markers in Triple-negative Breast Cancers. J. Breast Cancer 2009, 12, 4–13. [Google Scholar] [CrossRef]
- Martín, M.; Rodríguez-Lescure, Á.; Ruiz, A.; Alba, E.; Calvo, L.; Ruiz-Borrego, M.; Santaballa, A.; Rodríguez, C.; Crespo, C.; Abad, M.; et al. Molecular predictors of efficacy of adjuvant weekly paclitaxel in early breast cancer. Breast Cancer Res. Treat. 2009. [Epub ahead of print]. [Google Scholar]
- Tan, E.Y.; Yan, M.; Campo, L.; Han, C.; Takano, E.; Turley, H.; Candiloro, I.; Pezzella, F.; Gatter, K.C.; Millar, E.K.A.; et al. The key hypoxia regulated gene CAIX is upregulated in basal-like breast tumours and is associated with resistance to chemotherapy. Br. J. Cancer 2009, 100, 405–411. [Google Scholar] [CrossRef]
- Cheang, M.; Chia, S.K.; Tu, D.; Jiang, S.; Shepherd, L.E.; Pritchard, K.I.; Nielsen, T.O. Anthracyclines in basal breast cancer: The NCIC-CTG trial MA5 comparing adjuvant CMF to CEF. In Presented at the 2009 ASCO Annual Meeting, Orlando, FL, USA, May 2009.
- Voduc, D.; Nielsen, T. Basal and Triple-Negative Breast Cancers: Impact on Clinical Decision-Making and Novel Therapeutic Options. Clin. Breast Cancer 2008, 8, s171–s178. [Google Scholar] [CrossRef]
- Rakha, E.A.; Elsheikh, S.E.; Aleskandarany, M.A.; Habashi, H.O.; Green, A.R.; Powe, D.G.; El-Sayed, M.E.; Benhasouna, A.; Brunet, J.S.; Akslen, L.A.; et al. Triple-Negative Breast Cancer: Distinguishing between Basal and Nonbasal Subtypes. Clin. Cancer Res. 2009, 15, 2302–2310. [Google Scholar] [CrossRef]
- Laakso, M.; Loman, N.; Borg, A.; Isola, J. Cytokeratin 5/14-positive breast cancer: true basal phenotype confined to BRCA1 tumors. Mod. Pathol. 2005, 18, 1321–1328. [Google Scholar] [CrossRef]
- Potemski, P.; Kusinska, R.; Watala, C.; Pluciennik, E.; Bednarek, A.K.; Kordek, R. Prognostic Relevance of Basal Cytokeratin Expression in Operable Breast Cancer. Oncology 2005, 69, 478–485. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sayed, M.E.; Green, A.R.; Paish, E.C.; Lee, A.H.S.; Ellis, I.O. Breast carcinoma with basal differentiation: a proposal for pathology definition based on basal cytokeratin expression. Histopathology 2007, 50, 434–438. [Google Scholar] [CrossRef]
- Malzahn, K.; Mitze, M.; Thoenes, M.; Moll, R. Biological and prognostic significance of stratified epithelial cytokeratins in infiltrating ductal breast carcinomas. Virchows Arch. 1998, 433, 119–129. [Google Scholar] [CrossRef]
- Dairkee, S.H.; Puett, L.; Hackett, A.J. Expression of Basal and Luminal Epithelium-Specific Keratins in Normal, Benign, and Malignant Breast Tissue1. J. Natl. Cancer Inst. 1988, 80, 691–695. [Google Scholar] [CrossRef]
- Wetzels, R.H.; Kuijpers, H.J.; Lane, E.B.; Leigh, I.M.; Troyanovsky, S.M.; Holland, R.; van Haelst, U.J.; Ramaekers, F.C. Basal cell-specific and hyperproliferation-related keratins in human breast cancer. Am. J. Pathol. 1991, 138, 751–763. [Google Scholar]
- Wetzels, R.H.; Holland, R.; van Haelst, U.J.; Lane, E.B.; Leigh, I.M.; Ramaekers, F.C. Detection of basement membrane components and basal cell keratin 14 in noninvasive and invasive carcinomas of the breast. Am. J. Pathol. 1989, 134, 571–579. [Google Scholar]
- Chen, P.C.; Chen, C.K.; Nicastri, A.D.; Wait, R.B. Myoepithelial carcinoma of the breast with distant metastasis and accompanied by adenomyoepitheliomas. Histopathology 1994, 24, 543–548. [Google Scholar]
- Livasy, C.A.; Karaca, G.; Nanda, R.; Tretiakova, M.S.; Olopade, O.I.; Moore, D.T.; Perou, C.M. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod. Pathol. 2005, 19, 264–271. [Google Scholar]
- Thike, A.A.; Cheok, P.Y.; Jara-Lazaro, A.R.; Tan, B.; Tan, P.; Tan, P.H. Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod. Pathol. 2009, 23, 123–133. [Google Scholar]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef]
- Liu, Z.B.; Wu, J.; Ping, B.; Feng, L.Q.; Lu, J.S.; Shen, K.W.; Shen, Z.Z.; Shaol, Z.M. Basal cytokeratin expression in relation to immunohistochemical and clinical characterization in breast cancer patients with triple-negative phenotype. Tumori 2009, 95, 53–62. [Google Scholar]
- Chen, M.H.S.; Wai-Cheong Yip, G.; Tse, G.M.-K.; Moriya, T.; Lui, P.C.-W.; Zin, M.-L.; Bay, B.-H.; Tan, P.-H. Expression of basal keratins and vimentin in breast cancers of young women correlates with adverse pathologic parameters. Mod. Pathol. 2008, 21, 1183–1191. [Google Scholar] [CrossRef]
- Rodriguez-Pinilla, S.M.; Sarrio, D.; Honrado, E.; Moreno-Bueno, G.; Hardisson, D.; Calero, F.; Benitez, J.; Palacios, J. Vimentin and laminin expression is associated with basal-like phenotype in both sporadic and BRCA1-associated breast carcinomas. J. Clin. Pathol. 2007, 60, 1006–1012. [Google Scholar]
- Sarrio, D.; Rodriguez-Pinilla, S.M.; Hardisson, D.; Cano, A.; Moreno-Bueno, G.; Palacios, J. Epithelial-Mesenchymal Transition in Breast Cancer Relates to the Basal-like Phenotype. Cancer Res. 2008, 68, 989–997. [Google Scholar] [CrossRef]
- Rodriguez-Pinilla, S.M.; Sarrio, D.; Honrado, E.; Hardisson, D.; Calero, F.; Benitez, J.; Palacios, J. Prognostic Significance of Basal-Like Phenotype and Fascin Expression in Node-Negative Invasive Breast Carcinomas. Clin. Cancer Res. 2006, 12, 1533–1539. [Google Scholar] [CrossRef]
- Yoder, B.J.; Tso, E.; Skacel, M.; Pettay, J.; Tarr, S.; Budd, T.; Tubbs, R.R.; Adams, J.C.; Hicks, D.G. The Expression of Fascin, an Actin-Bundling Motility Protein, Correlates with Hormone Receptor-Negative Breast Cancer and a More Aggressive Clinical Course. Clin. Cancer Res. 2005, 11, 186–192. [Google Scholar]
- Caigang, L.; Bo, C.; Jun, Z.; Ruishan, Z.; Fan, Y.; Feng, J.; Huimian, X.; Ping, L. Clinical implications for nestin protein expression in breast cancer. Cancer Sci. 2009, 101, 815–819. [Google Scholar]
- Li, H.; Cherukuri, P.; Li, N.; Cowling, V.; Spinella, M.; Cole, M.; Godwin, A.K.; Wells, W.; DiRenzo, J. Nestin Is Expressed in the Basal/Myoepithelial Layer of the Mammary Gland and Is a Selective Marker of Basal Epithelial Breast Tumors. Cancer Res. 2007, 67, 501–510. [Google Scholar] [CrossRef]
- Parry, S.; Savage, K.; Marchio, C.; Reis-Filho, J.S. Nestin is expressed in basal-like and triple negative breast cancers. J. Clin. Pathol. 2008, 61, 1045–1050. [Google Scholar] [CrossRef]
- Charafe-Jauffret, E.; Ginestier, C.; Monville, F.; Finetti, P.; Adelaide, J.; Cervera, N.; Fekairi, S.; Xerri, L.; Jacquemier, J.; Birnbaum, D.; et al. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene 2005, 25, 2273–2284. [Google Scholar]
- Charafe-Jauffret, E.; Monville, F.; Bertucci, F.; Esterni, B.; Ginestier, C.; Finetti, P.; Cervera, N.; Geneix, J.; Hassanein, M.; Rabayrol, L.; et al. Moesin expression is a marker of basal breast carcinomas. Int. J. Cancer 2007, 121, 1779–1785. [Google Scholar] [CrossRef]
- Kusinska, R.; Kordek, R.; Pluciennik, E.; Bednarek, A.; Piekarski, J.; Potemski, P. Does vimentin help to delineate the so-called 'basal type breast cancer'? J. Exp. Clin. Cancer Res. 2009, 28, 118–126. [Google Scholar] [CrossRef]
- Ouban, A.; Ahmed, A.A. Claudins in human cancer: A review. Histol. Histopathol. 2010, 25, 83–90. [Google Scholar]
- Blanchard, A.; Skliris, G.; Watson, P.; Murphy, L.; Penner, C.; Tomes, L.; Young, T.; Leygue, E.; Myal, Y. Claudins 1, 3, and 4 protein expression in ER negative breast cancer correlates with markers of the basal phenotype. Virchows Arch. 2009, 454, 647–656. [Google Scholar] [CrossRef]
- Kulka, J.; Szász, A.; Németh, Z.; Madaras, L.; Schaff, Z.; Molnár, I.; Tőkés, A.-M. Expression of Tight Junction Protein Claudin-4 in Basal-Like Breast Carcinomas. Pathol. Oncol. Res. 2009, 15, 59–64. [Google Scholar] [CrossRef]
- Lanigan, F.; McKiernan, E.; Brennan, D.J.; Hegarty, S.; Millikan, R.C.; McBryan, J.; Jirstrom, K.; Landberg, G.; Martin, F.; Duffy, M.J.; et al. Increased claudin-4 expression is associated with poor prognosis and high tumour grade in breast cancer. Int. J. Cancer 2009, 124, 2088–2097. [Google Scholar] [CrossRef]
- Elsheikh, S.E.; Green, A.R.; Rakha, E.A.; Samaka, R.M.; Ammar, A.A.; Powe, D.; Reis-Filho, J.S.; Ellis, I.O. Caveolin 1 and Caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotype. Br. J. Cancer 2008, 99, 327–334. [Google Scholar] [CrossRef]
- Pinilla, S.; Honrado, E.; Hardisson, D.; Benítez, J.; Palacios, J. Caveolin-1 expression is associated with a basal-like phenotype in sporadic and hereditary breast cancer. Breast Cancer Res. Treat. 2006, 99, 85–90. [Google Scholar] [CrossRef]
- Savage, K.; Lambros, M.B.K.; Robertson, D.; Jones, R.L.; Jones, C.; Mackay, A.; James, M.; Hornick, J.L.; Pereira, E.M.; Milanezi, F.; et al. Caveolin 1 Is Overexpressed and Amplified in a Subset of Basal-like and Metaplastic Breast Carcinomas: A Morphologic, Ultrastructural, Immunohistochemical, and In situ Hybridization Analysis. Clin. Cancer Res. 2007, 13, 90–101. [Google Scholar] [CrossRef]
- Savage, K.; Leung, S.; Todd, S.; Brown, L.; Jones, R.; Robertson, D.; James, M.; Parry, S.; Rodrigues-Pinilla, S.; Huntsman, D.; et al. Distribution and significance of caveolin 2 expression in normal breast and invasive breast cancer: an immunofluorescence and immunohistochemical analysis. Breast Cancer Res. Treat. 2008, 110, 245–256. [Google Scholar] [CrossRef]
- Sloan, E.K.; Ciocca, D.R.; Pouliot, N.; Natoli, A.; Restall, C.; Henderson, M.A.; Fanelli, M.A.; Cuello-Carrion, F.D.; Gago, F.E.; Anderson, R.L. Stromal Cell Expression of Caveolin-1 Predicts Outcome in Breast Cancer. Am. J. Pathol. 2009, 174, 2035–2043. [Google Scholar] [CrossRef]
- Witkiewicz, A.K.; Dasgupta, A.; Sammons, S.; Er, O.; Potoczek, M.B.; Guiles, F.; Sotgia, F.; Brody, J.R.; Mitchell, E.P.; Lisanti, M.P. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biol. Ther. 2010, 2. Epub ahead of print. [Google Scholar]
- Humphries, M.J.; Reynolds, A. Cell-to-cell contact and extracellular matrix. Curr. Opin. Cell Biol. 2009, 21, 613–615. [Google Scholar] [CrossRef]
- Helleman, J.; Jansen, M.P.H.M.; Ruigrok-Ritstier, K.; van Staveren, I.L.; Look, M.P.; Meijer-van Gelder, M.E.; Sieuwerts, A.M.; Klijn, J.G.M.; Sleijfer, S.; Foekens, J.A.; et al. Association of an Extracellular Matrix Gene Cluster with Breast Cancer Prognosis and Endocrine Therapy Response. Clin. Cancer Res. 2008, 14, 5555–5564. [Google Scholar] [CrossRef]
- Lakhani, S.R.; Reis-Filho, J.S.; Fulford, L.; Penault-Llorca, F.; van der Vijver, M.; Parry, S.; Bishop, T.; Benitez, J.; Rivas, C.; Bignon, Y.-J.; et al. Prediction of BRCA1 Status in Patients with Breast Cancer Using Estrogen Receptor and Basal Phenotype. Clin. Cancer Res. 2005, 11, 5175–5180. [Google Scholar] [CrossRef]
- Jones, C.; Mackay, A.; Grigoriadis, A.; Cossu, A.; Reis-Filho, J.S.; Fulford, L.; Dexter, T.; Davies, S.; Bulmer, K.; Ford, E.; et al. Expression Profiling of Purified Normal Human Luminal and Myoepithelial Breast Cells: Identification of Novel Prognostic Markers for Breast Cancer. Cancer Res. 2004, 64, 3037–3045. [Google Scholar] [CrossRef]
- Rodrigues, L.R.; Lopes, N.; Sousa, B.; Vieira, D.; Milanezi, F.; Paulsson, M.; Lindmark-Mansson, H.; Teixeira, J.A.; Schmitt, F. Significance of osteopontin expression in human invasive breast tumour stroma. Open Breast Cancer J. 2009, 1, 1–9. [Google Scholar] [CrossRef]
- Wai, P.; Kuo, P. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008, 27, 103–118. [Google Scholar] [CrossRef]
- Carpenter, P.M.; Dao, A.V.; Arain, Z.S.; Chang, M.K.; Nguyen, H.P.; Arain, S.; Wang-Rodriguez, J.; Kwon, S.-Y.; Wilczynski, S.P. Motility Induction in Breast Carcinoma by Mammary Epithelial Laminin 332 (Laminin 5). Mol. Cancer Res. 2009, 7, 462–475. [Google Scholar] [CrossRef]
- Marinkovich, M.P. Laminin 332 in squamous-cell carcinoma. Nat. Rev. Cancer 2007, 7, 370–380. [Google Scholar] [CrossRef]
- Gilcrease, M.; Zhou, X.; Lu, X.; Woodward, W.; Hall, B.; Morrissey, P. Alpha6beta4 integrin crosslinking induces EGFR clustering and promotes EGF-mediated Rho activation in breast cancer. J. Exp. Clin. Cancer Res. 2009, 28, 67. [Google Scholar] [CrossRef]
- Lu, S.; Simin, K.; Khan, A.; Mercurio, A.M. Analysis of Integrin Beta4 Expression in Human Breast Cancer: Association with Basal-like Tumors and Prognostic Significance. Clin. Cancer Res. 2008, 14, 1050–1058. [Google Scholar] [CrossRef]
- Reis-Filho, J.S.; Steele, D.; Di Palma, S.; Jones, R.L.; Savage, K.; James, M.; Milanezi, F.; Schmitt, F.C.; Ashworth, A. Distribution and significance of nerve growth factor receptor (NGFR/p75NTR) in normal, benign and malignant breast tissue. Mod. Pathol. 2006, 19, 307–319. [Google Scholar] [CrossRef]
- Hasegawa, M.; Moritani, S.; Murakumo, Y.; Sato, T.; Hagiwara, S.; Suzuki, C.; Mii, S.; Jijiwa, M.; Enomoto, A.; Asai, N.; et al. CD109 expression in basal-like breast carcinoma. Pathol. Int. 2008, 58, 288–294. [Google Scholar] [CrossRef]
- Arnes, J.B.; Brunet, J.-S.; Stefansson, I.; Begin, L.R.; Wong, N.; Chappuis, P.O.; Akslen, L.A.; Foulkes, W.D. Placental Cadherin and the Basal Epithelial Phenotype of BRCA1-Related Breast Cancer. Clin. Cancer Res. 2005, 11, 4003–4011. [Google Scholar] [CrossRef]
- Paredes, J.; Lopes, N.; Milanezi, F.; Schmitt, F. P-cadherin and cytokeratin 5: useful adjunct markers to distinguish basal-like ductal carcinomas in situ. Virchows Arch. 2007, 450, 73–80. [Google Scholar] [CrossRef]
- Potemski, P.; Kusinska, R.; Kubiak, R.; Piekarski, J.H.; Pluciennik, E.; Bednarek, A.K.; Kordek, R. Relationship of P-cadherin expression to basal phenotype of breast carcinoma. Polish J. Pathol. 2007, 58, 183–188. [Google Scholar]
- Honeth, G.; Bendahl, P.-O.; Ringner, M.; Saal, L.; Gruvberger-Saal, S.; Lovgren, K.; Grabau, D.; Ferno, M.; Borg, A.; Hegardt, C. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. Treat. 2008, 10, R53. [Google Scholar] [CrossRef]
- Klingbeil, P.; Natrajan, R.; Everitt, G.; Vatcheva, R.; Marchio, C.; Palacios, J.; Buerger, H.; Reis-Filho, J.; Isacke, C. CD44 is overexpressed in basal-like breast cancers but is not a driver of 11p13 amplification. Breast Cancer Res. Treat. 2009, 120, 95–109. [Google Scholar]
- Wienke, D.; Davies, G.C.; Johnson, D.A.; Sturge, J.; Lambros, M.B.K.; Savage, K.; Elsheikh, S.E.; Green, A.R.; Ellis, I.O.; Robertson, D.; et al. The Collagen Receptor Endo180 (CD280) Is Expressed on Basal-like Breast Tumor Cells and Promotes Tumor Growth In vivo. Cancer Res. 2007, 67, 10230–10240. [Google Scholar] [CrossRef]
- Garcia, S.; Dalès, J.-P.; Charafe-Jauffret, E.; Carpentier-Meunier, S.; Andrac-Meyer, L.; Jacquemier, J.; Andonian, C.; Lavaut, M.-N.; Allasia, C.; Bonnier, P.; et al. Poor prognosis in breast carcinomas correlates with increased expression of targetable CD146 and c-Met and with proteomic basal-like phenotype. Human Pathol. 2007, 38, 830–841. [Google Scholar] [CrossRef]
- Graveel, C.R.; DeGroot, J.D.; Su, Y.; Koeman, J.; Dykema, K.; Leung, S.; Snider, J.; Davies, S.R.; Swiatek, P.J.; Cottingham, S.; et al. Met induces diverse mammary carcinomas in mice and is associated with human basal breast cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 12909–12914. [Google Scholar]
- Zabouo, G.; Imbert, A.-M.; Jacquemier, J.; Finetti, P.; Moreau, T.; Esterni, B.; Bertucci, F.; Chabannon, C. CD146 expression is associated with a poor prognosis in human breast tumors and with enhanced motility in breast cancer cell lines. Breast Cancer Res. 2009, 11, R1. [Google Scholar] [CrossRef]
- Ouhtit, A.; Gaur, R.L.; Abd Elmageed, Z.Y.; Fernando, A.; Thouta, R.; Trappey, A.K.; Abdraboh, M.E.; El-Sayyad, H.I.; Rao, P.; Raj, M.G.H. Towards understanding the mode of action of the multifaceted cell adhesion receptor CD146. Biochim. Biophys. Acta 2009, 1795, 130–136. [Google Scholar]
- Shih, L.M.; Hsu, M.Y.; Palazzo, J.P.; Herlyn, M. The cell-cell adhesion receptor Mel-CAM acts as a tumor suppressor in breast carcinoma. Am. J. Pathol. 1997, 151, 745–751. [Google Scholar]
- Arnes, J.B.; Begin, L.R.; Stefansson, I.; Brunet, J.S.; Nielsen, T.O.; Foulkes, W.D.; Akslen, L.A. Expression of epidermal growth factor receptor in relation to BRCA1 status, basal-like markers and prognosis in breast cancer. J. Clin. Pathol. 2009, 62, 139–146. [Google Scholar] [CrossRef]
- Viale, G.; Rotmensz, N.; Maisonneuve, P.; Bottiglieri, L.; Montagna, E.; Luini, A.; Veronesi, P.; Intra, M.; Torrisi, R.; Cardillo, A.; et al. Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res. Treat. 2009, 116, 317–328. [Google Scholar] [CrossRef]
- Nalwoga, H. Expression of EGFR and c-kit is associated with the basal-like phenotype in breast carcinomas of African women. Acta Pathol. Microbiol. Immunol. Scandinavica 2008, 116, 515–525. [Google Scholar] [CrossRef]
- Stratford, A.; Habibi, G.; Astanehe, A.; Jiang, H.; Hu, K.; Park, E.; Shadeo, A.; Buys, T.; Lam, W.; Pugh, T.; et al. Epidermal growth factor receptor (EGFR) is transcriptionally induced by the Y-box binding protein-1 (YB-1) and can be inhibited with Iressa in basal-like breast cancer, providing a potential target for therapy. Breast Cancer Res. 2007, 9, R61. [Google Scholar] [CrossRef]
- Linderholm, B.K.; Hellborg, H.; Johansson, U.; Elmberger, G.; Skoog, L.; Lehtio, J.; Lewensohn, R. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann. Oncol. 2009, 20, 1639–1646. [Google Scholar] [CrossRef]
- Ribeiro-Silva, A.; de Vale, F.R.; Zucoloto, S. Vascular endothelial growth factor expression in the basal subtype of breast carcinoma. Am. J. Clin. Pathol. 2006, 125, 512–518. [Google Scholar]
- Dent, S. F. The role of VEGF in triple-negative breast cancer: where do we go from here? Ann. Oncol. 2009, 20, 1615–1617. [Google Scholar] [CrossRef]
- Modjtahedi, H.; Essapen, S. Epidermal growth factor receptor inhibitors in cancer treatment: advances, challenges and opportunities. Anti-Cancer Drugs 2009, 20, 851–855. [Google Scholar] [CrossRef]
- Shahi, P.K.; Pineda, I.F. Tumoral Angiogenesis: Review of the Literature. Cancer Invest. 2008, 26, 104–108. [Google Scholar] [CrossRef]
- Sledge, G. VEGF-Targeting Therapy for Breast Cancer. J. Mammary Gland Biol. Neoplasia 2005, 10, 319–323. [Google Scholar] [CrossRef]
- Tsuda, H.; Morita, D.; Kimura, M.; Shinto, E.; Ohtsuka, Y.; Matsubara, O.; Inazawa, J.; Tamaki, K.; Mochizuki, H.; Tamai, S.; et al. Correlation of KIT and EGFR overexpression with invasive ductal breast carcinoma of the solid-tubular subtype, nuclear grade 3, and mesenchymal or myoepithelial differentiation. Cancer Sci. 2005, 96, 48–53. [Google Scholar] [CrossRef]
- Dickler, M.; Cobleigh, M.; Miller, K.; Klein, P.; Winer, E. Efficacy and safety of erlotinib in patients with locally advanced or metastatic breast cancer. Breast Cancer Res. Treat. 2009, 115, 115–121. [Google Scholar] [CrossRef]
- Dickler, M.N.; Rugo, H.S.; Eberle, C.A.; Brogi, E.; Caravelli, J.F.; Panageas, K.S.; Boyd, J.; Yeh, B.; Lake, D.E.; Dang, C.T.; et al. A Phase II Trial of Erlotinib in Combination with Bevacizumab in Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2008, 14, 7878–7883. [Google Scholar] [CrossRef]
- Flynn, J.F.; Wong, C.; Wu, J.M. Anti-EGFR therapy: mechanism and advances in clinical efficacy in breast cancer. J. Oncol. 2009, 2009, 526963. [Google Scholar]
- Modi, S.; D'Andrea, G.; Norton, L.; Yao, T.J.; Caravelli, J.; Rosen, P.; Hudis, C.; Seidman, A. A Phase I Study of Cetuximab/Paclitaxel in Patients with Advanced-Stage Breast Cancer. Clin. Breast Cancer 2006, 7, 270–277. [Google Scholar] [CrossRef]
- Carey, L.A.; Rugo, H.S.; Marcom, P.K.; Irvin, W., Jr.; Ferraro, M.; Burrows, E.; He, X.; Perou, C.M.; Winer, E.P. TBCRC 001: EGFR inhibition with cetuximab added to carboplatin in metastatic triple-negative (basal-like) breast cancer. In Presented at the 2008 ASCO Annual Meeting, Boston, MA, June 2008.
- Conte, P.; Guarneri, V. Triple-negative breast cancer: current management and future options. Eur. J. Cancer Suppl. 2009, 7, 14–18. [Google Scholar] [CrossRef]
- Chia, K.; Tutt, A. Triple-negative breast cancer: an update. Adv. Breast Cancer 2007, 4, 75–80. [Google Scholar]
- Saeki, R.; Kondoh, M.; Kakutani, H.; Tsunoda, S.-i.; Mochizuki, Y.; Hamakubo, T.; Tsutsumi, Y.; Horiguchi, Y.; Yagi, K. A Novel Tumor-Targeted Therapy Using a Claudin-4-Targeting Molecule. Mol. Pharmacol. 2009, 76, 918–926. [Google Scholar] [CrossRef]
- Rodriguez-Pinilla, S.M.; Sarrio, D.; Moreno-Bueno, G.; Rodriguez-Gil, Y.; Martinez, M.A.; Hernandez, L.; Hardisson, D.; Reis-Filho, J.S.; Palacios, J. Sox2: a possible driver of the basal-like phenotype in sporadic breast cancer. Mod. Pathol. 2007, 20, 474–481. [Google Scholar] [CrossRef]
- Ray, P.S.; Wang, J.; Qu, Y.; Shin-Sim, M.; Shamonki, J.; Liu, B.; Elashoff, D.; Hoon, D.S.; Giuliano, A.E.; Cui, X. Diagnostic and prognostic significance of FOXC1 expression in basal-like/triple-negative breast cancer. In Poster presented at the ASCO 2009 Breast Cancer Symposium, San Francisco, CA, USA, October 2009.
- Ray, P.S.; Wang, J.; Qu, Y.; Shin-Sim, M.; Shamonki, J.; Liu, B.; Hoon, D.S.; Giuliano, A.E.; Cui, X. Role of FOXC1 in regulation of basal-like/triple-negative breast cancer. In Presented at the 2009 ASCO Annual Meeting, Orlando, FL, USA, May 2009.
- Mani, S.A.; Yang, J.; Brooks, M.; Schwaninger, G.; Zhou, A.; Miura, N.; Kutok, J.L.; Hartwell, K.; Richardson, A.L.; Weinberg, R.A. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc. Natl. Acad. Sci. USA 2007, 104, 10069–10074. [Google Scholar] [CrossRef]
- Umemura, S.; Shirane, M.; Takekoshi, S.; Kusakabe, T.; Itoh, J.; Egashira, N.; Tokuda, Y.; Mori, K.; Osamura, Y.R. Overexpression of E2F-5 correlates with a pathological basal phenotype and a worse clinical outcome. Br. J. Cancer 2009, 100, 764–771. [Google Scholar] [CrossRef]
- Dahl, E.; En-Nia, A.; Wiesmann, F.; Krings, R.; Djudjaj, S.; Breuer, E.; Fuchs, T.; Wild, P.; Hartmann, A.; Dunn, S.; et al. Nuclear detection of Y-box protein-1 (YB-1) closely associates with progesterone receptor negativity and is a strong adverse survival factor in human breast cancer. BMC Cancer 2009, 9, 410–426. [Google Scholar] [CrossRef] [Green Version]
- Janz, M.; Harbeck, N.; Dettmar, P.; Berger, U.; Schmidt, A.; Jürchott, K.; Schmitt, M.; Royer, H.-D. Y-box factor YB-1 predicts drug resistance and patient outcome in breast cancer independent of clinically relevant tumor biologic factors HER2, uPA and PAI-1. Int. J. Cancer 2002, 97, 278–282. [Google Scholar] [CrossRef]
- Wang, X.; Chao, L.; Li, X.; Ma, G.; Chen, L.; Zang, Y.; Zhou, G. Elevated expression of phosphorylated c-Jun NH2-terminal kinase in basal-like and "triple-negative" breast cancers. Human Pathol. 2010, 41, 401–406. [Google Scholar] [CrossRef]
- Ribeiro-Silva, A.; Ramalho, L.N.Z.; Garcia, S.B.; Brandão, D.F.; Chahud, F.; Zucoloto, S. p63 correlates with both BRCA1 and cytokeratin 5 in invasive breast carcinomas: further evidence for the pathogenesis of the basal phenotype of breast cancer. Histopathology 2005, 47, 458–466. [Google Scholar] [CrossRef]
- Chae, B.J.; Bae, J.S.; Lee, A.; Park, W.C.; Seo, Y.J.; Song, B.J.; Kim, J.S.; Jung, S.S. p53 as a Specific Prognostic Factor in Triple-negative Breast Cancer. Jpn. J. Clin. Oncol. 2009, 39, 217–224. [Google Scholar] [CrossRef]
- Manie, E.; Vincent-Salomon, A.; Lehmann-Che, J.; Pierron, G.; Turpin, E.; Warcoin, M.; Gruel, N.; Lebigot, I.; Sastre-Garau, X.; Lidereau, R.; et al. High Frequency of TP53 Mutation in BRCA1 and Sporadic Basal-like Carcinomas but not in BRCA1 Luminal Breast Tumors. Cancer Res. 2009, 69, 663–671. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Brunet, J.-S.; Stefansson, I. M.; Straume, O.; Chappuis, P.O.; Begin, L.R.; Hamel, N.; Goffin, J.R.; Wong, N.; Trudel, M.; et al. The Prognostic Implication of the Basal-Like (Cyclin Ehigh/p27low/p53+/Glomeruloid-Microvascular-Proliferation+) Phenotype of BRCA1-Related Breast Cancer. Cancer Res. 2004, 64, 830–835. [Google Scholar] [CrossRef]
- Rakha, E.A.; Putti, T.C.; El-Rehim, D.M.A.; Paish, C.; Green, A.R.; Powe, D.G.; Lee, A.H.; Robertson, J.F.; Ellis, I.O. Morphological and immunophenotypic analysis of breast carcinomas with basal and myoepithelial differentiation. J. Pathol. 2006, 208, 495–506. [Google Scholar] [CrossRef]
- Yehiely, F.; Moyano, J.V.; Evans, J.R.; Nielsen, T.O.; Cryns, V.L. Deconstructing the molecular portrait of basal-like breast cancer. Trends Mol. Med. 2006, 12, 537–544. [Google Scholar] [CrossRef]
- Bolshakov, S.; Walker, C.M.; Strom, S.S.; Selvan, M.S.; Clayman, G.L.; El-Naggar, A.; Lippman, S.M.; Kripke, M.L.; Ananthaswamy, H.N. p53 Mutations in Human Aggressive and Nonaggressive Basal and Squamous Cell Carcinomas. Clin. Cancer Res. 2003, 9, 228–234. [Google Scholar]
- Soussi, T. p53 alterations in human cancer: more questions than answers. Oncogene 2007, 26, 2145–2156. [Google Scholar] [CrossRef]
- Alsner, J.; Jensen, V.; Kyndi, M.; Vrou Offersen, B.; Vu, P.; Borresen-Dale, A.-L.; Overgaard, J. A comparison between p53 accumulation determined by immunohistochemistry and TP53 mutations as prognostic variables in tumours from breast cancer patients. Acta Oncol. 2008, 47, 600–607. [Google Scholar] [CrossRef]
- Chappuis, P.O.; Donato, E.; Goffin, J.R.; Wong, N.; Begin, L.R.; Kapusta, L.R.; Brunet, J.S.; Porter, P.; Foulkes, W.D. Cyclin E expression in breast cancer: predicting germline BRCA1 mutations, prognosis and response to treatment. Ann. Oncol. 2005, 16, 735–742. [Google Scholar] [CrossRef]
- Signoretti, S.; Di Marcotullio, L.; Richardson, A.; Ramaswamy, S.; Isaac, B.; Rue, M.; Monti, F.; Loda, M.; Pagano, M. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J. Clin. Invest. 2002, 110, 633–641. [Google Scholar]
- Voduc, D.; Nielsen, T.O.; Cheang, M.C.; Foulkes, W.D. The combination of high cyclin E and Skp2 expression in breast cancer is associated with a poor prognosis and the basal phenotype. Human Pathol. 2008, 39, 1431–1437. [Google Scholar] [CrossRef]
- Gauthier, M.L.; Berman, H.K.; Miller, C.; Kozakeiwicz, K.; Chew, K.; Moore, D.; Rabban, J.; Chen, Y.Y.; Kerlikowske, K.; Tlsty, T.D. Abrogated Response to Cellular Stress Identifies DCIS Associated with Subsequent Tumor Events and Defines Basal-like Breast Tumors. Cancer Cell 2007, 12, 479–491. [Google Scholar] [CrossRef]
- Herschkowitz, J.; He, X.; Fan, C.; Perou, C. The functional loss of the retinoblastoma tumour suppressor is a common event in basal-like and luminal B breast carcinomas. Breast Cancer Res. 2008, 10, R75. [Google Scholar] [CrossRef]
- Subhawong, A.P.; Subhawong, T.; Nassar, H.; Kouprina, N.; Begum, S.; Vang, R.; Westra, W.H.; Argani, P. Most Basal-like Breast Carcinomas Demonstrate the Same Rb-/p16+ Immunophenotype as the HPV-related Poorly Differentiated Squamous Cell Carcinomas Which They Resemble Morphologically. Am. J. Surg. Pathol. 2009, 33, 163–175. [Google Scholar] [CrossRef]
- Yerushalmi, R.; Woods, R.; Ravdin, P.M.; Hayes, M.M.; Gelmon, K.A. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010, 11, 174–183. [Google Scholar] [CrossRef]
- Livasy, C.A.; Perou, C.M.; Karaca, G.; Cowan, D.W.; Maia, D.; Jackson, S.; Tse, C.-K.; Nyante, S.; Millikan, R.C. Identification of a basal-like subtype of breast ductal carcinoma in situ. Human Pathol. 2007, 38, 197–204. [Google Scholar] [CrossRef]
- Wirapati, P.; Sotiriou, C.; Kunkel, S.; Farmer, P.; Pradervand, S.; Haibe-Kains, B.; Desmedt, C.; Ignatiadis, M.; Sengstag, T.; Schutz, F.; et al. Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008, 10, R65. [Google Scholar] [CrossRef]
- Desmedt, C.; Haibe-Kains, B.; Wirapati, P.; Buyse, M.; Larsimont, D.; Bontempi, G.; Delorenzi, M.; Piccart, M.; Sotiriou, C. Biological Processes Associated with Breast Cancer Clinical Outcome Depend on the Molecular Subtypes. Clinical Cancer Res. 2008, 14, 5158–5165. [Google Scholar] [CrossRef]
- Ashworth, A. A Synthetic Lethal Therapeutic Approach: Poly(ADP) Ribose Polymerase Inhibitors for the Treatment of Cancers Deficient in DNA Double-Strand Break Repair. J. Clin. Oncol. 2008, 26, 3785–3790. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Stefansson, I.M.; Chappuis, P.O.; Begin, L.R.; Goffin, J.R.; Wong, N.; Trudel, M.; Akslen, L.A. Germline BRCA1 Mutations and a Basal Epithelial Phenotype in Breast Cancer. J. Natl. Cancer Inst. 2003, 95, 1482–1485. [Google Scholar] [CrossRef]
- Silver, D.P.; Richardson, A.L.; Eklund, A.C.; Wang, Z.C.; Szallasi, Z.; Li, Q.; Juul, N.; Leong, C.-O.; Calogrias, D.; Buraimoh, A.; et al. Efficacy of Neoadjuvant Cisplatin in Triple-Negative Breast Cancer. J. Clin. Oncol. 2010, 28, 1145–1153. [Google Scholar] [CrossRef]
- Turner, N.C.; Reis-Filho, J.S.; Russell, A.M.; Springall, R.J.; Ryder, K.; Steele, D.; Savage, K.; Gillett, C.E.; Schmitt, F.C.; Ashworth, A.; et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene 2006, 26, 2126–2132. [Google Scholar]
- Gilbert, P.M.; Mouw, J.K.; Unger, M.A.; Lakins, J.N.; Gbegnon, M.K.; Clemmer, V.B.; Benezra, M.; Licht, J.D.; Boudreau, N.J.; Tsai, K.K.C.; et al. HOXA9 regulates BRCA1 expression to modulate human breast tumor phenotype. J. Clin. Invest. 2010, 120, 1535–1550. [Google Scholar] [CrossRef]
- Lacroix, M.; Leclercq, G. The “portrait” of hereditary breast cancer. Breast Cancer Res. Treat. 2005, 89, 297–304. [Google Scholar] [CrossRef]
- Lakhani, S.R.; O'Hare, M.J.; Ashworth, A. Profiling familial breast cancer. Nat. Med. 2001, 7, 408–410. [Google Scholar] [CrossRef]
- Hosey, A.M.; Gorski, J.J.; Murray, M.; Quinn, J.E.; Chung, W.Y.; Stewart, G.E.; James, C.R.; Farragher, S.M.; Mulligan, J.M.; Scott, A.N.; et al. Molecular Basis for Estrogen Receptor {alpha} Deficiency in BRCA1-Linked Breast Cancer. J. Natl. Cancer Inst. 2007, 99, 1683–1694. [Google Scholar] [CrossRef]
- Gorski, J.; James, C.; Quinn, J.; Stewart, G.; Staunton, K.; Buckley, N.; McDyer, F.; Kennedy, R.; Wilson, R.; Mullan, P.; et al. BRCA1 transcriptionally regulates genes associated with the basal-like phenotype in breast cancer. Breast Cancer Res. Treat. 2009. [Google Scholar] [CrossRef]
- Liu, X.; Holstege, H.; van der Gulden, H.; Treur-Mulder, M.; Zevenhoven, J.; Velds, A.; Kerkhoven, R.M.; van Vliet, M.H.; Wessels, L.F.A.; Peterse, J.L.; et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 12111–12116. [Google Scholar] [CrossRef]
- McCarthy, A.; Savage, K.; Gabriel, A.; Naceur, C.; Reis-Filho, J.S.; Ashworth, A. A mouse model of basal-like breast carcinoma with metaplastic elements. J. Pathol. 2007, 211, 389–398. [Google Scholar] [CrossRef]
- Tan, A.R.; Swain, S.M. Therapeutic Strategies for Triple-Negative Breast Cancer. Cancer J. 2008, 14, 343–351. [Google Scholar] [CrossRef]
- Ashworth, A. A Synthetic Lethal Therapeutic Approach: Poly(ADP) Ribose Polymerase Inhibitors for the Treatment of Cancers Deficient in DNA Double-Strand Break Repair. J. Clin. Oncol. 2008, 26, 3785–3790. [Google Scholar] [CrossRef]
- Stebbing, J.; Ellis, P.; Tutt, A. PARP inhibitors in BRCA1-/BRCA2-associated and triple-negative breast cancers. Future Oncol. 2010, 6, 485–486. [Google Scholar] [CrossRef]
- Fong, P.C.; Boss, D.S.; Carden, C.P.; Roelvink, M.; De Greve, J.; Gourley, C.M.; Carmichael, J.; De Bono, J.S.; Schellens, J.H.M.; Kaye, S.B. AZD2281 (KU-0059436), a PARP (poly ADP-ribose polymerase) inhibitor with single agent anticancer activity in patients with BRCA deficient ovarian cancer: Results from a phase I study. In Presented at the 2008 ASCO Annual Meeting, Boston, MA, USA, June 2008.
- Yap, T.A.; Boss, D.S.; Fong, P.C.; Roelvink, M.; Tutt, A.; Carmichael, J.; O'connor, M.J.; Kaye, S.B.; Schellens, J.H.M.; de Bono, J.S. First in human phase I pharmacokinetic and pharmacodynamic study of KU-0059436 (Ku), a small molecule inhibitor of poly ADP-ribose polymerase (PARP) in cancer patients, including BRCA1/2 mutation carriers. In Presented at the 2007 ASCO Annual Meeting, Chicago, IL, USA, June 2007.
- Walter, O.; Prasad, M.; Lu, S.; Quinlan, R.M.; Edmiston, K.L.; Khan, A. IMP3 is a novel biomarker for triple negative invasive mammary carcinoma associated with a more aggressive phenotype. Human Pathol. 2009, 40, 1528–1533. [Google Scholar] [CrossRef]
- Nalwoga, H.; Arnes, J.B.; Wabinga, H.; Akslen, L.A. Expression of aldehyde dehydrogenase 1 (ALDH1) is associated with basal-like markers and features of aggressive tumours in African breast cancer. Br. J. Cancer 2010, 102, 369–375. [Google Scholar] [CrossRef]
- Resetkova, E.; Reis-Filho, J.; Jain, R.; Mehta, R.; Thorat, M.; Nakshatri, H.; Badve, S. Prognostic impact of ALDH1 in breast cancer: a story of stem cells and tumor microenvironment. Breast Cancer Res. Treat. 2009. [Google Scholar] [CrossRef]
- Otterbach, F.; Callies, R.; Adamzik, M.; Kimmig, R.; Siffert, W.; Schmid, K.; Bankfalvi, A. Aquaporin 1 (AQP1) expression is a novel characteristic feature of a particularly aggressive subgroup of basal-like breast carcinomas. Breast Cancer Res. Treat. 2009, 120, 67–76. [Google Scholar]
- Arai, K.; Takano, S.; Teratani, T.; Ito, Y.; Yamada, T.; Nozawa, R. S100A8 and S100A9 Overexpression Is Associated with Poor Pathological Parameters in Invasive Ductal Carcinoma of the Breast. Curr. Cancer Drug Targets 2008, 8, 243–252. [Google Scholar] [CrossRef]
- Arai, K.; Teratani, T.; Kuruto-Niwa, R.; Yamada, T.; Nozawa, R. S100A9 expression in invasive ductal carcinoma of the breast: S100A9 expression in adenocarcinoma is closely associated with poor tumour differentiation. Eur. J. Cancer 2004, 40, 1179–1187. [Google Scholar] [CrossRef]
- Skaland, I.; Janssen, E.A.M.; Gudlaugsson, E.; Hui Ru Guo, L.; Baak, J.P.A. The prognostic value of the proliferation marker Phosphohistone H3 (PPH3) in luminal, basal-like and triple negative phenotype invasive lymph node-negative breast cancer. Cell. Oncol. 2009, 31, 261–271. [Google Scholar]
- Kuroda, H.; Ishida, F.; Nakai, M.; Ohnisi, K.; Itoyama, S. Basal cytokeratin expression in relation to biological factors in breast cancer. Human Pathol. 2008, 39, 1744–1750. [Google Scholar] [CrossRef]
- Lancashire, L.; Powe, D.; Reis-Filho, J.; Rakha, E.; Lemetre, C.; Weigelt, B.; Abdel-Fatah, T.; Green, A.; Mukta, R.; Blamey, R.; et al. A validated gene expression profile for detecting clinical outcome in breast cancer using artificial neural networks. Breast Cancer Res. Treat. 2009, 120, 83–93. [Google Scholar]
- Tang, X.Y.; Umemura, S.; Tsukamoto, H.; Kumaki, N.; Tokuda, Y.; Osamura, R.Y. Overexpression of fatty acid binding protein-7 correlates with basal-like subtype of breast cancer. Pathol. Res. Prac. 2010, 206, 98–101. [Google Scholar] [CrossRef]
- Moyano, J.V.; Evans, J.R.; Chen, F.; Lu, M.; Werner, M.E.; Yehiely, F.; Diaz, L.K.; Turbin, D.; Karaca, G.; Wiley, E.; et al. alphaB-Crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J. Clin. Invest. 2006, 116, 261–270. [Google Scholar]
- Sitterding, S.M.; Wiseman, W.R.; Schiller, C.L.; Luan, C.; Chen, F.; Moyano, J.V.; Watkin, W.G.; Wiley, E.L.; Cryns, V.L.; Diaz, L.K. alphaB-crystallin: A novel marker of invasive basal-like and metaplastic breast carcinomas. Ann. Diagn. Pathol. 2008, 12, 33–40. [Google Scholar] [CrossRef]
- Pal, S.; Childs, B.; Pegram, M. Emergence of nonanthracycline regimens in the adjuvant treatment of breast cancer. Breast Cancer Res. Treat. 2010, 119, 25–32. [Google Scholar]
- Choi, W.W.L.; Weisenburger, D.D.; Greiner, T.C.; Piris, M.A.; Banham, A.H.; Delabie, J.; Braziel, R.M.; Geng, H.; Iqbal, J.; Lenz, G.; et al. A New Immunostain Algorithm Classifies Diffuse Large B-Cell Lymphoma into Molecular Subtypes with High Accuracy. Clin. Cancer Res. 2009, 15, 5494–5502. [Google Scholar] [CrossRef]
- Geiss, G.K.; Bumgarner, R.E.; Birditt, B.; Dahl, T.; Dowidar, N.; Dunaway, D.L.; Fell, H.P.; Ferree, S.; George, R.D.; Grogan, T.; et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotech. 2008, 26, 317–325. [Google Scholar] [CrossRef]
- Paik, S.; Shak, S.; Tang, G.; Kim, C.; Baker, J.; Cronin, M.; Baehner, F.L.; Walker, M.G.; Watson, D.; Park, T.; et al. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. N. Engl. J. Med. 2004, 351, 2817–2826. [Google Scholar] [CrossRef]
- Rimsza, L.M.; LeBlanc, M.L.; Unger, J.M.; Miller, T.P.; Grogan, T.M.; Persky, D.O.; Martel, R.R.; Sabalos, C.M.; Seligmann, B.; Braziel, R.M.; et al. Gene expression predicts overall survival in paraffin-embedded tissues of diffuse large B-cell lymphoma treated with R-CHOP. Blood 2008, 112, 3425–3433. [Google Scholar] [CrossRef]
- Sotiriou, C.; Pusztai, L. Gene-Expression Signatures in Breast Cancer. N. Engl. J. Med. 2009, 360, 790–800. [Google Scholar] [CrossRef]
- Schneider, B.P.; Winer, E.P.; Foulkes, W.D.; Garber, J.; Perou, C.M.; Richardson, A.; Sledge, G.W.; Carey, L.A. Triple-Negative Breast Cancer: Risk Factors to Potential Targets. Clin. Cancer Res. 2008, 14, 8010–8018. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Choo, J.R.; Nielsen, T.O. Biomarkers for Basal-like Breast Cancer. Cancers 2010, 2, 1040-1065. https://doi.org/10.3390/cancers2021040
Choo JR, Nielsen TO. Biomarkers for Basal-like Breast Cancer. Cancers. 2010; 2(2):1040-1065. https://doi.org/10.3390/cancers2021040
Chicago/Turabian StyleChoo, Jennifer R., and Torsten O. Nielsen. 2010. "Biomarkers for Basal-like Breast Cancer" Cancers 2, no. 2: 1040-1065. https://doi.org/10.3390/cancers2021040
APA StyleChoo, J. R., & Nielsen, T. O. (2010). Biomarkers for Basal-like Breast Cancer. Cancers, 2(2), 1040-1065. https://doi.org/10.3390/cancers2021040




