Modulation of Cancer-Associated Fibroblasts via the miR-624-5p/FAP Axis Drives Progression and Metastasis in Non-Small Cell Lung Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. 68Ga (Gallium)-FAP Inhibitor (FAPI) PET/CT Imaging of NSCLC Patients
2.2. Cell Lines
2.3. Fibroblast Isolation and Culture of NSCLC Patients
2.4. Cell Immunofluorescence (IF) and FISH
2.5. Bioinformatic Analysis
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assay
2.7. miRNA Mimic and Inhibitor Transfection
2.8. Cell Proliferation Assay
2.9. Collagen Contraction Assay
2.10. Conditioned Medium (CM) for Fibroblasts and Coculture of CAFs and Cancer Cells
2.11. Cell Migration and Invasion Assay
2.12. Dual-Luciferase Reporter Assay
2.13. Western Blotting Analysis
2.14. Statistical Analyses
3. Results
3.1. 68Ga-FAPI-04 PET/CT Imaging of NSCLC Patients Demonstrates That FAP Is Associated with Tumor Progression and Metastasis
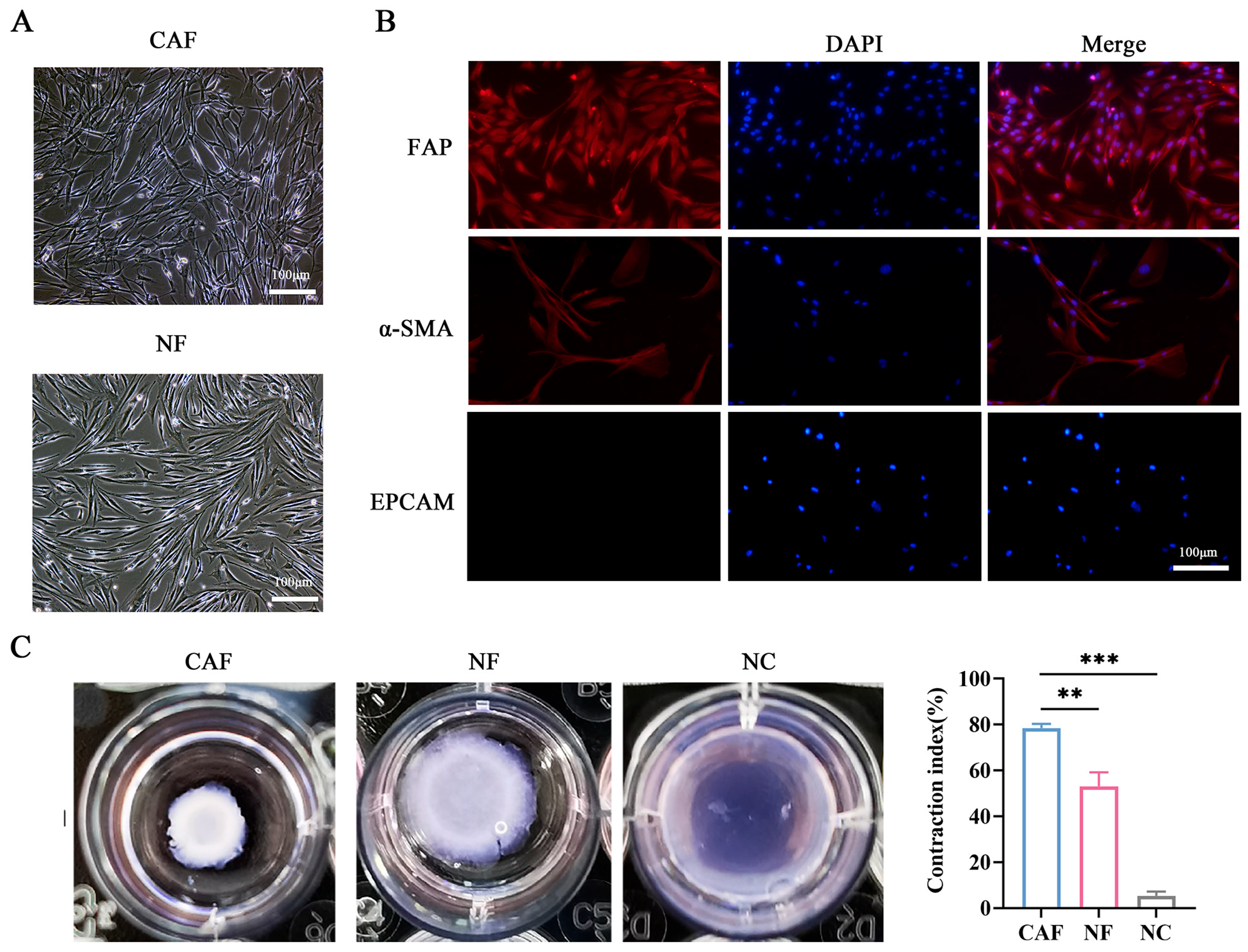
3.2. Isolation and Identification of CAFs and NFs in NSCLC Patients
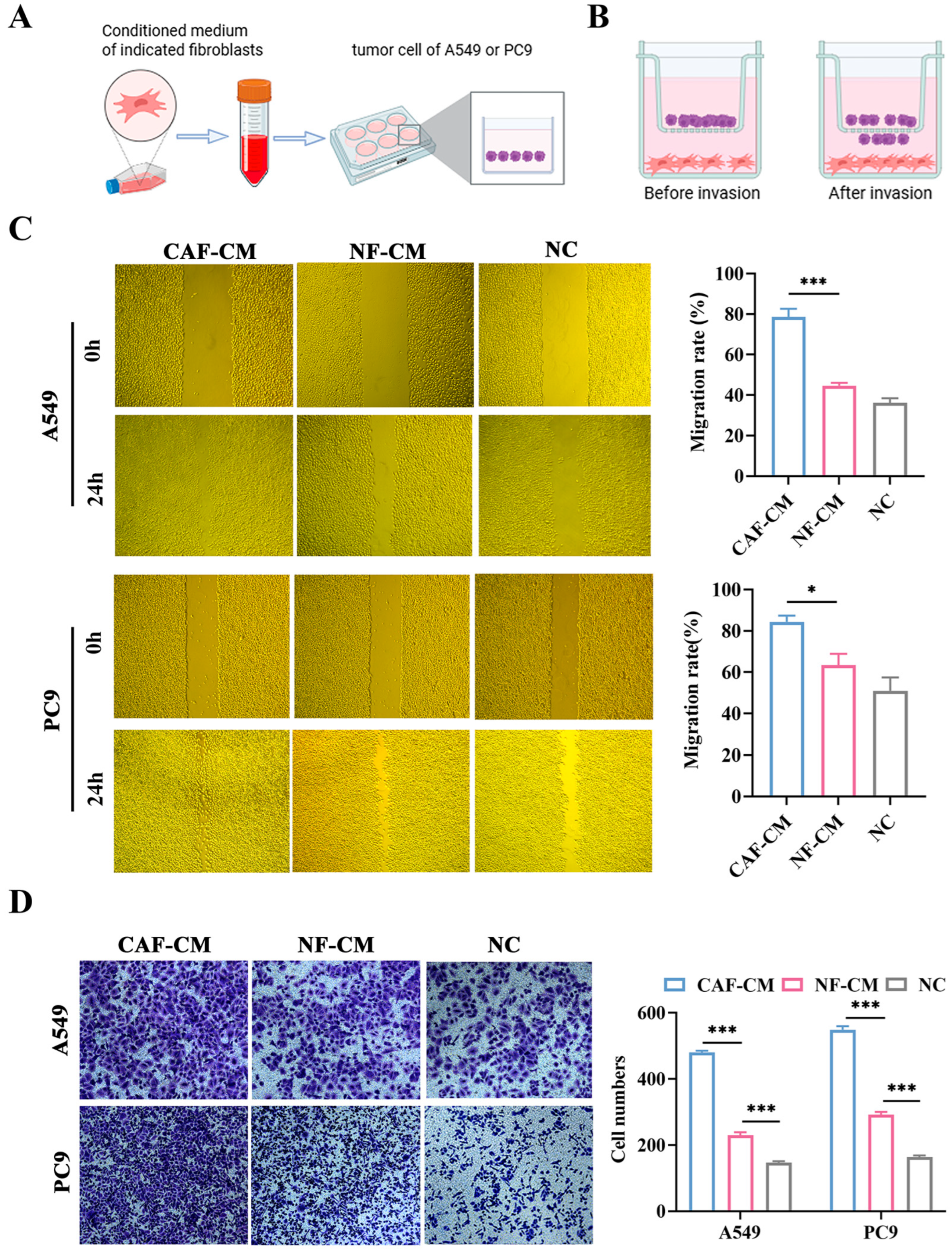
3.3. CAFs Promote the Migration and Invasion of A549 and PC9 Cells
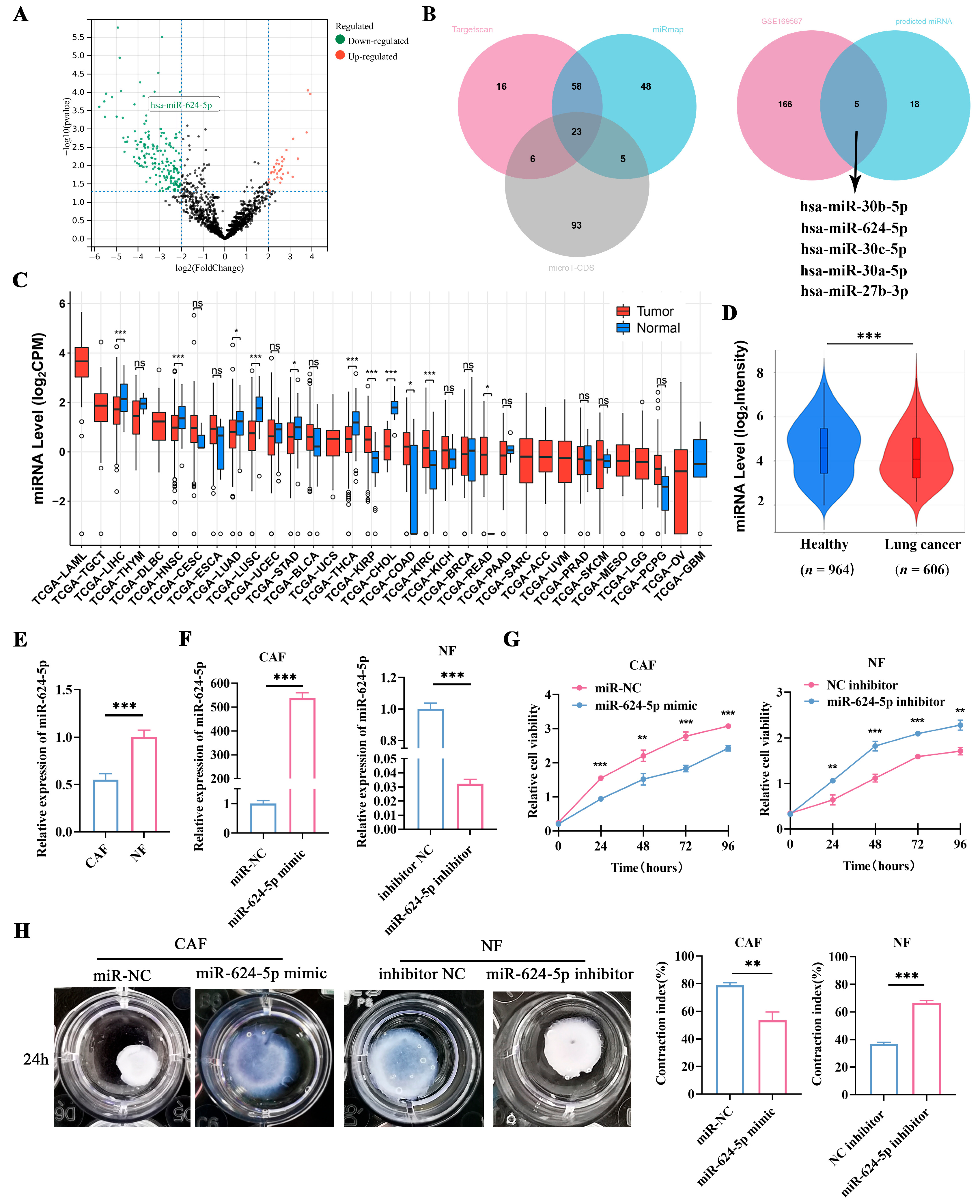
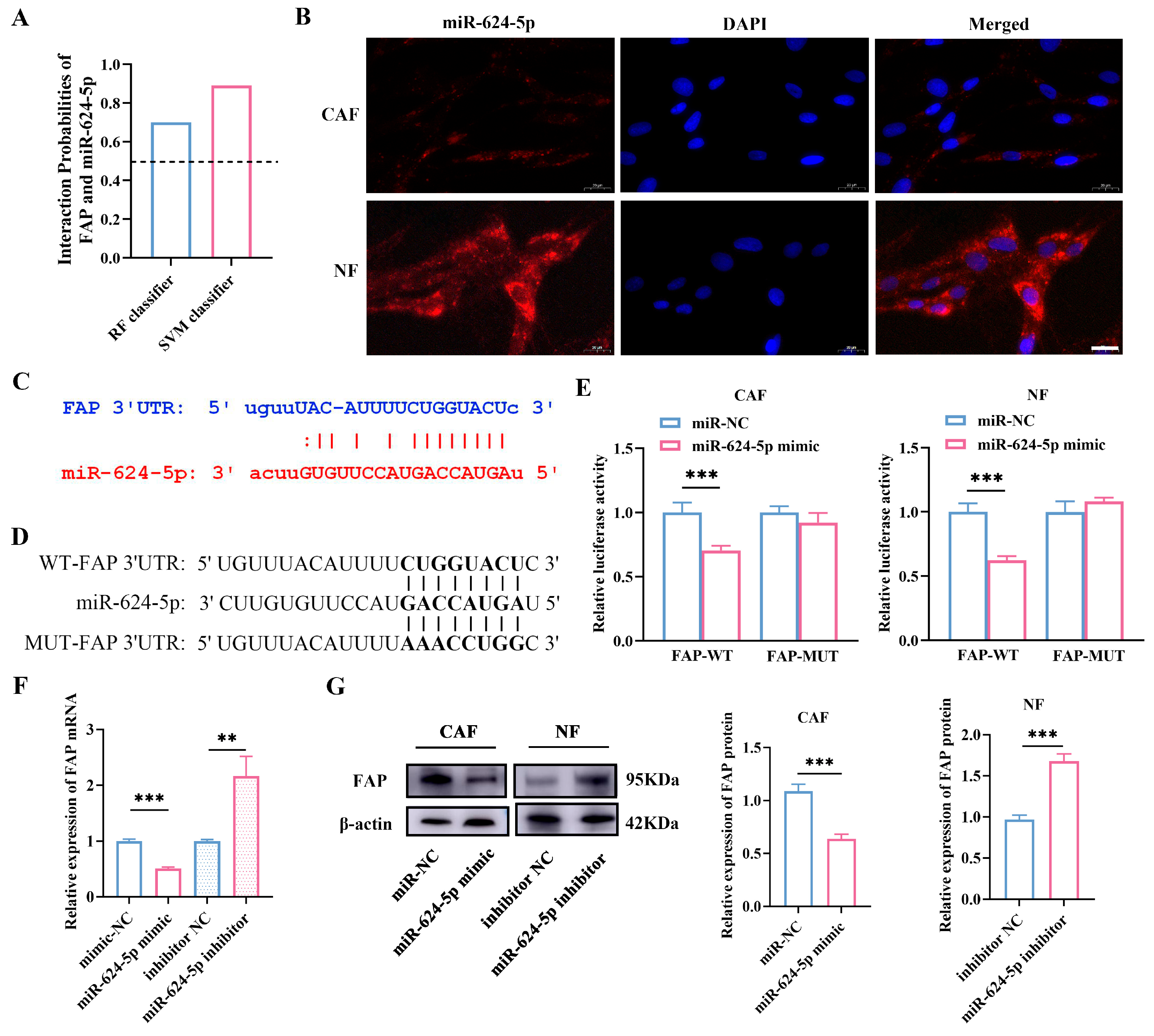
3.4. miR-624-5p Is Downregulated in CAFs of NSCLC and Inhibits CAF Activation
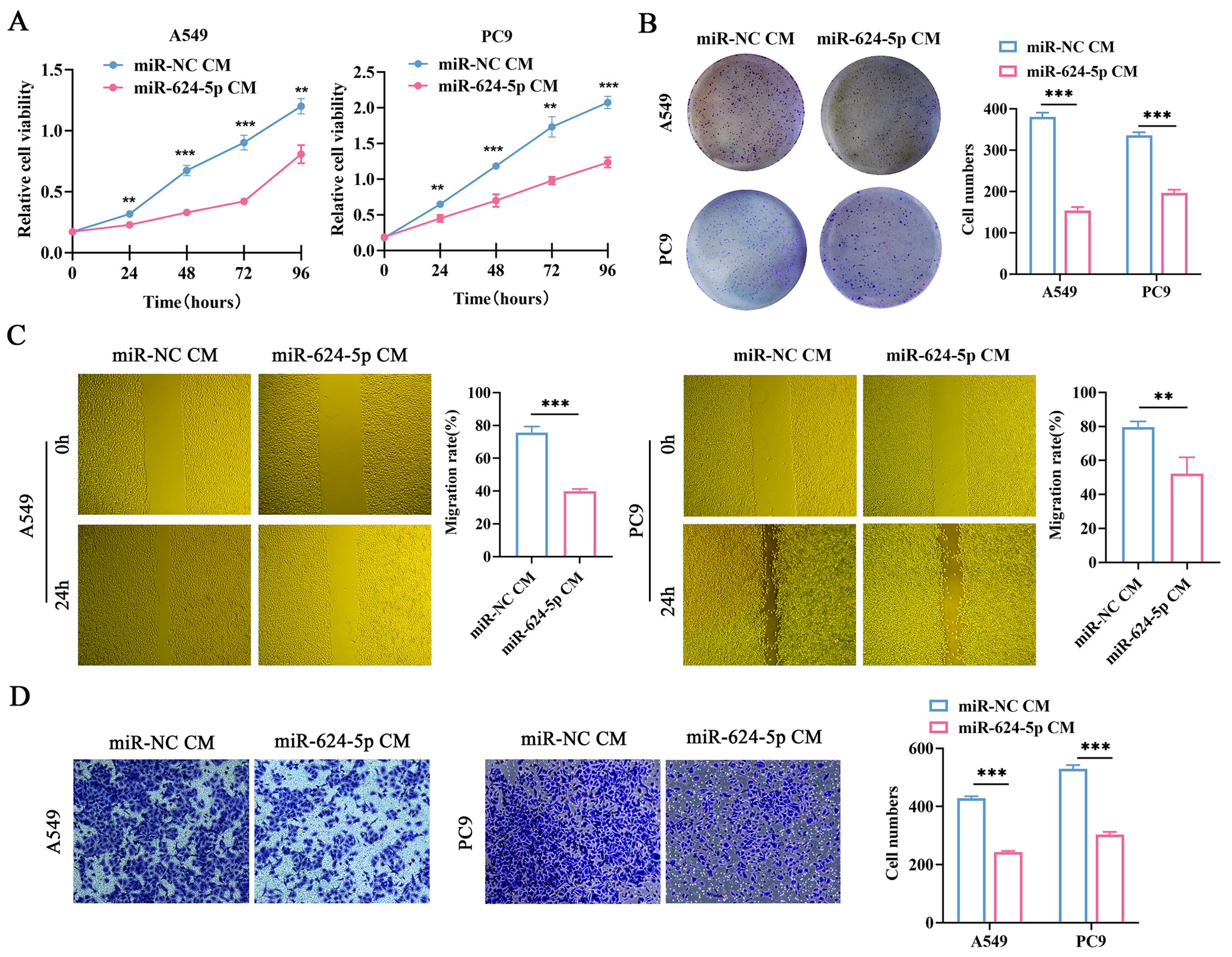
3.5. Overexpression of miR-624-5p in CAFs Inhibited the Proliferation, Migration, and Invasion of NSCLC Cells
3.6. miR-624-5p Inhibits CAF Activation by Downregulating FAP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAFs | Cancer-associated fibroblasts |
| NFs | Normal fibroblasts |
| NSCLC | Non-small cell lung cancer |
| TME | Tumor microenvironment |
| FAP | Fibroblast activation protein |
| FAPI | Fibroblast activation protein inhibitor |
| PET | Positron emission tomography |
| MIP | Maximum intensity projection |
| SUVmax | Maximum standardized uptake value |
| SUVmean | Mean standardized uptake value |
| MiRNA | MicroRNA |
| qRT-PCR | Real-time quantitative polymerase chain reaction |
| FISH | Fluorescence in situ hybridization |
| CAF-CM | CAF-derived conditioned medium |
| NF-CM | NF-derived conditioned medium |
| UTR | Untranslated region |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Qu, C.; Zhou, P.; Zhou, Q.; Li, D.; Wu, X.; Yang, L. Extracellular vesicles activated cancer-associated fibroblasts promote lung cancer metastasis through mitophagy and mtDNA transfer. J. Exp. Clin. Cancer Res. 2024, 43, 158. [Google Scholar] [CrossRef]
- Chhabra, Y.; Weeraratna, A.T. Fibroblasts in cancer: Unity in heterogeneity. Cell 2023, 186, 1580–1609. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wu, Z.; Qi, Y.; Wu, B.; Zhu, X. The metastasizing mechanisms of lung cancer: Recent advances and therapeutic challenges. Biomed. Pharmacother. 2021, 138, 111450. [Google Scholar] [CrossRef]
- Gu, X.; Zhu, Y.; Su, J.; Wang, S.; Su, X.; Ding, X.; Jiang, L.; Fei, X.; Zhang, W. Lactate-induced activation of tumor-associated fibroblasts and IL-8-mediated macrophage recruitment promote lung cancer progression. Redox Biol. 2024, 74, 103209. [Google Scholar] [CrossRef]
- Park, J.E.; Lenter, M.C.; Zimmermann, R.N.; Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Fibroblast Activation Protein, a Dual Specificity Serine Protease Expressed in Reactive Human Tumor Stromal Fibroblasts. J. Biol. Chem. 1999, 274, 36505–36512. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Weiner, L.M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020, 39, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Deng, Y.; Guo, H.; Ren, Z.; He, Y.; Ren, X.; Huang, L.-B.; Zhang, W.-H.; Chen, H.-N.; Shu, Y.; et al. Single-cell and spatial transcriptomics profile the interaction of SPP1+ macrophages and FAP+ fibroblasts in non-small cell lung cancer. Transl. Lung Cancer Res. 2025, 14, 2646–2669. [Google Scholar] [CrossRef]
- Wu, H.; Xiang, Z.; Huang, G.; He, Q.; Song, J.; Dou, R.; Yang, C.; Wang, S.; Xiong, B. BGN/FAP/STAT3 positive feedback loop mediated mutual interaction between tumor cells and mesothelial cells contributes to peritoneal metastasis of gastric cancer. Int. J. Biol. Sci. 2023, 19, 465–483. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Q.; Liu, Z.; Luo, X.; Fan, Y.; Liu, Y.; Zhang, Y.; Hua, S.; Fu, Q.; Zhao, M.; et al. Downregulation of FAP suppresses cell proliferation and metastasis through PTEN/PI3K/AKT and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death Dis. 2014, 5, e1155. [Google Scholar] [CrossRef]
- Liu, T.; Huang, C.; Sun, L.; Chen, Z.; Ge, Y.; Ji, W.; Chen, S.; Zhao, Y.; Wang, M.; Wang, D.; et al. FAP+ gastric cancer mesenchymal stromal cells via paracrining INHBA and remodeling ECM promote tumor progression. Int. Immunopharmacol. 2024, 144, 113697. [Google Scholar] [CrossRef] [PubMed]
- Monsky, W.L.; Lin, C.Y.; Aoyama, A.; Kelly, T.; Akiyama, S.K.; Mueller, S.C.; Chen, W.T. A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res. 1994, 54, 5702–5710. [Google Scholar]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Xu, Q.; Pandupuspitasari, N.S.; Khan, F.A.; Wu, D.; Sun, F.; Huang, C. Non-coding RNAs-glycolysis axis in cancer therapy resistance: Insight into mechanism to therapeutic solution. Biochem. Pharmacol. 2025, 242, 117286. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, T.; Mazzarelli, F.; Cuttano, R.; Dama, E.; Melocchi, V.; Afanga, M.K.; Perrone, R.M.; Graziano, P.; Bianchi, F. Unveiling the origin and functions of diagnostic circulating microRNAs in lung cancer. Br. J. Cancer 2025, 132, 947–956. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Fu, L.; Shi, M.; Hu, G.; Du, F.; Wang, Z.; Xiao, Y.; Zhang, Y.; Li, Y. Role of exosomal non-coding RNAs in cancer-associated fibroblast-mediated therapy resistance (Review). Int. J. Oncol. 2025, 67, 68. [Google Scholar] [CrossRef]
- Zhuang, J.; Shen, L.; Li, M.; Sun, J.; Hao, J.; Li, J.; Zhu, Z.; Ge, S.; Zhang, D.; Guo, H.; et al. Cancer-Associated Fibroblast-Derived miR-146a-5p Generates a Niche That Promotes Bladder Cancer Stemness and Chemoresistance. Cancer Res. 2023, 83, 1611–1627. [Google Scholar] [CrossRef]
- Song, X.; Li, T.; Zhou, W.; Feng, C.; Zhou, Z.; Chen, Y.; Li, D.; Chen, L.; Zhao, J.; Zhang, Y.; et al. CAF-derived exosomal miR-196b-5p after androgen deprivation therapy promotes epithelial-mesenchymal transition in prostate cancer cells through HOXC8/NF-κB signaling pathway. Biol. Direct 2025, 20, 80. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, W.; Tang, P.; Jiang, D.; Gu, C.; Huang, Y.; Gong, F.; Rong, Y.; Qian, D.; Chen, J.; et al. miR-624-5p promoted tumorigenesis and metastasis by suppressing hippo signaling through targeting PTPRB in osteosarcoma cells. J. Exp. Clin. Cancer Res. 2019, 38, 488. [Google Scholar] [CrossRef]
- Yang, L.; Tan, W.; Wei, Y.; Xie, Z.; Li, W.; Ma, X.; Wang, Q.; Li, H.; Zhang, Z.; Shang, C.; et al. CircLIFR suppresses hepatocellular carcinoma progression by sponging miR-624-5p and inactivating the GSK-3β/β-catenin signaling pathway. Cell Death Dis. 2022, 13, 464. [Google Scholar] [CrossRef]
- Luo, L.; Huang, J.; Chen, D.; Yang, H.; Deng, Y.; He, Y.; Lin, J.; Zhao, R.; Wang, X.; Su, Z.; et al. Airway basal stem cell-derived extracellular vesicles drive ECM remodeling and suppress fibroblasts activation via the miR-30a-5p/FAP axis in benign tracheal stenosis. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef]
- Bartoszewska, E.; Misiąg, P.; Czapla, M.; Rakoczy, K.; Tomecka, P.; Filipski, M.; Wawrzyniak-Dzierżek, E.; Choromańska, A. The Role of microRNAs in Lung Cancer: Mechanisms, Diagnostics and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 3736. [Google Scholar] [CrossRef]
- Wang, B.; Gu, B.; Gao, L.; Ma, C.; Li, X.; Wang, Y.; Hu, J.; Wang, N.; Xiang, L.; Yu, Y.; et al. SERPINE1 Facilitates Metastasis in Gastric Cancer Through Anoikis Resistance and Tumor Microenvironment Remodeling. Small 2025, 21, e2500136. [Google Scholar] [CrossRef] [PubMed]
- Cords, L.; Engler, S.; Haberecker, M.; Rüschoff, J.H.; Moch, H.; de Souza, N.; Bodenmiller, B. Cancer-associated fibroblast phenotypes are associated with patient outcome in non-small cell lung cancer. Cancer Cell 2024, 42, 396–412.e5. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, H.; Zou, Y.; Zhang, H.; Wang, Y.; Ren, X.; Wang, H.; Xu, Y.; Li, J.; Tang, H.; et al. Distinct fibroblast subpopulations associated with bone, brain or intrapulmonary metastasis in advanced non-small-cell lung cancer. Clin. Transl. Med. 2024, 14, e1605. [Google Scholar] [CrossRef]
- Mori, Y.; Dendl, K.; Cardinale, J.; Kratochwil, C.; Giesel, F.L.; Haberkorn, U. FAPI PET: Fibroblast Activation Protein Inhibitor Use in Oncologic and Nononcologic Disease. Radiology 2023, 306, e220749. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Song, S.; Zou, S.; Kuang, D.; Wang, D.; Min, X.; Feng, Z.; Zhu, Y.; Cheng, Z.; Cheng, S.; et al. Assessment of viable tumours by [68Ga]Ga-FAPI-04 PET/CT after local regional treatment in patients with hepatocellular carcinoma. Eur. J. Nucl. Med. 2025, 52, 2132–2144. [Google Scholar] [CrossRef]
- Wang, L.; Tang, G.; Hu, K.; Liu, X.; Zhou, W.; Li, H.; Huang, S.; Han, Y.; Chen, L.; Zhong, J.; et al. Comparison of (68)Ga-FAPI and (18)F-FDG PET/CT in the Evaluation of Advanced Lung Cancer. Radiology 2022, 303, 191–199. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, L.; Li, P.; Lu, J.; Ren, J.; Yan, S.; Wu, H.; Yuan, S.; Fu, Z.; Yu, J. FAPI Compared with FDG PET/CT for Diagnosis of Primary and Metastatic Lung Cancer. Radiology 2023, 308, e222785. [Google Scholar] [CrossRef]
- Mona, C.E.; Benz, M.R.; Hikmat, F.; Grogan, T.R.; Lueckerath, K.; Razmaria, A.; Riahi, R.; Slavik, R.; Girgis, M.D.; Carlucci, G.; et al. Correlation of 68Ga-FAPi-46 PET Biodistribution with FAP Expression by Immunohistochemistry in Patients with Solid Cancers: Interim Analysis of a Prospective Translational Exploratory Study. J. Nucl. Med. 2021, 63, 1021–1026. [Google Scholar] [CrossRef]
- Knuutila, J.S.; Riihilä, P.; Nissinen, L.; Heiskanen, L.; Kallionpää, R.E.; Pellinen, T.; Kähäri, V.M. Cancer-associated fibroblast activation predicts progression, metastasis, and prognosis of cutaneous squamous cell carcinoma. Int. J. Cancer 2024, 155, 1112–1127. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Jia, J.; Cui, X.; Li, Z.; Wu, A. FAP promotes metastasis and chemoresistance via regulating YAP1 and macrophages in mucinous colorectal adenocarcinoma. iScience 2023, 26, 106600. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ning, Z.; Ma, L.; Liu, W.; Shao, C.; Shu, Y.; Shen, H. Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Mol. Cancer 2017, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jia, Y.; Wang, J.; Chen, X.; Han, J.; Zhen, S.; Yin, S.; Lv, W.; Yu, F.; Wang, J.; et al. circNOX4 activates an inflammatory fibroblast niche to promote tumor growth and metastasis in NSCLC via FAP/IL-6 axis. Mol. Cancer 2024, 23, 47. [Google Scholar] [CrossRef]
- Brennen, W.N.; Thorek, D.L.J.; Jiang, W.; Krueger, T.E.; Antony, L.; Denmeade, S.R.; Isaacs, J.T. Overcoming stromal barriers to immuno-oncological responses via fibroblast activation protein-targeted therapy. Immunotherapy 2021, 13, 155–175. [Google Scholar] [CrossRef]
- Nilforoushan, N.; Khavaran, A.; Palihati, M.; Patel, Y.; Giarratana, A.O.; Das, J.P.; Capaccione, K.M. Cancer-Associated Fibroblasts: Clinical Applications in Imaging and Therapy. Tomography 2025, 11, 143. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kawasaki, T.; Tatsumi, K.; Okaya, T.; Sato, S.; Shimada, A.; Misawa, T.; Hatano, R.; Morimoto, C.; Kasuya, Y.; et al. Transcriptome Analysis of Fibroblasts in Hypoxia-Induced Vascular Remodeling: Functional Roles of CD26/DPP4. Int. J. Mol. Sci. 2024, 25, 12599. [Google Scholar] [CrossRef]
- Enz, N.; Vliegen, G.; De Meester, I.; Jungraithmayr, W. CD26/DPP4—A potential biomarker and target for cancer therapy. Pharmacol. Ther. 2019, 198, 135–159. [Google Scholar] [CrossRef]
- Hui, Y.; Xu, Z.; Li, J.; Kuang, L.; Zhong, Y.; Tang, Y.; Wei, J.; Zhou, H.; Zheng, T. Nonenzymatic function of DPP4 promotes diabetes-associated cognitive dysfunction through IGF-2R/PKA/SP1/ERp29/IP3R2 pathway-mediated impairment of Treg function and M1 microglia polarization. Metabolism 2023, 138, 155340. [Google Scholar] [CrossRef]
- Privé, B.M.; Boussihmad, M.A.; Timmermans, B.; van Gemert, W.A.; Peters, S.M.B.; Derks, Y.H.W.; van Lith, S.A.M.; Mehra, N.; Nagarajah, J.; Heskamp, S.; et al. Fibroblast activation protein-targeted radionuclide therapy: Background, opportunities, and challenges of first (pre)clinical studies. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1906–1918. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wu, J.; Cao, W.; Chen, Q.; Shao, Z.; Hu, C.; Zhang, Y.; Weng, W.; Meng, T.; Meng, X.; et al. A novel FAP-targeting antibody-exatecan conjugate improves immune checkpoint blockade by reversing immunosuppressive microenvironment in pancreatic cancer. Oncogene 2025, 44, 4114–4129. [Google Scholar] [CrossRef] [PubMed]

| Clinicopathological Parameter | Number of Cases (Proportion) |
|---|---|
| Sex | |
| Male | 36 (59.0%) |
| Female | 25 (41.0%) |
| Age at diagnosis | |
| <60 | 13 (21.3%) |
| ≥60 | 48 (78.7%) |
| Smoking history | |
| Yes | 26 (42.6%) |
| No | 35 (53.4%) |
| Histological type | |
| Adenocacinoma | 41 (67.2%) |
| Squamous cell carcinoma | 15 (24.6%) |
| Others | 5 (8.2%) |
| TNM Stage | |
| I | 18 (29.6%) |
| II | 11 (18.0%) |
| III | 24 (39.3%) |
| IV | 8 (13.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zhao, Y.; Zhen, S.; Li, X.; Chen, X.; Zhang, X.; Zhao, X.; Liu, L. Modulation of Cancer-Associated Fibroblasts via the miR-624-5p/FAP Axis Drives Progression and Metastasis in Non-Small Cell Lung Cancer. Cancers 2026, 18, 279. https://doi.org/10.3390/cancers18020279
Zhao Y, Zhen S, Li X, Chen X, Zhang X, Zhao X, Liu L. Modulation of Cancer-Associated Fibroblasts via the miR-624-5p/FAP Axis Drives Progression and Metastasis in Non-Small Cell Lung Cancer. Cancers. 2026; 18(2):279. https://doi.org/10.3390/cancers18020279
Chicago/Turabian StyleZhao, Yan, Shuman Zhen, Xiaoxu Li, Xiaolin Chen, Xue Zhang, Xinming Zhao, and Lihua Liu. 2026. "Modulation of Cancer-Associated Fibroblasts via the miR-624-5p/FAP Axis Drives Progression and Metastasis in Non-Small Cell Lung Cancer" Cancers 18, no. 2: 279. https://doi.org/10.3390/cancers18020279
APA StyleZhao, Y., Zhen, S., Li, X., Chen, X., Zhang, X., Zhao, X., & Liu, L. (2026). Modulation of Cancer-Associated Fibroblasts via the miR-624-5p/FAP Axis Drives Progression and Metastasis in Non-Small Cell Lung Cancer. Cancers, 18(2), 279. https://doi.org/10.3390/cancers18020279






