Cellular Senescence as a Risk Factor in Head and Neck Cancer—Diagnostic and Therapeutic Perspective
Simple Summary
Abstract
1. Introduction
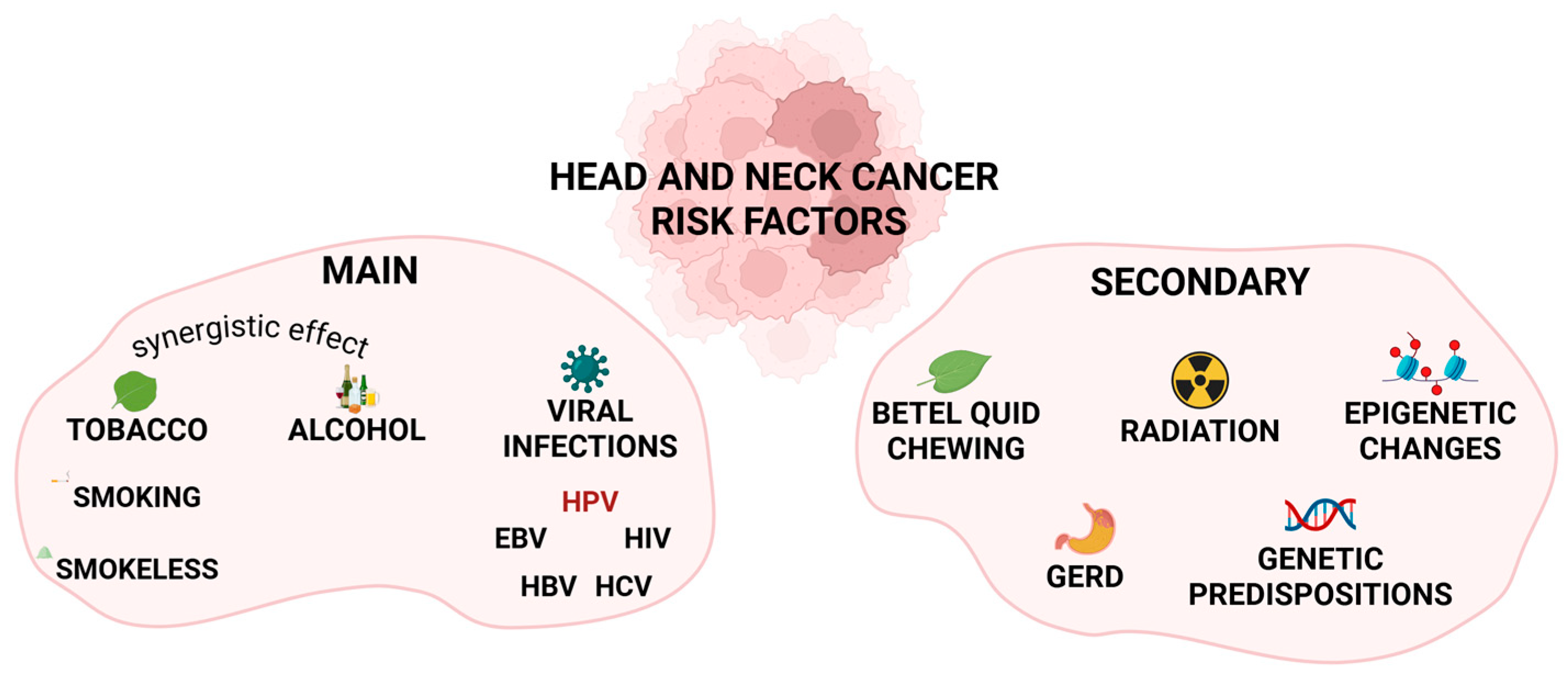
2. HNC Risk Factors
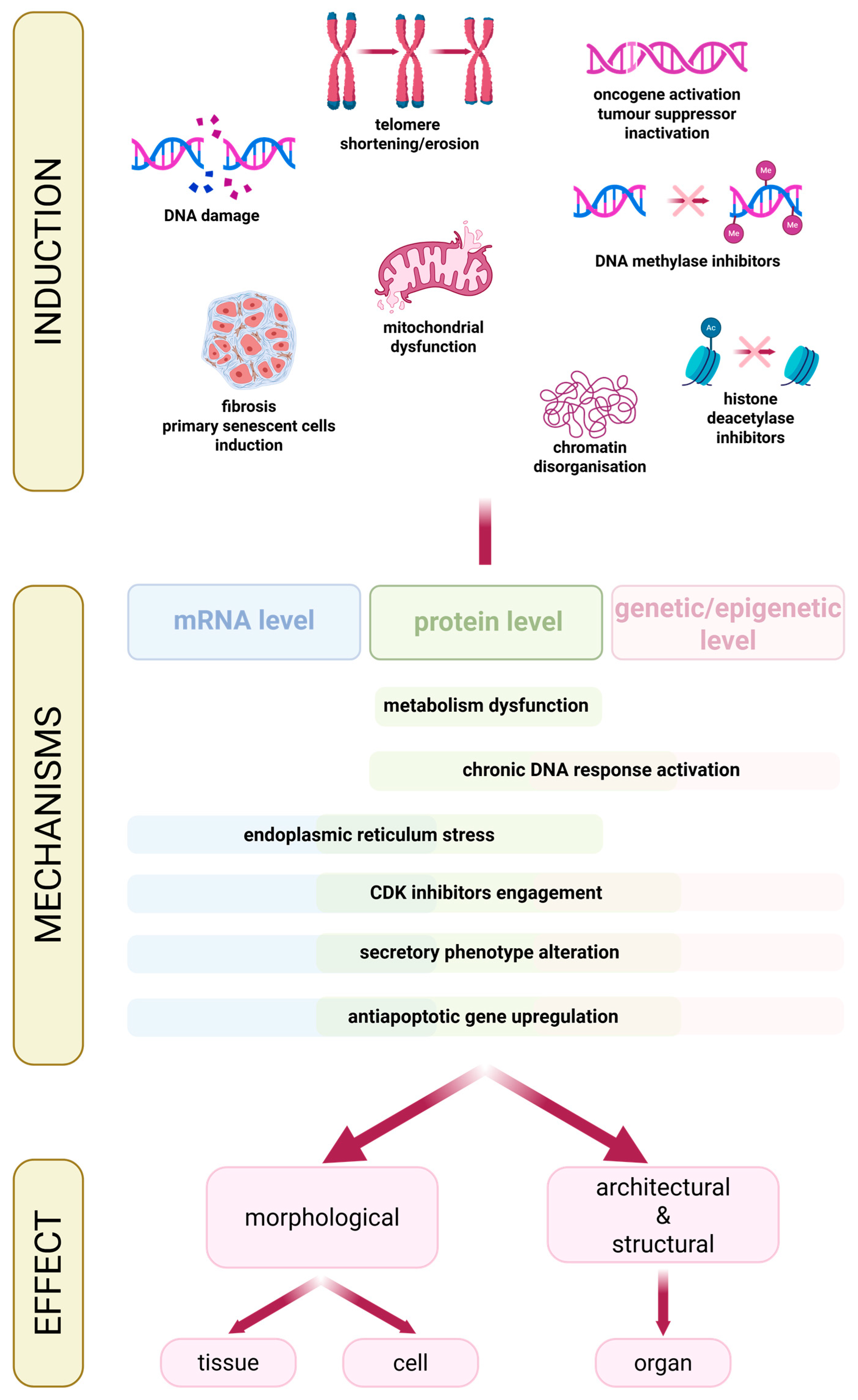
3. Cellular Senescence
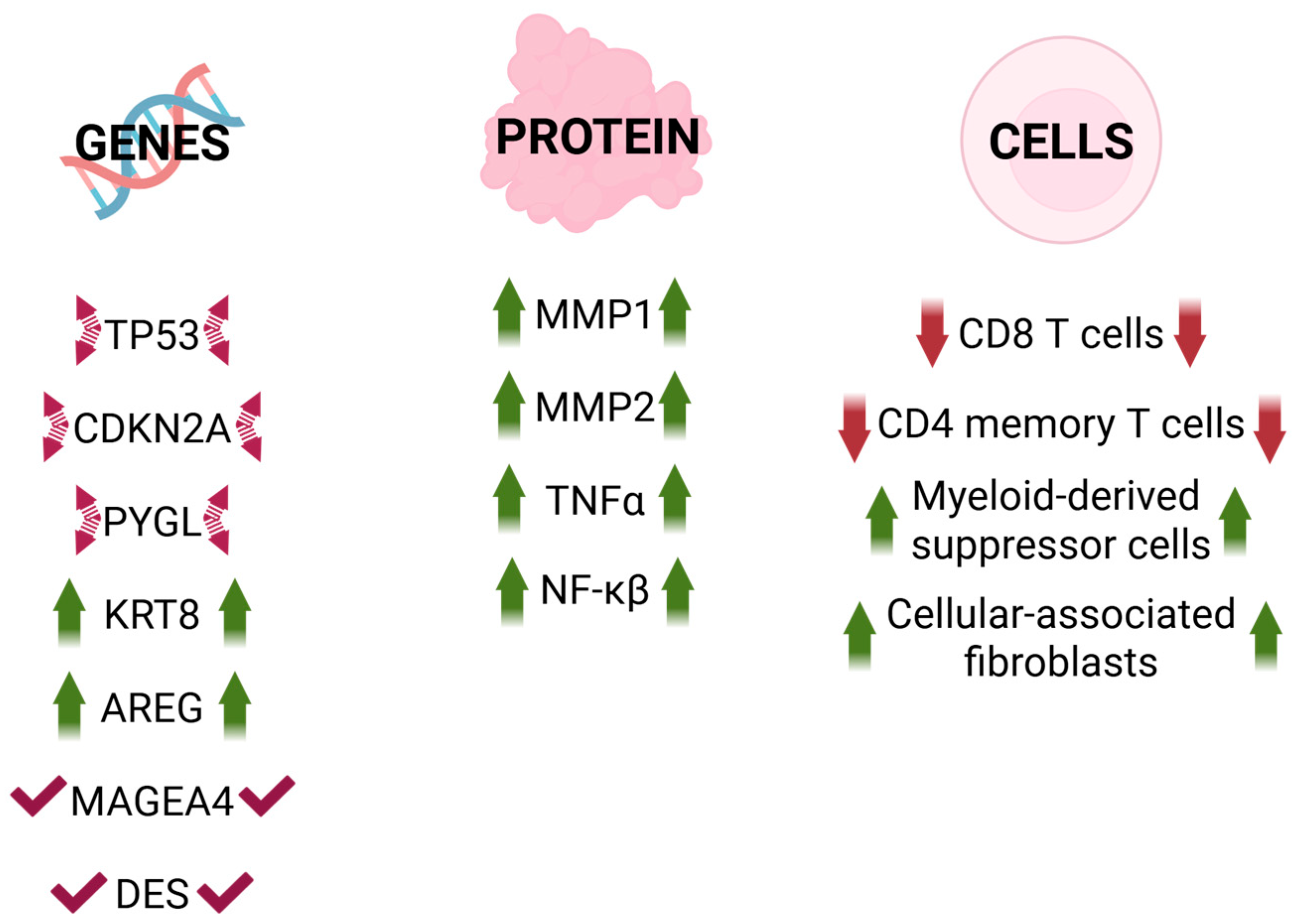
4. Senescence in Head and Neck Cancer
- -
- -
- KRT8 (Keratin 8): primary component of the intermediate filament cytoskeleton mainly in simple epithelial tissues. It is frequently dysregulated during cancer progression and metastasis. Overexpression enhances cell proliferation and migration in gastric and lung cancers, whereas reduced expression significantly inhibits cell proliferation, migration, and epithelial–mesenchymal transition (EMT). Additionally, it is identified as a pan-cancer early biomarker [70,75,76,77].
- -
- AREG (Amphiregulin): epidermal growth factor receptor (EGFR) ligand. It plays a critical role in several aspects of cancerogenesis, including cancer cell growth, invasion, metastasis, angiogenesis, and resistance to apoptosis. It is considered a critical component of the signaling pathways that drive the induction of senescence [70,78,79].
- -
- MAGEA4: a member of the melanoma-associated antigen (MAGE) family, highly expressed in various tumor tissues but exhibiting low levels in normal tissues (excluding testis and placenta). High expression of MAGEA4 is associated with poor outcomes in cancer [80].
- -
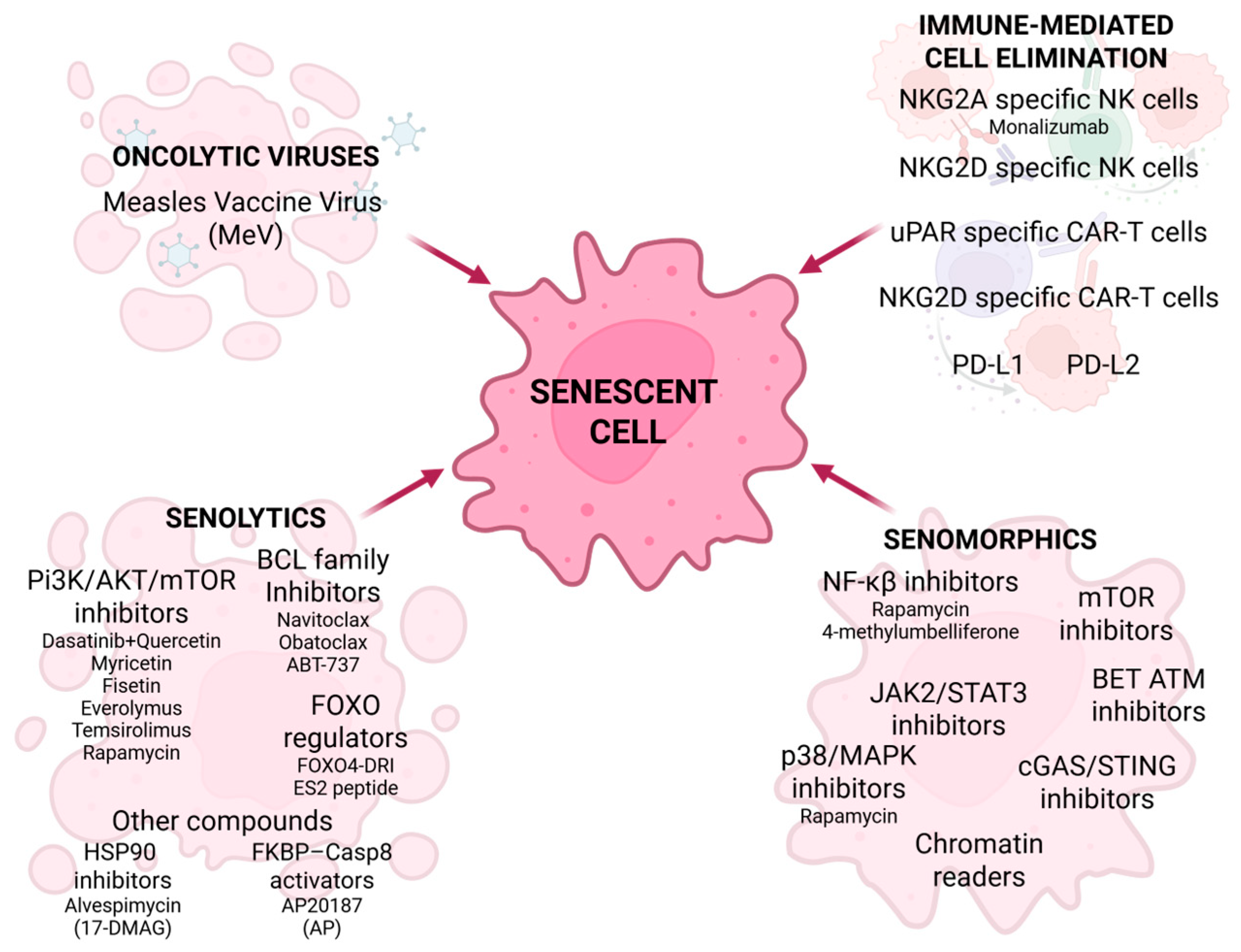
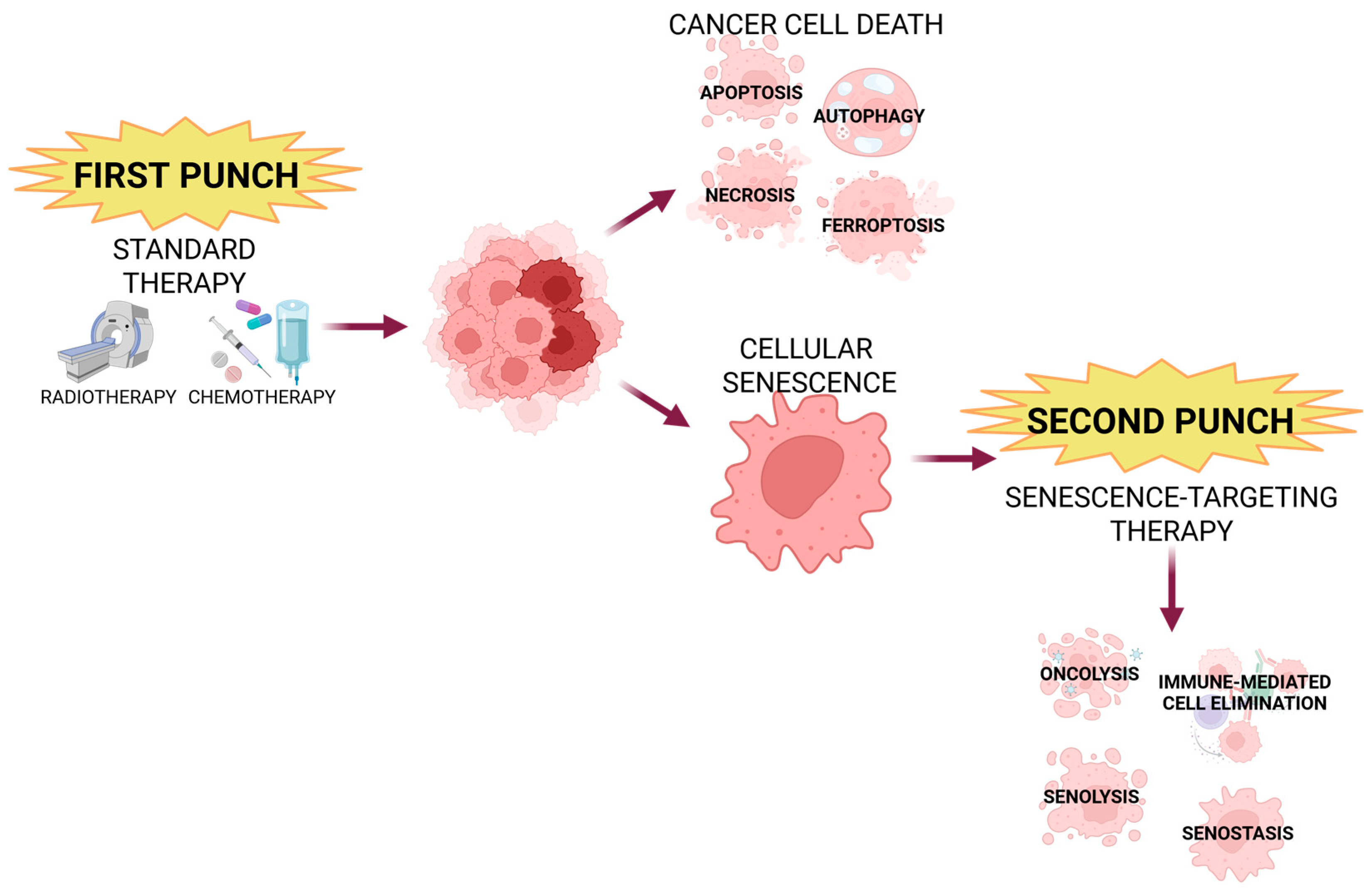
5. Current Senescence-Associated Cancer Treatments
6. Senotherapy in HNC-Clinical Trials, Limitations, and Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.D.; Grandis, J.R. The Molecular Pathogenesis of Head and Neck Cancer. Cancer Biol. Ther. 2010, 9, 1–7. [Google Scholar] [CrossRef]
- Luo, J.; Sun, T.; Liu, Z.; Liu, Y.; Liu, J.; Wang, S.; Shi, X.; Zhou, H. Persistent Accumulation of Therapy-Induced Senescent Cells: An Obstacle to Long-Term Cancer Treatment Efficacy. Int. J. Oral Sci. 2025, 17, 59. [Google Scholar] [CrossRef]
- Wyss, A.B.; Hashibe, M.; Lee, Y.-C.A.; Chuang, S.-C.; Muscat, J.; Chen, C.; Schwartz, S.M.; Smith, E.; Zhang, Z.-F.; Morgenstern, H.; et al. Smokeless Tobacco Use and the Risk of Head and Neck Cancer: Pooled Analysis of US Studies in the INHANCE Consortium. Am. J. Epidemiol. 2016, 184, 703–716. [Google Scholar] [CrossRef]
- Janbaz, K.H.; Qadir, M.I.; Basser, H.T.; Bokhari, T.H.; Ahmad, B. Review Risk for Oral Cancer from Smokeless Tobacco. Wspolczesna Onkol. Oncol. 2014, 3, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Di Credico, G.; Edefonti, V.; Polesel, J.; Pauli, F.; Torelli, N.; Serraino, D.; Negri, E.; Luce, D.; Stucker, I.; Matsuo, K.; et al. Joint Effects of Intensity and Duration of Cigarette Smoking on the Risk of Head and Neck Cancer: A Bivariate Spline Model Approach. Oral Oncol. 2019, 94, 47–57. [Google Scholar] [CrossRef]
- Gislon, L.C.; Curado, M.P.; López, R.V.M.; De Oliveira, J.C.; Vasconcelos De Podestá, J.R.; Ventorin Von Zeidler, S.; Brennan, P.; Kowalski, L.P. Risk Factors Associated with Head and Neck Cancer in Former Smokers: A Brazilian Multicentric Study. Cancer Epidemiol. 2022, 78, 102143. [Google Scholar] [CrossRef]
- Stanfill, S.B.; Connolly, G.N.; Zhang, L.; Jia, L.T.; Henningfield, J.E.; Richter, P.; Lawler, T.S.; Ayo-Yusuf, O.A.; Ashley, D.L.; Watson, C.H. Global Surveillance of Oral Tobacco Products: Total Nicotine, Unionised Nicotine and Tobacco-Specific N-Nitrosamines. Tob. Control 2011, 20, e2. [Google Scholar] [CrossRef] [PubMed]
- Ji, E.H.; Sun, B.; Zhao, T.; Shu, S.; Chang, C.H.; Messadi, D.; Xia, T.; Zhu, Y.; Hu, S. Characterization of Electronic Cigarette Aerosol and Its Induction of Oxidative Stress Response in Oral Keratinocytes. PLoS ONE 2016, 11, e0154447. [Google Scholar] [CrossRef]
- Sancilio, S.; Gallorini, M.; Cataldi, A.; Di Giacomo, V. Cytotoxicity and Apoptosis Induction by E-Cigarette Fluids in Human Gingival Fibroblasts. Clin. Oral Investig. 2016, 20, 477–483. [Google Scholar] [CrossRef]
- Ganapathy, V.; Manyanga, J.; Brame, L.; McGuire, D.; Sadhasivam, B.; Floyd, E.; Rubenstein, D.A.; Ramachandran, I.; Wagener, T.; Queimado, L. Electronic Cigarette Aerosols Suppress Cellular Antioxidant Defenses and Induce Significant Oxidative DNA Damage. PLoS ONE 2017, 12, e0177780. [Google Scholar] [CrossRef]
- Robin, H.P.; Trudeau, C.N.; Robbins, A.J.; Chung, E.J.; Rahman, E.; Strickland, O.L.G.; Jordan, S.; Licari, F.W.; Winden, D.R.; Reynolds, P.R.; et al. Inflammation and Invasion in Oral Squamous Cell Carcinoma Cells Exposed to Electronic Cigarette Vapor Extract. Front. Oncol. 2022, 12, 917862. [Google Scholar] [CrossRef]
- Tsai, K.Y.F.; Hirschi Budge, K.M.; Lepre, A.P.; Rhees, M.S.; Ajdaharian, J.; Geiler, J.; Epperson, D.G.; Astle, K.J.; Winden, D.R.; Arroyo, J.A.; et al. Cell Invasion, RAGE Expression, and Inflammation in Oral Squamous Cell Carcinoma (OSCC) Cells Exposed to E-cigarette Flavoring. Clin. Exp. Dent. Res. 2020, 6, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.; Rahimy, M.; Korrapati, A.; Xuan, Y.; Zou, A.E.; Krishnan, A.R.; Tsui, T.; Aguilera, J.A.; Advani, S.; Crotty Alexander, L.E.; et al. Electronic Cigarettes Induce DNA Strand Breaks and Cell Death Independently of Nicotine in Cell Lines. Oral Oncol. 2016, 52, 58–65. [Google Scholar] [CrossRef]
- Tommasi, S.; Caliri, A.W.; Caceres, A.; Moreno, D.E.; Li, M.; Chen, Y.; Siegmund, K.D.; Besaratinia, A. Deregulation of Biologically Significant Genes and Associated Molecular Pathways in the Oral Epithelium of Electronic Cigarette Users. Int. J. Mol. Sci. 2019, 20, 738. [Google Scholar] [CrossRef]
- Manyanga, J.; Ganapathy, V.; Bouharati, C.; Mehta, T.; Sadhasivam, B.; Acharya, P.; Zhao, D.; Queimado, L. Electronic Cigarette Aerosols Alter the Expression of Cisplatin Transporters and Increase Drug Resistance in Oral Cancer Cells. Sci. Rep. 2021, 11, 1821. [Google Scholar] [CrossRef]
- Kundu, A.; Sachdeva, K.; Feore, A.; Sanchez, S.; Sutton, M.; Seth, S.; Schwartz, R.; Chaiton, M. Evidence Update on the Cancer Risk of Vaping E-Cigarettes: A Systematic Review. Tob. Induc. Dis. 2025, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Dal Maso, L.; Torelli, N.; Biancotto, E.; Di Maso, M.; Gini, A.; Franchin, G.; Levi, F.; La Vecchia, C.; Serraino, D.; Polesel, J. Combined Effect of Tobacco Smoking and Alcohol Drinking in the Risk of Head and Neck Cancers: A Re-Analysis of Case–Control Studies Using Bi-Dimensional Spline Models. Eur. J. Epidemiol. 2016, 31, 385–393. [Google Scholar] [CrossRef]
- Wang, M.; McIntee, E.J.; Cheng, G.; Shi, Y.; Villalta, P.W.; Hecht, S.S. Identification of DNA Adducts of Acetaldehyde. Chem. Res. Toxicol. 2000, 13, 1149–1157. [Google Scholar] [CrossRef]
- Scoccianti, C.; Cecchini, M.; Anderson, A.S.; Berrino, F.; Boutron-Ruault, M.-C.; Espina, C.; Key, T.J.; Leitzmann, M.; Norat, T.; Powers, H.; et al. European Code against Cancer 4th Edition: Alcohol Drinking and Cancer. Cancer Epidemiol. 2015, 39, S67–S74. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.Y.; Han, K.; Shin, D.W.; Yoo, J.E.; Cho, M.H.; Jeon, K.H.; Kim, D.; Hong, S.; Jun, J.K. Alcohol Drinking Pattern and Risk of Head and Neck Cancer: A Nationwide Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 11204. [Google Scholar] [CrossRef]
- Fujioka, K.; Shibamoto, T. Determination of Toxic Carbonyl Compounds in Cigarette Smoke. Environ. Toxicol. 2006, 21, 47–54. [Google Scholar] [CrossRef]
- Launoy, G.; Milan, C.; Faivre, J.; Pienkowski, P.; Gignoux, M. Tobacco Type and Risk of Squamous Cell Cancer of the Oesophagus in Males: A French Multicentre Case-Control Study. Int. J. Epidemiol. 2000, 29, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Di Credico, G.; Polesel, J.; Dal Maso, L.; Pauli, F.; Torelli, N.; Luce, D.; Radoï, L.; Matsuo, K.; Serraino, D.; Brennan, P.; et al. Alcohol Drinking and Head and Neck Cancer Risk: The Joint Effect of Intensity and Duration. Br. J. Cancer 2020, 123, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Reports of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2014.
- Al-Soneidar, W.A.; Harper, S.; Madathil, S.A.; Schlecht, N.F.; Nicolau, B. Do Cutaneous Human Papillomavirus Genotypes Affect Head and Neck Cancer? Evidence and Bias-Correction from a Case-Control Study. Cancer Epidemiol. 2022, 79, 102205. [Google Scholar] [CrossRef]
- Smith, E.M.; Ritchie, J.M.; Pawlita, M.; Rubenstein, L.M.; Haugen, T.H.; Turek, L.P.; Hamsikova, E. Human Papillomavirus Seropositivity and Risks of Head and Neck Cancer. Int. J. Cancer 2007, 120, 825–832. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Anderson, W.F.; Gillison, M.L. Incidence Trends for Human Papillomavirus–Related and –Unrelated Oral Squamous Cell Carcinomas in the United States. J. Clin. Oncol. 2008, 26, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef]
- Gupta, I.; Ghabreau, L.; Al-Thawadi, H.; Yasmeen, A.; Vranic, S.; Al Moustafa, A.-E.; Malki, M.I. Co-Incidence of Human Papillomaviruses and Epstein–Barr Virus Is Associated With High to Intermediate Tumor Grade in Human Head and Neck Cancer in Syria. Front. Oncol. 2020, 10, 1016. [Google Scholar] [CrossRef]
- Heawchaiyaphum, C.; Malat, P.; Pientong, C.; Roytrakul, S.; Yingchutrakul, Y.; Aromseree, S.; Suebsasana, S.; Mahalapbutr, P.; Ekalaksananan, T. The Dual Functions of Andrographolide in the Epstein–Barr Virus-Positive Head-and-Neck Cancer Cells: The Inhibition of Lytic Reactivation of the Epstein–Barr Virus and the Induction of Cell Death. Int. J. Mol. Sci. 2023, 24, 15867. [Google Scholar] [CrossRef]
- Hung, S.-H.; Yang, T.-H.; Cheng, Y.-F.; Chen, C.-S.; Lin, H.-C. Associations of Head and Neck Cancer with Hepatitis B Virus and Hepatitis C Virus Infection. Cancers 2023, 15, 4510. [Google Scholar] [CrossRef] [PubMed]
- Dhull, A.K.; Atri, R.; Dhankhar, R.; Chauhan, A.K.; Kaushal, V. Major Risk Factors in Head and Neck Cancer: A Retrospective Analysis of 12-Year Experiences. World J. Oncol. 2018, 9, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Carioli, G.; Santucci, C.; Bertuccio, P.; Gallus, S.; Garavello, W.; Negri, E.; La Vecchia, C. Global Trends in Oral and Pharyngeal Cancer Incidence and Mortality. Int. J. Cancer 2020, 147, 1040–1049. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Tail, Y.-H.; Wang, W.-C.; Chen, C.-Y.; Kao, Y.-H.; Chen, Y.-K.; Chen, C.-H. Malignant Transformation in 5071 Southern Taiwanese Patients with Potentially Malignant Oral Mucosal Disorders. BMC Oral Health 2014, 14, 99. [Google Scholar] [CrossRef]
- Eells, A.C.; Mackintosh, C.; Marks, L.; Marino, M.J. Gastroesophageal Reflux Disease and Head and Neck Cancers: A Systematic Review and Meta-Analysis. Am. J. Otolaryngol. 2020, 41, 102653. [Google Scholar] [CrossRef]
- Liao, Y.; Hsu, C.; Leu, C.; Lai, S.; Huang, Y.; Hsieh, M.; Chen, T.; Chen, C.; Wang, C.; Yang, T.; et al. Radiation-induced Sarcoma of Head and Neck: Clinical Characteristics and Molecular Signatures. Head Neck 2023, 45, 638–646. [Google Scholar] [CrossRef]
- He, Y.; Ji, P.; Li, Y.; Wang, R.; Ma, H.; Yuan, H. Genetic Variants Were Associated With the Prognosis of Head and Neck Squamous Carcinoma. Front. Oncol. 2020, 10, 372. [Google Scholar] [CrossRef]
- Scully, C.; Field, J.K.; Tanzawa, H. Genetic Aberrations in Oral or Head and Neck Squamous Cell Carcinoma (SCCHN): 1. Carcinogen Metabolism, DNA Repair and Cell Cycle Control. Oral Oncol. 2000, 36, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Agents Classified by the IARC Monographs, Volumes 1–137. Available online: https://monographs.iarc.who.int/agents-classified-by-the-iarc (accessed on 5 December 2024).
- d’Adda di Fagagna, F.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA Damage Checkpoint Response in Telomere-Initiated Senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef]
- Stein, G.H.; Drullinger, L.F.; Soulard, A.; Dulić, V. Differential Roles for Cyclin-Dependent Kinase Inhibitors P21 and P16 in the Mechanisms of Senescence and Differentiation in Human Fibroblasts. Mol. Cell. Biol. 1999, 19, 2109–2117. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Sun, Y.; Coppé, J.-P.; Lam, E.W.-F. Cellular Senescence: The Sought or the Unwanted? Trends Mol. Med. 2018, 24, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular Senescence and Senolytics: The Path to the Clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Ungvari, Z.; Ungvari, A.; Fekete, M.; Kiss, C.; Győrffy, B. Senescence-Related Genes as Prognostic Indicators in Breast Cancer Survival. GeroScience 2024, 47, 2995–3006. [Google Scholar] [CrossRef] [PubMed]
- Lehoczki, A.; Menyhart, O.; Andrikovics, H.; Fekete, M.; Kiss, C.; Mikala, G.; Ungvari, Z.; Győrffy, B. Prognostic Impact of a Senescence Gene Signature in Multiple Myeloma. GeroScience 2025, 47, 5025–5037. [Google Scholar] [CrossRef]
- Cummings, S.R.; Lui, L.-Y.; Zaira, A.; Mau, T.; Fielding, R.A.; Atkinson, E.J.; Patel, S.; LeBrasseur, N. Biomarkers of Cellular Senescence and Major Health Outcomes in Older Adults. GeroScience 2024, 47, 3407–3415. [Google Scholar] [CrossRef] [PubMed]
- Shaban, H.A.; Gasser, S.M. Dynamic 3D Genome Reorganization during Senescence: Defining Cell States through Chromatin. Cell Death Differ. 2025, 32, 9–15. [Google Scholar] [CrossRef]
- Krtolica, A.; Parrinello, S.; Lockett, S.; Desprez, P.-Y.; Campisi, J. Senescent Fibroblasts Promote Epithelial Cell Growth and Tumorigenesis: A Link between Cancer and Aging. Proc. Natl. Acad. Sci. USA 2001, 98, 12072–12077. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Zhu, Z.-Y.; Ma, T.; Zhang, L.; Chen, J.; Jiang, J.-W.; Lu, C.-H.; Ding, Y.-T.; Guan, W.-X.; Yi, N.; et al. TP53 Mutation-Related Senescence Is an Indicator of Hepatocellular Carcinoma Patient Outcomes from Multiomics Profiles. Smart Med. 2023, 2, e20230005. [Google Scholar] [CrossRef]
- Morton, J.P.; Timpson, P.; Karim, S.A.; Ridgway, R.A.; Athineos, D.; Doyle, B.; Jamieson, N.B.; Oien, K.A.; Lowy, A.M.; Brunton, V.G.; et al. Mutant P53 Drives Metastasis and Overcomes Growth Arrest/Senescence in Pancreatic Cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 246–251. [Google Scholar] [CrossRef]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Däbritz, J.H.M.; Zhao, Z.; Yu, Y.; Dörr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-Associated Reprogramming Promotes Cancer Stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhu, Y.; Nandi, S.P.; Lee, M.; Watari, K.; Bareng, B.; Ohira, M.; Liu, Y.; Sakane, S.; Carlessi, R.; et al. FBP1 Controls Liver Cancer Evolution from Senescent MASH Hepatocytes. Nature 2025, 637, 461–469. [Google Scholar] [CrossRef]
- Toussaint, O.; Dumont, P.; Remacle, J.; Dierick, J.-F.; Pascal, T.; Frippiat, C.; Magalhaes, J.P.; Zdanov, S.; Chainiaux, F. Stress-Induced Premature Senescence or Stress-Induced Senescence-Like Phenotype: One In Vivo Reality, Two Possible Definitions? Sci. World J. 2002, 2, 575013. [Google Scholar] [CrossRef]
- Di Micco, R.; Fumagalli, M.; Cicalese, A.; Piccinin, S.; Gasparini, P.; Luise, C.; Schurra, C.; Garre’, M.; Giovanni Nuciforo, P.; Bensimon, A.; et al. Oncogene-Induced Senescence Is a DNA Damage Response Triggered by DNA Hyper-Replication. Nature 2006, 444, 638–642. [Google Scholar] [CrossRef]
- Herr, L.M.; Schaffer, E.D.; Fuchs, K.F.; Datta, A.; Brosh, R.M. Replication Stress as a Driver of Cellular Senescence and Aging. Commun. Biol. 2024, 7, 616. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic Ras. Provokes Premature Cell Senescence Associated with Accumulation of P53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Dulić, V.; Beney, G.-E.; Frebourg, G.; Drullinger, L.F.; Stein, G.H. Uncoupling between Phenotypic Senescence and Cell Cycle Arrest in Aging P21-Deficient Fibroblasts. Mol. Cell. Biol. 2000, 20, 6741–6754. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Y.; Xu, C.; Liang, Y.; Zhao, Y.; He, Q.; Li, J.; Chen, K.; Qiao, H.; Liu, N.; et al. Chemotherapy-Induced Senescence Reprogramming Promotes Nasopharyngeal Carcinoma Metastasis by circRNA-Mediated PKR Activation. Adv. Sci. 2023, 10, 2205668. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Fuhrmann-Stroissnigg, H.; Dai, H.M.; Ling, Y.Y.; Stout, M.B.; Pirtskhalava, T.; Giorgadze, N.; Johnson, K.O.; Giles, C.B.; et al. Identification of a Novel Senolytic Agent, Navitoclax, Targeting the Bcl-2 Family of Anti-Apoptotic Factors. Aging Cell 2016, 15, 428–435. [Google Scholar] [CrossRef]
- Schoetz, U.; Klein, D.; Hess, J.; Shnayien, S.; Spoerl, S.; Orth, M.; Mutlu, S.; Hennel, R.; Sieber, A.; Ganswindt, U.; et al. Early Senescence and Production of Senescence-Associated Cytokines Are Major Determinants of Radioresistance in Head-and-Neck Squamous Cell Carcinoma. Cell Death Dis. 2021, 12, 1162. [Google Scholar] [CrossRef]
- Ostrowska, K.; Niewinski, P.; Piotrowski, I.; Ostapowicz, J.; Koczot, S.; Suchorska, W.M.; Golusiński, P.; Masternak, M.M.; Golusiński, W. Senescence in Head and Neck Squamous Cell Carcinoma: Relationship between Senescence-Associated Secretory Phenotype (SASP) mRNA Expression Level and Clinicopathological Features. Clin. Transl. Oncol. 2024, 26, 1022–1032. [Google Scholar] [CrossRef]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-Associated Fibroblasts Induce Epithelial–Mesenchymal Transition of Bladder Cancer Cells through Paracrine IL-6 Signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef]
- He, Y.; Qiu, Y.; Yang, X.; Lu, G.; Zhao, S.-S. Remodeling of Tumor Microenvironment by Cellular Senescence and Immunosenescence in Cervical Cancer. Semin. Cancer Biol. 2025, 108, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; Fridman, J.S.; Yang, M.; Lee, S.; Baranov, E.; Hoffman, R.M.; Lowe, S.W. A Senescence Program Controlled by P53 and p16INK4a Contributes to the Outcome of Cancer Therapy. Cell 2002, 109, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Domen, A.; Deben, C.; Verswyvel, J.; Flieswasser, T.; Prenen, H.; Peeters, M.; Lardon, F.; Wouters, A. Cellular Senescence in Cancer: Clinical Detection and Prognostic Implications. J. Exp. Clin. Cancer Res. 2022, 41, 360. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.; Gan, R.-H.; Yuan, S.; Lan, T.; Zheng, D.; Lu, Y.-G. IL-8 Activates Fibroblasts to Promote the Invasion of HNSCC Cells via STAT3-MMP1. Cell Death Discov. 2024, 10, 65. [Google Scholar] [CrossRef]
- Lee, Y.C.; Nam, Y.; Kim, M.; Kim, S.I.; Lee, J.-W.; Eun, Y.-G.; Kim, D. Prognostic Significance of Senescence Related Tumor Microenvironment Genes in Head and Neck Squamous Cell Carcinoma. Aging 2023, 16, 985–1001. [Google Scholar] [CrossRef]
- Deng, L.; Mi, J.; Ruan, X.; Zhang, G.; Pan, Y.; Wang, R. Identification and Analysis of Senescence-Related Genes in Head and Neck Squamous Cell Carcinoma by a Comprehensive Bioinformatics Approach. Mediat. Inflamm. 2022, 2022, 4007469. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, M.; Zanoni, M.; Pirini, F.; Tumedei, M.M.; Ravaioli, S.; Rapposelli, I.G.; Frassineti, G.L.; Bravaccini, S. Pancreatic Cancer and Cellular Senescence: Tumor Microenvironment under the Spotlight. Int. J. Mol. Sci. 2021, 23, 254. [Google Scholar] [CrossRef]
- Hagiwara, Y.; McGhee, J.R.; Fujihashi, K.; Kobayashi, R.; Yoshino, N.; Kataoka, K.; Etani, Y.; Kweon, M.-N.; Tamura, S.; Kurata, T.; et al. Protective Mucosal Immunity in Aging Is Associated with Functional CD4+ T Cells in Nasopharyngeal-Associated Lymphoreticular Tissue1. J. Immunol. 2003, 170, 1754–1762. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, H.; Xu, G.; Li, X.; Chen, W.; Lun, Y.; Zhang, J. Comprehensive Analysis of Cellular Senescence and Immune Microenvironment in Papillary Thyroid Carcinoma. Aging 2024, 16, 2866–2886. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, X.; Yi, S.; Liu, G.; Liu, Y.; Ling, Y. Six Glycolysis-Related Genes as Prognostic Risk Markers Can Predict the Prognosis of Patients with Head and Neck Squamous Cell Carcinoma. Biomed. Res. Int. 2021, 2021, 8824195. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Pan, B.; Zheng, C.; Hong, L.; Han, W. KRT8 Serves as a Novel Biomarker for LUAD and Promotes Metastasis and EMT via NF-κB Signaling. Front. Oncol. 2022, 12, 875146. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Liu, Y.; Ding, F.; Ni, Y.; Shao, S. High KRT8 Expression Promotes Tumor Progression and Metastasis of Gastric Cancer. Cancer Sci. 2017, 108, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.K.D.; Limaye, M.; Schaffert, S.; West, R.; Ozawa, M.G.; Chu, P.; Nair, V.S.; Koong, A.C.; Khatri, P. A Multi-Scale Integrated Analysis Identifies KRT8 as a Pan-Cancer Early Biomarker. Pac. Symp. Biocomput. 2021, 26, 297–308. [Google Scholar]
- Pommer, M.; Kuphal, S.; Bosserhoff, A.K.; Pommer, M.; Kuphal, S.; Bosserhoff, A.K. Amphiregulin Regulates Melanocytic Senescence. Cells 2021, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Busser, B.; Sancey, L.; Brambilla, E.; Coll, J.-L.; Hurbin, A. The Multiple Roles of Amphiregulin in Human Cancer. Biochim. Biophys. Acta 2011, 1816, 119–131. [Google Scholar] [CrossRef]
- Zhu, W.; Yi, Q.; Chen, Z.; Wang, J.; Zhong, K.; Ouyang, X.; Yang, K.; Jiang, B.; Zhong, J.; Zhong, J. Exploring the Role and Mechanisms of MAGEA4 in Tumorigenesis, Regulation, and Immunotherapy. Mol. Med. 2025, 31, 43. [Google Scholar] [CrossRef]
- Paulin, D.; Li, Z. Desmin: A Major Intermediate Filament Protein Essential for the Structural Integrity and Function of Muscle. Exp. Cell Res. 2004, 301, 1–7. [Google Scholar] [CrossRef]
- Rajabian, N.; Choudhury, D.; Ikhapoh, I.; Saha, S.; Kalyankar, A.S.; Mehrotra, P.; Shahini, A.; Breed, K.; Andreadis, S.T. Reversine Ameliorates Hallmarks of Cellular Senescence in Human Skeletal Myoblasts via Reactivation of Autophagy. Aging Cell 2023, 22, e13764. [Google Scholar] [CrossRef]
- Lee, M.K.; Woo, S.R.; Noh, J.K.; Min, S.; Kong, M.; Lee, Y.C.; Ko, S.-G.; Eun, Y.-G. Prognostic Significance of SASP-Related Gene Signature of Radiation Therapy in Head and Neck Squamous Cell Carcinoma. Mol. Cancer Ther. 2024, 23, 1348–1359. [Google Scholar] [CrossRef]
- Hassona, Y.; Cirillo, N.; Heesom, K.; Parkinson, E.K.; Prime, S.S. Senescent Cancer-Associated Fibroblasts Secrete Active MMP-2 That Promotes Keratinocyte Dis-Cohesion and Invasion. Br. J. Cancer 2014, 111, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, E.K.; James, E.L.; Prime, S.S. Senescence-Derived Extracellular Molecules as Modulators of Oral Cancer Development: A Mini-Review. Gerontology 2016, 62, 417–424. [Google Scholar] [CrossRef]
- Hassona, Y.; Cirillo, N.; Lim, K.P.; Herman, A.; Mellone, M.; Thomas, G.J.; Pitiyage, G.N.; Parkinson, E.K.; Prime, S.S. Progression of Genotype-Specific Oral Cancer Leads to Senescence of Cancer-Associated Fibroblasts and Is Mediated by Oxidative Stress and TGF-β. Carcinogenesis 2013, 34, 1286–1295. [Google Scholar] [CrossRef]
- Prime, S.S.; Cirillo, N.; Hassona, Y.; Lambert, D.W.; Paterson, I.C.; Mellone, M.; Thomas, G.J.; James, E.N.L.; Parkinson, E.K. Fibroblast Activation and Senescence in Oral Cancer. J. Oral Pathol. Med. 2017, 46, 82–88. [Google Scholar] [CrossRef]
- Dańczak-Pazdrowska, A.; Gornowicz-Porowska, J.; Polańska, A.; Krajka-Kuźniak, V.; Stawny, M.; Gostyńska, A.; Rubiś, B.; Nourredine, S.; Ashiqueali, S.; Schneider, A.; et al. Cellular Senescence in Skin-related Research: Targeted Signaling Pathways and Naturally Occurring Therapeutic Agents. Aging Cell 2023, 22, e13845. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; González-Maroto, C.; Tavassoli, M. Crosstalk between CAFs and Tumour Cells in Head and Neck Cancer. Cell Death Discov. 2024, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, C.; Sun, H.; Li, Q.; Hu, J.; Jiang, T.; Zhou, S. Identification of Several Senescence-associated Genes Signature in Head and Neck Squamous Cell Carcinoma. Clin. Lab. Anal. 2022, 36, e24555. [Google Scholar] [CrossRef]
- Andreikos, D.; Kyrodimos, E.; Kotsinas, A.; Chrysovergis, A.; Papacharalampous, G.X. The Association between Telomere Length and Head and Neck Cancer Risk: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 9000. [Google Scholar] [CrossRef]
- Lagunas, A.M.; Francis, M.; Maniar, N.B.; Nikolova, G.; Wu, J.; Crowe, D.L. Paracrine Interaction of Cancer Stem Cell Populations Is Regulated by the Senescence-Associated Secretory Phenotype (SASP). Mol. Cancer Res. 2019, 17, 1480–1492. [Google Scholar] [CrossRef]
- Chen, L.; Lin, J.; Wen, Y.; Lan, B.; Xiong, J.; Fu, Y.; Chen, Y.; Chen, C. A Senescence-Related lncRNA Signature Predicts Prognosis and Reflects Immune Landscape in HNSCC. Oral Oncol. 2024, 149, 106659. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.; Hameed, Y.; Kiani, M.N.; Aftab, A. Common Features between Aging and Cancer: A Narrative Review. Aging Adv. 2024, 1, 118–134. [Google Scholar] [CrossRef]
- Sieben, C.J.; Sturmlechner, I.; Van De Sluis, B.; Van Deursen, J.M. Two-Step Senescence-Focused Cancer Therapies. Trends Cell Biol. 2018, 28, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, K.; Herbet, M.; Murias, M.; Piątkowska-Chmiel, I. Senolytics: Charting a New Course or Enhancing Existing Anti-Tumor Therapies? Cell. Oncol. 2024, 48, 351–371. [Google Scholar] [CrossRef]
- Zingoni, A.; Antonangeli, F.; Sozzani, S.; Santoni, A.; Cippitelli, M.; Soriani, A. The Senescence Journey in Cancer Immunoediting. Mol. Cancer 2024, 23, 68. [Google Scholar] [CrossRef]
- Meyer, M.; Fourie, C.; Van Der Merwe, H.; Botha, H.; Engelbrecht, A.-M. Targeting Treatment Resistance in Cervical Cancer: A New Avenue for Senolytic Therapies. Adv. Med. Sci. 2025, 70, 33–43. [Google Scholar] [CrossRef]
- Xiao, S.; Qin, D.; Hou, X.; Tian, L.; Yu, Y.; Zhang, R.; Lyu, H.; Guo, D.; Chen, X.-Z.; Zhou, C.; et al. Cellular Senescence: A Double-Edged Sword in Cancer Therapy. Front. Oncol. 2023, 13, 1189015. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic Drugs: From Discovery to Translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Wang, X.; Fukumoto, T.; Noma, K. Therapeutic Strategies Targeting Cellular Senescence for Cancer and Other Diseases. J. Biochem. 2024, 175, 525–537. [Google Scholar] [CrossRef]
- Romesser, P.B.; Lowe, S.W. The Potent and Paradoxical Biology of Cellular Senescence in Cancer. Annu. Rev. Cancer Biol. 2023, 7, 207–228. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Viel, T.A. Potential of Natural Senolytic Compounds in Eliminating Senescent Cells and Alleviating Age-Related Skin Deterioration: A Narrative Review. Aging Adv. 2024, 1, 143–153. [Google Scholar] [CrossRef]
- Chambers, C.R.; Ritchie, S.; Pereira, B.A.; Timpson, P. Overcoming the Senescence-associated Secretory Phenotype (SASP): A Complex Mechanism of Resistance in the Treatment of Cancer. Mol. Oncol. 2021, 15, 3242–3255. [Google Scholar] [CrossRef]
- Wagner, V.; Gil, J. Senescence as a Therapeutically Relevant Response to CDK4/6 Inhibitors. Oncogene 2020, 39, 5165–5176. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Liu, G.-H.; Chao, W.-Y.; Shi, C.-S.; Lin, C.-Y.; Lim, Y.-P.; Lu, C.-H.; Lai, P.-Y.; Chen, H.-R.; Lee, Y.-R. Piperlongumine Suppresses Proliferation of Human Oral Squamous Cell Carcinoma through Cell Cycle Arrest, Apoptosis and Senescence. Int. J. Mol. Sci. 2016, 17, 616. [Google Scholar] [CrossRef] [PubMed]
- Baar, M.P.; Brandt, R.M.C.; Putavet, D.A.; Klein, J.D.D.; Derks, K.W.J.; Bourgeois, B.R.M.; Stryeck, S.; Rijksen, Y.; Van Willigenburg, H.; Feijtel, D.A.; et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 2017, 169, 132–147.e16. [Google Scholar] [CrossRef]
- Xiao, C.; Beitler, J.J.; Peng, G.; Levine, M.E.; Conneely, K.N.; Zhao, H.; Felger, J.C.; Wommack, E.C.; Chico, C.E.; Jeon, S.; et al. Epigenetic Age Acceleration, Fatigue, and Inflammation in Patients Undergoing Radiation Therapy for Head and Neck Cancer: A Longitudinal Study. Cancer 2021, 127, 3361–3371. [Google Scholar] [CrossRef]
- Shahbandi, A.; Rao, S.G.; Anderson, A.Y.; Frey, W.D.; Olayiwola, J.O.; Ungerleider, N.A.; Jackson, J.G. BH3 Mimetics Selectively Eliminate Chemotherapy-Induced Senescent Cells and Improve Response in TP53 Wild-Type Breast Cancer. Cell Death Differ. 2020, 27, 3097–3116. [Google Scholar] [CrossRef]
- Ahmadinejad, F.; Bos, T.; Hu, B.; Britt, E.; Koblinski, J.; Souers, A.J.; Leverson, J.D.; Faber, A.C.; Gewirtz, D.A.; Harada, H. Senolytic-Mediated Elimination of Head and Neck Tumor Cells Induced Into Senescence by Cisplatin. Mol. Pharmacol. 2022, 101, 168–180. [Google Scholar] [CrossRef]
- Kamada, K.; Kurio, N.; Mouri, Y.; Kudo, Y. Combination Treatment with Hyaluronic Acid Synthesis and Bcl-2 Inhibitors Induces Senolytic Elimination of Oral Squamous Cell Carcinoma Cells in Vitro. J. Oral Maxillofac. Surg. Med. Pathol. 2025, 37, 289–296. [Google Scholar] [CrossRef]
- Hu, Q.; Peng, J.; Jiang, L.; Li, W.; Su, Q.; Zhang, J.; Li, H.; Song, M.; Cheng, B.; Xia, J.; et al. Metformin as a Senostatic Drug Enhances the Anticancer Efficacy of CDK4/6 Inhibitor in Head and Neck Squamous Cell Carcinoma. Cell Death Dis. 2020, 11, 925. [Google Scholar] [CrossRef]
- Samaraweera, L.; Adomako, A.; Rodriguez-Gabin, A.; McDaid, H.M. A Novel Indication for Panobinostat as a Senolytic Drug in NSCLC and HNSCC. Sci. Rep. 2017, 7, 1900. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Silva, J.P.N.; Pinto, B.; Monteiro, L.; Silva, P.M.A.; Bousbaa, H. Combination Therapy as a Promising Way to Fight Oral Cancer. Pharmaceutics 2023, 15, 1653. [Google Scholar] [CrossRef]
- Demurtas, S.; Cena, H.; Benazzo, M.; Gabanelli, P.; Porcelli, S.; Preda, L.; Bortolotto, C.; Bertino, G.; Mauramati, S.; Veneroni, M.V.; et al. Head and Neck Cancer (HNC) Prehabilitation: Advantages and Limitations. J. Clin. Med. 2024, 13, 6176. [Google Scholar] [CrossRef]
- Zhang, Y. Senolytics to Improve Physical and Cognitive Function in Older Adults with Multiple Sclerosis; NCBI: Bethesda, MD, USA, 2025.
- Washington University School of Medicine Phase II Clinical Trial to Evaluate the Safety and Feasibility of Senolytic Therapy in Alzheimer’s Disease; clinicaltrials.gov. 2025. Available online: https://www.clinicaltrials.gov/study/NCT04685590 (accessed on 12 December 2025).
- Hickson, L.J. Senescence, Frailty, and Mesenchymal Stem Cell Functionality in Chronic Kidney Disease: Effect of Senolytic Agents; clinicaltrials.gov. 2025. Available online: https://www.clinicaltrials.gov/study/NCT02848131 (accessed on 12 December 2025).
- Stone, A.V. Targeting Senescence to Reduce Osteoarthritis Pain and cartilagE Breakdown (ROPE); NCBI: Bethesda, MD, USA, 2022.
- Khosla, S. Targeting Cellular Senescence With Senolytics to Improve Skeletal Health in Older Humans: A Phase 2, Single-Center, 20-Week, Open-Label, Randomized Controlled Trial; NCBI: Bethesda, MD, USA, 2024.
- Fan, S. Phase II Trial of Tislelizumab, an Anti-PD-1 Monoclonal Antibody, in Combination with Dasatinib and Quercetin, as a Novel Neoadjuvant Pre-Surgical Therapy for Head and Neck Squamous Cell Carcinoma; NCBI: Bethesda, MD, USA, 2024.
- Liu, N.; Wu, J.; Deng, E.; Zhong, J.; Wei, B.; Cai, T.; Xie, Z.; Duan, X.; Fu, S.; Osei-Hwedieh, D.O.; et al. Immunotherapy and Senolytics in Head and Neck Squamous Cell Carcinoma: Phase 2 Trial Results. Nat. Med. 2025, 31, 3047–3061. [Google Scholar] [CrossRef]
- Jun, J.-I.; Lau, L.F. The Matricellular Protein CCN1 Induces Fibroblast Senescence and Restricts Fibrosis in Cutaneous Wound Healing. Nat. Cell Biol. 2010, 12, 676–685. [Google Scholar] [CrossRef]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.-M.; Vijg, J.; Van Steeg, H.; Dollé, M.E.T.; et al. An Essential Role for Senescent Cells in Optimal Wound Healing through Secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef]
- von Joest, M.; Chen, C.; Douché, T.; Chantrel, J.; Chiche, A.; Gianetto, Q.G.; Matondo, M.; Li, H. Amphiregulin Mediates Non-Cell-Autonomous Effect of Senescence on Reprogramming. Cell Rep. 2022, 40, 111074. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Cañamero, M.; Maraver, A.; Gómez-López, G.; Contreras, J.; Murillo-Cuesta, S.; Rodríguez-Baeza, A.; Varela-Nieto, I.; Ruberte, J.; Collado, M.; et al. Programmed Cell Senescence during Mammalian Embryonic Development. Cell 2013, 155, 1104–1118. [Google Scholar] [CrossRef]
- Marin, I.; Boix, O.; Garcia-Garijo, A.; Sirois, I.; Caballe, A.; Zarzuela, E.; Ruano, I.; Attolini, C.S.-O.; Prats, N.; López-Domínguez, J.A.; et al. Cellular Senescence Is Immunogenic and Promotes Antitumor Immunity. Cancer Discov. 2023, 13, 410–431. [Google Scholar] [CrossRef]
- Kang, T.-W.; Yevsa, T.; Woller, N.; Hoenicke, L.; Wuestefeld, T.; Dauch, D.; Hohmeyer, A.; Gereke, M.; Rudalska, R.; Potapova, A.; et al. Senescence Surveillance of Pre-Malignant Hepatocytes Limits Liver Cancer Development. Nature 2011, 479, 547–551. [Google Scholar] [CrossRef]
- Childs, B.G.; Bussian, T.J.; Baker, D.J. Cellular Identification and Quantification of Senescence-Associated β-Galactosidase Activity In Vivo. In Cellular Senescence: Methods and Protocols; Demaria, M., Ed.; Springer: New York, NY, USA, 2019; pp. 31–38. ISBN 978-1-4939-8931-7. [Google Scholar]
- Turinetto, V.; Giachino, C. Multiple Facets of Histone Variant H2AX: A DNA Double-Strand-Break Marker with Several Biological Functions. Nucleic Acids Res. 2015, 43, 2489–2498. [Google Scholar] [CrossRef]
- Evangelou, K.; Gorgoulis, V.G. Sudan Black B, The Specific Histochemical Stain for Lipofuscin: A Novel Method to Detect Senescent Cells. In Oncogene-Induced Senescence: Methods and Protocols; Nikiforov, M.A., Ed.; Springer: New York, NY, USA, 2017; pp. 111–119. ISBN 978-1-4939-6670-7. [Google Scholar]
- Zhou, R.; Xie, X.; Qin, Z.; Li, X.; Liu, J.; Li, H.; Zheng, Q.; Luo, Y. Cytosolic dsDNA Is a Novel Senescence Marker Associated with Pyroptosis Activation. Tissue Cell 2021, 72, 101554. [Google Scholar] [CrossRef]
- Chan, J.; Rubbi, L.; Pellegrini, M. DNA Methylation Entropy Is a Biomarker for Aging. Aging 2025, 17, 685–698. [Google Scholar] [CrossRef]
- López-Domínguez, J.A.; Rodríguez-López, S.; Ahumada-Castro, U.; Desprez, P.-Y.; Konovalenko, M.; Laberge, R.-M.; Cárdenas, C.; Villalba, J.M.; Campisi, J. Cdkn1a Transcript Variant 2 Is a Marker of Aging and Cellular Senescence. Aging 2021, 13, 13380–13392. [Google Scholar] [CrossRef] [PubMed]
- Kwak, I.H.; Kim, H.S.; Choi, O.R.; Ryu, M.S.; Lim, I.K. Nuclear Accumulation of Globular Actin as a Cellular Senescence Marker. Cancer Res. 2004, 64, 572–580. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, E.W.; Croteau, D.L.; Bohr, V.A. Heterochromatin: An Epigenetic Point of View in Aging. Exp. Mol. Med. 2020, 52, 1466–1474. [Google Scholar] [CrossRef]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2021, 11, 630186. [Google Scholar] [CrossRef] [PubMed]
- de Mera-Rodríguez, J.A.; Álvarez-Hernán, G.; Gañán, Y.; Martín-Partido, G.; Rodríguez-León, J.; Francisco-Morcillo, J. Is Senescence-Associated β-Galactosidase a Reliable in Vivo Marker of Cellular Senescence During Embryonic Development? Front. Cell Dev. Biol. 2021, 9, 623175. [Google Scholar] [CrossRef] [PubMed]
- Jannone, G.; Rozzi, M.; Najimi, M.; Decottignies, A.; Sokal, E.M. An Optimized Protocol for Histochemical Detection of Senescence-Associated Beta-Galactosidase Activity in Cryopreserved Liver Tissue. J. Histochem. Cytochem. 2020, 68, 269–278. [Google Scholar] [CrossRef]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-Associated β-Galactosidase Is Lysosomal β-Galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of Cellular Senescence. Telomere Shortening as a Marker of Cellular Senescence. Aging 2016, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kohli, J.; Demaria, M. Senescent Cells in Cancer Therapy: Friends or Foes? Trends Cancer 2020, 6, 838–857. [Google Scholar] [CrossRef]
- Dong, Z.; Luo, Y.; Yuan, Z.; Tian, Y.; Jin, T.; Xu, F. Cellular Senescence and SASP in Tumor Progression and Therapeutic Opportunities. Mol. Cancer 2024, 23, 181. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espín, D.; Rovira, M.; Galiana, I.; Giménez, C.; Lozano-Torres, B.; Paez-Ribes, M.; Llanos, S.; Chaib, S.; Muñoz-Martín, M.; Ucero, A.C.; et al. A Versatile Drug Delivery System Targeting Senescent Cells. EMBO Mol. Med. 2018, 10, EMMM201809355. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Stachowiak, M.; Kostrzewa, M.; Golusinski, W.; Golusinski, P.; Golusinska-Kardach, E.; Masternak, M.M.; Rubiś, B. Cellular Senescence as a Risk Factor in Head and Neck Cancer—Diagnostic and Therapeutic Perspective. Cancers 2026, 18, 87. https://doi.org/10.3390/cancers18010087
Stachowiak M, Kostrzewa M, Golusinski W, Golusinski P, Golusinska-Kardach E, Masternak MM, Rubiś B. Cellular Senescence as a Risk Factor in Head and Neck Cancer—Diagnostic and Therapeutic Perspective. Cancers. 2026; 18(1):87. https://doi.org/10.3390/cancers18010087
Chicago/Turabian StyleStachowiak, Magdalena, Magdalena Kostrzewa, Wojciech Golusinski, Pawel Golusinski, Ewelina Golusinska-Kardach, Michal M. Masternak, and Błażej Rubiś. 2026. "Cellular Senescence as a Risk Factor in Head and Neck Cancer—Diagnostic and Therapeutic Perspective" Cancers 18, no. 1: 87. https://doi.org/10.3390/cancers18010087
APA StyleStachowiak, M., Kostrzewa, M., Golusinski, W., Golusinski, P., Golusinska-Kardach, E., Masternak, M. M., & Rubiś, B. (2026). Cellular Senescence as a Risk Factor in Head and Neck Cancer—Diagnostic and Therapeutic Perspective. Cancers, 18(1), 87. https://doi.org/10.3390/cancers18010087







