Clear Cell Renal Cell Carcinoma Metastasis to the Thyroid: A Narrative Review of the Literature
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Inclusion/Exclusion Criteria
- •
- Participants: Male or female patients regardless of age and race with metastatic ccRCC to the thyroid.
- •
- Interventions: Any treatment intervention (surgery, chemotherapy, radiotherapy, immunotherapy, and targeted therapy) concerning primary ccRCC or thyroid metastasis was acceptable.
- •
- Comparison: We were interested in pointing out the various different therapeutic approaches (novel and classical), rather than comparing them.
- •
- Outcome: Interval from primary diagnosis of ccRCC to thyroid metastases and/or presence of disease relapse after treatment of thyroid metastases.
2.2. Literature Search Strategy
2.3. Data Tabulation and Extraction
2.4. Tools for Data Processing and Statistical Analysis
3. Results
3.1. Study Selection
3.2. Demographic, Clinical, and Imaging Features
3.3. History of ccRCC
3.4. Treatment Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Tu, W.; Cang, S. Frequency, incidence and survival outcomes of clear cell renal cell carcinoma in the United States from 1973 to 2014: A SEER-based analysis. Medicine 2019, 98, e16684. [Google Scholar] [CrossRef]
- Larcher, A.; Campi, R.; Bex, A.; Bray, F.; Bukavina, L.; Jonasch, E.; Jemal, A.; Marston Linehan, W.; Marandino, L.; Mir, M.C.; et al. Epidemiology of Renal Cancer: Incidence, Mortality, Survival, Genetic Predisposition, and Risk Factors. Eur. Urol. 2025, 88, 341–358. [Google Scholar] [CrossRef]
- Weiss, L.; Harlos, J.P.; Torhorst, J.; Gunthard, B.; Hartveit, F.; Svendsen, E.; Huang, W.L.; Grundmann, E.; Eder, M.; Zwicknagl, M.; et al. Metastatic patterns of renal carcinoma: An analysis of 687 necropsies. J. Cancer Res. Clin. Oncol. 1988, 114, 605–612. [Google Scholar] [CrossRef]
- Bonsib, S.M. Renal veins and venous extension in clear cell renal cell carcinoma. Mod. Pathol. 2007, 20, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Olsen, M.R.; Hudes, G.R.; Burke, J.M.; Edenfield, W.J.; Wilding, G.; Agarwal, N.; Thompson, J.A.; Cella, D.; et al. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J. Clin. Oncol. 2012, 30, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Staehler, M.; Negrier, S.; Chevreau, C.; Desai, A.A.; Rolland, F.; et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J. Clin. Oncol. 2009, 27, 3312–3318. [Google Scholar] [CrossRef] [PubMed]
- Iesalnieks, I.; Machens, A.; Bures, C.; Krenz, D.; Winter, H.; Vorländer, C.; Bareck, E.; Alesina, P.F.; Musholt, T.; Steinmüller, T.; et al. Local recurrence in the neck and survival after thyroidectomy for metastatic renal cell carcinoma. Ann. Surg. Oncol. 2015, 22, 1798–1805. [Google Scholar] [CrossRef]
- Nguyen, M.; He, G.; Lam, A.K.Y. Clinicopathological and Molecular Features of Secondary Cancer (Metastasis) to the Thyroid and Advances in Management. Int. J. Mol. Sci. 2022, 23, 3242. [Google Scholar] [CrossRef]
- Battistella, E.; Pomba, L.; Mattara, G.; Franzato, B.; Toniato, A. Metastases to the thyroid gland: Review of incidence, clinical presentation, diagnostic problems and surgery, our experience. J. Endocrinol. Investig. 2020, 43, 1555–1560. [Google Scholar] [CrossRef]
- Rodrigo-Gómez, L.; Pardal-Refoyo, J.L.; Batuecas-Caletrío, A. Prevalence of metastatic tumors in the thyroid gland. Systematic review and meta-analysis. Rev. ORL 2021, 12, 67–83. [Google Scholar] [CrossRef]
- Duggal, N.M.; Horattas, M.C. Metastatic renal cell carcinoma to the thyroid gland. Endocr. Pract. 2008, 14, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Alberto, F.P.; Luis, M.M.; Alberto, E.S.; Kyriakos, G.; Javier, R.V.A.; Enrique, H.A. Thyroid metastasis of clear renal cell carcinoma: A case report and review of the literature. Pan Afr. Med. J. 2024, 49, 26. [Google Scholar] [CrossRef]
- Jia, H.Y.; Chen, J.; Zhai, Z.X.; Fan, W.W.; Yuan, S.J.; Liu, Q.; Yan, X.H.; Shen, Q.Q.; Liu, L.P. Characterization of thyroid metastasis from clear cell renal cell carcinoma on ultrasonography: A report of three cases and literature review. Eur. Thyroid. J. 2023, 12, e230121. [Google Scholar] [CrossRef]
- Khaddour, K.; Marernych, N.; Ward, W.L.; Liu, J.; Pappa, T. Characteristics of clear cell renal cell carcinoma metastases to the thyroid gland: A systematic review. World J. Clin. Cases 2019, 7, 3474–3485. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Wohlin, C.; Kalinowski, M.; Romero Felizardo, K.; Mendes, E. Successful combination of database search and snowballing for identification of primary studies in systematic literature studies. Inf. Softw. Technol. 2022, 147, 106908. [Google Scholar] [CrossRef]
- R Foundation. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 4 August 2025).
- Abbassi, Z.; Strano, F.; Koliakos, E.; Thomopoulos, T.; Christodoulou, M. Thyroid Gland Hemorrhage in a Patient with Past Medical History of Renal Clear Cell Carcinoma: Report of a Very Rare Case. Am. J. Case Rep. 2018, 19, 920–923. [Google Scholar] [CrossRef]
- Abdel-Aziz, Y.; Hammad, T.; Nawras, M.; Abdulwahid, H.; Nawras, A. Metastatic Renal Cell Cancer to Thyroid Diagnosed by Endoscopic Ultrasound Guided Fine Needle Aspiration Technique. Case Rep. Gastrointest. Med. 2017, 2017, 6725297. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hadi, S.; Beauchamp, F.D.; Cheverez-Ocasio, J.; Pascual-Marrero, J.; Portela-Arraiza, J.C.; Correa-Rivas, M. Late-Onset Metastatic Renal Cell Carcinoma to the Thyroid Gland: An Unusual Airway Emergency. Am. J. Case Rep. 2022, 23, e934814. [Google Scholar] [CrossRef]
- Albandar, H.J.; Roberto, E.S.; See, J.R.H.; Sabiers, J.H. Arteriovenous malformation and thyroid metastasis from underlying renal cell carcinoma, an unusual presentation of malignancy: A case report. Oncol. Lett. 2017, 13, 3323–3327. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Aggamy, M.A.; Joudeh, A.A.; Alabdullatif, H.A.; Alzahrani, H.; Gomha, M.A. Metastasectomy of Sequential Asynchronous Metastatic Renal Cell Carcinoma to the Pancreas, Thyroid, Skin, Contralateral Kidney, and Lung with Cumulative Survival Beyond 10 Years: A Case Report and Clinicopathologic Review. Am. J. Case Rep. 2021, 22, e931696. [Google Scholar] [CrossRef]
- Babar, M.; Hamdani, S.; Liu, C.; Vedula, J.; Schnapp, D.S. Metachronous renal cell carcinoma with metastasis to the urinary bladder, and distant organs, 28 years after radical nephrectomy: A case report. BMC Urol. 2019, 19, 136. [Google Scholar] [CrossRef]
- Bayraktar, Z.; Albayrak, S. Metastasis of renal cell carcinoma to the thyroid gland 9 years after nephrectomy: A case report and literature review. Arch. Ital. Urol. Androl. 2017, 89, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, A.; Tiscornia-Wasserman, P.G. Cytology diagnosis of metastatic clear cell renal cell carcinoma, synchronous to pancreas, and metachronous to thyroid and contralateral adrenal: Report of a case and literature review. Diagn. Cytopathol. 2017, 45, 161–167. [Google Scholar] [CrossRef]
- Bruckschen, F.; Gerharz, C.D.; Sagir, A. Renal cell carcinoma with unusual metachronous metastasis up to 22 years after nephrectomy: Two case reports. J. Med. Case Rep. 2021, 15, 490. [Google Scholar] [CrossRef]
- Cesaretti, M.; Trotta, M.; Varaldo, E.; Ansaldo, G.; Leale, I.; Borgonovo, G. Metastases to the thyroid gland from renal cancer. Tumori J. 2013, 99, e107–e110. [Google Scholar] [CrossRef] [PubMed]
- Chara, L.; Rodríguez, B.; Holgado, E.; Ramírez, N.; Fernández-Rañada, I.; Mohedano, N.; Arcediano, A.; García, I.; Cassinello, J. An unusual metastatic renal cell carcinoma with maintained complete response to sunitinib treatment. Case Rep. Oncol. 2011, 4, 583–586. [Google Scholar] [CrossRef]
- Chin, C.J.; Franklin, J.H.; Moussa, M.; Chin, J.L. Metastasis from renal cell carcinoma to the thyroid 12 years after nephrectomy. CMAJ 2011, 183, 1398–1399. [Google Scholar] [CrossRef] [PubMed]
- Citgez, B.; Uludag, M.; Yetkin, G.; Gurbulak, E.K.; Ozguven, B.Y.; Citgez, S.; Yalcin, V. Thyroid metastasis of renal cell carcinoma. World J. Endocr. Surg. 2011, 3, 93–95. [Google Scholar] [CrossRef]
- Connolly, C.E. Renal cell metastasis to the thyroid gland: An emerging phenomenon. Int. J. Surg. Case Rep. 2018, 45, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Demir, L.; Erten, C.; Somali, I.; Can, A.; Dirican, A.; Bayoglu, V.; Kucukzeybek, Y.; Altinboga, A.A.; Ermete, M.; Oztop, R.M.; et al. Metastases of renal cell carcinoma to the larynx and thyroid: Two case reports on metastasis developing years after nephrectomy. Can. Urol. Assoc. J. 2012, 6, E209–E212. [Google Scholar] [CrossRef] [PubMed]
- Di Furia, M.; Della Penna, A.; Salvatorelli, A.; Clementi, M.; Guadagni, S. A single thyroid nodule revealing early metastases from clear cell renal carcinoma: Case report and review of literature. Int. J. Surg. Case Rep. 2017, 34, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, V.; D’Antonio, A.; Caleo, A.; Valvano, L. Metastatic renal cell carcinoma to the thyroid gland 24 years after the primary tumour. BMJ Case Rep. 2013, 2013, bcr2012007569. [Google Scholar] [CrossRef]
- Falcone, R.; Ramundo, V.; Lamartina, L.; Ascoli, V.; Bosco, D.; Di Gioia, C.; Montesano, T.; Biffoni, M.; Bononi, M.; Giacomelli, L.; et al. Sonographic Presentation of Metastases to the Thyroid Gland: A Case Series. J. Endocr. Soc. 2018, 2, 855–859. [Google Scholar] [CrossRef]
- Fei, W.U.; Chengwei, X.I.A.; Rui, H.A.I.; Chen, X.; Meirong, L.I.; Qingxi, G.U.O.; Shanshan, L.I.U.; Zhou, X. Papillary thyroid carcinoma with clear cell renal cell carcinoma metastasized to the thyroid gland: A case report. Oncol. Lett. 2023, 26, 528. [Google Scholar] [CrossRef]
- Foppiani, L.; Massollo, M.; Del Monte, P.; Bandelloni, R.; Arlandini, A.; Piccardo, A. Late-onset metastasis of renal cell carcinoma into a hot thyroid nodule: An uncommon finding not to be overlooked. Case Rep. Endocrinol. 2015, 2015, 268714. [Google Scholar] [CrossRef]
- García-Trujillo, A.O.; Feriz-Bonelo, K.M.; Guzmán-Gómez, G.E.; Tejada-Marín, J.W.; Figueroa-Agudelo, F.N.; Arrunátegui-Ramírez, A.M.; Estupiñán-Caicedo, D.M.; Guerra-Soto, M.A. Isolated metastasis in the thyroid gland secondary to renal cell carcinoma: Case report. Salud Uninorte 2024, 40, 339–351. [Google Scholar] [CrossRef]
- Gawlik, C.; Lane, J.; Horattas, M. Tumor-to-tumor spread: A case report and literature review of renal cell carcinoma metastasis into thyroid cancer. World J. Surg. Oncol. 2023, 21, 362. [Google Scholar] [CrossRef]
- Geisbush, T.R.; Dymon, Z.; Gabriel, M.S.; Yedavalli, V. A Multimodal and Pathological Analysis of a Renal Cell Carcinoma Metastasis to the Thyroid Gland 11 Years Post Nephrectomy. J. Radiol. Case Rep. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Gheorghiu, M.L.; Iorgulescu, R.; Vrabie, C.D.; Tupea, C.C.; Ursu, H.I. Thyroid Metastasis from Clear Cell Carcinoma of The Kidney 16 Years After Nephrectomy. Acta Endocrinol. 2016, 12, 80–84. [Google Scholar] [CrossRef]
- Habibullah, A.H.; Abdelmonim, S.K.; Aldajani, A.; Rajab, M.; Alessa, M.; Alkaf, H. Rare presentation of metastatic renal cell carcinoma to thyroid gland: A case report. Ann. Med. Surg. 2020, 56, 194–196. [Google Scholar] [CrossRef]
- Hryshchyshyn, A.; Bahrii, A.; Khimich, S.; Bohush, H.; Botsun, P.; Chuba, V. Renal cell carcinoma metastasis to the thyroid gland: A case report. J. Med. Case Rep. 2024, 18, 606. [Google Scholar] [CrossRef]
- Jha, P.; Shekhar, M.; Wan, J.; Mari-Aparici, C. Diffuse thyroid metastases and bilateral internal jugular vein tumor thrombus from renal cell cancer. Radiol. Case Rep. 2016, 11, 434–437. [Google Scholar] [CrossRef]
- Khalafi-Nezhad, A.; Zamani, A.; Amini, M.; Negahban, S. A case report of renal cell carcinoma metastasis revealed through late-onset thyroid nodules. Cancer Rep. 2024, 7, e2113. [Google Scholar] [CrossRef]
- Khan, M.S.; Iyer, V.B.; Varshney, N. A Rare Case of Metastasis to the Thyroid Gland from Renal Clear Cell Carcinoma 11 Years after Nephrectomy and Concurrent Primary Esophageal Carcinoma. Case Rep. Oncol. Med. 2018, 2018, 3790106. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Deen, S.; Ramshankar, V.; Majhi, U. Palliative thyroidectomy in the setting of a metastatic renal cell carcinoma. Indian J. Nucl. Med. 2014, 29, 273–275. [Google Scholar] [CrossRef]
- Lee, J.G.; Yang, Y.; Kim, K.S.; Hyun, C.L.; Lee, J.S.; Koh, G.; Lee, D. A case of metastatic renal cell carcinoma to thyroid gland. Chonnam Med. J. 2011, 47, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, H.; Xiao, W.; Li, J.; Jiang, M.; Sheng, J. Metastasis of clear cell renal cell carcinoma to hyalinizing trabecular tumor of the thyroid: A case report. Oncol. Lett. 2025, 29, 105. [Google Scholar] [CrossRef] [PubMed]
- Lo, X.; Tam, T.K.; Cheung, S.Y.; Leong, H.T. Metastatic renal cell carcinoma presenting with thyroid nodule and Addisonian crisis: Tumour-to-tumour metastasis. Surg. Pract. 2015, 19, 181–183. [Google Scholar] [CrossRef]
- Macedo-Alves, D.; Koch, P.; Soares, V.; Gouveia, P.; Honavar, M.; Taveira-Gomes, A. Thyroid metastasis from renal cell carcinoma-A case report after 9 years. Int. J. Surg. Case Rep. 2015, 16, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Medas, F.; Calò, P.G.; Lai, M.L.; Tuveri, M.; Pisano, G.; Nicolosi, A. Renal cell carcinoma metastasis to thyroid tumor: A case report and review of the literature. J. Med. Case Rep. 2013, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Mosavi Toomatari, S.B.; Ghasemi-Rad, M. Metastasis from renal cell carcinoma to thyroid presenting as rapidly growing neck mass. Int. J. Surg. Case Rep. 2014, 5, 1110–1112. [Google Scholar] [CrossRef]
- Nixon, I.J.; Whitcher, M.; Glick, J.; Palmer, F.L.; Shaha, A.R.; Shah, J.P.; Patel, S.G.; Ganly, I. Surgical management of metastases to the thyroid gland. Ann. Surg. Oncol. 2011, 18, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Okada, A.; Guan, K.; Tauchi-Nishi, P. Metastatic neoplasms to the thyroid diagnosed by fine-needle aspiration/core needle biopsy: Clinicopathologic and cytomorphologic correlation. CytoJournal 2017, 14, 16. [Google Scholar] [CrossRef]
- Ramírez-Plaza, C.P.; Domínguez-López, M.E.; Blanco-Reina, F. Thyroid metastasis as initial presentation of clear cell renal carcinoma. Int. J. Surg. Case Rep. 2015, 10, 101–103. [Google Scholar] [CrossRef]
- Ricci, I.; Barillaro, F.; Conti, E.; Intersimone, D.; Dessanti, P.; Aschele, C. Clear-cell renal cell carcinoma single thyroid metastasis: A single-center retrospective analysis and review of the literature. Arch. Ital. Urol. Androl. 2021, 93, 68–70. [Google Scholar] [CrossRef]
- Sarkar, P.K.; Nissanka-Jayasuria, E.; Eraibey, M.; Kommu, S. Internist’s tumour into thyroid: A case report. J. West. Afr. Coll. Surg. 2024, 14, 348–351. [Google Scholar] [CrossRef]
- Shepherd, M.; Lohmann, J.; Nodit, L.; Vaghaiwalla, T.; Mancini, M. Case Report: Metastatic renal cell carcinoma to the thyroid- A rare encounter. Front. Surg. 2022, 9, 1000425. [Google Scholar] [CrossRef]
- Shi, J.L.; Zhou, J.Q.; Li, J.P. Renal clear cell carcinoma with thyroid and parotid metastases: A case report. Oncol. Lett. 2015, 10, 2617–2619. [Google Scholar] [CrossRef]
- Sindoni, A.; Rizzo, M.; Tuccari, G.; Ieni, A.; Barresi, V.; Calbo, L.; Cucinotta, E.; Mallamace, A.; Trimarchi, F.; Benvenga, S. Thyroid metastases from clear cell renal carcinoma 18years after nephrectomy. Ann. D’endocrinol 2010, 71, 127–130. [Google Scholar] [CrossRef]
- Solmaz, A.; Muhammedoglu, A.; Altinay, S.; Ercetin, C.; Yavuz, E.; Gulcicek, O.B.; Yalcin, S.; Erbil, Y. Isolated thyroid metastasis from renal cell carcinoma. Turk. J. Surg. 2017, 33, 110–112. [Google Scholar] [CrossRef]
- Song, O.K.; Koo, J.S.; Kwak, J.Y.; Moon, H.J.; Yoon, J.H.; Kim, E.K. Metastatic renal cell carcinoma in the thyroid gland: Ultrasonographic features and the diagnostic role of core needle biopsy. Ultrasonography 2017, 36, 252–259. [Google Scholar] [CrossRef]
- Surov, A.; Machens, A.; Holzhausen, H.J.; Spielmann, R.P.; Dralle, H. Radiological features of metastases to the thyroid. Acta Radiol. 2016, 57, 444–450. [Google Scholar] [CrossRef]
- Tadisina, S.; Sami, F.; Mettman, D.; Ridella, M. A Rare Case of Tumor-to-Tumor Metastasis: Renal Cell Carcinoma Metastasis to Papillary Thyroid Carcinoma. JCEM Case Rep. 2024, 2, luae081. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, L.; Wang, X.; Zhang, J.; Zhan, W.; Zhou, W. Metastases to the thyroid gland: Ultrasonographic findings and diagnostic value of fine-needle aspiration cytology. Front. Oncol. 2022, 12, 939965. [Google Scholar] [CrossRef]
- Tian, P.; Du, W.; Liu, X.; Xu, W.; Rong, X.; Zhang, Z.; Wang, Y. Ultrasonographic characteristics of thyroid metastasis from clear cell renal cell carcinoma: A case report. Medicine 2020, 99, e23070. [Google Scholar] [CrossRef] [PubMed]
- Valdez, C.; Rezaei, M.K.; Hendricks, F.; Knoll, S.M. Metastatic renal cell carcinoma to the thyroid 23 years after nephrectomy. Urol. Case Rep. 2014, 2, 129–130. [Google Scholar] [CrossRef]
- Vandemergel, X. Solitary Intrathyroid Metastasis Occurring 23 Years after Resection of Renal Cell Carcinoma. Case Rep. Endocrinol. 2021, 2021, 2735256. [Google Scholar] [CrossRef] [PubMed]
- Velez Torres, J.M.; Briski, L.M.; Martinez Duarte, E.; Sadow, P.M.; Kerr, D.A.; Kryvenko, O.N. Metastatic Clear Cell Renal Cell Carcinoma Involving the Thyroid Gland: A Clinicopathologic Study of 17 Patients. Int. J. Surg. Pathol. 2022, 30, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.; Affandi, K.; Tan, G.; Muhammad, R. Metastasis within a metastasis to the thyroid: A rare phenomenon. Indian J. Pathol. Microbiol. 2017, 60, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Tan, D.; Liu, B.; Han, X.; Jin, X.; Shen, D.; Zhou, L.; Shen, J. Kidney cancer with thyroid metastasis combined with thyroid carcinoma, a case report. BMC Endocr. Disord. 2023, 23, 95. [Google Scholar] [CrossRef]
- Xu, S.; Xu, J.; Yu, C.; Zeng, Y.; Tang, L.; Tang, M.; Yu, T.; Sun, Z.; Zhang, X. Case report: Report of a rare encounter: Metastasis of renal cell carcinoma to the thyroid. Front. Oncol. 2024, 14, 1350043. [Google Scholar] [CrossRef]
- Yamauchi, M.; Kai, K.; Shibamiya, N.; Shimazu, R.; Monji, M.; Suzuki, K.; Kakinoki, H.; Tobu, S.; Kuratomi, Y. Didactic surgical experience of thyroid metastasis from renal cell carcinoma: A case report. World J. Clin. Cases 2018, 6, 1018–1023. [Google Scholar] [CrossRef]
- Kefeli, M.; Mete, O. An Unusual Solitary Thyroid Nodule with Bloody Follicles: Metastatic Renal Cell Carcinoma Within an Infiltrative Follicular Variant Papillary Carcinoma. Endocr. Pathol. 2016, 27, 171–174. [Google Scholar] [CrossRef]
- Hellums, R.N.; Kovatch, K.J.; Friscia, M.E.; Schwartz, T.R.; Pellitteri, P.K. Metastatic renal cell carcinoma to the thyroid with cervicothoracic venous tumor thrombosis. Head Neck 2023, 45, E31–E35. [Google Scholar] [CrossRef] [PubMed]
- Al Abdrabalnabi, A.A.; AlQattan, A.S.; Algarni, S.; Mashhour, M.; Al-Qahtani, M. Metastatic renal cell carcinoma to the pancreas, thyroid, & subcutaneous tissue 13 years after Radical nephrectomy: A case report. Int. J. Surg. Case Rep. 2019, 60, 183–185. [Google Scholar] [CrossRef]
- Badawi, F.; Meliti, A. Tumor-to-Tumor Metastasis of Renal Cell Carcinoma to a Follicular Variant of Papillary Thyroid Carcinoma: A Case Report and Literature Review. Cureus 2022, 14, e23742. [Google Scholar] [CrossRef]
- Cilengir, A.H.; Kalayci, T.O.; Duygulu, G.; Rezanko, T.A.; İnci, M.F. Metastasis of Renal Clear Cell Carcinoma to Thyroid Gland Mimicking Adenomatous Goiter. Pol. J. Radiol. 2016, 81, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Hirokawa, M.; Yabuta, T.; Fukushima, M.; Masuoka, H.; Higashiyama, T.; Kihara, M.; Ito, Y.; Miya, A.; Amino, N.; et al. Metastatic carcinoma to the thyroid gland from renal cell carcinoma: Role of ultrasonography in preoperative diagnosis. Thyroid. Res. 2015, 8, 4. [Google Scholar] [CrossRef]
- Moradi Tabriz, H.; Eftekhar Javadi, A.; Zandnejadi, A. A Rare Case of Metastasis of Renal Clear Cell Carcinoma to the Thyroid Gland, Presenting as a Goiter Nodule, Three Years After Nephrectomy. Iran. J. Pathol. 2020, 15, 342–345. [Google Scholar] [CrossRef]
- Xie, W.T.; Wang, Y.Q.; Du, Z.S.; Chen, Y.J.; Wu, Y.; Zhu, D.D.; Tang, L.A. Ultrasonographic Findings and Prognosis of Metastases to the Thyroid Gland. Ultrason. Imaging 2023, 45, 219–226. [Google Scholar] [CrossRef]
- Zamarrón, C.; Abdulkader, I.; Areses, M.C.; García-Paz, V.; León, L.; Cameselle-Teijeiro, J. Metastases of renal cell carcinoma to the thyroid gland with synchronous benign and malignant follicular cell-derived neoplasms. Case Rep. Oncol. Med. 2013, 2013, 485025. [Google Scholar] [CrossRef] [PubMed]
- Lieder, A.; Guenzel, T.; Lebentrau, S.; Schneider, C.; Franzen, A. Diagnostic relevance of metastatic renal cell carcinoma in the head and neck: An evaluation of 22 cases in 671 patients. Int. Braz. J. Urol. 2017, 43, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Paner, G.P.; Stadler, W.M.; Hansel, D.E.; Montironi, R.; Lin, D.W.; Amin, M.B. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur. Urol. 2018, 73, 560–569. [Google Scholar] [CrossRef]
- D’Angelo, F.A.; Magistri, P.; Antolino, L.; Socciarelli, F. Granulomatous reaction within the thyroid metastases of a renal cell carcinoma. Int. J. Surg. Pathol. 2014, 22, 87–89. [Google Scholar] [CrossRef]
- Aydogdu, Y.F.; Gülçek, E.; Büyükkasap, Ç.; Bostanci, H. Outcomes of thyroidectomy for secondary thyroid malignancies, a single center experience. Discov. Oncol. 2024, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Balta, H.; Kocaman, N.; Ucer, O. A rare but very serious disease: Tumors metastasizing to the thyroid in the light of cases. Tissue Cell 2022, 79, 101958. [Google Scholar] [CrossRef]
- Moghaddam, P.A.; Cornejo, K.M.; Khan, A. Metastatic carcinoma to the thyroid gland: A single institution 20-year experience and review of the literature. Endocr. Pathol. 2013, 24, 116–124. [Google Scholar] [CrossRef]
- Russell, J.O.; Yan, K.; Burkey, B.; Scharpf, J. Nonthyroid Metastasis to the Thyroid Gland: Case Series and Review with Observations by Primary Pathology. Otolaryngol. Head Neck Surg. 2016, 155, 961–968. [Google Scholar] [CrossRef]
- Tjahjono, R.; Phung, D.; Gurney, H.; Gupta, R.; Riffat, F.; Palme, C.E. Thyroid gland metastasis from renal cell carcinoma: A case series and literature review. ANZ J. Surg. 2021, 91, 708–715. [Google Scholar] [CrossRef]
- Jackson, G.; Fino, N.; Bitting, R.L. Clinical Characteristics of Patients With Renal Cell Carcinoma and Metastasis to the Thyroid Gland. Clin. Med. Insights Oncol. 2017, 11, 1179554917743981. [Google Scholar] [CrossRef]
- Kaliszewski, K.; Szkudlarek, D.; Kasperczak, M.; Nowak, Ł. Clear cell renal carcinoma metastasis mimicking primary thyroid tumor. Pol. Arch. Intern. Med. 2019, 129, 211–214. [Google Scholar] [CrossRef]
- Chen, H.; Nicol, T.L.; Udelsman, R. Clinically significant, isolated metastatic disease to the thyroid gland. World J. Surg. 1999, 23, 177–180; discussion 181. [Google Scholar] [CrossRef]
- Heffess, C.S.; Wenig, B.M.; Thompson, L.D. Metastatic renal cell carcinoma to the thyroid gland: A clinicopathologic study of 36 cases. Cancer 2002, 95, 1869–1878. [Google Scholar] [CrossRef]
- Iesalnieks, I.; Winter, H.; Bareck, E.; Sotiropoulos, G.C.; Goretzki, P.E.; Klinkhammer-Schalke, M.; Bröckner, S.; Trupka, A.; Anthuber, M.; Rupprecht, H.; et al. Thyroid metastases of renal cell carcinoma: Clinical course in 45 patients undergoing surgery. Assessment of factors affecting patients’ survival. Thyroid. 2008, 18, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Beutner, U.; Leowardi, C.; Bork, U.; Lüthi, C.; Tarantino, I.; Pahernik, S.; Wente, M.N.; Büchler, M.W.; Schmied, B.M.; Müller, S.A. Survival after renal cell carcinoma metastasis to the thyroid: Single center experience and systematic review of the literature. Thyroid 2015, 25, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Nezami, B.G.; MacLennan, G.T. Clear Cell Renal Cell Carcinoma: A Comprehensive Review of its Histopathology, Genetics, and Differential Diagnosis. Int. J. Surg. Pathol. 2025, 33, 265–280. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Yang, Z. Treatment strategies for clear cell renal cell carcinoma: Past, present and future. Front. Oncol. 2023, 13, 1133832. [Google Scholar] [CrossRef] [PubMed]
- Rathmell, W.K.; Rumble, R.B.; Van Veldhuizen, P.J.; Al-Ahmadie, H.; Emamekhoo, H.; Hauke, R.J.; Louie, A.V.; Milowsky, M.I.; Molina, A.M.; Rose, T.L.; et al. Management of Metastatic Clear Cell Renal Cell Carcinoma: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2957–2995. [Google Scholar] [CrossRef]
- Cimino-Mathews, A.; Sharma, R.; Netto, G.J. Diagnostic use of PAX8, CAIX, TTF-1, and TGB in metastatic renal cell carcinoma of the thyroid. Am. J. Surg. Pathol. 2011, 35, 757–761. [Google Scholar] [CrossRef]
- Calzolari, F.; Sartori, P.V.; Talarico, C.; Parmeggiani, D.; Beretta, E.; Pezzullo, L.; Bovo, G.; Sperlongano, P.; Monacelli, M.; Lucchini, R.; et al. Surgical treatment of intrathyroid metastases: Preliminary results of a multicentric study. Anticancer Res. 2008, 28, 2885–2888. [Google Scholar]
- Machens, A.; Dralle, H. Outcome after thyroid surgery for metastasis from renal cell cancer. Surgery 2010, 147, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, M.K.; Gharib, H.; Goellner, J.R.; Van Heerden, J.A. Metastasis to the thyroid gland: A report of 43 cases. Cancer 1997, 79, 574–578. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
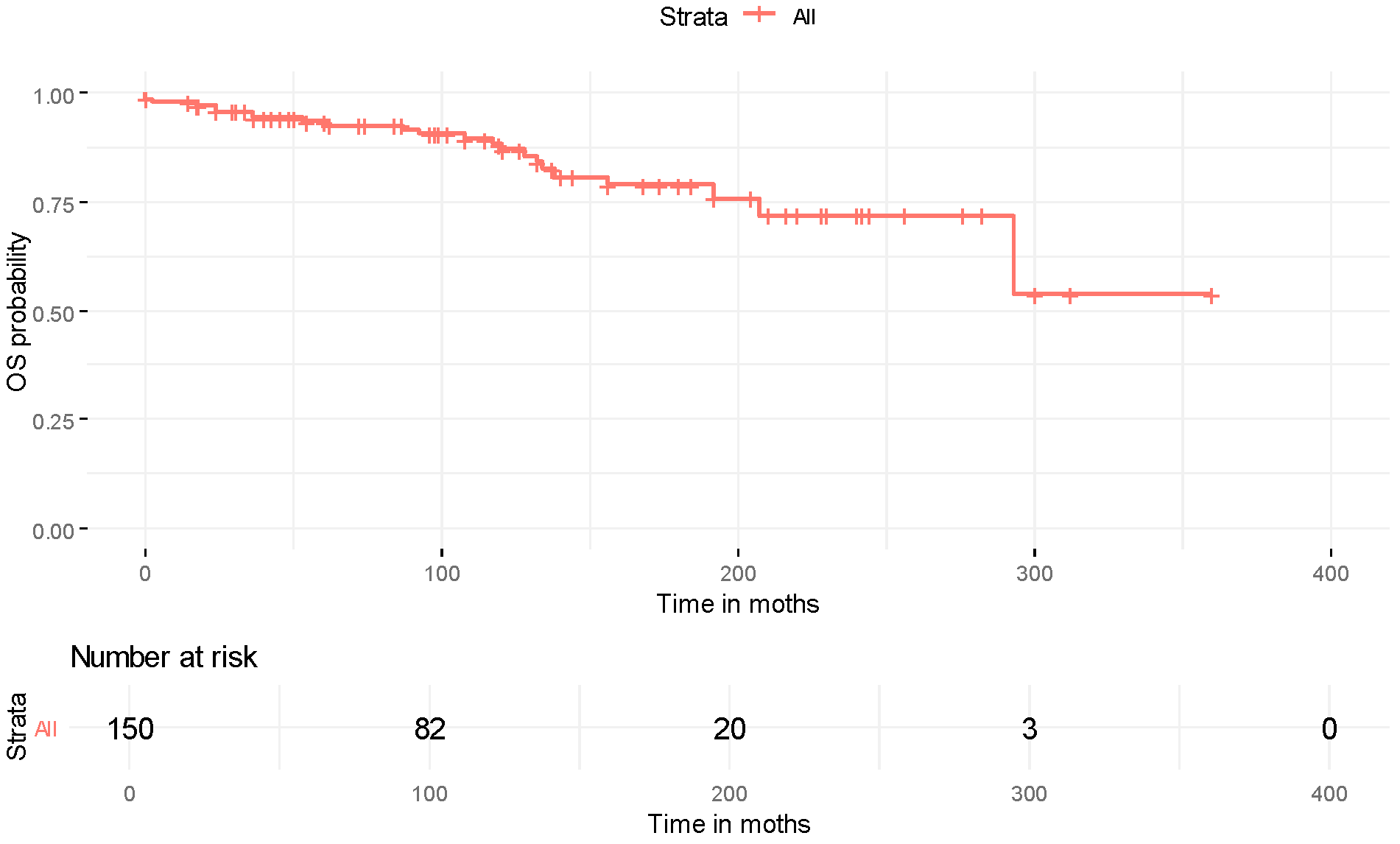
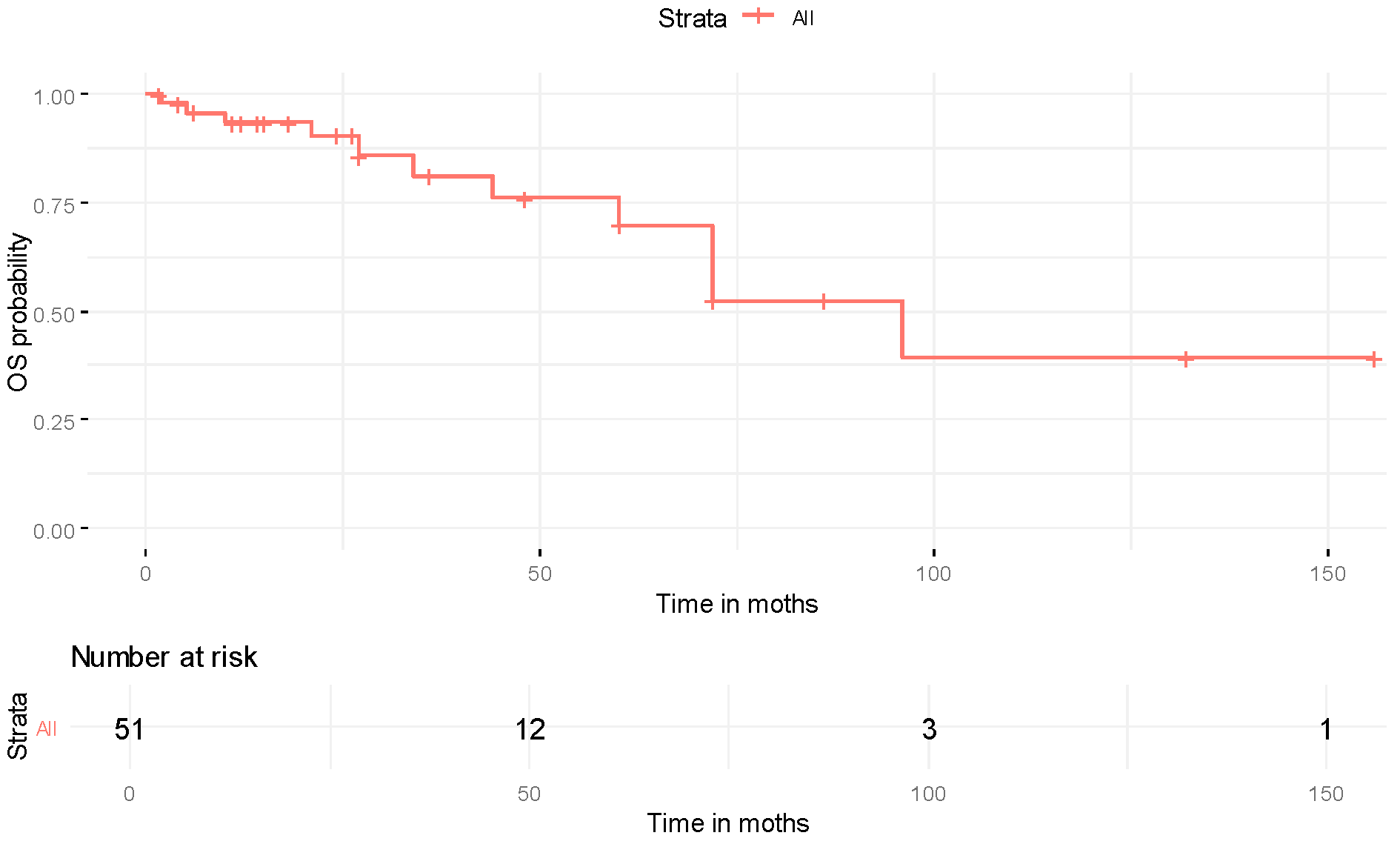
| Characteristic | Overall (N = 189) |
|---|---|
| Gender | |
| Female | 88 (46.6%) |
| Male | 97 (51.3%) |
| Missing | 4 (2.1%) |
| Age | |
| Mean (SD) | 65.1 (10.3) |
| Median [Min, Max] | 65.0 [39.0, 88.0] |
| Synchronous neoplasia (other) | |
| No | 65 (34.4%) |
| Yes | 16 (8.5%) |
| Missing | 108 (57.1%) |
| Non-thyroid-metastasis (prior) | |
| No | 64 (33.9%) |
| Yes | 16 (8.5%) |
| Missing | 109 (57.7%) |
| Non-thyroid-metastasis (synchronous) | |
| No | 65 (34.3%) |
| Yes | 15 (7.9%) |
| Missing | 109 (57.8%) |
| Follow-up after thyroid metastasis (months) | |
| Mean (SD) | 35.6 (35.3) |
| Median [Min, Max] | 24.0 [1.50, 156] |
| Missing | 138 (73.0%) |
| Larger tumor dimension (cm) | |
| Mean (SD) | 3.83 (2.03) |
| Median [Min, Max] | 3.50 [0.800, 10.0] |
| Missing | 91 (48.1%) |
| Solitary (S)/Multiple (M) | |
| M | 29 (15.3%) |
| S | 97 (51.3%) |
| Missing | 63 (33.3%) |
| Laterality (R: right, L: left, B: both lobes) | |
| B | 26 (13.8%) |
| Isthmus | 1 (0.5%) |
| L | 38 (20.1%) |
| L and isthmus | 1 (0.5%) |
| R | 47 (24.9%) |
| Missing | 76 (40.2%) |
| Stage | |
| I | 20 (10.6%) |
| II | 5 (2.6%) |
| III | 12 (6.3%) |
| IV | 7 (3.7%) |
| Missing | 145 (76.8%) |
| Grade (WHO/ISUP or Fuhrman) on diagnosis | |
| 1 | 4 (2.1%) |
| 2 | 20 (10.6%) |
| 3 | 14 (7.4%) |
| 4 | 3 (1.6%) |
| Missing | 148 (78.3%) |
| Kidney disease treatment | |
| Partial nephrectomy | 3 (1.5%) |
| Total nephrectomy | 37 (19.6%) |
| Radical nephrectomy | 30 (16%) |
| Total nephrectomy and contralateral adrenalectomy | 1 (0.5%) |
| Missing | 118 (62.4%) |
| Outcome | |
| A | 26 (13.8%) * |
| AWD | 10 (5.3%) |
| AWtD | 44 (23.3%) |
| D | 26 (13.8%) |
| DOD | 13 (6.9%) |
| Missing | 70 (36.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Samaras, M.G.; Pouliakis, A.; Skaretzos, K.; Boutas, I.; Kontogeorgi, A.; Dimas, D.T.; Ieronimaki, A.-I.; Zanelli, M.; Palicelli, A.; Zizzo, M.; et al. Clear Cell Renal Cell Carcinoma Metastasis to the Thyroid: A Narrative Review of the Literature. Cancers 2026, 18, 57. https://doi.org/10.3390/cancers18010057
Samaras MG, Pouliakis A, Skaretzos K, Boutas I, Kontogeorgi A, Dimas DT, Ieronimaki A-I, Zanelli M, Palicelli A, Zizzo M, et al. Clear Cell Renal Cell Carcinoma Metastasis to the Thyroid: A Narrative Review of the Literature. Cancers. 2026; 18(1):57. https://doi.org/10.3390/cancers18010057
Chicago/Turabian StyleSamaras, Menelaos G., Abraham Pouliakis, Konstantinos Skaretzos, Ioannis Boutas, Adamantia Kontogeorgi, Dionysios T. Dimas, Argyro-Ioanna Ieronimaki, Magda Zanelli, Andrea Palicelli, Maurizio Zizzo, and et al. 2026. "Clear Cell Renal Cell Carcinoma Metastasis to the Thyroid: A Narrative Review of the Literature" Cancers 18, no. 1: 57. https://doi.org/10.3390/cancers18010057
APA StyleSamaras, M. G., Pouliakis, A., Skaretzos, K., Boutas, I., Kontogeorgi, A., Dimas, D. T., Ieronimaki, A.-I., Zanelli, M., Palicelli, A., Zizzo, M., Broggi, G., Caltabiano, R., Salzano, S., & Koufopoulos, N. I. (2026). Clear Cell Renal Cell Carcinoma Metastasis to the Thyroid: A Narrative Review of the Literature. Cancers, 18(1), 57. https://doi.org/10.3390/cancers18010057










