Paediatric Thyroid Carcinoma: The Genetic Revolution and Its Implications for Therapy and Outcomes
Simple Summary
Abstract
1. Introduction
2. Somatic Gene Alterations in Differentiated Thyroid Carcinomas
2.1. RET
2.2. NTRK
2.3. ALK
2.4. BRAF
2.5. TERT
2.6. RAS
2.7. Pax-8/PPAR-Gamma
3. Germline P/LPV Associated with Predisposition to Thyroid Carcinomas
3.1. PTEN Hamartoma Tumour Syndrome
3.2. DICER1 Syndrome
3.3. Carney Complex Type 1
3.4. Familial Adenomatous Polyposis
3.5. Werner Syndrome
3.6. Multiple Endocrine Neoplasia Type 2
4. Germline Molecular Testing for Cancer Predisposition Syndromes
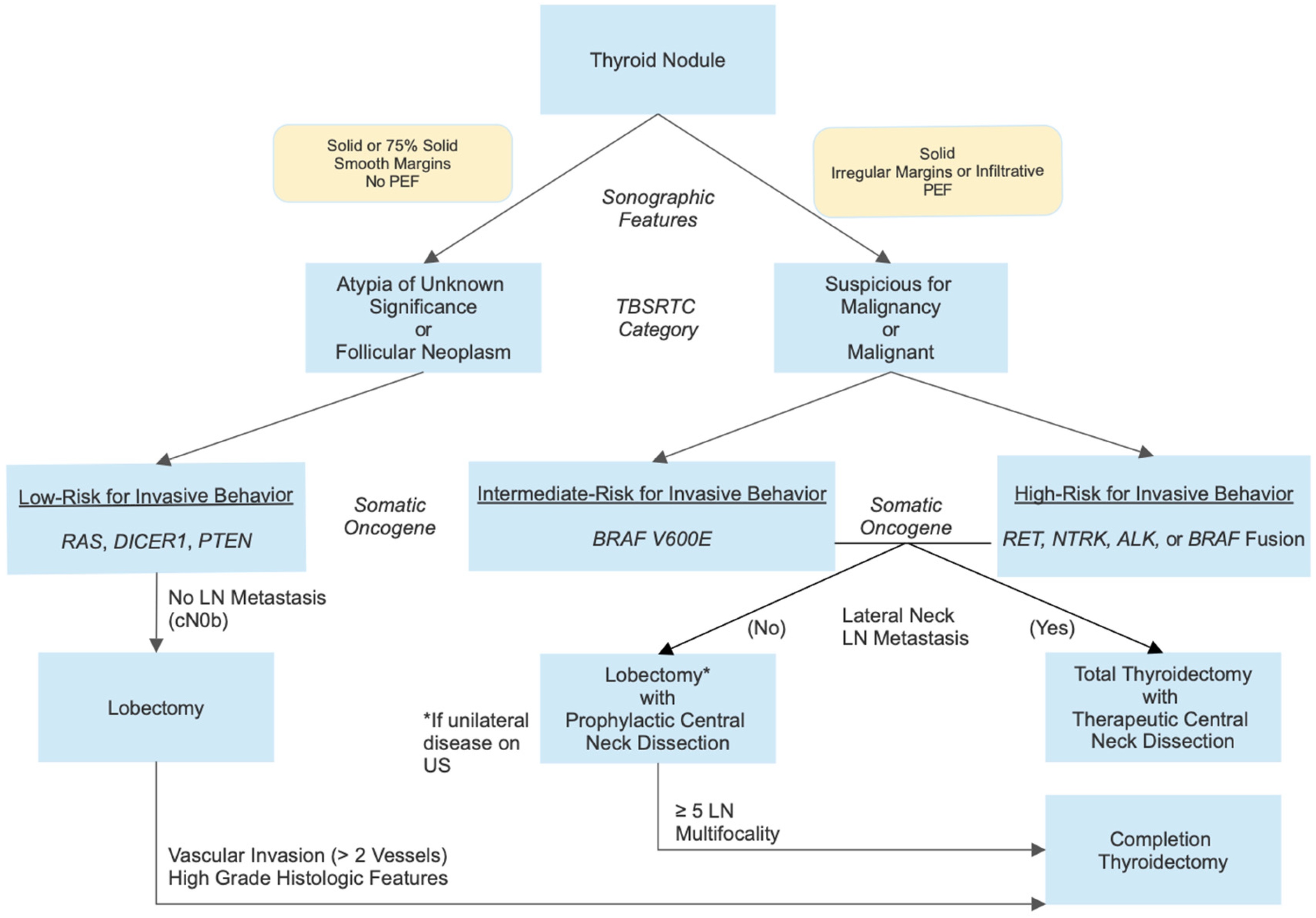
5. Molecular Testing of Thyroid Nodule FNA Samples
6. The Value of a Molecular Diagnosis Post-Operatively
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Richman, D.M.; Benson, C.B.; Doubilet, P.M.; Peters, H.E.; Huang, S.A.; Asch, E.; Wassner, A.J.; Smith, J.R.; Cherella, C.E.; Frates, M.C. Thyroid Nodules in Pediatric Patients: Sonographic Characteristics and Likelihood of Cancer. Radiology 2018, 288, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Lebbink, C.A.; Links, T.P.; Czarniecka, A.; Dias, R.P.; Elisei, R.; Izatt, L.; Krude, H.; Lorenz, K.; Luster, M.; Newbold, K.; et al. 2022 European Thyroid Association Guidelines for the management of pediatric thyroid nodules and differentiated thyroid carcinoma. Eur. Thyroid. J. 2022, 11, e220146. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Ly, S.; Castroneves, L.A.; Frates, M.C.; Benson, C.B.; Feldman, H.A.; Wassner, A.J.; Smith, J.R.; Marqusee, E.; Alexander, E.K.; et al. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J. Clin. Endocrinol. Metab. 2013, 98, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- Cherella, C.E.; Wassner, A.J. Pediatric thyroid cancer: Recent developments. Best. Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101715. [Google Scholar] [CrossRef]
- Bauer, A.J. Molecular Genetics of Thyroid Cancer in Children and Adolescents. Endocrinol. Metab. Clin. North. Am. 2017, 46, 389–403. [Google Scholar] [CrossRef]
- Francis, G.L.; Waguespack, S.G.; Bauer, A.J.; Angelos, P.; Benvenga, S.; Cerutti, J.M.; Dinauer, C.A.; Hamilton, J.; Hay, I.D.; Luster, M.; et al. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2015, 25, 716–759. [Google Scholar] [CrossRef]
- Handkiewicz-Junak, D.; Niedziela, M.; Lewinski, A.; Bosowski, A.; Chmielik, E.; Czarniecka, A.; Dedecjus, M.; Dembowska-Baginska, B.; Gawlik-Starzyk, A.; Gorecki, W.; et al. Diagnostics and treatment of differentiated thyroid carcinoma in children—Guidelines of the Polish National Scientific Societies, 2024 Update. Endokrynol. Pol. 2024, 75, 565–591. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Hensley, S.G.; Hu, M.I.; Bassett, R.L.; Ying, A.K.; Zafereo, M.E.; Perrier, N.D.; Busaidy, N.L.; Hyde, S.M.; Grubbs, E.G.; Waguespack, S.G. Pediatric Medullary Thyroid Carcinoma: Clinical Presentations and Long-Term Outcomes in 144 Patients over 6 Decades. J. Clin. Endocrinol. Metab. 2024, 109, 2256–2268. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Gallant, J.N.; Chen, S.C.; Ortega, C.A.; Rohde, S.L.; Belcher, R.H.; Netterville, J.L.; Baregamian, N.; Wang, H.; Liang, J.; Ye, F.; et al. Evaluation of the Molecular Landscape of Pediatric Thyroid Nodules and Use of a Multigene Genomic Classifier in Children. JAMA Oncol. 2022, 8, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Pekova, B.; Sykorova, V.; Dvorakova, S.; Vaclavikova, E.; Moravcova, J.; Katra, R.; Astl, J.; Vlcek, P.; Kodetova, D.; Vcelak, J.; et al. RET, NTRK, ALK, BRAF, and MET Fusions in a Large Cohort of Pediatric Papillary Thyroid Carcinomas. Thyroid 2020, 30, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.S.; Alswailem, M.; Alswailem, A.A.; Al-Hindi, H.; Goljan, E.; Alsudairy, N.; Abouelhoda, M. Genetic Alterations in Pediatric Thyroid Cancer Using a Comprehensive Childhood Cancer Gene Panel. J. Clin. Endocrinol. Metab. 2020, 105, 3324–3334. [Google Scholar] [CrossRef]
- Franco, A.T.; Ricarte-Filho, J.C.; Isaza, A.; Jones, Z.; Jain, N.; Mostoufi-Moab, S.; Surrey, L.; Laetsch, T.W.; Li, M.M.; DeHart, J.C.; et al. Fusion Oncogenes Are Associated with Increased Metastatic Capacity and Persistent Disease in Pediatric Thyroid Cancers. J. Clin. Oncol. 2022, 40, 1081–1090. [Google Scholar] [CrossRef]
- Stosic, A.; Fuligni, F.; Anderson, N.D.; Davidson, S.; de Borja, R.; Acker, M.; Forte, V.; Campisi, P.; Propst, E.J.; Wolter, N.E.; et al. Diverse Oncogenic Fusions and Distinct Gene Expression Patterns Define the Genomic Landscape of Pediatric Papillary Thyroid Carcinoma. Cancer Res. 2021, 81, 5625–5637. [Google Scholar] [CrossRef]
- Guleria, P.; Srinivasan, R.; Rana, C.; Agarwal, S. Molecular Landscape of Pediatric Thyroid Cancer: A Review. Diagnostics 2022, 12, 3136. [Google Scholar] [CrossRef]
- Rangel-Pozzo, A.; Sisdelli, L.; Cordioli, M.I.V.; Vaisman, F.; Caria, P.; Mai, S.; Cerutti, J.M. Genetic Landscape of Papillary Thyroid Carcinoma and Nuclear Architecture: An Overview Comparing Pediatric and Adult Populations. Cancers 2020, 12, 3146. [Google Scholar] [CrossRef]
- Mostoufi-Moab, S.; Labourier, E.; Sullivan, L.; LiVolsi, V.; Li, Y.; Xiao, R.; Beaudenon-Huibregtse, S.; Kazahaya, K.; Adzick, N.S.; Baloch, Z.; et al. Molecular Testing for Oncogenic Gene Alterations in Pediatric Thyroid Lesions. Thyroid 2018, 28, 60–67. [Google Scholar] [CrossRef]
- de Sousa, M.S.A.; Nunes, I.N.; Christiano, Y.P.; Sisdelli, L.; Cerutti, J.M. Genetic alterations landscape in paediatric thyroid tumours and/or differentiated thyroid cancer: Systematic review. Rev. Endocr. Metab. Disord. 2024, 25, 35–51. [Google Scholar] [CrossRef]
- Morton, L.M.; Karyadi, D.M.; Stewart, C.; Bogdanova, T.I.; Dawson, E.T.; Steinberg, M.K.; Dai, J.; Hartley, S.W.; Schonfeld, S.J.; Sampson, J.N.; et al. Radiation-related genomic profile of papillary thyroid carcinoma after the Chernobyl accident. Science 2021, 372, eabg2538. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Q.; Zhang, K.; Liang, Y.; Ren, F.; Zhang, J.; Kan, C.; Han, F.; Sun, X. NTRK fusions in thyroid cancer: Pathology and clinical aspects. Crit. Rev. Oncol. Hematol. 2023, 184, 103957. [Google Scholar] [CrossRef] [PubMed]
- Ricarte-Filho, J.C.; Halada, S.; O’Neill, A.; Casado-Medrano, V.; Laetsch, T.W.; Franco, A.T.; Bauer, A.J. The clinical aspect of NTRK-fusions in pediatric papillary thyroid cancer. Cancer Genet. 2022, 262, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Nies, M.; Vassilopoulou-Sellin, R.; Bassett, R.L.; Yedururi, S.; Zafereo, M.E.; Cabanillas, M.E.; Sherman, S.I.; Links, T.P.; Waguespack, S.G. Distant Metastases from Childhood Differentiated Thyroid Carcinoma: Clinical Course and Mutational Landscape. J. Clin. Endocrinol. Metab. 2021, 106, e1683–e1697. [Google Scholar] [CrossRef] [PubMed]
- Shih, K.P.; Lee, Y.C.; Tsai, J.J.; Lin, S.H.; Liu, C.Y.; Li, W.S.; Li, C.F.; Hang, J.F. Clinicopathologic Features and Cytologic Correlation of ALK-Rearranged Papillary Thyroid Carcinoma: A Series of Eight Cases. Endocr. Pathol. 2024, 35, 134–146. [Google Scholar] [CrossRef]
- Lee, Y.A.; Lee, H.; Im, S.W.; Song, Y.S.; Oh, D.Y.; Kang, H.J.; Won, J.K.; Jung, K.C.; Kwon, D.; Chung, E.J.; et al. NTRK and RET fusion-directed therapy in pediatric thyroid cancer yields a tumor response and radioiodine uptake. J. Clin. Investig. 2021, 131, e144847. [Google Scholar] [CrossRef]
- Ji, J.H.; Oh, Y.L.; Hong, M.; Yun, J.W.; Lee, H.W.; Kim, D.; Ji, Y.; Kim, D.H.; Park, W.Y.; Shin, H.T.; et al. Identification of Driving ALK Fusion Genes and Genomic Landscape of Medullary Thyroid Cancer. PLoS Genet. 2015, 11, e1005467. [Google Scholar] [CrossRef]
- Hillier, K.; Hughes, A.; Shamberger, R.C.; Shusterman, S.; Perez-Atayde, A.R.; Wassner, A.J.; Iafrate, A.J.; Dubuc, A.; Janeway, K.A.; Rothenberg, S.M.; et al. A Novel ALK Fusion in Pediatric Medullary Thyroid Carcinoma. Thyroid 2019, 29, 1704–1707. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Nikiforov, Y.E. Radiation-induced thyroid cancer: What we have learned from chernobyl. Endocr. Pathol. 2006, 17, 307–317. [Google Scholar] [CrossRef]
- Mitsutake, N.; Saenko, V. Molecular pathogenesis of pediatric thyroid carcinoma. J. Radiat. Res. 2021, 62, i71–i77. [Google Scholar] [CrossRef]
- Givens, D.J.; Buchmann, L.O.; Agarwal, A.M.; Grimmer, J.F.; Hunt, J.P. BRAF V600E does not predict aggressive features of pediatric papillary thyroid carcinoma. Laryngoscope 2014, 124, E389–E393. [Google Scholar] [CrossRef] [PubMed]
- Henke, L.E.; Perkins, S.M.; Pfeifer, J.D.; Ma, C.; Chen, Y.; DeWees, T.; Grigsby, P.W. BRAF V600E mutational status in pediatric thyroid cancer. Pediatr. Blood Cancer 2014, 61, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Matsuse, M.; Mitsutake, N. TERT promoter mutations in thyroid cancer. Endocr. J. 2023, 70, 1035–1049. [Google Scholar] [CrossRef]
- Yu, P.; Qu, N.; Zhu, R.; Hu, J.; Han, P.; Wu, J.; Tan, L.; Gan, H.; He, C.; Fang, C.; et al. TERT accelerates BRAF mutant-induced thyroid cancer dedifferentiation and progression by regulating ribosome biogenesis. Sci. Adv. 2023, 9, eadg7125. [Google Scholar] [CrossRef]
- Chakraborty, D.; Shakya, S.; Ballal, S.; Agarwal, S.; Bal, C. BRAF V600E and TERT promoter mutations in paediatric and young adult papillary thyroid cancer and clinicopathological correlation. J. Pediatr. Endocrinol. Metab. 2020, 33, 1465–1474. [Google Scholar] [CrossRef]
- Oishi, N.; Kondo, T.; Nakazawa, T.; Mochizuki, K.; Inoue, T.; Kasai, K.; Tahara, I.; Yabuta, T.; Hirokawa, M.; Miyauchi, A.; et al. Frequent BRAF (V600E) and Absence of TERT Promoter Mutations Characterize Sporadic Pediatric Papillary Thyroid Carcinomas in Japan. Endocr. Pathol. 2017, 28, 103–111. [Google Scholar] [CrossRef]
- Geng, J.; Liu, Y.; Guo, Y.; Wang, H.; Tai, J.; Jin, Y.; Zhang, J.; Yu, Y.; Wang, S.; Song, Y.; et al. Correlation between TERT C228T and clinic-pathological features in pediatric papillary thyroid carcinoma. Sci. China Life Sci. 2019, 62, 1563–1571. [Google Scholar] [CrossRef]
- Kondo, T.; Ezzat, S.; Asa, S.L. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat. Rev. Cancer 2006, 6, 292–306. [Google Scholar] [CrossRef]
- Raman, P.; Koenig, R.J. Pax-8-PPAR-gamma fusion protein in thyroid carcinoma. Nat. Rev. Endocrinol. 2014, 10, 616–623. [Google Scholar] [CrossRef]
- Vuong, H.G.; Kondo, T.; Oishi, N.; Nakazawa, T.; Mochizuki, K.; Miyauchi, A.; Hirokawa, M.; Katoh, R. Paediatric follicular thyroid carcinoma-indolent cancer with low prevalence of RAS mutations and absence of PAX8-PPARG fusion in a Japanese population. Histopathology 2017, 71, 760–768. [Google Scholar] [CrossRef]
- Paulson, V.A.; Rudzinski, E.R.; Hawkins, D.S. Thyroid Cancer in the Pediatric Population. Genes 2019, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- van der Tuin, K.; Ruano, D.; Knijnenburg, J.; van der Luijt, R.B.; Morreau, H.; Links, T.P.; Hes, F.J.; Dutch Pediatric Thyroid Cancer, C. Clinically Relevant Germline Variants in Children with Nonmedullary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2024, 109, e2214–e2221. [Google Scholar] [CrossRef] [PubMed]
- Balinisteanu, I.; Panzaru, M.C.; Caba, L.; Ungureanu, M.C.; Florea, A.; Grigore, A.M.; Gorduza, E.V. Cancer Predisposition Syndromes and Thyroid Cancer: Keys for a Short Two-Way Street. Biomedicines 2023, 11, 2143. [Google Scholar] [CrossRef] [PubMed]
- Capezzone, M.; Marchisotta, S.; Cantara, S.; Busonero, G.; Brilli, L.; Pazaitou-Panayiotou, K.; Carli, A.F.; Caruso, G.; Toti, P.; Capitani, S.; et al. Familial non-medullary thyroid carcinoma displays the features of clinical anticipation suggestive of a distinct biological entity. Endocr. Relat. Cancer 2008, 15, 1075–1081. [Google Scholar] [CrossRef]
- Cameselle-Teijeiro, J.M.; Erickson, L.A.; LiVolsi, V.; Nose, V. Endocrine and Neuroendocrine Tumours. In WHO Classification of Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2022; Volume 10, Available online: https://tumourclassification.iarc.who.int/chaptercontent/53/168 (accessed on 3 March 2025).
- Milani, D.; Dolci, A.; Muller, I.; Pavesi, M.A.; Runza, L.; Kuhn, E.; Natacci, F.; Peissel, B.; Ricci, M.T.; Despini, L.; et al. Thyroid findings in pediatric and adult patients with PTEN hamartoma tumor syndrome: A retrospective analysis, and literature review. Endocrine 2023, 81, 98–106. [Google Scholar] [CrossRef]
- Smith, J.R.; Liu, E.; Church, A.J.; Asch, E.; Cherella, C.E.; Srivastava, S.; Kamihara, J.; Wassner, A.J. Natural History of Thyroid Disease in Children with PTEN Hamartoma Tumor Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, e1121–e1130. [Google Scholar] [CrossRef]
- Hendricks, L.A.J.; Hoogerbrugge, N.; Schuurs-Hoeijmakers, J.H.M.; Vos, J.R. A review on age-related cancer risks in PTEN hamartoma tumor syndrome. Clin. Genet. 2021, 99, 219–225. [Google Scholar] [CrossRef]
- Pilarski, R.; Burt, R.; Kohlman, W.; Pho, L.; Shannon, K.M.; Swisher, E. Cowden syndrome and the PTEN hamartoma tumor syndrome: Systematic review and revised diagnostic criteria. J. Natl. Cancer Inst. 2013, 105, 1607–1616. [Google Scholar] [CrossRef]
- Tan, M.H.; Mester, J.; Peterson, C.; Yang, Y.; Chen, J.L.; Rybicki, L.A.; Milas, K.; Pederson, H.; Remzi, B.; Orloff, M.S.; et al. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am. J. Hum. Genet. 2011, 88, 42–56. [Google Scholar] [CrossRef]
- Baran, J.A.; Tsai, S.D.; Isaza, A.; Brodeur, G.M.; MacFarland, S.P.; Zelley, K.; Adams, D.M.; Franco, A.T.; Bauer, A.J. The Clinical Spectrum of PTEN Hamartoma Tumor Syndrome: Exploring the Value of Thyroid Surveillance. Horm. Res. Paediatr. 2020, 93, 634–642. [Google Scholar] [CrossRef]
- Tischkowitz, M.; Colas, C.; Pouwels, S.; Hoogerbrugge, N.; PHTS Guideline Development Group; The European Reference Network, GENTURIS. Cancer Surveillance Guideline for individuals with PTEN hamartoma tumour syndrome. Eur. J. Hum. Genet. 2020, 28, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.A.P.; MacFarland, S.P.; Perrino, M.R.; Mitchell, S.G.; Kamihara, J.; Nelson, A.T.; Mallinger, P.H.R.; Brzezinski, J.J.; Maxwell, K.N.; Woodward, E.R.; et al. Update on Pediatric Surveillance Recommendations for PTEN Hamartoma Tumor Syndrome, DICER1-Related Tumor Predisposition, and Tuberous Sclerosis Complex. Clin. Cancer Res. 2025, 31, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, A.; Baitamouni, S.; Liu, D.; Yehia, L.; Anthony, K.; McCarther, A.; Tischkowitz, M.; MacFarland, S.P.; Ngeow, J.; Hoogerbrugge, N.; et al. Cancer and Overgrowth Manifestations of PTEN Hamartoma Tumour Syndrome: Management Recommendations from the International PHTS Consensus Guidelines Working Group. Clin. Cancer Res. 2025, 31, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.A.P.; Williams, G.M.; Kamihara, J.; Stewart, D.R.; Harris, A.K.; Bauer, A.J.; Turner, J.; Shah, R.; Schneider, K.; Schneider, K.W.; et al. DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clin. Cancer Res. 2018, 24, 2251–2261. [Google Scholar] [CrossRef]
- Gonzalez, I.A.; Stewart, D.R.; Schultz, K.A.P.; Field, A.P.; Hill, D.A.; Dehner, L.P. DICER1 tumor predisposition syndrome: An evolving story initiated with the pleuropulmonary blastoma. Mod. Pathol. 2022, 35, 4–22. [Google Scholar] [CrossRef]
- Khan, N.E.; Bauer, A.J.; Schultz, K.A.P.; Doros, L.; Decastro, R.M.; Ling, A.; Lodish, M.B.; Harney, L.A.; Kase, R.G.; Carr, A.G.; et al. Quantification of Thyroid Cancer and Multinodular Goiter Risk in the DICER1 Syndrome: A Family-Based Cohort Study. J. Clin. Endocrinol. Metab. 2017, 102, 1614–1622. [Google Scholar] [CrossRef]
- Spaulding, S.L.; Maayah, M.; Dinauer, C.A.; Prasad, M.; Darbinyan, A.; Morotti, R.; Christison-Lagay, E.R. Molecular Genetics Augment Cytopathologic Evaluation and Surgical Planning of Pediatric Thyroid Nodules. J. Pediatr. Surg. 2024, 59, 975–980. [Google Scholar] [CrossRef]
- Wasserman, J.D.; Sabbaghian, N.; Fahiminiya, S.; Chami, R.; Mete, O.; Acker, M.; Wu, M.K.; Shlien, A.; de Kock, L.; Foulkes, W.D. DICER1 Mutations Are Frequent in Adolescent-Onset Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2018, 103, 2009–2015. [Google Scholar] [CrossRef]
- Lee, Y.A.; Im, S.W.; Jung, K.C.; Chung, E.J.; Shin, C.H.; Kim, J.I.; Park, Y.J. Predominant DICER1 Pathogenic Variants in Pediatric Follicular Thyroid Carcinomas. Thyroid 2020, 30, 1120–1131. [Google Scholar] [CrossRef]
- Sachdev, C.; Gattani, R.G.; Agrawal, J. Carney Complex and Its Association with Thyroid Cancer, Molecular Pathway, and Treatment. Cureus 2023, 15, e48503. [Google Scholar] [CrossRef]
- Carney, J.A.; Lyssikatos, C.; Seethala, R.R.; Lakatos, P.; Perez-Atayde, A.; Lahner, H.; Stratakis, C.A. The Spectrum of Thyroid Gland Pathology in Carney Complex: The Importance of Follicular Carcinoma. Am. J. Surg. Pathol. 2018, 42, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, P.; Davaro, E.P.; Doan, J.V.; Ising, M.E.; Evans, N.R.; Phillips, N.J.; Lai, J.; Guzman, M.A. Familial Adenomatous Polyposis Syndrome: An Update and Review of Extraintestinal Manifestations. Arch. Pathol. Lab. Med. 2019, 143, 1382–1398. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, M.; Barbesino, G.; Faquin, W.; Chan-Smutko, G.; Patel, D.; Shannon, K.M.; Daniels, G.H.; Chung, D.C. Prevalence of thyroid cancer in familial adenomatous polyposis syndrome and the role of screening ultrasound examinations. Clin. Gastroenterol. Hepatol. 2007, 5, 367–373. [Google Scholar] [CrossRef]
- Lam, A.K.; Fridman, M. Characteristics of cribriform morular variant of papillary thyroid carcinoma in post-Chernobyl affected region. Hum. Pathol. 2018, 74, 170–177. [Google Scholar] [CrossRef]
- MacFarland, S.P.; Becktell, K.; Schneider, K.W.; Kuiper, R.P.; Lesmana, H.; Meade, J.; Nichols, K.E.; Porter, C.C.; Savage, S.A.; Schultz, K.A.; et al. Pediatric Cancer Screening in Hereditary Gastrointestinal Cancer Risk Syndromes: An Update from the AACR Childhood Cancer Predisposition Working Group. Clin. Cancer Res. 2024, 30, 4566–4571. [Google Scholar] [CrossRef]
- Oshima, J.; Sidorova, J.M.; Monnat, R.J., Jr. Werner syndrome: Clinical features, pathogenesis and potential therapeutic interventions. Ageing Res. Rev. 2017, 33, 105–114. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Sugano, H.; Matsumoto, T.; Furuichi, Y.; Miller, R.W.; Goto, M. Unusual features of thyroid carcinomas in Japanese patients with Werner syndrome and possible genotype–phenotype relations to cell type and race. Cancer 1999, 85, 1345–1352. [Google Scholar] [CrossRef]
- Mathiesen, J.S.; Effraimidis, G.; Rossing, M.; Rasmussen, A.K.; Hoejberg, L.; Bastholt, L.; Godballe, C.; Oturai, P.; Feldt-Rasmussen, U. Multiple endocrine neoplasia type 2: A review. Semin. Cancer Biol. 2022, 79, 163–179. [Google Scholar] [CrossRef]
- Taylor-Miller, T.; Tucker, K.; Sugo, E.; Anazodo, A.; Mowat, D. Clues for Early Diagnosis of MEN2B Syndrome Before Medullary Thyroid Carcinoma. Pediatrics 2024, 154, e2022059517. [Google Scholar] [CrossRef]
- Castinetti, F.; Waguespack, S.G.; Machens, A.; Uchino, S.; Hasse-Lazar, K.; Sanso, G.; Else, T.; Dvorakova, S.; Qi, X.P.; Elisei, R.; et al. Natural history, treatment, and long-term follow up of patients with multiple endocrine neoplasia type 2B: An international, multicentre, retrospective study. Lancet Diabetes Endocrinol. 2019, 7, 213–220. [Google Scholar] [CrossRef]
- Ali, S.Z.; Baloch, Z.W.; Cochand-Priollet, B.; Schmitt, F.C.; Vielh, P.; VanderLaan, P.A. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023, 33, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Cherella, C.E.; Hollowell, M.L.; Smith, J.R.; Zendejas, B.; Modi, B.P.; Cibas, E.S.; Wassner, A.J. Subtype of atypia on cytology and risk of malignancy in pediatric thyroid nodules. Cancer Cytopathol. 2022, 130, 330–335. [Google Scholar] [CrossRef]
- Sipos, J.A.; Ringel, M.D. Molecular testing in thyroid cancer diagnosis and management. Best. Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101680. [Google Scholar] [CrossRef] [PubMed]
- Livhits, M.J.; Zhu, C.Y.; Kuo, E.J.; Nguyen, D.T.; Kim, J.; Tseng, C.H.; Leung, A.M.; Rao, J.; Levin, M.; Douek, M.L.; et al. Effectiveness of Molecular Testing Techniques for Diagnosis of Indeterminate Thyroid Nodules: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Steward, D.L.; Carty, S.E.; Sippel, R.S.; Yang, S.P.; Sosa, J.A.; Sipos, J.A.; Figge, J.J.; Mandel, S.; Haugen, B.R.; Burman, K.D.; et al. Performance of a Multigene Genomic Classifier in Thyroid Nodules with Indeterminate Cytology: A Prospective Blinded Multicenter Study. JAMA Oncol. 2019, 5, 204–212. [Google Scholar] [CrossRef]
- Lai, S.T.; Bauer, A.J. Approach to the Pediatric Patient with Thyroid Nodules. J. Clin. Endocrinol. Metab. 2025, 10, dgaf090. [Google Scholar] [CrossRef]
- Alexander, E.K.; Kennedy, G.C.; Baloch, Z.W.; Cibas, E.S.; Chudova, D.; Diggans, J.; Friedman, L.; Kloos, R.T.; LiVolsi, V.A.; Mandel, S.J.; et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N. Engl. J. Med. 2012, 367, 705–715. [Google Scholar] [CrossRef]
- Patel, K.N.; Angell, T.E.; Babiarz, J.; Barth, N.M.; Blevins, T.; Duh, Q.Y.; Ghossein, R.A.; Harrell, R.M.; Huang, J.; Kennedy, G.C.; et al. Performance of a Genomic Sequencing Classifier for the Preoperative Diagnosis of Cytologically Indeterminate Thyroid Nodules. JAMA Surg. 2018, 153, 817–824. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Ohori, N.P.; Hodak, S.P.; Carty, S.E.; LeBeau, S.O.; Ferris, R.L.; Yip, L.; Seethala, R.R.; Tublin, M.E.; Stang, M.T.; et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: A prospective analysis of 1056 FNA samples. J. Clin. Endocrinol. Metab. 2011, 96, 3390–3397. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Wald, A.I.; Roy, S.; Durso, M.B.; Nikiforov, Y.E. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J. Clin. Endocrinol. Metab. 2013, 98, E1852–E1860. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Mercurio, S.; Wald, A.I.; Barbi de Moura, M.; Callenberg, K.; Santana-Santos, L.; Gooding, W.E.; Yip, L.; Ferris, R.L.; Nikiforov, Y.E. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer 2018, 124, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Lupo, M.A.; Walts, A.E.; Sistrunk, J.W.; Giordano, T.J.; Sadow, P.M.; Massoll, N.; Campbell, R.; Jackson, S.A.; Toney, N.; Narick, C.M.; et al. Multiplatform molecular test performance in indeterminate thyroid nodules. Diagn. Cytopathol. 2020, 48, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.T.; Labourier, E.; Ablordeppey, K.K.; Surrey, L.F.; Mostoufi-Moab, S.; Isaza, A.; Adzick, N.S.; Kazahaya, K.; Kumar, G.; Bauer, A.J. miRNA expression can classify pediatric thyroid lesions and increases the diagnostic yield of mutation testing. Pediatr. Blood Cancer 2020, 67, e28276. [Google Scholar] [CrossRef] [PubMed]
- Mollen, K.P.; Shaffer, A.D.; Yip, L.; Monaco, S.E.; Huyett, P.; Viswanathan, P.; Witchel, S.F.; Duvvuri, U.; Simons, J.P. Unique Molecular Signatures Are Associated with Aggressive Histology in Pediatric Differentiated Thyroid Cancer. Thyroid 2022, 32, 236–244. [Google Scholar] [CrossRef]
- Nicholson, K.J.; Roberts, M.S.; McCoy, K.L.; Carty, S.E.; Yip, L. Molecular Testing Versus Diagnostic Lobectomy in Bethesda III/IV Thyroid Nodules: A Cost-Effectiveness Analysis. Thyroid 2019, 29, 1237–1243. [Google Scholar] [CrossRef]
- National Institute for Health and Care. Thyroid Cancer: Assessment and Management. In Evidence Review for Molecular Testing [NICE Guideline No. 230]; NICE: Manchester, UK, 2022; Available online: https://www.nice.org.uk/guidance/ng230 (accessed on 3 March 2025).
- Lau, L.M.S.; Khuong-Quang, D.A.; Mayoh, C.; Wong, M.; Barahona, P.; Ajuyah, P.; Senapati, A.; Nagabushan, S.; Sherstyuk, A.; Altekoester, A.K.; et al. Precision-guided treatment in high-risk pediatric cancers. Nat. Med. 2024, 30, 1913–1922. [Google Scholar] [CrossRef]
- Yang, A.T.; Lai, S.T.; Laetsch, T.W.; Bhatti, T.; Baloch, Z.; Surrey, L.F.; Franco, A.T.; Ricarte-Filho, J.C.M.; Mostoufi-Moab, S.; Adzick, N.S.; et al. Molecular landscape and therapeutic strategies in pediatric differentiated thyroid carcinoma. Endocr. Rev. 2025, 10, bnaf003. [Google Scholar] [CrossRef]
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef]
- Subbiah, V.; Hu, M.I.; Wirth, L.J.; Schuler, M.; Mansfield, A.S.; Curigliano, G.; Brose, M.S.; Zhu, V.W.; Leboulleux, S.; Bowles, D.W.; et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): A multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021, 9, 491–501. [Google Scholar] [CrossRef]
- Morgenstern, D.A.; Casanova, M.; van Tilburg, C.M.; Ziegler, D.S.; Campbell, M.; Watt, T.C.; Pappo, A.S.; Laetsch, T.W.; Liu, D.; Liming, K.; et al. Safety and efficacy of selpercatinib in pediatric patients with RET-altered solid tumors: Updated results from LIBRETTO-121. J. Clin. Oncol. 2024, 42, 10022. [Google Scholar] [CrossRef]
- Kazahaya, K.; Prickett, K.K.; Paulson, V.A.; Dahl, J.P.; Manning, S.C.; Rudzinski, E.R.; Rastatter, J.C.; Parikh, S.R.; Hawkins, D.S.; Brose, M.S.; et al. Targeted Oncogene Therapy Before Surgery in Pediatric Patients with Advanced Invasive Thyroid Cancer at Initial Presentation: Is It Time for a Paradigm Shift? JAMA Otolaryngol. Head. Neck Surg. 2020, 146, 748–753. [Google Scholar] [CrossRef] [PubMed]
- FDA. RETEVMO® (Selpercatinib) Capsules, for Oral Use. U.S. Patent; Updated 12/2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/218160s004lbl.pdf (accessed on 25 April 2025).
- Waguespack, S.G.; Drilon, A.; Lin, J.J.; Brose, M.S.; McDermott, R.; Almubarak, M.; Bauman, J.; Casanova, M.; Krishnamurthy, A.; Kummar, S.; et al. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. Eur. J. Endocrinol. 2022, 186, 631–643. [Google Scholar] [CrossRef] [PubMed]
- FDA. VITRAKVI® (Larotrectinib) Capsules, for Oral Use. U.S. Patent; Updated 11/2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/211710s009lbl.pdf (accessed on 25 April 2025).
- Bowles, D.W.; Bazhenova, L.; Hescot, S.; Folprecht, G.; Daga, H.; Massarelli, E.; Conley, A.P.; Lamartina, L.; Lin, J.; Ahn, E.; et al. Entrectinib in patients with ntrk fusion-positive (ntrk-fp) thyroid cancer: Updated data from startrk-2. Endocr. Abstr. 2022, 84, OP03-14. [Google Scholar] [CrossRef]
- Gouda, M.A.; Subbiah, V. Expanding the Benefit: Dabrafenib/Trametinib as Tissue-Agnostic Therapy for BRAF V600E-Positive Adult and Pediatric Solid Tumors. Am. Soc. Clin. Oncol. Educ. Book. 2023, 43, e404770. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.C.; Cabanillas, M.E.; Boran, A.; et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: Updated analysis from the phase II ROAR basket study. Ann. Oncol. 2022, 33, 406–415. [Google Scholar] [CrossRef]
- Van Tilburg, C.; Albert, C.M.; Bielack, S.; DuBois, S.G.; Federman, N.; Georger, B.; RNagasubramanian, R.; Pappo, A.S.; Norenburg, R.; Dima, L.; et al. Abstract: Efficacy and safety of larotrectinib in pediatric patients with non-central nervous system tropomysin receptor kinase fusion-positive cancer: And expanded dataset. Pediatr. Blood Cancer 2021, 68 (Suppl. S5), e29349. [Google Scholar] [CrossRef]
- Mascarenhas, L.; van Tilburg, C.M.; Doz, F.; Zwaan, C.M.; Albert, C.M.; Blattman, C.; Geoerger, B.; DuBois, S.G.; Federman, N.; Nagasubramanian, R.; et al. Efficacy and safety of larotrectinib in pediatric patients with tropomyosin receptor kinase (TRK) fusion-positive cancer: An expanded dataset. J. Clin. Oncol. 2022, 40, 10030. [Google Scholar] [CrossRef]
- Chuk, M.K.; Widemann, B.C.; Minard, C.G.; Liu, X.; Kim, A.; Bernhardt, M.B.; Kudgus, R.A.; Reid, J.M.; Voss, S.D.; Blaney, S.; et al. A phase 1 study of cabozantinib in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1211, a report from the Children’s Oncology Group. Pediatr. Blood Cancer 2018, 65, e27077. [Google Scholar] [CrossRef]
- Chesover, A.D.; Vali, R.; Hemmati, S.H.; Wasserman, J.D. Lung Metastasis in Children with Differentiated Thyroid Cancer: Factors Associated with Diagnosis and Outcomes of Therapy. Thyroid 2021, 31, 50–60. [Google Scholar] [CrossRef]
- Sugino, K.; Nagahama, M.; Kitagawa, W.; Ohkuwa, K.; Uruno, T.; Matsuzu, K.; Suzuki, A.; Tomoda, C.; Hames, K.Y.; Akaishi, J.; et al. Distant Metastasis in Pediatric and Adolescent Differentiated Thyroid Cancer: Clinical Outcomes and Risk Factor Analyses. J. Clin. Endocrinol. Metab. 2020, 105, e3981–e3988. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; Alswailem, M.; Moria, Y.; Almutairi, R.; Alotaibi, M.; Murugan, A.K.; Qasem, E.; Alghamdi, B.; Al-Hindi, H. Lung Metastasis in Pediatric Thyroid Cancer: Radiological Pattern, Molecular Genetics, Response to Therapy, and Outcome. J. Clin. Endocrinol. Metab. 2019, 104, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Waguespack, S.G.; Tewari, S.O.; Busaidy, N.L.; Zafereo, M.E. Larotrectinib Before Initial Radioactive Iodine Therapy in Pediatric TRK Fusion-Positive Papillary Thyroid Carcinoma: Time to Reconsider the Treatment Paradigm for Distantly Metastatic Disease? JCO Precis. Oncol. 2022, 6, e2100467. [Google Scholar] [CrossRef] [PubMed]
- Groussin, L.; Theodon, H.; Bessiene, L.; Bricaire, L.; Bonnet-Serrano, F.; Cochand-Priollet, B.; Leroy, K.; Garinet, S.; Pasmant, E.; Zerbit, J.; et al. Redifferentiating Effect of Larotrectinib in NTRK-Rearranged Advanced Radioactive-Iodine Refractory Thyroid Cancer. Thyroid 2022, 32, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Sayehli, C.; Hanscheid, H.; Higuchi, T.; Serfling, S.E.; Fassnacht, M.; Goebeler, M.E.; Buck, A.K.; Kroiss, M. Successful combination of selpercatinib and radioiodine after pretherapeutic dose estimation in RET-altered thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1833–1834. [Google Scholar] [CrossRef]
- Syed, A.R.; Gorana, A.; Nohr, E.; Yuan, X.K.; Amin, M.P.; Ghaznavi, S.; Lamb, D.; McIntyre, J.; Eszlinger, M.; Paschke, R. Predictors of radioiodine (RAI)-avidity restoration for NTRK fusion-positive RAI-resistant metastatic thyroid cancers. Eur. Thyroid. J. 2024, 13, e230227. [Google Scholar] [CrossRef]
- Weiler, D.; Perez Lago, M.D.S. Successful radioiodine redifferentiation with selpercatinib in RET fusion-positive papillary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3467–3468. [Google Scholar] [CrossRef]
| Diagnostic Category | ROM Mean (Range) | |
|---|---|---|
| I | Nondiagnostic | 14% (0–33) |
| II | Benign a | 6% (0–27) |
| III | Atypia of undetermined significance | 28% (11–54) |
| IV | Follicular neoplasm b | 50% (28–100) |
| V | Suspicious for malignancy | 81% (40–100) |
| VI | Malignant | 98% (86–100) |
| Tier | Molecular Alteration | Risk of Invasive Disease | Surgical Approach | |
|---|---|---|---|---|
| 1 | Point mutations
| Low | Lobectomy without central neck dissection provided no evidence of LN metastasis on US | Completion thyroidectomy if >2 vessel vascular invasion or high-grade histologic features |
| 2 | Point mutations
| Intermediate | Total thyroidectomy with prophylactic/therapeutic central neck dissection. Consider lobectomy with prophylactic central neck dissection if no evidence of LN metastasis on US. | Completion thyroidectomy if multifocality or ≥5 positive LNs |
| 3 | Fusions
| High | ||
| Agent | Molecular Target(s) | Histology | n | Age (Years) | Response | Citation |
|---|---|---|---|---|---|---|
| Selpercatinib | RET | MTC | 14 | 2–20 2–20 | ORR 83.3% | [92] |
| PTC | 10 | ORR 100% | ||||
| 2-year PFS 92.4% | ||||||
| Larotrectinib | NTRK | Agnostic | 78 | 0.1–17.8 | ORR 88% | [100] |
| 94 | 0–18 | ORR 84% | [101] | |||
| PTC | 2 | 6–13 | PR 50%, CR 50% | [95] | ||
| Cabozantinib | VEGFR2, RET, MET, FLT3, NTRK, AXL | MTC | 5 | 1 | PR 40% | [102] |
| Agent | Molecular Target(s) | Histology | Age (Years) | Location(s) | Status | ClinicalTrials.gov ID |
|---|---|---|---|---|---|---|
| Larotrectinib | NTRK | DTC | ≥1 | USA | Recruiting | NCT05783323 |
| Repotrectinib | ALK, ROS1, NTRK | Agnostic | ≥12 | International | Recruiting | NCT03093116 |
| Pralsetinib | RET | Agnostic | 0.5–21 | International | Active, not recruiting | NCT03899792 |
| Pralsetinib | RET | Thyroid carcinoma | ≥12 | USA | Active, not recruiting | NCT04759911 |
| Pralsetinib | RET | DTC | ≥12 | USA | Recruiting | NCT05668962 |
| Pralsetinib | RET | Agnostic | ≥12 | International | Active, not recruiting | NCT03157128 |
| Oncogene-specific kinase inhibitors | NTRK, RET, ALK, BRAFV600E | DTC | ≥0 | USA, Australia | Recruiting | NCT05024929 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanderniet, J.A.; Fuentes-Bolanos, N.A.; Cho, Y.H.; Tucker, K.M.; Anazodo, A.; Bauer, A.J.; Benitez-Aguirre, P.Z. Paediatric Thyroid Carcinoma: The Genetic Revolution and Its Implications for Therapy and Outcomes. Cancers 2025, 17, 1549. https://doi.org/10.3390/cancers17091549
Vanderniet JA, Fuentes-Bolanos NA, Cho YH, Tucker KM, Anazodo A, Bauer AJ, Benitez-Aguirre PZ. Paediatric Thyroid Carcinoma: The Genetic Revolution and Its Implications for Therapy and Outcomes. Cancers. 2025; 17(9):1549. https://doi.org/10.3390/cancers17091549
Chicago/Turabian StyleVanderniet, Joel A., Noemi A. Fuentes-Bolanos, Yoon Hi Cho, Katherine M. Tucker, Antoinette Anazodo, Andrew J. Bauer, and Paul Z. Benitez-Aguirre. 2025. "Paediatric Thyroid Carcinoma: The Genetic Revolution and Its Implications for Therapy and Outcomes" Cancers 17, no. 9: 1549. https://doi.org/10.3390/cancers17091549
APA StyleVanderniet, J. A., Fuentes-Bolanos, N. A., Cho, Y. H., Tucker, K. M., Anazodo, A., Bauer, A. J., & Benitez-Aguirre, P. Z. (2025). Paediatric Thyroid Carcinoma: The Genetic Revolution and Its Implications for Therapy and Outcomes. Cancers, 17(9), 1549. https://doi.org/10.3390/cancers17091549





