Differential Infiltration of T-Cell Populations in Tumor and Liver Tissues Predicts Recurrence-Free Survival in Surgically Resected Hepatocellular Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Samples
2.2. Flow Cytometry
2.3. Statistical Analysis
3. Results
3.1. Differential Expressions of Tissue-Resident Markers Among T Cells Between Tumor and Background Liver Tissues
3.2. Associations Between Clinical or Pathologic Characteristics and Each T-Cell Subpopulation Within Tumor and Background Liver Tissues
3.3. Clinical Relevance of Relative Infiltration of Each T-Cell Subpopulation Within Tumor and Background Liver Tissues in Patients with HCC with Resection
3.4. Expressions of Exhaustion and Cytotoxicity Markers of Each T-Cell Subpopulation Within Tumor and Background Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Alc | Alcohol-related liver disease |
| AFP | Alpha fetoprotein |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| CI | Confidence interval |
| CTL | Cytotoxic T lymphocyte |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| HR | Hazard ratio |
| MFI | Mean fluorescence intensity |
| MHC | Major histocompatibility complex |
| PD-1 | Programmed death protein 1 |
| NKG2D | Natural killer group 2 member D |
| NT | Non-tumor |
| OS | Overall survival |
| PIVKA-II | Protein induced by vitamin K absence or antagonist-II |
| RFS | Recurrence-free survival |
| T-tissue | Tumor tissue |
| TME | Tumor microenvironment |
| TRM | Tissue-resident memory T cells |
| TCGA | The Cancer Genome Atlas |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Korean Liver Cancer, A.; National Cancer Center, K. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 583–705. [Google Scholar] [CrossRef]
- Kim, N.R.; Rho, S.Y.; Navarro, J.; An, C.; Han, D.H.; Choi, J.S.; Kim, M.J.; Choi, G.H. Additional nodules detected using EOB-MRI in patients with resectable single hepatocellular carcinoma: An implication for active treatment strategy. J. Liver Cancer 2024, 24, 92–101. [Google Scholar] [CrossRef]
- Huang, Y.; Su, Y.; Chen, Y.; Xu, J.; Zhu, L.; Wei, H.; Qin, S.; Peng, Y.; Qi, L. Risk factors and nomogram predictive models for postsurgical progression/hyperprogression recurrence in hepatocellular carcinoma with macroscopic vascular invasion. World J. Surg. Oncol. 2024, 22, 305. [Google Scholar] [CrossRef]
- St Paul, M.; Ohashi, P.S. The Roles of CD8(+) T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020, 30, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M.; Topalian, S.L. The role of CD4+ T cell responses in antitumor immunity. Curr. Opin. Immunol. 1998, 10, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Ma, N.; Yang, Y.; Ma, Y.; Wang, F.; Wang, Y.; Wei, J.; Chen, H.; Tartarone, A.; et al. Progenitor-like exhausted SPRY1+CD8+ T cells potentiate responsiveness to neoadjuvant PD-1 blockade in esophageal squamous cell carcinoma. Cancer Cell 2023, 41, 1852–1870.E9. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, F.; Liu, Y.; Zhang, Z.; Hu, H.; Ning, S.; Zhang, H.; Chen, X.; Liao, Z.; Zhang, Z. Targeting TM4SF1 promotes tumor senescence enhancing CD8+ T cell cytotoxic function in hepatocellular carcinoma. Clin. Mol. Hepatol. 2024, 31, 489–508. [Google Scholar] [CrossRef]
- Ran, L.F.; Xie, X.P.; Xia, J.Z.; Xie, F.L.; Fan, Y.M.; Wu, F. T-lymphocytes from focused ultrasound ablation subsequently mediate cellular antitumor immunity after adoptive cell transfer immunotherapy. Front. Immunol. 2023, 14, 1155229. [Google Scholar] [CrossRef]
- Suzuki, H.; Mishra, S.; Paul, S.; Hoshida, Y. Molecular and immune landscape of hepatocellular carcinoma for therapeutic development. J. Liver Cancer 2024, 25, 9–18. [Google Scholar] [CrossRef]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B.A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S.K.; Poon, C.; Gant, K.L.; et al. Immune checkpoint therapy-current perspectives and future directions. Cell 2023, 186, 1652–1669. [Google Scholar] [CrossRef]
- Buggert, M.; Price, D.A.; Mackay, L.K.; Betts, M.R. Human circulating and tissue-resident memory CD8(+) T cells. Nat. Immunol. 2023, 24, 1076–1086. [Google Scholar] [CrossRef]
- Tang, K.; Li, X.; Mo, J.; Chen, Y.; Huang, C.; Li, T.; Luo, T.; Zhong, Z.; Jiang, Y.; Yang, D.; et al. CD69 serves as a potential diagnostic and prognostic biomarker for hepatocellular carcinoma. Sci. Rep. 2023, 13, 7452. [Google Scholar] [CrossRef] [PubMed]
- Skon, C.N.; Lee, J.Y.; Anderson, K.G.; Masopust, D.; Hogquist, K.A.; Jameson, S.C. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 2013, 14, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, B.G.; Pallett, L.J.; Li, X.; Davies, S.P.; Amin, O.E.; Gill, U.S.; Kucykowicz, S.; Patel, A.M.; Aliazis, K.; Liu, Y.S.; et al. The human liver microenvironment shapes the homing and function of CD4(+) T-cell populations. Gut 2022, 71, 1399–1411. [Google Scholar] [CrossRef]
- Li, Y.; Gu, Y.; Yang, P.; Wang, Y.; Yu, X.; Li, Y.; Jin, Z.; Xu, L. CD69 is a Promising Immunotherapy and Prognosis Prediction Target in Cancer. Immunotargets Ther. 2024, 13, 1–14. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, B.; Goswami, S.; Meng, L.; Zhang, D.; Cao, C.; Li, T.; Zhu, F.; Ma, L.; Zhang, Z.; et al. PD1(Hi) CD8(+) T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.J.; Lee, Y.H.; Pan, L.; Lai, L.; Chua, C.; Wasser, M.; Lim, T.K.H.; Yeong, J.; Toh, H.C.; Lee, S.Y.; et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut 2019, 68, 916–927. [Google Scholar] [CrossRef]
- Hack, S.P.; Spahn, J.; Chen, M.; Cheng, A.L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020, 16, 975–989. [Google Scholar] [CrossRef]
- Yim, S.Y.; Lee, S.H.; Baek, S.W.; Sohn, B.; Jeong, Y.S.; Kang, S.H.; Park, K.; Park, H.; Lee, S.S.; Kaseb, A.O.; et al. Genomic biomarkers to predict response to atezolizumab plus bevacizumab immunotherapy in hepatocellular carcinoma: Insights from the IMbrave150 trial. Clin. Mol. Hepatol. 2024, 30, 807–823. [Google Scholar] [CrossRef]
- Sohn, W.; Lee, Y.S.; Kim, S.S.; Kim, J.H.; Jin, Y.J.; Kim, G.A.; Sung, P.S.; Yoo, J.J.; Chang, Y.; Lee, E.J.; et al. KASL clinical practice guidelines for the management of metabolic dysfunction-associated steatotic liver disease 2025. Clin. Mol. Hepatol. 2025, 31, S1–S31. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Nakashima, O.; Kutami, R.; Yamamoto, O.; Kojiro, M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology 1998, 27, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; He, J.C.; Yang, Y.; Wang, J.M.; Qian, Y.W.; Yang, T.; Ji, L. The Prognostic Value of Tumor-infiltrating Lymphocytes in Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Sci. Rep. 2017, 7, 7525. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Xu, X.; Qian, Y.; Xue, W.; Wang, Y.; Du, J.; Jin, L.; Tan, Y. Prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: A meta-analysis. Medicine 2018, 97, e13301. [Google Scholar] [CrossRef]
- Park, D.J.; Sung, P.S.; Kim, J.H.; Lee, G.W.; Jang, J.W.; Jung, E.S.; Bae, S.H.; Choi, J.Y.; Yoon, S.K. EpCAM-high liver cancer stem cells resist natural killer cell-mediated cytotoxicity by upregulating CEACAM1. J. Immunother. Cancer 2020, 8, e000301. [Google Scholar] [CrossRef]
- Sung, P.S.; Jang, J.W. Natural Killer Cell Dysfunction in Hepatocellular Carcinoma: Pathogenesis and Clinical Implications. Int. J. Mol. Sci. 2018, 19, 3648. [Google Scholar] [CrossRef]
- Simoni, Y.; Becht, E.; Fehlings, M.; Loh, C.Y.; Koo, S.L.; Teng, K.W.W.; Yeong, J.P.S.; Nahar, R.; Zhang, T.; Kared, H.; et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018, 557, 575–579. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, J.W.; Choi, Y.J.; Rha, M.S.; Koh, J.Y.; Kim, K.H.; Kim, C.G.; Lee, Y.J.; Kim, A.R.; Park, J.; et al. Functions of human liver CD69(+)CD103(-)CD8(+) T cells depend on HIF-2alpha activity in healthy and pathologic livers. J. Hepatol. 2020, 72, 1170–1181. [Google Scholar] [CrossRef]
- Chu, Y.; Liao, J.; Li, J.; Wang, Y.; Yu, X.; Wang, J.; Xu, X.; Xu, L.; Zheng, L.; Xu, J.; et al. CD103(+) tumor-infiltrating lymphocytes predict favorable prognosis in patients with esophageal squamous cell carcinoma. J. Cancer 2019, 10, 5234–5243. [Google Scholar] [CrossRef]
- Barsch, M.; Salie, H.; Schlaak, A.E.; Zhang, Z.; Hess, M.; Mayer, L.S.; Tauber, C.; Otto-Mora, P.; Ohtani, T.; Nilsson, T.; et al. T-cell exhaustion and residency dynamics inform clinical outcomes in hepatocellular carcinoma. J. Hepatol. 2022, 77, 397–409. [Google Scholar] [CrossRef]
- Chen, L.; Huang, H.; Huang, Z.; Chen, J.; Liu, Y.; Wu, Y.; Li, A.; Ge, J.; Fang, Z.; Xu, B.; et al. Prognostic values of tissue-resident CD8(+)T cells in human hepatocellular carcinoma and intrahepatic cholangiocarcinoma. World J. Surg. Oncol. 2023, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Mita, Y.; Kimura, M.Y.; Hayashizaki, K.; Koyama-Nasu, R.; Ito, T.; Motohashi, S.; Okamoto, Y.; Nakayama, T. Crucial role of CD69 in anti-tumor immunity through regulating the exhaustion of tumor-infiltrating T cells. Int. Immunol. 2018, 30, 559–567. [Google Scholar] [CrossRef]
- Han, J.W.; Kim, J.H.; Kim, D.H.; Jang, J.W.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; Ahn, J.; Yang, H.; Sung, P.S. Higher Number of Tumor-Infiltrating PD-L1+ Cells Is Related to Better Response to Multikinase Inhibitors in Hepatocellular Carcinoma. Diagnostics 2023, 13, 1453. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.X.; Lao, X.M.; Wang, X.Y.; Ren, Y.Z.; Lu, Y.T.; Shi, W.; Wang, Y.Z.; Wu, C.Y.; Xu, L.; Chen, M.S.; et al. Pericancerous cross-presentation to cytotoxic T lymphocytes impairs immunotherapeutic efficacy in hepatocellular carcinoma. Cancer Cell 2024, 42, 2082–2097.E10. [Google Scholar] [CrossRef]
- Kumar, B.V.; Ma, W.; Miron, M.; Granot, T.; Guyer, R.S.; Carpenter, D.J.; Senda, T.; Sun, X.; Ho, S.H.; Lerner, H.; et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017, 20, 2921–2934. [Google Scholar] [CrossRef]
- Park, S.L.; Gebhardt, T.; Mackay, L.K. Tissue-Resident Memory T Cells in Cancer Immunosurveillance. Trends Immunol. 2019, 40, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Pallett, L.J.; Maini, M.K. Liver-resident memory T cells: Life in lockdown. Semin. Immunopathol. 2022, 44, 813–825. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Li, P.; Luo, J.; Qin, R.; Peng, Q.; Li, B.; Wei, X.; Wang, T.; Shi, H.; et al. Single-cell analysis reveals HBV-specific PD-1(+)CD8(+) TRM cells in tumor borders are associated with HBV-related hepatic damage and fibrosis in HCC patients. J. Exp. Clin. Cancer Res. 2023, 42, 152. [Google Scholar] [CrossRef]
- Park, J.G.; Roh, P.R.; Kang, M.W.; Cho, S.W.; Hwangbo, S.; Jung, H.D.; Kim, H.U.; Kim, J.H.; Yoo, J.S.; Han, J.W.; et al. Intrahepatic IgA complex induces polarization of cancer-associated fibroblasts to matrix phenotypes in the tumor microenvironment of HCC. Hepatology 2024, 80, 1074–1086. [Google Scholar] [CrossRef]
- Kumar, B.V.; Kratchmarov, R.; Miron, M.; Carpenter, D.J.; Senda, T.; Lerner, H.; Friedman, A.; Reiner, S.L.; Farber, D.L. Functional heterogeneity of human tissue-resident memory T cells based on dye efflux capacities. JCI Insight 2018, 3, e123568. [Google Scholar] [CrossRef]
- Dumauthioz, N.; Labiano, S.; Romero, P. Tumor Resident Memory T Cells: New Players in Immune Surveillance and Therapy. Front. Immunol. 2018, 9, 2076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, Z.; Zhang, F.; Meadows, G.G. Alcohol consumption and antitumor immunity: Dynamic changes from activation to accelerated deterioration of the immune system. Adv. Exp. Med. Biol. 2015, 815, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Ratna, A.; Mandrekar, P. Alcohol and Cancer: Mechanisms and Therapies. Biomolecules 2017, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhou, J.; Xu, H.; Li, Y.; Ma, S.; Qiao, H.; Zeng, K.; Wang, Q.; Ouyang, J.; Liu, Y.; et al. Alcohol reshapes a liver premetastatic niche for cancer by extra- and intrahepatic crosstalk-mediated immune evasion. Mol. Ther. 2023, 31, 2662–2680. [Google Scholar] [CrossRef]
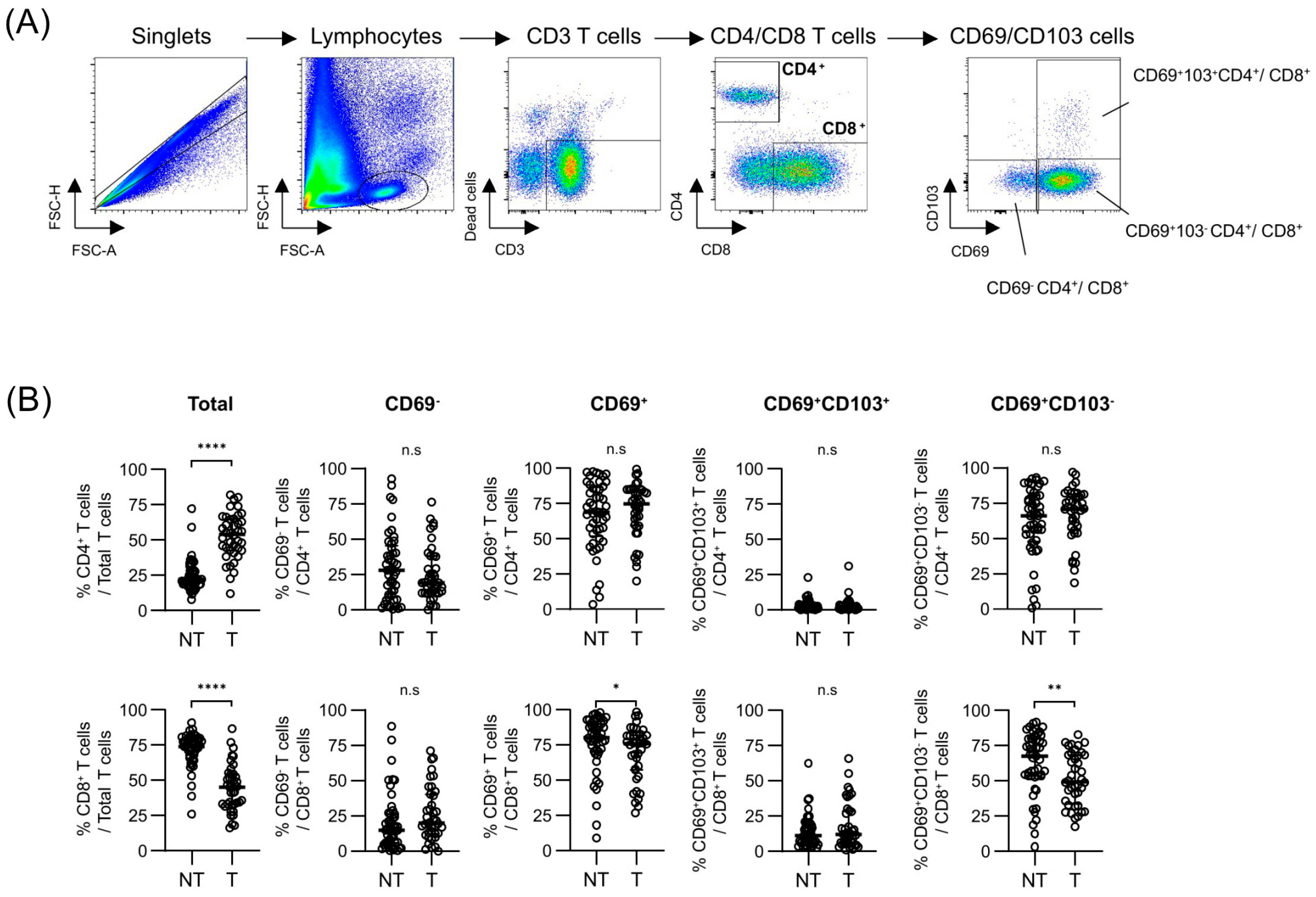


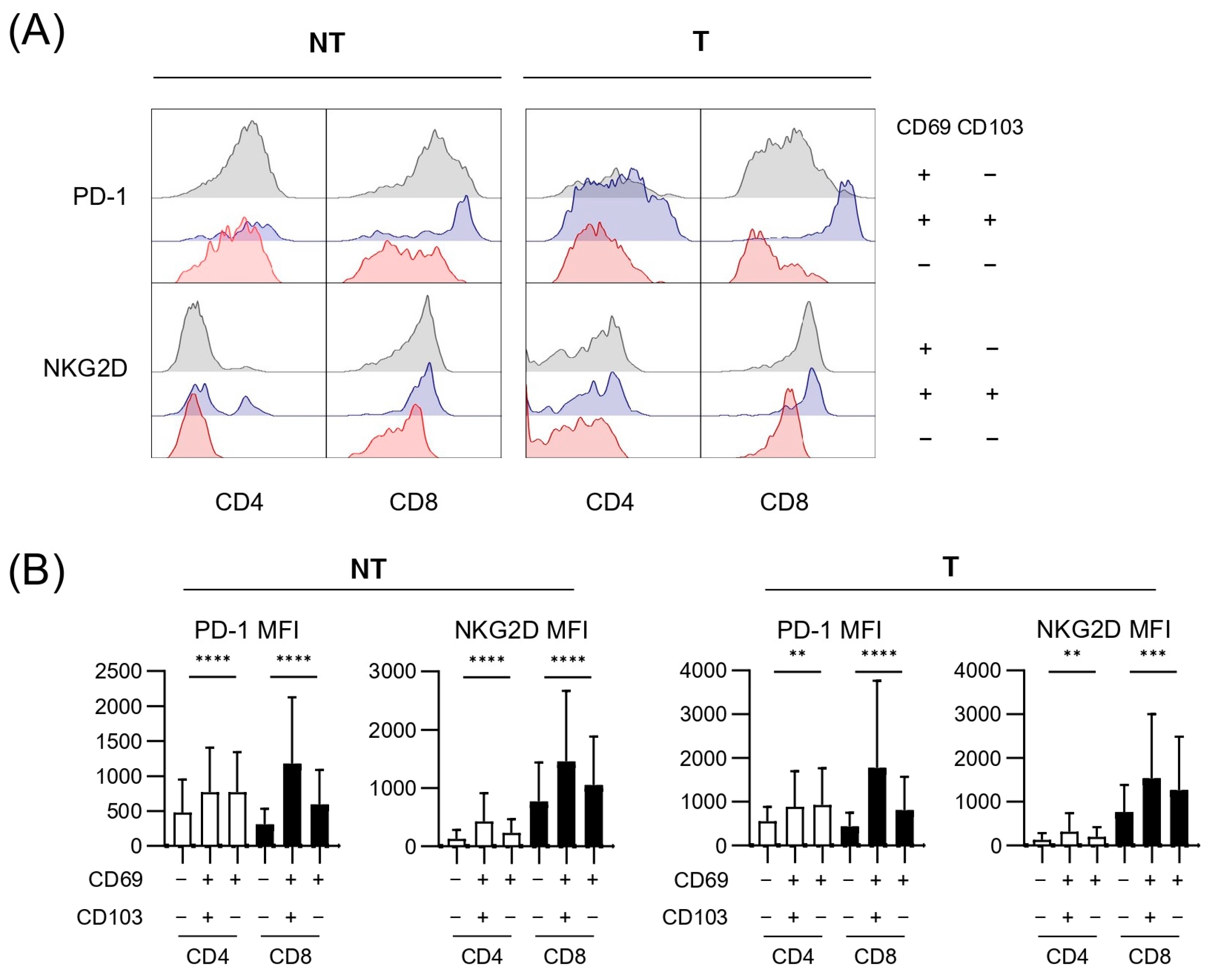
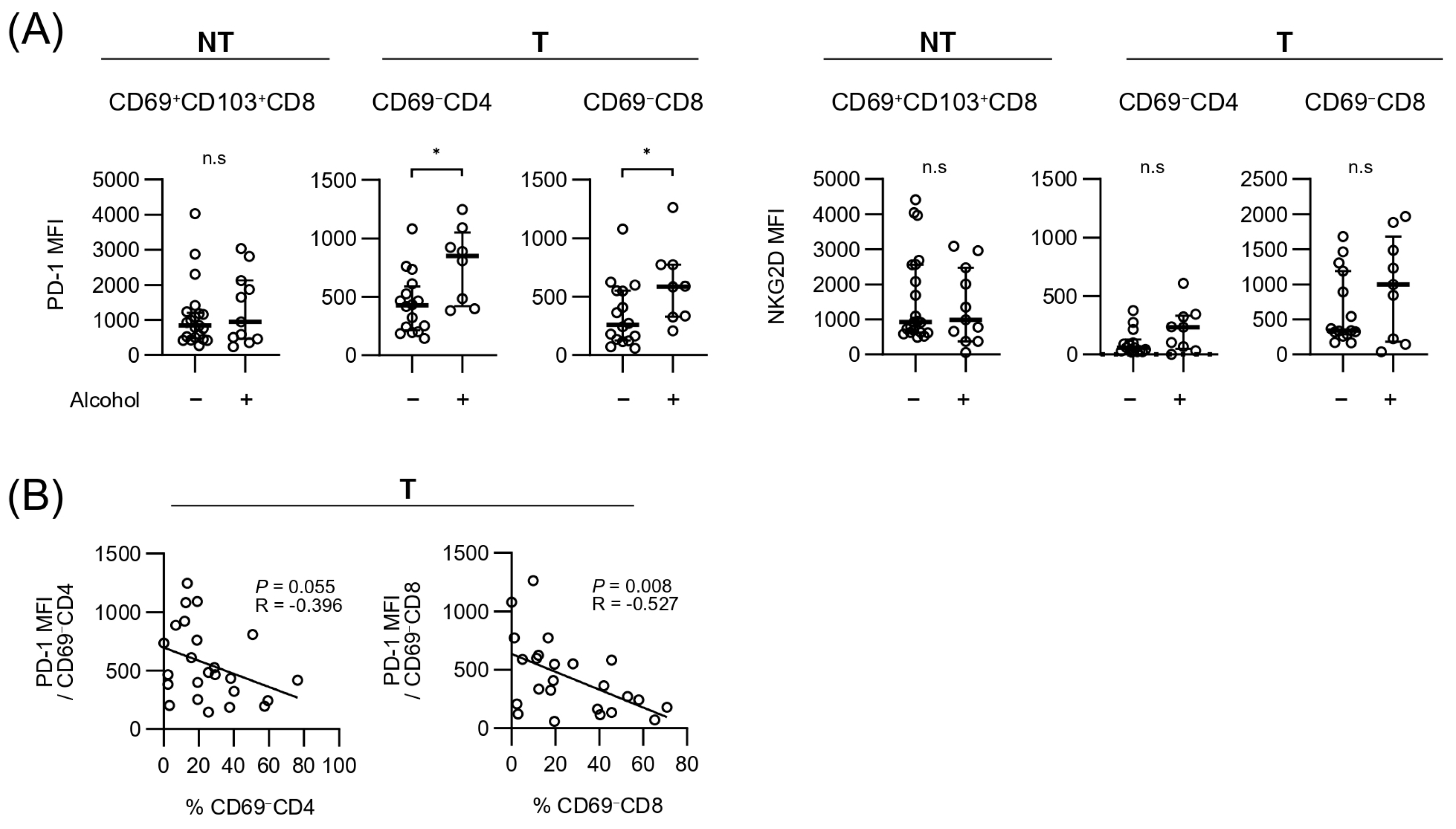
| Total (n = 57) | |
|---|---|
| Age (y) | 62.7 ± 8.8 |
| Male gender (n, %) | 43 (75) |
| Etiology | |
| HBV (n, %) | 28 (49) |
| Alcohol (n, %) | 9 (16) |
| HBV + Alc (n, %) | 12 (21) |
| MASH (n, %) | 4 (7) |
| Unknown (n, %) | 4 (7) |
| AST (U/L) | 32.0 (23.0–39.0) |
| ALT (U/L) | 29.0 (20.0–45.0) |
| Total bilirubin (mg/dL) | 0.7 (0.5–1.0) |
| Albumin (g/dL) | 4.4 (4.1–4.6) |
| AFP (ng/mL) | 4.5 (2.7–47.5) |
| PIVKA-II (mAU/mL) | 43.0 (20.0–274.0) |
| Tumor number | 1.0 (1.0–1.0) |
| Largest tumor size | 3.7 (2.0–5.6) |
| Worst tumor grade | 3.0 (2.0–3.0) |
| Major tumor grade | 2.0 (2.0–2.0) |
| Microvascular invasion (n, %) | 19 (33) |
| Pathologic T stage | 1.0 (1.0–2.0) |
| Non-tumor/Tumor Tissue/ Clinicopathological | Population | HR (95% CI) | p | cutoff |
|---|---|---|---|---|
| NT | CD69−CD4 | 0.98 (0.93–1.02) | 0.294 | |
| NT | CD69+CD4 | 1.03 (0.98–1.07) | 0.275 | |
| NT | CD69+CD103+CD4 | 0.98 (0.89–1.08) | 0.712 | |
| NT | CD69+CD103−CD4 | 1.03 (0.98–1.09) | 0.184 | |
| NT | CD69−CD8 | 0.96 (0.89–1.03) | 0.258 | |
| NT | CD69+CD8 | 1.97 (1.04–3.71) | 0.037 | 85.6 |
| NT | CD69+CD103+CD8 | 0.41 (0.23–0.73) | 0.002 | 3.7 |
| NT | CD69+CD103−CD8 | 2.14 (1.33–3.44) | <0.001 | 82.3 |
| T | CD69−CD4 | 0.48 (0.32–0.72) | <0.001 | 11.5 |
| T | CD69+CD4 | 2.23 (1.44–3.45) | <0.001 | 85.4 |
| T | CD69+CD103+CD4 | 1.89 (1.19–3.01) | 0.007 | 1.6 |
| T | CD69+CD103−CD4 | 1.93 (1.22–3.05) | 0.005 | 81.2 |
| T | CD69−CD8 | 0.46 (0.26–0.81) | 0.007 | 19.5 |
| T | CD69+CD8 | 2.15 (1.33–3.49) | 0.002 | 76.5 |
| T | CD69+CD103+CD8 | 1.63 (1.01–2.65) | 0.049 | 41.1 |
| T | CD69+CD103−CD8 | 1.74 (1.04–2.93) | 0.036 | 75.3 |
| Clinicopathological | Age | 1.00 (0.97–1.03) | 0.964 | |
| Clinicopathological | Male gender | 1.41 (0.46–4.30) | 0.756 | |
| Clinicopathological | Viral etiology | 0.67 (0.31–1.42) | 0.295 | |
| Clinicopathological | Alcohol etiology | 1.34 (0.27–6.66) | 0.72 | |
| Clinicopathological | AST | 1.00 (0.99–1.01) | 0.901 | |
| Clinicopathological | ALT | 0.99 (0.98–1.01) | 0.473 | |
| Clinicopathological | Total bilirubin | 0.86 (0.31–2.34) | 0.761 | |
| Clinicopathological | Albumin | 0.56 (0.28–1.13) | 0.107 | |
| Clinicopathological | INR | 1.15 (0.09–14.76) | 0.913 | |
| Clinicopathological | AFP | 1.86 (1.18–2.93) | 0.007 | 102 |
| Clinicopathological | PIVKA-II | 2.07 (1.26–3.39) | 0.004 | 493 |
| Clinicopathological | Tumor number | 1.19 (0.52–2.72) | 0.688 | |
| Clinicopathological | Largest tumor size | 2.82 (1.47–5.41) | 0.002 | 6.9 |
| Clinicopathological | Worst tumor grade | 1.45 (0.75–2.81) | 0.264 | |
| Clinicopathological | Major tumor grade | 1.67 (0.80–3.49) | 0.172 | |
| Clinicopathological | microvascular invasion | 1.55 (0.47–5.11) | 0.469 | |
| Clinicopathological | Pathologic T stage | 1.94 (1.22–3.08) | 0.005 | 3 |
| Clinicopathological | Histologic liver fibrosis | 1.18 (0.59–2.39) | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, E.J.; Choi, H.J.; You, Y.K.; Seo, D.H.; Kwon, M.H.; Yang, K.; Lee, J.; Jang, J.W.; Yoon, S.K.; Han, J.W.; et al. Differential Infiltration of T-Cell Populations in Tumor and Liver Tissues Predicts Recurrence-Free Survival in Surgically Resected Hepatocellular Carcinoma. Cancers 2025, 17, 1548. https://doi.org/10.3390/cancers17091548
Jang EJ, Choi HJ, You YK, Seo DH, Kwon MH, Yang K, Lee J, Jang JW, Yoon SK, Han JW, et al. Differential Infiltration of T-Cell Populations in Tumor and Liver Tissues Predicts Recurrence-Free Survival in Surgically Resected Hepatocellular Carcinoma. Cancers. 2025; 17(9):1548. https://doi.org/10.3390/cancers17091548
Chicago/Turabian StyleJang, Eun Ji, Ho Joong Choi, Young Kyoung You, Deok Hwa Seo, Mi Hyun Kwon, Keungmo Yang, Jaejun Lee, Jeong Won Jang, Seung Kew Yoon, Ji Won Han, and et al. 2025. "Differential Infiltration of T-Cell Populations in Tumor and Liver Tissues Predicts Recurrence-Free Survival in Surgically Resected Hepatocellular Carcinoma" Cancers 17, no. 9: 1548. https://doi.org/10.3390/cancers17091548
APA StyleJang, E. J., Choi, H. J., You, Y. K., Seo, D. H., Kwon, M. H., Yang, K., Lee, J., Jang, J. W., Yoon, S. K., Han, J. W., & Sung, P. S. (2025). Differential Infiltration of T-Cell Populations in Tumor and Liver Tissues Predicts Recurrence-Free Survival in Surgically Resected Hepatocellular Carcinoma. Cancers, 17(9), 1548. https://doi.org/10.3390/cancers17091548






