Clinical Outcomes and Prognostic Factors for Extramammary Paget’s Disease Treated with Radiation Therapy: A Multi-Institutional Observational Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Pretreatment Evaluation
2.2. RT
2.3. Combination Therapy
2.4. Follow-Up Evaluation and Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
3.2. Tumor Responses
3.3. Outcomes
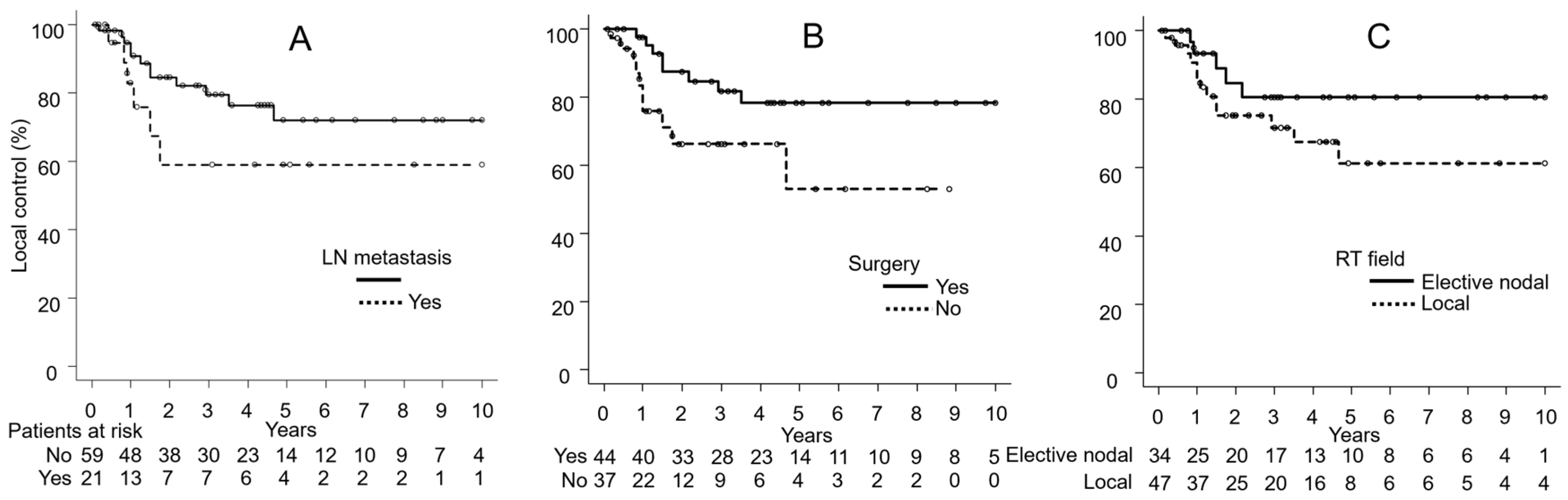
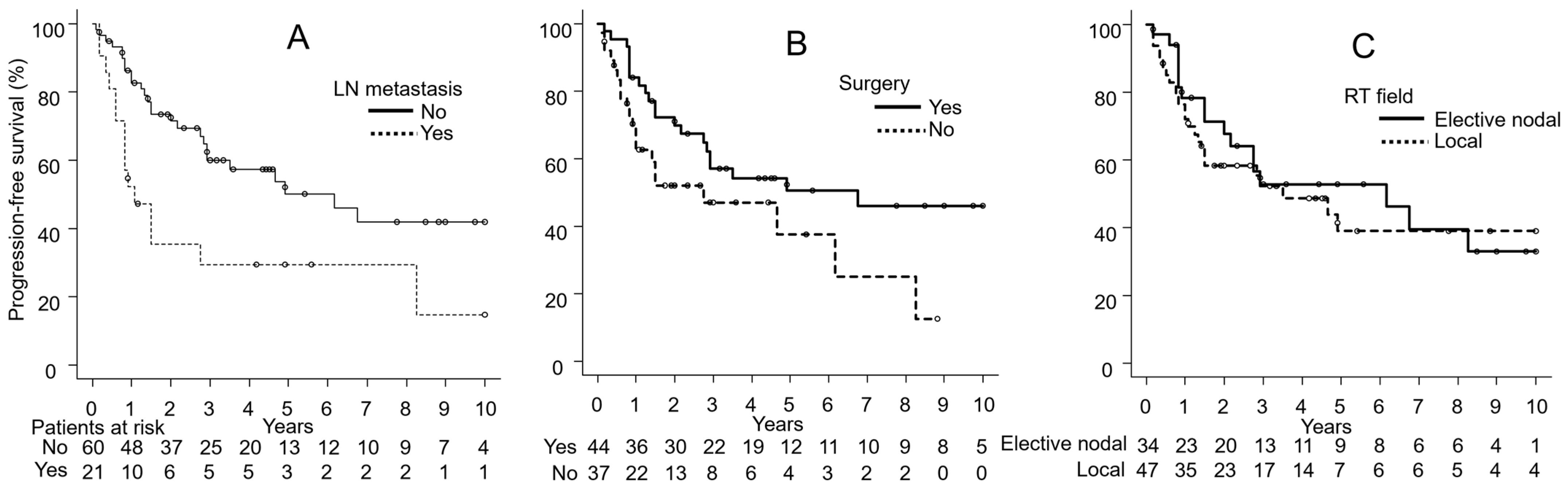
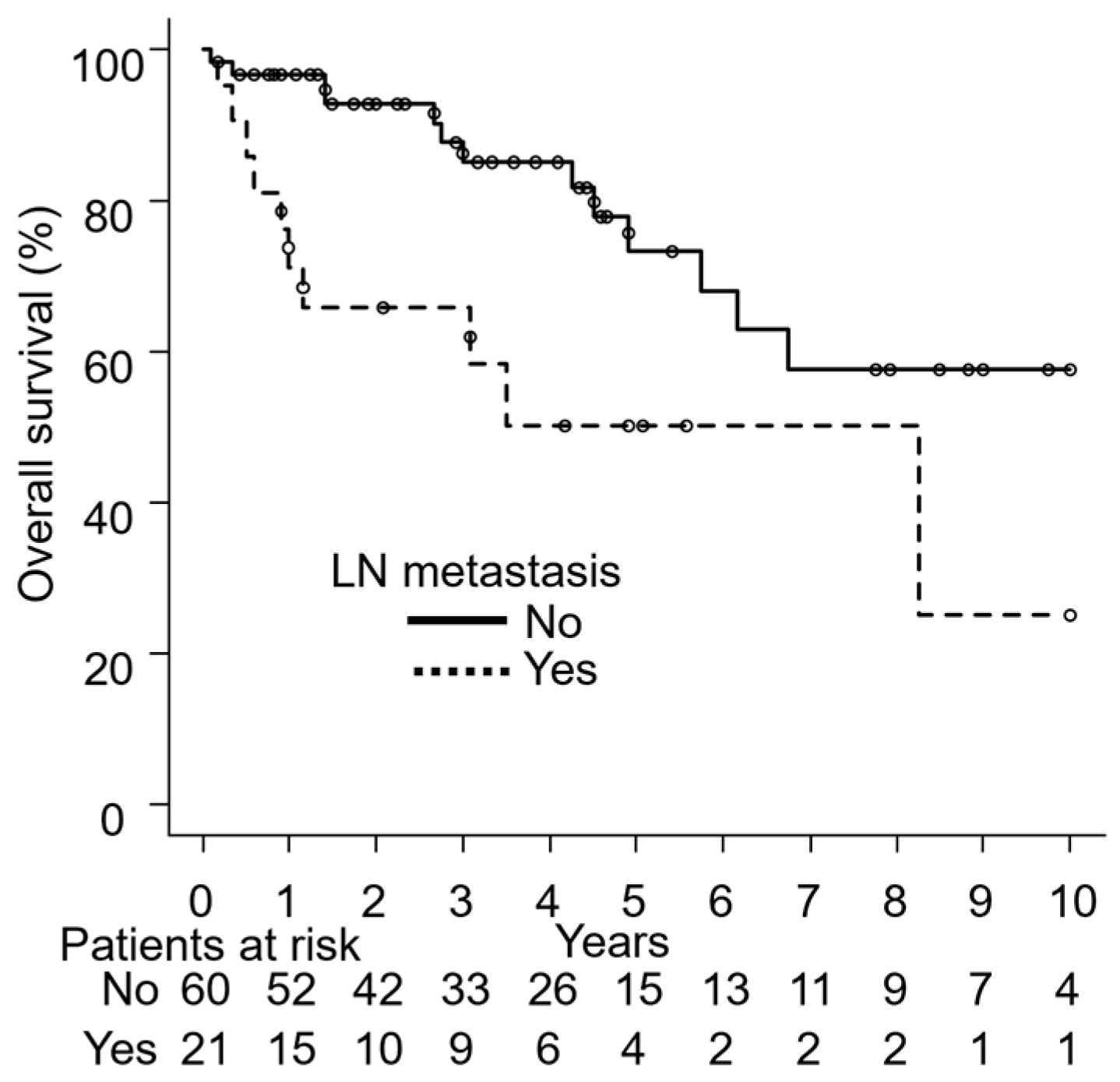
3.4. Univariate Analyses
3.5. Multivariate Analyses
3.6. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMPD | Extramammary Paget’s disease |
| RT | Radiation therapy |
| LC | Local control |
| PFS | Progression-free survival |
| OS | Overall survival |
| LN | Lymph node |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| PET | Positron emission tomograph |
| GTV | Gross tumor volume |
| CTV | Clinical target volume |
| PTV | Planning target volume |
| IMRT | Intensity-modulated radiation therapy |
| 3DCRT | Three-dimensional conformal radiation therapy |
| EQD2 | Equivalent dose in 2 Gy fractions |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| CR | Complete response |
| PR | Partial response |
| PD | Progressive disease |
| SD | Stable disease |
| NCI-CTCAE | National Cancer Institute Common Terminology Criteria for Adverse Events |
| CI | Confidence interval |
| HR | Hazard ratio |
References
- Kanitakis, J. Mammary and Extramammary Paget’s Disease. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, V.; Davidson, E.J.; Davies-Humphreys, J. Extramammary Paget’s Disease. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Bagby, C.M.; MacLennan, G.T. Extramammary Paget’s Disease of the Penis and Scrotum. J. Urol. 2009, 182, 2908–2909. [Google Scholar] [CrossRef] [PubMed]
- Karam, A.; Dorigo, O. Increased Risk and Pattern of Secondary Malignancies in Patients with Invasive Extramammary Paget Disease. Br. J. Dermatol. 2014, 170, 661–671. [Google Scholar] [CrossRef]
- Ishizuki, S.; Nakamura, Y. Extramammary Paget’s Disease: Diagnosis, Pathogenesis, and Treatment with Focus on Recent Developments. Curr. Oncol. 2021, 28, 2969–2986. [Google Scholar] [CrossRef] [PubMed]
- Hatta, N.; Yamada, M.; Hirano, T.; Fujimoto, A.; Morita, R. Extramammary Paget’s Disease: Treatment, Prognostic Factors and Outcome in 76 Patients. Br. J. Dermatol. 2008, 158, 313–318. [Google Scholar] [CrossRef]
- Siesling, S.; Elferink, M.A.G.; van Dijck, J.A.A.M.; Pierie, J.P.E.N.; Blokx, W.A.M. Epidemiology and Treatment of Extramammary Paget Disease in the Netherlands. Eur. J. Surg. Oncol. 2007, 33, 951–955. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Z.; Pan, S.; Liu, J.; Zuo, S.; Wang, P. The Clinical Characteristics and Prognostic Factors of Primary Extramammary Paget’s Disease Treated with Surgery in Anogenital Regions: A Large Population Study from the SEER Database and Our Centre. J. Clin. Med. 2023, 12, 582. [Google Scholar] [CrossRef]
- Ohara, K.; Fujisawa, Y.; Yoshino, K.; Kiyohara, Y.; Kadono, T.; Murata, Y.; Uhara, H.; Hatta, N.; Uchi, H.; Matsushita, S.; et al. A Proposal for a TNM Staging System for Extramammary Paget Disease: Retrospective Analysis of 301 Patients with Invasive Primary Tumors. J. Dermatol. Sci. 2016, 83, 234–239. [Google Scholar] [CrossRef]
- Kodama, S.; Kaneko, T.; Saito, M.; Yoshiya, N.; Honma, S.; Tanaka, K. A Clinicopathologic Study of 30 Patients with Paget’s Disease of the Vulva. Gynecol. Oncol. 1995, 56, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Feuer, G.A.; Shevchuk, M.; Calanog, A. Vulvar Paget’s Disease: The Need to Exclude an Invasive Lesion. Gynecol. Oncol. 1990, 38, 81–89. [Google Scholar] [CrossRef]
- Fanning, J.; Lambert, H.C.L.; Hale, T.M.; Morris, P.C.; Schuerch, C. Paget’s Disease of the Vulva: Prevalence of Associated Vulvar Adenocarcinoma, Invasive Paget’s Disease, and Recurrence after Surgical Excision. Am. J. Obstet. Gynecol. 1999, 180, 24–27. [Google Scholar] [CrossRef]
- Sawada, Y.; Bito, T.; Kabashima, R.; Yoshiki, R.; Hino, R.; Nakamura, M.; Shiraishi, M.; Tokura, Y. Ectopic Extramammary Paget’s Disease: Case Report and Literature Review. Acta Derm. Venereol. 2010, 90, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, Y.; Yoshino, K.; Kiyohara, Y.; Kadono, T.; Murata, Y.; Uhara, H.; Hatta, N.; Uchi, H.; Matsushita, S.; Takenouchi, T.; et al. The Role of Sentinel Lymph Node Biopsy in the Management of Invasive Extramammary Paget’s Disease: Multi-Center, Retrospective Study of 151 Patients. J. Dermatol. Sci. 2015, 79, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.S.; Yang, C.H.; Chen, C.H. Extramammary Paget’s Disease of the Unilateral Axilla: A Review of Seven Cases in a 20-Year Experience. Int. J. Dermatol. 2011, 50, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Koike, I.; Wada, H.; Miyagi, E.; Kasuya, T.; Kaizu, H.; Matsui, T.; Mukai, Y.; Ito, E.; Inoue, T. Radiation Therapy for Extramammary Paget’s Disease: Treatment Outcomes and Prognostic Factors. Ann. Oncol. 2014, 25, 291–297. [Google Scholar] [CrossRef]
- Besa, P.; Rich, T.A.; Delclos, L.; Edwards, C.L.; Ota, D.M.; Wharton, J.T. Clinical Original Contribution Extramammary Paget’s Disease of the Perineal Skin: Role of Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 73–78. [Google Scholar] [CrossRef]
- Itonaga, T.; Nakayama, H.; Okubo, M.; Mikami, R.; Nogi, S.; Tajima, Y.; Sugahara, S.; Tokuuye, K. Radiotherapy in Patients with Extramammary Paget’s Disease—Our Own Experience and Review of the Literature. Oncol. Res. Treat. 2014, 37, 18–22. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Casà, C.; Macchia, G.; Pesce, A.; Garganese, G.; Gui, B.; Perotti, G.; Gentileschi, S.; Inzani, F.; Autorino, R.; et al. The Role of Radiotherapy in Extramammary Paget Disease: A Systematic Review. Int. J. Gynecol. Cancer 2018, 28, 829–839. [Google Scholar] [CrossRef]
- Brown, R.S.D.; Lankester, K.J.; McCormack, M.; Power, D.A.; Spittle, M.F. Radiotherapy for Perianal Paget’s Disease. Clin. Oncol. 2002, 14, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Stockdale, A.D. Radiotherapy: An Effective Treatment for Extramammary Paget’s Disease. Clin. Oncol. 1991, 3, 3–5. [Google Scholar] [CrossRef]
- Hata, M.; Koike, I.; Wada, H.; Minagawa, Y.; Kasuya, T.; Matsui, T.; Suzuki, R.; Takano, S.; Inoue, T. Radiation Therapy for Lymph Node Metastasis from Extramammary Paget’s Disease. J. Eur. Acad. Dermatology Venereol. 2014, 28, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Xie, M.; Fu, S.; Guo, J.; Peng, Y.; Cai, Z.; Jiang, Y.; Zheng, D.; Wang, Z. Survival Analysis of Patients with Invasive Extramammary Paget Disease: Implications of Anatomic Sites. BMC Cancer 2018, 18, 403. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Y. Prognostic Value of Treatment Options for Extramammary Paget’s Disease: A SEER Database Analysis. Transl. Cancer Res. 2021, 10, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Koike, I.; Wada, H.; Miyagi, E.; Odagiri, K.; Minagawa, Y.; Kasuya, T.; Kaizu, H.; Inoue, T. Definitive Radiation Therapy for Extramammary Paget’s Disease. Anticancer Res. 2012, 32, 3315–3320. [Google Scholar]
- Mann, J.; Lavaf, A.; Tejwani, A.; Ross, P.; Ashamalla, H. Perianal Paget Disease Treated Definitively with Radiotherapy. Curr. Oncol. 2012, 19, 496–500. [Google Scholar] [CrossRef]
- Hata, M.; Koike, I.; Wada, H.; Miyagi, E.; Kasuya, T.; Kaizu, H.; Mukai, Y.; Inoue, T. Postoperative Radiation Therapy for Extramammary Paget’s Disease. Br. J. Dermatol. 2015, 172, 1014–1020. [Google Scholar] [CrossRef]
- Tran, M.N.; Harvey, J.A. Extensive Extramammary Paget’s Disease With Underlying Perianal Adenocarcinoma: The Role of Neoadjuvant Radiation Treatment. Cureus 2020, 2, e11966. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Park, W.; Lee, J.; Cho, E.Y.; Moon, G.H. Aggressive Clinical Course of Extramammary Paget Disease after Radiotherapy. Radiat. Oncol. J. 2014, 32, 95–98. [Google Scholar] [CrossRef]
- Ha, D.-L.; Ha, G.U.; Kim, J.Y.; Han, M.-H.; Lee, H.J.; Hong, D.G.; Lee, J.E.; Chung, H.Y.; Lee, S.-J. Five- and 10-Year Survival in Extramammary Paget’s Disease: A Focus on Wide Local Excision. Clin. Oncol. 2024, 20, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Kaku-Ito, Y.; Furue, M.; Ito, T. The Outcome of Chemotherapy for Metastatic Extramammary Paget’s Disease. J. Clin. Med. 2021, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Sohn, B.S.; Kim, J.; Kim, M.; Hong, J.Y.; Lee, J.; Park, S.E.; Kim, H.; Lee, H.J.; Kang, E.J.; Lee, S.I.; et al. Treatment Outcomes of Advanced/Metastatic Extramammary Paget’s Disease in Korean Patients: KCSG-RC20-06. Cancer Med. 2023, 12, 15159–15175. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, Y.; Umebayashi, Y.; Otsuka, F. Metastatic Extramammary Paget’s Disease Successfully Controlled with Tumour Dormancy Therapy Using Docetaxel. Br. J. Dermatol. 2006, 154, 375–376. [Google Scholar] [CrossRef]
- Mochitomi, Y.; Sakamoto, R.; Gushi, A.; Hashiguchi, T.; Mera, K.; Matsushita, S.; Nishi, M.; Kanzaki, T.; Kanekura, T. Extramammary Paget’s Disease/Carcinoma Successfully Treated with a Combination Chemotherapy: Report of Two Cases. J. Dermatol. 2005, 32, 632–637. [Google Scholar] [CrossRef]
- Kariya, K.; Tsuji, T.; Schwartz, R.A. Trial of Low-Dose 5-Fluorouracil/Cisplatin Therapy for Advanced Extramammary Paget’s Disease. Dermatol. Surg. 2004, 30, 341–344. [Google Scholar] [CrossRef]
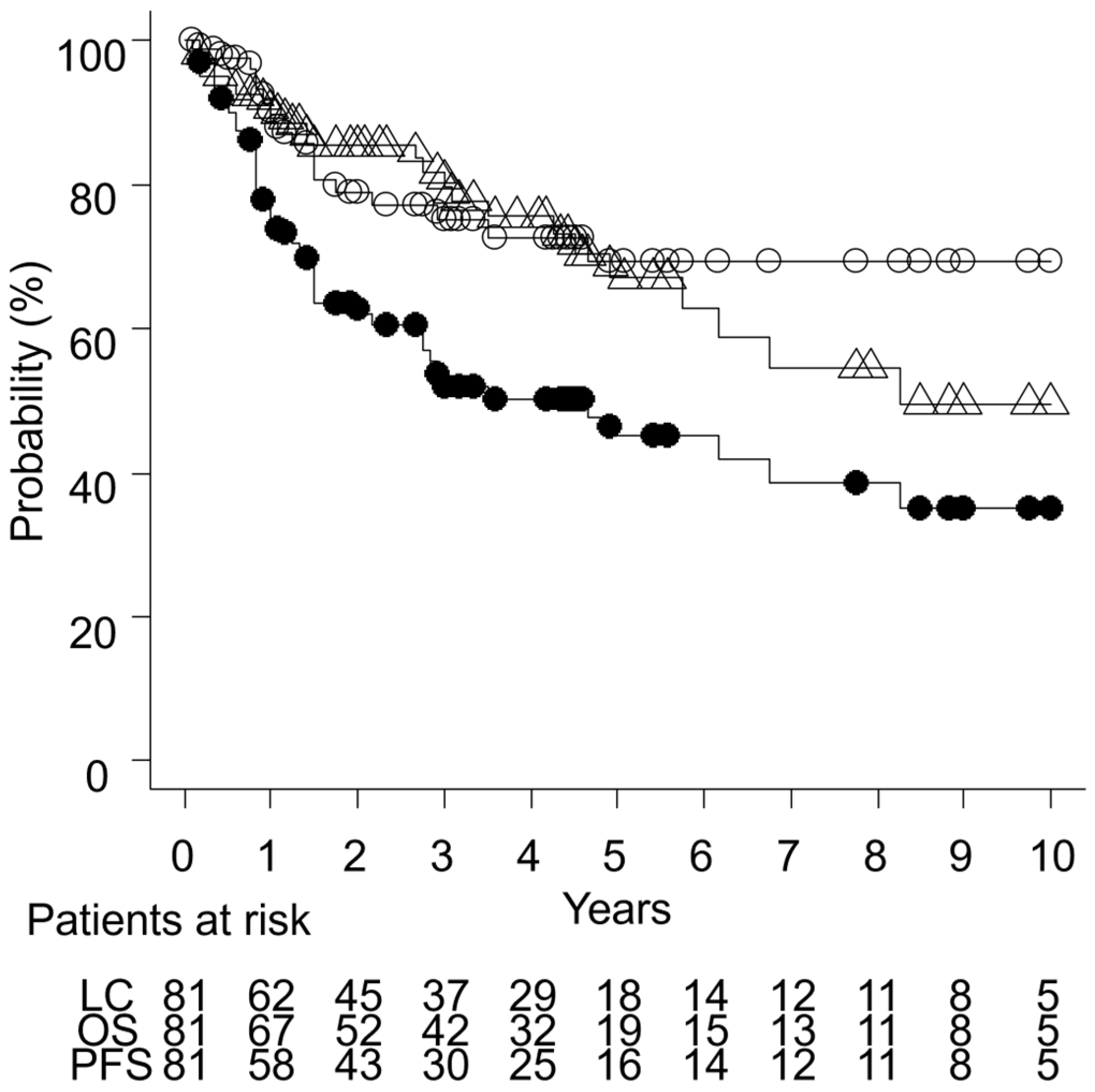
| Characteristic | n = 81 |
|---|---|
| Age (years) | 78 (50–95) |
| <78/≥78 | 35 (43%)/46 (57%) |
| Sex | |
| Male/female | 34 (42%)/47 (58%) |
| PS | |
| 0/1/2/3/missing | 27 (33%)/35 (43%)/14 (17%)/2 (2%)/3 (4%) |
| Primary tumor site | |
| Genital/perianal/inguinal/axillary/genital to perianal | 71 (88%)/4 (5%)/1 (1%)/1 (1%)/4 (5%) |
| Tumor size (cm) | 6 (0.6–20) |
| <10/≥10 | 65 (80%)/16 (20%) |
| Tumor thickness (mm) | |
| <4/≥4 | 58 (72%)/23 (28%) |
| Lymph node metastases | |
| No/yes | 60 (74%)/21 (26%) |
| Treatment methods | |
| RT | 30 (37%) |
| RT + surgery | 39 (48%) |
| RT + chemotherapy * | 7 (9%) |
| RT + surgery + chemotherapy | 5 (6%) |
| RT field | |
| Local/elective nodal | 47 (58%)/34 (42%) |
| RT methods | |
| IMRT or 3DCRT/electron beams alone | 51 (63%)/30 (37%) |
| Total dose (Gy) | 56 (30–69) |
| EQD2 (Gy) | 56 (30–71) |
| Characteristic | Variable | n | Local Control | Progression-Free Survival | Overall Survival | |||
|---|---|---|---|---|---|---|---|---|
| 3-Year Rate (%) | p-Value | 3-Year Rate (%) | p-Value | 3-Year Rate (%) | p-Value | |||
| Age (years) | <78 | 35 | 79 | 0.46 | 57 | 0.18 | 84 | 0.20 |
| ≥78 | 46 | 72 | 48 | 75 | ||||
| Sex | Female | 47 | 78 | 0.42 | 58 | 0.62 | 77 | 0.55 |
| Male | 34 | 71 | 44 | 84 | ||||
| PS | 0, 1 | 62 | 78 | 0.64 | 53 | 0.45 | 83 | 0.73 |
| ≥2 | 16 | 85 | 62 | 63 | ||||
| Tumor size (cm) | <10 | 67 | 75 | 0.59 | 51 | 0.86 | 79 | 0.42 |
| ≥10 | 14 | 75 | 56 | 85 | ||||
| Tumor thickness (mm) | <4 | 58 | 79 | 0.63 | 58 | 0.31 | 80 | 0.92 |
| ≥4 | 23 | 64 | 38 | 80 | ||||
| Lymph node metastasis | Yes | 21 | 59 | 0.18 | 30 | 0.005 | 66 | 0.006 |
| No | 60 | 80 | 60 | 85 | ||||
| Surgery | Yes | 44 | 82 | 0.04 | 57 | 0.051 | 85 | 0.09 |
| No | 37 | 66 | 47 | 72 | ||||
| RT field | Local | 47 | 72 | 0.18 | 52 | 0.62 | 77 | 0.89 |
| Elective nodal | 34 | 80 | 53 | 83 | ||||
| RT methods | IMRT or 3DCRT | 51 | 83 | 0.06 | 51 | 0.35 | 86 | 0.18 |
| Electron beams only | 30 | 65 | 55 | 76 | ||||
| EQD2 (Gy) | ≥56 | 45 | 81 | 0.20 | 52 | 0.56 | 77 | 0.13 |
| <56 | 36 | 68 | 52 | 84 | ||||
| Variable | Local Control | Progression-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (years) | |||||||||
| 0: <78 | 0.86 | 0.28–2.66 | 0.80 | 1.09 | 0.54–2.23 | 0.81 | 1.40 | 0.56–3.51 | 0.47 |
| 1: ≥78 | |||||||||
| PS | |||||||||
| 0:0, 1 | 0.85 | 0.27–2.63 | 0.77 | 0.62 | 0.31–1.23 | 0.17 | 0.57 | 0.24–1.38 | 0.22 |
| 1:2- | |||||||||
| Tumor size (cm) | |||||||||
| 0:<10 | 2.58 | 0.69–9.65 | 0.16 | 1.49 | 0.63–3.53 | 0.37 | 0.85 | 0.23–3.14 | 0.81 |
| 1: ≥10 | |||||||||
| Tumor thickness (mm) | |||||||||
| 0: <4 | 2.45 | 0.75–8.03 | 0.14 | 1.57 | 0.77–3.21 | 0.22 | 1.21 | 0.47–3.11 | 0.69 |
| 1: ≥4 | |||||||||
| Lymph node metastasis | |||||||||
| 0: No | 5.56 | 1.52–20.39 | 0.01 | 3.77 | 1.74–8.18 | 0.001 | 3.96 | 1.51–10.37 | 0.005 |
| 1: Yes | |||||||||
| Surgery | |||||||||
| 0: Yes | 4.29 | 1.27–14.49 | 0.02 | 2.13 | 1.02–4.42 | 0.04 | 1.83 | 0.72–4.65 | 0.20 |
| 1: No | |||||||||
| RT field | |||||||||
| 0: Elective nodal | 8.54 | 1.85–39.48 | 0.006 | 2.46 | 1.11–5.45 | 0.03 | 1.77 | 0.66–4.77 | 0.26 |
| 1: Local | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niwa, M.; Tomita, N.; Ishiyama, H.; Kaneko, H.; Oshima, Y.; Takano, H.; Matsuo, M.; Kuno, M.; Miyakawa, A.; Otsuka, S.; et al. Clinical Outcomes and Prognostic Factors for Extramammary Paget’s Disease Treated with Radiation Therapy: A Multi-Institutional Observational Study. Cancers 2025, 17, 1507. https://doi.org/10.3390/cancers17091507
Niwa M, Tomita N, Ishiyama H, Kaneko H, Oshima Y, Takano H, Matsuo M, Kuno M, Miyakawa A, Otsuka S, et al. Clinical Outcomes and Prognostic Factors for Extramammary Paget’s Disease Treated with Radiation Therapy: A Multi-Institutional Observational Study. Cancers. 2025; 17(9):1507. https://doi.org/10.3390/cancers17091507
Chicago/Turabian StyleNiwa, Masanari, Natsuo Tomita, Hiromichi Ishiyama, Hijiri Kaneko, Yukihiko Oshima, Hirota Takano, Masayuki Matsuo, Mayu Kuno, Akifumi Miyakawa, Shinya Otsuka, and et al. 2025. "Clinical Outcomes and Prognostic Factors for Extramammary Paget’s Disease Treated with Radiation Therapy: A Multi-Institutional Observational Study" Cancers 17, no. 9: 1507. https://doi.org/10.3390/cancers17091507
APA StyleNiwa, M., Tomita, N., Ishiyama, H., Kaneko, H., Oshima, Y., Takano, H., Matsuo, M., Kuno, M., Miyakawa, A., Otsuka, S., Takaoka, T., Okazaki, D., Torii, A., Kita, N., Takano, S., Nakamura, M., Kato, H., Morita, A., & Hiwatashi, A. (2025). Clinical Outcomes and Prognostic Factors for Extramammary Paget’s Disease Treated with Radiation Therapy: A Multi-Institutional Observational Study. Cancers, 17(9), 1507. https://doi.org/10.3390/cancers17091507







