68Ga-Trivehexin: Current Status of αvβ6-Integrin Imaging and Perspectives
Simple Summary
Abstract
1. Introduction
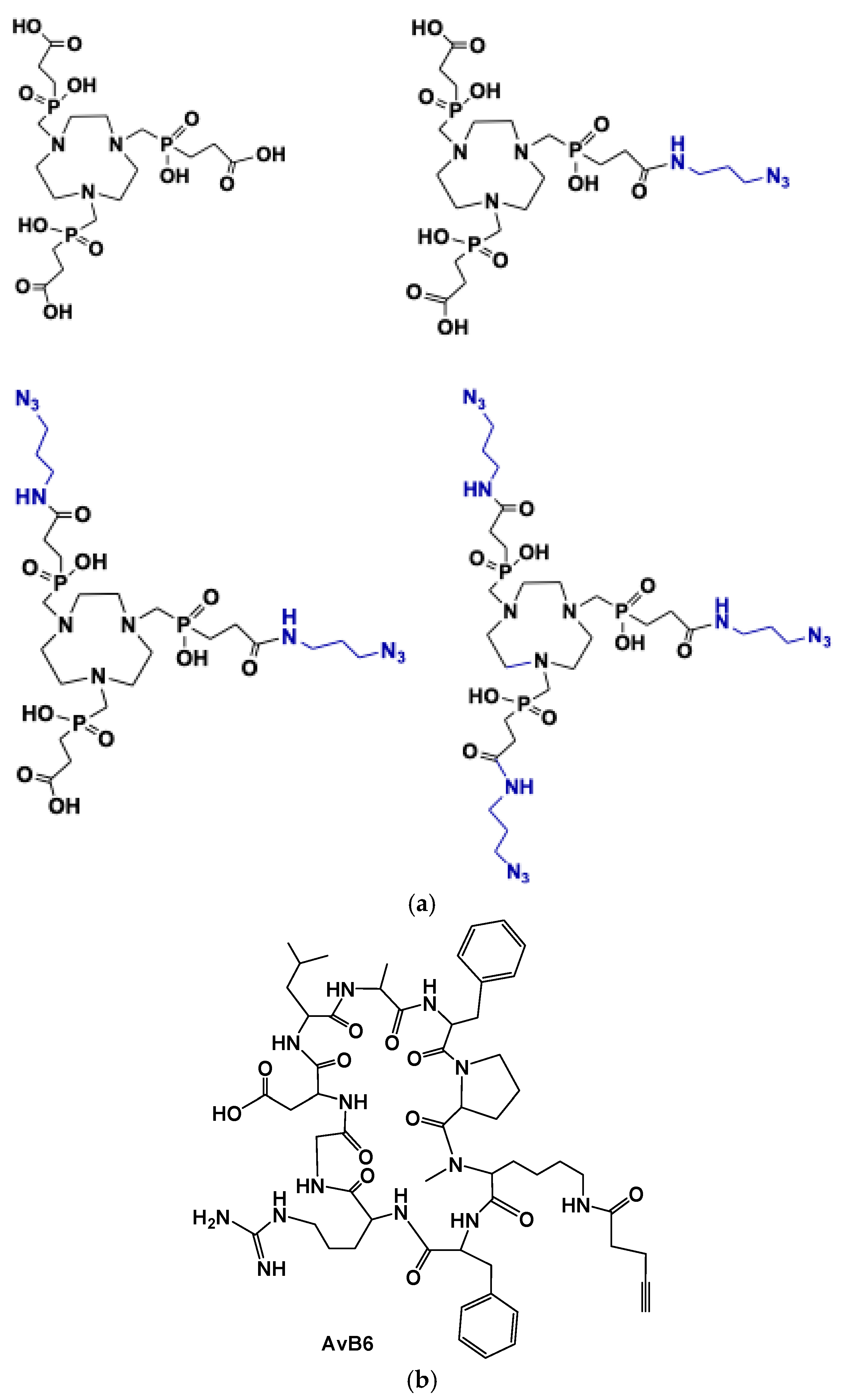
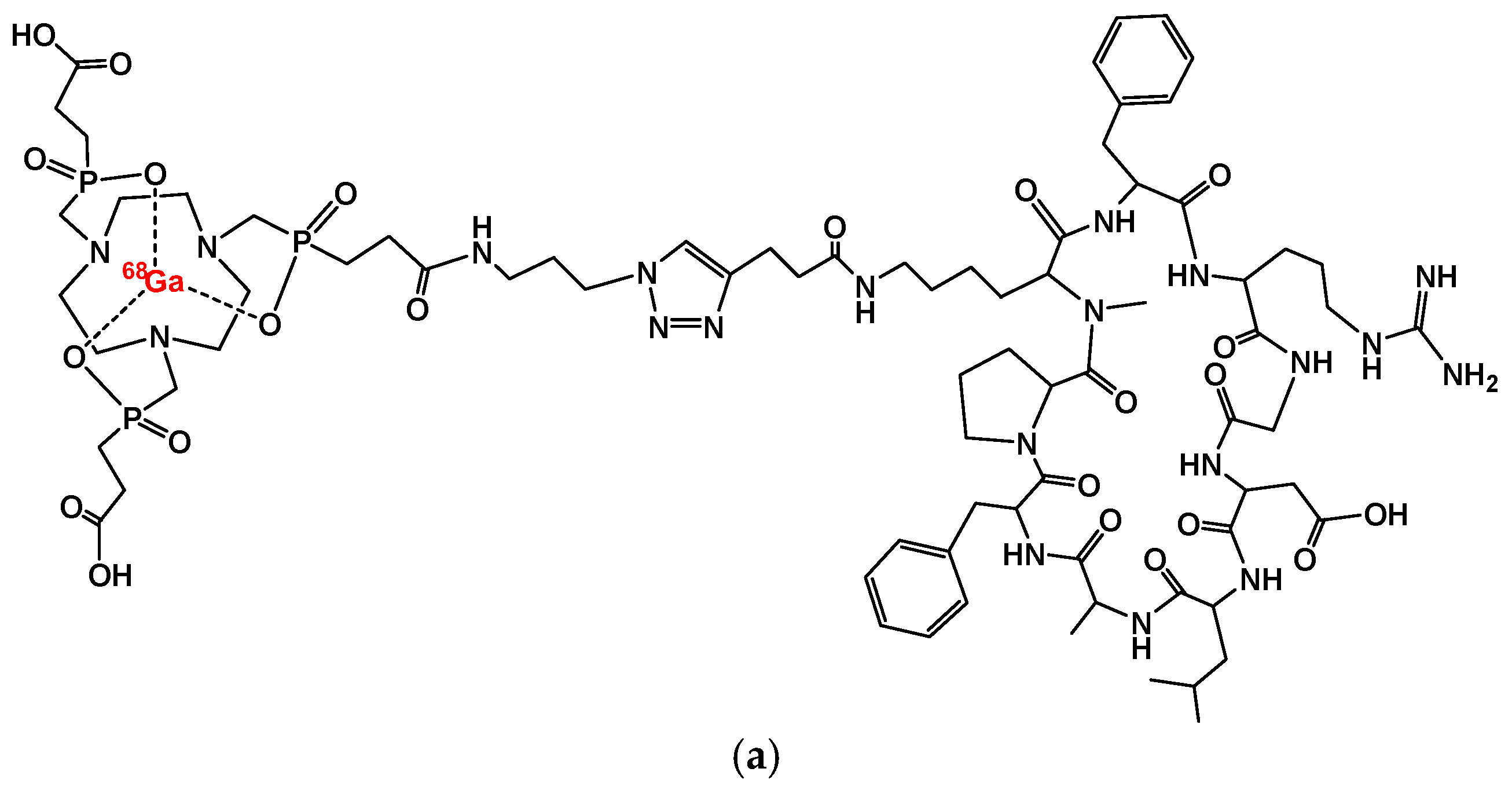
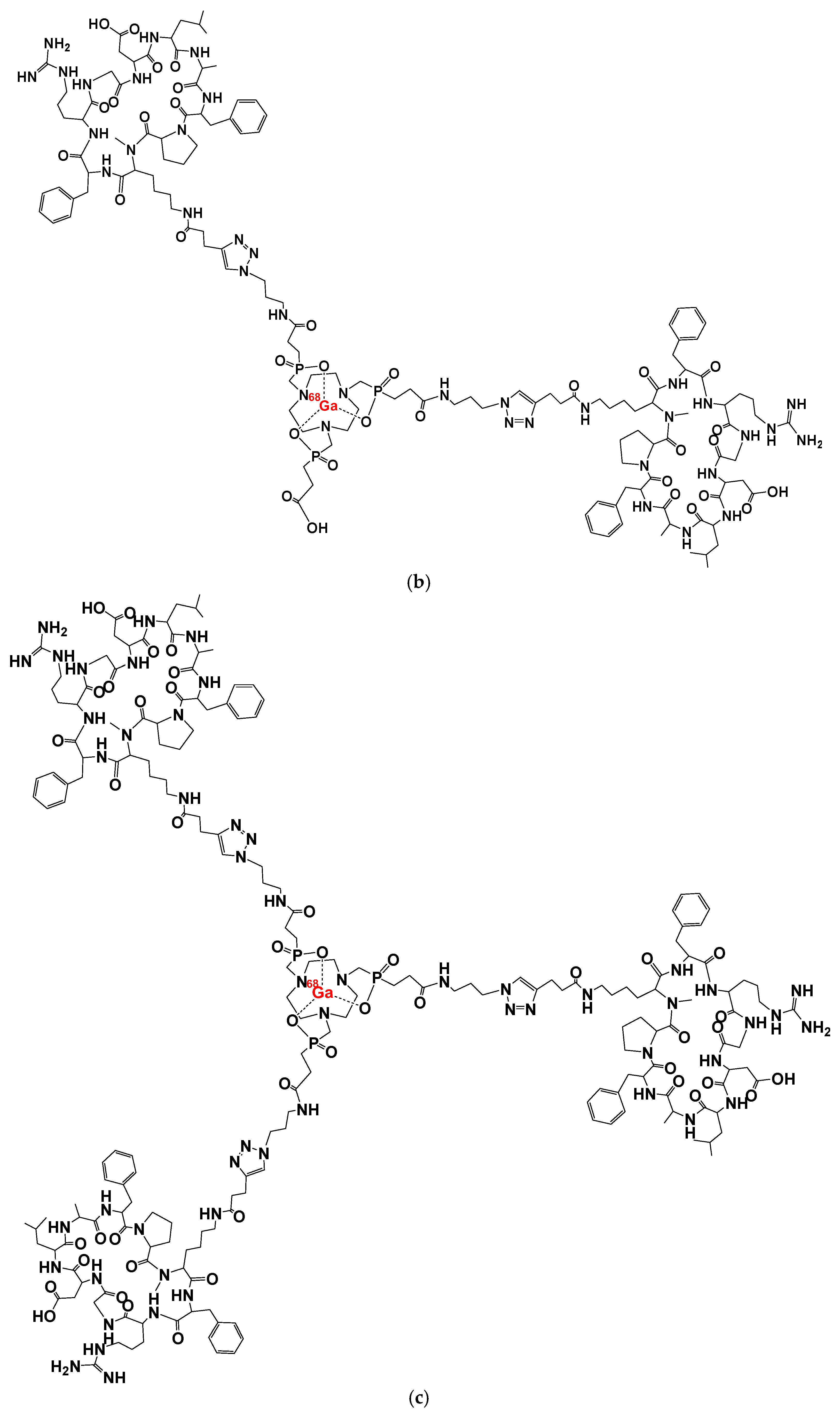
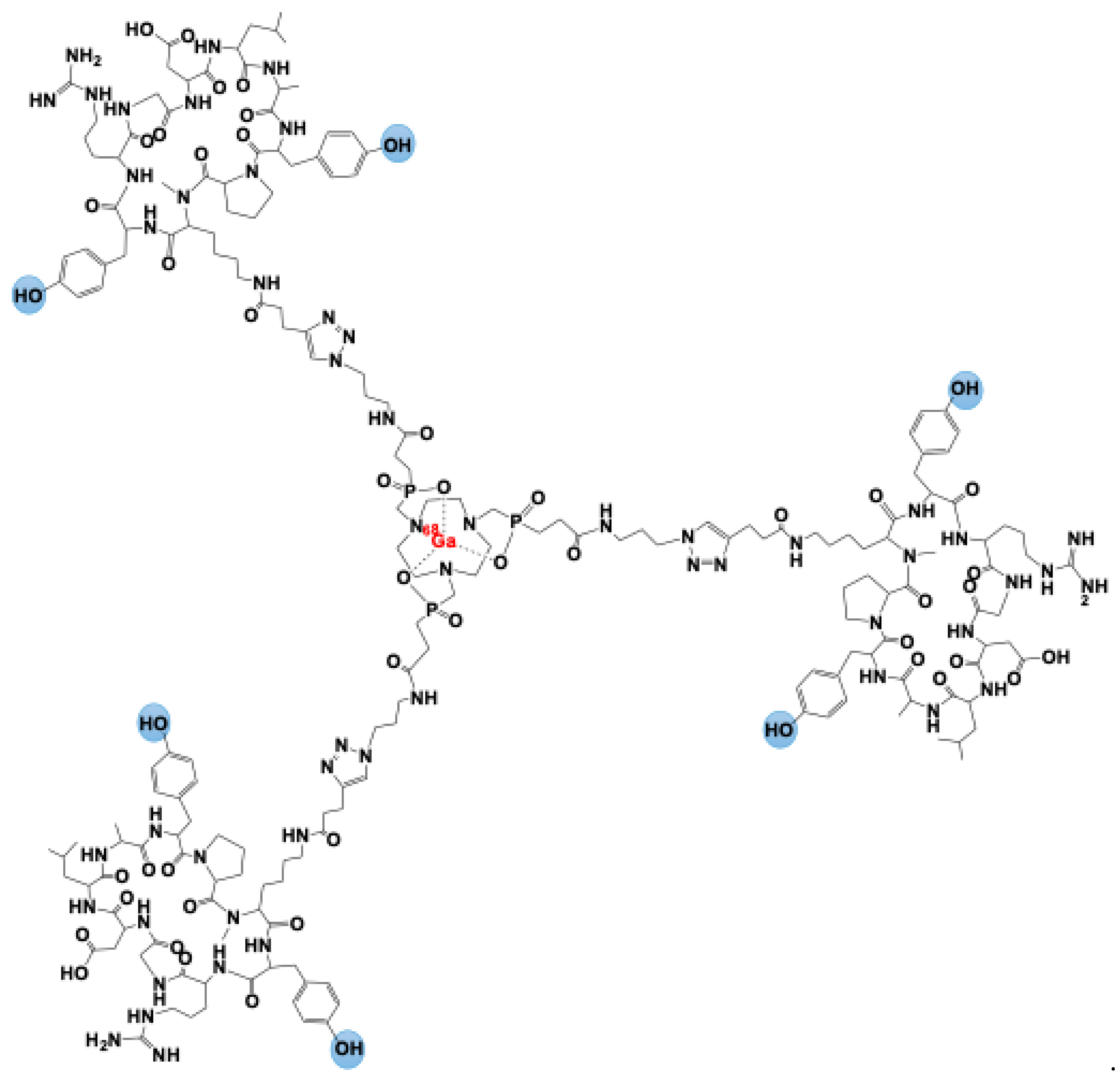
2. [68Ga]Ga-Trivehexin: A Radiochemical Insight
3. [68Ga]Ga-Trivehexin: Normal Biodistribution and Dosimetry
4. [68Ga]Ga-Trivehexin: Preliminary Clinical Experiences
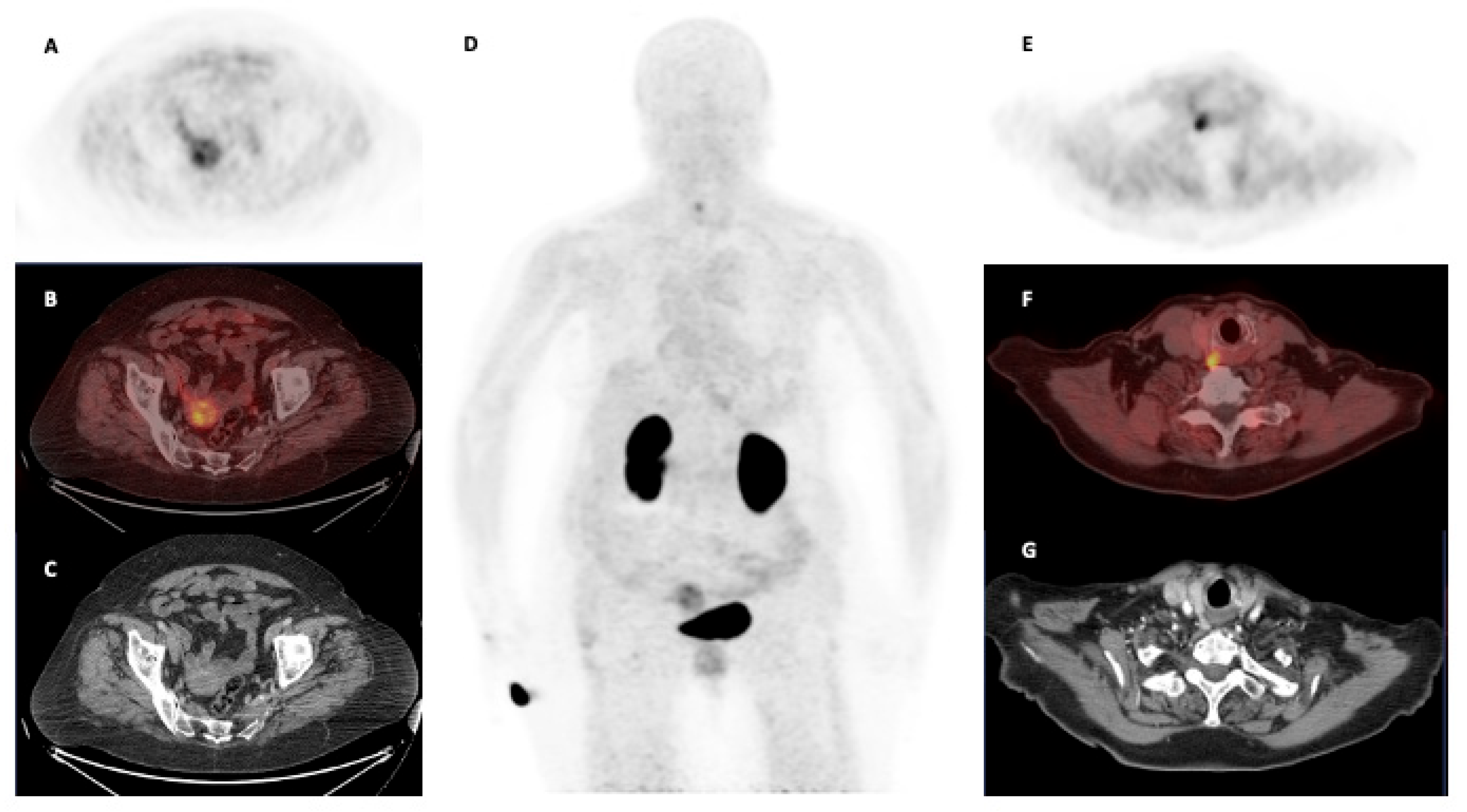
4.1. Oncological Diseases
4.2. Non-Oncological Diseases
| Author | Country | Year | Clinical Setting | Disease | N. of Patients | Injected Activity (MBq) | Time of Scan (mins p.i.) | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Quigley et al. [41] | Germany | 2021 | Oncological | PDAC | 1 | 87 | 70 | Focal radiotracer uptake at the PDAC of the pancreatic head. |
| Quigley et al. [20] | Germany | 2022 | Oncological | HNSCC, salivary duct carcinoma, PDAC | 4 | 172–142–135–105 | 61–120 | [68Ga]Ga-Trivehexin PET/CT detected the same oncological lesions visible at [18F]FDG PET/CT but with lower inflammatory findings in HNSCC and salivary duct carcinoma. In PDAC, [68Ga]Ga-Trivehexin PET/CT revealed more liver metastases than CT. |
| Thakral et al. [36] | India | 2023 | Oncological | HNC, PDAC | 20 | 110 (84–185) | 10, 60, 90 | The scan performed 60 min post injection demonstrated the higher radiotracer uptake. |
| Rehm et al. [43] | Germany | 2024 | Oncological | HNSCC, PDAC | 1 | 146 | 60 | [68Ga]Ga-Trivehexin PET/CT detected a brain metastasis from HNSCC. |
| Das et al. [42] | India—Germany | 2023 | Oncological | HNC, PDAC | 32 | 1.85–2.22 per kg | 60 | [68Ga]Ga-Trivehexin PET/CT showed a sensitivity and specificity of 92.5% and 100%, respectively. Uptake intensity showed a positive correlation with αvβ6-integrin expression at IHC. |
| Marafi et al. [44] | Kuwait | 2024 | Oncological | NSCLC | 1 | – | – | [68Ga]Ga-Trivehexin PET/CT detected brain metastases from NSCLC. |
| Singh et al. [45] | India | 2024 | Oncological | Ovarian carcinoma | 1 | – | – | [68Ga]Ga-Trivehexin PET/CT may be useful to define disease extension in metastatic ovarian carcinoma. |
| Wu et al. [47] | China | 2024 | Oncological | Bronchial mucoepidermoid carcinoma | 1 | – | – | [68Ga]Ga-Trivehexin PET demonstrated higher TBR than [18F]FDG PET/CT in a patient with bronchial mucoepidermoid carcinoma (10.1 vs. 2.5, respectively). |
| Novruzov et al. [49] | Azerbaijan | 2024 | Oncological | SPPNP | 1 | – | – | [68Ga]Ga-Trivehexin PET/CT was negative in a patient with SPPNP with high uptake at [18F]FDG and [68Ga]Ga-FAPI PET/CT. |
| Rehm et al. [39] | Germany | 2024 | Oncological | PDAC | 44 | 139 (84–160) | Early dynamic: 45 min acquisition in list mode; Late scan: 62 (55–85) | [68Ga]Ga-Trivehexin PET/CT demonstrated high accuracy for the detection of αvβ6-integrin expression in pancreatic cancer and metastases (lymph node, liver, peritoneal and other sites). The dynamic acquisition showed that the latest images (acquired 45 min p.i) had the higher TBR. |
| Kuyumcu et al. [56] | Turkey | 2024 | Non-oncological | PHP | 13 | 185 | Early scan: 30 Late scan: 50–90 | In comparison to [99mTc]Tc-MIBI scintigraphy-SPECT/CT, [68Ga]Ga-Trivehexin PET/CT identified 7 additional parathyroid lesions. [68Ga]Ga-Trivehexin PET/CT identified hyperfunctioning parathyroids in 2 out of 3 PHP patients negative at [18F]fluorocholine PET/CT. |
| Singhal et al. [46] | India | 2025 | Oncological | DTC | 1 | – | – | [68Ga]Ga-Trivehexin PET/CT was more accurate than [18F]FDG PET/CT to define disease extension in a patient with papillary thyroid carcinoma. |
| Kömek et al. [48] | Turkey | 2025 | Oncological | BC | 1 | – | – | [68Ga]Ga-Trivehexin PET/CT detected the primary BC, left internal mammary and axillary lymph node metastases with a higher tracer uptake than [18F]FDG PET/CT. Moreover, ruled out disease involvement in mediastinal [18F]FDG-avid lymph nodes, confirmed reactive at cytology. |
| Emerson et al. [50] | India | 2025 | Oncological | HNSCC | 1 | – | – | [68Ga]Ga-Trivehexin PET/CT detected HNSCC of the oral cavity. |
| Wu et al. [51] | China | 2025 | Oncological/Non-oncological | NSCLC, IPF | 1 | – | – | [68Ga]Ga-Trivehexin PET/CT showed intense uptake in correspondence to a mucinous lung adenocarcinoma (with only faint uptake at [18F]FDG PET/CT) and IPF. |
| Kuyumcu et al. [53] | Turkey | 2025 | Oncological/Non-oncological | NSCLC, IPF | 1 | – | – | A mucinous lung cancer demonstrated higher uptake at [68Ga]Ga-Trivehexin than at [18F]FDG PET/CT. [68Ga]Ga-Trivehexin demonstrated diffuse intense uptake in IPF. |
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawal, I.O.; Abubakar, S.O.; Ndlovu, H.; Mokoala, K.M.G.; More, S.S.; Sathekge, M.M. Advances in Radioligand Theranostics in Oncology. Mol. Diagn. Ther. 2024, 28, 265–289. [Google Scholar] [CrossRef] [PubMed]
- Vaz, S.C.; Woll, J.P.P.; Cardoso, F.; Groheux, D.; Cook, G.J.R.; Ulaner, G.A.; Jacene, H.; Rubio, I.T.; Schoones, J.W.; Peeters, M.-J.V.; et al. Joint EANM-SNMMI Guideline on the Role of 2-[18F]FDG PET/CT in No Special Type Breast Cancer: (Endorsed by the ACR, ESSO, ESTRO, EUSOBI/ESR, and EUSOMA). Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2706–2732. [Google Scholar] [CrossRef] [PubMed]
- Urso, L.; Panareo, S.; Castello, A.; Ambrosio, M.R.; Zatelli, M.C.; Caracciolo, M.; Tonini, E.; Valpiani, G.; Boschi, A.; Uccelli, L.; et al. Glucose Metabolism Modification Induced by Radioligand Therapy with [177Lu]Lu/[90Y]Y-DOTATOC in Advanced Neuroendocrine Neoplasms: A Prospective Pilot Study within FENET-2016 Trial. Pharmaceutics 2022, 14, 2009. [Google Scholar] [CrossRef] [PubMed]
- Urso, L.; Quartuccio, N.; Caracciolo, M.; Evangelista, L.; Schirone, A.; Frassoldati, A.; Arnone, G.; Panareo, S.; Bartolomei, M. Impact on the Long-Term Prognosis of FDG PET/CT in Luminal-A and Luminal-B Breast Cancer. Nucl. Med. Commun. 2022, 43, 212–219. [Google Scholar] [CrossRef]
- Castello, A.; Rossi, S.; Lopci, E. 18F-FDG PET/CT in Restaging and Evaluation of Response to Therapy in Lung Cancer: State of the Art. Curr. Radiopharm. 2020, 13, 228–237. [Google Scholar] [CrossRef]
- Casali, M.; Lauri, C.; Altini, C.; Bertagna, F.; Cassarino, G.; Cistaro, A.; Erba, A.P.; Ferrari, C.; Mainolfi, C.G.; Palucci, A.; et al. State of the Art of 18F-FDG PET/CT Application in Inflammation and Infection: A Guide for Image Acquisition and Interpretation. Clin. Transl. Imaging 2021, 9, 299–339. [Google Scholar] [CrossRef]
- Evangelista, L.; Zattoni, F.; Burei, M.; Bertin, D.; Borsatti, E.; Baresic, T.; Farsad, M.; Trenti, E.; Bartolomei, M.; Panareo, S.; et al. A Prospective Randomized Multicenter Study on the Impact of [18F]F-Choline PET/CT Versus Conventional Imaging for Staging Intermediate- to High-Risk Prostate Cancer. J. Nucl. Med. 2024, 65, 1013–1020. [Google Scholar] [CrossRef]
- Chondrogiannis, S.; Marzola, M.C.; Rubello, D. 18F-DOPA PET/Computed Tomography Imaging. PET Clin. 2014, 9, 307–321. [Google Scholar] [CrossRef]
- Dondi, F.; Gazzilli, M.; Viganò, G.L.; Pisani, A.R.; Ferrari, C.; Rubini, G.; Bertagna, F. The Role of 11C-Methionine PET Imaging for the Evaluation of Lymphomas: A Systematic Review. Hematol. Rep. 2024, 16, 752–768. [Google Scholar] [CrossRef]
- Calais, J.; Ceci, F.; Eiber, M.; Hope, T.A.; Hofman, M.S.; Rischpler, C.; Bach-Gansmo, T.; Nanni, C.; Savir-Baruch, B.; Elashoff, D.; et al. 18F-Fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in Patients with Early Biochemical Recurrence after Prostatectomy: A Prospective, Single-Centre, Single-Arm, Comparative Imaging Trial. Lancet Oncol. 2019, 20, 1286–1294. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lau, W.F.E.; Hicks, R.J. Somatostatin Receptor Imaging with 68Ga DOTATATE PET/CT: Clinical Utility, Normal Patterns, Pearls, and Pitfalls in Interpretation. Radiographics 2015, 35, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-Specific Membrane Antigen PET-CT in Patients with High-Risk Prostate Cancer before Curative-Intent Surgery or Radiotherapy (proPSMA): A Prospective, Randomised, Multicentre Study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Urso, L.; Bianconi, F.; Palumbo, B.; Marzola, M.C.; Evangelista, L.; Schillaci, O. Radiomics and Theranostics with Molecular and Metabolic Probes in Prostate Cancer: Toward a Personalized Approach. Expert Rev. Mol. Diagn. 2023, 23, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; De Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Duan, X.; Xia, L.; Zhang, Z.; Ren, Y.; Pomper, M.G.; Rowe, S.P.; Li, X.; Li, N.; Zhang, N.; Zhu, H.; et al. First-in-Human Study of the Radioligand 68Ga-N188 Targeting Nectin-4 for PET/CT Imaging of Advanced Urothelial Carcinoma. Clin. Cancer Res. 2023, 29, 3395–3407. [Google Scholar] [CrossRef]
- Lindner, T.; Giesel, F.L.; Kratochwil, C.; Serfling, S.E. Radioligands Targeting Fibroblast Activation Protein (FAP). Cancers 2021, 13, 5744. [Google Scholar] [CrossRef]
- Filippi, L.; Urso, L.; Schillaci, O.; Evangelista, L. [18F]-FDHT PET for the Imaging of Androgen Receptor in Prostate and Breast Cancer: A Systematic Review. Diagnostics 2023, 13, 2613. [Google Scholar] [CrossRef]
- Kimura, R.H.; Iagaru, A.; Guo, H.H. Mini Review of First-in-Human Integrin Avβ6 PET Tracers. Front. Nucl. Med. 2023, 3, 1271208. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every Step of the Way: Integrins in Cancer Progression and Metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Quigley, N.G.; Steiger, K.; Hoberück, S.; Czech, N.; Zierke, M.A.; Kossatz, S.; Pretze, M.; Richter, F.; Weichert, W.; Pox, C.; et al. PET/CT Imaging of Head-and-Neck and Pancreatic Cancer in Humans by Targeting the “Cancer Integrin” Avβ6 with Ga-68-Trivehexin. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1136–1147. [Google Scholar] [CrossRef]
- Pierschbacher, M.D.; Ruoslahti, E. Cell Attachment Activity of Fibronectin Can Be Duplicated by Small Synthetic Fragments of the Molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Steiger, K.; Quigley, N.G.; Groll, T.; Richter, F.; Zierke, M.A.; Beer, A.J.; Weichert, W.; Schwaiger, M.; Kossatz, S.; Notni, J. There Is a World beyond Avβ3-Integrin: Multimeric Ligands for Imaging of the Integrin Subtypes Avβ6, Avβ8, Avβ3, and A5β1 by Positron Emission Tomography. EJNMMI Res. 2021, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Notni, J. RGD Forever!-Past, Present, and Future of a 3-Letter-Code in Radiopharmacy and Life Sciences. Pharmaceuticals 2022, 16, 56. [Google Scholar] [CrossRef]
- Schottelius, M.; Laufer, B.; Kessler, H.; Wester, H.-J. Ligands for Mapping αv β3 -Integrin Expression in Vivo. Acc. Chem. Res. 2009, 42, 969–980. [Google Scholar] [CrossRef]
- Liu, S.; Chen, S.; Zeng, J. TGF-β Signaling: A Complex Role in Tumorigenesis (Review). Mol. Med. Rep. 2017, 17, 699–704. [Google Scholar] [CrossRef]
- Niu, J.; Li, Z. The Roles of Integrin Avβ6 in Cancer. Cancer Lett. 2017, 403, 128–137. [Google Scholar] [CrossRef]
- Horan, G.S.; Wood, S.; Ona, V.; Li, D.J.; Lukashev, M.E.; Weinreb, P.H.; Simon, K.J.; Hahm, K.; Allaire, N.E.; Rinaldi, N.J.; et al. Partial Inhibition of Integrin Alpha(v)Beta6 Prevents Pulmonary Fibrosis without Exacerbating Inflammation. Am. J. Respir. Crit. Care Med. 2008, 177, 56–65. [Google Scholar] [CrossRef]
- Li, S.; McGuire, M.J.; Lin, M.; Liu, Y.-H.; Oyama, T.; Sun, X.; Brown, K.C. Synthesis and Characterization of a High-Affinity {alpha}v{beta}6-Specific Ligand for in Vitro and in Vivo Applications. Mol. Cancer Ther. 2009, 8, 1239–1249. [Google Scholar] [CrossRef]
- Hausner, S.H.; Abbey, C.K.; Bold, R.J.; Gagnon, M.K.; Marik, J.; Marshall, J.F.; Stanecki, C.E.; Sutcliffe, J.L. Targeted In Vivo Imaging of Integrin Avβ6 with an Improved Radiotracer and Its Relevance in a Pancreatic Tumor Model. Cancer Res. 2009, 69, 5843–5850. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; Hong, Y.; Kimura, R.H.; Ma, X.; Liu, H.; Qin, C.; Hu, X.; Hayes, T.R.; Benny, P.; et al. 99m Tc-Labeled Cystine Knot Peptide Targeting Integrin αv β6 for Tumor SPECT Imaging. Mol. Pharm. 2014, 11, 1208–1217. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Ma, T.; Sun, X.; Shi, J.; Jia, B.; Sun, Y.; Zhan, J.; Zhang, H.; Zhu, Z.; et al. Integrin αv β6 –Targeted SPECT Imaging for Pancreatic Cancer Detection. J. Nucl. Med. 2014, 55, 989–994. [Google Scholar] [CrossRef] [PubMed]
- John, A.E.; Luckett, J.C.; Tatler, A.L.; Awais, R.O.; Desai, A.; Habgood, A.; Ludbrook, S.; Blanchard, A.D.; Perkins, A.C.; Jenkins, R.G.; et al. Preclinical SPECT/CT Imaging of Avβ6 Integrins for Molecular Stratification of Idiopathic Pulmonary Fibrosis. J. Nucl. Med. 2013, 54, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, O.V.; Marelli, U.K.; Kapp, T.G.; Di Leva, F.S.; Di Maro, S.; Nieberler, M.; Reuning, U.; Schwaiger, M.; Novellino, E.; Marinelli, L.; et al. Stable Peptides Instead of Stapled Peptides: Highly Potent αvβ6-Selective Integrin Ligands. Angew. Chem. Int. Ed. 2016, 55, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Reich, D.; Maltsev, O.V.; Kapp, T.G.; Steiger, K.; Hoffmann, F.; Esposito, I.; Weichert, W.; Kessler, H.; Wester, H.-J. In Vivo PET Imaging of the Cancer Integrin Avβ6 Using68 Ga-Labeled Cyclic RGD Nonapeptides. J. Nucl. Med. 2017, 58, 671–677. [Google Scholar] [CrossRef]
- Quigley, N.G.; Steiger, K.; Richter, F.; Weichert, W.; Hoberück, S.; Kotzerke, J.; Notni, J. Tracking a TGF-β Activator in Vivo: Sensitive PET Imaging of Avβ8-Integrin with the Ga-68-Labeled Cyclic RGD Octapeptide Trimer Ga-68-Triveoctin. EJNMMI Res. 2020, 10, 133. [Google Scholar] [CrossRef]
- Thakral, P.; Das, S.S.; Dhiman, S.; Manda, D.; Virupakshappa, C.B.; Malik, D.; Sen, I. Validation of In-House Kit-Like Synthesis of68 Ga-Trivehexin and Its Biodistribution for Targeting the Integrin Avβ6 Expressing Tumors. Cancer Biother. Radiopharm. 2023, 38, 468–474. [Google Scholar] [CrossRef]
- Solanki, R.; Mittal, B.R.; Kumar, R.; Singh, H.; Sharma, A. Incidental Detection of 68 Ga-DOTA-RGD-2 Uptake in Uterine Fibroid. Clin. Nucl. Med. 2024, 49, e17–e18. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Carnevale, A.; Pellegrino, F.; Stefano, G.D.; Bonelli, C.; Renzulli, M.; Giganti, M.; Carrafiello, G. Uterine Myomas: Extravascular Treatment. Semin. Ultrasound CT MRI 2021, 42, 56–74. [Google Scholar] [CrossRef]
- Rehm, J.; Winzer, R.; Pretze, M.; Müller, J.; Notni, J.; Hempel, S.; Distler, M.; Folprecht, G.; Kotzerke, J. Avβ6-Integrin Targeted PET/CT Imaging in Pancreatic Cancer Patients Using 68Ga-Trivehexin. Front. Nucl. Med. 2024, 4, 1487602. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, Y.; Zhu, J.; Wu, H.; Wu, J.; Li, L.; Huang, J.; Xiao, Z.; He, Y. Fully-Automated Production of [68Ga]Ga-Trivehexin for Clinical Application and Its Biodistribution in Healthy Volunteers. Front. Oncol. 2024, 14, 1445415. [Google Scholar] [CrossRef]
- Quigley, N.G.; Czech, N.; Sendt, W.; Notni, J. PET/CT Imaging of Pancreatic Carcinoma Targeting the “Cancer Integrin” Avβ6. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4107–4108. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Ahlawat, S.; Thakral, P.; Malik, D.; Simecek, J.; Cb, V.; Koley, M.; Gupta, J.; Sen, I. Potential Efficacy of 68Ga-Trivehexin PET/CT and Immunohistochemical Validation of Avβ6 Integrin Expression in Patients With Head and Neck Squamous Cell Carcinoma and Pancreatic Ductal Adenocarcinoma. Clin. Nucl. Med. 2024, 49, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Winzer, R.; Notni, J.; Hempel, S.; Distler, M.; Folprecht, G.; Kotzerke, J. Concomitant Metastatic Head-and-Neck Cancer and Pancreatic Cancer Assessed by Avβ6-Integrin PET/CT Using 68Ga-Trivehexin: Incidental Detection of a Brain Metastasis. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3469–3471. [Google Scholar] [CrossRef] [PubMed]
- Marafi, F.; Esmail, A.A.; Alfeeli, M.A.; Sadeq, A. 68Ga-Trivehexin PET/CT in Metastatic Non–Small Cell Lung Cancer to the Brain. Clin. Nucl. Med. 2024, 49, 971–972. [Google Scholar] [CrossRef]
- Singh, P.; Agrawal, K.; Emerson, R.; Baranwal, A.; Patro, P.S.S.; Parida, G.K. “Cancer Integrin” Avβ6 Imaging With 68Ga-Trivehexin PET/CT in Assessment of Ovarian Carcinoma. Clin. Nucl. Med. 2024, 49, e619–e621. [Google Scholar] [CrossRef]
- Singhal, T.; Agrawal, K.; Mandal, S.; Parida, G.K. Cancer-Specific Integrin Imaging With 68Ga-Trivehexin: A Potential Imaging for Accurate Staging of Thyroid Malignancy. Clin. Nucl. Med. 2025, 50, e168–e170. [Google Scholar] [CrossRef]
- Wu, H.; Li, L.; Xiao, Z.; Li, C.; He, Y. Avβ6-Integrin Targeted [68Ga]Ga-Trivehexin PET/CT Imaging of a Rare Bronchial Mucoepidermoid Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 1291–1292. [Google Scholar] [CrossRef]
- Kömek, H.; Güzel, Y.; Kaplan, İ.; Yilmaz, E.E.; Can, C. Superiority of 68Ga-Trivehexin PET/CT Over 18F-FDG PET/CT in the Evaluation of Lymph Nodes in Patients With Breast Cancer. Clin. Nucl. Med. 2025, 50, e175–e177. [Google Scholar] [CrossRef]
- Novruzov, F.; Mehdi, E.; Aliyeva, N.; Orucova, P.; Simecek, J.; Aliyev, J. The True Negative [68Ga]Ga-Trivehexin PET/CT in Solid Pseudopapillary Neoplasm of Pancreas, Mimicking Pancreatic Adenocarcinoma in [18F]FDG and [68Ga]Ga-FAPI Scans. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 1942–1943. [Google Scholar] [CrossRef]
- Emerson, R.; Agrawal, K.; Singh, P.; Patro, P.S.S.; Kumar, N. Cancer Integrin Imaging With 68Ga-Trivehexin PET-CT in Head and Neck Squamous Cell Carcinoma Improves Diagnostic Accuracy. Clin. Nucl. Med. 2025, 50, e180–e181. [Google Scholar] [CrossRef]
- Wu, H.; Li, L.; Xiao, Z.; Chen, Q.; Li, C.; He, Y. [68Ga]Ga-Trivehexin PET/CT Imaging of Integrin-Avβ6 Expression in Concomitant Mucinous Lung Adenocarcinoma and Idiopathic Pulmonary Fibrosis. Eur. J. Nucl. Med. Mol. Imaging 2025. [Google Scholar] [CrossRef] [PubMed]
- Moss, B.J.; Ryter, S.W.; Rosas, I.O. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 515–546. [Google Scholar] [CrossRef] [PubMed]
- Kuyumcu, S.; Denizmen Zorba, D.; Özkan, Z.G. 68Ga-Trivehexin PET/CT Uptake in Malignant and Fibrotic Lung Tissue: Refining Diagnostic Applications. Eur. J. Nucl. Med. Mol. Imaging 2025. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- Mapelli, P.; Busnardo, E.; Magnani, P.; Freschi, M.; Picchio, M.; Gianolli, L.; Messa, C. Incidental Finding of Parathyroid Adenoma with 11C-Choline PET/CT. Clin. Nucl. Med. 2012, 37, 593–595. [Google Scholar] [CrossRef]
- Kuyumcu, S.; Denizmen, D.; Has-Simsek, D.; Poyanli, A.; Uzum, A.K.; Buyukkaya, F.; Isik, E.G.; Onder, S.; Aksakal, N.; Ozkan, Z.G.; et al. 68Ga-Trivehexin PET/CT: A Promising Novel Tracer for Primary Hyperparathyroidism. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3912–3923. [Google Scholar] [CrossRef]
- Treglia, G.; Piccardo, A.; Paone, G.; Trimboli, P.; Imperiale, A. [18F]Fluorocholine PET/CT as First-Line vs. Second-Line Imaging Method to Localize Parathyroid Adenomas in Primary Hyperparathyroidism: “Game, Set, and Match.” Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3596–3599. [Google Scholar] [CrossRef]
- Caldarella, C.; De Risi, M.; Massaccesi, M.; Miccichè, F.; Bussu, F.; Galli, J.; Rufini, V.; Leccisotti, L. Role of 18F-FDG PET/CT in Head and Neck Squamous Cell Carcinoma: Current Evidence and Innovative Applications. Cancers 2024, 16, 1905. [Google Scholar] [CrossRef]
- Murakami, K. FDG-PET for Hepatobiliary and Pancreatic Cancer: Advances and Current Limitations. World J. Clin. Oncol. 2011, 2, 229–236. [Google Scholar] [CrossRef]
- Wu, L.; Hu, J.; Hua, J.; Liu, M.; Chen, J.; Xu, J. Diagnostic Value of Diffusion-weighted Magnetic Resonance Imaging Compared with Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography for Pancreatic Malignancy: A Meta-analysis Using a Hierarchical Regression Model. J. Gastro. Hepatol. 2012, 27, 1027–1035. [Google Scholar] [CrossRef]
- Wang, L.; Dong, P.; Wang, W.G.; Tian, B.L. Positron Emission Tomography Modalities Prevent Futile Radical Resection of Pancreatic Cancer: A Meta-Analysis. Int. J. Surg. 2017, 46, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; El-Hashash, A.H.K. Cell-Based Therapy for Idiopathic Pulmonary Fibrosis. Stem Cell Investig. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Chisholm, A.; Collard, H.R.; Flaherty, K.R.; Myers, J.; Raghu, G.; Walsh, S.L.F.; White, E.S.; Richeldi, L. The Diagnosis of Idiopathic Pulmonary Fibrosis: Current and Future Approaches. Lancet Respir. Med. 2017, 5, 61–71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urso, L.; Napolitano, R.; Speltri, G.; Tuncel, M.; Badrane, I.; Uccelli, L.; Porto, F.; Martini, P.; Niorettini, A.; Cittanti, C.; et al. 68Ga-Trivehexin: Current Status of αvβ6-Integrin Imaging and Perspectives. Cancers 2025, 17, 1504. https://doi.org/10.3390/cancers17091504
Urso L, Napolitano R, Speltri G, Tuncel M, Badrane I, Uccelli L, Porto F, Martini P, Niorettini A, Cittanti C, et al. 68Ga-Trivehexin: Current Status of αvβ6-Integrin Imaging and Perspectives. Cancers. 2025; 17(9):1504. https://doi.org/10.3390/cancers17091504
Chicago/Turabian StyleUrso, Luca, Rebecca Napolitano, Giorgia Speltri, Murat Tuncel, Ilham Badrane, Licia Uccelli, Francesca Porto, Petra Martini, Alessandro Niorettini, Corrado Cittanti, and et al. 2025. "68Ga-Trivehexin: Current Status of αvβ6-Integrin Imaging and Perspectives" Cancers 17, no. 9: 1504. https://doi.org/10.3390/cancers17091504
APA StyleUrso, L., Napolitano, R., Speltri, G., Tuncel, M., Badrane, I., Uccelli, L., Porto, F., Martini, P., Niorettini, A., Cittanti, C., Bartolomei, M., & Boschi, A. (2025). 68Ga-Trivehexin: Current Status of αvβ6-Integrin Imaging and Perspectives. Cancers, 17(9), 1504. https://doi.org/10.3390/cancers17091504









