Inflammation, Immunosuppression, and Immunotherapy in Pancreatic Cancer—Where Are We Now?
Simple Summary
Abstract
1. Introduction
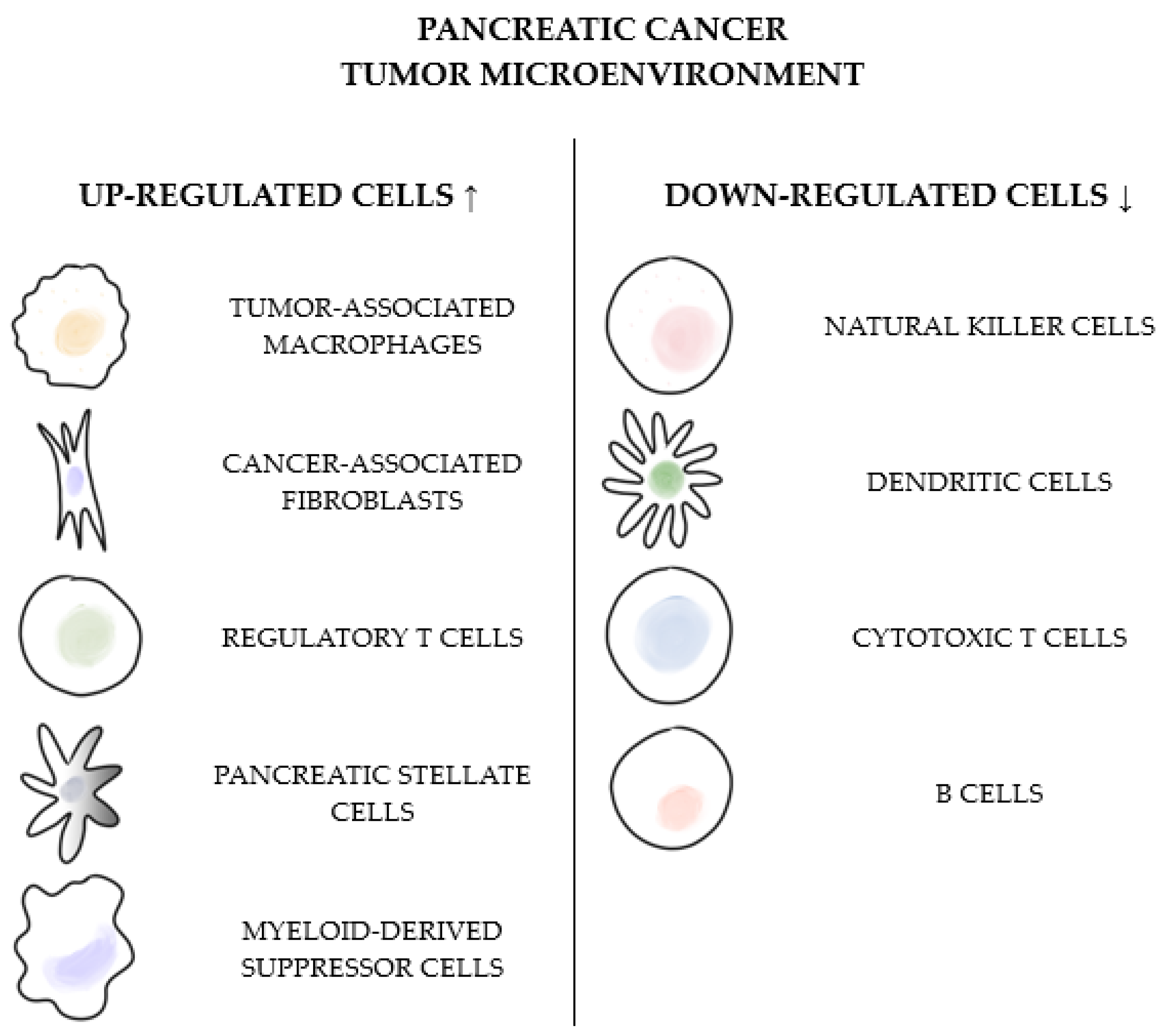
2. Inflammation and Immunosuppression
3. Inflammatory Markers
| Parameter | How Counted | Cut-Off Value | No of Patients | Time of Measurement | Association Between High Parameters and Outcomes | Ref. |
|---|---|---|---|---|---|---|
| CAR | CRP (mg/L) ÷ albumin (g/dL) | 0.18 | 595 | Pretreatment Post-chemotherapy | Shorter OS | [54] |
| 0.06 | 163 | Preoperative | Shorter OS and DFS | [45] | ||
| 0.34 | 142 | 14 postoperative days | Shorter OS and RFS | [46] | ||
| 3.85 | 302 | Pretreatment | Shorter OS and PFS | [55] | ||
| 0.09 | 143 | Preoperative | Shorter OS | [56] | ||
| 0.03 | 113 | Preoperative | Shorter OS and RFS | [44] | ||
| 0.4 | 1294 | Pretreatment | Shorter OS | [42] | ||
| CRP/ pre-albumin | CRP (mg/dl) ÷ prealbumin (mg/dL) | 1.3 | 20 | Preoperative | Shorter RFS | [48] |
| CALLY | [albumin (g/L) × lymphocyte count] ÷ [CRP (mg/L) × 104] | 1.03 | 121 | Preoperative | Lower rates of postoperative complications, longer OS | [51] |
| 3.00 | 307 | Longer OS and DFS | [53] | |||
| 1.90 | 461 | Longer OS and RFS | [52] | |||
| SII | [platelet count × neutrophil count] ÷ lymphocyte count | 400–900 | 2132 (meta-analysis), 1749 (meta-analysis) | Pretreatment | Shorter OS, DFS, and PFS | [39,40] |
| SIRI | [neutrophil count × monocyte count] ÷ lymphocyte count | 0.69–2.35 | 1160 (meta-analysis) | Pretreatment | Shorter OS and PFS | [41] |
| LMR | lymphocyte count ÷ monocyte count | 2.05–4.62 | 2557 (meta-analysis) | Pretreatment | Longer OS, DFS, RFS | [35] |
| 1.60–5.00 | 4019 (meta-analysis) | Preoperative | Longer OS | [36] | ||
| NLR | neutrophil count ÷ lymphocyte count | 2.00–5.00 | 8252 (meta-analysis) | Pretreatment | Shorter OS and DFS | [29] |
| PLR | platelet count ÷ lymphocyte count | 126–300 | 3182 (meta-analysis) | Pretreatment | Shorter OS and PFS | [32] |
| IBI | [lymphocyte count + monocyte count + neutrophil count + platelet count] ÷ CRP (mg/L) | 30 | 1294 | Pretreatment | Longer OS | [42] |
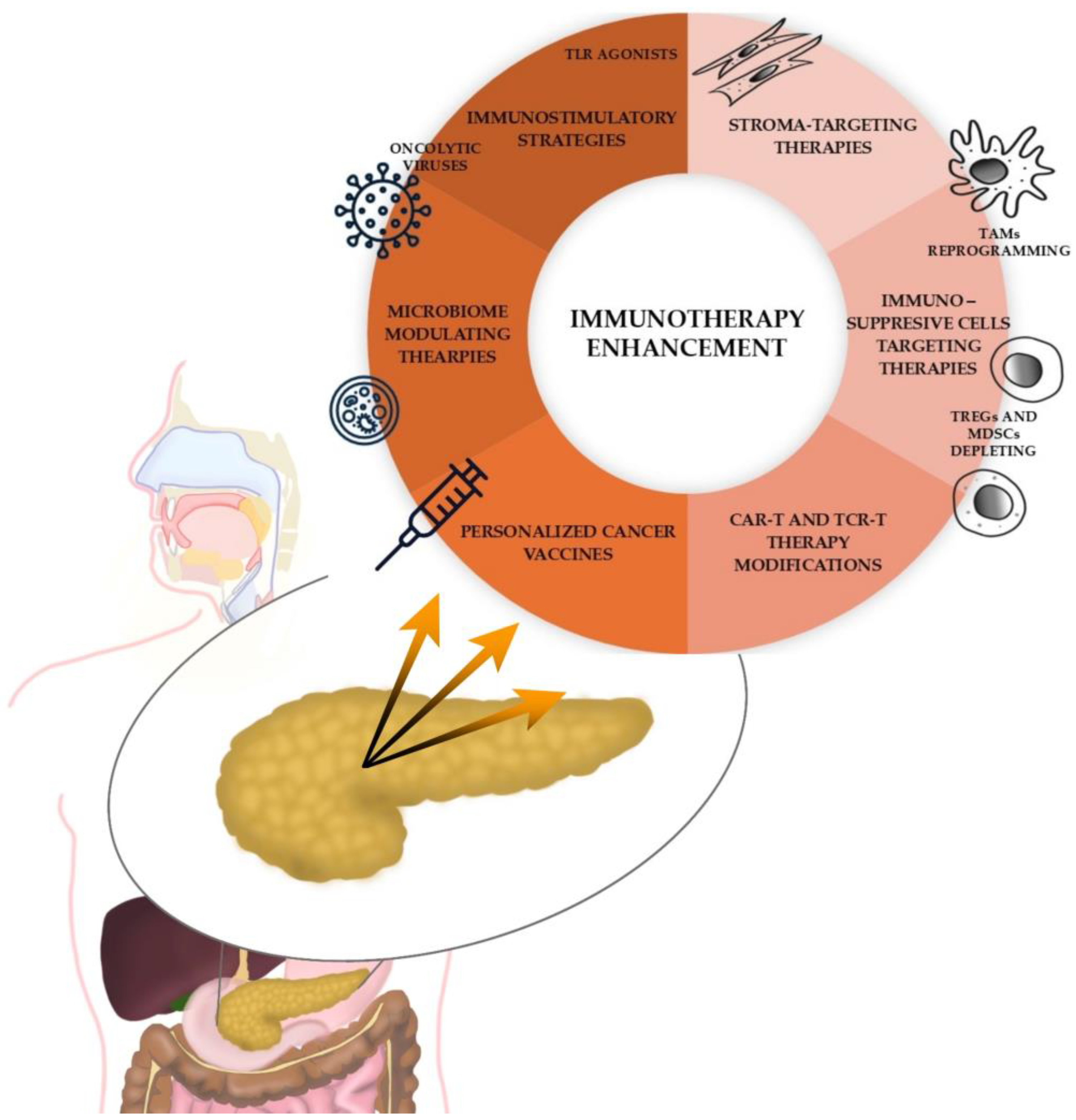
4. Immunotherapy
4.1. Current Immunotherapy Options in PC
4.2. Why Is Single-Agent Immunotherapy Not Effective in PC?
4.3. Clinical Trials
4.4. Vaccine-Based Immunotherapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| PC | Pancreatic cancer |
| OS | Overall survival |
| NCCN | National Comprehensive Cancer Network |
| mFOLFIRINOX | Modified FOLFIRINOX |
| RT | Radiotherapy |
| MSI | Microsatellite instability |
| MSI-H | High microsatellite instability |
| dMMR | Mismatch repair deficiency |
| TMB | Tumor mutational burden |
| PARP | Poly(ADP-ribose) Polymerase |
| NTRK | Neurotrophic tropomyosin receptor kinase |
| PD-L1 | Programmed cell death ligand 1 |
| TME | Tumor microenvironment |
| MDSCs | Myeloid-derived suppressor cells |
| Tregs | Regulatory T-cells |
| FDA | U.S. Food and Drug Administration (FDA) |
| ESMO | European Society for Medical Oncology |
| PS | Performance status |
| TMB-H | High tumor mutational burden |
| ICI | Immune checkpoint inhibitor |
| TAMs | Tumor-associated macrophages |
| TLR | Toll-like receptor |
| IFN-I | Type I Interferon |
| DPP | Dipeptidyl peptidase |
| FAP | Fibroblast activation protein |
| NLR | Neutrophil-to-lymphocyte ratio |
| DFS | Disease-free survival |
| EMT | Epithelial-to-mesenchymal transition |
| PLR | Platelet-to-lymphocyte ratio |
| PFS | Progression-free survival |
| LMR | Lymphocyte-to-monocyte ratio |
| RFS | Recurrence-free survival |
| SII | Immune–inflammation index |
| SIRI | Systemic inflammation response index |
| IBI | Inflammatory benchmark index |
| CAR | CRP/albumin ratio |
| mGPS | Modified Glasgow prognostic score |
| PNI | Prognostic index |
| CALLY index | CRP-albumin-lymphocyte index |
| GNRI | Geriatric nutritional risk index |
| CONUT status | Controlling nutritional status |
| PDAC | Pancreatic ductal adenocarcinoma |
| PDE5 | Phosphodiesterase type 5 |
| CXCL | C-X-C motif chemokine ligand |
| MHC | Major histocompatibility complex |
| CAF | Cancer-associated fibroblasts |
| PSC | Pancreatic stellate cell |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| NK | Natural killer |
| DC | Dendritic cells |
References
- Wang, S.; Zheng, R.; Li, J.; Zeng, H.; Li, L.; Chen, R.; Sun, K.; Han, B.; Bray, F.; Wei, W.; et al. Global, regional, and national lifetime risks of developing and dying from gastrointestinal cancers in 185 countries: A population-based systematic analysis of GLOBOCAN. Lancet Gastroenterol. Hepatol. 2024, 9, 229–237. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2025. Atlanta: American Cancer Society. 2025. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2025-cancer-facts-figures.html (accessed on 12 March 2025).
- Stoffel, E.M.; Brand, R.E.; Goggins, M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology 2023, 164, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Chen, X.; Fan, B.; Zeng, L.; Zhou, Z.; Mao, Z.; Shen, Q. Multidisciplinary team diagnosis and treatment of pancreatic cancer: Current landscape and future prospects. Front. Oncol. 2023, 13, 1077605. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 2.2025). Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 20 February 2025).
- Kwaśniewska, D.; Fudalej, M.; Nurzyński, P.; Badowska-Kozakiewicz, A.; Czerw, A.; Cipora, E.; Sygit, K.; Bandurska, E.; Deptała, A. How A Patient with Resectable or Borderline Resectable Pancreatic Cancer should Be Treated-A Comprehensive Review. Cancers 2023, 15, 4275. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Stocken, D.D.; Bassi, C.; Ghaneh, P.; Cunningham, D.; Goldstein, D.; Padbury, R.; Moore, M.J.; Gallinger, S.; Mariette, C.; et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA 2010, 304, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Melisi, D.; Macarulla, T.; Pazo Cid, R.; Chandana, S.R.; De La Fouchardière, C.; Dean, A.; Kiss, I.; Lee, W.J.; Goetze, T.O.; et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): A randomised, open-label, phase 3 trial. Lancet 2023, 402, 1272–1281. [Google Scholar] [CrossRef]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- Huguet, F.; Dabout, V.; Rivin Del Campo, E.; Gaujoux, S.; Bachet, J.B. The role of radiotherapy in locally advanced pancreatic cancer. Br. J. Radiol. 2021, 94, 20210044. [Google Scholar] [CrossRef]
- Fudalej, M.; Kwaśniewska, D.; Nurzyński, P.; Badowska-Kozakiewicz, A.; Mękal, D.; Czerw, A.; Sygit, K.; Deptała, A. New Treatment Options in Metastatic Pancreatic Cancer. Cancers 2023, 15, 2327. [Google Scholar] [CrossRef]
- Storandt, M.H.; Tran, N.; Martin, N.; Jatoi, A. Pembrolizumab near the end of life in patients with metastatic pancreatic cancer: A multi-site consecutive series to examine survival and patient treatment burden. Cancer Immunol. Immunother. 2023, 72, 2515–2520. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.; Pecoraro, C.; Panzeca, G.; Xu, G.; Roeten, M.S.F.; Cascioferro, S.; Giovannetti, E.; Diana, P.; Parrino, B. 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Nortopsentin Derivatives against Pancreatic Ductal Adenocarcinoma: Synthesis, Cytotoxic Activity, and Inhibition of CDK1. Mar. Drugs 2023, 21, 412. [Google Scholar] [CrossRef] [PubMed]
- Neerasa, J.; Kim, B.; Chung, H. Novel dual-targeting PROTAC degraders of GSK-3β and CDK5: A promising approach for pancreatic cancer treatment. Bioorganic Med. Chem. 2025, 120, 118085. [Google Scholar] [CrossRef]
- Rudloff, U. Emerging kinase inhibitors for the treatment of pancreatic ductal adenocarcinoma. Expert. Opin. Emerg. Drugs 2022, 27, 345–368. [Google Scholar] [CrossRef] [PubMed]
- Rosellini, M.; Marchetti, A.; Mollica, V.; Rizzo, A.; Santoni, M.; Massari, F. Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat. Rev. Urol. 2023, 20, 133–157. [Google Scholar] [CrossRef]
- Bear, A.S.; Vonderheide, R.H.; O’Hara, M.H. Challenges and Opportunities for Pancreatic Cancer Immunotherapy. Cancer Cell 2020, 38, 788–802. [Google Scholar] [CrossRef]
- Shadhu, K.; Xi, C. Inflammation and pancreatic cancer: An updated review. Saudi J. Gastroenterol. 2019, 25, 3–13. [Google Scholar] [CrossRef]
- Fan, Z.; Luo, G.; Gong, Y.; Xu, H.; Qian, Y.; Deng, S.; Huang, Q.; Yang, C.; Cheng, H.; Jin, K.; et al. Prognostic Value of the C-Reactive Protein/Lymphocyte Ratio in Pancreatic Cancer. Ann. Surg. Oncol. 2020, 27, 4017–4025. [Google Scholar] [CrossRef]
- Fudalej, M.; Cichowska, I.; Badowska-Kozakiewicz, A.; Deptała, A. The prevalence and impact of overweight and hypertension among patients with pancreatic cancer. Oncol. Clin. Pract. 2024, 21, 15–27. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 2015, 36, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Wang, H.; Zhang, S.; Wei, Y.; Liu, S. The Interplay Between Inflammation and Stromal Components in Pancreatic Cancer. Front. Immunol. 2022, 13, 850093. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the association of chronic inflammation and cancer: Insights and implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef]
- Pęczek, P.; Gajda, M.; Rutkowski, K.; Fudalej, M.; Deptała, A.; Badowska-Kozakiewicz, A.M. Cancer-associated inflammation: Pathophysiology and clinical significance. J. Cancer Res. Clin. Oncol. 2023, 149, 2657–2672. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Q.; Fan, J.; Cheng, S.; Ding, W.; Hua, Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis containing 8252 patients. Clin. Chim. Acta 2018, 479, 181–189. [Google Scholar] [CrossRef]
- Gaida, M.M.; Steffen, T.G.; Günther, F.; Tschaharganeh, D.F.; Felix, K.; Bergmann, F.; Schirmacher, P.; Hänsch, G.M. Polymorphonuclear neutrophils promote dyshesion of tumor cells and elastase-mediated degradation of E-cadherin in pancreatic tumors. Eur. J. Immunol. 2012, 42, 3369–3380. [Google Scholar] [CrossRef]
- Bausch, D.; Pausch, T.; Krauss, T.; Hopt, U.T.; Fernandez-del-Castillo, C.; Warshaw, A.L.; Thayer, S.P.; Keck, T. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis 2011, 14, 235–243. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, S.; Fathy, A.H.; Qian, H.; Zhao, Y. Prognostic value of platelet-to-lymphocyte ratio in pancreatic cancer: A comprehensive meta-analysis of 17 cohort studies. Onco Targets Ther. 2018, 11, 1899–1908. [Google Scholar] [CrossRef]
- Menter, D.G.; Tucker, S.C.; Kopetz, S.; Sood, A.K.; Crissman, J.D.; Honn, K.V. Platelets and cancer: A casual or causal relationship: Revisited. Cancer Metastasis Rev. 2014, 33, 231–269. [Google Scholar] [CrossRef] [PubMed]
- Boone, B.A.; Zenati, M.S.; Rieser, C.; Hamad, A.; Al-Abbas, A.; Zureikat, A.H.; Hogg, M.E.; Neal, M.D.; Zeh, H.J., 3rd. Risk of Venous Thromboembolism for Patients with Pancreatic Ductal Adenocarcinoma Undergoing Preoperative Chemotherapy Followed by Surgical Resection. Ann. Surg. Oncol. 2019, 26, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.-j.; Ma, J.-y.; Hu, G. Lymphocyte-to-monocyte ratio in pancreatic cancer: Prognostic significance and meta-analysis. Clin. Chim. Acta 2018, 481, 142–146. [Google Scholar] [CrossRef]
- Li, H.; Peng, S.; An, R.; Du, N.; Wu, H.; Zhen, X.; Gao, Y.; Li, Z.; Min, J. The prognostic role of lymphocyte-to-monocyte ratio in patients with resectable pancreatic cancer: A systematic review and meta-analysis. PeerJ 2024, 12, e17585. [Google Scholar] [CrossRef] [PubMed]
- Goto, W.; Kashiwagi, S.; Asano, Y.; Takada, K.; Takahashi, K.; Hatano, T.; Takashima, T.; Tomita, S.; Motomura, H.; Hirakawa, K.; et al. Predictive value of lymphocyte-to-monocyte ratio in the preoperative setting for progression of patients with breast cancer. BMC Cancer 2018, 18, 1137. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef]
- Li, X.; Lin, H.; Ouyang, R.; Yang, Y.; Peng, J. Prognostic significance of the systemic immune-inflammation index in pancreatic carcinoma patients: A meta-analysis. Biosci. Rep. 2021, 41, BSR20204401. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Z.; Wang, Z.; Yue, C.; Hu, W.; Lu, H. Prognostic value of systemic immune-inflammation index in patients with pancreatic cancer: A meta-analysis. Clin. Exp. Med. 2022, 22, 637–646. [Google Scholar] [CrossRef]
- Shen, H.; Zuo, F. Prognostic role of systemic inflammation response index (SIRI) in patients with pancreatic cancer: A meta-analysis. Front. Oncol. 2024, 14, 1465279. [Google Scholar] [CrossRef]
- Neumann, C.C.M.; Schneider, F.; Hilfenhaus, G.; Vecchione, L.; Felsenstein, M.; Ihlow, J.; Geisel, D.; Sander, S.; Pratschke, J.; Stintzing, S.; et al. Inflammation-Based Prognostic Scores in Pancreatic Cancer Patients-A Single-Center Analysis of 1294 Patients within the Last Decade. Cancers 2023, 15, 2367. [Google Scholar] [CrossRef]
- Badowska-Kozakiewicz, A.; Fudalej, M.; Kwaśniewska, D.; Durlik, M.; Nasierowska-Guttmejer, A.; Mormul, A.; Włoszek, E.; Czerw, A.; Banaś, T.; Deptała, A. Diabetes Mellitus and Pancreatic Ductal Adenocarcinoma—Prevalence, Clinicopathological Variables, and Clinical Outcomes. Cancers 2022, 14, 2840. [Google Scholar] [CrossRef] [PubMed]
- Haruki, K.; Shiba, H.; Shirai, Y.; Horiuchi, T.; Iwase, R.; Fujiwara, Y.; Furukawa, K.; Misawa, T.; Yanaga, K. The C-reactive Protein to Albumin Ratio Predicts Long-Term Outcomes in Patients with Pancreatic Cancer After Pancreatic Resection. World J. Surg. 2016, 40, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, Y.; Miyazaki, K.; Yoshikawa, M.; Yamada, S.; Saito, Y.; Ikemoto, T.; Imura, S.; Morine, Y.; Shimada, M. Value of the CRP-albumin ratio in patients with resectable pancreatic cancer. J. Med. Investig. 2021, 68, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Yamashita, Y.-i.; Hashimoto, D.; Nakagawa, S.; Umezaki, N.; Yamao, T.; Tsukamoto, M.; Kitano, Y.; Yamamura, K.; Miyata, T.; et al. Clinical usefulness of postoperative C-reactive protein/albumin ratio in pancreatic ductal adenocarcinoma. Am. J. Surg. 2018, 216, 111–115. [Google Scholar] [CrossRef]
- Unal, D.; Orhan, O.; Eroglu, C.; Kaplan, B. Prealbumin is a more sensitive marker than albumin to assess the nutritional status in patients undergoing radiotherapy for head and neck cancer. Contemp. Oncol./Współczesna Onkol. 2013, 17, 276–280. [Google Scholar] [CrossRef]
- Kwon, C.H.; Seo, H.I.; Kim, D.U.; Han, S.Y.; Kim, S.; Lee, N.K.; Hong, S.B.; Ahn, J.H.; Park, Y.M.; Noh, B.G. Clinical significance of C-reactive protein-to-prealbumin ratio in predicting early recurrence in resectable pancreatic cancer. Korean J. Clin. Oncol. 2023, 19, 11–17. [Google Scholar] [CrossRef]
- Müller, L.; Hahn, F.; Mähringer-Kunz, A.; Stoehr, F.; Gairing, S.J.; Michel, M.; Foerster, F.; Weinmann, A.; Galle, P.R.; Mittler, J.; et al. Immunonutritive Scoring for Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization: Evaluation of the CALLY Index. Cancers 2021, 13, 5018. [Google Scholar] [CrossRef]
- Zhu, D.; Lin, Y.-D.; Yao, Y.-Z.; Qi, X.-J.; Qian, K.; Lin, L.-Z. Negative association of C-reactive protein-albumin-lymphocyte index (CALLY index) with all-cause and cause-specific mortality in patients with cancer: Results from NHANES 1999–2018. BMC Cancer 2024, 24, 1499. [Google Scholar] [CrossRef]
- Angın, Y.S.; Şendil, A.M.; Zengin, A.; Ceylan, C.; Bal, C.; Kılıç, M.; Ulaş, M. Pancreatic Cancer and the Prognostic Value of the CALLY Index: A Comparative Analysis. Indian J. Surg. 2025. [Google Scholar] [CrossRef]
- Kawahara, S.; Aoyama, T.; Murakawa, M.; Kanemoto, R.; Matsushita, N.; Hashimoto, I.; Kamiya, M.; Maezawa, Y.; Kobayashi, S.; Ueno, M.; et al. Clinical usefulness of C-reactive protein-albumin-lymphocyte (CALLY) index as a prognostic biomarker in patients undergoing surgical resection of pancreatic cancer. Langenbecks Arch. Surg. 2024, 409, 317. [Google Scholar] [CrossRef]
- Matsui, S.; Kato, Y.; Ohgi, K.; Ashida, R.; Yamada, M.; Otsuka, S.; Uesaka, K.; Sugiura, T. Prognostic impact of the CALLY index in patients with resectable pancreatic cancer. Surg. Oncol. Insight 2025, 2, 100119. [Google Scholar] [CrossRef]
- Fan, Z.; Fan, K.; Gong, Y.; Huang, Q.; Yang, C.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; Luo, G.; et al. The CRP/Albumin Ratio Predicts Survival And Monitors Chemotherapeutic Effectiveness In Patients With Advanced Pancreatic Cancer. Cancer Manag. Res. 2019, 11, 8781–8788. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.Y.; Kim, D.S.; Kang, E.J.; Kim, J.S.; Choi, Y.J.; Oh, S.C.; Seo, J.H.; Kim, J.S. Inflammatory markers as prognostic indicators in pancreatic cancer patients who underwent gemcitabine-based palliative chemotherapy. Korean J. Intern. Med. 2020, 35, 171–184. [Google Scholar] [CrossRef]
- Ikuta, S.; Aihara, T.; Yamanaka, N. Preoperative C-reactive protein to albumin ratio is a predictor of survival after pancreatic resection for pancreatic ductal adenocarcinoma. Asia Pac. J. Clin. Oncol. 2019, 15, e109–e114. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, M.; Zhang, D.; Chen, M.; Zhu, D. Clinical cancer immunotherapy: Current progress and prospects. Front. Immunol. 2022, 13, 961805. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Farhangnia, P.; Khorramdelazad, H.; Nickho, H.; Delbandi, A.-A. Current and future immunotherapeutic approaches in pancreatic cancer treatment. J. Hematol. Oncol. 2024, 17, 40. [Google Scholar] [CrossRef]
- Sakakida, T.; Ishikawa, T.; Doi, T.; Morita, R.; Kataoka, S.; Miyake, H.; Yamaguchi, K.; Moriguchi, M.; Sogame, Y.; Yasuda, H.; et al. Genomic profile and clinical features of MSI-H and TMB-high pancreatic cancers: Real-world data from C-CAT database. J. Gastroenterol. 2024, 59, 145–156. [Google Scholar] [CrossRef]
- Maio, M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; De Jesus Acosta, A.; Doi, T.; Longo, F.; et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: Updated analysis from the phase II KEYNOTE-158 study. Ann. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef]
- Le, D.T.; Diaz, L.A., Jr.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.H.; Kavan, P.; et al. Pembrolizumab for previously treated, microsatellite instability-high/mismatch repair-deficient advanced colorectal cancer: Final analysis of KEYNOTE-164. Eur. J. Cancer 2023, 186, 185–195. [Google Scholar] [CrossRef]
- André, T.; Berton, D.; Curigliano, G.; Sabatier, R.; Tinker, A.V.; Oaknin, A.; Ellard, S.; de Braud, F.; Arkenau, H.T.; Trigo, J.; et al. Antitumor Activity and Safety of Dostarlimab Monotherapy in Patients With Mismatch Repair Deficient Solid Tumors: A Nonrandomized Controlled Trial. JAMA Netw. Open 2023, 6, e2341165. [Google Scholar] [CrossRef]
- Schenker, M.; Burotto, M.; Richardet, M.; Ciuleanu, T.; Goncalves, A.; Steeghs, N.; Schöffski, P.; Ascierto, P.A.; Maio, M.; Lugowska, I.; et al. Abstract CT022: CheckMate 848: A randomized, open-label, phase 2 study of nivolumab in combination with ipilimumab or nivolumab monotherapy in patients with advanced or metastatic solid tumors of high tumor mutational burden. Cancer Res. 2022, 82, CT022. [Google Scholar] [CrossRef]
- Murakami, T.; Hiroshima, Y.; Matsuyama, R.; Homma, Y.; Hoffman, R.M.; Endo, I. Role of the tumor microenvironment in pancreatic cancer. Ann. Gastroenterol. Surg. 2019, 3, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, M.T.; Antignani, A.; Fitzgerald, D.J. Managing the TME to improve the efficacy of cancer therapy. Front. Immunol. 2022, 13, 954992. [Google Scholar] [CrossRef] [PubMed]
- Hartupee, C.; Nagalo, B.M.; Chabu, C.Y.; Tesfay, M.Z.; Coleman-Barnett, J.; West, J.T.; Moaven, O. Pancreatic cancer tumor microenvironment is a major therapeutic barrier and target. Front. Immunol. 2024, 15, 1287459. [Google Scholar] [CrossRef]
- Ullman, N.A.; Burchard, P.R.; Dunne, R.F.; Linehan, D.C. Immunologic Strategies in Pancreatic Cancer: Making Cold Tumors Hot. J. Clin. Oncol. 2022, 40, 2789–2805. [Google Scholar] [CrossRef]
- Foucher, E.D.; Ghigo, C.; Chouaib, S.; Galon, J.; Iovanna, J.; Olive, D. Pancreatic Ductal Adenocarcinoma: A Strong Imbalance of Good and Bad Immunological Cops in the Tumor Microenvironment. Front. Immunol. 2018, 9, 1044. [Google Scholar] [CrossRef]
- Chen, G.; Wu, K.; Li, H.; Xia, D.; He, T. Role of hypoxia in the tumor microenvironment and targeted therapy. Front. Oncol. 2022, 12, 961637. [Google Scholar] [CrossRef]
- Imamura, T.; Ashida, R.; Ohshima, K.; Uesaka, K.; Sugiura, T.; Ohgi, K.; Yamada, M.; Otsuka, S.; Hatakeyama, K.; Nagashima, T.; et al. Characterization of pancreatic cancer with ultra-low tumor mutational burden. Sci. Rep. 2023, 13, 4359. [Google Scholar] [CrossRef]
- Haddaoui, H.E.; van Eijck, C.W.; Doukas, M.; van den Bosch, T.P.; van Koetsveld, P.M.; Hofland, L.J.; Mustafa, D.A.; van Eijck, C.H. Rintatolimod: A potential treatment in patients with pancreatic cancer expressing Toll-like receptor 3. Am. J. Cancer Res. 2023, 13, 2657–2669. [Google Scholar]
- AIM ImmunoTech Reports Positive Preliminary Data in Phase 1b/2 Study of Ampligen and Imfinzi as a Combination Therapy for Late-Stage Pancreatic Cancer. Available online: https://aimimmuno.com/aim-immunotech-reports-positive-preliminary-data-in-phase-1b-2-study-of-ampligen-and-imfinzi-as-a-combination-therapy-for-late-stage-pancreatic-cancer/ (accessed on 11 March 2025).
- Gehris, J.; Ervin, C.; Hawkins, C.; Womack, S.; Churillo, A.M.; Doyle, J.; Sinusas, A.J.; Spinale, F.G. Fibroblast activation protein: Pivoting cancer/chemotherapeutic insight towards heart failure. Biochem. Pharmacol. 2024, 219, 115914. [Google Scholar] [CrossRef]
- Weinberg, B.A.; Lekan, A.; Fitzgerald, A.; Malchiodi, Z.; Gutierrez, M.; Tesfaye, A.A.; Tan, M.T.; Noel, M.S.; He, A.R.; Mukherji, R.; et al. Phase II trial of BXCL701 and pembrolizumab in patients with metastatic pancreatic ductal adenocarcinoma (EXPEL-PANC): Preliminary findings. J. Clin. Oncol. 2024, 42, LBA4132. [Google Scholar] [CrossRef]
- Adhikary, P.; Chakrabarti, J.; Resmi, M.P.; Wang, J.; Sohal, D.; Shroff, R.T.; Ahmad, S.A.; Zavros, Y. Abstract 233: Cabozantinib changes the landscape of the pancreatic tumor microenvironment and improves the efficacy of pembrolizumab. Cancer Res. 2024, 84, 233. [Google Scholar] [CrossRef]
- Su, J.; Zhang, J.; Wu, Y.; Ni, C.; Ding, Y.; Cai, Z.; Xu, M.; Lai, M.; Wang, J.; Lin, S.; et al. Cabozantinib in combination with immune checkpoint inhibitors for renal cell carcinoma: A systematic review and meta-analysis. Front. Pharmacol. 2024, 15, 1322473. [Google Scholar] [CrossRef] [PubMed]
- Dilly, J.; Hoffman, M.T.; Abbassi, L.; Li, Z.; Paradiso, F.; Parent, B.D.; Hennessey, C.J.; Jordan, A.C.; Morgado, M.; Dasgupta, S.; et al. Mechanisms of Resistance to Oncogenic KRAS Inhibition in Pancreatic Cancer. Cancer Discov. 2024, 14, 2135–2161. [Google Scholar] [CrossRef] [PubMed]
- Sethna, Z.; Guasp, P.; Reiche, C.; Milighetti, M.; Ceglia, N.; Patterson, E.; Lihm, J.; Payne, G.; Lyudovyk, O.; Rojas, L.A.; et al. RNA neoantigen vaccines prime long-lived CD8+ T cells in pancreatic cancer. Nature 2025, 639, 1042–1051. [Google Scholar] [CrossRef]
- Igarashi, Y.; Sasada, T. Cancer Vaccines: Toward the Next Breakthrough in Cancer Immunotherapy. J. Immunol. Res. 2020, 2020, 5825401. [Google Scholar] [CrossRef]
- Lutz, E.R.; Wu, A.A.; Bigelow, E.; Sharma, R.; Mo, G.; Soares, K.; Solt, S.; Dorman, A.; Wamwea, A.; Yager, A.; et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol. Res. 2014, 2, 616–631. [Google Scholar] [CrossRef]
- Tsujikawa, T.; Kumar, S.; Borkar, R.N.; Azimi, V.; Thibault, G.; Chang, Y.H.; Balter, A.; Kawashima, R.; Choe, G.; Sauer, D.; et al. Quantitative Multiplex Immunohistochemistry Reveals Myeloid-Inflamed Tumor-Immune Complexity Associated with Poor Prognosis. Cell Rep. 2017, 19, 203–217. [Google Scholar] [CrossRef]
- Heumann, T.; Judkins, C.; Li, K.; Lim, S.J.; Hoare, J.; Parkinson, R.; Cao, H.; Zhang, T.; Gai, J.; Celiker, B.; et al. A platform trial of neoadjuvant and adjuvant antitumor vaccination alone or in combination with PD-1 antagonist and CD137 agonist antibodies in patients with resectable pancreatic adenocarcinoma. Nat. Commun. 2023, 14, 3650. [Google Scholar] [CrossRef]
- Cao, J.; Jin, Y.; Li, W.; Zhang, B.; He, Y.; Liu, H.; Xia, N.; Wei, H.; Yan, J. DNA vaccines targeting the encoded antigens to dendritic cells induce potent antitumor immunity in mice. BMC Immunol. 2013, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, R.; Debnath, D.; Hartley, M.L.; Noel, M.S. The Role of Immunotherapy in Pancreatic Cancer. Curr. Oncol. 2022, 29, 6864–6892. [Google Scholar] [CrossRef]
- Asimgil, H.; Ertetik, U.; Çevik, N.C.; Ekizce, M.; Doğruöz, A.; Gökalp, M.; Arık-Sever, E.; Istvanffy, R.; Friess, H.; Ceyhan, G.O.; et al. Targeting the undruggable oncogenic KRAS: The dawn of hope. JCI Insight 2022, 7, e153688. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.A.; Zhang, H.X.; Jiang, Y.N.; Luo, W.H. Cancer vaccines: Targeting KRAS-driven cancers. Expert. Rev. Vaccines 2020, 19, 163–173. [Google Scholar] [CrossRef]
- Bannoura, S.F.; Uddin, M.H.; Nagasaka, M.; Fazili, F.; Al-Hallak, M.N.; Philip, P.A.; El-Rayes, B.; Azmi, A.S. Targeting KRAS in pancreatic cancer: New drugs on the horizon. Cancer Metastasis Rev. 2021, 40, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Wainberg, Z.A.; Weekes, C.D.; Furqan, M.; Kasi, P.M.; Devoe, C.E.; Leal, A.D.; Chung, V.; Basturk, O.; VanWyk, H.; et al. Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: The phase 1 AMPLIFY-201 trial. Nat. Med. 2024, 30, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.D.; Heumann, T.R.; Berg, M.; Ferguson, A.; Lim, S.J.; Wang, H.; Nauroth, J.; Laheru, D.; Jaffee, E.M.; Azad, N.S.; et al. A phase I study of a mutant KRAS-targeted long peptide vaccine combined with ipilimumab/nivolumab in resected pancreatic cancer and MMR-proficient metastatic colorectal cancer. J. Clin. Oncol. 2023, 41, TPS814. [Google Scholar] [CrossRef]
- Ni, L. Advances in mRNA-Based Cancer Vaccines. Vaccines 2023, 11, 1599. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral vectored vaccines: Design, development, preventive and therapeutic applications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 149. [Google Scholar] [CrossRef]
- Le, D.T.; Picozzi, V.J.; Ko, A.H.; Wainberg, Z.A.; Kindler, H.; Wang-Gillam, A.; Oberstein, P.; Morse, M.A.; Zeh, H.J., 3rd; Weekes, C.; et al. Results from a Phase IIb, Randomized, Multicenter Study of GVAX Pancreas and CRS-207 Compared with Chemotherapy in Adults with Previously Treated Metastatic Pancreatic Adenocarcinoma (ECLIPSE Study). Clin. Cancer Res. 2019, 25, 5493–5502. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, T.; Crocenzi, T.; Durham, J.N.; Sugar, E.A.; Wu, A.A.; Onners, B.; Nauroth, J.M.; Anders, R.A.; Fertig, E.J.; Laheru, D.A.; et al. Evaluation of Cyclophosphamide/GVAX Pancreas Followed by Listeria-Mesothelin (CRS-207) with or without Nivolumab in Patients with Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 3578–3588. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.E.; Zhang, Z.; Mitchell, J.T.; Charmsaz, S.; Hernandez, A.G.; Coyne, E.M.; Shin, S.M.; Vargas Carvajal, D.C.; Sidiropoulos, D.N.; Cho, Y.; et al. Phosphodiesterase-5 inhibition collaborates with vaccine-based immunotherapy to reprogram myeloid cells in pancreatic ductal adenocarcinoma. JCI Insight 2024, 9, e179292. [Google Scholar] [CrossRef] [PubMed]
| Combination | Phase | Indication | Identifier |
|---|---|---|---|
| DARATUMUMAB (anti-CD38) + KRAS vaccine + NIVOLUMAB (anti-PD-1) | Phase II | Advanced PDAC—second-line treatment * Mutant KRAS in codon 12 (12A, C, D, R, S, V) or 13D | NCT06015724 |
| DURVALUMAB (anti-PD-1) + RINTATOLIMOD (TLR-3 agonist) | Phase I, Phase II | Metastatic PDAC—first-line treatment | NCT05927142 |
| DURVALUMAB (anti-PD-1) + OLECLUMAB (anti-CD73) | Phase II | Resectable PDAC—treatment before the surgery | NCT06060405 |
| PEMBROLIZUMAB (anti-PD-1) + TALABOSTAT (DPP8/9 and FAP inhibitor) | Phase II | Metastatic PDAC—second-line treatment | NCT05558982 |
| PENPULIMAB (anti-PD-1) + ANLOTINIB (multi-targeting TKI) + chemotherapy (gemcitabine + nab-paclitaxel) | Phase II | Metastatic PC—first-line treatment | NCT06051851 |
| BOTENSILIMAB (CTLA-4 inhibitor) + chemotherapy (gemcitabine + nab-paclitaxel) | Phase II | Metastatic PDAC—second-line treatment | NCT05630183 |
| TADALAFIL (PDE5 inhibitor) + PEMBROLIZUMAB (anti-PD-1) + IPILIMUMAB (CTLA-4 inhibitor) + CRS-207 (Listeria monocytogenes vaccine) | Phase II | Metastatic PDAC—second- or later-line treatment | NCT05014776 |
| AVELUMAB (anti-PD-L1) + PEPINEMAB (SEMA4D inhibitor) | Phase I, Phase II | Metastatic PDAC—second-line treatment | NCT05102721 |
| Chemotherapy + SBRT + NIVOLUMAB (anti-PD-1) + IPILIMUMAB (CTLA-4 inhibitor) | Phase I | Metastatic PDAC—first-line treatment | NCT05088889 |
| Neoadjuvant chemotherapy (mFOLFIRINOX + PEMBROLIZUMAB (anti-PD-1) + surgery + adjuvant chemotherapy (mFOLFIRINOX) + PEMBROLIZUMAB | Phase II | Resectable PDAC—treatment before and after the surgery | NCT05132504 |
| CABOZANTINIB (multi-targeting TKI) + PEMBROLIZUMAB (anti-PD-1) | Phase II | Metastatic PDAC—second- or later-line treatment | NCT05052723 |
| LEVANTINIB (multi-targeting TKI) + PEMBROLIZUMAB (anti-PD-1) | Phase II | Metastatic/Unresectable PDAC—maintenance (PR/SD after 16 weeks of 1st- or 2nd-line treatment) | NCT04887805 |
| NIRAPARIB (PARP inhibitor) + DOSTARLIMAB (anti-PD-1) | Phase II | Metastatic PDAC—second- or later-line treatment * germline or somatic mutations BRCA1/2, PALB2, BARD1, RAD51C, or RAD51D | NCT04493060 |
| OLAPARIB (PARP inhibitor) + PEMBROLIZUMAB (anti-PD-1) | Phase II | Metastatic PDAC * dMMR or TMB > 4 Mutations/Mb | NCT05093231 |
| XH001 (neoantigen cancer vaccine) + IPILIMUMAB (CTLA-4 inhibitor) + chemotherapy (gemcitabine + capecitabine) | Phase I, Phase II | Resectable PDAC—treatment after the surgery | NCT06353646 |
| Irreversible electroporation + PEMBROLIZUMAB (anti-PD-1) | Phase I | Locally advanced unresectable PC—treatment after chemotherapy and ablative stereotactic magnetic resonance image-guided adaptive radiation therapy | NCT06378047 |
| ANLOTINIB (multi-targeting TKI) + BENMELSTOBART (anti-PD-L1) + chemotherapy (gemcitabine + nab-paclitaxel) | Phase II | Metastatic PC—first-line treatment | NCT06621095 |
| SBRT + BOTENSILIMAB (Fc-enhanced anti-CTLA-4) + BALSTILIMAB (anti-PD-1) | Phase II | Metastatic PDAC—second- or later-line treatment | NCT06843551 |
| AGEN1423 (anti-CD73-TGF-β-trap) + BOTENSILIMAB (Fc-enhanced anti-CTLA4) | Phase II | Advanced PDAC—second- or later-line treatment | NCT05632328 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fudalej, M.; Krupa, K.; Badowska-Kozakiewicz, A.; Deptała, A. Inflammation, Immunosuppression, and Immunotherapy in Pancreatic Cancer—Where Are We Now? Cancers 2025, 17, 1484. https://doi.org/10.3390/cancers17091484
Fudalej M, Krupa K, Badowska-Kozakiewicz A, Deptała A. Inflammation, Immunosuppression, and Immunotherapy in Pancreatic Cancer—Where Are We Now? Cancers. 2025; 17(9):1484. https://doi.org/10.3390/cancers17091484
Chicago/Turabian StyleFudalej, Marta, Kamila Krupa, Anna Badowska-Kozakiewicz, and Andrzej Deptała. 2025. "Inflammation, Immunosuppression, and Immunotherapy in Pancreatic Cancer—Where Are We Now?" Cancers 17, no. 9: 1484. https://doi.org/10.3390/cancers17091484
APA StyleFudalej, M., Krupa, K., Badowska-Kozakiewicz, A., & Deptała, A. (2025). Inflammation, Immunosuppression, and Immunotherapy in Pancreatic Cancer—Where Are We Now? Cancers, 17(9), 1484. https://doi.org/10.3390/cancers17091484





