A Systematic Analysis of Expression and Function of RAS GTPase-Activating Proteins (RASGAPs) in Urological Cancers: A Mini-Review
Simple Summary
Abstract
1. Introduction
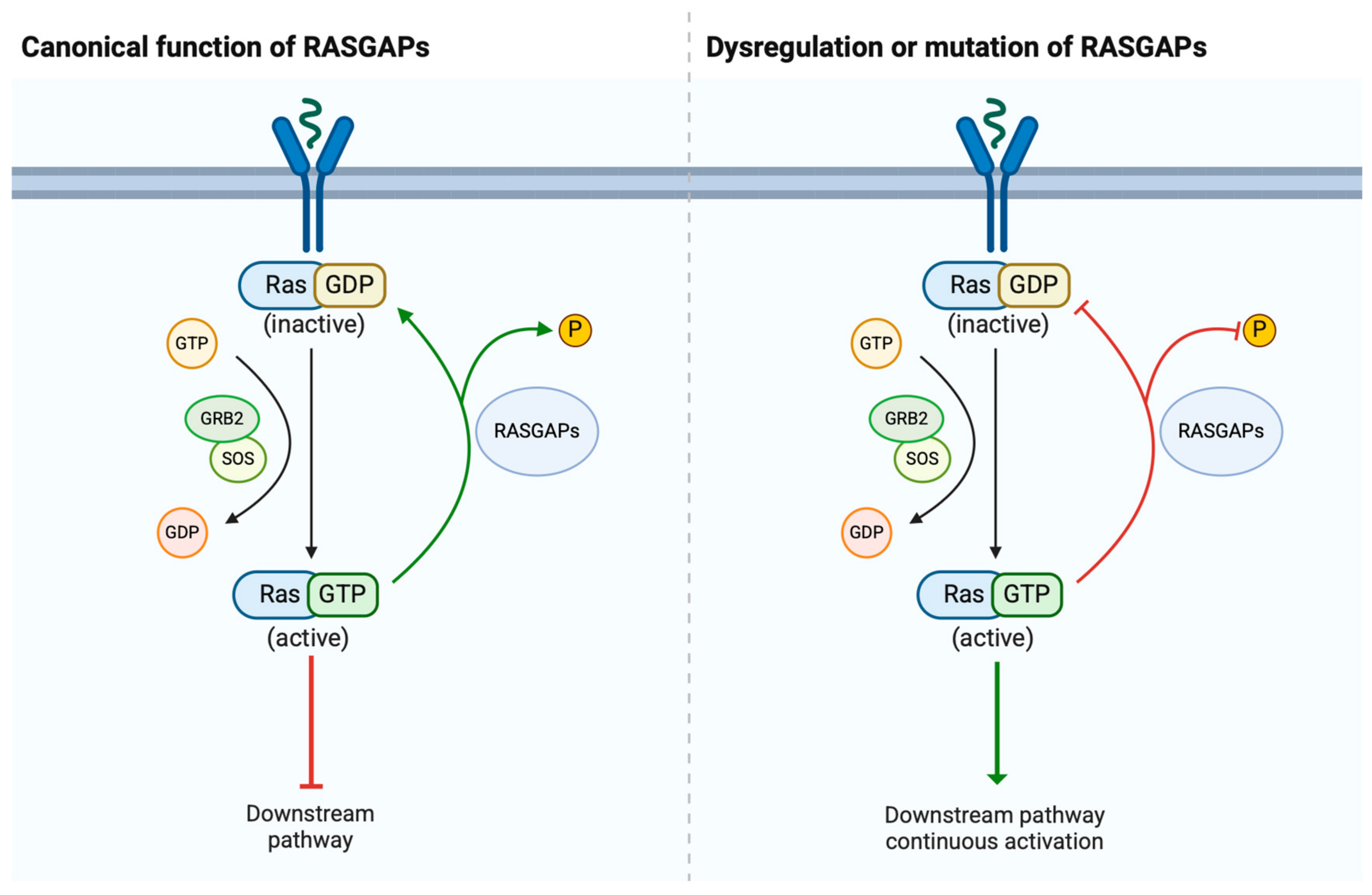
2. Structure and Physiological Roles of RASGAPs
2.1. Structure of RASGAPs
2.2. Physiological Roles of RASGAPs
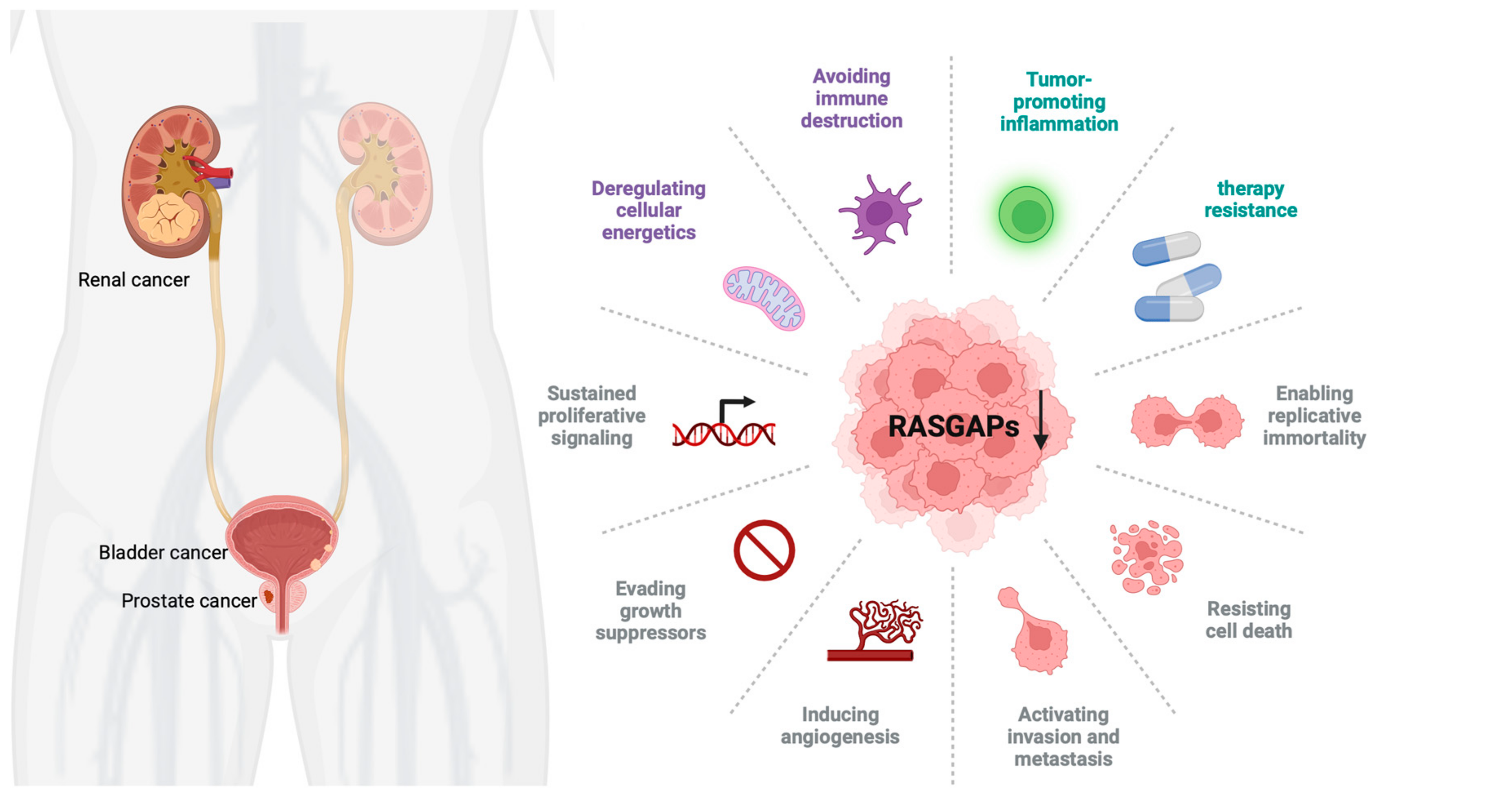
3. Dysregulation of RASGAPs Promotes Urological Cancers
3.1. RASGAPs and Prostate Cancer
3.2. RASGAPs and Bladder Cancer
3.3. RASGAPs and Renal Cancer
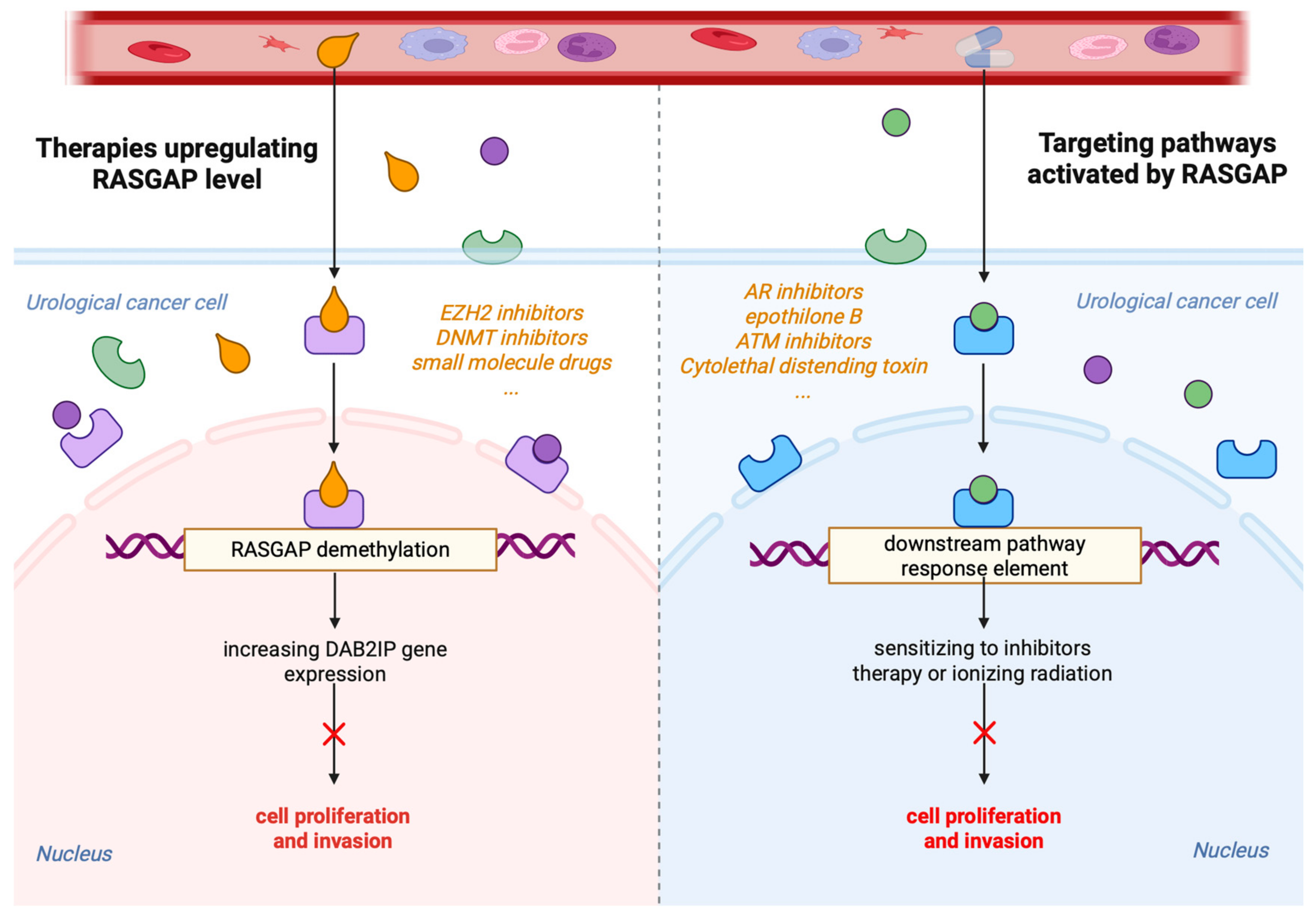
4. Targeting the RASGAPs Is a Significant Strategy in Cancer Therapy
5. Perspectives on the Future of RASGAPs
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADPC | Androgen-dependent prostate cancer |
| ADTs | Androgen deprivation therapies |
| AKT | Protein Kinase B (also known as PKB) |
| AR | Androgen receptor |
| ARHGAP | Rho GTPase-Activating Protein |
| ASK1-JNK | Apoptosis Signal-regulating Kinase 1–c-Jun N-terminal Kinase |
| ATM | Ataxia-Telangiectasia Mutated |
| BCa | Bladder cancer |
| CAFs | Cancer-associated fibroblasts |
| CDT | Cytolethal distending toxin |
| CRPC | Castration-resistant prostate cancer |
| CPR | Proline-rich domain in C terminal |
| DAB2IP | Disabled-2 Interacting Protein |
| DC | Dendritic cell |
| DNMT1/3A | DNA Methyltransferase 1/3A |
| EMT | Epithelial-to-mesenchymal transition |
| Enz | Enzalutamide |
| ERE | Estrogen-response element |
| ERβ | Estrogen receptor beta |
| ERK1/2 | Extracellular Signal-Regulated Kinase 1/2 |
| GEFs | Guanine nucleotide exchange factors |
| GSK3β | Glycogen Synthase Kinase 3 Beta |
| GSK126 | EZH2 inhibitor (specific compound) |
| HIF1α | Hypoxia-Inducible Factor 1-alpha |
| HIF2α | Hypoxia-Inducible Factor 2-alpha |
| HNRNPU | Heterogeneous Nuclear Ribonucleoprotein U |
| IQGAP1 | IQ motif-containing GTPase-Activating Protein 1 |
| IR | Ionizing radiation |
| KIF3A | Kinesin family member 3A |
| lncRNAs | Long non-coding RNAs |
| MAPK | Mitogen-Activated Protein Kinase |
| MEK | Mitogen-Activated Protein Kinase Kinase |
| MIBC | Muscle-invasive bladder cancer |
| mTOR | Mammalian target of rapamycin |
| mutp53 | Mutant p53 proteins |
| NF-κB | Nuclear factor kappa B |
| NF1 | Neurofibromin 1 |
| NMIBC | Non-muscle-invasive bladder cancer |
| NSCLC | Non-small-cell lung cancer |
| OPHN1 | Oligophrenin 1 |
| PAG | Phosphoprotein Associated with Glycosphingolipid Microdomains 1 |
| PARP-1 | Poly (ADP-ribose) Polymerase 1 |
| PCa | Prostate cancer |
| PD-L1 | Programmed Death-Ligand 1 |
| PI3K | Phosphoinositide 3-Kinase |
| PROX1 | Prospero Homeobox 1 |
| RASA1/2/3/4 | Ras GTPase-Activating Protein family members |
| RASAL1/2/3 | Ras Activator-like proteins |
| RASGAPs | RAS GTPase-Activating Proteins |
| RCC | Renal cell carcinoma |
| RSK1 | Ribosomal S6 kinase 1 |
| SPRED2 | Sprouty-Related EVH1 Domain-Containing Protein 2 |
| SYNGAP1 | SYNaptic GTPase Activating Protein 1 |
| TCC | Transitional cell carcinoma |
| TGFβ | Transforming growth factor beta |
| Th1/Th2 | T-helper 1/T-helper 2 cells |
| Treg | Regulatory T cell |
| UTR | Untranslated region |
| VEGF | Vascular endothelial growth factor |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. RAS oncogenes: The first 30 years. Nat. Rev. Cancer 2003, 3, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, H. RAS signaling in carcinogenesis, cancer therapy and resistance mechanisms. J. Hematol. Oncol. 2024, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Vigil, D.; Cherfils, J.; Rossman, K.L.; Der, C.J. Ras superfamily GEFs and GAPs: Validated and tractable targets for cancer therapy? Nat. Rev. Cancer 2010, 10, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Simanshu, D.K.; Nissley, D.V.; McCormick, F. RAS Proteins and Their Regulators in Human Disease. Cell 2017, 170, 17–33. [Google Scholar] [CrossRef]
- Liu, L.; Xu, C.; Hsieh, J.T.; Gong, J.; Xie, D. DAB2IP in cancer. Oncotarget 2016, 7, 3766–3776. [Google Scholar] [CrossRef]
- Bellazzo, A.; Di Minin, G.; Collavin, L. Block one, unleash a hundred. Mechanisms of DAB2IP inactivation in cancer. Cell Death Differ. 2017, 24, 15–25. [Google Scholar] [CrossRef]
- Stites, E.C.; Trampont, P.C.; Ma, Z.; Ravichandran, K.S. Network analysis of oncogenic Ras activation in cancer. Science 2007, 318, 463–467. [Google Scholar] [CrossRef]
- Trahey, M.; McCormick, F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science 1987, 238, 542–545. [Google Scholar] [CrossRef]
- Martin, G.A.; Viskochil, D.; Bollag, G.; McCabe, P.C.; Crosier, W.J.; Haubruck, H.; Conroy, L.; Clark, R.; O’Connell, P.; Cawthon, R.M.; et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell 1990, 63, 843–849. [Google Scholar] [CrossRef]
- Cox, A.D.; Der, C.J. The dark side of Ras: Regulation of apoptosis. Oncogene 2003, 22, 8999–9006. [Google Scholar] [CrossRef] [PubMed]
- Bernards, A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim. Biophys. Acta 2003, 1603, 47–82. [Google Scholar] [CrossRef]
- Bernards, A.; Settleman, J. GAP control: Regulating the regulators of small GTPases. Trends Cell Biol. 2004, 14, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.L.; Rehmann, H.; Wittinghofer, A. GEFs and GAPs: Critical elements in the control of small G proteins. Cell 2007, 129, 865–877. [Google Scholar] [CrossRef]
- Ahmadian, M.R.; Kiel, C.; Stege, P.; Scheffzek, K. Structural fingerprints of the Ras-GTPase activating proteins neurofibromin and p120GAP. J. Mol. Biol. 2003, 329, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Scheffzek, K.; Ahmadian, M.R.; Kabsch, W.; Wiesmüller, L.; Lautwein, A.; Schmitz, F.; Wittinghofer, A. The Ras-RasGAP complex: Structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 1997, 277, 333–338. [Google Scholar] [CrossRef]
- Pylayeva-Gupta, Y.; Grabocka, E.; Bar-Sagi, D. RAS oncogenes: Weaving a tumorigenic web. Nat. Rev. Cancer 2011, 11, 761–774. [Google Scholar] [CrossRef]
- Prior, I.A.; Lewis, P.D.; Mattos, C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012, 72, 2457–2467. [Google Scholar] [CrossRef]
- Feng, M.; Bao, Y.; Li, Z.; Li, J.; Gong, M.; Lam, S.; Wang, J.; Marzese, D.M.; Donovan, N.; Tan, E.Y.; et al. RASAL2 activates RAC1 to promote triple-negative breast cancer progression. J. Clin. Investig. 2014, 124, 5291–5304. [Google Scholar] [CrossRef]
- Lin, Y.; Pal, D.S.; Banerjee, P.; Banerjee, T.; Qin, G.; Deng, Y.; Borleis, J.; Iglesias, P.A.; Devreotes, P.N. Ras suppression potentiates rear actomyosin contractility-driven cell polarization and migration. Nat. Cell Biol. 2024, 26, 1062–1076. [Google Scholar] [CrossRef]
- Song, F.; Dai, Q.; Grimm, M.O.; Steinbach, D. The Antithetic Roles of IQGAP2 and IQGAP3 in Cancers. Cancers 2023, 15, 1115. [Google Scholar] [CrossRef] [PubMed]
- Fincham, V.J.; Chudleigh, A.; Frame, M.C. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J. Cell Sci. 1999, 112 Pt 6, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, I.J.; Chiang, S.H.; Chang, L.; Vollenweider, D.; Watson, R.T.; Inoue, M.; Pessin, J.E.; Saltiel, A.R. Gapex-5, a Rab31 guanine nucleotide exchange factor that regulates Glut4 trafficking in adipocytes. Cell Metab. 2007, 5, 59–72. [Google Scholar] [CrossRef]
- Noguchi, T.; Matozaki, T.; Inagaki, K.; Tsuda, M.; Fukunaga, K.; Kitamura, Y.; Kitamura, T.; Shii, K.; Yamanashi, Y.; Kasuga, M. Tyrosine phosphorylation of p62(Dok) induced by cell adhesion and insulin: Possible role in cell migration. EMBO J. 1999, 18, 1748–1760. [Google Scholar] [CrossRef]
- Hobbs, G.A.; Der, C.J.; Rossman, K.L. RAS isoforms and mutations in cancer at a glance. J. Cell Sci. 2016, 129, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Schubbert, S.; Shannon, K.; Bollag, G. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer 2007, 7, 295–308. [Google Scholar] [CrossRef]
- Mustachio, L.M.; Chelariu-Raicu, A.; Szekvolgyi, L.; Roszik, J. Targeting KRAS in Cancer: Promising Therapeutic Strategies. Cancers 2021, 13, 1204. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Liu, Y.; Shi, Z.; Xuan, Z.; Yang, K.; Xu, C.; Bai, Y.; Fu, M.; Xiao, Q.; et al. The Crucial Role of AR-V7 in Enzalutamide-Resistance of Castration-Resistant Prostate Cancer. Cancers 2022, 14, 4877. [Google Scholar] [CrossRef]
- Niu, Y.; Guo, C.; Wen, S.; Tian, J.; Luo, J.; Wang, K.; Tian, H.; Yeh, S.; Chang, C. ADT with antiandrogens in prostate cancer induces adverse effect of increasing resistance, neuroendocrine differentiation and tumor metastasis. Cancer Lett. 2018, 439, 47–55. [Google Scholar] [CrossRef]
- Mishra, R.; Haldar, S.; Suchanti, S.; Bhowmick, N.A. Epigenetic changes in fibroblasts drive cancer metabolism and differentiation. Endocr. Relat. Cancer 2019, 26, R673–R688. [Google Scholar] [CrossRef]
- Chen, H.; Toyooka, S.; Gazdar, A.F.; Hsieh, J.T. Epigenetic regulation of a novel tumor suppressor gene (hDAB2IP) in prostate cancer cell lines. J. Biol. Chem. 2003, 278, 3121–3130. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tu, S.W.; Hsieh, J.T. Down-regulation of human DAB2IP gene expression mediated by polycomb Ezh2 complex and histone deacetylase in prostate cancer. J. Biol. Chem. 2005, 280, 22437–22444. [Google Scholar] [CrossRef] [PubMed]
- Duggan, D.; Zheng, S.L.; Knowlton, M.; Benitez, D.; Dimitrov, L.; Wiklund, F.; Robbins, C.; Isaacs, S.D.; Cheng, Y.; Li, G.; et al. Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene DAB2IP. J. Natl. Cancer Inst. 2007, 99, 1836–1844. [Google Scholar] [CrossRef]
- Wu, K.; Xie, D.; Zou, Y.; Zhang, T.; Pong, R.C.; Xiao, G.; Fazli, L.; Gleave, M.; He, D.; Boothman, D.A.; et al. The mechanism of DAB2IP in chemoresistance of prostate cancer cells. Clin. Cancer Res. 2013, 19, 4740–4749. [Google Scholar] [CrossRef]
- Min, J.; Zaslavsky, A.; Fedele, G.; McLaughlin, S.K.; Reczek, E.E.; De Raedt, T.; Guney, I.; Strochlic, D.E.; Macconaill, L.E.; Beroukhim, R.; et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat. Med. 2010, 16, 286–294. [Google Scholar] [CrossRef]
- Wang, B.; Huang, J.; Zhou, J.; Hui, K.; Xu, S.; Fan, J.; Li, L.; Wang, X.; Hsieh, J.T.; He, D.; et al. DAB2IP regulates EMT and metastasis of prostate cancer through targeting PROX1 transcription and destabilizing HIF1α protein. Cell. Signal. 2016, 28, 1623–1630. [Google Scholar] [CrossRef]
- Xie, D.; Gore, C.; Liu, J.; Pong, R.C.; Mason, R.; Hao, G.; Long, M.; Kabbani, W.; Yu, L.; Zhang, H.; et al. Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc. Natl. Acad. Sci. USA 2010, 107, 2485–2490. [Google Scholar] [CrossRef]
- Xie, D.; Gore, C.; Zhou, J.; Pong, R.C.; Zhang, H.; Yu, L.; Vessella, R.L.; Min, W.; Hsieh, J.T. DAB2IP coordinates both PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc. Natl. Acad. Sci. USA 2009, 106, 19878–19883. [Google Scholar] [CrossRef]
- Wu, K.; Liu, J.; Tseng, S.F.; Gore, C.; Ning, Z.; Sharifi, N.; Fazli, L.; Gleave, M.; Kapur, P.; Xiao, G.; et al. The role of DAB2IP in androgen receptor activation during prostate cancer progression. Oncogene 2014, 33, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wu, S.; Chong, Y.; Guan, B.; Li, L.; He, D.; Wang, X.; Wang, B.; Wu, K. DAB2IP regulates intratumoral testosterone synthesis and CRPC tumor growth by ETS1/AKR1C3 signaling. Cell. Signal. 2022, 95, 110336. [Google Scholar] [CrossRef]
- Valentino, E.; Bellazzo, A.; Di Minin, G.; Sicari, D.; Apollonio, M.; Scognamiglio, G.; Di Bonito, M.; Botti, G.; Del Sal, G.; Collavin, L. Mutant p53 potentiates the oncogenic effects of insulin by inhibiting the tumor suppressor DAB2IP. Proc. Natl. Acad. Sci. USA 2017, 114, 7623–7628. [Google Scholar] [CrossRef]
- Kong, Z.; Xie, D.; Boike, T.; Raghavan, P.; Burma, S.; Chen, D.J.; Habib, A.A.; Chakraborty, A.; Hsieh, J.T.; Saha, D. Downregulation of human DAB2IP gene expression in prostate cancer cells results in resistance to ionizing radiation. Cancer Res. 2010, 70, 2829–2839. [Google Scholar] [CrossRef]
- Kong, Z.; Raghavan, P.; Xie, D.; Boike, T.; Burma, S.; Chen, D.; Chakraborty, A.; Hsieh, J.T.; Saha, D. Epothilone B confers radiation dose enhancement in DAB2IP gene knock-down radioresistant prostate cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1210–1218. [Google Scholar] [CrossRef]
- De Florian Fania, R.; Bellazzo, A.; Collavin, L. An update on the tumor-suppressive functions of the RasGAP protein DAB2IP with focus on therapeutic implications. Cell Death Differ. 2024, 31, 844–854. [Google Scholar] [CrossRef]
- Moon, H.; Ruelcke, J.E.; Choi, E.; Sharpe, L.J.; Nassar, Z.D.; Bielefeldt-Ohmann, H.; Parat, M.O.; Shah, A.; Francois, M.; Inder, K.L.; et al. Diet-induced hypercholesterolemia promotes androgen-independent prostate cancer metastasis via IQGAP1 and caveolin-1. Oncotarget 2015, 6, 7438–7453. [Google Scholar] [CrossRef]
- Cerutti, C.; Lucotti, S.; Menendez, S.T.; Reymond, N.; Garg, R.; Romero, I.A.; Muschel, R.; Ridley, A.J. IQGAP1 and NWASP promote human cancer cell dissemination and metastasis by regulating β1-integrin via FAK and MRTF/SRF. Cell Rep. 2024, 43, 113989. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhuang, R.L.; Yu, S.L.; Xie, Z.X.; Peng, S.R.; Li, Z.A.; Li, B.H.; Xie, J.J.; Li, Y.N.; Li, K.W.; et al. Cancer-associated fibroblasts regulate mitochondrial metabolism and inhibit chemosensitivity via ANGPTL4-IQGAP1 axis in prostate cancer. J. Adv. Res. 2024. [Google Scholar] [CrossRef]
- Ernst, T.; Hergenhahn, M.; Kenzelmann, M.; Cohen, C.D.; Bonrouhi, M.; Weninger, A.; Klären, R.; Gröne, E.F.; Wiesel, M.; Güdemann, C.; et al. Decrease and gain of gene expression are equally discriminatory markers for prostate carcinoma: A gene expression analysis on total and microdissected prostate tissue. Am. J. Pathol. 2002, 160, 2169–2180. [Google Scholar] [CrossRef]
- Xie, Y.; Yan, J.; Cutz, J.C.; Rybak, A.P.; He, L.; Wei, F.; Kapoor, A.; Schmidt, V.A.; Tao, L.; Tang, D. IQGAP2, A candidate tumour suppressor of prostate tumorigenesis. Biochim. Biophys. Acta 2012, 1822, 875–884. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, L.; Tao, L. Downregulation of IQGAP2 Correlates with Prostate Cancer Recurrence and Metastasis. Transl. Oncol. 2019, 12, 236–244. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, H.; Yi, X.; Zhang, T.; Wei, Q.; Li, H.; Ai, J. Tumor-antigens and immune landscapes identification for prostate adenocarcinoma mRNA vaccine. Mol. Cancer 2021, 20, 160. [Google Scholar] [CrossRef] [PubMed]
- Berndt, S.I.; Wang, Z.; Yeager, M.; Alavanja, M.C.; Albanes, D.; Amundadottir, L.; Andriole, G.; Beane Freeman, L.; Campa, D.; Cancel-Tassin, G.; et al. Two susceptibility loci identified for prostate cancer aggressiveness. Nat. Commun. 2015, 6, 6889. [Google Scholar] [CrossRef] [PubMed]
- Kolfschoten, I.G.; van Leeuwen, B.; Berns, K.; Mullenders, J.; Beijersbergen, R.L.; Bernards, R.; Voorhoeve, P.M.; Agami, R. A genetic screen identifies PITX1 as a suppressor of RAS activity and tumorigenicity. Cell 2005, 121, 849–858. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, S.; Gu, Y.; Liang, H.; He, F.; Wang, X.; He, D.; Wu, K. RASAL2 regulates the cell cycle and cyclin D1 expression through PI3K/AKT signalling in prostate tumorigenesis. Cell Death Discov. 2022, 8, 275. [Google Scholar] [CrossRef]
- Tailor, K.; Paul, J.; Ghosh, S.; Kumari, N.; Kwabi-Addo, B. RASAL2 suppresses the proliferative and invasive ability of PC3 prostate cancer cells. Oncotarget 2021, 12, 2489–2499. [Google Scholar] [CrossRef]
- Mishra, R.; Haldar, S.; Placencio, V.; Madhav, A.; Rohena-Rivera, K.; Agarwal, P.; Duong, F.; Angara, B.; Tripathi, M.; Liu, Z.; et al. Stromal epigenetic alterations drive metabolic and neuroendocrine prostate cancer reprogramming. J. Clin. Investig. 2018, 128, 4472–4484. [Google Scholar] [CrossRef]
- Kachroo, N.; Valencia, T.; Warren, A.Y.; Gnanapragasam, V.J. Evidence for downregulation of the negative regulator SPRED2 in clinical prostate cancer. Br. J. Cancer 2013, 108, 597–601. [Google Scholar] [CrossRef]
- Lorenzo, C.; McCormick, F. SPRED proteins and their roles in signal transduction, development, and malignancy. Genes. Dev. 2020, 34, 1410–1421. [Google Scholar] [CrossRef] [PubMed]
- Billuart, P.; Bienvenu, T.; Ronce, N.; des Portes, V.; Vinet, M.C.; Zemni, R.; Roest Crollius, H.; Carrié, A.; Fauchereau, F.; Cherry, M.; et al. Oligophrenin-1 encodes a rhoGAP protein involved in X-linked mental retardation. Nature 1998, 392, 923–926. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Li, S.; Sun, F.; Wang, G.; Wei, D.; Yang, T.; Gu, S. Androgen deprivation-induced OPHN1 amplification promotes castration-resistant prostate cancer. Oncol. Rep. 2022, 47, 3. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, H.; Chen, J.; Wang, J.; Liu, J.; Jiang, Y. Targeting Akt in cancer for precision therapy. J. Hematol. Oncol. 2021, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, Y.; Gong, M.; Pei, F.; Zheng, J. Phosphoprotein associated with glycosphingolipid microdomains 1 inhibits the proliferation and invasion of human prostate cancer cells in vitro through suppression of Ras activation. Oncol. Rep. 2012, 28, 606–614. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Lv, M.; Shang, S.; Liu, K.; Wang, Y.; Xu, P.; Song, H.; Zhang, J.; Sun, Z.; Yan, Y.; Zhu, Z.; et al. Revitalizing Bacillus Calmette-Guérin Immunotherapy for Bladder Cancer: Nanotechnology and Bioengineering Approaches. Pharmaceutics 2024, 16, 1067. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Akbani, R.; Broom, B.M.; Wang, W.; Verhaak, R.G.W.; McConkey, D.; Lerner, S.; Morgan, M.; Creighton, C.J.; Smith, C.; et al. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef]
- Goebell, P.J.; Knowles, M.A. Bladder cancer or bladder cancers? Genetically distinct malignant conditions of the urothelium. Urol. Oncol. 2010, 28, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Beukers, W.; Hercegovac, A.; Zwarthoff, E.C. HRAS mutations in bladder cancer at an early age and the possible association with the Costello Syndrome. Eur. J. Hum. Genet. 2014, 22, 837–839. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Jou, Y.C.; Tsai, Y.S.; Chen, S.Y.; Hsieh, H.Y.; Tsai, H.T.; Tzai, T.S. Loss of DAB2IP expression in human urothelial carcinoma is associated with poorer recurrence-free survival. Virchows Arch. 2016, 468, 733–740. [Google Scholar] [CrossRef]
- Shen, Y.J.; Kong, Z.L.; Wan, F.N.; Wang, H.K.; Bian, X.J.; Gan, H.L.; Wang, C.F.; Ye, D.W. Downregulation of DAB2IP results in cell proliferation and invasion and contributes to unfavorable outcomes in bladder cancer. Cancer Sci. 2014, 105, 704–712. [Google Scholar] [CrossRef]
- Kunze, E.; Von Bonin, F.; Werner, C.; Wendt, M.; Schlott, T. Transitional cell carcinomas and nonurothelial carcinomas of the urinary bladder differ in the promoter methylation status of the caveolin-1, hDAB2IP and p53 genes, but not in the global methylation of Alu elements. Int. J. Mol. Med. 2006, 17, 3–13. [Google Scholar] [CrossRef][Green Version]
- Ou, Z.; Wang, Y.; Chen, J.; Tao, L.; Zuo, L.; Sahasrabudhe, D.; Joseph, J.; Wang, L.; Yeh, S. Estrogen receptor β promotes bladder cancer growth and invasion via alteration of miR-92a/DAB2IP signals. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, B.; Hui, K.; Zeng, J.; Fan, J.; Wang, X.; Hsieh, J.-T.; He, D.; Wu, K. miR-92b targets DAB2IP to promote EMT in bladder cancer migration and invasion. Oncol. Rep. 2016, 36, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Sun, P.; Hu, J.; Feng, H.; Li, M.; Liu, G.; Pan, Y.; Feng, Y.; Xu, Y.; Feng, K.; et al. miRNA-556-3p promotes human bladder cancer proliferation, migration and invasion by negatively regulating DAB2IP expression. Int. J. Oncol. 2017, 50, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wang, B.; Chen, Y.; Zhou, J.; Huang, J.; Hui, K.; Zeng, J.; Zhu, J.; Zhang, K.; Li, L.; et al. DAB2IP regulates the chemoresistance to pirarubicin and tumor recurrence of non-muscle invasive bladder cancer through STAT3/Twist1/P-glycoprotein signaling. Cell. Signal. 2015, 27, 2515–2523. [Google Scholar] [CrossRef]
- He, H.; Chang, R.; Zhang, T.; Yang, C.; Kong, Z. ATM mediates DAB2IP-deficient bladder cancer cell resistance to ionizing radiation through the p38MAPK and NF-κB signaling pathway. Mol. Med. Rep. 2017, 16, 1216–1222. [Google Scholar] [CrossRef]
- Aaltonen, V.; Boström, P.J.; Söderström, K.O.; Hirvonen, O.; Tuukkanen, J.; Nurmi, M.; Laato, M.; Peltonen, J. Urinary bladder transitional cell carcinogenesis is associated with down-regulation of NF1 tumor suppressor gene in vivo and in vitro. Am. J. Pathol. 1999, 154, 755–765. [Google Scholar] [CrossRef]
- Shi, Z.D.; Hao, L.; Han, X.X.; Wu, Z.X.; Pang, K.; Dong, Y.; Qin, J.X.; Wang, G.Y.; Zhang, X.M.; Xia, T.; et al. Targeting HNRNPU to overcome cisplatin resistance in bladder cancer. Mol. Cancer 2022, 21, 37. [Google Scholar] [CrossRef]
- Hui, K.; Gao, Y.; Huang, J.; Xu, S.; Wang, B.; Zeng, J.; Fan, J.; Wang, X.; Yue, Y.; Wu, S.; et al. RASAL2, a RAS GTPase-activating protein, inhibits stemness and epithelial-mesenchymal transition via MAPK/SOX2 pathway in bladder cancer. Cell Death Dis. 2017, 8, e2600. [Google Scholar] [CrossRef]
- Hui, K.; Wu, S.; Yue, Y.; Gu, Y.; Guan, B.; Wang, X.; Hsieh, J.T.; Chang, L.S.; He, D.; Wu, K. RASAL2 inhibits tumor angiogenesis via p-AKT/ETS1 signaling in bladder cancer. Cell. Signal. 2018, 48, 38–44. [Google Scholar] [CrossRef]
- Hensel, J.; Duex, J.E.; Owens, C.; Dancik, G.M.; Edwards, M.G.; Frierson, H.F.; Theodorescu, D. Patient Mutation Directed shRNA Screen Uncovers Novel Bladder Tumor Growth Suppressors. Mol. Cancer Res. 2015, 13, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kim, Y.H.; Jeong, P.; Piao, X.M.; Byun, Y.J.; Kang, H.W.; Kim, W.T.; Lee, J.Y.; Kim, I.Y.; Moon, S.K.; et al. Diagnostic value of combined IQGAP3/BMP4 and IQGAP3/FAM107A expression ratios in urinary cell-free DNA for discriminating bladder cancer from hematuria. Urol. Oncol. 2019, 37, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kim, Y.H.; Jeong, P.; Piao, X.M.; Byun, Y.J.; Seo, S.P.; Kang, H.W.; Kim, W.T.; Lee, J.Y.; Ryu, D.H.; et al. Urinary Cell-Free DNA IQGAP3/BMP4 Ratio as a Prognostic Marker for Non-Muscle-Invasive Bladder Cancer. Clin. Genitourin. Cancer 2019, 17, e704–e711. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Guo, X.B.; Zhao, B. CDC42 Regulates Cell Proliferation and Apoptosis in Bladder Cancer via the IQGAP3-Mediated Ras/ERK Pathway. Biochem. Genet. 2022, 60, 2383–2398. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Kotolloshi, R.; Gajda, M.; Hölzer, M.; Grimm, M.O.; Steinbach, D. Reduced IQGAP2 Promotes Bladder Cancer through Regulation of MAPK/ERK Pathway and Cytokines. Int. J. Mol. Sci. 2022, 23, 13508. [Google Scholar] [CrossRef]
- Yang, C.; Wu, S.; Mou, Z.; Zhou, Q.; Zhang, Z.; Chen, Y.; Ou, Y.; Chen, X.; Dai, X.; Xu, C.; et al. Transcriptomic Analysis Identified ARHGAP Family as a Novel Biomarker Associated With Tumor-Promoting Immune Infiltration and Nanomechanical Characteristics in Bladder Cancer. Front. Cell Dev. Biol. 2021, 9, 657219. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Zheng, J.; Li, Z.; Li, S.; Wang, K.; Chen, X. Liquid biopsy at the frontier in renal cell carcinoma: Recent analysis of techniques and clinical application. Mol. Cancer 2023, 22, 37. [Google Scholar] [CrossRef]
- Gray, R.E.; Harris, G.T. Renal Cell Carcinoma: Diagnosis and Management. Am. Fam. Physician 2019, 99, 179–184. [Google Scholar]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef]
- Linehan, W.M.; Ricketts, C.J. The metabolic basis of kidney cancer. Semin. Cancer Biol. 2013, 23, 46–55. [Google Scholar] [CrossRef]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Z.; Pei, X.; Lai, J.; Qu, W. DAB2IP stabilizes p27(Kip1) via suppressing PI3K/AKT signaling in clear cell renal cell carcinoma. Funct. Integr. Genom. 2023, 23, 326. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, J.; Wu, K.; Yun, E.J.; Kapur, P.; Pong, R.C.; Du, Y.; Wang, B.; Authement, C.; Hernandez, E.; et al. Loss of DAB2IP in RCC cells enhances their growth and resistance to mTOR-targeted therapies. Oncogene 2016, 35, 4663–4674. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Lin, C.J.; Dang, A.; Hernandez, E.; Guo, J.; Chen, W.M.; Allison, J.; Kim, N.; Kapur, P.; Brugarolas, J.; et al. Downregulation of Human DAB2IP Gene Expression in Renal Cell Carcinoma Results in Resistance to Ionizing Radiation. Clin. Cancer Res. 2019, 25, 4542–4551. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Dang, A.; Hernandez, E.; Hsieh, J.T. DAB2IP modulates primary cilia formation associated with renal tumorigenesis. Neoplasia 2021, 23, 169–180. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Wang, Y.; Pan, Y.; Han, X.; Peng, B.; Zhang, X.; Niu, S.; Wang, H.; Ye, Q.; et al. DMDRMR promotes angiogenesis via antagonizing DAB2IP in clear cell renal cell carcinoma. Cell Death Dis. 2022, 13, 456. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Zhou, J.; Lin, C.J.; Xu, S.; Santoyo, J.; Hernandez, E.; Lai, C.H.; Lin, H.; He, D.; Hsieh, J.T. The network of DAB2IP-miR-138 in regulating drug resistance of renal cell carcinoma associated with stem-like phenotypes. Oncotarget 2017, 8, 66975–66986. [Google Scholar] [CrossRef]
- Yeh, C.R.; Ou, Z.Y.; Xiao, G.Q.; Guancial, E.; Yeh, S. Infiltrating T cells promote renal cell carcinoma (RCC) progression via altering the estrogen receptor β-DAB2IP signals. Oncotarget 2015, 6, 44346–44359. [Google Scholar] [CrossRef]
- Hui, K.; Yue, Y.; Wu, S.; Gu, Y.; Guan, B.; Wang, X.; Hsieh, J.T.; Chang, L.S.; He, D.; Wu, K. The expression and function of RASAL2 in renal cell carcinoma angiogenesis. Cell Death Dis. 2018, 9, 881. [Google Scholar] [CrossRef]
- Tan, D.; Miao, D.; Zhao, C.; Shi, J.; Lv, Q.; Lu, F.; Ruan, H.; Xiong, Z.; Zhang, X. N6-methyladenosine-modified ALDH9A1 modulates lipid accumulation and tumor progression in clear cell renal cell carcinoma through the NPM1/IQGAP2/AKT signaling pathway. Cell Death Dis. 2024, 15, 520. [Google Scholar] [CrossRef]
- Vanli, G.; Sempoux, C.; Widmann, C. The caspase-3/p120 RasGAP stress-sensing module reduces liver cancer incidence but does not affect overall survival in gamma-irradiated and carcinogen-treated mice. Mol. Carcinog. 2017, 56, 1680–1684. [Google Scholar] [CrossRef]
- Kitajima, S.; Barbie, D.A. RASA1/NF1-Mutant Lung Cancer: Racing to the Clinic? Clin. Cancer Res. 2018, 24, 1243–1245. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.K.; Olsen, S.N.; Dake, B.; De Raedt, T.; Lim, E.; Bronson, R.T.; Beroukhim, R.; Polyak, K.; Brown, M.; Kuperwasser, C.; et al. The RasGAP gene, RASAL2, is a tumor and metastasis suppressor. Cancer Cell 2013, 24, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Arafeh, R.; Qutob, N.; Emmanuel, R.; Keren-Paz, A.; Madore, J.; Elkahloun, A.; Wilmott, J.S.; Gartner, J.J.; Di Pizio, A.; Winograd-Katz, S.; et al. Recurrent inactivating RASA2 mutations in melanoma. Nat. Genet. 2015, 47, 1408–1410. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.D.; Fesik, S.W.; Kimmelman, A.C.; Luo, J.; Der, C.J. Drugging the undruggable RAS: Mission possible? Nat. Rev. Drug Discov. 2014, 13, 828–851. [Google Scholar] [CrossRef]
- Papke, B.; Der, C.J. Drugging RAS: Know the enemy. Science 2017, 355, 1158–1163. [Google Scholar] [CrossRef]
- Ostrem, J.M.; Shokat, K.M. Direct small-molecule inhibitors of KRAS: From structural insights to mechanism-based design. Nat. Rev. Drug Discov. 2016, 15, 771–785. [Google Scholar] [CrossRef]
- Ostrem, J.M.; Peters, U.; Sos, M.L.; Wells, J.A.; Shokat, K.M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 2013, 503, 548–551. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Wang, X.; Li, J.; Wei, J.; Wang, Y.; Song, W.; Zhang, Z. MiR-1266 promotes cell proliferation, migration and invasion in cervical cancer by targeting DAB2IP. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3623–3630. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Zhang, Q.; Lin, R. miR-182 contributes to cell proliferation, invasion and tumor growth in colorectal cancer by targeting DAB2IP. Int. J. Biochem. Cell Biol. 2019, 111, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, R.; Wang, H.; Liu, Q. Hypoxia-driven miR-1307-3p promotes hepatocellular carcinoma cell proliferation and invasion by modulating DAB2 interacting protein. Pathol. Res. Pract. 2022, 237, 154066. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, L.; Wang, X.; Jin, H. RNA-based therapeutics: An overview and prospectus. Cell Death Dis. 2022, 13, 644. [Google Scholar] [CrossRef]
- Schade, A.E.; Kuzmickas, R.; Rodriguez, C.L.; Mattioli, K.; Enos, M.; Gardner, A.; Cichowski, K. Combating castration-resistant prostate cancer by co-targeting the epigenetic regulators EZH2 and HDAC. PLoS Biol. 2023, 21, e3002038. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.; Zhang, H.; Li, H.; Zhang, M.; Wang, H.; Zhang, M.; Qiu, P.; Zhang, R.; Liu, J. DNMT3A facilitates colorectal cancer progression via regulating DAB2IP mediated MEK/ERK activation. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166353. [Google Scholar] [CrossRef]
- Chandarlapaty, S. Negative feedback and adaptive resistance to the targeted therapy of cancer. Cancer Discov. 2012, 2, 311–319. [Google Scholar] [CrossRef]
- Ku, S.Y.; Gleave, M.E.; Beltran, H. Towards precision oncology in advanced prostate cancer. Nat. Rev. Urol. 2019, 16, 645–654. [Google Scholar] [CrossRef]
- Tortorella, E.; Giantulli, S.; Sciarra, A.; Silvestri, I. AR and PI3K/AKT in Prostate Cancer: A Tale of Two Interconnected Pathways. Int. J. Mol. Sci. 2023, 24, 2046. [Google Scholar] [CrossRef]
- Lai, C.H.; Chang, C.S.; Liu, H.H.; Tsai, Y.S.; Hsu, F.M.; Yu, Y.L.; Lai, C.K.; Gandee, L.; Pong, R.C.; Hsu, H.W.; et al. Sensitization of radio-resistant prostate cancer cells with a unique cytolethal distending toxin. Oncotarget 2014, 5, 5523–5534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shen, Y.; Chen, Y.; Hsieh, J.T.; Kong, Z. The ATM inhibitor KU55933 sensitizes radioresistant bladder cancer cells with DAB2IP gene defect. Int. J. Radiat. Biol. 2015, 91, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, J.; Shifrut, E.; Kale, N.; Nyberg, W.A.; Blaeschke, F.; Chen, Y.Y.; Li, Z.; Bapat, S.P.; Diolaiti, M.E.; O’Leary, P.; et al. RASA2 ablation in T cells boosts antigen sensitivity and long-term function. Nature 2022, 609, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Ruscetti, M.; Leibold, J.; Bott, M.J.; Fennell, M.; Kulick, A.; Salgado, N.R.; Chen, C.C.; Ho, Y.J.; Sanchez-Rivera, F.J.; Feucht, J.; et al. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science 2018, 362, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Corcoran, R.B. Targeting Alterations in the RAF-MEK Pathway. Cancer Discov. 2019, 9, 329–341. [Google Scholar] [CrossRef]
- Downward, J. RAS Synthetic Lethal Screens Revisited: Still Seeking the Elusive Prize? Clin. Cancer Res. 2015, 21, 1802–1809. [Google Scholar] [CrossRef]
- Yue, X.; Wu, F.; Li, Y.; Liu, J.; Boateng, M.; Mandava, K.; Zhang, C.; Feng, Z.; Gao, J.; Hu, W. Gain of function mutant p53 protein activates AKT through the Rac1 signaling to promote tumorigenesis. Cell Cycle 2020, 19, 1338–1351. [Google Scholar] [CrossRef]
- Di Minin, G.; Bellazzo, A.; Dal Ferro, M.; Chiaruttini, G.; Nuzzo, S.; Bicciato, S.; Piazza, S.; Rami, D.; Bulla, R.; Sommaggio, R.; et al. Mutant p53 reprograms TNF signaling in cancer cells through interaction with the tumor suppressor DAB2IP. Mol. Cell 2014, 56, 617–629. [Google Scholar] [CrossRef]
- Sidransky, D.; Von Eschenbach, A.; Tsai, Y.C.; Jones, P.; Summerhayes, I.; Marshall, F.; Paul, M.; Green, P.; Hamilton, S.R.; Frost, P.; et al. Identification of p53 gene mutations in bladder cancers and urine samples. Science 1991, 252, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Prior, I.A.; Hood, F.E.; Hartley, J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020, 80, 2969–2974. [Google Scholar] [CrossRef]
| Tumor Type | RASGAPs | Exp. | Biological Effect | Mechanism | Ref. |
|---|---|---|---|---|---|
| PCa | DAB2IP | +++ | DAB2IP loss promoted PCa EMT and metastasis | Tumor suppressor: targeted GSK3β/β-catenin; targeted NF-κB signaling; targeted PROX1/HIF1α | [35,36,37] |
| DAB2IP loss accelerated PCa growth in vivo | Tumor suppressor: targeted PI3K-AKT and ASK1-JNK pathway | [38] | |||
| DAB2IP loss contributed to the development of CRPC | Tumor suppressor: targeted testosterone synthesis and AR signaling | [39,40] | |||
| DAB2IP loss increased cell proliferation and invasiveness | Tumor suppressor: mut p53 enhanced insulin-induced AKT1 activation by binding and inhibiting DAB2IP | [41] | |||
| DAB2IP loss promoted resistance to ionizing radiation | Tumor suppressor: enhanced DSB repair, robust G(2)-M checkpoint control, and resistance to apoptosis and | [42,43,44] | |||
| IQGAP1 | +++ | IQGAP1 promoted cancer cell dissemination and metastasis | Oncogene: regulated β1-integrin via FAK and MRTF/SRF | [46] | |
| IQGAP1 promoted PCa tumor growth and increased chemoresistance | Oncogene: activated by ANGPTL4 in CAFs to activate Raf-MEK-ERK-PGC1α axis and drive mitochondrial biogenesis and OXPHOS metabolism | [47] | |||
| IQGAP2 | +++ | IQGAP2 downregulation was associated with high Gleason score, recurrence and metastasis | Tumor suppressor: activated AKT signaling | [50] | |
| IQGAP3 | + | IQGAP3 was positively correlated with infiltration of B cells, macrophages and dendritic cells | - | [51] | |
| RASA1 | ++ | RASA1 was positively associated with aggressive PCa and Gleason score | - | [52] | |
| RASAL1 | + | RASAL1 inhibited tumorigenicity of human primary cells | - | [53] | |
| RASAL2 | + | RASAL2 promoted tumor cell proliferation, the transition from G1 to S phase in vitro and tumor growth in vivo | Oncogene: activated PI3K/AKT/cyclin D1 pathway | [54] | |
| RASAL2 overexpression inhibited cell proliferation and invasion and induced an S phase plus G2/M phase cell cycle arrest | Tumor suppressor: downregulated TNFα expression | [55] | |||
| RASAL3 | + | Epigenetic silencing of RASAL3 promoted lethal PCa growth and the development of resistance to ADT | Tumor suppressor: expressed in prostatic CAFS; activated Ras signaling and drived macropinocytosis-mediated glutamine synthesis; ADT promoted RASAL3 epigenetic silencing and glutamine secretion | [56] | |
| BCa | DAB2IP | +++ | DAB2IP-deficient BCa cells promoted chemoresistance and tumor recurrence in NMIBC | Tumor suppressor: targeted STAT3 phosphorylation and transactivation; elevated Twist1 and P-glycoprotein expression | [75] |
| DAB2IP-knockdown induced BCa resistance to IR | Tumor suppressor: elevated expression of ATM; inhibited MAPK and NF-κB signaling pathways | [76] | |||
| NF1 | ++ | Knockout of HNRNPU enhanced cisplatin sensitivity by regulating NF1 expression | - | [78] | |
| RASAL1 | + | RASAL1 inhibited the tumorigenicity of human primary cells | - | [53] | |
| RASAL2 | + | RASAL2 BCa tumorigenesis and distant metastasis in vivo | Tumor suppressor: inhibited MAPK/SOX2 signaling and BCa stemness and EMT | [79] | |
| Knockdown of RASAL2 promoted angiogenesis in BCa | Tumor suppressor: targeted RASAL2-AKT-ETS1/VEGFA signaling axis | [80] | |||
| IQGAP1 | +++ | IQGAP1 inhibited cancer growth | Tumor suppressor: regulated TGFβ signaling | [81] | |
| IQGAP2 | + | Reduced IQGAP2 promoted tumor proliferation, migration, invasion and EMT | Tumor suppressor: regulated MAPK/ERK pathway and reduced cytokines | [85] | |
| IQGAP3 | ++ | IQGAP3 inhibited apoptosis and promoted BCa cells proliferation | Oncogene: activated RAS/ERK signaling | [84] | |
| RCC | DAB2IP | +++ | DAB2IP knockdown increased cell proliferation, promoted cell cycle progression in G1/S phase | Tumor suppressor: regulated the phosphorylation level of AKT and p27 | [93] |
| Loss of DAB2IP enhanced RCC sensitivity to growth factor stimulation and resistance to mTOR inhibitors | Tumor suppressor: targeted ERK/RSK1 and PI3K/mTOR pathways; induced HIF-2α expression; repressed p21/WAF1 transcription | [94] | |||
| Loss of DAB2IP elevated PARP-1 protein levels; RCC acquired IR-resistance | Tumor suppressor: DAB2IP acted as a scaffold to assemble a ternary complex with PARP-1 and E3 ubiquitin ligases, facilitating PARP-1 ubiquitination and subsequent proteasomal degradation | [95] | |||
| DAB2IP-KIF3A complex suppressed renal tumorigenesis | Tumor suppressor: KIF3A interacted with the N-terminal PH domain of DAB2IP; extended KIF3A’s half-life and facilitates ciliogenesis | [96] | |||
| DAB2IP loss drived angiogenesis and conferring sunitinib resistance in RCC | Tumor suppressor: DMDRMR/miR-378a-5p/DAB2IP axis; targeted VEGFA/VEGFR2 signaling | [97] | |||
| DAB2IP-mediated miR-138 in modulating RCC stem-like phenotypes | Tumor suppressor: DAB2IP loss interact with DNMT1 to facilitate promoter methylation of miR-138; miR-138 could suppress ABCA13 and EZH2 | [98] | |||
| DAB2IP inhibition promoted RCC cell invasion | Tumor suppressor: targetd infiltrating T cells/ERβ/DAB2IP signals | [99] | |||
| RASAL2 | ++ | RASAL2 inhibited angiogenesis | Tumor suppressor: decreased the expression of VEGFA through p-GSK3β/c-FOS pathway | [100] | |
| IQGAP2 | ++ | ALDH9A1 deficiency promoted tumor proliferation, invasion, migration, and lipid ac-cumulation in RCC through downregulating IQGAP2 | Tumor suppressor; involved in ALDH9A1-NPM1-IQGAP2-AKT axis | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Wang, G.; Gao, G.; Xia, H.; Jiao, L.; Wu, K. A Systematic Analysis of Expression and Function of RAS GTPase-Activating Proteins (RASGAPs) in Urological Cancers: A Mini-Review. Cancers 2025, 17, 1485. https://doi.org/10.3390/cancers17091485
Song H, Wang G, Gao G, Xia H, Jiao L, Wu K. A Systematic Analysis of Expression and Function of RAS GTPase-Activating Proteins (RASGAPs) in Urological Cancers: A Mini-Review. Cancers. 2025; 17(9):1485. https://doi.org/10.3390/cancers17091485
Chicago/Turabian StyleSong, Hao, Guojing Wang, Guoqiang Gao, Huayu Xia, Lianying Jiao, and Kaijie Wu. 2025. "A Systematic Analysis of Expression and Function of RAS GTPase-Activating Proteins (RASGAPs) in Urological Cancers: A Mini-Review" Cancers 17, no. 9: 1485. https://doi.org/10.3390/cancers17091485
APA StyleSong, H., Wang, G., Gao, G., Xia, H., Jiao, L., & Wu, K. (2025). A Systematic Analysis of Expression and Function of RAS GTPase-Activating Proteins (RASGAPs) in Urological Cancers: A Mini-Review. Cancers, 17(9), 1485. https://doi.org/10.3390/cancers17091485





