The Role of CA125 and HE4 in Uterine Sarcomas: Beyond Diagnosis and Prognosis—A Systematic Review and Case Series from a Single Institution
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Literature Review
- Original studies (prospective, retrospective, observational, or case series).
- Studies analyzing the role of CA125 in uterine sarcomas (diagnosis, prognosis, monitoring).
- Studies published in English.
- Studies based only on carcinosarcomas, due to their partially epithelial origin.
- Studies on extrauterine sarcomas.
- Studies not reporting data on CA125/HE4 and uterine sarcomas.
- Reviews, letters to the editor, and abstracts without complete data.
- Author and year of publication.
- Study type (retrospective, prospective, case report, etc.).
- Number of patients included.
- Role of CA125 or HE4 (diagnosis, prognosis, disease monitoring).
- Main findings.
Risk of Bias
- Good quality: 3 or 4 stars in the selection domain AND 1 or 2 stars in the comparability domain AND 2 or 3 stars in the outcome/exposure domain.
- Fair quality: 2 stars in the selection domain AND 1 or 2 stars in the comparability domain AND 2 or 3 stars in the outcome/exposure domain.
- Poor quality: 0 or 1 star in the selection domain OR 0 stars in the comparability domain OR 0 or 1 stars in the outcome/exposure domain.
2.2. Case Series
Statistical Analysis
3. Results
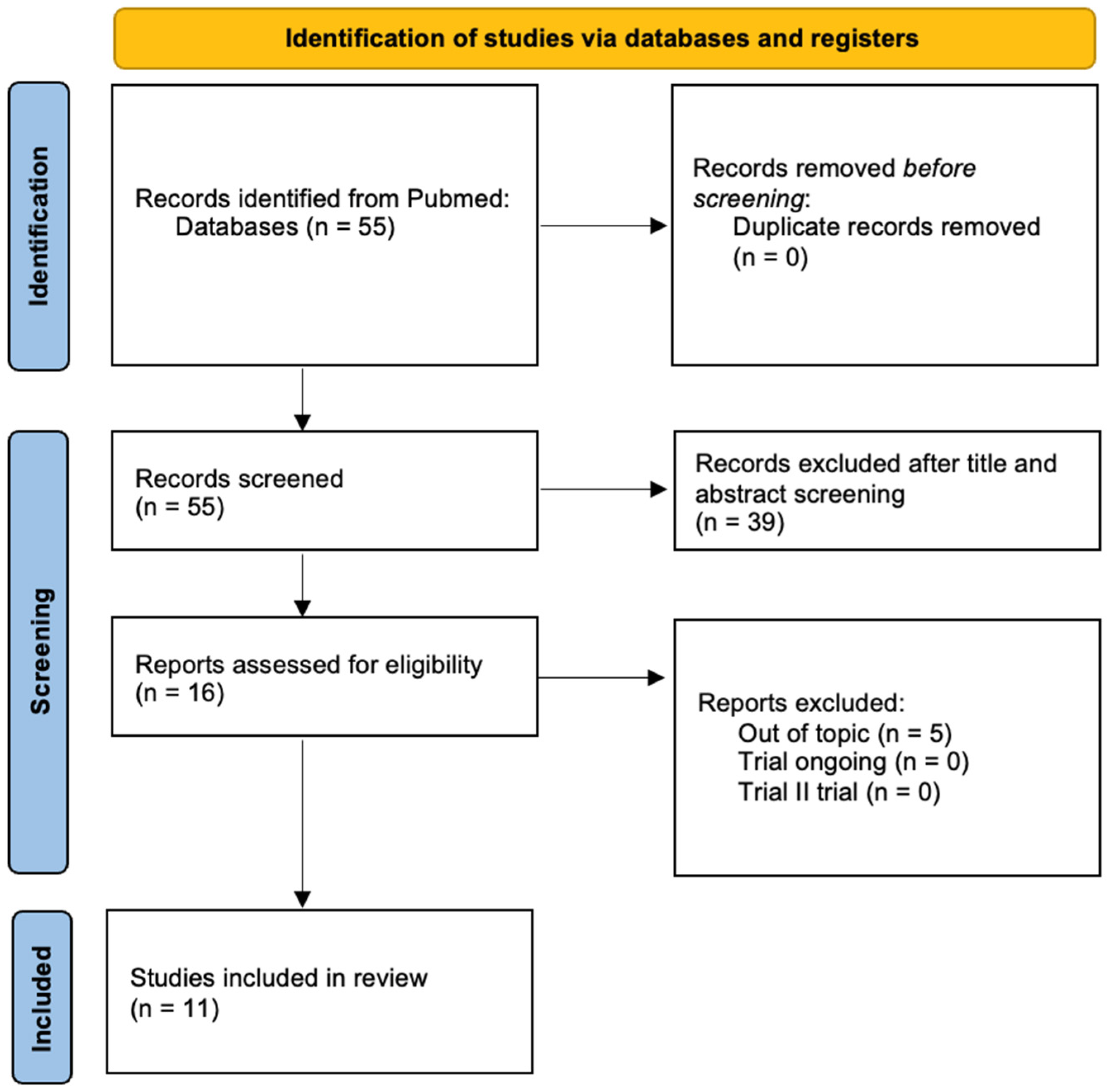
3.1. Systematic Literature Review
3.2. Case Series
3.2.1. Clinical and Histopathological Characteristics
3.2.2. Diagnosis: Role of CA125 and HE4
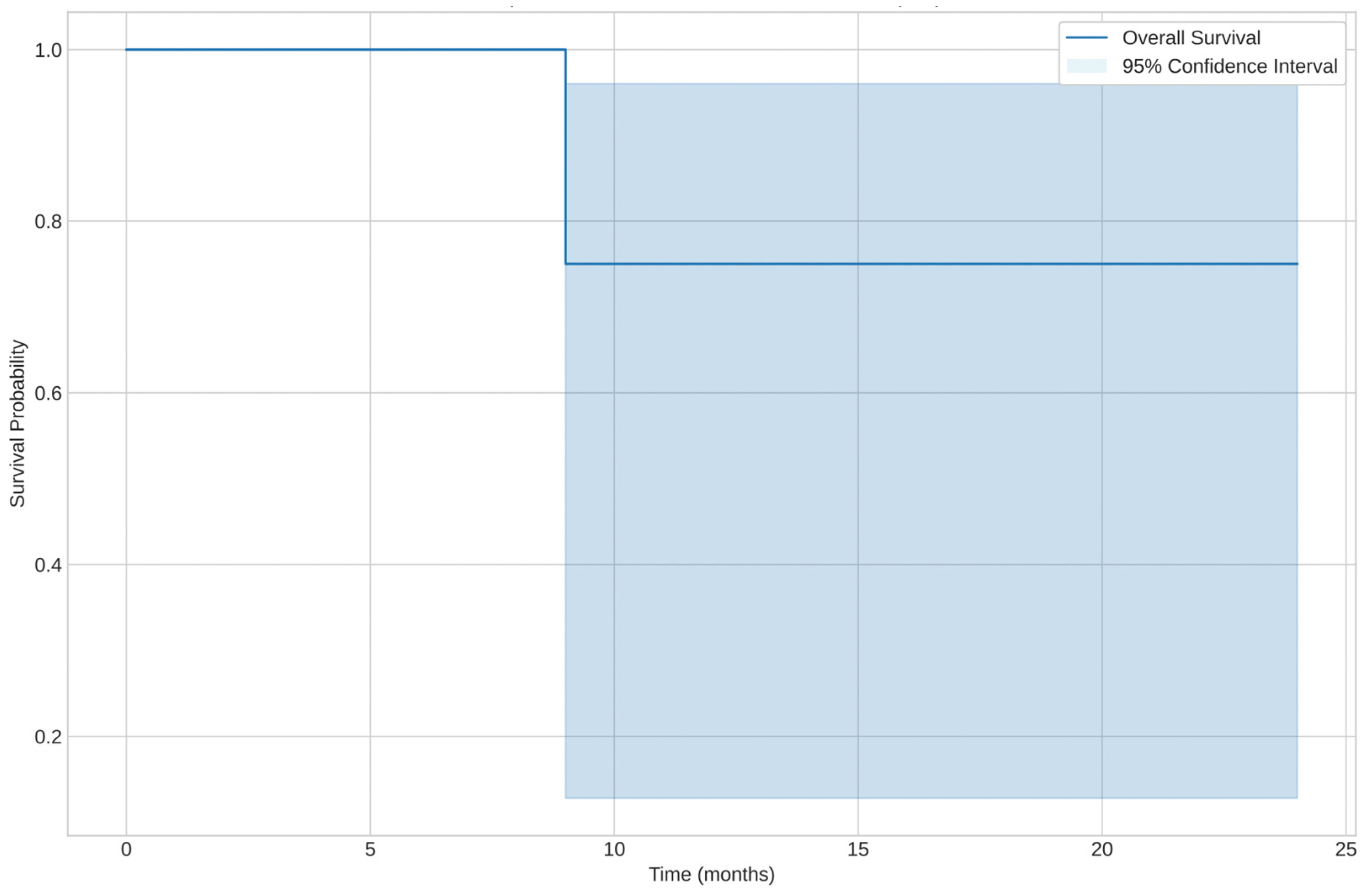
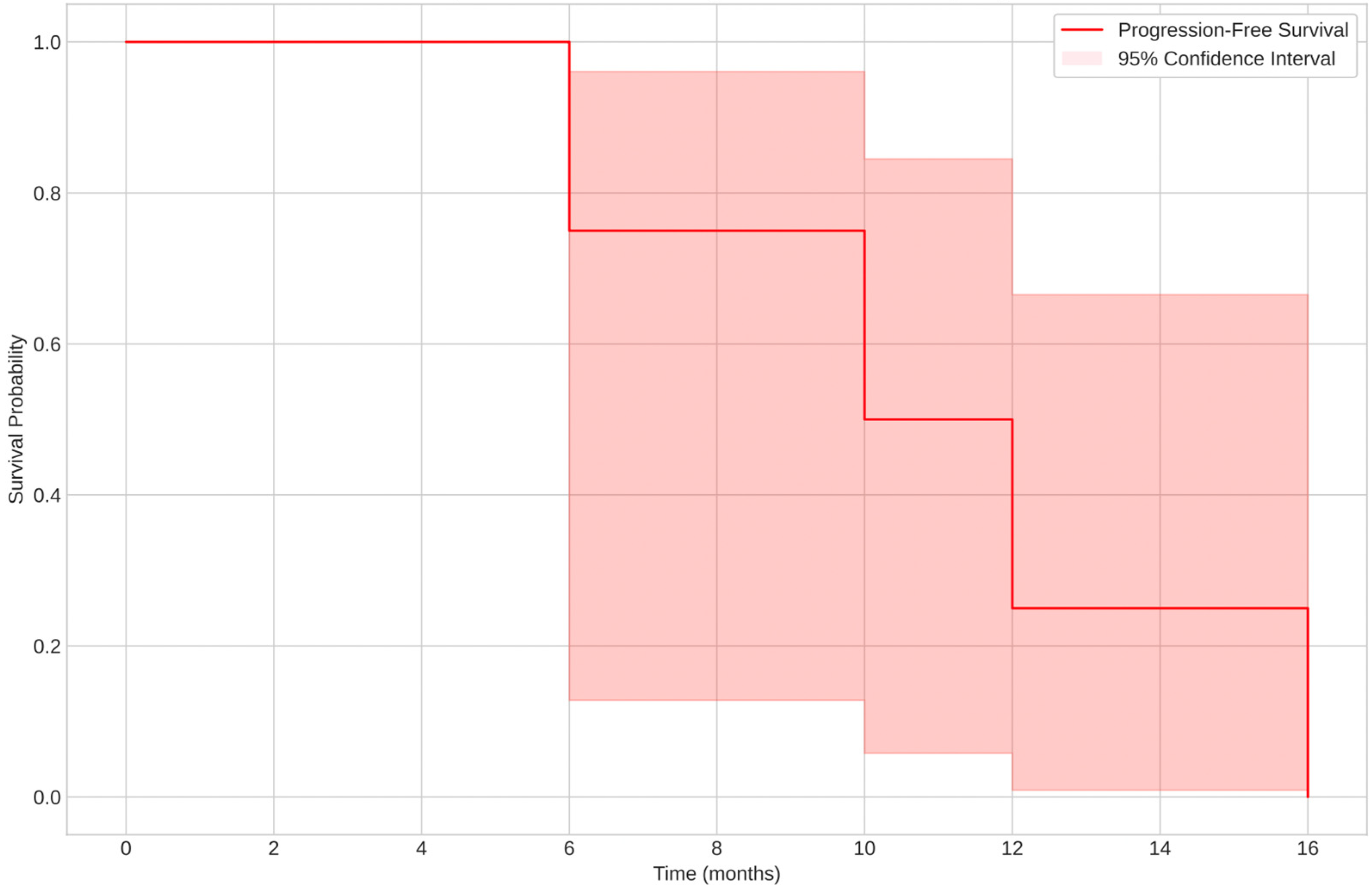
3.2.3. Prognosis: Overall Survival (OS) and Progression-Free Survival (PFS)
- Spindle cell leiomyosarcoma had the shortest PFS, with a median of 13 months (range: 9–94 months).
- The low-grade subtype exhibited a longer PFS (median: 12 months), while the high-grade subtype had a PFS of 45 months.
- Spindle cell leiomyosarcoma showed a median OS of 171 months for a censored case (patient still alive) and 13 months for a non-censored case (deceased patient).
- Overall survival was highest for the spindle cell leiomyosarcoma subtype, with one censored case at 171 months.
4. Discussion
4.1. Strengths and Limitations
4.2. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AIOM. Linee Guida Sarcomi dei Tessuti Molli e GIST. Available online: https://www.aiom.it/linee-guida-aiom-2024-sarcomi-dei-tessuti-molli-e-gist/ (accessed on 14 March 2025).
- Ray-Coquard, I.; Casali, P.G.; Croce, S.; Fennessy, F.M.; Fischerova, D.; Jones, R.; Sanfilippo, R.; Zapardiel, I.; Amant, F.; Blay, J.Y.; et al. ESGO/EURACAN/GCIG Guidelines for the Management of Patients with Uterine Sarcomas. Int. J. Gynecol. Cancer 2024, 34, 1499–1521. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.S.; Al Rashid, A.A.; Ladumor, S.B.; Mohamed, M.A.; Kambal, A.S.; Saloum, N.; Mohamed, S.E.M.K.; Al Hyassat, S.; Singh, R. The Role of Multiparametric MRI in Differentiating Uterine Leiomyosarcoma from Benign Degenerative Leiomyoma and Leiomyoma Variants: A Retrospective Analysis. Clin. Radiol. 2023, 78, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Al Khuri, M.; Al Salmi, I.; Al Ajmi, H.; Al Hadidi, A.; Alabousi, A.; Haider, E.; Vasudev, P.; Al Salmi, A.; Jose, S.; Alrahbi, N.; et al. Validating the Diagnostic Accuracy of an MRI-Based Scoring System for Differentiating Benign Uterine Leiomyomas from Leiomyosarcomas. Int. J. Gynecol. Cancer 2024, 34, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Shah, J.P.; Kumar, S.; Bryant, C.S.; Munkarah, A.; Morris, R.T. Ovarian and Uterine Carcinosarcomas: A Comparative Analysis of Prognostic Variables and Survival Outcomes. Int. J. Gynecol. Cancer 2010, 20, 888–894. [Google Scholar] [CrossRef]
- Vilos, G.A.; Allaire, C.; Laberge, P.Y.; Leyland, N.; Special Contributors. The Management of Uterine Leiomyomas. J. Obstet. Gynaecol. Can. 2015, 37, 157–178. [Google Scholar] [CrossRef]
- Capriglione, S.; Plotti, F.; Miranda, A.; Lopez, S.; Scaletta, G.; Moncelli, M.; Luvero, D.; De Cicco Nardone, C.; Terranova, C.; Montera, R.; et al. Further Insight into Prognostic Factors in Endometrial Cancer: The New Serum Biomarker HE4. Expert Rev. Anticancer Ther. 2017, 17, 9–18. [Google Scholar] [CrossRef]
- Plotti, F.; Scaletta, G.; Terranova, C.; Montera, R.; De Cicco Nardone, C.; Luvero, D.; Rossini, G.; Gatti, A.; Schirò, T.; Moncelli, M.; et al. The Role of Human Epididymis Protein 4 as a Biomarker in Gynecologic Malignancies. Minerva Ginecol. 2019, 71, 36–43. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, S.; Jin, Y.; Zhao, Y.; Wang, Y. Roles of CA125 in Diagnosis, Prediction, and Oncogenesis of Ovarian Cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188503. [Google Scholar] [CrossRef]
- Elorriaga, M.Á.; Neyro, J.L.; Mieza, J.; Cristóbal, I.; Llueca, A. Biomarkers in Ovarian Pathology: From Screening to Diagnosis. Review of the Literature. J. Pers. Med. 2021, 11, 1115. [Google Scholar] [CrossRef]
- The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 23 April 2025).
- WHO Classification of Tumours of Soft Tissue and Bone. Available online: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Soft-Tissue-And-Bone-2013 (accessed on 14 March 2025).
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C., Jr.; Skates, S.J. A Novel Multiple Marker Bioassay Utilizing HE4 and CA125 for the Prediction of Ovarian Cancer in Patients with a Pelvic Mass. Gynecol. Oncol. 2009, 112, 40–46. [Google Scholar] [CrossRef]
- Moore, R.G.; Jabre-Raughley, M.; Brown, A.K.; Robison, K.M.; Miller, M.C.; Allard, W.J.; Kurman, R.J.; Bast, R.C.; Skates, S.J. Comparison of a Novel Multiple Marker Assay vs. the Risk of Malignancy Index for the Prediction of Epithelial Ovarian Cancer in Patients with a Pelvic Mass. Am. J. Obstet. Gynecol. 2010, 203, 228.e1–228.e6. [Google Scholar] [CrossRef] [PubMed]
- Juang, C.M.; Yen, M.S.; Horng, H.C.; Twu, N.F.; Yu, H.C.; Hsu, W.L. Potential Role of Preoperative Serum CA125 for the Differential Diagnosis Between Uterine Leiomyoma and Uterine Leiomyosarcoma. Eur. J. Gynaecol. Oncol. 2006, 27, 370–374. [Google Scholar] [PubMed]
- Das, S.; Srivastava, S.; Srivastava, P.; Prasad, N.; Roy, M.; Sarkar, I. A Rare Case of Aggressive Uterine Leiomyosarcoma: A Case Report. Pan Afr. Med. J. 2024, 49, 10. [Google Scholar] [CrossRef]
- Yilmaz, N.; Sahin, I.; Kilic, S.; Ozgu, E.; Gungor, T.; Bilge, U. Assessment of the Predictivity of Preoperative Serum CA125 in the Differential Diagnosis of Uterine Leiomyoma and Uterine Sarcoma in the Turkish Female Population. Eur. J. Gynaecol. Oncol. 2009, 30, 412–414. [Google Scholar]
- Kim, H.S.; Han, K.H.; Chung, H.H.; Kim, J.W.; Park, N.H.; Song, Y.S.; Kang, S.B. Neutrophil to Lymphocyte Ratio for Preoperative Diagnosis of Uterine Sarcomas: A Case-Matched Comparison. Eur. J. Surg. Oncol. 2010, 36, 691–698. [Google Scholar] [CrossRef]
- Yang, L.; Cai, Y.; Wang, Y.; Huang, Y.; Zhang, C.; Ma, H.; Zhou, J.G. Fibroblast Growth Factor 23 Is a Potential Prognostic Biomarker in Uterine Sarcoma. Technol. Cancer Res. Treat. 2024, 23, 15330338241245924. [Google Scholar] [CrossRef] [PubMed]
- Visnovsky, J.; Kudela, E.; Slavik, P.; Krkoska, M.; Buocik, P.; Szepe, P.; Danko, J. Survival and Risk Factors Associated with Uterine Sarcomas and Carcinosarcomas in Stage I and II. Neuro Endocrinol. Lett. 2015, 36, 750–757. [Google Scholar]
- Zhang, Y.Y.; Li, Y.; Qin, M.; Cai, Y.; Jin, Y.; Pan, L.Y. High-Grade Endometrial Stromal Sarcoma: A Retrospective Study of Factors Influencing Prognosis. Cancer Manag. Res. 2019, 11, 831–837. [Google Scholar] [CrossRef]
- Menczer, J.; Schreiber, L.; Berger, E.; Ben-Shem, E.; Golan, A.; Levy, T. CA125 Expression in the Tissue of Uterine Leiomyosarcoma. Isr. Med. Assoc. J. 2014, 16, 697–699. [Google Scholar]
- Duk, J.M.; Bouma, J.; Burger, G.T.N.; Nap, M.; De Bruijn, H.W.A. CA125 in Serum and Tumor from Patients with Uterine Sarcoma. Int. J. Gynecol. Cancer 1994, 4, 156–160. [Google Scholar] [CrossRef]
- Vigone, A.; Giana, M.; Surico, D.; Leutner, M.; Surico, N. Massive Myxoid Leiomyosarcoma of the Uterus. Int. J. Gynecol. Cancer 2005, 15, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Patsner, B.; Mann, W.J. Use of Serum CA-125 in Monitoring Patients with Uterine Sarcoma: A Preliminary Report. Cancer 1988, 62, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Funston, G.; Hamilton, W.; Abel, G.; Crosbie, E.J.; Rous, B.; Walter, F.M. The Diagnostic Performance of CA125 for the Detection of Ovarian and Non-Ovarian Cancer in Primary Care: A Population-Based Cohort Study. PLoS Med. 2020, 17, e1003295. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, M.A.; Sandhu, N.; Høgdall, C.; Christensen, I.J.; Nedergaard, L.; Lundvall, L.; Engelholm, S.A.; Pedersen, A.T.; Hartwell, D.; Lydolph, M.; et al. Evaluation of HE4, CA125, Risk of Ovarian Malignancy Algorithm (ROMA) and Risk of Malignancy Index (RMI) as Diagnostic Tools of Epithelial Ovarian Cancer in Patients with a Pelvic Mass. Gynecol. Oncol. 2012, 127, 379–383. [Google Scholar] [CrossRef]
- Plotti, F.; Guzzo, F.; Schirò, T.; Terranova, C.; Nardone, C.D.C.; Montera, R.; Luvero, D.; Scaletta, G.; Lopez, S.; Capriglione, S.; et al. Role of Human Epididymis Protein 4 (HE4) in Detecting Recurrence in CA125 Negative Ovarian Cancer Patients. Int. J. Gynecol. Cancer 2019, 29, 768–771. [Google Scholar] [CrossRef]
- Angioli, R.; Capriglione, S.; Scaletta, G.; Aloisi, A.; Miranda, A.; De Cicco Nardone, C.; Terranova, C.; Plotti, F. The Role of HE4 in Endometrial Cancer Recurrence: How to Choose the Optimal Follow-Up Program. Tumour Biol. 2016, 37, 4973–4978. [Google Scholar] [CrossRef]
- Capriglione, S.; Plotti, F.; Miranda, A.; Ricciardi, R.; Scaletta, G.; Aloisi, A.; Guzzo, F.; Montera, R.; Angioli, R. Utility of Tumor Marker HE4 as Prognostic Factor in Endometrial Cancer: A Single-Center Controlled Study. Tumour Biol. 2015, 36, 4151–4156. [Google Scholar] [CrossRef]
| Author, Year | Journal | Country | Study Design | Number of Pts 1 | Role of CA125 | Main Findings |
|---|---|---|---|---|---|---|
| Juang C.M. et al., 2006 [15] | Eur J Gynaecol Oncol | Taiwan | Comparative study | 42 | Diagnosis | Preoperative serum CA125 had a potential role in the differential diagnosis between early-stage and advanced-stage uterine leiomyosarcoma. |
| Das S. et al., 2024 [16] | Pan Afr Med | India | Case report | 1 | Diagnosis | CA125 level and LDH level were elevated. |
| Yilmaz N. et al., 2009 [17] | Eur J Gynaecol Oncol | Turkey | Retrospective study | 26 | Diagnosis | In the differential diagnosis of myoma and uterine sarcoma, the preoperative serum CA125 level did not have any predictivity. There was no association between staging and CA125 in uterine sarcomas. |
| Kim H.S. et al., 2010 [18] | Eur J Surg Oncol | Republic of Korea | Observational study | 20 | Diagnosis | There was no difference in the values of serum CA125 levels between preoperative and recurrence times; preoperative and end-of-the-study times; response evaluation and end-of-the-study times, compared with values of the NLR. |
| Yang L. et al., 2024 [19] | Technol Cancer Res Treat | China | Comparative study | 57 | Prognosis | Only CA125 and tumor recurrence remained significant predictors of OS, while only tumor relapse had prognostic significance for PFS (p < 0.05) |
| Visnovsky J. et al., 2015 [20] | Neuro Endocrinol Lett | Slovakia | Retrospective study | 29 | Prognosis | Highly statistically significant values for the inverse correlation between survival and tumor size, positive lymph nodes, high mitotic activity, vascular invasion, positive peritoneal cytology, elevated CA125, smoking, and BMI in sarcoma (p < 0.001 for all factors). |
| Zhang Y. et al., 2019 [21] | Cancer Manag Res. | China | Retrospective observational study | 40 | Prognosis | The probability of death increased by 1.234-fold (95% CI: 1.032–1.476) or 1.016-fold (95% CI: 1.004–1.028) for each unit of increase in the minimum or average value of CA125. |
| Menczer J. et al., 2014 [22] | Isr Med Assoc J | Israel | Retrospective study | 17 | Diagnosis | CA125 was not immunohistochemically expressed in the tissue of any LMS tumors examined. The origin of elevated serum CA125 in some of these tumors is therefore not in its tissue and remains unknown. |
| Duk J.M. et al., 1994 [23] | Int J Gynecol Cancer | Netherlands | Observational prospective study | 33 | Diagnosis | Sarcoma cells were completely negative for CA125, whereas positivity was observed in the epithelial component of mixed Müllerian tumors. The source of the elevated serum CA125 levels in patients with uterine sarcoma may be stimulated mesothelial cells. |
| Vigon A. et al., 2005 [24] | IJGC | Italy | Case report | 1 | Diagnosis | The level of serum CA125 was high at diagnosis, within normal limits after the fifth cycle of chemotherapy, and subsequently increased again at recurrence. |
| Patsner B. et al., 1988 [25] | Cancer | New York state | Observational study | 11 | Diagnosis | Elevated preoperative serum CA125 levels were found. Serial levels during chemotherapy inconsistently reflected response to treatment and proved to have limited clinical value. |
| Study | Year | Selection (*/4) | Comparability (*/2) | Exposure (*/3) | Total (*/9) | Estimated Risk of Bias |
|---|---|---|---|---|---|---|
| Juang et al. [15] | 2006 | *** | * | ** | 6 | Good |
| Yilmaz et al. [17] | 2009 | *** | * | * | 5 | Fair |
| Kim et al. [18] | 2010 | **** | ** | ** | 8 | Good |
| Yang et al. [19] | 2024 | **** | ** | ** | 8 | Good |
| Visnovsky et al. [20] | 2015 | ** | * | ** | 5 | Fair |
| Zhang et al. [21] | 2019 | *** | ** | ** | 7 | Good |
| Menczer et al. [22] | 2014 | ** | * | * | 4 | Poor |
| Duk et al. [23] | 1994 | ** | * | * | 4 | Poor |
| Patsner et al. [25] | 1988 | * | - | * | 2 | Poor |
| Patient | Age at Diagnosis (years) | Menopause | Parity | Family History for Gyn Cancer | BMI | Histology | CA125 (U/mL) | HE4 (pmol/L) | ROMA SCORE | OS (months) | PFS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | No | 0 | No | 22 | Leiomyosarcoma conventional type | 76 | 162 | 59.38% | 8+ | - |
| 2 | 43 | No | 1 | No | 21 | Low-grade leiomyosarcoma | 86 | 50 | 8.24% | 12+ | - |
| 3 | 56 | Yes | 1 | No | 30 | Undifferentiated leiomyosarcoma | 17 | 36 | 9.2% | 12+ | - |
| 4 | 44 | No | 2 | No | 22 | Undifferentiated leiomyosarcoma | 86 | 444 | 94.2% | 24+ | - |
| 5 | 70 | Yes | 1 | No | 22 | Undifferentiated leiomyosarcoma | 37 | 103 | 34.83% | 30+ | - |
| 6 | 58 | Yes | 0 | Yes | 27 | Leiomyosarcoma conventional type | 68 | 37 | 22.34% | 17+ | - |
| 7 | 55 | Yes | 0 | No | 31 | Myxoid leiomyosarcoma | 27 | 115 | 32.24% | 27+ | - |
| 8 | 54 | Yes | 0 | No | 37 | Spindle cell leiomyosarcoma | 31 | 29 | 11.16% | 47+ | - |
| 9 | 54 | Yes | 0 | No | 20 | Leiomyosarcoma conventional type | 206 | 122 | 69.13% | 13 | 13 |
| 10 | 55 | Yes | 2 | Yes | 31 | Low-grade leiomyosarcoma | 15 | 42 | 9.79% | 98+ | - |
| 11 | 51 | Yes | 0 | No | 24 | High-grade leiomyosarcoma | 20 | 45 | 12.58% | 57 | 45 |
| 12 | 80 | Yes | 2 | No | 33 | Leiomyosarcoma conventional type | 27 | 250 | 51.62% | 8 | 8 |
| 13 | 43 | No | 0 | No | 20 | Leiomyosarcoma conventional type | 132 | 179 | 65.74% | 118 | 94 |
| 14 | 83 | Yes | 3 | No | 26 | Spindle cell leiomyosarcoma | 78 | 234 | 68.41% | 3+ | - |
| 15 | 51 | No | 2 | No | 25 | Leiomyosarcoma conventional type | 156 | 187 | 68.27% | 9 | 9 |
| 16 | 46 | No | 2 | No | 42 | Leiomyosarcoma conventional type | 120 | 221 | 75.9% | 171+ | - |
| Clinical Characteristics | OS (p-Value) | PFS (p-Value) |
|---|---|---|
| Age at diagnosis | 0.03 | 0.04 |
| Menopause | 0.06 | 0.08 |
| Parity | 0.15 | 0.2 |
| Family history for gyn cancer | 0.5 | 0.4 |
| BMI | 0.07 | 0.09 |
| Histology | 0.04 | 0.04 |
| CA125 | 0.1 | 0.12 |
| HE4 | 0.12 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cundari, G.B.; Feole, L.; Terranova, C.; De Cicco Nardone, C.; Montera, R.; Luvero, D.; Guzzo, F.; Martinelli, A.; Di Donato, V.; Angioli, R.; et al. The Role of CA125 and HE4 in Uterine Sarcomas: Beyond Diagnosis and Prognosis—A Systematic Review and Case Series from a Single Institution. Cancers 2025, 17, 1473. https://doi.org/10.3390/cancers17091473
Cundari GB, Feole L, Terranova C, De Cicco Nardone C, Montera R, Luvero D, Guzzo F, Martinelli A, Di Donato V, Angioli R, et al. The Role of CA125 and HE4 in Uterine Sarcomas: Beyond Diagnosis and Prognosis—A Systematic Review and Case Series from a Single Institution. Cancers. 2025; 17(9):1473. https://doi.org/10.3390/cancers17091473
Chicago/Turabian StyleCundari, Gianna Barbara, Laura Feole, Corrado Terranova, Carlo De Cicco Nardone, Roberto Montera, Daniela Luvero, Federica Guzzo, Arianna Martinelli, Violante Di Donato, Roberto Angioli, and et al. 2025. "The Role of CA125 and HE4 in Uterine Sarcomas: Beyond Diagnosis and Prognosis—A Systematic Review and Case Series from a Single Institution" Cancers 17, no. 9: 1473. https://doi.org/10.3390/cancers17091473
APA StyleCundari, G. B., Feole, L., Terranova, C., De Cicco Nardone, C., Montera, R., Luvero, D., Guzzo, F., Martinelli, A., Di Donato, V., Angioli, R., & Plotti, F. (2025). The Role of CA125 and HE4 in Uterine Sarcomas: Beyond Diagnosis and Prognosis—A Systematic Review and Case Series from a Single Institution. Cancers, 17(9), 1473. https://doi.org/10.3390/cancers17091473







