Small Bowel Tumors: A 7-Year Study in a Tertiary Care Hospital
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
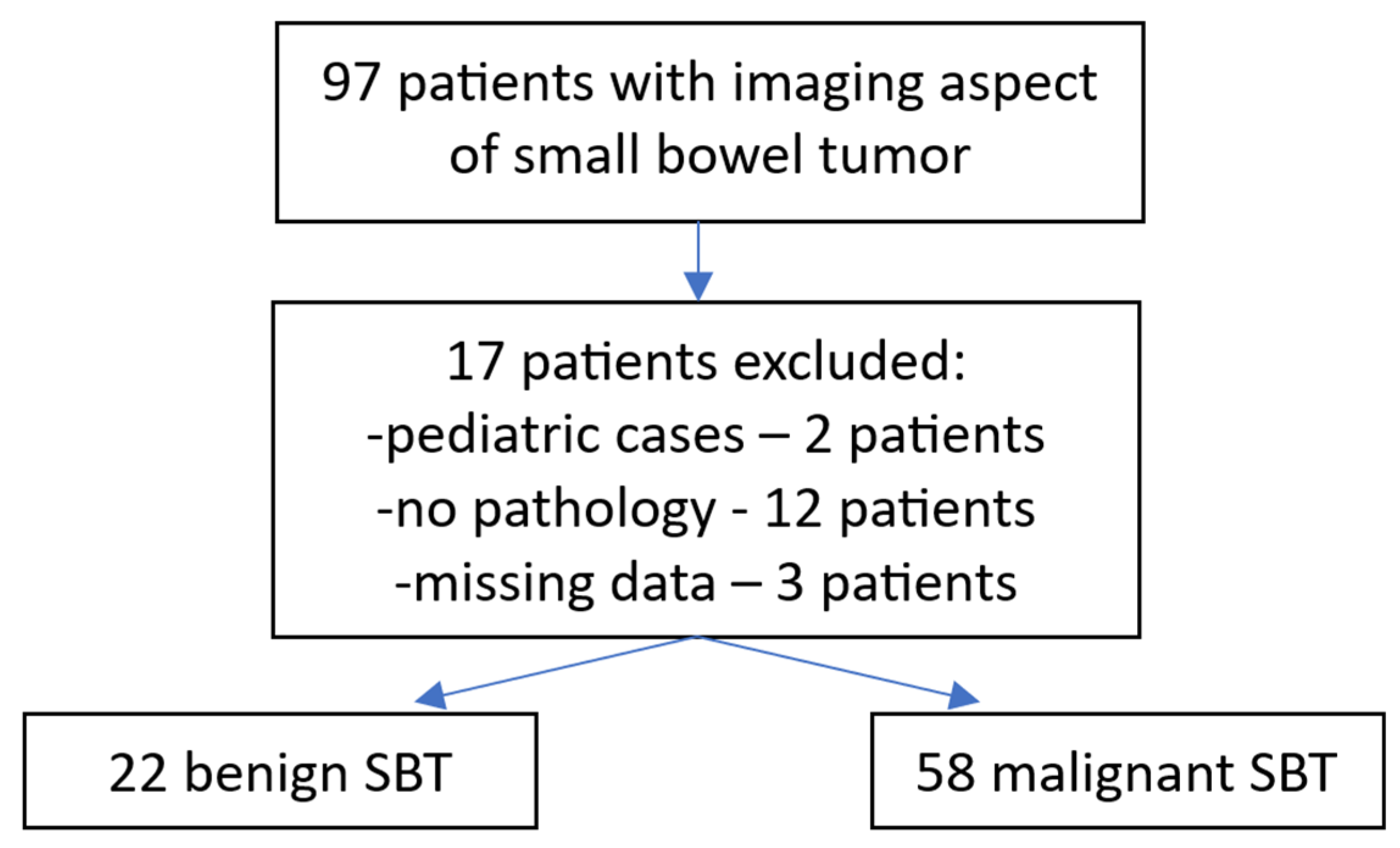
2.1. Patient Selection
2.2. Data Collection and Statistical Analysis
3. Results
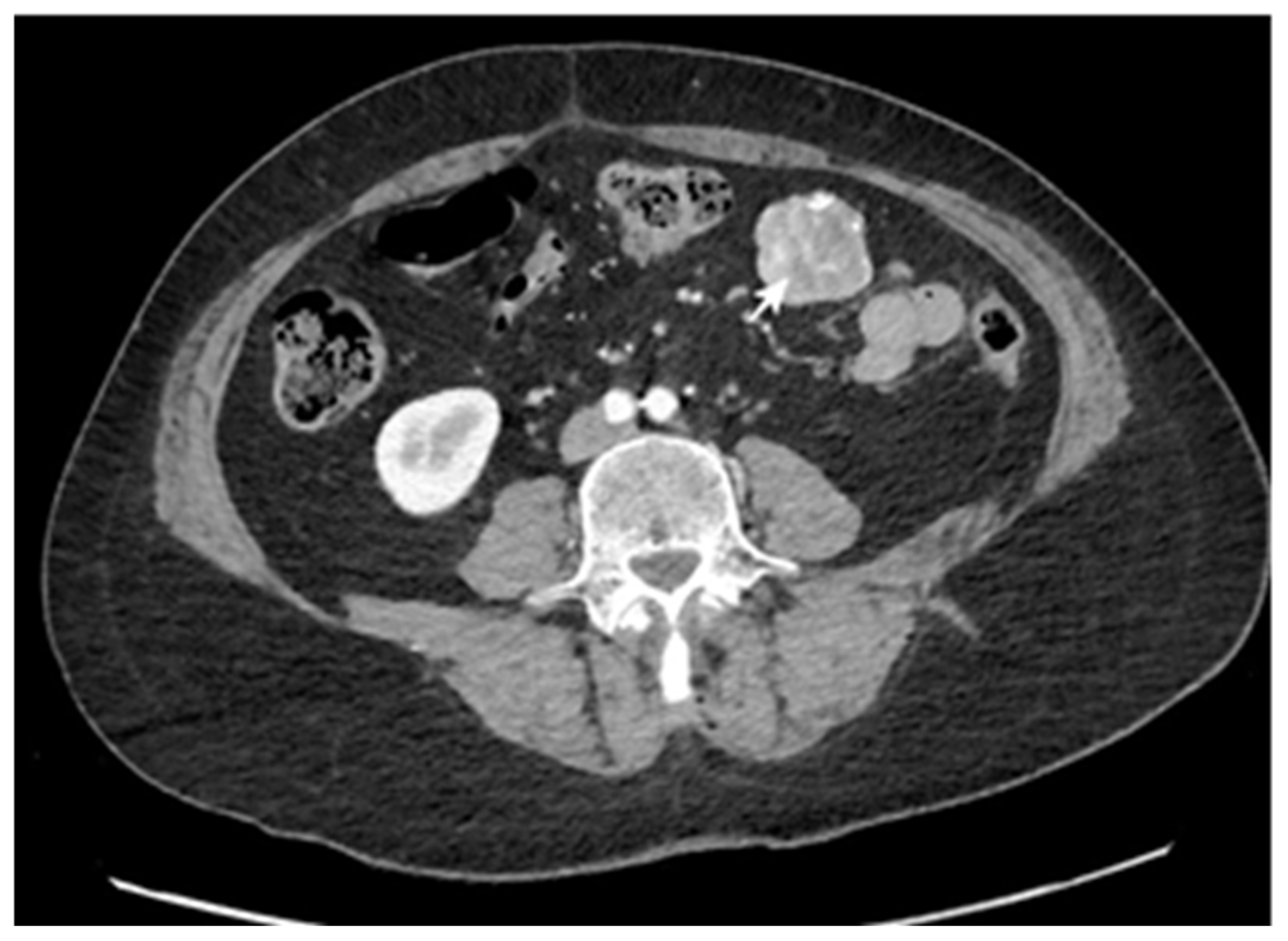
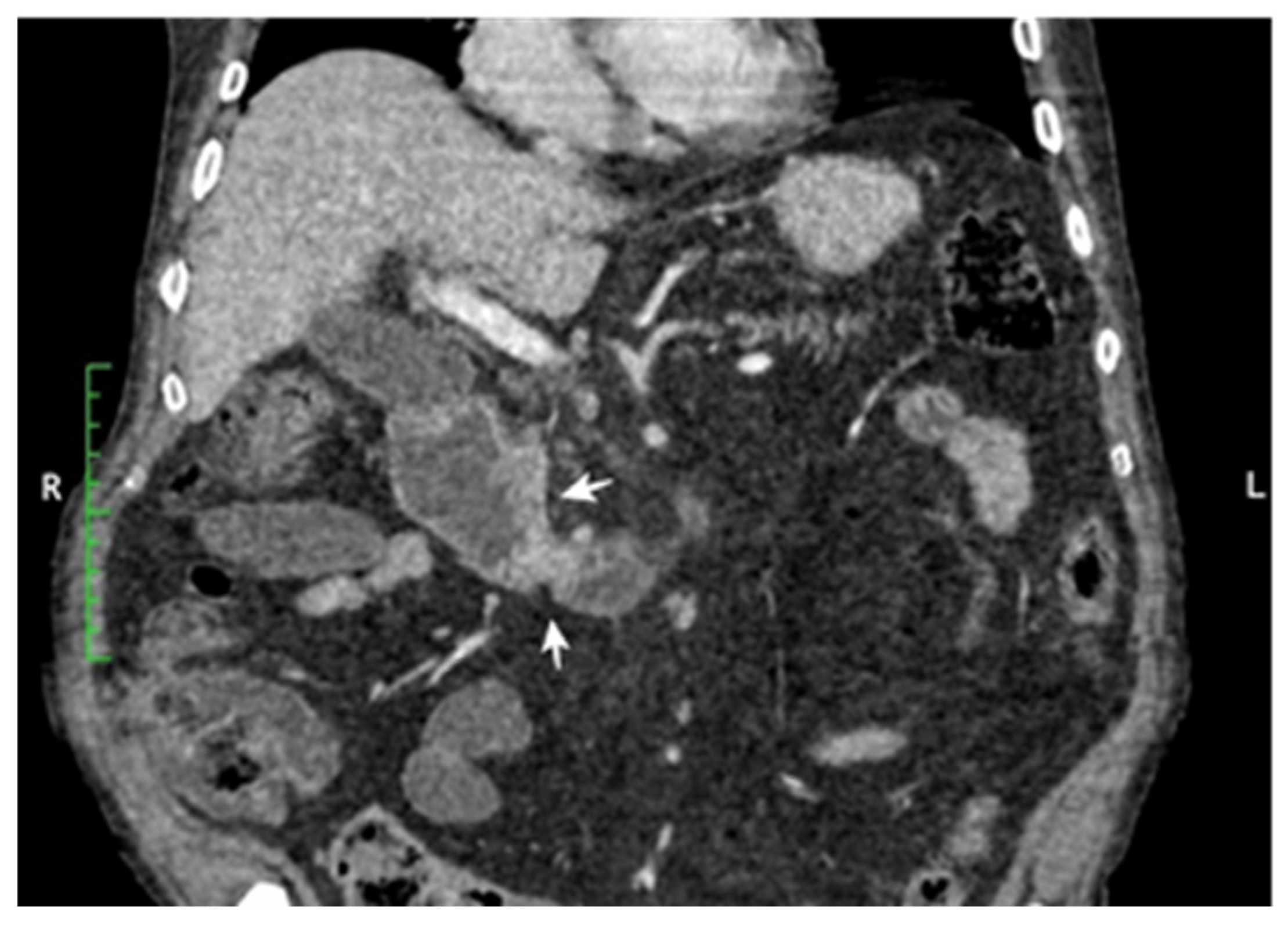
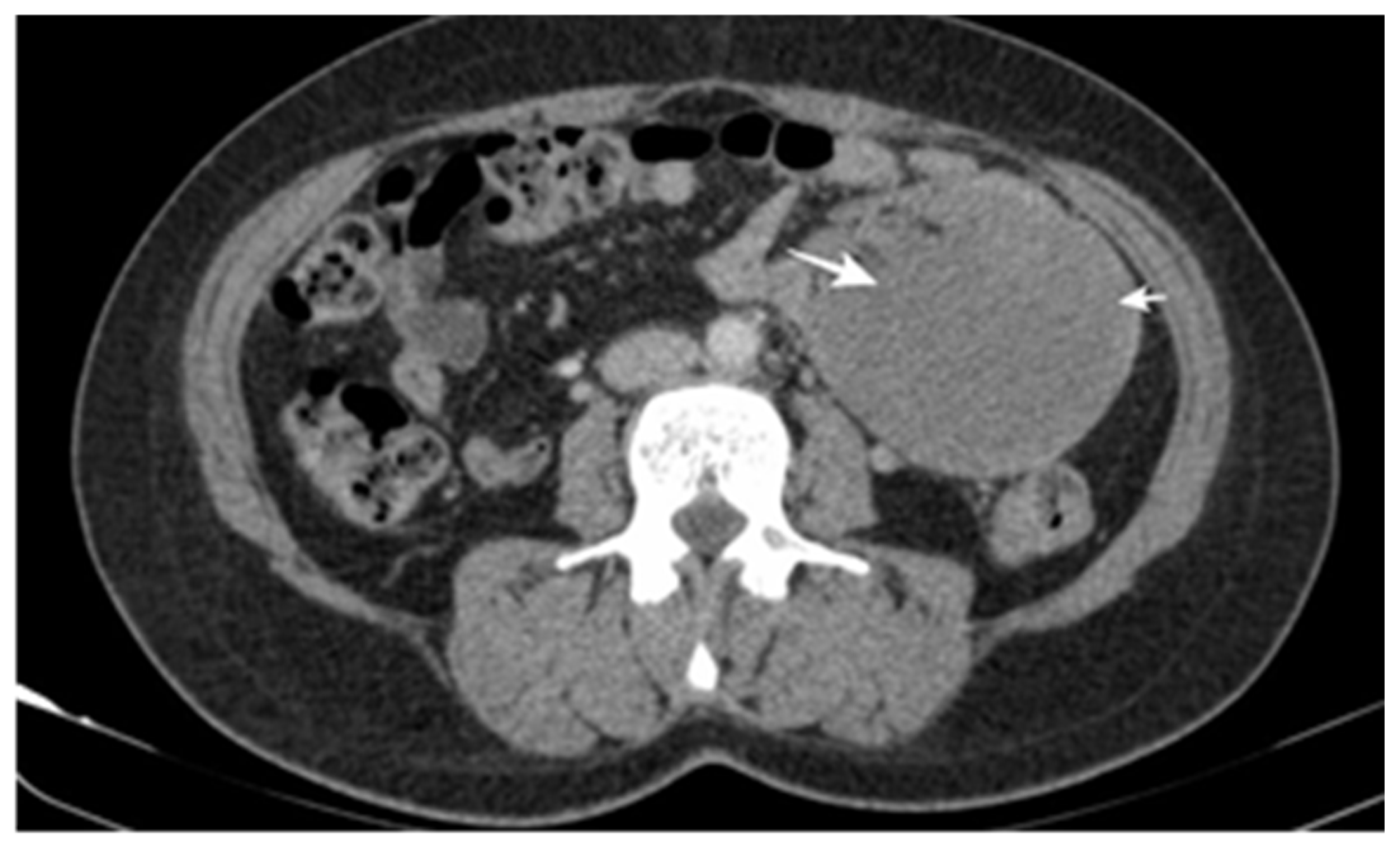
3.1. Main Characteristics of Patients
3.2. Treatment Particularities
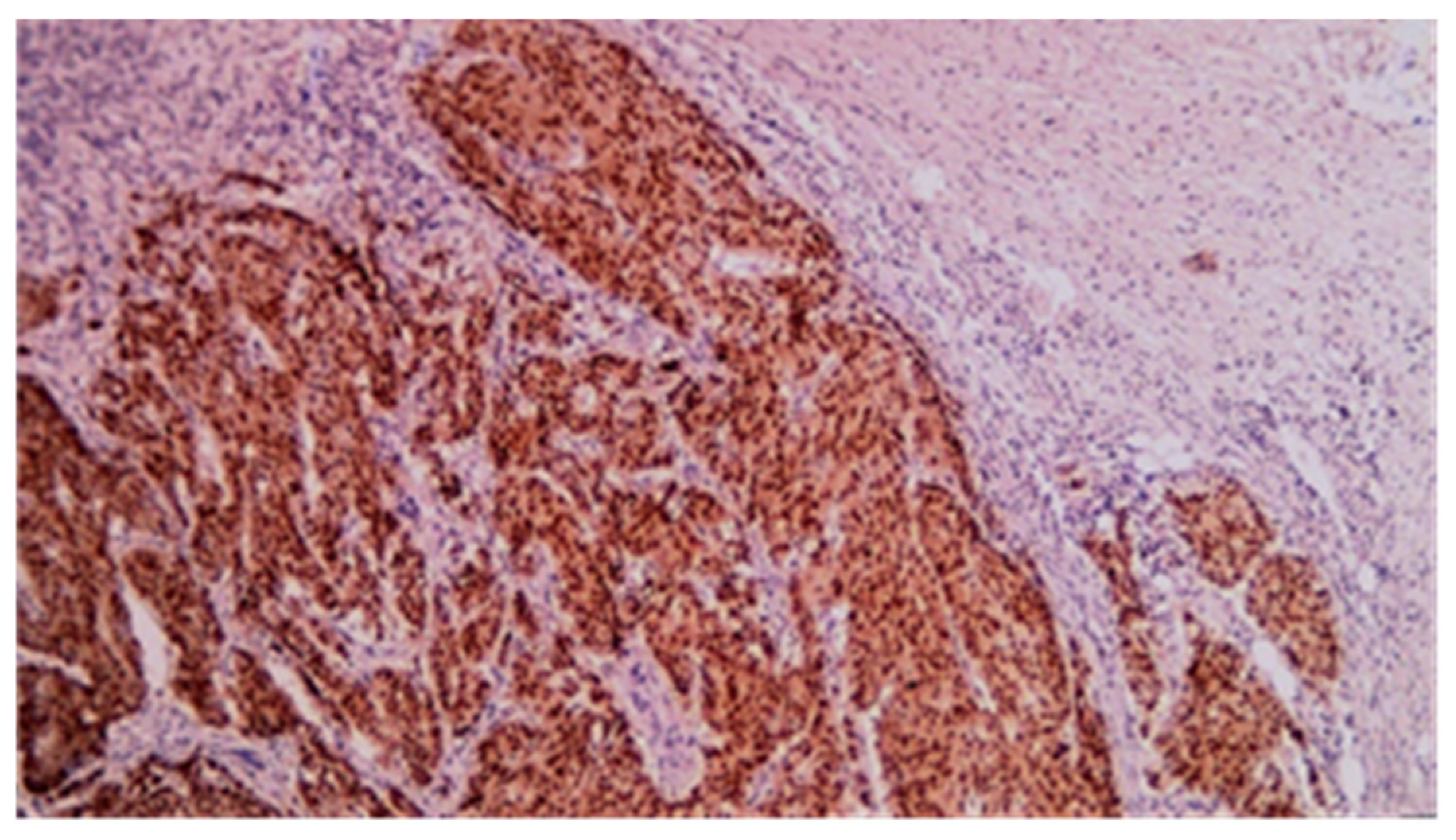
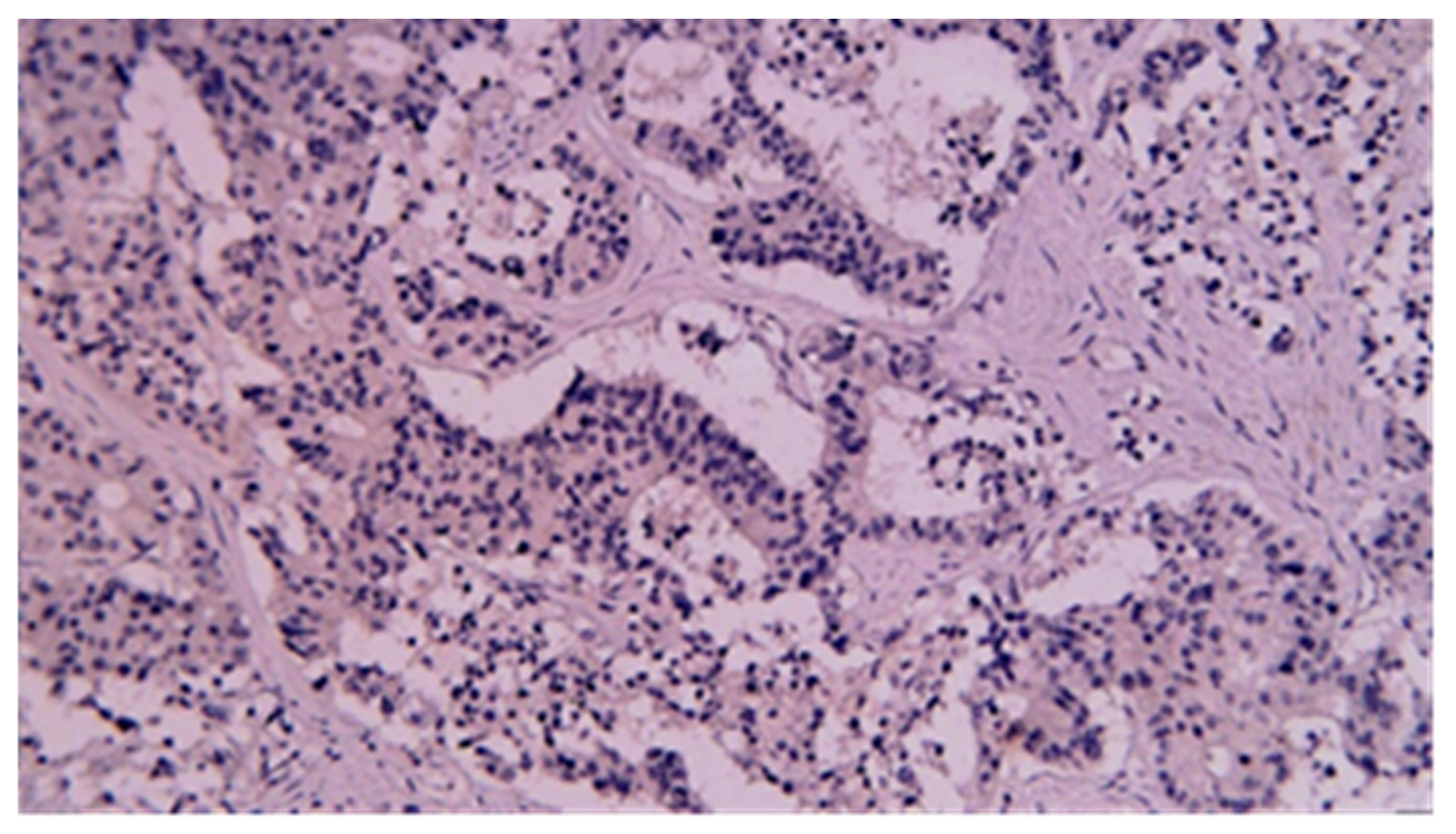
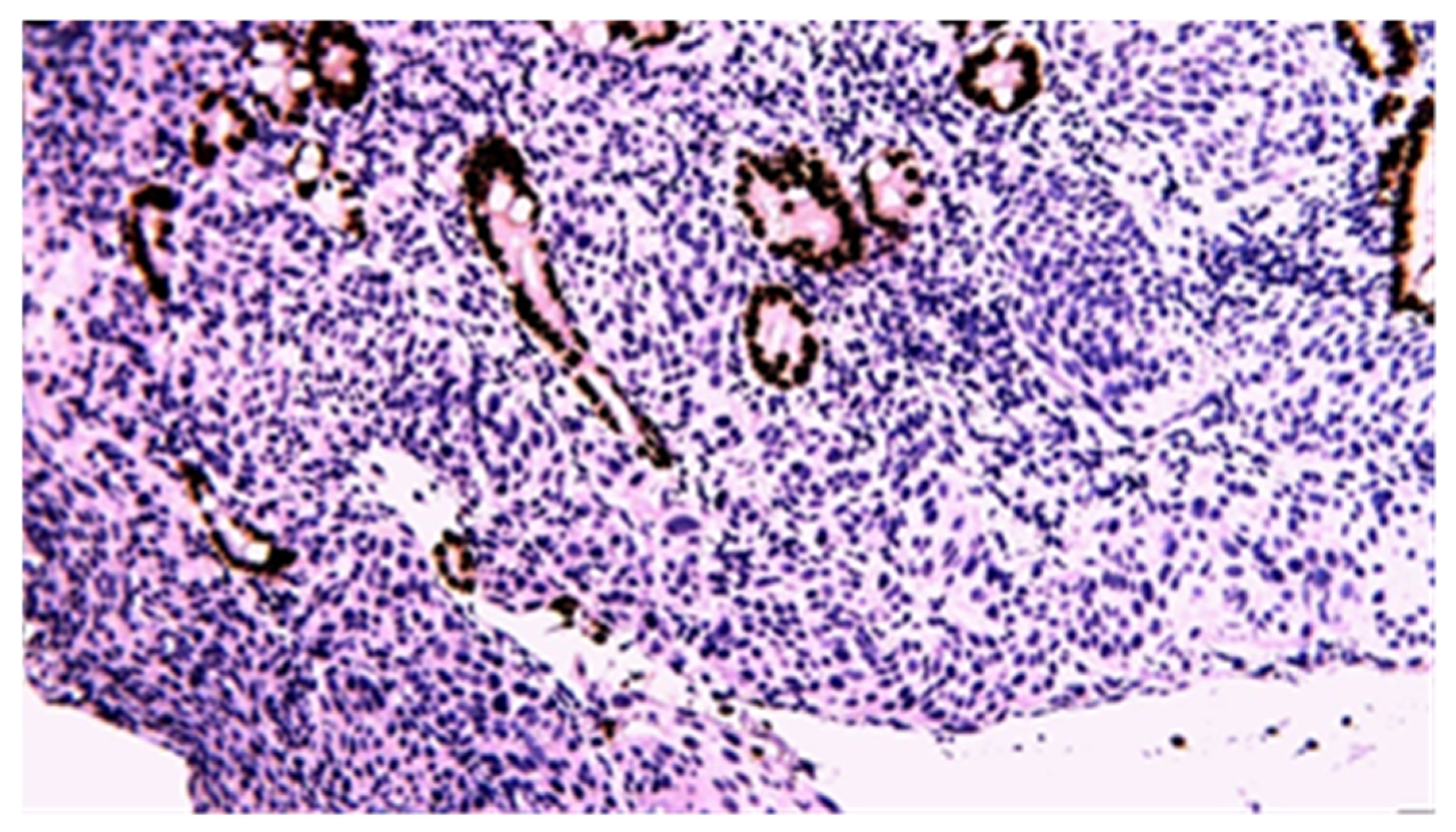
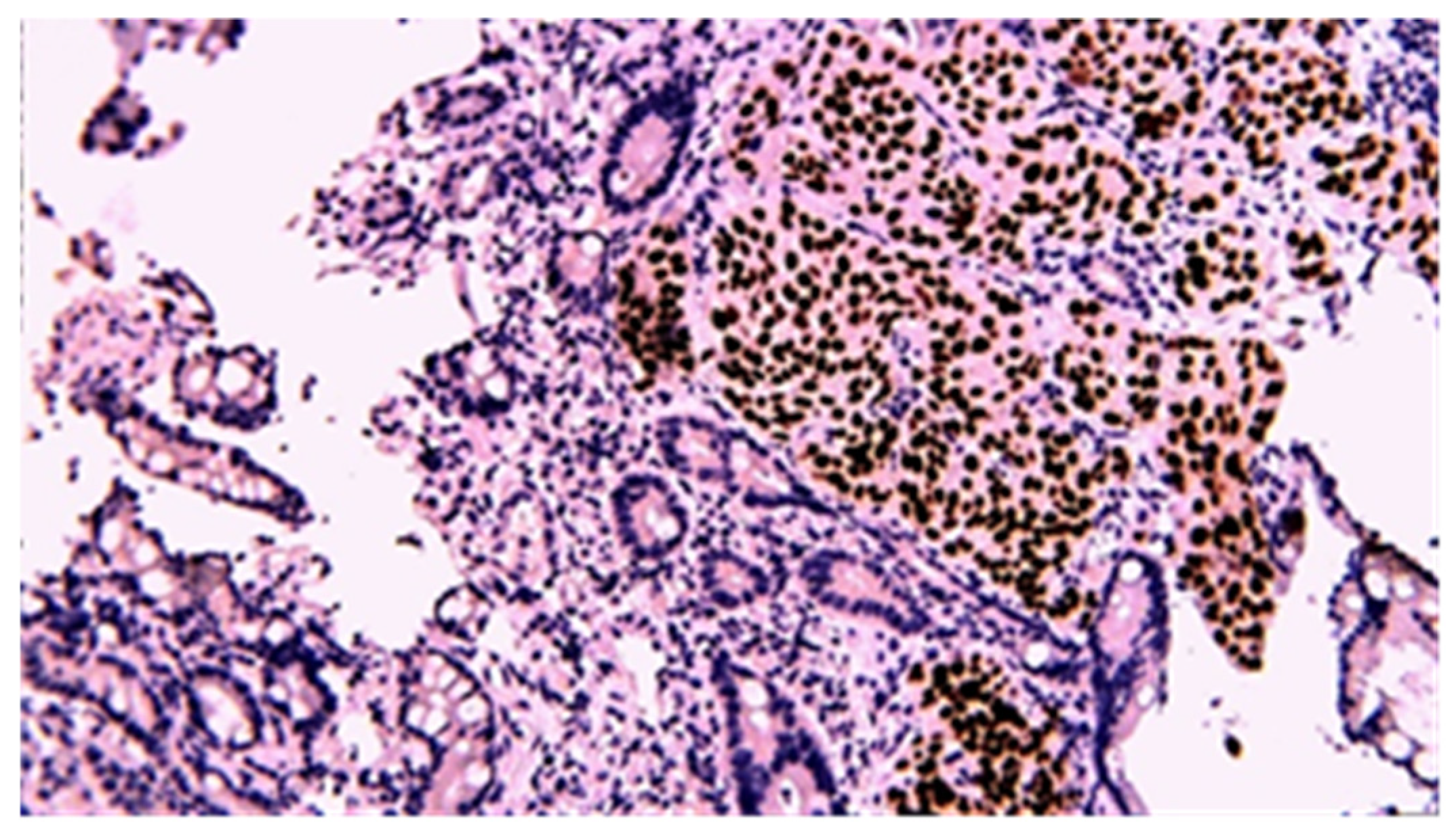
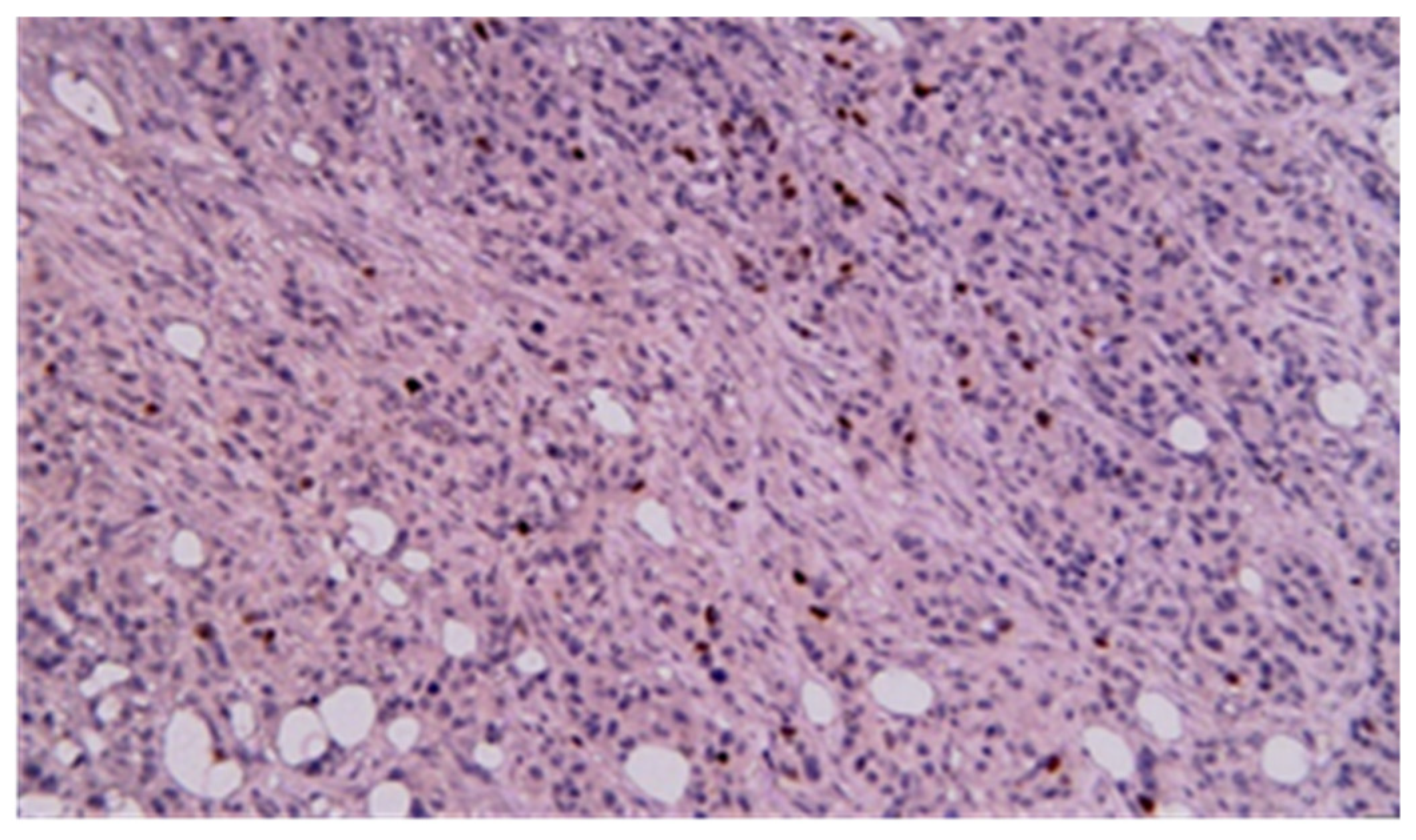
3.3. Immunohistochemistry
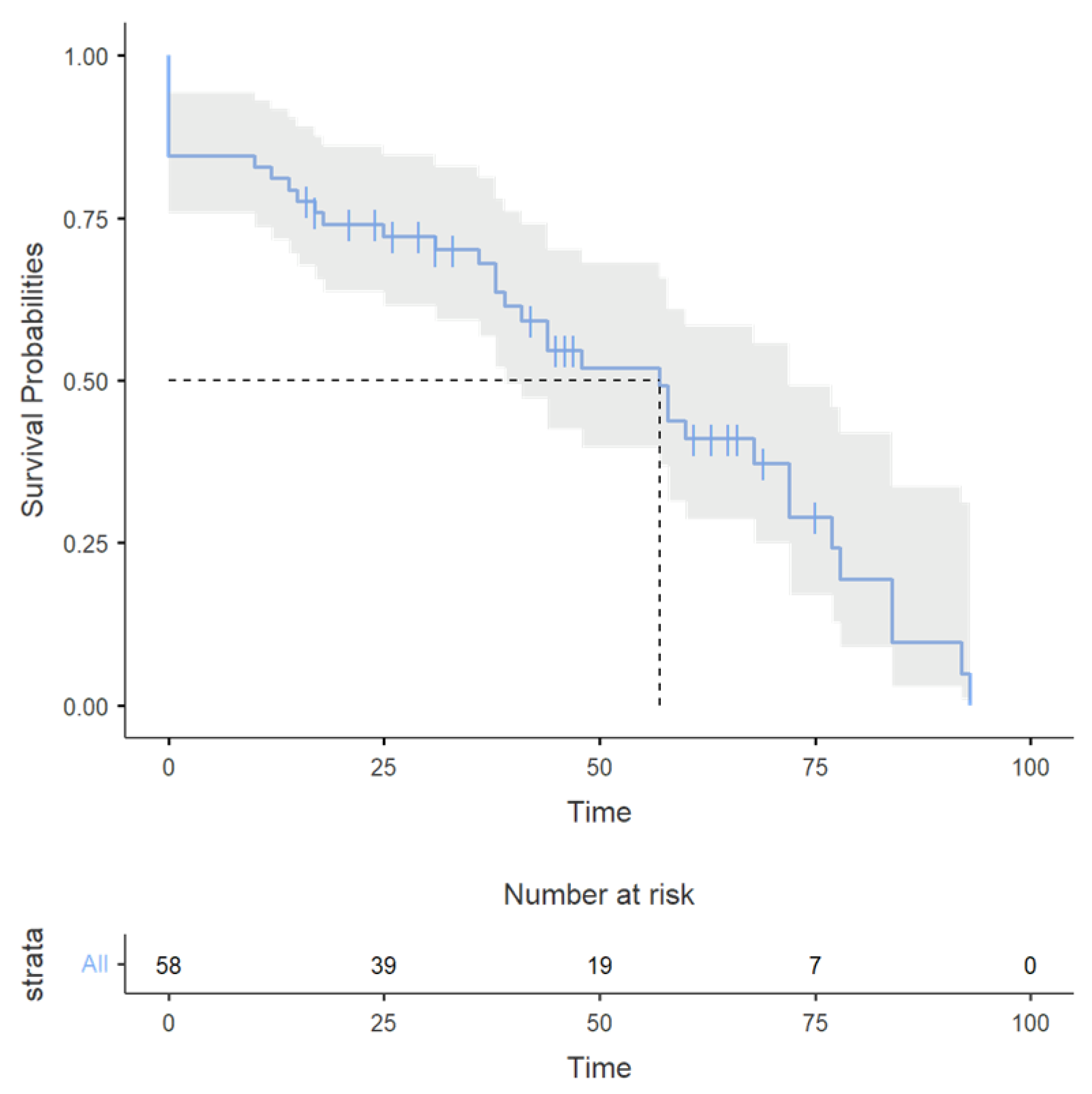
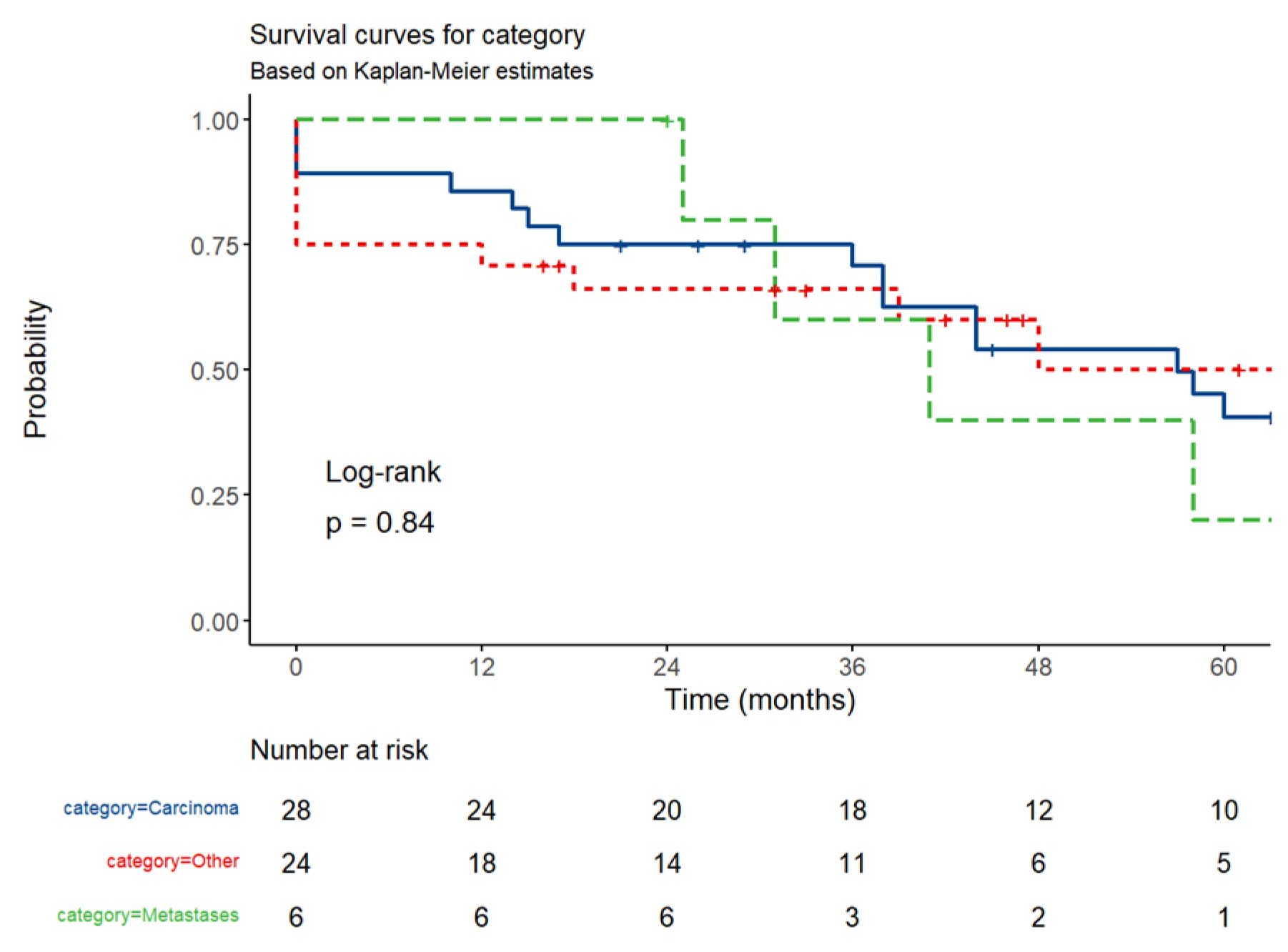
3.4. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheung, D.Y.; Choi, M.-G. Current Advance in Small Bowel Tumors. Clin. Endosc. 2011, 44, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kye, B.H.; Lee, J.I.; Kim, S.H.; Kim, H.J.; Kang, W.K.; Oh, S.T. Clinicopathological Features of Primary Jejunoileal Tumors. J. Korean Soc. Coloproctology 2010, 26, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, J.W. Small Bowel Malignancies in Patients Undergoing Capsule Endoscopy for Iron Deficiency Anemia. Diagnostics 2021, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Safatle-Ribeiro, A.V.; Ribeiro, U., Jr. Impact of enteroscopy on diagnosis and management of small bowel tumors. Chin. J. Cancer Res. 2020, 32, 319–333. [Google Scholar] [CrossRef]
- Sahin, E. Emergent approach to small bowel tumors: Diagnosis and treatment. Turk. J. Trauma Emerg. Surg. 2024, 30, 155–159. [Google Scholar] [CrossRef]
- Pan, S.Y. Epidemiology of cancer of the small intestine. World J. Gastrointest. Oncol. 2011, 3, 33–42. [Google Scholar] [CrossRef]
- Cardoso, H.; Rodrigues, J.T.; Marques, M.; Ribeiro, A.; Vilas-Boas, F.; Santos-Antunes, J.; Rodrigues-Pinto, E.; Silva, M.; Maia, J.C.; Macedo, G. Malignant Small Bowel Tumors: Diagnosis, Management and Prognosis. Acta Medica Port. 2015, 28, 448–456. [Google Scholar] [CrossRef]
- Shah, P.P.; Kothari, S. Jejunal Adenocarcinoma—A Case Report with Review. Indian J. Surg. 2011, 75, 82–85. [Google Scholar] [CrossRef][Green Version]
- Krishnamurthy, P.; Varghese, S.E.; Gopalswamy, N.; Hillman, N.; Ali, S.A. Small-bowel adenocarcinoma: Case report and review of literature on diagnosis of small-bowel tumors. Gastroenterol. Hepatol. 2007, 3, 129–135. [Google Scholar]
- Obleagă, C.V.; Streba, C.T.; Mirea, C.S.; Vîlcea, I.D.; Florescu, D.N.; Ciorbagiu, M.C.; Turcu, T.; Florescu, M.M.; Șerbănescu, M.S.; Mehedințeanu, A.-M.; et al. Primitive Resectable Small Bowel Cancer Clinical–Pathological Analysis: A 10-Year Retrospective Study in a General Surgery Unit. Cancers 2024, 16, 3713. [Google Scholar] [CrossRef]
- Mirea, C.S.; Ciorbagiu, M.C.; Obleaga, C.V.; Moraru, E.; Mogoanta, S.S.; Ciurea, R.N.; Foarfa, M.C.; Vilcea, A.M.; Vilcea, I.D. Stage IV duodenal GIST requiring emergency pancreaticoduodenectomy—Diagnosis difficulties and therapeutic options. Rom. J. Morphol. Embryol. 2018, 59, 543–548. [Google Scholar] [PubMed]
- Nandedkar, S.; Trivedi, K.; Malukani, K.; Kk, T. Primary squamous cell carcinoma of the small intestine. J. Cancer Res. Ther. 2013, 9, 739–740. [Google Scholar] [CrossRef] [PubMed]
- Terada, T. Malignant tumors of the small intestine: A histopathologic study of 41 cases among 1,312 consecutive specimens of small intestine. Int. J. Clin. Exp. Pathol. 2012, 5, 203–209. [Google Scholar] [PubMed]
- Mumtaz, S.; Ahmad, Z.; Fatima, S.; Qureshi, A. Squamous cell carcinoma in the small intestine. BMJ Case Rep. 2011, 2011, bcr0120113762. [Google Scholar] [CrossRef]
- Wang, F.-D.; Wang, Z.-W.; Xue, H.-D.; Wu, H.-W.; Zhang, Y.; Yu, J.-C.; Jin, Z.-Y. Primary Squamous Cell Carcinoma of the Small Intestine. Chin. Med. J. 2016, 129, 2131–2133. [Google Scholar] [CrossRef]
- Platt, C.C.; Haboubi, N.Y.; Schofield, P.F. Primary squamous cell carcinoma of the terminal ileum. J. Clin. Pathol. 1991, 44, 253–254. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, L.; Zhang, J.-X.; Pan, Y.-S. Rare squamous cell carcinoma of the jejunum causing perforated peritonitis: A case report. World J. Gastrointest. Oncol. 2022, 14, 2295–2301. [Google Scholar] [CrossRef]
- Sun, D.S.; Shin, O.R.; Ku, Y.M.; Kim, Y.-S.; Seo, K.-J. Squamous cell carcinoma of the small bowel manifesting as a jejunal perforation: A case report. Int. J. Clin. Exp. Pathol. 2014, 7, 6345–6349. [Google Scholar]
- Soni, S.; Elhence, P.; Varshney, V.K.; Suman, S. Primary squamous cell carcinoma of the ampulla of Vater: Management and review of the literature. BMJ Case Rep. 2021, 14, e236477. [Google Scholar] [CrossRef]
- Bolanaki, H.; Giatromanolaki, A.; Sivridis, E.; Karayiannakis, A.J. Primary squamous cell carcinoma of the ampulla of Vater. JOP 2014, 15, 42–45. [Google Scholar] [CrossRef]
- Battal, M.; Bostancı, O.; Basak, T.; Kartal, K.; Ekiz, F. Pure Squamous Cell Carcinoma of the Duodenum. Case Rep. Surg. 2015, 2015, 714640. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, N.; Nakabayashi, Y.; Dairaku, K.; Tomori, K.; Hiramoto, Y.; Kurihara, K. Intestinal obstruction caused by primary adenosquamous cell carcinoma of the small intestine: A case report and review of the literature. Mol. Clin. Oncol. 2018, 10, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kumar, A.; Gowda, V.; Sahai, R.; Kumar, S.; Kishore, S. An adding up of an exceptionally rare case report: Primary Squamous cell carcinoma of Jejunum from North India. J. Cancer Res. Ther. 2023, 19, S451–S453. [Google Scholar] [CrossRef]
- Jadhav, M.; Nivash, T.H. Unveiling a Jejunal Leiomyosarcoma Presenting as a Gastrointestinal Stromal Tumor: A Case Report. Cureus 2024, 16, e66973. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Williams, E.S.; Leffall, L.D. Primary malignant small bowel tumors: An atypical abdominal emergency. J. Natl. Med. Assoc. 1995, 87, 276–279. [Google Scholar]
- Farhat, M.H.; Shamseddine, A.I.; Barada, K.A. Small Bowel Tumors: Clinical Presentation, Prognosis, and Outcome in 33 Patients in a Tertiary Care Center. J. Oncol. 2008, 2008, 212067. [Google Scholar] [CrossRef]
- Beltran, M.A.; Cruces, K.S. Primary tumors of jejunum and ileum as a cause of intestinal obstruction: A case control study. Int. J. Surg. 2007, 5, 183–191. [Google Scholar] [CrossRef]
- Kogo, H.; Shimanuki, K.; Iwao, T.; Yoshida, H. Small bowel GIST with hemorrhagic shock diagnosed by capsule endoscopy and double-balloon endoscopy, angiography-guided hemostasis, and laparoscopic-assisted resection. Clin. Case Rep. 2021, 9, e04240. [Google Scholar] [CrossRef]
- Turan, M.; Karadayi, K.; Duman, M.; Ozer, H.; Arici, S.; Yildirir, C.; Koçak, O.; Sen, M. Small bowel tumors in emergency surgery. Ulus. Travma Acil Cerrahi Derg. 2010, 16, 327–333. [Google Scholar]
- Chou, J.-W.; Feng, C.-L.; Lai, H.-C.; Tsai, C.-C.; Chen, S.-H.; Hsu, C.-H.; Cheng, K.-S.; Peng, C.-Y.; Chung, P.-K. Obscure Gastrointestinal Bleeding Caused by Small Bowel Lipoma. Intern. Med. 2008, 47, 1601–1603. [Google Scholar] [CrossRef][Green Version]
- Al-Swaiti, G.T.; Al-Qudah, M.H.; Al-Doud, M.A.; Al-Bdour, A.R.; Al-Nizami, W. Spontaneous perforation of jejunal gastrointestinal stromal tumor: A case report. Int. J. Surg. Case Rep. 2020, 73, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Livengood, J.C.; Fenoglio, M.E. Gastrointestinal Hemorrhage from a Small Bowel Polypoid Hemangioma. JSLS J. Soc. Laparoendosc. Surg. 2002, 6, 179–180. [Google Scholar]
- Gil Heo, T. Solitary jejunal cavernous hemangioma causing intermittent melena: A case report. Int. J. Surg. Case Rep. 2021, 84, 106121. [Google Scholar] [CrossRef]
- Abboud, B. Vanek’s tumor of the small bowel in adults. World J. Gastroenterol. 2015, 21, 4802–4808. [Google Scholar] [CrossRef]
- Rockey, D.C.; Altayar, O.; Falck-Ytter, Y.; Kalmaz, D. AGA Technical Review on Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology 2020, 159, 1097–1119. [Google Scholar] [CrossRef]
- Yin, M.; Lin, J.; Liu, L.; Gao, J.; Xu, W.; Yu, C.; Qu, S.; Liu, X.; Qian, L.; Xu, C.; et al. Development of a Deep Learning Model for Malignant Small Bowel Tumors Survival: A SEER-Based Study. Diagnostics 2022, 12, 1247. [Google Scholar] [CrossRef]
- Dolu, S.; Onem, S.; Htway, Z.; Hajıyev, F.; Bilgen, A.; Binicier, H.C.; Kalemoglu, E.; Sagol, O.; Akarsu, M. Endoscopic and histological characteristics of small bowel tumors diagnosed by double-balloon enteroscopy. Clin. Endosc. 2023, 56, 83–91. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, L.; Zhang, T.; Sun, P.; Sun, J.; Zhou, J.; Wang, L.; Fan, R.; Wang, Z.; Cheng, S.; et al. Clinical characteristics of small bowel tumors diagnosed by double-balloon endoscopy: Experience from a Chinese tertiary hospital. Turk. J. Gastroenterol. 2020, 31, 30–35. [Google Scholar] [CrossRef]
- Robles, E.P.-C.; Delgado, P.E.; Conesa, P.B.; Andrés, B.M.; Guggiana, M.F.; Mateos, E.A.; Caballero, M.F.; Agudo, J.L.R.; Martínez, S.C.; Latorre, R.; et al. Role of double-balloon enteroscopy in malignant small bowel tumors. World J. Gastrointest. Endosc. 2015, 7, 652–658. [Google Scholar] [CrossRef]
- Chung, C.-S.; Tai, C.-M.; Huang, T.-Y.; Chang, C.-W.; Chen, K.-C.; Tseng, C.-M.; Wang, H.-Y.; Chu, C.-H.; Wu, J.-M.; Chen, Y.; et al. Small bowel tumors: A digestive endoscopy society of Taiwan (DEST) multicenter enteroscopy-based epidemiologic study. J. Formos. Med. Assoc. 2018, 117, 705–710. [Google Scholar] [CrossRef]
- Ko, M.; Yen, C.; Yen, H. Obscure gastrointestinal bleeding with negative abdominal computed tomography study: The importance of enteroscopy for early diagnosis of small bowel malignancy. JGH Open 2019, 4, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.S.; Song, J.H.; Nam, K.; Kim, S.-E.; Jeong, E.S.; Kim, J.H.; Jeon, S.R. Discordance Rate and Risk Factor of Other Diagnostic Modalities for Small Bowel Tumors Detected by Device-Assisted Enteroscopy: A Korean Association for the Study of Intestinal Disease (KASID) Multicenter Study. Gut Liver 2024, 18, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.; Yin, A.; Hu, W.; Hu, W.; Zhao, L.; Zhao, L.; Ding, Y.; Ding, Y.; Yu, H.; Yu, H. Diagnosis and therapy using double-balloon endoscopy for small bowel disease: Experience from a Chinese tertiary hospital. J. Int. Med. Res. 2020, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B. Application of double-balloon enteroscopy for small bowel tumors. Clin. Endosc. 2023, 56, 53–54. [Google Scholar] [CrossRef]
- Nishimura, N.; Mizuno, M.; Shimodate, Y.; Doi, A.; Mouri, H.; Matsueda, K.; Yamamoto, H. The Role of Double-balloon Enteroscopy in the Diagnosis and Surgical Treatment of Metastatic Small Bowel Tumors. Intern. Med. 2018, 57, 1209–1212. [Google Scholar] [CrossRef]
- Banciu, C.; Munteanu, A.; Aprotosoaie, A.; Fabian, R.; Dobrescu, A.; Vaduva, A.; Fabian, A.; Soica, I.; Ivan, V.; Sima, L. Obscure Bleeding from a Metastatic Small Bowel Tumor Diagnosed Using Motorized Spiral Enteroscopy: A Case Study and a Literature Review. Diagnostics 2024, 14, 904. [Google Scholar] [CrossRef]
- Chen, W.-G. Double-balloon enteroscopy in small bowel tumors: A Chinese single-center study. World J. Gastroenterol. 2013, 19, 3665–3671. [Google Scholar] [CrossRef]
- Fantasia, S.; Valdivia, P.C.; Kayali, S.; Koulaouzidis, G.; Pennazio, M.; Koulaouzidis, A. The Role of Capsule Endoscopy in the Diagnosis and Management of Small Bowel Tumors: A Narrative Review. Cancers 2024, 16, 262. [Google Scholar] [CrossRef]
- Vere, C.C.; Foarfă, C.; Streba, C.T.; Cazacu, S.; Pârvu, D.; Ciurea, T. Videocapsule endoscopy and single balloon enteroscopy: Novel diagnostic techniques in small bowel pathology. Rom. J. Morphol. Pathol. 2009, 50, 467–474. [Google Scholar]
- Pongprasobchai, S.; Chitsaeng, S.; Tanwandee, T.; Manatsathit, S.; Kachintorn, U. Yield, etiologies and outcomes of capsule endoscopy in Thai patients with obscure gastrointestinal bleeding. World J. Gastrointest. Endosc. 2013, 5, 122–127. [Google Scholar] [CrossRef]
- Zagorowicz, E.S. Small bowel tumors detected and missed during capsule endoscopy: Single center experience. World J. Gastroenterol. 2013, 19, 9043–9048. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.Y.; Kim, J.S.; Shim, K.-N.; Choi, M.-G.; Korean Gut Image Study Group. The Usefulness of Capsule Endoscopy for Small Bowel Tumors. Clin. Endosc. 2016, 49, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Lee, O.Y.; Jeen, Y.T.; Lim, C.Y.; Cheung, D.Y.; Cheon, J.H.; Ye, B.D.; Song, H.J.; Do, J.H.; Lee, K.J.; et al. Indications for Detection, Completion, and Retention Rates of Small Bowel Capsule Endoscopy Based on the 10-Year Data from the Korean Capsule Endoscopy Registry. Clin. Endosc. 2015, 48, 399–404. [Google Scholar] [CrossRef]
- Kim, E.R. Roles of Capsule Endoscopy and Device-Assisted Enteroscopy in the Diagnosis and Treatment of Small-Bowel Tumors. Clin. Endosc. 2020, 53, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Hong, S.N.; Jang, H.J.; Jeon, S.R.; Cha, J.M.; Park, S.J.; Byeon, J.S.; Ko, B.M.; Kim, E.R.; Choi, H.; et al. Clinical Efficacy of Various Diagnostic Tests for Small Bowel Tumors and Clinical Features of Tumors Missed by Capsule Endoscopy. Gastroenterol. Res. Pr. 2015, 2015, 623208. [Google Scholar] [CrossRef]
- Shyung, M.G.; Green, J.; Min, M.; Schlieve, C.R.; Patel, K.; Cahan, M.; Cave, D. Carcinoids and Capsules: A Case Series Highlighting the Utility of Capsule Endoscopy in Patients With Small Bowel Carcinoids. Gastroenterol. Res. 2017, 10, 347–351. [Google Scholar] [CrossRef]
- Ribeiro, I.; Pinho, R.; Rodrigues, A.; Fernandes, C.; Silva, J.; Ponte, A.; Tente, D.; Carvalho, J. The Importance of Alternative Diagnostic Modalities in the Diagnosis of Small Bowel Tumors After a Negative Capsule Endoscopy. GE Port. J. Gastroenterol. 2015, 22, 112–116. [Google Scholar] [CrossRef]
- Sulbaran, M.; de Moura, E.; Bernardo, W.; Morais, C.; Oliveira, J.; Bustamante-Lopez, L.; Sakai, P.; Mönkemüller, K.; Safatle-Ribeiro, A. Overtube-assisted enteroscopy and capsule endoscopy for the diagnosis of small-bowel polyps and tumors: A systematic review and meta-analysis. Endosc. Int. Open 2016, 04, E151–E163. [Google Scholar] [CrossRef]
- Fujita, M.; Manabe, N.; Honda, K.; Murao, T.; Osawa, M.; Kawai, R.; Akiyama, T.; Shiotani, A.; Haruma, K.; Hata, J. Usefulness of Ultrasonography for Diagnosis of Small Bowel Tumors. Medicine 2015, 94, e1464. [Google Scholar] [CrossRef]
- Faggian, A.; Fracella, M.R.; D’Alesio, G.; Alabiso, M.E.; Berritto, D.; Feragalli, B.; Miele, V.; Iasiello, F.; Grassi, R. Small-Bowel Neoplasms: Role of MRI Enteroclysis. Gastroenterol. Res. Pr. 2015, 2016, 9686815. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Sun, H.-L.; Luo, J.-H.; Ni, J.-Y.; Chen, D.; Jiang, X.-Y.; Zhou, J.-X.; Xu, L.-F. Interventional digital subtraction angiography for small bowel gastrointestinal stromal tumors with bleeding. World J. Gastroenterol. 2014, 20, 17955–17961. [Google Scholar] [CrossRef] [PubMed]
- Shyung, L.-R.; Lin, S.-C.; Shih, S.-C.; Chang, W.-H.; Chu, C.-H.; Wang, T.-E. Proposed Scoring System to Determine Small Bowel Mass Lesions Using Capsule Endoscopy. J. Formos. Med. Assoc. 2009, 108, 533–538. [Google Scholar] [CrossRef]
- Yang, Y.J. The Future of Capsule Endoscopy: The Role of Artificial Intelligence and Other Technical Advancements. Clin. Endosc. 2020, 53, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Alemanni, L.V.; Fabbri, S.; Rondonotti, E.; Mussetto, A. Recent developments in small bowel endoscopy: The “black box” is now open! Clin. Endosc. 2022, 55, 473–479. [Google Scholar] [CrossRef]
- Mărgăritescu, N.D.; Ciobanu, M.O.; Nemeş, R.N.; Ghelase, Ş.M.; Pleşea, R.M.; Georgescu, I.; Voinea, B.; Pleşea, I.E.; Chiuțu, L.C. The morphological profile of small bowel tumors—Our experience. Rom. J. Morphol. Embryol. 2016, 57, 1241–1252. [Google Scholar]
- Tarcoveanu, E.; Georgescum, S.; Vasilescu, A.; Danila, N.; Lupascu, C.; Dimofte, G.; Neacsu, C.N.; Moldovanu, R. Small bowel tumours from barium meal to capsule endoscopy and from open to laparoscopic approach. Chirurgia 2011, 106, 451–464. [Google Scholar]
- Negoi, I.; Paun, S.; Hostiuc, S.; Stoica, B.; Tanase, I.; Negoi, R.I.; Beuran, M. Most small bowel cancers are revealed by a complication. Einstein 2015, 13, 500–505. [Google Scholar] [CrossRef]
- Oweira, H.; Abdel-Rahman, O.; Mehrabi, A.; Reissfelder, C. Assessment of the external validity of the AJCC 8th staging system for small intestinal adenocarcinoma: A time to reconsider the role of tumor location? J. Gastrointest. Oncol. 2019, 10, 421–428. [Google Scholar] [CrossRef]
- Sánchez-Ramón, A.; Cerino-Palomino, V.; Medina-Franco, H. Tumores de intestino delgado: Experiencia en el Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”. Rev. Gastroenterol. Mex. 2012, 77, 181–185. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Zheng, C.; Chen, Y.; Xu, Q.; Ma, J.; Yuan, W.; Jiang, Q.; Zhao, Y.; Zhang, J.; Che, X.; et al. Clinicopathologic features, surgical treatments, and outcomes of small bowel tumors: A retrospective study in China. Int. J. Surg. 2017, 43, 145–154. [Google Scholar] [CrossRef]
- Rondonotti, E.; Pennazio, M.; Toth, E.; Menchen, P.; Riccioni, M.E.; De Palma, G.D.; Scotto, F.; De Looze, D.; Pachofsky, T.; Tacheci, I.; et al. Small-bowel neoplasms in patients undergoing video capsule endoscopy: A multicenter European study. Endoscopy 2008, 40, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Yoo, A.Y.; Lee, B.J.; Kim, W.S.; Kim, S.M.; Kim, S.H.; Joo, M.K.; Kim, H.J.; Park, J.-J. Clinicopathological Features of Small Bowel Tumors Diagnosed by Video Capsule Endoscopy and Balloon-Assisted Enteroscopy: A Single Center Experience. Clin. Endosc. 2021, 54, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Dabaja, B.S.; Suki, D.; Pro, B.; Bonnen, M.; Ajani, J. Adenocarcinoma of the small bowel: Presentation, prognostic factors, and outcome of 217 patients. Cancer 2004, 101, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Han, S.L.; Cheng, J.; Zhou, H.Z.; Guo, S.C.; Jia, Z.R.; Wang, P.F. Surgically treated primary malignant tumor of small bowel: A clinical analysis. World J. Gastroenterol. 2010, 16, 1527–1532. [Google Scholar] [CrossRef]
- Teufel, A.; Meindl-Beinker, N.M.; Hösel, P.; Gerken, M.; Roig, A.; Ebert, M.P.; Herr, W.; Scheiter, A.; Pauer, A.; Schlitt, H.J.; et al. Characteristics and outcome of patients with small bowel adenocarcinoma (SBA). J. Cancer Res. Clin. Oncol. 2023, 149, 4579–4590. [Google Scholar] [CrossRef]
- Tian, J.; Liu, J.; Guo, C.; Yang, X.; Yang, Y.; Gou, H.; Qiu, M.; Cao, D. Prognostic factors and treatment outcomes in patients with non-ampullary small bowel adenocarcinoma: Long-term analysis. Medicine 2019, 98, e15381. [Google Scholar] [CrossRef]
- Li, X.; Ying, H.; Cheng, Y.; Zhao, L.; Zhao, S.; Bai, C.; Zhou, J. Clinicopathological features and treatment outcomes of metastatic or locally unresectable small bowel adenocarcinoma. J. BUON 2019, 24, 2539–2545. [Google Scholar]

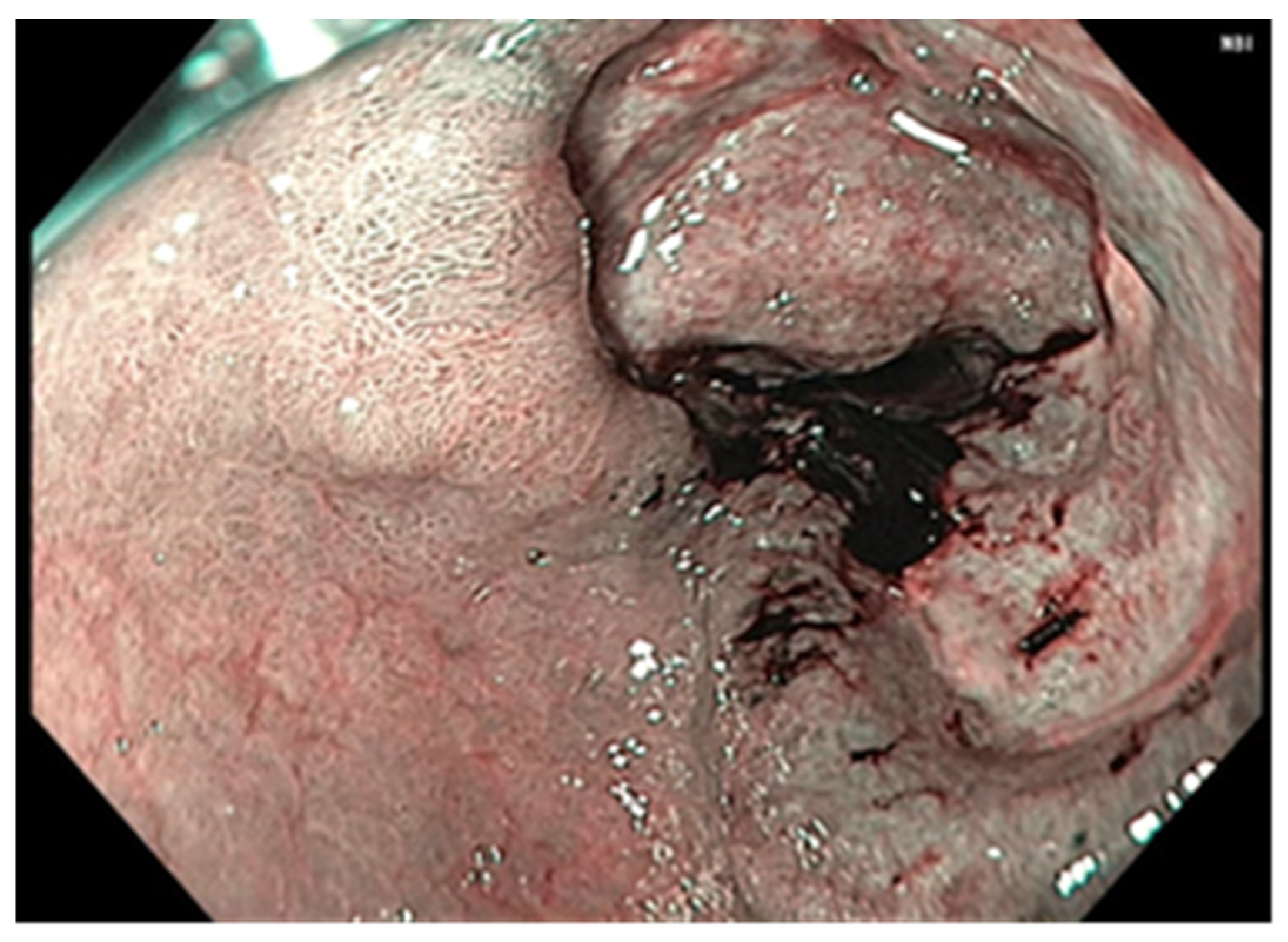
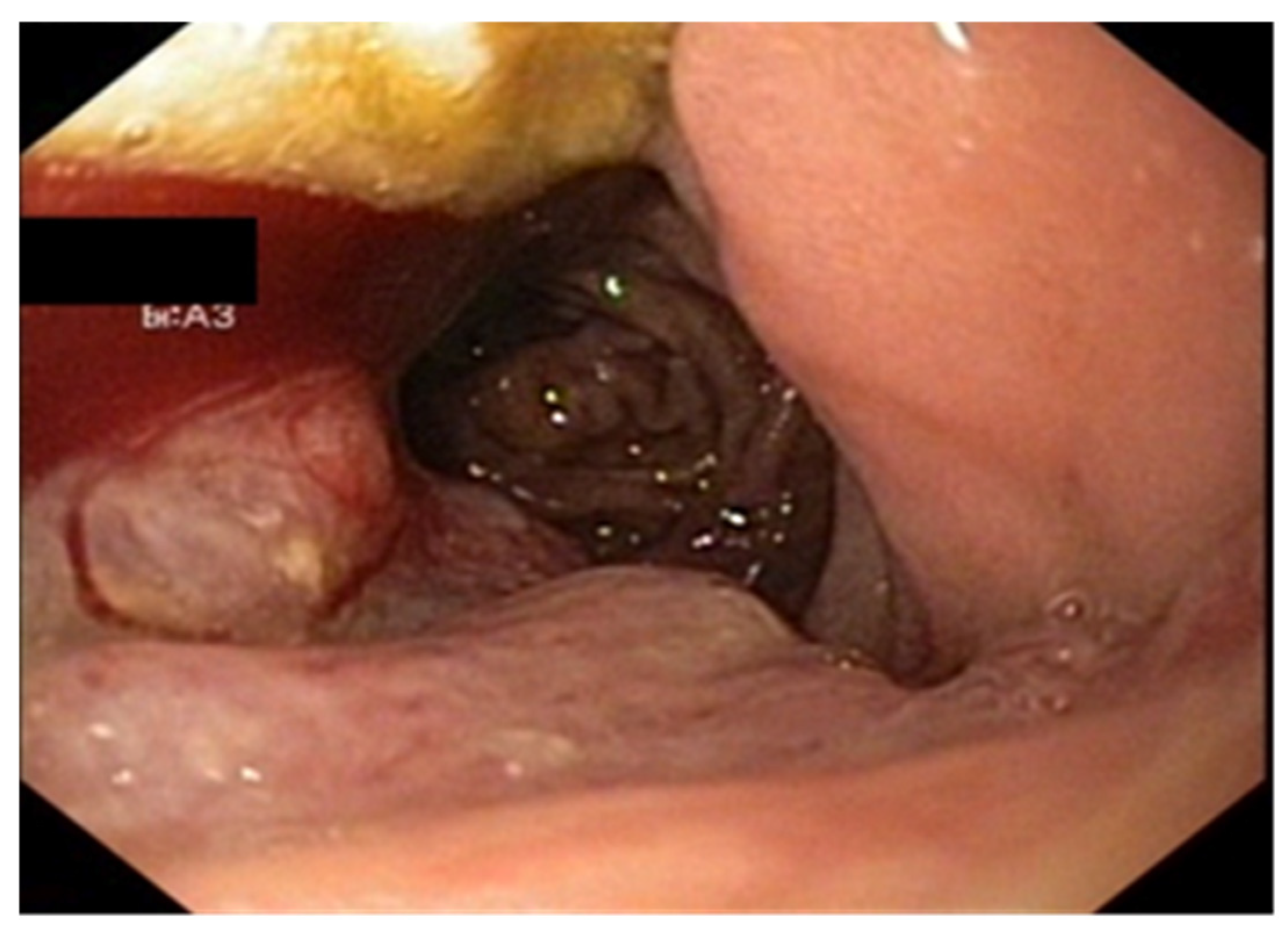
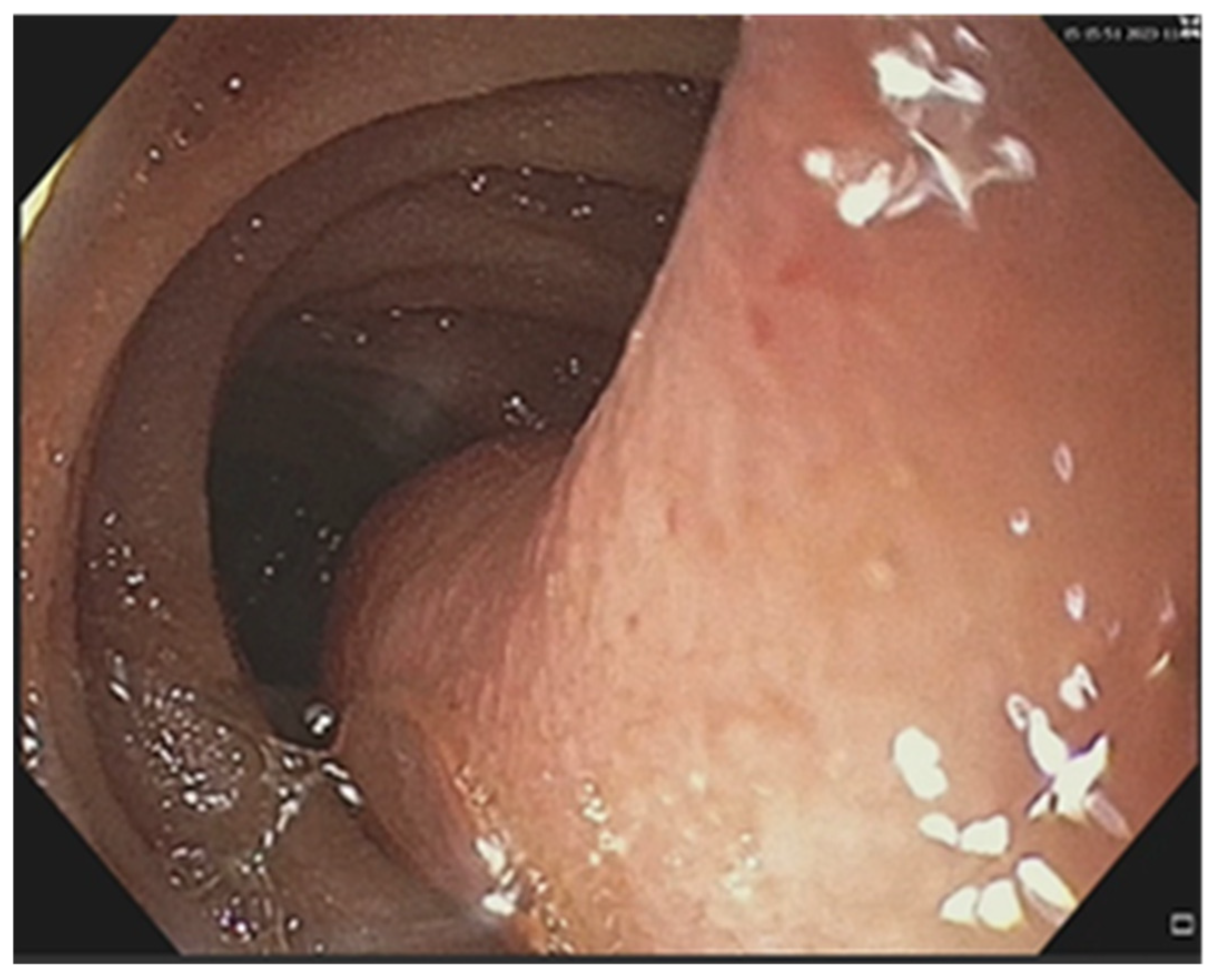
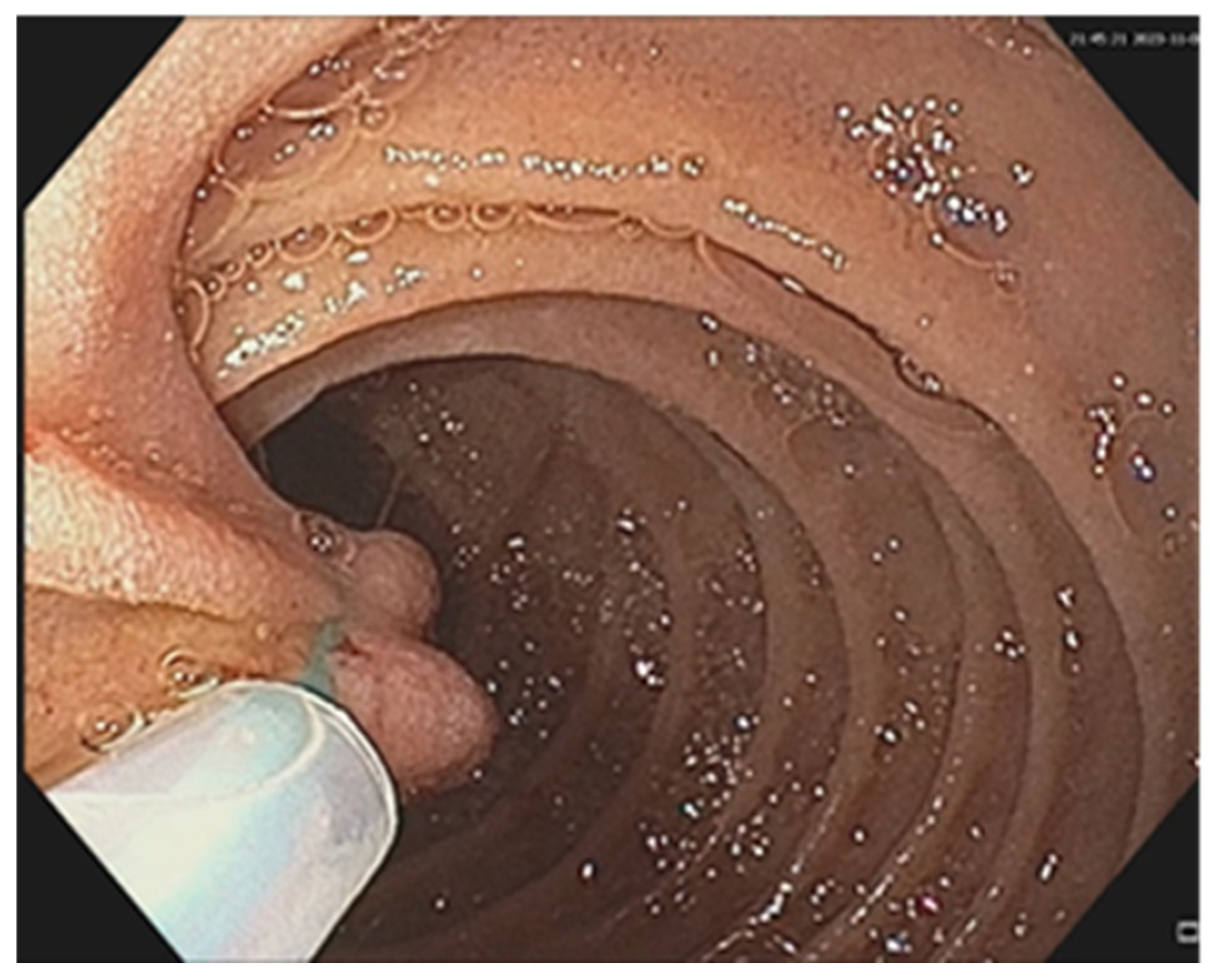
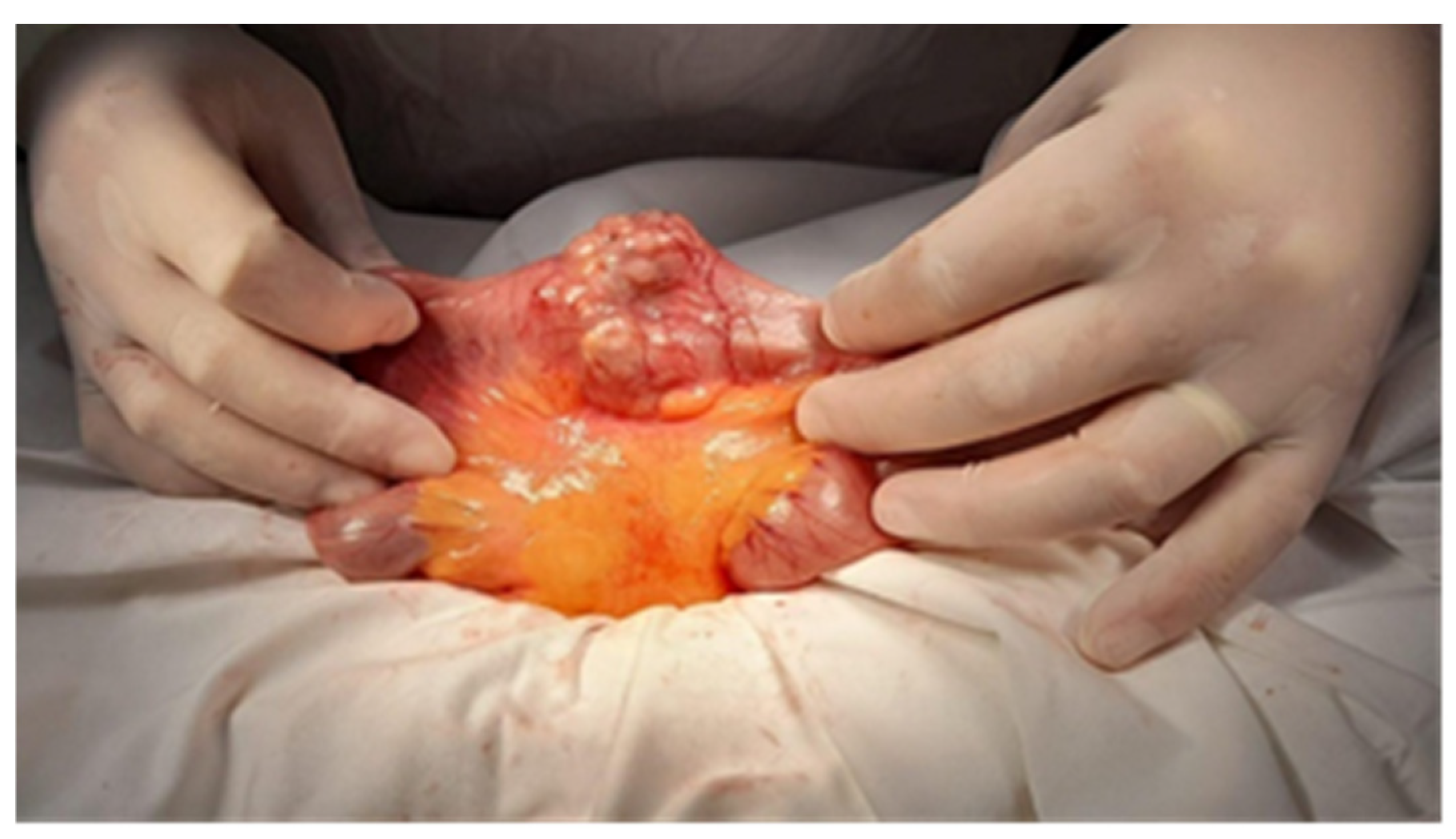
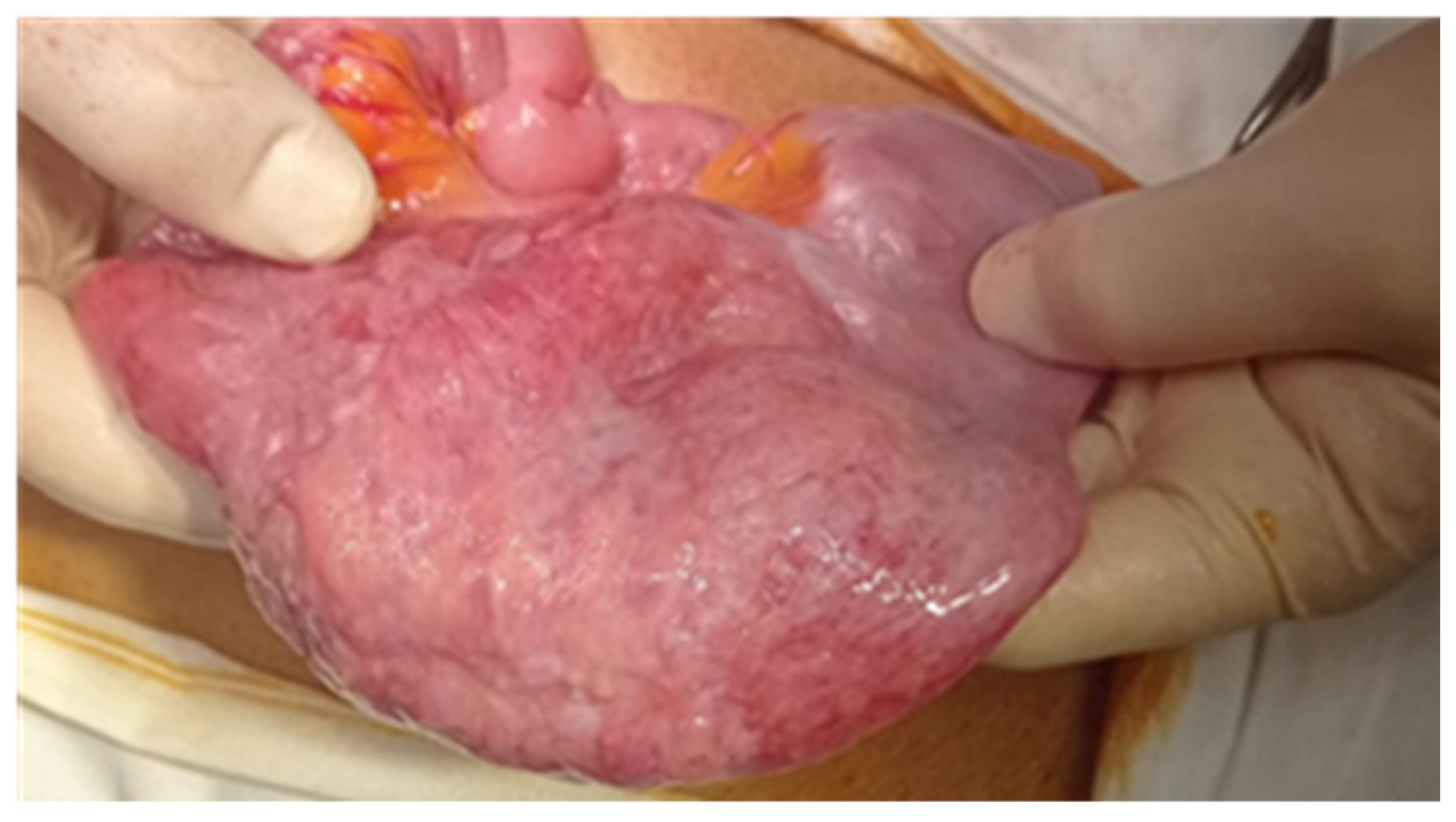
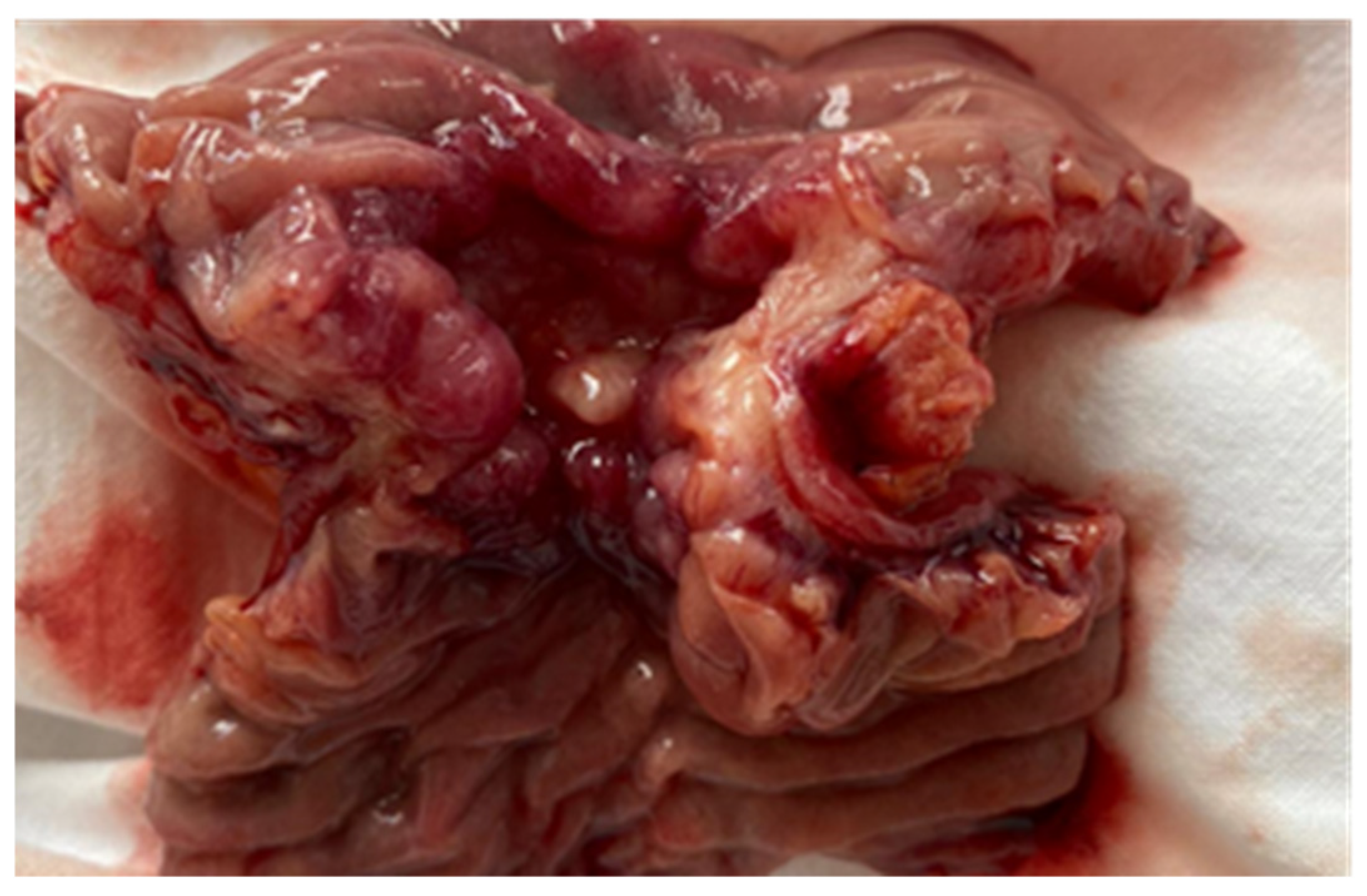
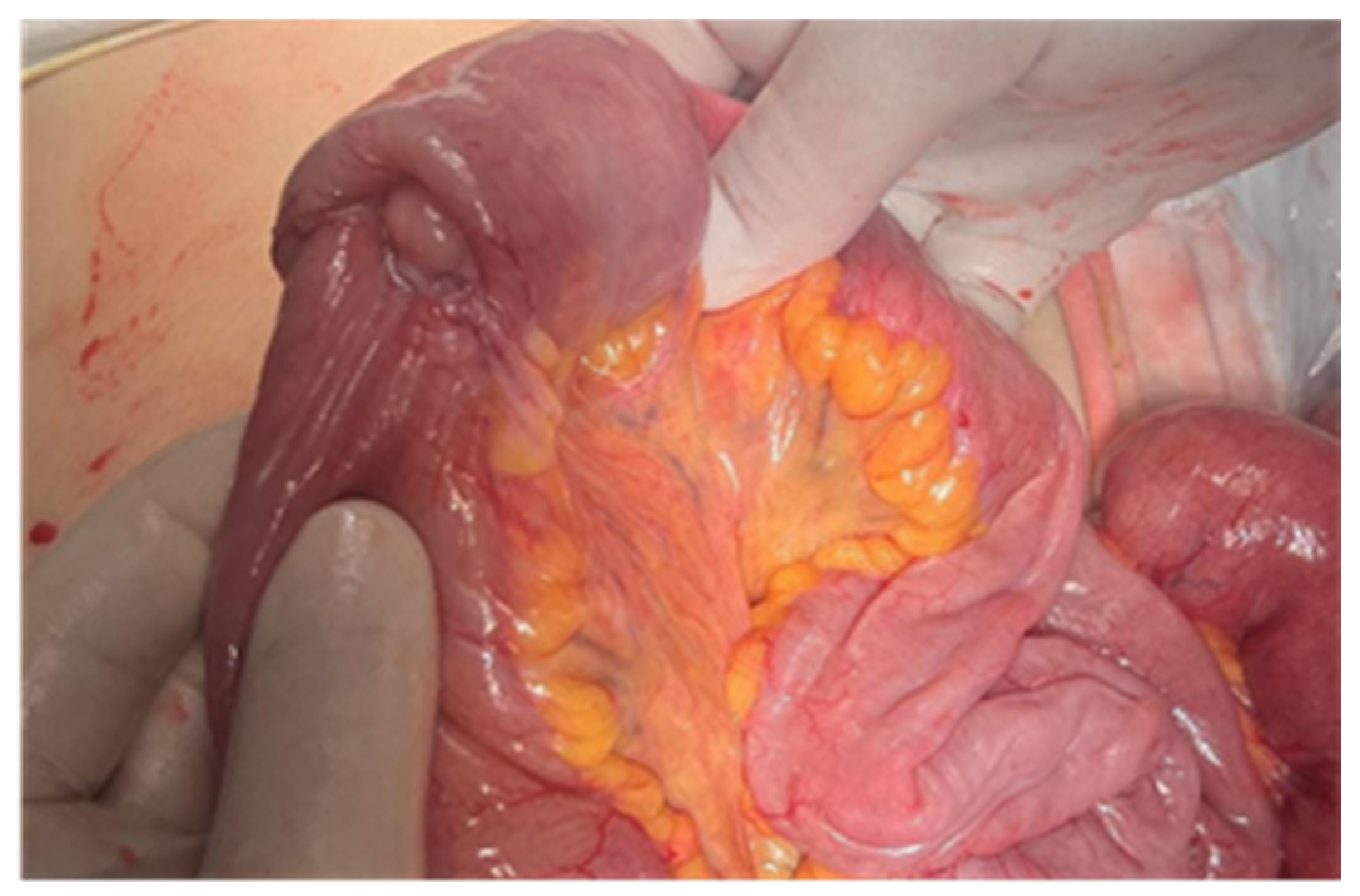

| No (%) | |
|---|---|
| Year of diagnosis | |
| 2017/2018/2019/2020/2021/2022/2023 | 6/13/11/5/14/11/20 |
| Age mean ± SD (min-max) | |
| Benign | 63.1 ± 9.4 (47–78) |
| Malignant | 64 ± 11.6 (34–85) |
| M/F (%) | |
| Benign | 14/8 (63.6) |
| Malignant | 36/22 (62.1) |
| Complains | |
| Site | |
| Duodenum | 28 (35) |
| Jejunum | 21 (26.4) |
| Jejuno-ileum | 3 (3.8) |
| Ileum | 23 (28.8) |
| Multiple | 5 (6.4) |
| Symptoms at admission | |
| Abdominal pain | 43 (53.8) |
| Nausea, vomiting | 25 (31.3) |
| Constipation/diarrhea | 13/1 (16.3/1.2) |
| Bleeding | 12 (15) |
| Anemia | 8 (10) |
| Weight loss | 3 (3.8) |
| Fatigue | 4 (5) |
| No symptoms | 6 (7.5) |
| Complications | |
| Occlusion | 12 (15) |
| Invagination | 6 (7.5) |
| Perforation | 4 (5) |
| CT scan number | 48 (60) |
| Tumor (%) | 39 (81.3) |
| Lymph nodes (% from malignant tumors) | 12 (33.3) |
| Metastasis (% from malignant tumors) | 22 (37.9) |
| Transabdominal ultrasound [No (%)] | 21 (26.3) |
| Tumor | 4 (19) |
| Endoscopy | |
| Upper digestive endoscopy | 34 (42.5) |
| Lower digestive endoscopy | 12 (15) |
| VCE | 4 (5) |
| Surgery | 52 (62.6) |
| Radical | 47 (58.8) |
| Palliative | 10 (12.5) |
| Laparoscopy with biopsy | 16 (20) |
| Endoscopic resection | 7 (8.8) |
| Pathology No (%) | |
| Benign | 22 (27.5) |
| GIST | 8 (36.4) |
| Fibroid inflammatory | 6 (27.5) |
| Epithelial (adenoma, hyperplastic) | 5 (22.7) |
| Lipoma | 1 (4.5) |
| Hemangioma | 1 (4.5) |
| Hamartoma | 1 (4.5) |
| Malignant | 58 (72.5) |
| Primitive carcinoma | 28 (48.3) |
| Adenocarcinoma | 24 (41.4) |
| Squamous cell carcinoma (1 mixed) | 2 (3.4) |
| Undifferentiated carcinoma | 1 (1.7) |
| Carcinosarcoma | 1 (1.7) |
| Lymphoma | 8 (13.8) |
| NET | 6 (10.3) |
| GIST | 6 (10.3) |
| Metastasis | 6 (10.3) |
| Carcinoma (1 from squamous cell carcinoma) | 3 (5.2) |
| Melanoma | 3 (5.2) |
| Grading (primary malignant SBT) | |
| G2 | 11 |
| G3 | 6 |
| G4 | 1 |
| Staging (primary malignant SBT) | |
| pT3 | 7 |
| pT4 | 5 |
| pN0 | 5 |
| pN1 | 4 |
| pN2 | 1 |
| M1/M0 | 14/22 |
| Invasion (primary malignant SBT) | |
| Vascular | 6 |
| Lymphatic | 3 |
| Perineural | 6 |
| In-hospital death [No (%)] | 12 (10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazacu, S.M.; Cârțu, D.; Popescu, M.; Streba, L.; Ungureanu, B.S.; Iovănescu, V.F.; Cimpoeru, M.; Mirea, C.S.; Surlin, V.M.; Mogoantă, S.; et al. Small Bowel Tumors: A 7-Year Study in a Tertiary Care Hospital. Cancers 2025, 17, 1465. https://doi.org/10.3390/cancers17091465
Cazacu SM, Cârțu D, Popescu M, Streba L, Ungureanu BS, Iovănescu VF, Cimpoeru M, Mirea CS, Surlin VM, Mogoantă S, et al. Small Bowel Tumors: A 7-Year Study in a Tertiary Care Hospital. Cancers. 2025; 17(9):1465. https://doi.org/10.3390/cancers17091465
Chicago/Turabian StyleCazacu, Sergiu Marian, Dan Cârțu, Mihai Popescu, Liliana Streba, Bogdan Silviu Ungureanu, Vlad Florin Iovănescu, Mihai Cimpoeru, Cecil Sorin Mirea, Valeriu Marian Surlin, Stelian Mogoantă, and et al. 2025. "Small Bowel Tumors: A 7-Year Study in a Tertiary Care Hospital" Cancers 17, no. 9: 1465. https://doi.org/10.3390/cancers17091465
APA StyleCazacu, S. M., Cârțu, D., Popescu, M., Streba, L., Ungureanu, B. S., Iovănescu, V. F., Cimpoeru, M., Mirea, C. S., Surlin, V. M., Mogoantă, S., & Florescu, M. M. (2025). Small Bowel Tumors: A 7-Year Study in a Tertiary Care Hospital. Cancers, 17(9), 1465. https://doi.org/10.3390/cancers17091465







