Suppression of Ovarian Cancer Cell Proliferation Is Associated with Upregulation of Cell-Matrix Adhesion Programs and Integrin-β4-Induced Cell Protection from Cisplatin
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. 3D Spheroid Formation and Matrigel Supplementation
2.3. Cell Proliferation Analysis
2.4. Patient-Derived Organoids
2.4.1. Organoid Derivation and Culture
2.4.2. RNA Sequencing of Patient-Derived Organoids
2.4.3. Scatter Plot and Heatmap Analyses
2.4.4. Drug Assays in Patient-Derived Organoids
2.4.5. Data Analysis and Statistics
2.5. Bioinformatics Analysis of TCGA Ovarian Cancer Transcriptomic Data
2.5.1. Data Retrieval and Preprocessing
2.5.2. t-SNE Visualization of Transcriptomic Data
2.5.3. Gene Set Variation Analysis (GSVA) and Correlation Analysis
2.5.4. Analysis of Cell Cycle Gene Expression by ITGB4 Levels in Ovarian Cancer
2.5.5. Gene Ontology (GO) Enrichment Analysis
2.5.6. Heatmap of RNA-Seq Differential Gene Expression (DEG)
2.6. RNA-Seq of Palbociclib-Treated Spheroids
2.6.1. Cell Treatment and Spheroid Formation
2.6.2. RNA Extraction and Quality Assessment
2.6.3. RNA Sequencing Workflow
2.6.4. Differential Expression Analysis
2.6.5. Gene Set Enrichment Analysis
2.7. Plasmids and Cloning
2.7.1. Plasmid Transformation and Expansion
2.7.2. Lentivirus Production
2.8. Western Blot Analysis
Quantification of Western Blot Data
2.9. Flow Cytometry
2.10. Statistical Analysis
3. Results
3.1. Examinations of Ovarian Cancer TCGA Datasets Reveal an Inverse Correlation Between Cell-Matrix Adhesion and Cell Cycle Gene Expression
3.2. ITGB4 mRNA Expression Changes with Cell Cycle Genes in Ovarian Cancer Patients
3.3. Integrin β4 Protein Expression Inversely Correlates with OC Cell Proliferation
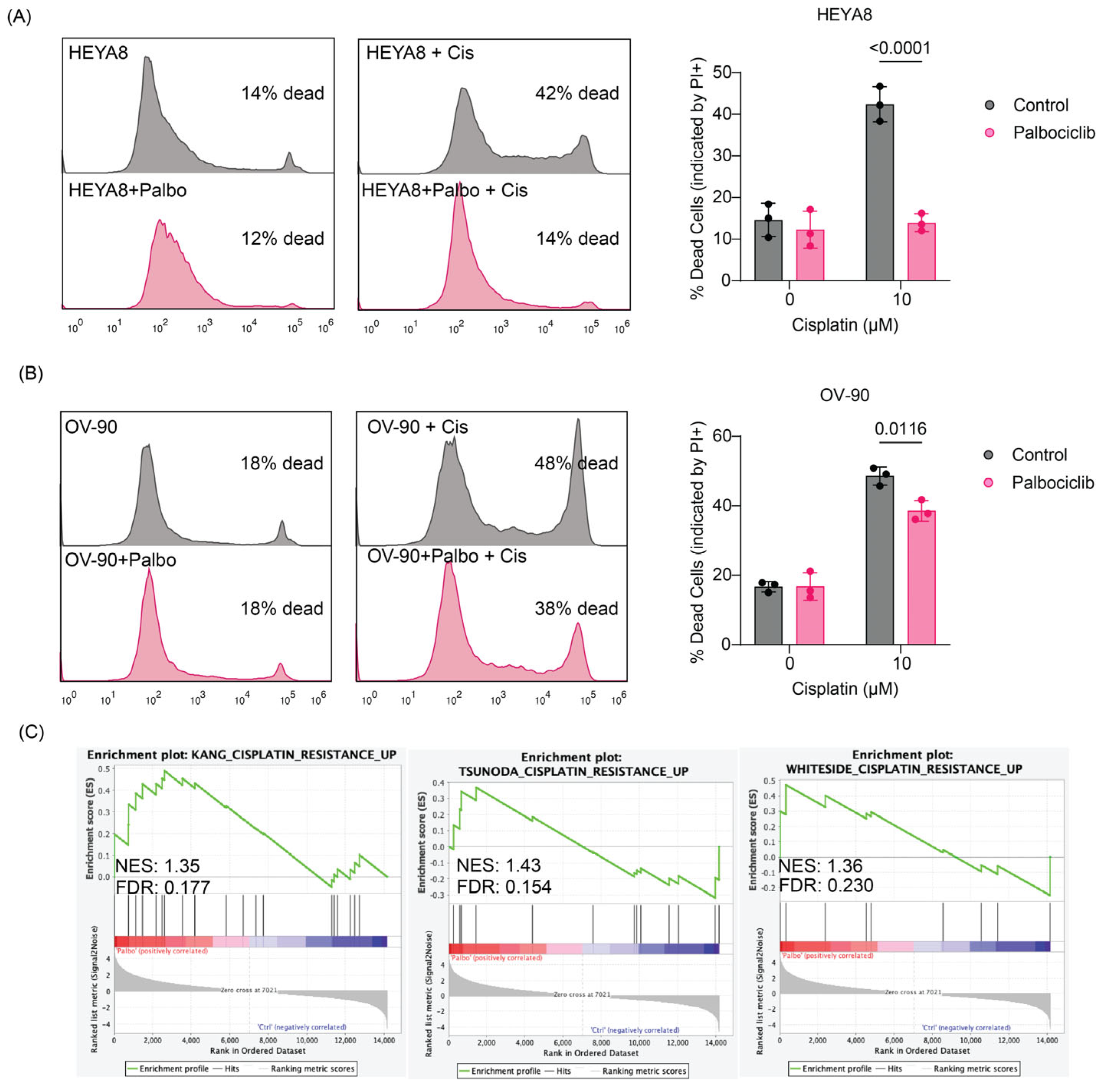
3.4. Inhibition of OC Cell Proliferation with Palbociclib Activates Cell-ECM Adhesion Programs, Expression of Integrin β4 and Induces Protection from Cisplatin
3.5. Palbociclib Treatment Modulates Cisplatin Sensitivity in Patient-Derived Ovarian Cancer Organoids
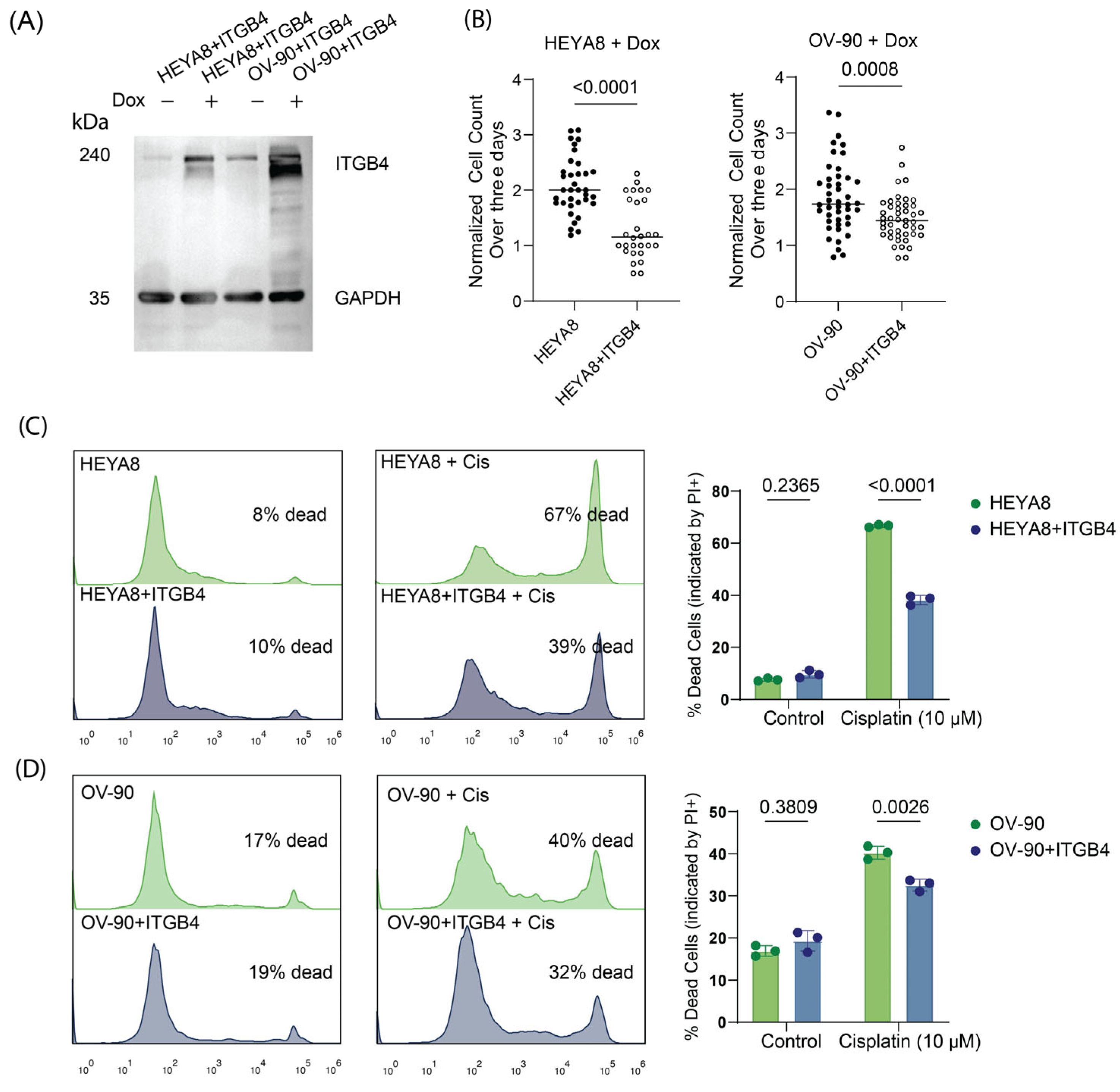
3.6. Overexpression of Integrin β4 Modulates Chemotherapy Response and Reduces Proliferation in Ovarian Cancer Cells
4. Discussion
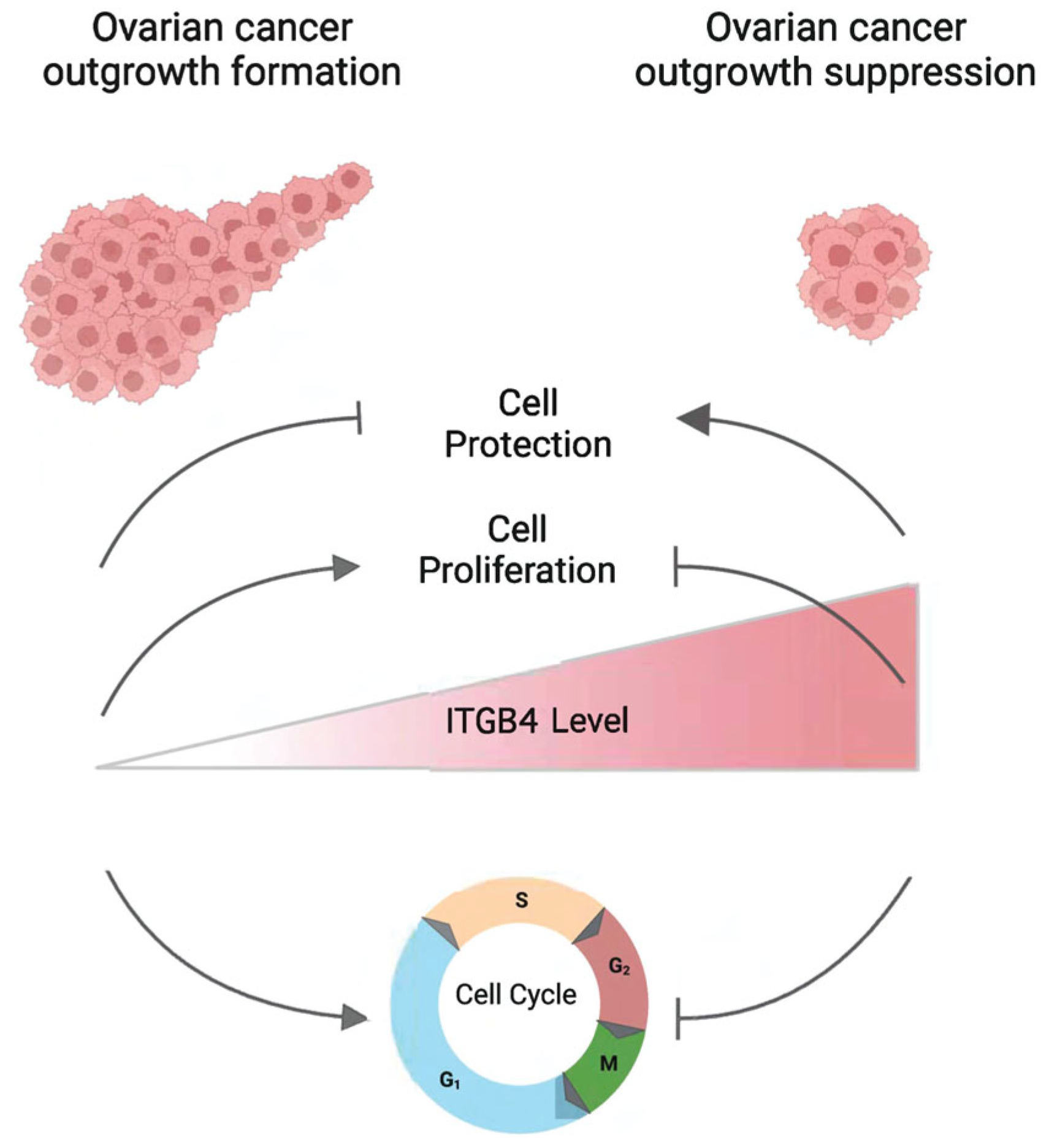
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garsed, D.W.; Pandey, A.; Fereday, S.; Kennedy, C.J.; Takahashi, K.; Alsop, K.; Hamilton, P.T.; Hendley, J.; Chiew, Y.E.; Traficante, N.; et al. The genomic and immune landscape of long-term survivors of high-grade serous ovarian cancer. Nat. Genet. 2022, 54, 1853–1864. [Google Scholar] [CrossRef]
- Alshehri, S.; Pavlovic, T.; Farsinejad, S.; Behboodi, P.; Quan, L.; Centeno, D.; Kung, D.; Rezler, M.; Lee, W.; Jasinski, P.; et al. Extracellular Matrix Modulates Outgrowth Dynamics in Ovarian Cancer. Adv. Biol. 2022, 6, e2200197. [Google Scholar] [CrossRef]
- Farsinejad, S.; Cattabiani, T.; Muranen, T.; Iwanicki, M. Ovarian Cancer Dissemination—A Cell Biologist’s Perspective. Cancers 2019, 11, 1957. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Pylayeva, Y.; Pepe, A.; Yoshioka, T.; Muller, W.J.; Inghirami, G.; Giancotti, F.G. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 2006, 126, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Muranen, T.; Iwanicki, M.P.; Curry, N.L.; Hwang, J.; DuBois, C.D.; Coloff, J.L.; Hitchcock, D.S.; Clish, C.B.; Brugge, J.S.; Kalaany, N.Y. Starved epithelial cells uptake extracellular matrix for survival. Nat. Commun. 2017, 8, 13989. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Ren, W.; Cui, Q.; Liang, H.; Yang, C.; Liu, W.; Wang, X.; Liu, X.; Shi, Y.; Feng, J.; et al. Integrin beta4 promotes DNA damage-related drug resistance in triple-negative breast cancer via TNFAIP2/IQGAP1/RAC1. eLife 2023, 12, RP88483. [Google Scholar] [CrossRef]
- Uphoff, C.C.; Drexler, H.G. Detection of mycoplasma contaminations. Methods Mol. Biol. 2005, 290, 13–23. [Google Scholar] [CrossRef]
- Kopper, O.; de Witte, C.J.; Lohmussaar, K.; Valle-Inclan, J.E.; Hami, N.; Kester, L.; Balgobind, A.V.; Korving, J.; Proost, N.; Begthel, H.; et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 2019, 25, 838–849. [Google Scholar] [CrossRef]
- Kessler, M.; Hoffmann, K.; Brinkmann, V.; Thieck, O.; Jackisch, S.; Toelle, B.; Berger, H.; Mollenkopf, H.J.; Mangler, M.; Sehouli, J.; et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat. Commun. 2015, 6, 8989. [Google Scholar] [CrossRef] [PubMed]
- Maenhoudt, N.; Defraye, C.; Boretto, M.; Jan, Z.; Heremans, R.; Boeckx, B.; Hermans, F.; Arijs, I.; Cox, B.; Van Nieuwenhuysen, E.; et al. Developing Organoids from Ovarian Cancer as Experimental and Preclinical Models. Stem Cell Rep. 2020, 14, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Hanzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdottir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstrale, M.; Laurila, E.; et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research, N. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Zeller, T.; Munnich, I.A.; Windisch, R.; Hilger, P.; Schewe, D.M.; Humpe, A.; Kellner, C. Perspectives of targeting LILRB1 in innate and adaptive immune checkpoint therapy of cancer. Front. Immunol. 2023, 14, 1240275. [Google Scholar] [CrossRef]
- Hoshino, A.; Boutboul, D.; Zhang, Y.; Kuehn, H.S.; Hadjadj, J.; Ozdemir, N.; Celkan, T.; Walz, C.; Picard, C.; Lenoir, C.; et al. Gain-of-function IKZF1 variants in humans cause immune dysregulation associated with abnormal T/B cell late differentiation. Sci. Immunol. 2022, 7, eabi7160. [Google Scholar] [CrossRef]
- Jacob, M.; Chang, L.; Pure, E. Fibroblast activation protein in remodeling tissues. Curr. Mol. Med. 2012, 12, 1220–1243. [Google Scholar] [CrossRef]
- Kang, H.C.; Kim, I.J.; Park, J.H.; Shin, Y.; Ku, J.L.; Jung, M.S.; Yoo, B.C.; Kim, H.K.; Park, J.G. Identification of genes with differential expression in acquired drug-resistant gastric cancer cells using high-density oligonucleotide microarrays. Clin. Cancer Res. 2004, 10, 272–284. [Google Scholar] [CrossRef]
- Tsunoda, T.; Koga, H.; Yokomizo, A.; Tatsugami, K.; Eto, M.; Inokuchi, J.; Hirata, A.; Masuda, K.; Okumura, K.; Naito, S. Inositol 1,4,5-trisphosphate (IP3) receptor type1 (IP3R1) modulates the acquisition of cisplatin resistance in bladder cancer cell lines. Oncogene 2005, 24, 1396–1402. [Google Scholar] [CrossRef]
- Whiteside, M.A.; Chen, D.T.; Desmond, R.A.; Abdulkadir, S.A.; Johanning, G.L. A novel time-course cDNA microarray analysis method identifies genes associated with the development of cisplatin resistance. Oncogene 2004, 23, 744–752. [Google Scholar] [CrossRef]
- Stewart, R.L.; O’Connor, K.L. Clinical significance of the integrin alpha6beta4 in human malignancies. Lab. Investig. 2015, 95, 976–986. [Google Scholar] [CrossRef]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Thangavel, C.; Boopathi, E.; Liu, Y.; Haber, A.; Ertel, A.; Bhardwaj, A.; Addya, S.; Williams, N.; Ciment, S.J.; Cotzia, P.; et al. RB Loss Promotes Prostate Cancer Metastasis. Cancer Res. 2017, 77, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.S.; Witkiewicz, A.K. The Strange Case of CDK4/6 Inhibitors: Mechanisms, Resistance, and Combination Strategies. Trends Cancer 2017, 3, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Thura, M.; Al-Aidaroos, A.Q.; Gupta, A.; Chee, C.E.; Lee, S.C.; Hui, K.M.; Li, J.; Guan, Y.K.; Yong, W.P.; So, J.; et al. Author Correction: PRL3-zumab as an immunotherapy to inhibit tumors expressing PRL3 oncoprotein. Nat. Commun. 2021, 12, 6431. [Google Scholar] [CrossRef]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, K.; Liu, P.; Geng, Y.; Wang, B.; Gan, W.; Guo, J.; Wu, F.; Chin, Y.R.; Berrios, C.; et al. Inhibition of Rb Phosphorylation Leads to mTORC2-Mediated Activation of Akt. Mol. Cell 2016, 62, 929–942. [Google Scholar] [CrossRef]
- Mercurio, A.M.; Bachelder, R.E.; Bates, R.C.; Chung, J. Autocrine signaling in carcinoma: VEGF and the alpha6beta4 integrin. Semin Cancer Biol. 2004, 14, 115–122. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Wang, L.; Agyin, J.; Tang, Y.; Lin, S.; Yeh, I.T.; De, K.; Sun, L.Z. Doxorubicin in combination with a small TGFbeta inhibitor: A potential novel therapy for metastatic breast cancer in mouse models. PLoS ONE 2010, 5, e10365. [Google Scholar] [CrossRef]
- Bierie, B.; Pierce, S.E.; Kroeger, C.; Stover, D.G.; Pattabiraman, D.R.; Thiru, P.; Liu Donaher, J.; Reinhardt, F.; Chaffer, C.L.; Keckesova, Z.; et al. Integrin-beta4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc. Natl. Acad. Sci. USA 2017, 114, E2337–E2346. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.M.; Busselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Huang Bartlett, C.; Cristofanilli, M. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 1672–1673. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.D.; Corcoran, R.B.; Engelman, J.A. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 2010, 28, 1075–1083. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farsinejad, S.; Centeno, D.; Savas-Carstens, J.; Polotaye, T.; Pavlovič, T.; Babvey, P.; Muranen, T.; Miedziarek, C.; Jasiński, P.; Dziabaszewska, E.; et al. Suppression of Ovarian Cancer Cell Proliferation Is Associated with Upregulation of Cell-Matrix Adhesion Programs and Integrin-β4-Induced Cell Protection from Cisplatin. Cancers 2025, 17, 1472. https://doi.org/10.3390/cancers17091472
Farsinejad S, Centeno D, Savas-Carstens J, Polotaye T, Pavlovič T, Babvey P, Muranen T, Miedziarek C, Jasiński P, Dziabaszewska E, et al. Suppression of Ovarian Cancer Cell Proliferation Is Associated with Upregulation of Cell-Matrix Adhesion Programs and Integrin-β4-Induced Cell Protection from Cisplatin. Cancers. 2025; 17(9):1472. https://doi.org/10.3390/cancers17091472
Chicago/Turabian StyleFarsinejad, Sadaf, Daniel Centeno, Jan Savas-Carstens, Teagan Polotaye, Tonja Pavlovič, Pouria Babvey, Taru Muranen, Cezary Miedziarek, Piotr Jasiński, Elżbieta Dziabaszewska, and et al. 2025. "Suppression of Ovarian Cancer Cell Proliferation Is Associated with Upregulation of Cell-Matrix Adhesion Programs and Integrin-β4-Induced Cell Protection from Cisplatin" Cancers 17, no. 9: 1472. https://doi.org/10.3390/cancers17091472
APA StyleFarsinejad, S., Centeno, D., Savas-Carstens, J., Polotaye, T., Pavlovič, T., Babvey, P., Muranen, T., Miedziarek, C., Jasiński, P., Dziabaszewska, E., Zaborowski, M. P., Lum, P. Y., Martin, L. A., & Iwanicki, M. P. (2025). Suppression of Ovarian Cancer Cell Proliferation Is Associated with Upregulation of Cell-Matrix Adhesion Programs and Integrin-β4-Induced Cell Protection from Cisplatin. Cancers, 17(9), 1472. https://doi.org/10.3390/cancers17091472





