Temporal Trends of Hyponatremia in Patients with Respiratory and Intrathoracic Cancers Treated with Chemotherapy and Immune Checkpoint Inhibitors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
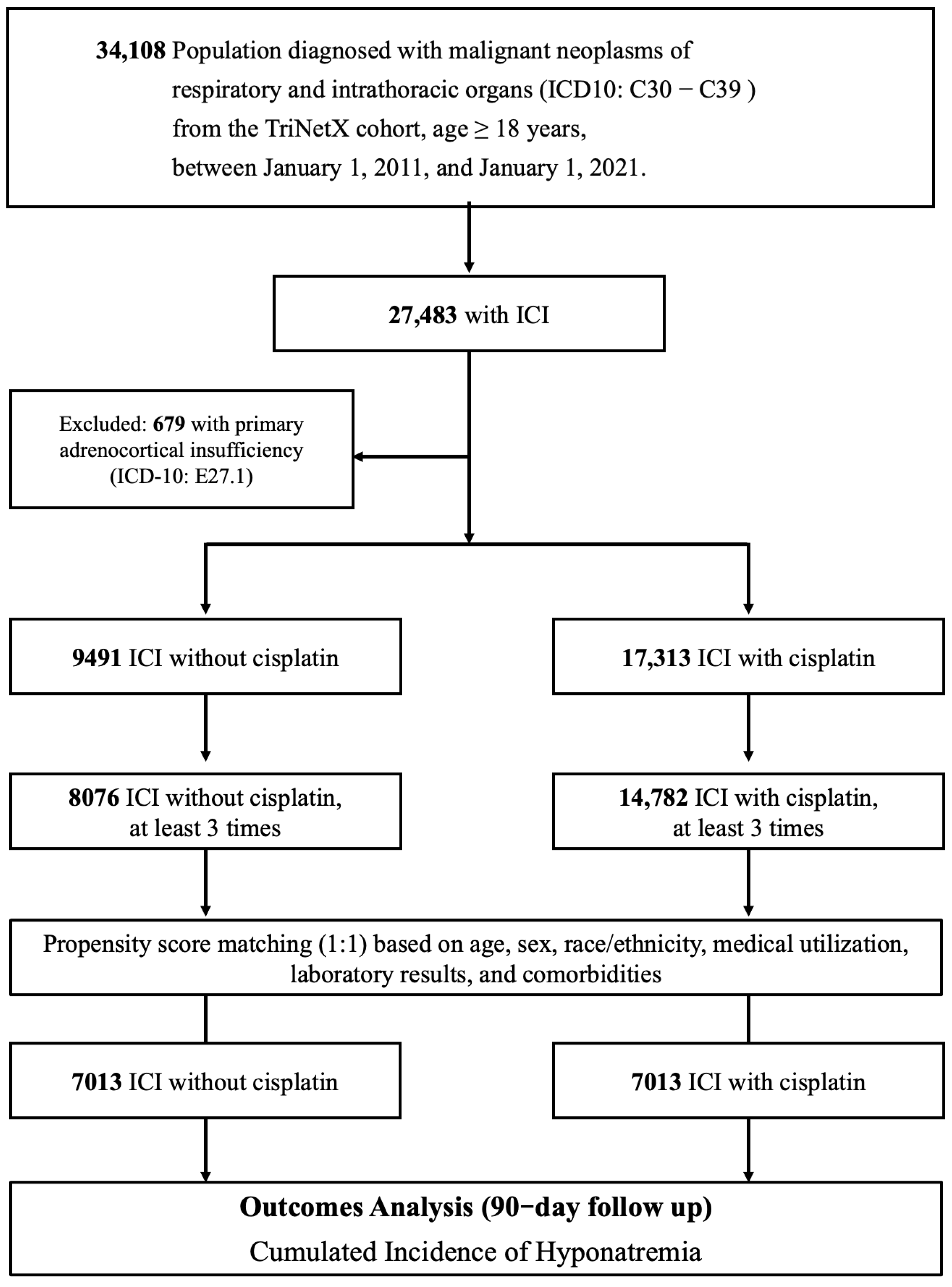
2.2. Study Cohorts
2.3. Index Date and Follow-Up Period
2.4. Data Analysis
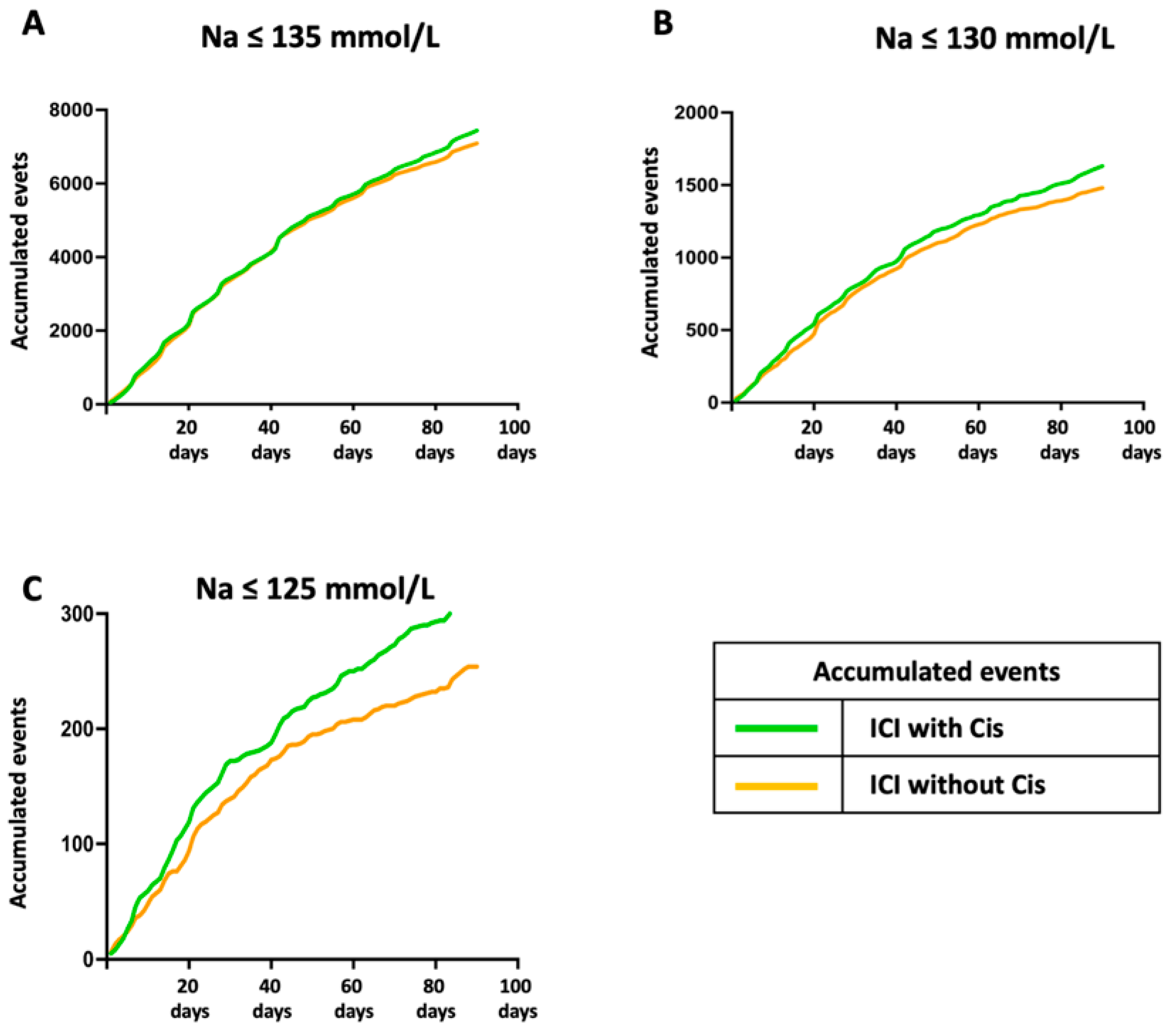
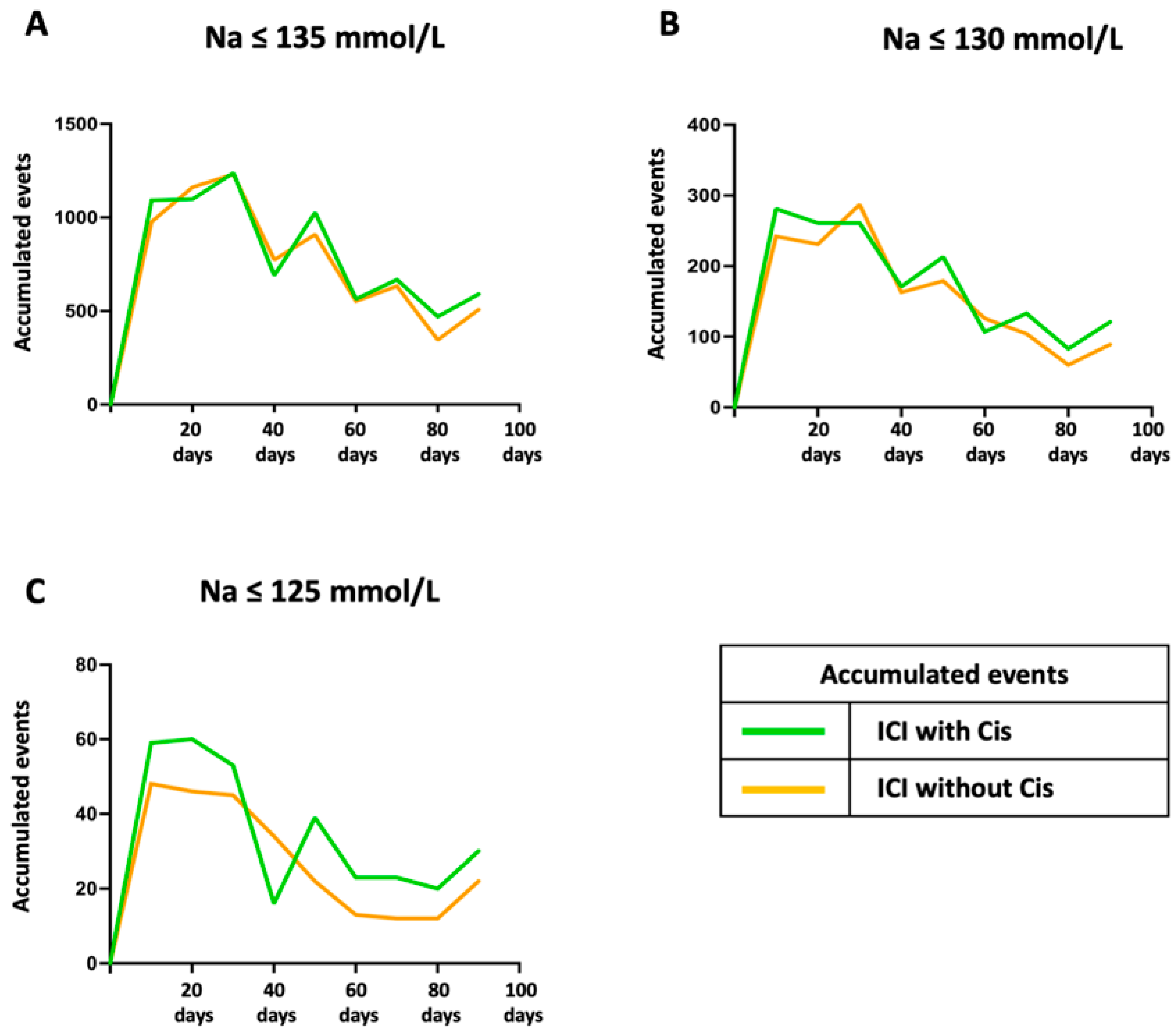
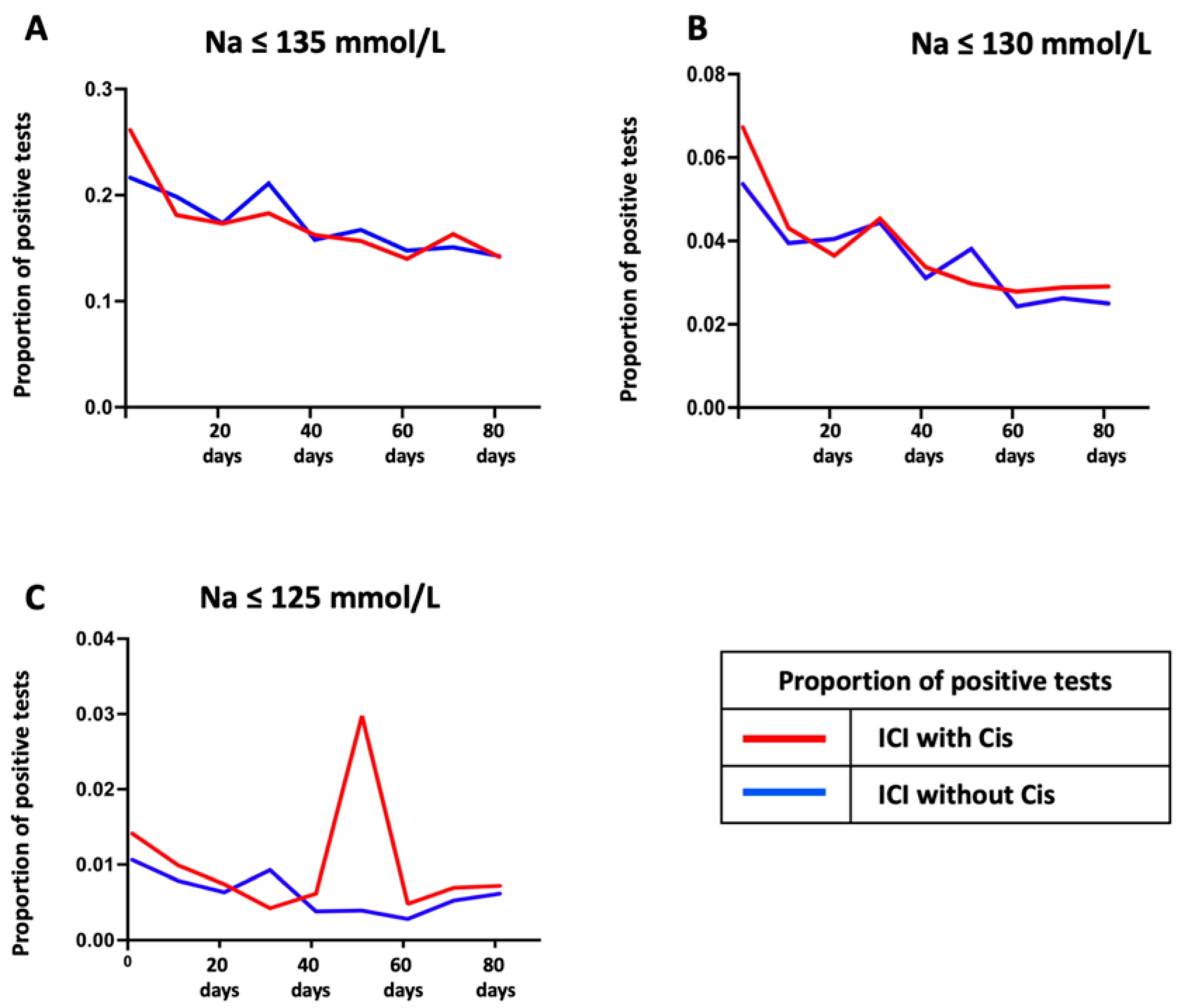
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTLA-4 | Cytotoxic T-lymphocyte associated protein 4 |
| eGFR | Directory of open access journals |
| HbA1c | Hemoglobin A1c |
| ICI | Immune checkpoint inhibitor |
| irAEs | Immune-related adverse events |
| LDL | Low-density lipoprotein |
| NSCLC | Non-small cell lung cancer |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| RSWS | Renal salt-wasting syndrome |
| SCLC | Small cell lung cancer |
| SIADH | Syndrome of inappropriate antidiuretic hormone secretion |
References
- Wojtukiewicz, M.Z.; Rek, M.M.; Karpowicz, K.; Górska, M.; Polityńska, B.; Wojtukiewicz, A.M.; Moniuszko, M.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Inhibitors of immune checkpoints-PD-1, PD-L1, CTLA-4-new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021, 40, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Franzin, R.; Netti, G.S.; Spadaccino, F.; Porta, C.; Gesualdo, L.; Stallone, G.; Castellano, G.; Ranieri, E. The Use of Immune Checkpoint Inhibitors in Oncology and the Occurrence of AKI: Where Do We Stand? Front. Immunol. 2020, 11, 574271. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B.A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S.K.; Poon, C.; Gant, K.L.; et al. Immune checkpoint therapy—Current perspectives and future directions. Cell 2023, 186, 1652–1669. [Google Scholar] [CrossRef]
- Atkins, M.B.; Clark, J.I.; Quinn, D.I. Immune checkpoint inhibitors in advanced renal cell carcinoma: Experience to date and future directions. Ann. Oncol. 2017, 28, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Doshi, S.M.; Shah, P.; Lei, X.; Lahoti, A.; Salahudeen, A.K. Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. Am. J. Kidney Dis. 2012, 59, 222–228. [Google Scholar] [CrossRef]
- Hansen, O.; Sørensen, P.; Hansen, K.H. The occurrence of hyponatremia in SCLC and the influence on prognosis: A retrospective study of 453 patients treated in a single institution in a 10-year period. Lung Cancer 2010, 68, 111–114. [Google Scholar] [CrossRef]
- Sandfeld-Paulsen, B.; Aggerholm-Pedersen, N.; Winther-Larsen, A. Hyponatremia in lung cancer: Incidence and prognostic value in a Danish population-based cohort study. Lung Cancer 2021, 153, 42–48. [Google Scholar] [CrossRef]
- Karaoğlan, B.B.; Yekedüz, E.; Yazgan, S.C.; Mocan, E.E.; Köksoy, E.B.; Yaşar, H.A.; Şenler, F.; Utkan, G.; Demirkazık, A.; Akbulut, H.; et al. Impact of low sodium values on survival outcomes of patients with cancer receiving immune checkpoint inhibitors. Immunotherapy 2024, 16, 821–828. [Google Scholar] [CrossRef]
- Sahay, M.; Sahay, R. Hyponatremia: A practical approach. Indian J. Endocrinol. Metab. 2014, 18, 760–771. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Osterlind, K.; Andersen, P.K. Prognostic factors in small cell lung cancer: Multivariate model based on 778 patients treated with chemotherapy with or without irradiation. Cancer Res. 1986, 46, 4189–4194. [Google Scholar] [PubMed]
- Allan, S.G.; Stewart, M.E.; Love, S.; Cornbleet, M.A.; Smyth, J.F.; Leonard, R.C. Prognosis at presentation of small cell carcinoma of the lung. Eur. J. Cancer 1990, 26, 703–705. [Google Scholar] [CrossRef]
- Bartalis, E.; Gergics, M.; Tinusz, B.; Földi, M.; Kiss, S.; Németh, D.; Solymár, M.; Szakács, Z.; Hegyi, P.; Mezösi, E.; et al. Prevalence and Prognostic Significance of Hyponatremia in Patients With Lung Cancer: Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 671951. [Google Scholar] [CrossRef]
- Selmer, C.; Madsen, J.C.; Torp-Pedersen, C.; Gislason, G.H.; Faber, J. Hyponatremia, all-cause mortality, and risk of cancer diagnoses in the primary care setting: A large population study. Eur. J. Intern. Med. 2016, 36, 36–43. [Google Scholar] [CrossRef]
- Brouns, S.H.; Dortmans, M.K.; Jonkers, F.S.; Lambooij, S.L.; Kuijper, A.; Haak, H.R. Hyponatraemia in elderly emergency department patients: A marker of frailty. Neth. J. Med. 2014, 72, 311–317. [Google Scholar] [PubMed]
- Holland-Bill, L.; Christiansen, C.F.; Heide-Jørgensen, U.; Ulrichsen, S.P.; Ring, T.; Jørgensen, J.O.; Sørensen, H.T. Hyponatremia and mortality risk: A Danish cohort study of 279 508 acutely hospitalized patients. Eur. J. Endocrinol. 2015, 173, 71–81. [Google Scholar] [CrossRef]
- Hayat, M.J.; Higgins, M. Understanding poisson regression. J. Nurs. Educ. 2014, 53, 207–215. [Google Scholar] [CrossRef]
- Seethapathy, H.; Rusibamayila, N.; Chute, D.F.; Lee, M.; Strohbehn, I.; Zubiri, L.; Faje, A.T.; Reynolds, K.L.; Jhaveri, K.D.; Sise, M.E. Hyponatremia and other electrolyte abnormalities in patients receiving immune checkpoint inhibitors. Nephrol. Dial. Transplant. 2021, 36, 2241–2247. [Google Scholar] [CrossRef]
- Iyer, A.V.; Krasnow, S.H.; Dufour, D.R.; Arcenas, A.S. Sodium-Wasting Nephropathy Caused by Cisplatin in a Patient with Small-Cell Lung Cancer. Clin. Lung Cancer 2003, 5, 187–189. [Google Scholar] [CrossRef]
- Bischoff, J.; Fries, C.; Heer, A.; Hoffmann, F.; Meyer, C.; Landsberg, J.; Fenske, W.K. It’s Not Always SIAD: Immunotherapy-Triggered Endocrinopathies Enter the Field of Cancer-Related Hyponatremia. J. Endocr. Soc. 2022, 6, bvac036. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.M.; Perazella, M.A. Immune Checkpoint Inhibitors and Immune-Related Adverse Renal Events. Kidney Int. Rep. 2020, 5, 1139–1148. [Google Scholar] [CrossRef]
- Donald, D.M.; Sherlock, M.; Thompson, C.J. Hyponatraemia and the syndrome of inappropriate antidiuresis (SIAD) in cancer. Endocr. Oncol. 2022, 2, R78–R89. [Google Scholar] [CrossRef]
- Desikan, S.P.; Varghese, R.; Kamoga, R.; Desikan, R. Acute hyponatremia from immune checkpoint inhibitor therapy for non–small cell lung cancer. Postgrad. Med. J. 2020, 96, 570–571. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Cao, L.; Joshi, P.; Sumoza, D. Renal salt-wasting syndrome in a patient with cisplatin-induced hyponatremia: Case report. Am. J. Clin. Oncol. 2002, 25, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Ezoe, Y.; Mizusawa, J.; Katayama, H.; Kataoka, K.; Muto, M. An integrated analysis of hyponatremia in cancer patients receiving platinum-based or nonplatinum-based chemotherapy in clinical trials (JCOG1405-A). Oncotarget 2018, 9, 6595–6606. [Google Scholar] [CrossRef][Green Version]
- Sandfeld-Paulsen, B.; Aggerholm-Pedersen, N.; Winther-Larsen, A. Hyponatremia as a prognostic factor in non-small cell lung cancer: A systematic review and meta-analysis. Transl. Lung Cancer Res. 2021, 10, 651–661. [Google Scholar] [CrossRef]
- Prat, P.; Bou, P.; Bosch, L.; Torrente, C. Pain related syndrome of inappropriate antidiuretic hormone secretion in a kitten. Top. Companion Anim. Med. 2024, 63, 100908. [Google Scholar] [CrossRef]
- Pillai, B.P.; Unnikrishnan, A.G.; Pavithran, P.V. Syndrome of inappropriate antidiuretic hormone secretion: Revisiting a classical endocrine disorder. Indian J. Endocrinol. Metab. 2011, 15 (Suppl. 3), S208–S215. [Google Scholar] [CrossRef] [PubMed]
- Jessel, S.; Weiss, S.A.; Austin, M.; Mahajan, A.; Etts, K.; Zhang, L.; Aizenbud, L.; Perdigoto, A.L.; Hurwitz, M.; Sznol, M.; et al. Immune Checkpoint Inhibitor-Induced Hypophysitis and Patterns of Loss of Pituitary Function. Front. Oncol. 2022, 12, 836859. [Google Scholar] [CrossRef]
- Corsello, S.M.; Barnabei, A.; Marchetti, P.; De Vecchis, L.; Salvatori, R.; Torino, F. Endocrine side effects induced by immune checkpoint inhibitors. J. Clin. Endocrinol. Metab. 2013, 98, 1361–1375. [Google Scholar] [CrossRef] [PubMed]
- Deligiorgi, M.V.; Siasos, G.; Vergadis, C.; Trafalis, D.T. Central diabetes insipidus related to anti-programmed cell-death 1 protein active immunotherapy. Int. Immunopharmacol. 2020, 83, 106427. [Google Scholar] [CrossRef]
- Angelousi, A.; Papalexis, P.; Karampela, A.; Marra, M.; Misthos, D.; Ziogas, D.; Gogas, H. Diabetes insipidus: A rare endocrine complication of immune check point inhibitors: A case report and literature review. Exp. Ther. Med. 2023, 25, 10. [Google Scholar] [CrossRef]
- Tan, M.H.; Iyengar, R.; Mizokami-Stout, K.; Yentz, S.; MacEachern, M.P.; Shen, L.Y.; Redman, B.; Gianchandani, R. Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: A scoping review of case reports. Clin. Diabetes Endocrinol. 2019, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Zhai, Y.; Ye, X.; Hu, F.; Xu, J.; Guo, X.; Zhuang, Y.; He, J. Endocrine toxicity of immune checkpoint inhibitors: A real-world study leveraging US Food and Drug Administration adverse events reporting system. J. Immunother. Cancer 2019, 7, 286. [Google Scholar] [CrossRef]
- Castillo, J.J.; Grohé, C.; Verbalis, J.G. Cancer-Related Hyponatremia: Enhancing Recognition and Improving Management. Clin. Lung Cancer 2016, 17, e29. [Google Scholar] [CrossRef]
- Rossi, G.; Pezzuto, A.; Sini, C.; Tuzi, A.; Citarella, F.; McCusker, M.G.; Nigro, O.; Tanda, E.; Russo, A. Concomitant medications during immune checkpoint blockage in cancer patients: Novel insights in this emerging clinical scenario. Crit. Rev. Oncol. Hematol. 2019, 142, 26–34. [Google Scholar] [CrossRef]
- Stuart, M.J.; Cuaso, C.; Miller, M.; Oski, F.A. Syndrome of recurrent increased secretion of antidiuretic hormone following multiple doses of vincristine. Blood 1975, 45, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Berardi, R.; Santoni, M.; Rinaldi, S.; Nunzi, E.; Smerilli, A.; Caramanti, M.; Morgese, F.; Torniai, M.; Savini, A.; Fiordoliva, I.; et al. Risk of Hyponatraemia in Cancer Patients Treated with Targeted Therapies: A Systematic Review and Meta-Analysis of Clinical Trials. PLoS ONE 2016, 11, e0152079. [Google Scholar] [CrossRef] [PubMed]
- Gross, P. Clinical management of SIADH. Ther. Adv. Endocrinol. Metab. 2012, 3, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Kafley, S.; Tamrakar, S.; Samra, M.; Shanmugar, S.; Gupta, I. Immune Checkpoint Inhibitors and Endocrine Disruption: A Case of Hyponatremia and Adrenal Insufficiency. Cureus 2024, 16, e70089. [Google Scholar] [CrossRef]
- Pham, P.C.; Reddy, P.; Qaqish, S.; Kamath, A.; Rodriguez, J.; Bolos, D.; Zalom, M.; Pham, P.T. Cisplatin-Induced Renal Salt Wasting Requiring over 12 Liters of 3% Saline Replacement. Case Rep. Nephrol. 2017, 2017, 8137078. [Google Scholar] [CrossRef]
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| ICI with Cisplatin (n = 14,782) | ICI Without Cisplatin Combination (n = 8076) | Std. Diff. | ICI with Cisplatin (n = 7013) | ICI Without Cisplatin Combination (n = 7013) | Std. Diff. | |
| Demographics | ||||||
| Age at index date (mean ± SD) | 65.3 ± 9.5 | 67.0 ± 10.5 | 0.167 | 66.7 ± 9.3 | 66.8 ± 10.4 | 0.004 |
| Male (%) | 52.1% | 55.0% | 0.059 | 53.5% | 53.6% | 0.002 |
| White (%) | 67.7% | 67.2% | 0.009 | 66.8% | 67.2% | 0.009 |
| Black or African American (%) | 10.0% | 9.0% | 0.034 | 10.3% | 9.8% | 0.018 |
| Comorbidities (%) | ||||||
| Hypertensive diseases | 26.5% | 25.4% | 0.026 | 24.5% | 25.8% | 0.032 |
| Chronic lower respiratory diseases | 23.1% | 17.8% | 0.132 | 18.6% | 19.2% | 0.015 |
| Diabetes mellitus | 10.2 | 10.2 | 0.132 | 9.5 | 10.4 | 0.029 |
| Ischemic heart diseases | 9.4 | 9.5 | 0.004 | 9.2 | 9.6 | 0.014 |
| Influenza and pneumonia | 7.1 | 6.1 | 0.041 | 6.0 | 6.5 | 0.021 |
| Malignant neoplasms of skin | 2.0 | 11.0 | 0.370 | 4.3 | 4.6 | 0.012 |
| Cerebrovascular diseases | 3.9 | 4.1 | 0.011 | 4.1 | 4.1 | 0.001 |
| Medications (%) | ||||||
| Blood glucose regulation agents | 17.2% | 13.2% | 0.111 | 13.2% | 13.7% | 0.013 |
| Antiarrhythmics | 31.1% | 23.9% | 0.163 | 24.8% | 25.2% | 0.009 |
| Beta-blockers | 12.6% | 13.8% | 0.036 | 13.2% | 13.8% | 0.019 |
| ACEI | 5.3% | 6.4% | 0.044 | 5.7% | 5.9% | 0.011 |
| ARB | 4.4% | 4.6% | 0.007 | 4.2% | 4.6% | 0.021 |
| CCB | 8.3% | 8.3% | <0.001 | 8.1% | 8.4% | 0.011 |
| Loop diuretics | 6.9% | 7.3% | 0.015 | 6.8% | 7.3% | 0.019 |
| Thiazides diuretics | 3.6% | 3.4% | 0.011 | 3.0% | 3.4% | 0.019 |
| Laboratory, Mean ± SD | ||||||
| Sodium, mmol/L | 137.5 ± 3.6 | 137.7 ± 3.7 | 0.063 | 137.5 ± 3.7 | 137.7 ± 3.8 | 0.031 |
| Potassium, mmol/L | 4.2 ± 0.5 | 4.2 ± 0.5 | 0.087 | 4.2 ± 0.5 | 4.2 ± 0.5 | 0.056 |
| Creatinine, mg/dL | 0.9 ± 0.4 | 1.0 ± 0.7 | 0.141 | 0.9 ± 0.4 | 1.0 ± 0.7 | 0.108 |
| Glucose, mg/dL | 119.1 ± 42.0 | 117.5 ± 42.2 | 0.037 | 120.0 ± 43.4 | 117.8 ± 42.6 | 0.051 |
| HbA1c, % | 6.9 ± 2.1 | 6.9 ± 2.1 | 0.013 | 6.8 ± 1.8 | 6.9 ± 2.2 | 0.070 |
| Albumin, g/dL | 3.6 ± 0.6 | 3.6 ± 0.6 | 0.052 | 3.6 ± 0.6 | 3.6 ± 0.6 | 0.070 |
| LDL, mg/dL | 93.6 ± 41.9 | 86.0 ± 36.1 | 0.193 | 91.6 ± 40.3 | 85.0 ± 37.1 | 0.171 |
| Hemoglobin, g/dL | 11.7 ± 2.0 | 11.9 ± 2.1 | 0.129 | 11.6 ± 2.0 | 11.8 ± 2.1 | 0.113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, K.-C.; Ho, C.-L.; Wang, J.; Zheng, C.-M.; Tsai, K.-W.; Hou, Y.-C.; Lu, C.-L. Temporal Trends of Hyponatremia in Patients with Respiratory and Intrathoracic Cancers Treated with Chemotherapy and Immune Checkpoint Inhibitors. Cancers 2025, 17, 1459. https://doi.org/10.3390/cancers17091459
Lu K-C, Ho C-L, Wang J, Zheng C-M, Tsai K-W, Hou Y-C, Lu C-L. Temporal Trends of Hyponatremia in Patients with Respiratory and Intrathoracic Cancers Treated with Chemotherapy and Immune Checkpoint Inhibitors. Cancers. 2025; 17(9):1459. https://doi.org/10.3390/cancers17091459
Chicago/Turabian StyleLu, Kuo-Cheng, Ching-Liang Ho, Joshua Wang, Cai-Mei Zheng, Kuo-Wang Tsai, Yi-Chou Hou, and Chien-Lin Lu. 2025. "Temporal Trends of Hyponatremia in Patients with Respiratory and Intrathoracic Cancers Treated with Chemotherapy and Immune Checkpoint Inhibitors" Cancers 17, no. 9: 1459. https://doi.org/10.3390/cancers17091459
APA StyleLu, K.-C., Ho, C.-L., Wang, J., Zheng, C.-M., Tsai, K.-W., Hou, Y.-C., & Lu, C.-L. (2025). Temporal Trends of Hyponatremia in Patients with Respiratory and Intrathoracic Cancers Treated with Chemotherapy and Immune Checkpoint Inhibitors. Cancers, 17(9), 1459. https://doi.org/10.3390/cancers17091459





