Single-Nucleotide Polymorphisms of BRCA1 and BRCA2 and Risk of Papillary Thyroid Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Selection and Genotyping of Single-Nucleotide Polymorphisms
2.3. Statistical Analysis
3. Results
3.1. Genotyping
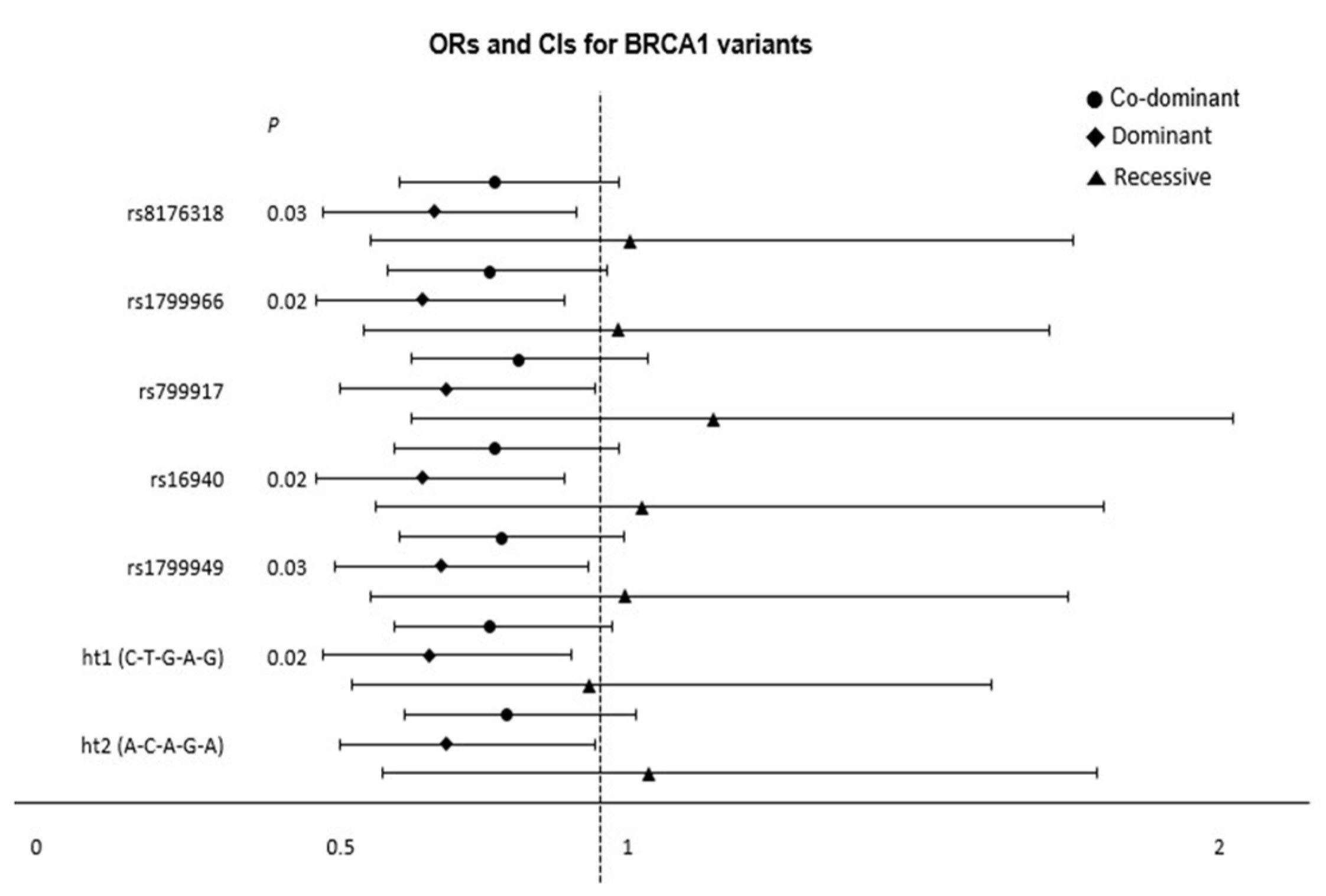
3.2. Logistic Regression Analysis of the Association of BRCA1 and BRCA2 Polymorphisms with Thyroid Cancer
3.3. Association of BRCA1 and BRCA2 Haplotypes with Thyroid Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Genes | Haplotype | rs8176318 | rs1799966 | rs799917 | rs16940 | rs1799949 | Frequency |
|---|---|---|---|---|---|---|---|
| BRCA1 | Ht a1 | C | T | G | A | G | 0.711 |
| Ht2 | A | C | A | G | A | 0.285 | |
| others | - | - | - | - | - | 0.003 | |
| rs1799943 | rs1799955 | rs15869 | |||||
| BRCA2 | Ht1 | G | A | A | 0.393 | ||
| Ht2 | A | G | A | 0.390 | |||
| Ht3 | G | A | C | 0.196 | |||
| Ht4 | G | G | A | 0.018 | |||
| others | - | - | - | 0.003 |
| Loci | Genotype | Genotype Distribution | Referent | Co-Dominant | Dominant | Recessive | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PTC a | Control | OR b (95% CI c) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| rs8176318 | CC | 271 (53.8%) | 131 (46.0%) | 1 | |||||||
| AC | 190 (37.7%) | 132 (46.3%) | 0.69 (0.51–0.94) | 0.0 2 * | 0.82 (0.66–1.03) | 0.10 | 0.72 (0.53–0.96) | 0.03 * | 1.05 (0.61–1.80) | 0.86 | |
| AA | 43 (8.5%) | 22 (7.7%) | 0.91 (0.52–1.59) | 0.74 | |||||||
| rs17-99966 | TT | 272 (53.5%) | 126 (45.0%) | 1 | |||||||
| CT | 192 (37.8%) | 132 (47.1%) | 0.87 (0.50–1.53) | 0.64 | 0.81 (0.64–1.01) | 0.07 | 0.70 (0.52–0.94) | 0.02 * | 1.03 (0.60–1.76) | 0.93 | |
| CC | 44 (8.7%) | 22 (7.9%) | 0.67 (0.49–0.91) | 0.01 * | |||||||
| rs799917 | GG | 275 (53.7%) | 135 (46.7%) | 1 | |||||||
| AG | 193 (37.7%) | 134 (46.4%) | 0.70 (0.52–0.95) | 0.02 * | 0.86 (0.68–1.08) | 0.19 | 0.74 (0.56–0.99) | 0.05 | 1.19(0.68–2.07) | 0.54 | |
| AA | 44 (8.6%) | 20 (6.9%) | 1.05 (0.59–1.86) | 0.88 | |||||||
| rs16940 | AA | 269 (53.1%) | 124 (44.8%) | 1 | |||||||
| AG | 194 (38.3%) | 132 (47.7%) | 0.67 (0.49–0.91) | 0.01 * | 0.82 (0.65–1.03) | 0.08 | 0.70 (0.52–0.94) | 0.02 * | 1.07 (0.62–1.85) | 0.81 | |
| GG | 44 (8.7%) | 21 (7.6%) | 0.91 (0.51–1.60) | 0.74 | |||||||
| rs1799949 | GG | 274 (53.5%) | 135 (46.2%) | 1 | |||||||
| AG | 195 (38.1%) | 135 (46.2%) | 0.70 (0.52–0.95) | 0.02 * | 0.83 (0.66–1.04) | 0.11 | 0.73 (0.55–0.98) | 0.03 * | 1.04 (0.61–1.79) | 0.88 | |
| AA | 43 (8.4%) | 22 (7.5%) | 0.91 (0.52–1.60) | 0.75 | |||||||
| Loci | Genotype | Genotype Distribution | Referent | Co-Dominant | Dominant | Recessive | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PTC a | Control | OR b (95% CI c) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| rs1799943 | GG | 200 (39.0%) | 97 (33.8%) | 1 | |||||||
| AG | 237 (46.2%) | 142 (49.5%) | 0.81 (0.59–1.12) | 0.21 | 0.87 (0.70–1.07) | 0.19 | 0.81 (0.59–1.09) | 0.16 | 0.88 (0.59–1.31) | 0.52 | |
| AA | 76 (14.8%) | 48 (16.7%) | 0.79 (0.51–1.23) | 0.30 | |||||||
| rs1799955 | AA | 191 (37.5%) | 91 (31.8%) | 1 | |||||||
| AG | 236 (46.3%) | 145 (50.7%) | 0.77 (0.56–1.07) | 0.12 | 0.88 (0.71–1.08) | 0.23 | 0.79 (0.58–1.07) | 0.12 | 0.95 (0.64–1.40) | 0.78 | |
| GG | 83 (16.3%) | 50 (17.5%) | 0.82 (0.53–1.27) | 0.37 | |||||||
| rs15869 | AA | 316 (64.6%) | 187 (64.5%) | 1 | |||||||
| AC | 152 (31.1%) | 94 (32.4%) | 0.94 (0.68–1.28) | 0.68 | 1.01 (0.78–1.31) | 0.93 | 0.97 (0.71–1.32) | 0.84 | 1.35 (0.61–3.00) | 0.45 | |
| CC | 21 (4.3%) | 9 (3.1%) | 1.33 (0.59–2.98) | 0.48 | |||||||
| Gene | Loci | Genotype | Genotype Distribution | Referent | Co-Dominant | Dominant | Recessive | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTC a | Control | OR b (95% CI c) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |||
| BRCA1 | ht d1 (C-T-G-A-G) | -/- | 276 (53.6%) | 135 (45.6%) | 1 | |||||||
| ht1/- | 195 (37.9%) | 137 (46.3%) | 0.69 (0.51–0.93) | 0.02 * | 0.81 (0.65–1.02) | 0.07 | 0.71 (0.53–0.95) | 0.02 * | 0.98 (0.58–1.66) | 0.95 | ||
| ht1/ht1 | 44 (8.5%) | 24 (8.1%) | 0.85 (0.49–1.47) | 0.56 | ||||||||
| ht d2 (A-C-A-G-A) | -/- | 276 (53.6%) | 138 (46.6%) | 1 | ||||||||
| ht2/- | 195 (37.9%) | 136 (45.9%) | 0.71 (0.52–0.96) | 0.03 * | 0.84 (0.67–1.06) | 0.14 | 0.74 (0.56–0.99) | 0.04 * | 1.08 (0.63–1.84) | 0.79 | ||
| ht2/ht2 | 44 (8.5%) | 22 (7.4%) | 0.95 (0.54–1.65) | 0.84 | ||||||||
| BRCA2 | ht e1 (G-A-A) | -/- | 193 (37.5%) | 113 (38.2%) | 1 | |||||||
| ht1/- | 229 (44.5%) | 144 (48.6%) | 0.94 (0.69–1.29) | 0.71 | 1.12 (0.91–1.38) | 0.28 | 1.04 (0.77–1.39) | 0.81 | 1.44 (0.96–2.16) | 0.07* | ||
| ht1/ht1 | 93 (18.1%) | 39 (13.2%) | 1.38 (0.88–2.14) | 0.15 | ||||||||
| ht e2 (A-G-A) | -/- | 201 (39.0%) | 99 (33.4%) | 1 | ||||||||
| ht2/- | 241 (46.8%) | 149 (50.3%) | 0.80 (0.58–1.10) | 0.17 | 0.86 (0.69–1.06) | 0.15 | 0.79 (0.59–1.07) | 0.13 | 0.86 (0.58–1.29) | 0.48 | ||
| ht2/ht2 | 73 (14.2%) | 48 (16.2%) | 0.77 (0.50–1.20) | 0.25 | ||||||||
| ht e3 (G-A-C) | -/- | 332 (64.5%) | 192 (64.9%) | 1 | ||||||||
| ht3/- | 161 (31.3%) | 95 (32.1%) | 0.96 (0.70–1.31) | 0.81 | 1.03 (0.80–1.34) | 0.81 | 1.00 (0.74–1.35) | 0.98 | 1.37 (0.62–3.03) | 0.43 | ||
| ht3/ht3 | 22 (4.3%) | 9 (3.0%) | 1.36 (0.61–3.02) | 0.44 | ||||||||
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.R.; Morris, L.G.; Davies, L. The thyroid cancer epidemic, 2017 perspective. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Lortet-Tieulent, J.; Franceschi, S.; Dal Maso, L.; Vaccarella, S. Thyroid cancer “epidemic” also occurs in low- and middle-income countries. Int. J. Cancer 2019, 144, 2082–2087. [Google Scholar] [CrossRef]
- Davies, L.; Morris, L.G.; Haymart, M.; Chen, A.Y.; Goldenberg, D.; Morris, J.; Ogilvie, J.B.; Terris, D.J.; Netterville, J.; Wong, R.J.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: The Increasing Incidence of Thyroid Cancer. Endocr. Pract. 2015, 21, 686–696. [Google Scholar] [CrossRef]
- Schonfeld, S.J.; Lee, C.; Berrington de Gonzalez, A. Medical exposure to radiation and thyroid cancer. Clin. Oncol. 2011, 23, 244–250. [Google Scholar] [CrossRef]
- LiVolsi, V.A.; Abrosimov, A.A.; Bogdanova, T.; Fadda, G.; Hunt, J.L.; Ito, M.; Rosai, J.; Thomas, G.A.; Williams, E.D. The Chernobyl thyroid cancer experience: Pathology. Clin. Oncol. 2011, 23, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Zidane, M.; Cazier, J.B.; Chevillard, S.; Ory, C.; Schlumberger, M.; Dupuy, C.; Deleuze, J.F.; Boland, A.; Haddy, N.; Lesueur, F.; et al. Genetic susceptibility to radiation-related differentiated thyroid cancers: A systematic review of literature. Endocr. Relat. Cancer 2019, 26, R583–R596. [Google Scholar] [CrossRef]
- Iglesias, M.L.; Schmidt, A.; Ghuzlan, A.A.; Lacroix, L.; Vathaire, F.; Chevillard, S.; Schlumberger, M. Radiation exposure and thyroid cancer: A review. Arch. Endocrinol. Metab. 2017, 61, 180–187. [Google Scholar] [CrossRef]
- Bang, H.S.; Choi, M.H.; Kim, C.S.; Choi, S.J. Gene expression profiling in undifferentiated thyroid carcinoma induced by high-dose radiation. J. Radiat. Res. 2016, 57, 238–249. [Google Scholar] [CrossRef]
- Wojcicka, A.; Czetwertynska, M.; Swierniak, M.; Dlugosinska, J.; Maciag, M.; Czajka, A.; Dymecka, K.; Kubiak, A.; Kot, A.; Płoski, R.; et al. Variants in the ATM-CHEK2-BRCA1 axis determine genetic predisposition and clinical presentation of papillary thyroid carcinoma. Genes Chromosomes Cancer 2014, 53, 516–523. [Google Scholar] [CrossRef]
- Lin, H.T.; Liu, F.C.; Lin, S.F.; Kuo, C.F.; Chen, Y.Y.; Yu, H.P. Familial Aggregation and Heritability of Nonmedullary Thyroid Cancer in an Asian Population: A Nationwide Cohort Study. J. Clin. Endocrinol. Metab. 2020, 105, dgaa191. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, J.; Thorleifsson, G.; Sigurdsson, J.K.; Stefansdottir, L.; Jonasson, J.G.; Gudjonsson, S.A.; Gudbjartsson, D.F.; Masson, G.; Johannsdottir, H.; Halldorsson, G.H.; et al. A genome-wide association study yields five novel thyroid cancer risk loci. Nat. Commun. 2017, 8, 14517. [Google Scholar] [CrossRef] [PubMed]
- Jendrzejewski, J.; Liyanarachchi, S.; Nagy, R.; Senter, L.; Wakely, P.E.; Thomas, A.; Nabhan, F.; He, H.; Li, W.; Sworczak, K.; et al. Papillary Thyroid Carcinoma: Association Between Germline DNA Variant Markers and Clinical Parameters. Thyroid 2016, 26, 1276–1284. [Google Scholar] [CrossRef]
- Hwangbo, Y.; Lee, E.K.; Son, H.Y.; Im, S.W.; Kwak, S.J.; Yoon, J.W.; Kim, M.J.; Kim, J.; Choi, H.S.; Ryu, C.H.; et al. Genome-Wide Association Study Reveals Distinct Genetic Susceptibility of Thyroid Nodules from Thyroid Cancer. J. Clin. Endocrinol. Metab. 2018, 103, 4384–4394. [Google Scholar] [CrossRef] [PubMed]
- Saenko, V.A.; Rogounovitch, T.I. Genetic Polymorphism Predisposing to Differentiated Thyroid Cancer: A Review of Major Findings of the Genome-Wide Association Studies. Endocrinol. Metab. 2018, 33, 164–174. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Tang, X.; Li, M.; Shi, F. Association between hTERT Polymorphisms and Female Papillary Thyroid Carcinoma. Recent. Pat. Anticancer. Drug Discov. 2019, 14, 268–279. [Google Scholar] [CrossRef]
- Shen, M.L.; Xiao, A.; Yin, S.J.; Wang, P.; Lin, X.Q.; Yu, C.B.; He, G.H. Associations between UGT2B7 polymorphisms and cancer susceptibility: A meta-analysis. Gene 2019, 706, 115–123. [Google Scholar] [CrossRef]
- Jendrzejewski, J.; Liyanarachchi, S.; Eiterman, A.; Thomas, A.; He, H.; Nagy, R.; Senter, L.; Sworczak, K.; de la Chapelle, A. Fine mapping of 14q13 reveals novel variants associated with different histological subtypes of papillary thyroid carcinoma. Int. J. Cancer 2019, 144, 503–512. [Google Scholar] [CrossRef]
- Son, H.Y.; Hwangbo, Y.; Yoo, S.K.; Im, S.W.; Yang, S.D.; Kwak, S.J.; Park, M.S.; Kwak, S.H.; Cho, S.W.; Ryu, J.S.; et al. Genome-wide association and expression quantitative trait loci studies identify multiple susceptibility loci for thyroid cancer. Nat. Commun. 2017, 8, 15966. [Google Scholar] [CrossRef]
- Gatzidou, E.; Michailidi, C.; Tseleni-Balafouta, S.; Theocharis, S. An epitome of DNA repair related genes and mechanisms in thyroid carcinoma. Cancer Lett. 2010, 290, 139–147. [Google Scholar] [CrossRef]
- Sandler, J.E.; Huang, H.; Zhao, N.; Wu, W.; Liu, F.; Ma, S.; Udelsman, R.; Zhang, Y. Germline Variants in DNA Repair Genes, Diagnostic Radiation, and Risk of Thyroid Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Pandith, A.A.; Masoodi, S.R.; Khan, S.H.; Rather, T.A.; Andrabi, K.I.; Mudassar, S. Significant association of TP53 Arg72Pro polymorphism in susceptibility to differentiated thyroid cancer. Cancer Biomark. 2015, 15, 459–465. [Google Scholar] [CrossRef]
- Halkova, T.; Dvorakova, S.; Sykorova, V.; Vaclavikova, E.; Vcelak, J.; Vlcek, P.; Sykorova, P.; Kodetova, D.; Betka, J.; Lastuvka, P.; et al. Polymorphisms in selected DNA repair genes and cell cycle regulating genes involved in the risk of papillary thyroid carcinoma. Cancer Biomark. 2016, 17, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Morillo-Bernal, J.; Fernandez, L.P.; Santisteban, P. FOXE1 regulates migration and invasion in thyroid cancer cells and targets ZEB1. Endocr. Relat. Cancer 2020, 27, 137–151. [Google Scholar] [CrossRef]
- Lonjou, C.; Damiola, F.; Moissonnier, M.; Durand, G.; Malakhova, I.; Masyakin, V.; Le Calvez-Kelm, F.; Cardis, E.; Byrnes, G.; Kesminiene, A.; et al. Investigation of DNA repair-related SNPs underlying susceptibility to papillary thyroid carcinoma reveals MGMT as a novel candidate gene in Belarusian children exposed to radiation. BMC Cancer 2017, 17, 328. [Google Scholar] [CrossRef] [PubMed]
- Streff, H.; Profato, J.; Ye, Y.; Nebgen, D.; Peterson, S.K.; Singletary, C.; Arun, B.K.; Litton, J.K. Cancer Incidence in First- and Second-Degree Relatives of BRCA1 and BRCA2 Mutation Carriers. Oncologist 2016, 21, 869–874. [Google Scholar] [CrossRef]
- Deng, C.X. BRCA1: Cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 2006, 34, 1416–1426. [Google Scholar] [CrossRef]
- Venkitaraman, A.R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002, 108, 171–182. [Google Scholar] [CrossRef]
- Pellegrini, L.; Yu, D.S.; Lo, T.; Anand, S.; Lee, M.; Blundell, T.L.; Venkitaraman, A.R. Insights into DNA recombination from the structure of a RAD51-BRCA2 complex. Nature 2002, 420, 287–293. [Google Scholar] [CrossRef]
- Pouptsis, A.; Swafe, L.; Patwardhan, M.; Stavraka, C. Surgical and Systemic Treatment of Hereditary Breast Cancer: A Mini-Review with a Focus on BRCA1 and BRCA2 Mutations. Front. Oncol. 2020, 10, 553080. [Google Scholar] [CrossRef]
- Pal, T.; Hamel, N.; Vesprini, D.; Sanders, K.; Mitchell, M.; Quercia, N.; Ng Cheong, N.; Murray, A.; Foulkes, W.; Narod, S.A. Double primary cancers of the breast and thyroid in women: Molecular analysis and genetic implications. Fam. Cancer 2001, 1, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Yadav, S.; Ogunleye, F.; Zakalik, D. Male BRCA mutation carriers: Clinical characteristics and cancer spectrum. BMC Cancer 2018, 18, 179. [Google Scholar] [CrossRef]
- Figueiredo, J.C.; Brooks, J.D.; Conti, D.V.; Poynter, J.N.; Teraoka, S.N.; Malone, K.E.; Bernstein, L.; Lee, W.D.; Duggan, D.J.; Siniard, A.; et al. Risk of contralateral breast cancer associated with common variants in BRCA1 and BRCA2: Potential modifying effect of BRCA1/BRCA2 mutation carrier status. Breast Cancer Res. Treat. 2011, 127, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Du, X.; Zhang, F.; Yuan, S. Association between BRCA1 polymorphisms rs799917 and rs1799966 and breast cancer risk: A meta-analysis. J. Int. Med. Res. 2019, 47, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Ricks-Santi, L.; McDonald, J.T.; Gold, B.; Dean, M.; Thompson, N.; Abbas, M.; Wilson, B.; Kanaan, Y.; Naab, T.J.; Dunston, G. Next Generation Sequencing Reveals High Prevalence of BRCA1 and BRCA2 Variants of Unknown Significance in Early-Onset Breast Cancer in African American Women. Ethn. Dis. 2017, 27, 169–178. [Google Scholar] [CrossRef]
- Su, T.; Sun, H.; Lu, X.; He, C.; Xiao, L.; He, J.; Yang, Y.; Tang, Y. Genetic polymorphisms and haplotypes of BRCA1 gene associated with quality of life and survival among patients with non-small-cell lung cancer. Qual. Life Res. 2020, 29, 2631–2640. [Google Scholar] [CrossRef]
- Sapkota, Y.; Mackey, J.R.; Lai, R.; Franco-Villalobos, C.; Lupichuk, S.; Robson, P.J.; Kopciuk, K.; Cass, C.E.; Yasui, Y.; Damaraju, S. Assessing SNP-SNP interactions among DNA repair, modification and metabolism related pathway genes in breast cancer susceptibility. PLoS ONE 2014, 8, e64896. [Google Scholar] [CrossRef]
- Song, C.M.; Kwon, T.K.; Park, B.L.; Ji, Y.B.; Tae, K. Single nucleotide polymorphisms of ataxia telangiectasia mutated and the risk of papillary thyroid carcinoma. Environ. Mol. Mutagen. 2015, 56, 70–76. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Stephens, M.; Smith, N.J.; Donnelly, P. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet. 2001, 68, 978–989. [Google Scholar] [CrossRef]
- Yang, F.; Chen, F.; Xu, J.; Guan, X. Identification and frequency of the rs12516 and rs8176318 BRCA1 gene polymorphisms among different populations. Oncol. Lett. 2016, 11, 2481–2486. [Google Scholar] [CrossRef] [PubMed]
- Moynahan, M.E.; Pierce, A.J.; Jasin, M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 2001, 7, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Venkitaraman, A.R. How do mutations affecting the breast cancer genes BRCA1 and BRCA2 cause cancer susceptibility? DNA Repair 2019, 81, 102668. [Google Scholar] [CrossRef]
- Yoshida, K.; Miki, Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004, 95, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- Seo, J.H.; Cho, D.Y.; Ahn, S.H.; Yoon, K.S.; Kang, C.S.; Cho, H.M.; Lee, H.S.; Choe, J.J.; Choi, C.W.; Kim, B.S.; et al. BRCA1 and BRCA2 germline mutations in Korean patients with sporadic breast cancer. Hum. Mutat. 2004, 24, 350. [Google Scholar] [CrossRef]
- Xu, L.; Doan, P.C.; Wei, Q.; Liu, Y.; Li, G.; Sturgis, E.M. Association of BRCA1 functional single nucleotide polymorphisms with risk of differentiated thyroid carcinoma. Thyroid 2012, 22, 35–43. [Google Scholar] [CrossRef]
- Guo, N.; Qu, P.; Li, H.; Liu, L.; Jin, H.; Liu, R.; Zhang, Z.; Zhang, X.; Li, Y.; Lu, X.; et al. BRCA2 3′-UTR Polymorphism rs15869 Alters Susceptibility to Papillary Thyroid Carcinoma via Binding hsa-mir-1178-3p. Pharmgenomics Pers. Med. 2021, 14, 533–544. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, H.G.; Yoo, S.S.; Kang, Y.R.; Choi, Y.Y.; Lee, W.K.; Choi, J.E.; Jeon, H.S.; Shin, K.M.; Oh, I.J.; et al. Polymorphisms in DNA repair and apoptosis-related genes and clinical outcomes of patients with non-small cell lung cancer treated with first-line paclitaxel-cisplatin chemotherapy. Lung Cancer 2013, 82, 330–339. [Google Scholar] [CrossRef]
- Du, Y.; Su, T.; Zhao, L.; Tan, X.; Chang, W.; Zhang, H.; Cao, G. Associations of polymorphisms in DNA repair genes and MDR1 gene with chemotherapy response and survival of non-small cell lung cancer. PLoS ONE 2014, 9, e99843. [Google Scholar] [CrossRef]
- Huang, C.S.; Liu, C.Y.; Lu, T.P.; Huang, C.J.; Chiu, J.H.; Tseng, L.M.; Huang, C.C. Targeted Sequencing of Taiwanese Breast Cancer with Risk Stratification by the Concurrent Genes Signature: A Feasibility Study. J. Pers. Med. 2021, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xu, L.; Pan, L.; Xu, K.; Li, G. The functional BRCA1 rs799917 genetic polymorphism is associated with gastric cancer risk in a Chinese Han population. Tumour. Biol. 2015, 36, 393–397. [Google Scholar] [CrossRef]
- Mullany, L.E.; Wolff, R.K.; Herrick, J.S.; Buas, M.F.; Slattery, M.L. SNP Regulation of microRNA Expression and Subsequent Colon Cancer Risk. PLoS ONE 2015, 10, e0143894. [Google Scholar] [CrossRef]
- Heublein, S.; Mayr, D.; Meindl, A.; Angele, M.; Gallwas, J.; Jeschke, U.; Ditsch, N. Thyroid Hormone Receptors Predict Prognosis in BRCA1 Associated Breast Cancer in Opposing Ways. PLoS ONE 2015, 10, e0127072. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, M. Linkage disequilibrium--understanding the evolutionary past and mapping the medical future. Nat. Rev. Gene 2008, 9, 477–485. [Google Scholar] [CrossRef]
- Dhouioui, S.; Baroudi, S.; Zemni, I.; Mahdhi, F.; Najjari, A.; Chelbi, H.; Khiari, H.; Boujelbene, N.; Zidi, I. IL-10 polymorphism genotypes, haplotypes, and diplo-types are associated with colorectal cancer predisposition and outcome in Tunisian population. Heliyon 2024, 10, e34852. [Google Scholar] [CrossRef]
- Shu, J.; Zhang, M.; Dong, X.; Long, J.; Li, Y.; Tan, P.; He, T.; Giovannucci, E.L.; Zhang, X.; Zhou, Z.; et al. Vitamin D receptor gene polymorphisms, bioavailable 25-hydroxyvitamin D, and hepatocellular carcinoma survival. J. Natl. Cancer Inst. 2024, 116, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Santorsola, M.; Ianniello, M.; Ceccarelli, A.; Casillo, M.; Sabbatino, F.; Petrillo, N.; Cascella, M.; Caraglia, F.; Picone, C.; et al. Predictive significance of FGFR4 p.G388R polymorphism in metastatic colorectal cancer patients receiving trifluridine/tipiracil (TAS-102) treatment. J. Transl. Med. 2024, 22, 379. [Google Scholar] [CrossRef]
- Butkiewicz, D.; Krześniak, M.; Gdowicz-Kłosok, A.; Giglok, M.; Marszałek-Zeńczak, M.; Suwiński, R. Polymorphisms in EGFR Gene Predict Clinical Outcome in Unresectable Non-Small Cell Lung Cancer Treated with Radiotherapy and Platinum-Based Chemoradio-therapy. Int. J. Mol. Sci. 2021, 22, 5605. [Google Scholar] [CrossRef]
- Abdullah, M.I.; Junit, S.M.; Ng, K.L.; Jayapalan, J.J.; Karikalan, B.; Hashim, O.H. Papillary Thyroid Cancer: Genetic Alterations and Molecular Biomarker Investigations. Int. J. Med. Sci. 2019, 16, 450–460. [Google Scholar] [CrossRef]



| Gene | SNPID | Assay-on-Demand ID | Company |
|---|---|---|---|
| BRCA1 | rs8176318 | C_3178688_10 | Thermo Fisher Scientific, Waltham, MA, USA |
| rs1799966 | C_2615208_20 | ||
| rs799917 | C_2287943_10 | ||
| rs16940 | C_11415267_10 | ||
| rs1799949 | C_2615193_10 | ||
| BRCA2 | rs1799943 | C_3070446_10 | |
| rs1799955 | C_7605612_10 | ||
| rs15869 | C_807118_10 |
| Variable | PTC a Cases (n = 515) | Controls (n = 296) | p Value |
|---|---|---|---|
| Age (mean ± SD) | 47.43 ± 12.11 | 47.22 ± 14.20 | 0.829 |
| Age, Male (mean ± SD) | 46.88 ± 12.91 | 48.10 ± 13.31 | 0.489 |
| Age, Female (mean ± SD) | 47.61 ± 11.86 | 46.78 ± 14.65 | 0.458 |
| Gender | 0.003 | ||
| Male | 124 (24.1%) | 100 (33.8%) | |
| Female | 391 (75.9%) | 196 (66.2%) |
| Gene | Loci | Allele | Major Homozygosity | Heterozygosity | Minor Homozygosity | Total | MAF a | HWE b |
|---|---|---|---|---|---|---|---|---|
| BRCA1 | rs8176318 | C>A | 402 | 322 | 65 | 789 | 0.286 | 0.963 |
| rs1799966 | T>C | 398 | 324 | 66 | 788 | 0.289 | 0.996 | |
| rs799917 | G>A | 410 | 327 | 64 | 801 | 0.284 | 0.915 | |
| rs16940 | A>G | 393 | 326 | 65 | 784 | 0.291 | 0.821 | |
| rs1799949 | G>A | 409 | 330 | 65 | 804 | 0.286 | 0.891 | |
| BRCA2 | rs1799943 | G>A | 297 | 379 | 124 | 800 | 0.392 | 0.865 |
| rs1799955 | A>G | 282 | 381 | 133 | 796 | 0.406 | 0.822 | |
| rs15869 | A>C | 503 | 246 | 30 | 779 | 0.196 | 0.991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.M.; Kim, Y.J.; Cheong, H.S.; Ji, Y.B.; Tae, K. Single-Nucleotide Polymorphisms of BRCA1 and BRCA2 and Risk of Papillary Thyroid Carcinoma. Cancers 2025, 17, 1456. https://doi.org/10.3390/cancers17091456
Song CM, Kim YJ, Cheong HS, Ji YB, Tae K. Single-Nucleotide Polymorphisms of BRCA1 and BRCA2 and Risk of Papillary Thyroid Carcinoma. Cancers. 2025; 17(9):1456. https://doi.org/10.3390/cancers17091456
Chicago/Turabian StyleSong, Chang Myeon, Yun Jin Kim, Hyun Sub Cheong, Yong Bae Ji, and Kyung Tae. 2025. "Single-Nucleotide Polymorphisms of BRCA1 and BRCA2 and Risk of Papillary Thyroid Carcinoma" Cancers 17, no. 9: 1456. https://doi.org/10.3390/cancers17091456
APA StyleSong, C. M., Kim, Y. J., Cheong, H. S., Ji, Y. B., & Tae, K. (2025). Single-Nucleotide Polymorphisms of BRCA1 and BRCA2 and Risk of Papillary Thyroid Carcinoma. Cancers, 17(9), 1456. https://doi.org/10.3390/cancers17091456






