Clinical and Pathologic Characteristics of Cytologically Indeterminate Thyroid Nodules with Non-V600E BRAF Alterations
Simple Summary
Abstract
1. Introduction
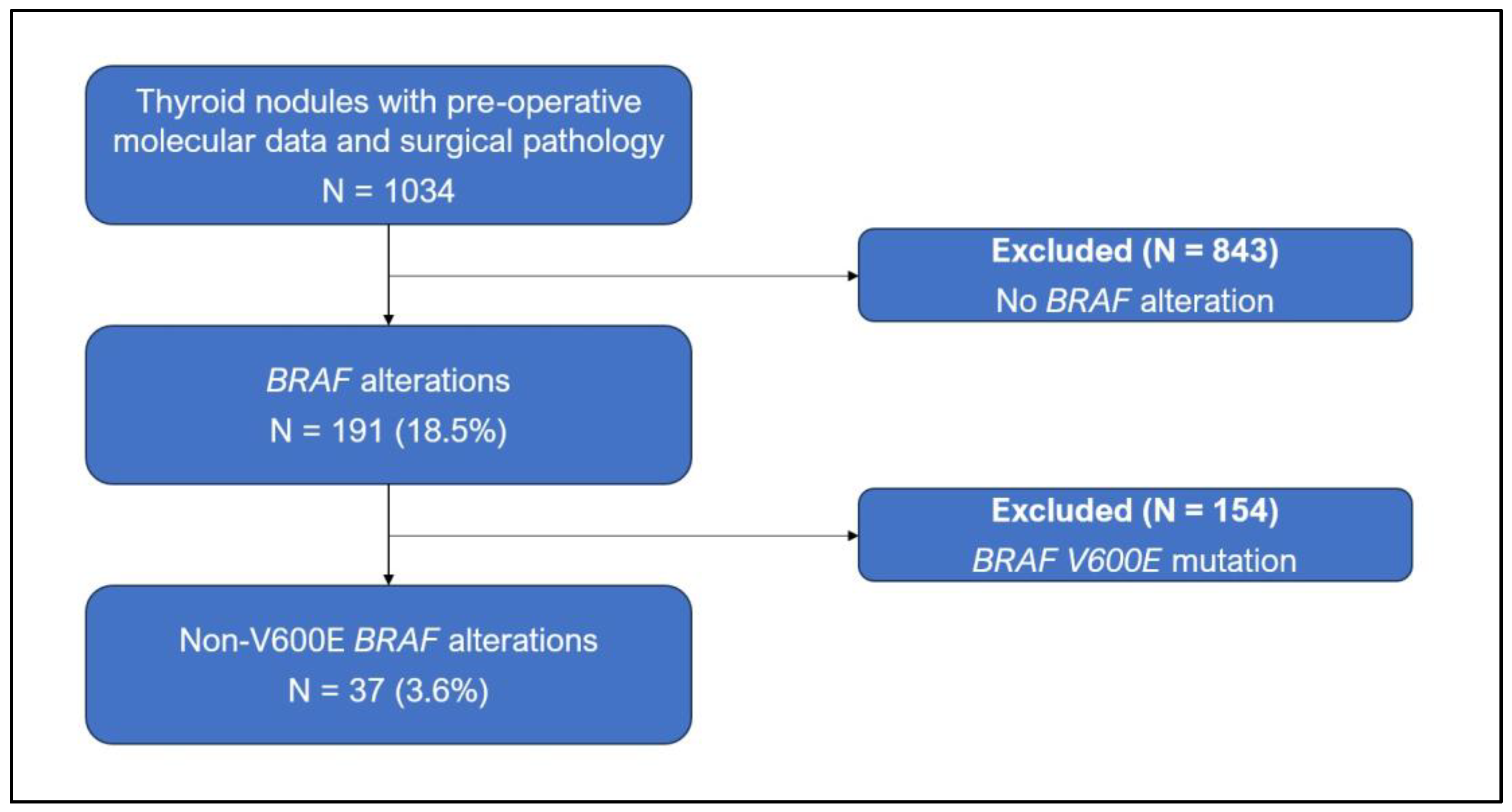
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Cytopathologic and Genetic Findings
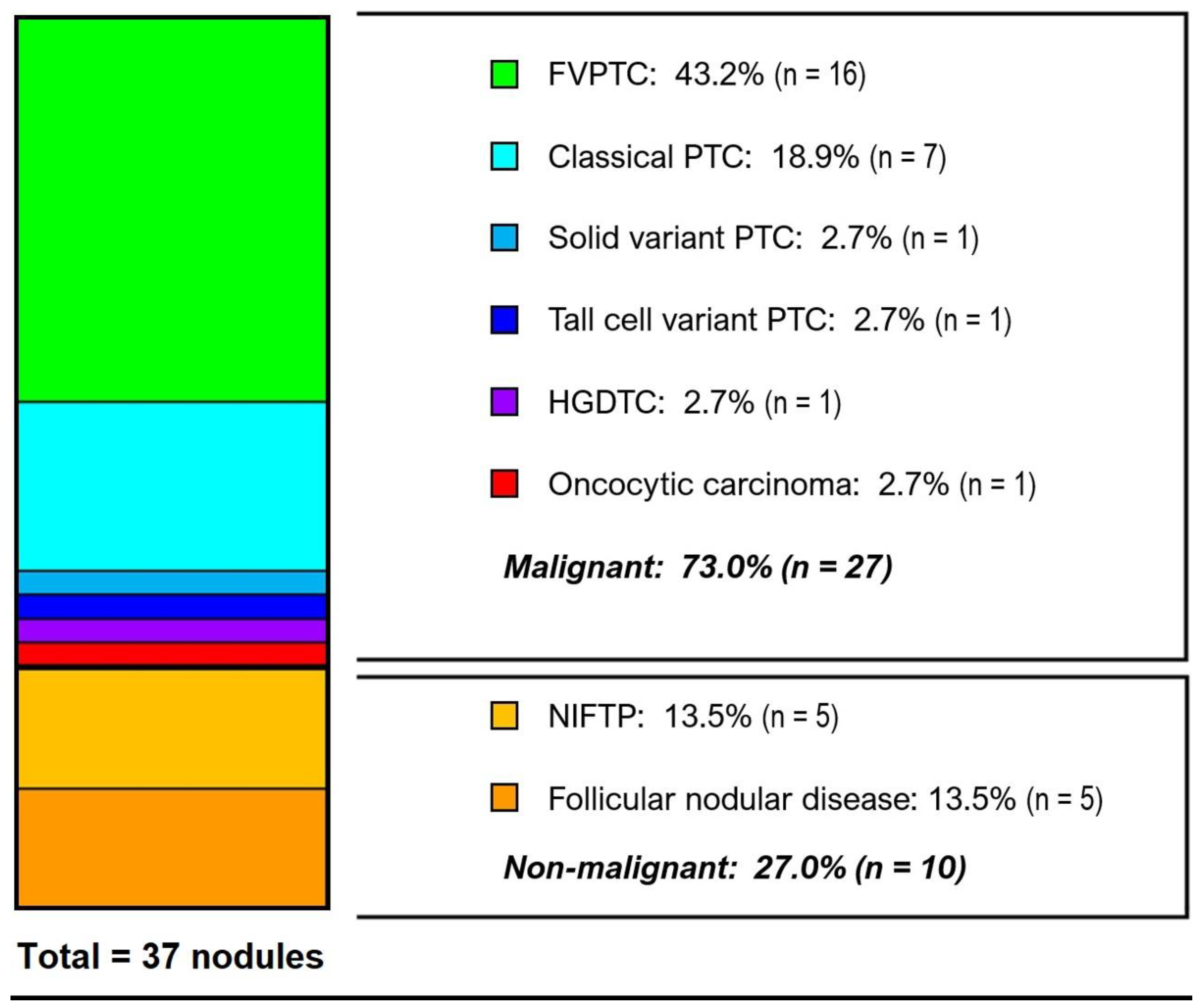
3.3. Histological Diagnoses
3.4. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guth, S.; Theune, U.; Aberle, J.; Galach, A.; Bamberger, C.M. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur. J. Clin. Investig. 2009, 39, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Sangalli, G.; Serio, G.; Zampatti, C.; Bellotti, M.; Lomuscio, G. Fine needle aspiration cytology of the thyroid: A comparison of 5469 cytological and final histological diagnoses. Cytopathology 2006, 17, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Grani, G.; Sponziello, M.; Filetti, S.; Durante, C. Thyroid nodules: Diagnosis and management. Nat. Rev. Endocrinol. 2024, 20, 715–728. [Google Scholar] [CrossRef]
- Khan, T.M.; Zeiger, M.A. Thyroid Nodule Molecular Testing: Is It Ready for Prime Time? Front. Endocrinol. 2020, 11, 590128. [Google Scholar] [CrossRef]
- Ferraz, C. Molecular testing for thyroid nodules: Where are we now? Rev. Endocr. Metab. Disord. 2024, 25, 149–159. [Google Scholar] [CrossRef]
- McMurtry, V.; Canberk, S.; Deftereos, G. Molecular testing in fine-needle aspiration of thyroid nodules. Diagn. Cytopathol. 2023, 51, 36–50. [Google Scholar] [CrossRef]
- Alexander, E.K.; Cibas, E.S. Diagnosis of thyroid nodules. Lancet Diabetes Endocrinol. 2022, 10, 533–539. [Google Scholar] [CrossRef]
- Giordano, T. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Macerola, E.; Poma, A.M.; Vignali, P.; Basolo, A.; Ugolini, C.; Torregrossa, L.; Ferruccio, S.; Basolo, F. Molecular genetics of follicular-derived thyroid cancer. Cancers 2021, 13, 1139. [Google Scholar] [CrossRef]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. BRAF mutation in thyroid cancer. Endocr. Relat. Cancer 2005, 12, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Nikiforova, M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, N.; Grone, J.; Uslar, V.; Tannapfel, A.; Weyhe, D. BRAF V600E mutation correlates with aggressive clinico-pathological features but does not influence tumor recurrence in papillary thyroid carcinoma—10-year single-center results. Gland Surg. 2020, 9, 1902–1913. [Google Scholar] [CrossRef]
- Xing, M.M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, M.; et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013, 309, 1493–1501. [Google Scholar] [CrossRef]
- Trimboli, P.; Treglia, G.; Condorelli, E.; Romanelli, F.; Crescenzi, A.; Bongiovanni, M.; Giovanella, L. BRAF-mutated carcinomas among thyroid nodules with prior indeterminate FNA report: A systematic review and meta-analysis. Clin. Endocrinol. 2016, 84, 315–320. [Google Scholar] [CrossRef]
- Kleiman, D.A.; Sporn, M.J.; Beninato, T.; Crowley, M.J.; Nguyen, A.; Uccelli, A.; Scognamiglio, T.; Zarnegar, R.; Fahey, T.J. Preoperative BRAF(V600E) mutation screening is unlikely to alter initial surgical treatment of patients with indeterminate thyroid nodules: A prospective case series of 960 patients. Cancer 2013, 119, 1495–1502. [Google Scholar] [CrossRef]
- Jinih, M.; Foley, N.; Osho, O.; Houlihan, L.; Toor, A.A.; Khan, J.Z.; Achakzai, A.A.; Redmond, H.P. BRAFV600E mutation as a predictor of thyroid malignancy in indeterminate nodules: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2017, 43, 1219–1227. [Google Scholar] [CrossRef]
- Owsley, J.; Stein, M.K.; Porter, J.; In, G.K.; Salem, M.; O’Day, S.; Elliott, A.; Poorman, K.; Gibney, G.; Vanderwalde, A. Prevalence of class I–III BRAF mutations among 114,662 cancer patients in a large genomic database. Exp. Biol. Med. 2021, 246, 31–39. [Google Scholar] [CrossRef]
- De Leo, A.; Serban, D.; Maloberti, T.; Sanza, V.; Coluccelli, S.; Altimari, A.; Gruppioni, E.; Chiarucci, F.; Carradini, A.G.; Repaci, A. Expanding the Spectrum of BRAF Non-V600E Mutations in Thyroid Nodules: Evidence-Based Data from a Tertiary Referral Centre. Int. J. Mol. Sci. 2023, 24, 4057. [Google Scholar] [CrossRef]
- Murugan, A.K.; Qasem, E.; Al-Hindi, H.; Shi, Y.; Alzahrani, A.S. Classical V600E and other non-hotspot BRAF mutations in adult differentiated thyroid cancer. J. Transl. Med. 2016, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, M.; Karunamurthy, A.; Chiosea, S.; Nikiforova, M.N.; Seethala, R.; Nikiforov, Y.E.; Coyne, C. Histopathologic and clinical characterization of thyroid tumors carrying the BRAFK601E mutation. Thyroid 2016, 26, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Tufano, R.P.; Teixeira, G.V.; Bishop, J.; Carson, K.A.; Xing, M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: A systematic review and meta-analysis. Medicine 2012, 91, 274–286. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Lepe, M.; Tolino, L.A.; Miller, M.E.; Ohori, N.P.; Wald, A.I.; Landau, M.S.; Kaya, C.; Malapelle, U.; Bellevi-cine, C.; et al. Thyroid Cytology Smear Slides: An Untapped Re-source for ThyroSeq Testing. Cancer Cytopathol. 2021, 129, 33–42. [Google Scholar] [CrossRef]
- Xing, M.; Clark, D.; Guan, H.; Ji, M.; Dackiw, A.; Carson, K.A.; Kim, M.; Tufaro, A.; Ladenson, P.; Zeiger, M.; et al. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J. Clin. Oncol. 2009, 27, 2977–2982. [Google Scholar] [CrossRef]
- Howell, G.M.; Nikiforova, M.N.; Carty, S.E.; Armstrong, M.J.; Hodak, S.P.; Stang, M.T.; McCoy, K.L.; Nikiforov, Y.E.; Yip, L. BRAF V600E mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann. Surg. Oncol. 2013, 20, 47–52. [Google Scholar] [CrossRef]
- Ciampi, R.; Knauf, J.A.; Kerler, R.; Gandhi, M.; Zhu, Z.; Nikiforova, M.N.; Rabes, H.M.; Fagin, J.A.; Nikiforov, Y.E. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J. Clin. Investig. 2005, 115, 94–101. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, E.S.; Kim, Y.S.; Won, N.H.; Chae, Y.S. BRAF mutation and AKAP9 expression in sporadic papillary thyroid carcinomas. Pathology 2006, 38, 201–204. [Google Scholar] [CrossRef]
- Yakushina, V.D.; Lerner, L.V.; Lavrov, A.V. Gene fusions in thyroid cancer. Thyroid 2018, 28, 158–167. [Google Scholar] [CrossRef]
- Sfreddo, H.J.; Koh, E.S.; Zhao, K.; Swartzwelder, C.E.; Untch, B.R.; Marti, J.L.; Roman, B.R.; Dublin, J.; Wang, R.S.; Xia, R.; et al. RAS-Mutated Cytologically Indeterminate Thyroid Nodules: Prevalence of Malignancy and Behavior Under Active Surveillance. Thyroid 2024, 34, 450–459. [Google Scholar] [CrossRef]
- Marcadis, A.R.; Valderrabano, P.; Ho, A.S.; Tepe, J.; Swartzwelder, C.E.; Byrd, S.; Sacks, W.L.; Untch, B.R.; Shaha, A.R.; Xu, B.; et al. Interinstitutional variation in predictive value of the ThyroSeq v2 genomic classifier for cytologically indeterminate thyroid nodules. Surgery 2019, 165, 17–24. [Google Scholar] [CrossRef] [PubMed]
| Age at Diagnosis (Years) | Treatment Type | ||
| Median (IQR) | 44 (36–58) | Total thyroidectomy | 17 (45.9%) |
| Range | 19–72 | Thyroid lobectomy | 17 (45.9%) |
| Isthmusectomy | 3 (8.1%) | ||
| Sex | |||
| Female | 27 (73.0%) | Final surgical pathology | |
| Male | 10 (27.0%) | Benign/non-malignant | 10 (27.0%) |
| Malignant | 27 (73.0%) | ||
| Nodule size on US (cm) | ATA low risk | 24 (64.9%) | |
| Median (IQR) | 1.8 (1.4–2.5) | ATA intermediate risk | 2 (5.4%) |
| <2 | 20 (54.1%) | ATA high risk | 1 (2.7%) |
| 2–4 | 16 (43.2%) | ||
| >4 | 1 (2.7%) | Histology | |
| Papillary carcinoma | 25 (67.6%) | ||
| Bethesda category | Follicular subtype | 16 (43.2%) | |
| III | 23 (62.2%) | Classical subtype | 7 (18.9%) |
| IV | 14 (37.8%) | Solid subtype | 1 (2.7%) |
| Tall cell subtype | 1 (2.7%) | ||
| Alteration type | NIFTP | 5 (13.5%) | |
| BRAF mutation | 32 (86.5%) | Follicular nodular disease | 5 (13.5%) |
| Class I | 0 (0%) | Oncocytic carcinoma | 1 (2.7%) |
| Class II | 25 (67.6%) | HGDTC | 1 (2.7%) |
| K601E | 17 (45.9%) | ||
| G469A | 4 (10.8%) | Gross extrathyroidal extension | 1 (2.7%) |
| N486_P490del | 1 (2.7%) | ||
| Class II + other * | 3 (8.1%) | Nodal disease | 1 (2.7%) |
| Class III | 0 (0%) | ||
| Unclassified † | 7 (18.9%) | Distant metastases | 0 (0%) |
| BRAF fusion | 4 (10.8%) | ||
| AGK–BRAF | 3 (8.1%) | Radioactive iodine | 3 (8.1%) |
| AKAP9–BRAF | 1 (2.7%) | ||
| BRAF-like gene expression ‡ | 1 (2.7%) | Recurrence | 0 (0%) |
| Duration of follow-up (months) | Deaths | 0 (0%) | |
| Median (IQR) | 27 (17.8–45.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Instrum, R.; Swartzwelder, C.E.; Ghossein, R.A.; Xu, B.; Givi, B.; Wong, R.J.; Untch, B.R.; Morris, L.G.T. Clinical and Pathologic Characteristics of Cytologically Indeterminate Thyroid Nodules with Non-V600E BRAF Alterations. Cancers 2025, 17, 741. https://doi.org/10.3390/cancers17050741
Instrum R, Swartzwelder CE, Ghossein RA, Xu B, Givi B, Wong RJ, Untch BR, Morris LGT. Clinical and Pathologic Characteristics of Cytologically Indeterminate Thyroid Nodules with Non-V600E BRAF Alterations. Cancers. 2025; 17(5):741. https://doi.org/10.3390/cancers17050741
Chicago/Turabian StyleInstrum, Ryan, Christina E. Swartzwelder, Ronald A. Ghossein, Bin Xu, Babak Givi, Richard J. Wong, Brian R. Untch, and Luc G. T. Morris. 2025. "Clinical and Pathologic Characteristics of Cytologically Indeterminate Thyroid Nodules with Non-V600E BRAF Alterations" Cancers 17, no. 5: 741. https://doi.org/10.3390/cancers17050741
APA StyleInstrum, R., Swartzwelder, C. E., Ghossein, R. A., Xu, B., Givi, B., Wong, R. J., Untch, B. R., & Morris, L. G. T. (2025). Clinical and Pathologic Characteristics of Cytologically Indeterminate Thyroid Nodules with Non-V600E BRAF Alterations. Cancers, 17(5), 741. https://doi.org/10.3390/cancers17050741





