Efficacy of Vascular Ligation for the Prevention of Intra- and Postoperative Bleeding in Transoral Robotic Surgery for Oropharyngeal Cancer
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Population
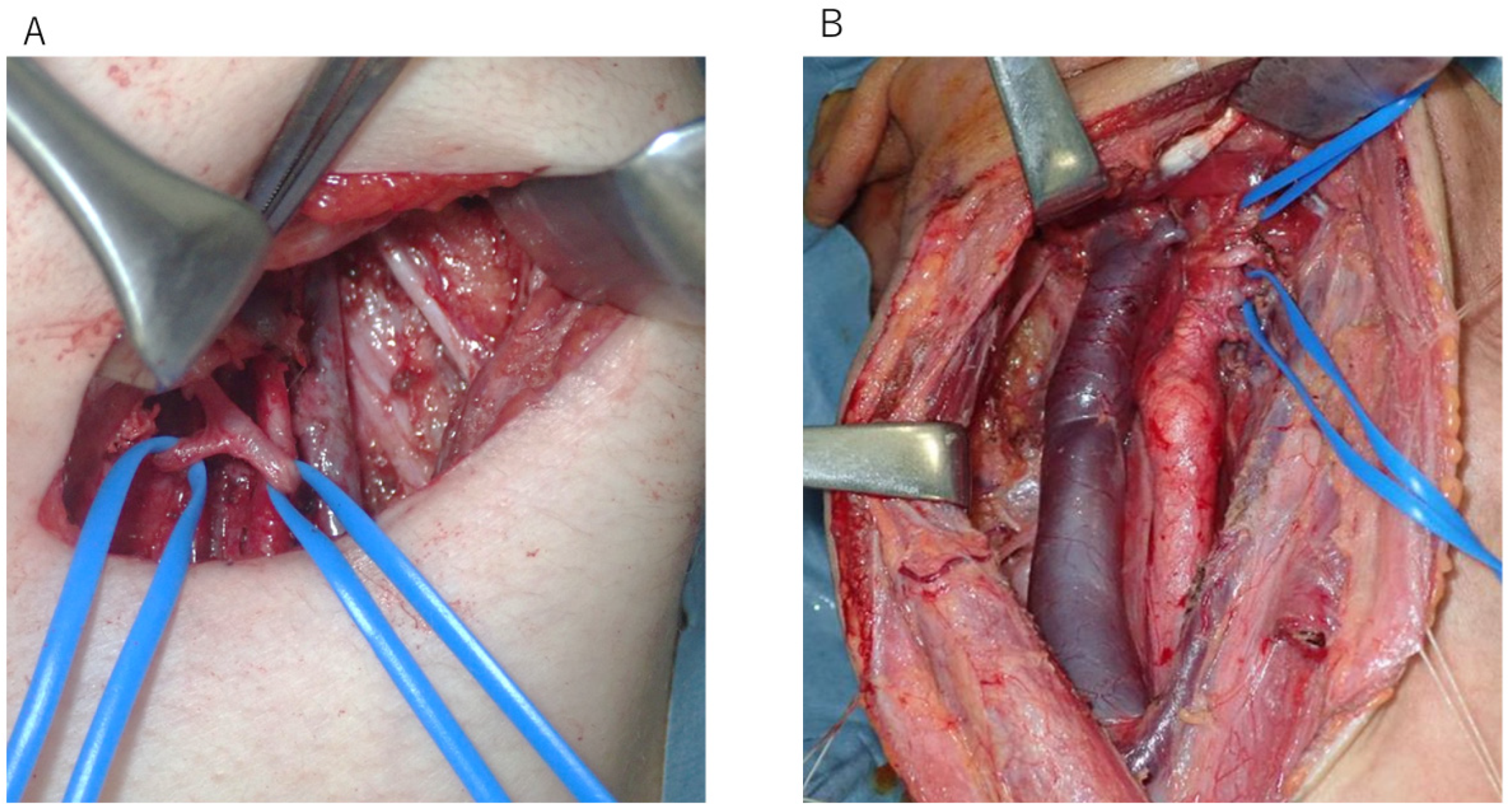
2.2. Surgical Procedure
2.3. Ethical Considerations
2.4. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. Assessment of Intra- and Postoperative Outcomes
3.3. Postoperative Bleeding After TORS
3.4. Late Cervical Lymph Node Metastasis After TORS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TORS | Transoral robotic surgery |
| ND | Neck dissection |
| CRT | Chemoradiotherapy |
| ENE | Extranodal extension |
| FOSS | Functional Outcome Swallowing Scale |
| SCC | Squamous cell carcinoma |
| RLN | Rouviere lymph node |
References
- Weinstein, G.S.; O’Malley, B.W., Jr.; Hockstein, N.G. Transoral robotic surgery: Supraglottic laryngectomy in a canine model. Laryngoscope 2005, 115, 1315–1319. [Google Scholar] [CrossRef]
- Sano, D.; Tateya, I.; Hori, R.; Ueda, T.; Mori, T.; Maruo, T.; Tsukahara, K.; Oridate, N. Transoral robotic surgery (TORS) in Japan: Procedures, advantages and current status. Jpn. J. Clin. Oncol. 2024, 9, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, K.; Hanyu, K.; Shimizu, A. Useful combination of Gehanno method, polyglycolic acid sheet and fibrin glue for prevention of postoperative dysfunction after soft palate resection with TORS. Auris Nasus Larynx 2024, 51, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Sano, D.; Shimizu, A.; Tateya, I.; Fujiwara, K.; Kishimoto, Y.; Maruo, T.; Fujimoto, Y.; Mori, T.; Kato, H.; Tsukahara, K.; et al. Current Status of Transoral Surgery for Patients With Early-Stage Pharyngeal and Laryngeal Cancers in Japan. Front. Oncol. 2021, 11, 804933. [Google Scholar] [CrossRef]
- Weinstein, G.S.; Quon, H.; Newman, H.J.; Chalian, J.A.; Malloy, K.; Lin, A.; Desai, A.; Livolsi, V.A.; Montone, K.T.; Cohen, K.R.; et al. Transoral robotic surgery alone for oropharyngeal cancer: An analysis of local control. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 628–634. [Google Scholar] [CrossRef]
- Williamson, A.; Moen, C.M.; Slim, M.A.M.; Warner, L.; O’Leary, B.; Paleri, V. Transoral robotic surgery without adjuvant therapy: A systematic review and meta-analysis of the association between surgical margins and local recurrence. Oral Oncol. 2023, 147, 106610. [Google Scholar] [CrossRef] [PubMed]
- Sethia, R.; Yumusakhuylu, A.C.; Ozbay, I.; Diavolitsis, V.; Brown, N.V.; Zhao, S.; Wei, L.; Old, M.; Agrawal, A.; Teknos, T.N.; et al. Quality of life outcomes of transoral robotic surgery with or without adjuvant therapy for oropharyngeal cancer. Laryngoscope 2018, 128, 403–411. [Google Scholar] [CrossRef]
- Meulemans, J.; Vanermen, M.; Goeleven, A.; Clement, P.; Nuyts, S.; Laenen, A.; Delaere, P.; Vander Poorten, V. Transoral robotic surgery (TORS) using the da Vinci Xi: Prospective analysis of feasibility, safety, and outcomes. Head Neck 2022, 44, 143–157. [Google Scholar] [CrossRef]
- Bollig, C.A.; Gilley, D.R.; Ahmad, J.; Jorgensen, J.B. Prophylactic arterial ligation following transoral robotic surgery: A systematic review and meta-analysis. Head Neck 2020, 42, 739–746. [Google Scholar] [CrossRef]
- Blom, M.; Zhang, H.; Tescher, A.; Dixon, B.; Magarey, M. Staged neck dissection prior to transoral robotic surgery for oropharyngeal cancer: Does it reduce post-operative complication rates? A multi-centre study of 104 patients. Eur. Arch. Otorhinolaryngol. 2023, 280, 5067–5072. [Google Scholar] [CrossRef]
- Pollei, T.R.; Hinni, M.L.; Moore, E.J.; Hayden, R.E.; Olsen, K.D.; Casler, J.D.; Walter, L.C. Analysis of postoperative bleeding and risk factors in transoral surgery of the oropharynx. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.C.; Theurer, J.; Prisman, E.; Read, N.; Berthelet, E.; Tran, E.; Fung, K.; de Almeida, J.R.; Bayley, A.; Goldstein, D.P.; et al. Randomized Trial of Radiotherapy Versus Transoral Robotic Surgery for Oropharyngeal Squamous Cell Carcinoma: Long-Term Results of the ORATOR Trial. J. Clin. Oncol. 2022, 40, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Campo, F.; Iocca, O.; De Virgilio, A.; Mazzola, F.; Mercante, G.; Pichi, B.; Holsinger, F.C.; Di Maio, P.; Ramella, S.; Pellini, R. Treatment of oropharyngeal squamous cell carcinoma: Is swallowing quality better after TORS or RT? Radiother. Oncol. 2023, 183, 109547. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.I.; Madsen, A.K.Ø.; Rubek, N.; Charabi, B.W.; Wessel, I.; Jensen, C.V.; Friborg, J.; von Buchwald, C. Dysphagia and QoL 3 Years After Treatment of Oropharyngeal Cancer With TORS or Radiotherapy. Laryngoscope 2023, 133, 1893–1898. [Google Scholar] [CrossRef]
- Charters, E.; Wu, R.; Milross, C.; Bogaardt, H.; Freeman-Sanderson, A.; Ballard, K.; Davies, S.; Oates, J.; Clark, J. Swallowing and communication outcomes following primary transoral robotic surgery. Head Neck 2021, 43, 2013–2023. [Google Scholar] [CrossRef]
- Costantino, A.; Sampieri, C.; De Virgilio, A.; Kim, S.H. Neo-adjuvant chemotherapy and transoral robotic surgery in locoregionally advanced oropharyngeal cancer. Eur. J. Surg. Oncol. 2023, 49, 107121. [Google Scholar] [CrossRef]
- Solimeno, L.S.; Park, Y.M.; Lim, J.Y.; Koh, Y.W.; Kim, S.H. Treatment outcomes of neoadjuvant chemotherapy and transoral robotic surgery in locoregionally advanced laryngopharyngeal carcinoma. Head Neck 2021, 43, 3429–3436. [Google Scholar] [CrossRef]
- Silver, J.A.; Bouganim, N.; Richardson, K.; Henry, M.; Mascarella, M.A.; Ramirez-GarciaLuna, J.; Golabi, N.; Mlynarek, A.M.; Zeitouni, A.; Hier, M.P.; et al. Quality of Life After Neoadjuvant Chemotherapy and Transoral Robotic Surgery for Oropharynx Cancer. JAMA Otolaryngol. Head Neck Surg. 2024, 150, 65–74. [Google Scholar] [CrossRef]
- Sampieri, C.; Costantino, A.; Pirola, F.; Kim, D.; Lee, K.; Kim, S.H. Neoadjuvant chemotherapy combined with transoral robotic surgery for stage III and IV laryngeal and hypopharyngeal carcinomas. Oral Oncol. 2023, 140, 106371. [Google Scholar] [CrossRef]
- Sampieri, C.; Cioccoloni, E.; Costantino, A.; Kim, D.; Lee, K.; Meccariello, G.; Cammaroto, G.; Vicini, C.; Kim, S.H. Neoadjuvant chemotherapy followed by transoral robotic surgery versus upfront surgery for locoregionally advanced oropharyngeal carcinoma: A propensity score matched analysis. Head Neck 2025, 47, 175–188. [Google Scholar] [CrossRef]
- Davies, J.C.; Husain, Z.; Day, T.A.; Graboyes, E.M.; Eskander, A. Perioperative Mortality Risk in Patients Undergoing Transoral Robotic Surgery for T1-T2 Oropharyngeal Squamous Cell Carcinoma: A National Cancer Database Study. Front. Oncol. 2022, 11, 808465. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.; Migliacci, J.; Karassawa Zanoni, D.; Boyle, J.O.; Singh, B.; Wong, R.J.; Patel, S.G.; Ganly, I. Haemorrhage following transoral robotic surgery. Clin. Otolaryngol. 2018, 43, 638–644. [Google Scholar] [CrossRef]
- Chia, S.H.; Gross, N.D.; Richmon, J.D. Surgeon experience and complications with Transoral Robotic Surgery (TORS). Otolaryngol. Head Neck Surg. 2013, 149, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.J.; Olsen, S.M.; Laborde, R.R.; García, J.J.; Walsh, F.J.; Price, D.L.; Janus, J.R.; Kasperbauer, J.L.; Olsen, K.D. Long-term functional and oncologic results of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Mayo Clin. Proc. 2012, 87, 219–225. [Google Scholar] [CrossRef]
- Laccourreye, O.; Malinvaud, D.; Garcia, D.; Ménard, M.; Hans, S.; Cauchois, R.; Bonfils, P. Postoperative hemorrhage after transoral oropharyngectomy for cancer of the lateral oropharynx. Ann. Otol. Rhinol. Laryngol. 2015, 124, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.; Goehring, L.; Tuller, E.; Oxford, L.; Lippert, D.; Britt, C. External Carotid Artery Ligation Versus Selective Artery Ligation in Transoral Surgery for Oropharyngeal Cancer. Otolaryngol. Head Neck Surg. 2024, 172, 942–946. [Google Scholar] [CrossRef]
- Gleysteen, J.; Troob, S.; Light, T.; Brickman, D.; Clayburgh, D.; Andersen, P.; Gross, N. The impact of prophylactic external carotid artery ligation on postoperative bleeding after transoral robotic surgery (TORS) for oropharyngeal squamous cell carcinoma. Oral Oncol. 2017, 70, 1–6. [Google Scholar] [CrossRef]
- Sharbel, D.D.; Abkemeier, M.; Sullivan, J.; Zimmerman, Z.; Albergotti, W.G.; Duvvuri, U.; Byrd, J.K. Transcervical arterial ligation for prevention of postoperative hemorrhage in transoral oropharyngectomy: Systematic review and meta-analysis. Head Neck 2021, 43, 334–344. [Google Scholar] [CrossRef]
- Zenga, J.; Suko, J.; Kallogjeri, D.; Pipkorn, P.; Nussenbaum, B.; Jackson, R.S. Postoperative hemorrhage and hospital revisit after transoral robotic surgery. Laryngoscope 2017, 127, 2287–2292. [Google Scholar] [CrossRef]
- Stokes, W.; Ramadan, J.; Lawson, G.; Ferris, F.R.L.; Holsinger, F.C.; Turner, M.T. Bleeding Complications After Transoral Robotic Surgery: A Meta-Analysis and Systematic Review. Laryngoscope 2021, 131, 95–105. [Google Scholar] [CrossRef]
| Characteristics | Group | All Patients (N = 35) | TORS (N = 20) | TORS + ND (IIa) + Vascular Ligation (N = 8) | TORS + ND (II–IV) + Vascular Ligation (N = 7) |
|---|---|---|---|---|---|
| Age, year median | 71.00 [30.00, 81.00] | 71.00 [59.00, 80.00] | 71.50 [30.00, 77.00] | 64.00 [37.00, 81.00] | |
| Sex, No. (%) | male | 29 (82.9) | 17 (85.0) | 6 (75.0) | 6 (85.7) |
| Female | 6 (17.1) | 3 (15.0) | 2 (25.0) | 1 (14.3) | |
| PS, No. (%) | 0 | 34 (97.1) | 19 (95.0) | 8 (100.0) | 7 (100.0) |
| 1 | 1 (2.9) | 1 (5.0) | 0 (0.0) | 0 (0.0) | |
| Perioperative anticoagulation, No. (%) | Yes | 7 (20.0) | 4 (20.0) | 2 (25.0) | 1 (14.3) |
| No | 28 (80.0) | 16 (80.0) | 6 (75.0) | 6 (85.7) | |
| History of head and neck cancer | Yes | 9 (25.7) | 7 (35.0) | 1 (12.5) | 1 (14.3) |
| No | 26 (74.3) | 13 (65.0) | 7 (87.5) | 6 (85.7) | |
| Prior radiation, No. (%) | Yes | 7 (20.0) | 5 (25.0) | 1 (12.5) | 1 (14.3) |
| No | 28 (80.0) | 15 (75.0) | 7 (87.5) | 6 (85.7) | |
| Primary site, No. (%) | Soft palate | 5 (14.3) | 4 (20.0) | 0 (0.0) | 1 (14.3) |
| Palatine tonsil | 17 (48.6) | 9 (45.0) | 3 (37.5) | 5 (71.4) | |
| Base of tongue | 10 (28.6) | 4 (20.0) | 5 (62.5) | 1 (14.3) | |
| Posterior wall | 3 (8.6) | 3 (15.0) | 0 (0.0) | 0 (0.0) | |
| Pathological diagnosis (%) | BCC | 1 (2.9) | 0 (0.0) | 0 (0.0) | 1 (14.3) |
| clear cell ca | 1 (2.9) | 0 (0.0) | 1 (12.5) | 0 (0.0) | |
| mucoeidermoid carcinoma | 1(2.9) | 1 (5.0) | 0 (0.0) | 0 (0.0) | |
| SCC | 25 (71.4) | 12 (60.0) | 7 (87.5) | 6 (85.7) | |
| SCCinsitu | 7 (20.0) | 7 (35.0) | 0 (0.0) | 0 (0.0) | |
| p16status, No. (%) | Positive | 13 (37.1) | 6 (30.0) | 2 (25.0) | 5 (71.4) |
| Negative | 22 (62.9) | 14 (70.0) | 6 (75.0) | 2 (28.6) | |
| cT (%), No. (%) | is | 7 (20.0) | 7 (35.0) | 0 (0.0) | 0 (0.0) |
| 1 | 11 (31.4) | 7 (35.0) | 2 (25.0) | 2 (28.6) | |
| 2 | 16 (45.7) | 6 (30.0) | 5 (62.5) | 5 (71.4) | |
| 4a | 1 (2.9) | 0 (0.0) | 1 (12.5) | 0 (0.0) | |
| cN (%), No. (%) | 0 | 28 (80.0) | 20 (100.0) | 8 (100.0) | 0 (0.0) |
| 1 | 5 (14.3) | 0 (0.0) | 0 (0.0) | 5 (71.4) | |
| 2 | 2 (5.7) | 0 (0.0) | 0 (0.0) | 2 (28.6) |
| Factor | Group | TORS (N = 20) | TORS + ND + Vascular Ligation (N = 15) | p Value |
|---|---|---|---|---|
| Total operation time, min | 77.5 [42.0, 132.0] | 202.0 [122.0, 398.0] | <0.001 | |
| Console time, min | 58.0 [40.0, 103.0] | 91.0 [68.0, 163.0] | <0.001 | |
| Total amount of blood loss, mL | 2.50 [0.0, 60.0] | 30.0 [0.0, 170.0] | 0.001 | |
| blood loss during console | 2.50 [0.0, 60.0] | 10.0 [0.0, 34.0] | 0.221 | |
| blood loss during ND | NA | 13.0 [0.0, 160.0] | - | |
| tumor size, mm | 16.50 [5.0, 39.0] | 26.0 [18.0, 40.0] | 0.012 | |
| Length of skin incision, cm | NA | 5.0 [3.00, 17.00] | - | |
| late cervical lymph node metastasis (%) | Yes | 2 (10.0) | 2 (13.3) | 1 |
| No | 18 (90.0) | 13 (86.7) | ||
| surgical margin (%) | Yes | 0 (0.0) | 1 (6.7) | 0.429 |
| No | 20 (100.0) | 14 (93.3) | ||
| pN (%) | 0 | 19 (100.0) | 5 (33.3) | <0.001 |
| 1 | 0 (0.0) | 5 (33.3) | ||
| 2 | 0 (0.0) | 5 (33.3) | ||
| pT (%) | is | 7 (35.0) | 0 (0.0) | <0.001 |
| 1 | 7 (35.0) | 0 (0.0) | ||
| 2 | 6 (30.0) | 13 (86.7) | ||
| 4 | 0 (0.0) | 2 (13.3) | ||
| SICU stay (%) | Yes | 1 (5.0) | 1 (6.7) | 1 |
| No | 19 (95.0) | 14 (93.3) | ||
| perioperative complications (%) | Yes | 4 (20.0) | 5 (33.3) | 0.451 |
| No | 16 (80.0) | 10 (66.7) | ||
| postoperative bleeding (%) | Yes | 1 (5.0) | 2 (13.3) | 0.565 |
| No | 19 (95.0) | 13 (86.7) | ||
| postoperative hospital stay, day | 6.0 [3.0, 14.0] | 10.0 [6.0, 32.0] | 0.015 | |
| period until peroral intake after surgery, day | 1.0 [1.0, 11.0] | 2.0 [1.0, 26.0] | 0.13 | |
| FOSS at 2 months after surgery (%) | 0 | 18 (100.0) | 13 (92.9) | 0.438 |
| 1 | 0 (0.0) | 1 (7.1) | ||
| Factor | Group | TORS + ND (IIa) + Vascular Ligation (N = 8) | TORS + ND (II–IV) + Vascular Ligation (N = 7) | p Value |
|---|---|---|---|---|
| Operation time, min | 172.5 [122.0, 261.0] | 307.0 [202.0, 398.0] | 0.003 | |
| Console time, min | 88.5 [68.0, 127.0] | 91.00 [78.0, 163.0] | 0.385 | |
| ND time, min | 84.0 [50.0, 134.0] | 199.0 [112.0, 269.0] | 0.003 | |
| Total amount of blood loss, mL | 15.0 [0.0, 48.0] | 43.0 [22.0, 170.0] | 0.032 | |
| blood loss during console, mL | 10.0 [0.0, 34.0] | 10.0 [0.0, 20.0] | 0.677 | |
| blood loss during ND, mL | 7.5 [0.0, 24.0] | 33.0 [10.0, 160.0] | 0.02 | |
| tumor size, mm | 25.0 [18.0, 40.0] | 26.0 [21.0, 37.0] | 0.816 | |
| Length of skin incision, cm | 4.0 [3.0, 5.0] | 12.0 [10.0, 17.0] | 0.001 | |
| late cervical lymph node metastasis (%) | Yes | 1 (12.5) | 1 (14.3) | 1 |
| No | 7 (87.5) | 6 (85.7) | ||
| surgical margin (%) | Yes | 0 (0.0) | 1 (14.3) | 0.467 |
| No | 8 (100.0) | 6 (85.7) | ||
| pN (%) | 0 | 5 (62.5) | 0 (0.0) | 0.068 |
| 1 | 2 (25.0) | 3 (42.9) | ||
| 2 | 1 (12.5) | 4 (57.1) | ||
| pT (%) | is | 0 (0.0) | 0 (0.0) | 1 |
| 1 | 0 (0.0) | 0 (0.0) | ||
| 2 | 7 (87.5) | 6 (85.7) | ||
| 4 | 1 (12.5) | 1 (14.3) | ||
| SICU stay (%) | Yes | 0 (0.0) | 1 (14.3) | 0.467 |
| No | 8 (100.0) | 6 (85.7) | ||
| perioperative complications (%) | Yes | 2 (25.0) | 3 (42.9) | 0.608 |
| No | 6 (75.0) | 4 (57.1) | ||
| postoperative bleeding (%) | Yes | 1 (12.5) | 1 (14.3) | 1 |
| No | 7 (87.5) | 6 (85.7) | ||
| postoperative hospital stay, day | 10.5 [6.0, 18.0] | 10.0 [7.0, 32.0] | 0.954 | |
| period until peroral intake after TORS, day | 2.0 [1.0, 11.0] | 2.0 [1.0, 26.0] | 0.507 | |
| FOSS at 2 months, after TORS (%) | 0 | 7 (87.5) | 6 (100.0) | 1 |
| 1 | 1 (12.5) | 0 (0.0) |
| Sex | Age | Primary Site | Bleeding Severity | Postoperative Day of Bleeding | Postoperative Hospital Stay | Prior Radiation | Perioperative Anticoagulation | Blood Loss During Console | Blood Loss During ND | ND + Vascular Ligation | pT | pN | p16 Status | Surgical Margin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | 80 | Palatine tonsil | minor | 10 | 9 | - | - | 2 | NA | - | 1 | 0 | positive | negative |
| Male | 63 | Base of tongue | intermediate | 14 | 6 | - | - | 0 | 13 | ND (IIa) + vascular ligation | 2 | 0 | positive | negative |
| Male | 73 | Base of tongue | minor | 14 | 10 | - | + | 15 | 10 | ND (II–IV) + vascular ligation | 2 | 2 | positive | negative |
| Sex | Age | Primary Site | The Time to Late Cervical Lymph Node Metastasis (Month) | The Late Cervical Lymph Node Site | ND + Vascular Ligation | Pathological Diagnosis | pT | pN | p16 Status | Surgical Margin |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | 71 | Palatine tonsil | 3 | IIa | - | SCC | 2 | 0 | positive | negative |
| Male | 80 | Palatine tonsil | 15 | IIa, III | - | SCC | 1 | 0 | positive | negative |
| Male | 77 | Palatine tonsil | 6 | IIb | ND (IIa) + vascular ligation | SCC | 4a | 2 | negative | negative |
| Male | 64 | Palatine tonsil | 18 | V, RLN | ND (II–IV) + vascular ligation | SCC | 2 | 1 | positive | negative |
| RLN: Rouviere lymph node | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, T.; Taruya, T.; Hattori, M.; Chikuie, N.; Sato, Y.; Hattori, T.; Hamamoto, T.; Ishino, T.; Takeno, S. Efficacy of Vascular Ligation for the Prevention of Intra- and Postoperative Bleeding in Transoral Robotic Surgery for Oropharyngeal Cancer. Cancers 2025, 17, 1446. https://doi.org/10.3390/cancers17091446
Ueda T, Taruya T, Hattori M, Chikuie N, Sato Y, Hattori T, Hamamoto T, Ishino T, Takeno S. Efficacy of Vascular Ligation for the Prevention of Intra- and Postoperative Bleeding in Transoral Robotic Surgery for Oropharyngeal Cancer. Cancers. 2025; 17(9):1446. https://doi.org/10.3390/cancers17091446
Chicago/Turabian StyleUeda, Tsutomu, Takayuki Taruya, Minoru Hattori, Nobuyuki Chikuie, Yuki Sato, Takayoshi Hattori, Takao Hamamoto, Takashi Ishino, and Sachio Takeno. 2025. "Efficacy of Vascular Ligation for the Prevention of Intra- and Postoperative Bleeding in Transoral Robotic Surgery for Oropharyngeal Cancer" Cancers 17, no. 9: 1446. https://doi.org/10.3390/cancers17091446
APA StyleUeda, T., Taruya, T., Hattori, M., Chikuie, N., Sato, Y., Hattori, T., Hamamoto, T., Ishino, T., & Takeno, S. (2025). Efficacy of Vascular Ligation for the Prevention of Intra- and Postoperative Bleeding in Transoral Robotic Surgery for Oropharyngeal Cancer. Cancers, 17(9), 1446. https://doi.org/10.3390/cancers17091446







