Durvalumab Prolongs Overall Survival, Whereas Radiation Dose Escalation > 66 Gy Might Improve Long-Term Local Control in Unresectable NSCLC Stage III: Updated Analysis of the Austrian Radio-Oncological Lung Cancer Study Association Registry (ALLSTAR)
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Radiochemoimmunotherapy
2.3. Endpoints and Statistics
3. Results
3.1. Patients
3.2. Radiochemoimmunotherapy
3.3. Local Control, Overall Survival, and Progression-Free Survival
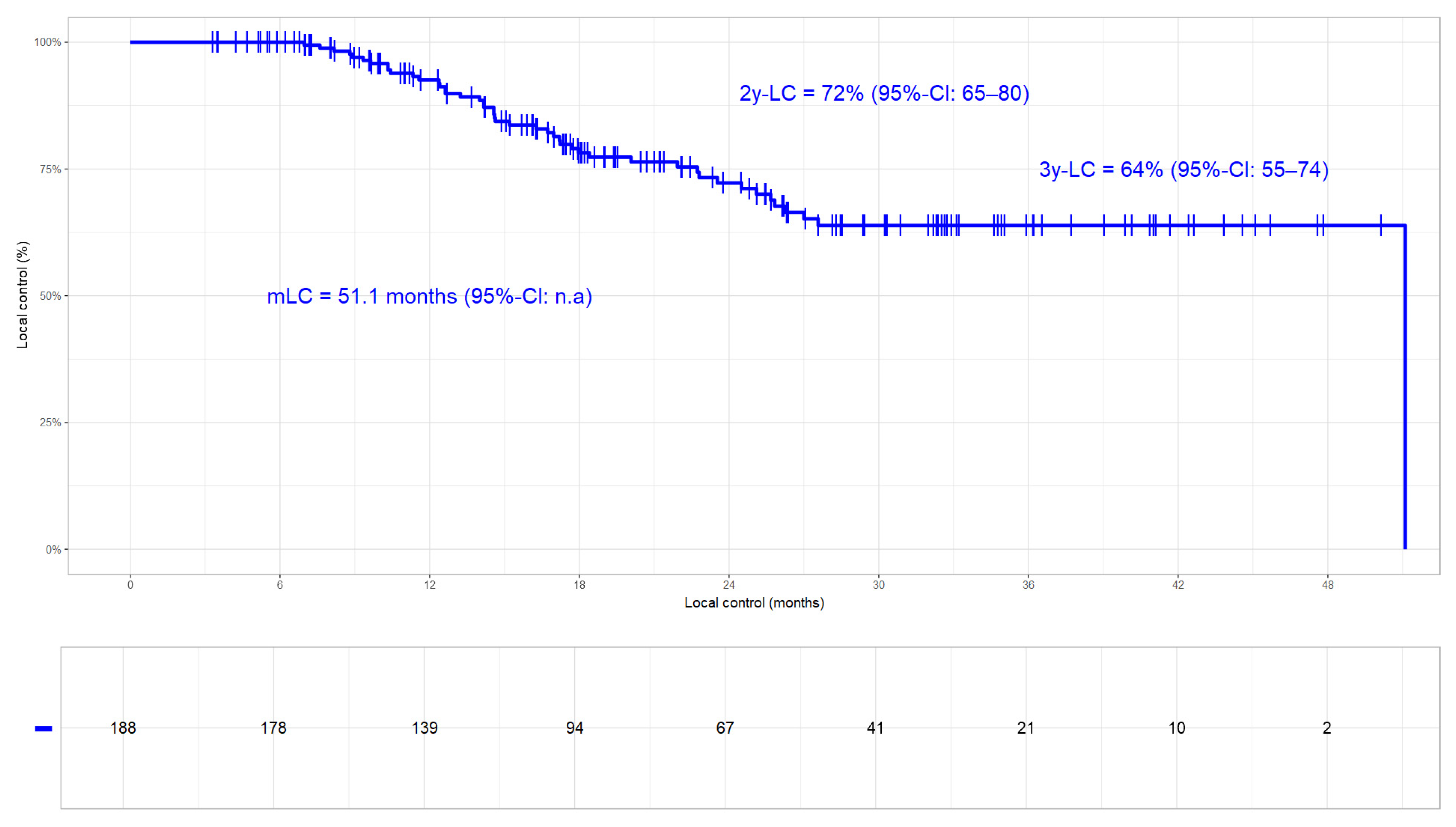
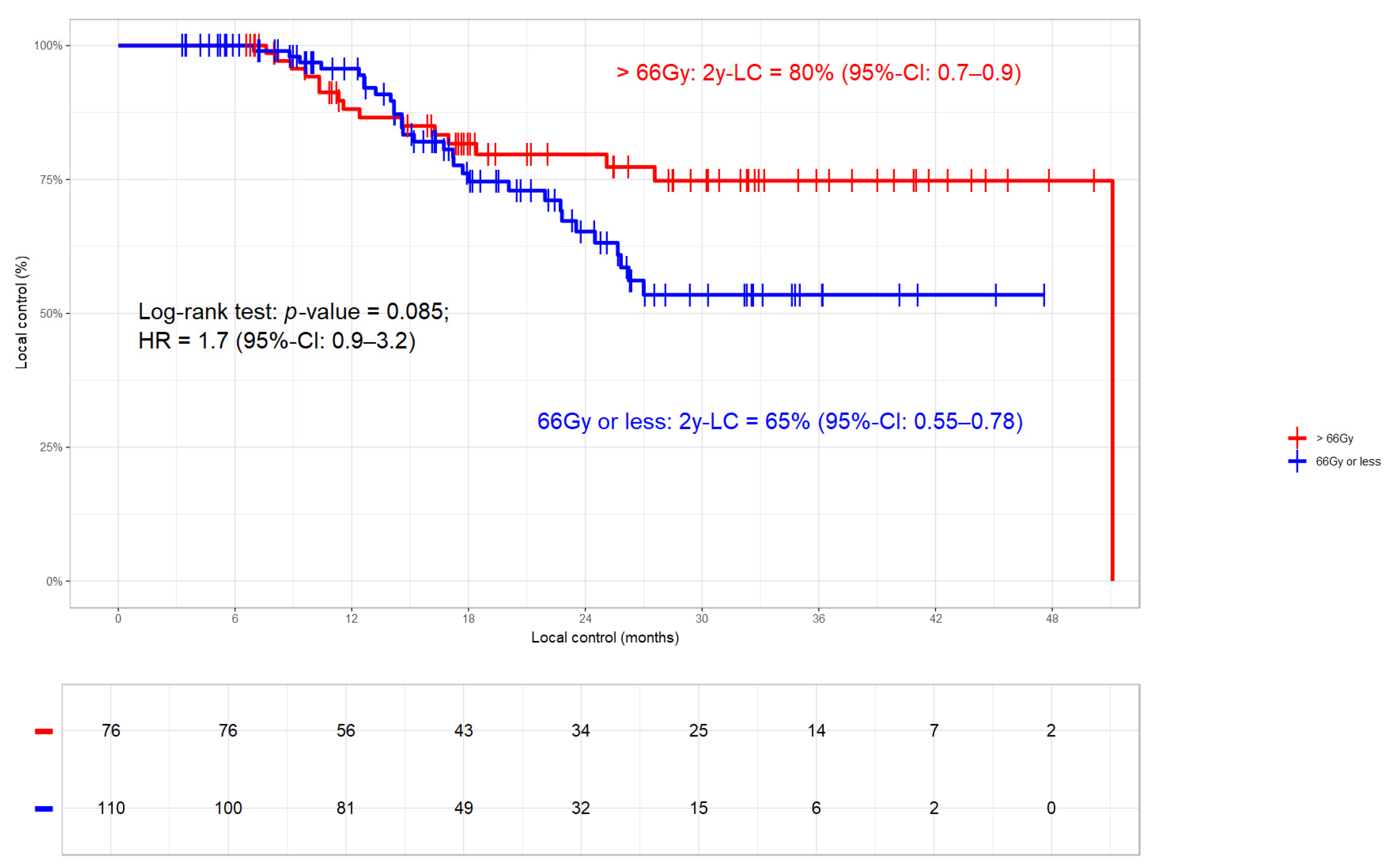
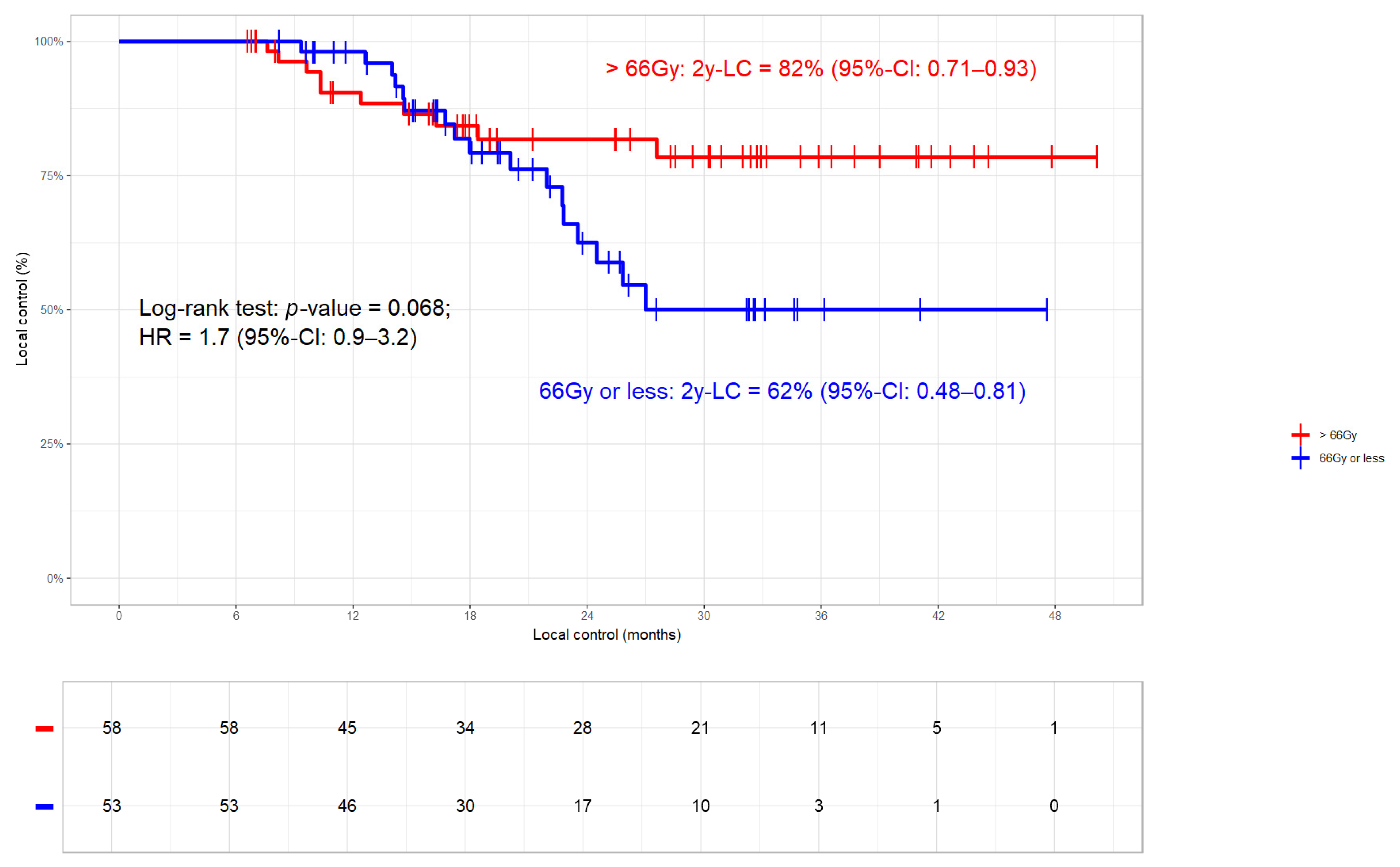
3.3.1. Local Control
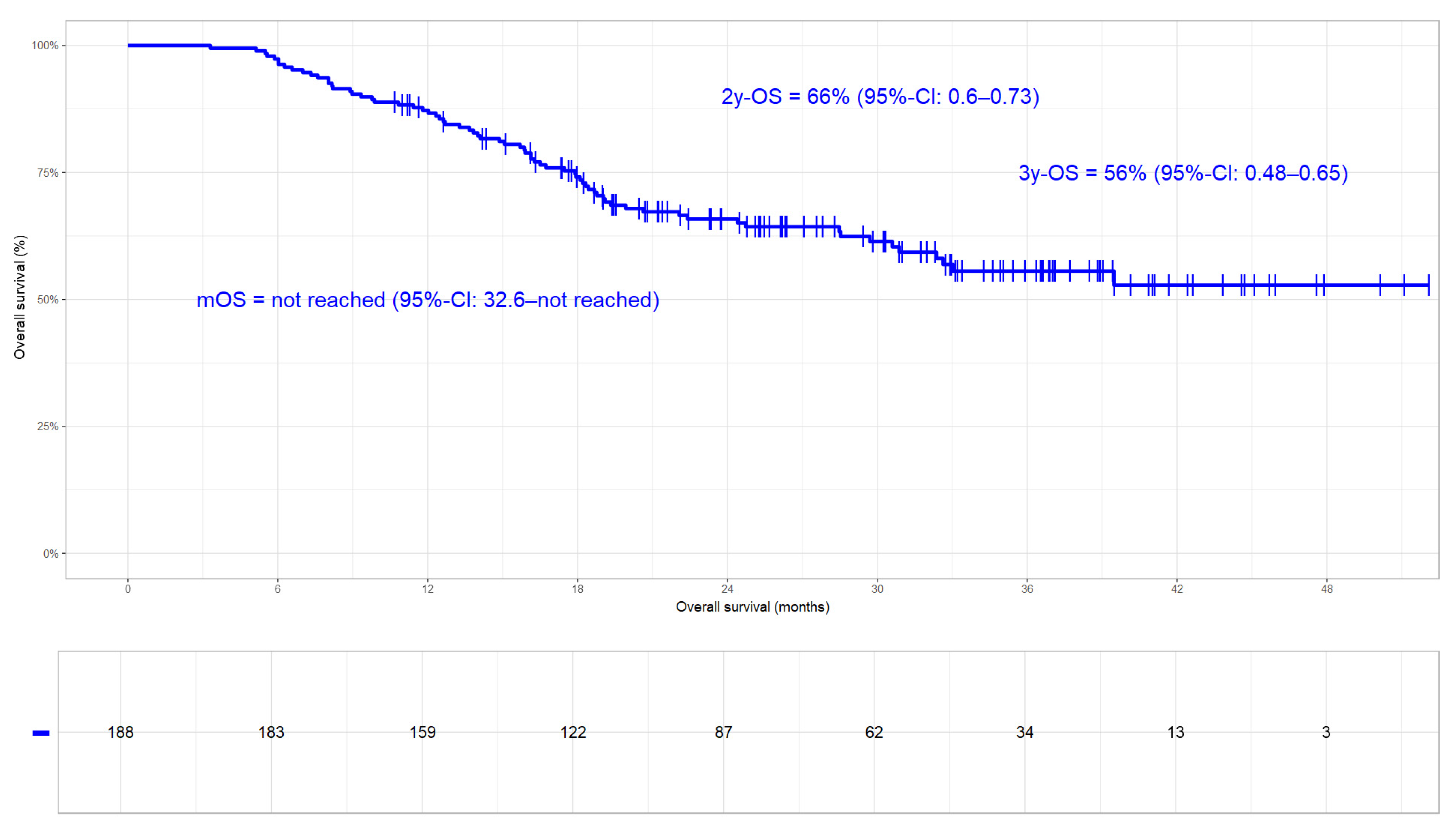
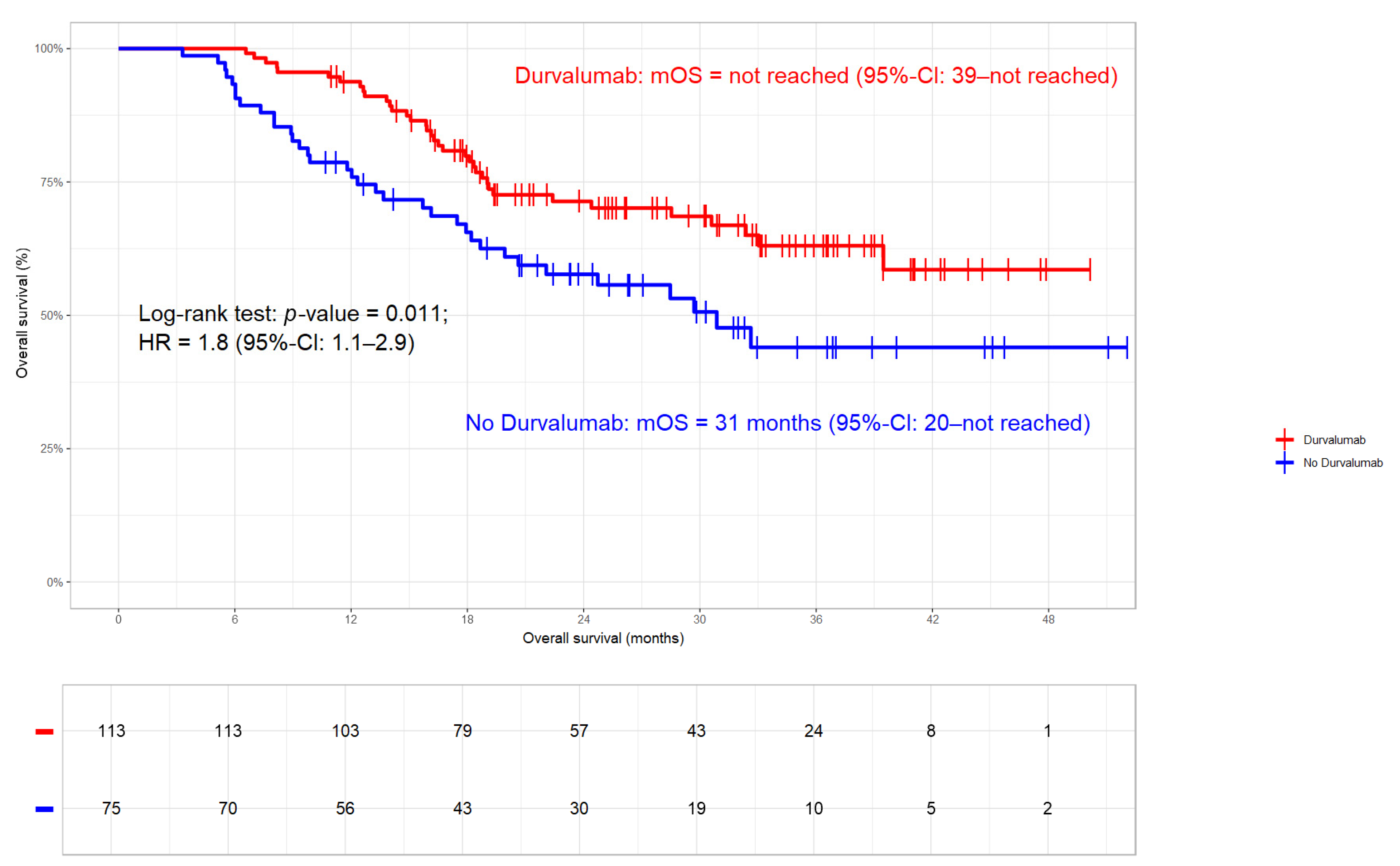
3.3.2. Overall Survival
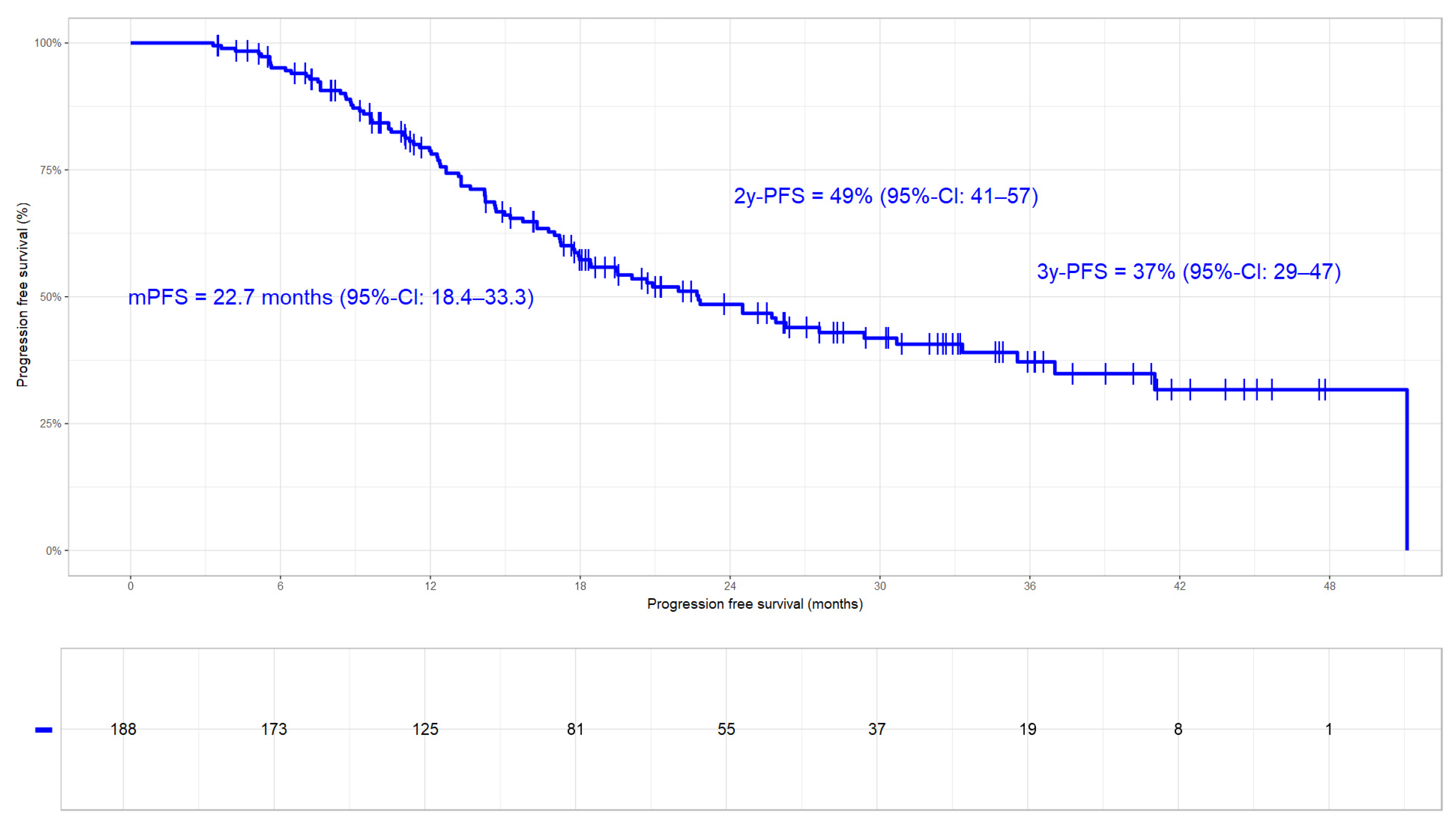
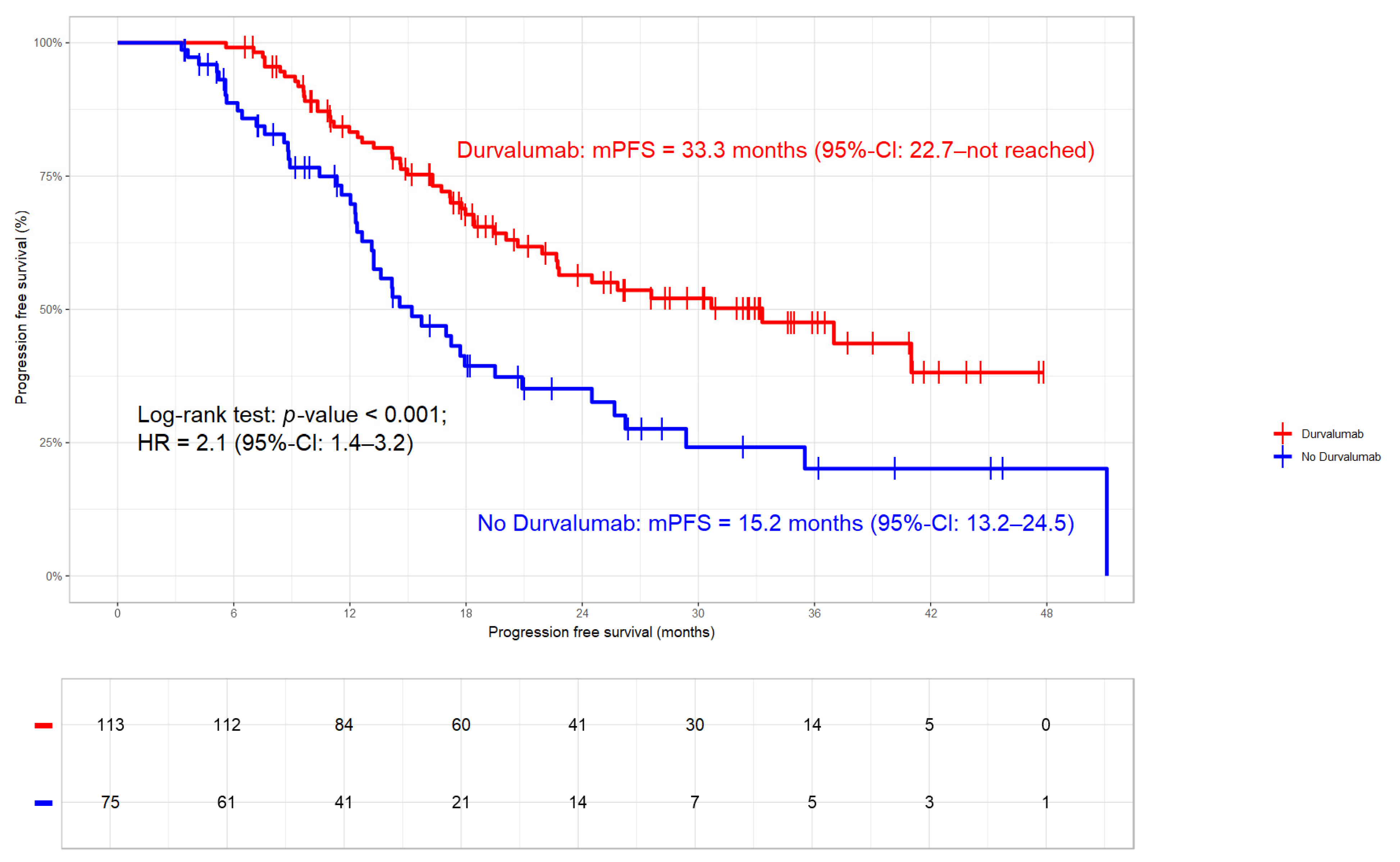
3.3.3. Progression Free Survival
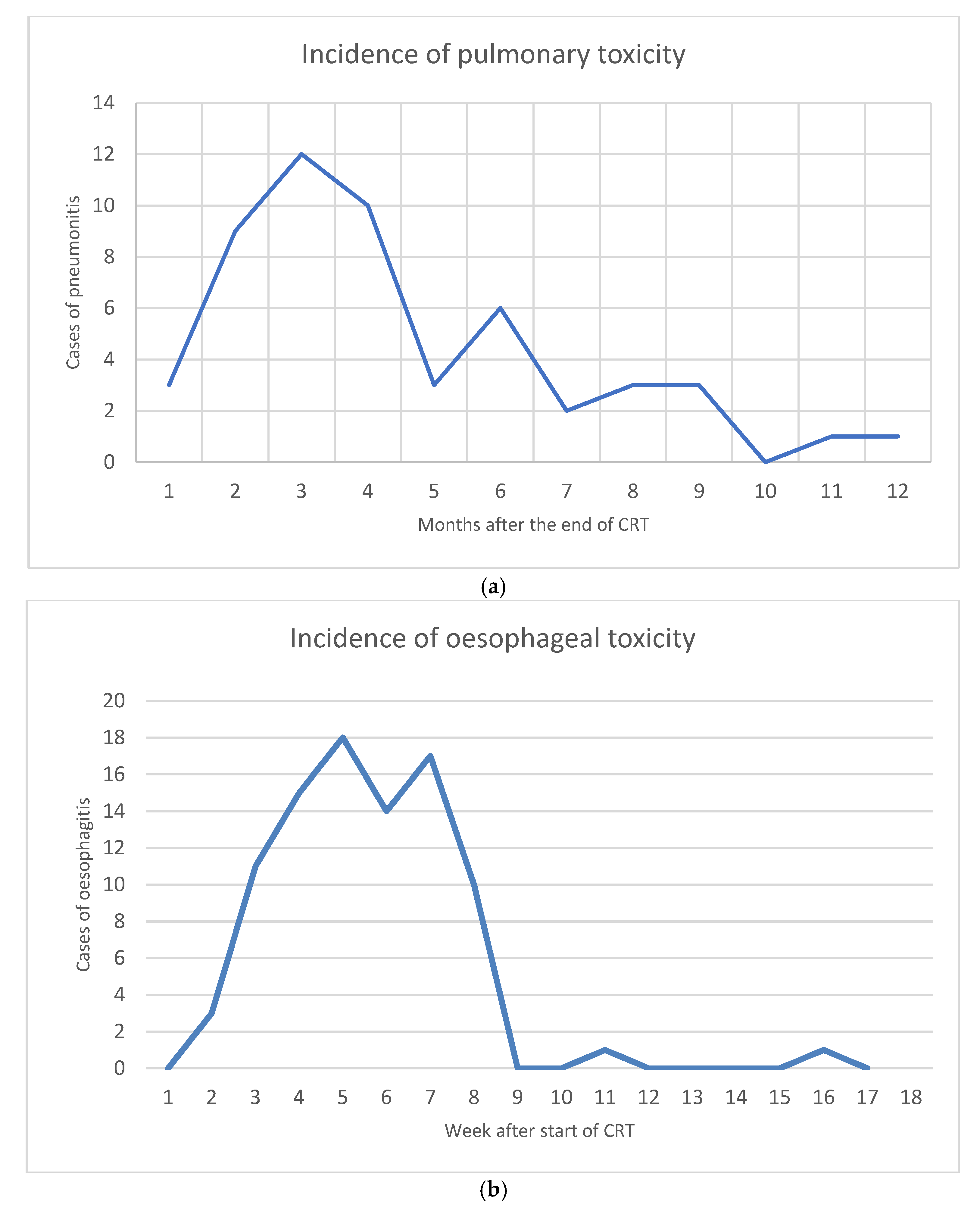
3.4. Toxicity
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AESI | adverse event of special interest |
| ALLSTAR | Austrian Radio-Oncological Lung Cancer Study Association Registry |
| CRIT | chemoradioimmunotherapy |
| CRT | chemoradiotherapy |
| cCRT | concomitant chemoradiotherapy |
| EAP | early-access programme |
| EQD2 | biologically equivalent dose in 2 Gy fractions |
| GTV | gross tumour volume |
| ICI | immune-checkpoint inhibitor |
| IMRT | intensity-modulated radiotherapy |
| LA-NSCLC | locally advanced non-small-cell lung cancer |
| LC | local control |
| MVA | multivariate analysis |
| OS | overall survival |
| PD-L1 | programmed death ligand 1 |
| PFS | progression-free survival |
| RCT | randomized control trial |
| RT | radiotherapy |
| RWD | real-world data |
| SCC | squamous cell carcinoma |
| SoC | standard of care |
| sCRT | sequential chemoradiotherapy |
| VMAT | volumetric arc therapy |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Aupérin, A.; Le Péchoux, C.; Rolland, E.; Curran, W.J.; Furuse, K.; Fournel, P.; Belderbos, J.; Clamon, G.; Ulutin, H.C.; Paulus, R.; et al. Meta-Analysis of Concomitant Versus Sequential Radiochemotherapy in Locally Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2010, 28, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes from the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Jazieh, A.R.; Onal, H.C.; Tan, D.S.W.; Soo, R.A.; Prabhash, K.; Kumar, A.; Huggenberger, R.; Robb, S.; Cho, B.-C. Real-World Treatment Patterns and Clinical Outcomes in Patients with Stage III NSCLC: Results of KINDLE, a Multicountry Observational Study. J. Thorac. Oncol. 2021, 16, 1733–1744. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Huang, Y.; Li, W.; Zhao, J.; Yang, Y.; Li, C.; Wang, L.; Bi, N. Real-World Safety and Efficacy of Consolidation Durvalumab After Chemoradiation Therapy for Stage III Non-small Cell Lung Cancer: A Systematic Review and Meta-analysis. Int. J. Radiat. Oncol. 2021, 112, 1154–1164. [Google Scholar] [CrossRef]
- Borghetti, P.; Volpi, G.; Facheris, G.; Cossali, G.; Mataj, E.; La Mattina, S.; Singh, N.; Imbrescia, J.; Bonù, M.L.; Tomasini, D.; et al. Unresectable stage III non-small cell lung cancer: Could durvalumab be safe and effective in real-life clinical scenarios? Results of a single-center experience. Front. Oncol. 2023, 13, 1208204. [Google Scholar] [CrossRef]
- Faehling, M.; Schumann, C.; Christopoulos, P.; Hoffknecht, P.; Alt, J.; Horn, M.; Eisenmann, S.; Schlenska-Lange, A.; Schütt, P.; Steger, F.; et al. Durvalumab after definitive chemoradiotherapy in locally advanced unresectable non-small cell lung cancer (NSCLC): Real-world data on survival and safety from the German expanded-access program (EAP). Lung Cancer 2020, 150, 114–122. [Google Scholar] [CrossRef]
- Girard, N.; Bar, J.; Garrido, P.; Garassino, M.C.; McDonald, F.; Mornex, F.; Filippi, A.R.; Smit, H.J.; Peters, S.; Field, J.K.; et al. Treatment Characteristics and Real-World Progression-Free Survival in Patients with Unresectable Stage III NSCLC Who Received Durvalumab After Chemoradiotherapy: Findings From the PACIFIC-R Study. J. Thorac. Oncol. 2022, 18, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Noh, J.M.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Ahn, M.-J.; Pyo, H.; Ahn, Y.C.; Park, K. Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer. Lung Cancer 2020, 146, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Kishi, N.; Matsuo, Y.; Shintani, T.; Ogura, M.; Mitsuyoshi, T.; Araki, N.; Fujii, K.; Okumura, S.; Nakamatsu, K.; Kishi, T.; et al. Recurrence patterns and progression-free survival after chemoradiotherapy with or without consolidation durvalumab for stage III non-small cell lung cancer. J. Radiat. Res. 2022, 64, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-K.; Oh, H.-J.; Kim, Y.-C.; Kim, Y.-H.; Ahn, S.-J.; Jeong, W.G.; Lee, J.Y.; Lee, J.C.; Choi, C.M.; Ji, W.; et al. Korean Real-World Data on Patients with Unresectable Stage III NSCLC Treated with Durvalumab After Chemoradiotherapy: PACIFIC-KR. J. Thorac. Oncol. 2023, 18, 1042–1054. [Google Scholar] [CrossRef]
- Preti, B.T.B.; Sanatani, M.S.; Breadner, D.; Lakkunarajah, S.; Scott, C.; Esmonde-White, C.; McArthur, E.; Rodrigues, G.; Chaudhary, M.; Mutsaers, A.; et al. Real-World Analysis of Durvalumab after Chemoradiation in Stage III Non-Small-Cell Lung Cancer. Curr. Oncol. 2023, 30, 7713–7721. [Google Scholar] [CrossRef]
- Filippi, A.; Bar, J.; Chouaid, C.; Christoph, D.; Field, J.; Fietkau, R.; Garassino, M.; Garrido, P.; Haakensen, V.; Kao, S.; et al. Real-world outcomes with durvalumab after chemoradiotherapy in patients with unresectable stage III NSCLC: Interim analysis of overall survival from PACIFIC-R. ESMO Open 2024, 9, 103464. [Google Scholar] [CrossRef]
- Rueda, A.G.; Taus, Á.; Álvarez, R.Á.; Bernabé-Caro, R.; Chara, L.; López-Brea, M.; Vilà, L.; González, M.Á.S.; Aldagalán, A.d.B.D.; Herrera, B.E.; et al. The S-REAL study: Spanish real-world data on unresectable stage III NSCLC patients treated with durvalumab after chemoradiotherapy. Clin. Transl. Oncol. 2024, 26, 1779–1789. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, J.J.; Soon, Y.Y.; Wong, A.; Aminkeng, F.; Ang, Y.; Asokumaran, Y.; Low, J.L.; Lee, M.; Choo, J.R.E.; et al. Real-world experience of consolidation durvalumab after concurrent chemoradiotherapy in stage III non-small cell lung cancer. Thorac. Cancer 2022, 13, 3152–3161. [Google Scholar] [CrossRef]
- Hong, K.S.; Choi, S.H.; Lee, S.Y.; Shin, K.-C.; Jang, J.G.; Kwon, Y.S.; Park, S.H.; Choi, K.-J.; Jung, C.Y.; Eom, J.S.; et al. Durvalumab Consolidation After Chemoradiotherapy in Elderly Patients with Unresectable Stage III NSCLC: A Real-World Multicenter Study. Clin. Lung Cancer 2024, 25, 354–364. [Google Scholar] [CrossRef]
- Abe, T.; Saito, S.; Iino, M.; Aoshika, T.; Ryuno, Y.; Ohta, T.; Igari, M.; Hirai, R.; Kumazaki, Y.; Miura, Y.; et al. Effect of durvalumab on local control after concurrent chemoradiotherapy for locally advanced non-small cell lung cancer in comparison with chemoradiotherapy alone. Thorac. Cancer 2020, 12, 245–250. [Google Scholar] [CrossRef]
- Zehentmayr, F.; Feurstein, P.; Ruznic, E.; Langer, B.; Grambozov, B.; Klebermass, M.; Hüpfel, H.; Feichtinger, J.; Minasch, D.; Heilmann, M.; et al. Durvalumab impacts progression-free survival while high-dose radiation >66 Gy improves local control without excess toxicity in unresectable NSCLC stage III: Real-world data from the Austrian radio-oncological lung cancer study association registry (ALLSTAR). Radiother. Oncol. 2024, 196, 110294. [Google Scholar] [CrossRef] [PubMed]
- Raben, D.; Rimner, A.; Senan, S.; Broadhurst, H.; Pellas, T.; Dennis, P.; Faivre-Finn, C. Patterns of Disease Progression with Durvalumab in Stage III Non-small Cell Lung Cancer (PACIFIC). Int. J. Radiat. Oncol. 2019, 105, 683. [Google Scholar] [CrossRef]
- Furuse, K.; Fukuoka, M.; Kawahara, M.; Nishikawa, H.; Takada, Y.; Kudoh, S.; Katagami, N.; Ariyoshi, Y. Phase III Study of Concurrent Versus Sequential Thoracic Radiotherapy in Combination with Mitomycin, Vindesine, and Cisplatin in Unresectable Stage III Non–Small-Cell Lung Cancer. J. Clin. Oncol. 1999, 17, 2692. [Google Scholar] [CrossRef]
- Fournel, P.; Robinet, G.; Thomas, P.; Souquet, P.-J.; Léna, H.; Vergnenégre, A.; Delhoume, J.-Y.; Le Treut, J.; Silvani, J.-A.; Dansin, E.; et al. Randomized Phase III Trial of Sequential Chemoradiotherapy Compared With Concurrent Chemoradiotherapy in Locally Advanced Non–Small-Cell Lung Cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique–Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J. Clin. Oncol. 2005, 23, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Zatloukal, P.; Petruzelka, L.; Zemanova, M.; Havel, L.; Janku, F.; Judas, L.; Kubik, A.; Krepela, E.; Fiala, P.; Pecen, L. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: A randomized study. Lung Cancer 2004, 46, 87–98. [Google Scholar] [CrossRef]
- Curran, W.J., Jr.; Paulus, R.; Langer, C.J.; Komaki, R.; Lee, J.S.; Hauser, S.; Movsas, B.; Wasserman, T.; Rosenthal, S.A.; Gore, E.; et al. Sequential vs Concurrent Chemoradiation for Stage III Non-Small Cell Lung Cancer: Randomized Phase III Trial RTOG 9410. J. Natl. Cancer Inst. 2011, 103, 1052–1460, Erratum in JNCI J. Natl. Cancer Inst. 2012, 104, 79. [Google Scholar] [CrossRef] [PubMed]
- Offin, M.; Shaverdian, N.; Rimner, A.; Lobaugh, S.; Shepherd, A.F.; Simone, C.B.; Gelblum, D.Y.; Wu, A.J.; Lee, N.; Kris, M.G.; et al. Clinical outcomes, local–regional control and the role for metastasis-directed therapies in stage III non-small cell lung cancers treated with chemoradiation and durvalumab. Radiother. Oncol. 2020, 149, 205–211. [Google Scholar] [CrossRef]
- Machtay, M.; Paulus, R.; Moughan, J.; Komaki, R.; Bradley, J.E.; Choy, H.; Albain, K.; Movsas, B.; Sause, W.T.; Curran, W.J. Defining Local-Regional Control and Its Importance in Locally Advanced Non-small Cell Lung Carcinoma. J. Thorac. Oncol. 2012, 7, 716–722. [Google Scholar] [CrossRef]
- Mauguen, A.; Le Péchoux, C.; Saunders, M.I.; Schild, S.E.; Turrisi, A.T.; Baumann, M.; Sause, W.T.; Ball, D.; Belani, C.P.; Bonner, J.A.; et al. Hyperfractionated or Accelerated Radiotherapy in Lung Cancer: An Individual Patient Data Meta-Analysis. J. Clin. Oncol. 2012, 30, 2788–2797. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Dörr, W.; Gahbauer, R.; Howell, R.W.; Joiner, M.C.; Jones, B.; Jones, D.T.; van der Kogel, A.J.; Wambersie, A.; Whitmore, G. Bioeffect modeling and equieffective dose concepts in radiation oncology—Terminology, quantities and units. Radiother. Oncol. 2012, 105, 266–268. [Google Scholar] [CrossRef]
- Bradley, J.D.; Bae, K.; Graham, M.V.; Byhardt, R.; Govindan, R.; Fowler, J.; Purdy, J.A.; Michalski, J.M.; Gore, E.; Choy, H. Primary Analysis of the Phase II Component of a Phase I/II Dose Intensification Study Using Three-Dimensional Conformal Radiation Therapy and Concurrent Chemotherapy for Patients With Inoperable Non–Small-Cell Lung Cancer: RTOG 0117. J. Clin. Oncol. 2010, 28, 2475–2480. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Dafni, U.; Boyer, M.; De Ruysscher, D.; Faivre-Finn, C.; Felip, E.; Garrido, P.; Girard, N.; Guckenberger, M.; Haanen, J.; et al. Position of a panel of international lung cancer experts on the approval decision for use of durvalumab in stage III non-small-cell lung cancer (NSCLC) by the Committee for Medicinal Products for Human Use (CHMP). Ann. Oncol. 2019, 30, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Bruni, A.; Scotti, V.; Borghetti, P.; Vagge, S.; Cozzi, S.; D’angelo, E.; Levra, N.G.; Fozza, A.; Taraborrelli, M.; Piperno, G.; et al. Corrigendum: A Real-World, Multicenter, Observational Retrospective Study of Durvalumab After Concomitant or Sequential Chemoradiation for Unresectable Stage III Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 802949. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, M.; Allen, A.; Cai, L.; Xiao, Y.; Chander, P. 116P Real-world outcomes with durvalumab (durva) after chemoradiotherapy (CRT) in patients with unresectable stage III NSCLC (SPOTLIGHT). Ann. Oncol. 2022, 33, S86. [Google Scholar] [CrossRef]
- Friedes, C.; Iocolano, M.; Lee, S.H.; Li, B.; Duan, L.; Levin, W.P.; Cengel, K.A.; Sun, L.L.; Aggarwal, C.; Marmarelis, M.E.; et al. Patterns of Failure, Low-Volume Relapse, and Subsequent Ablative Management in Locally Advanced Non-Small Cell Lung Cancer Treated with Definitive Chemoradiation and Consolidation Immune Checkpoint Inhibitors. Int. J. Radiat. Oncol. 2023, 118, 1435–1444. [Google Scholar] [CrossRef]
- Waterhouse, D.; Yong, C.; Frankart, A.; Brannman, L.; Mulrooney, T.; Robert, N.; Aguilar, K.M.; Ndukum, J.; Cotarla, I. Durvalumab real-world treatment patterns and outcomes in patients with stage III non-small-cell lung cancer treated in a US community setting. Future Oncol. 2023, 19, 1905–1916. [Google Scholar] [CrossRef]
- Landman, Y.; Jacobi, O.; Kurman, N.; Yariv, O.; Peretz, I.; Rotem, O.; Dudnik, E.; Zer, A.; Allen, A.M. Durvalumab after concurrent chemotherapy and high-dose radiotherapy for locally advanced non-small cell lung cancer. OncoImmunology 2021, 10, 1959979. [Google Scholar] [CrossRef]
- Wass, R.; Hochmair, M.; Kaiser, B.; Grambozov, B.; Feurstein, P.; Weiß, G.; Moosbrugger, R.; Sedlmayer, F.; Lamprecht, B.; Studnicka, M.; et al. Durvalumab after Sequential High Dose Chemoradiotherapy versus Standard of Care (SoC) for Stage III NSCLC: A Bi-Centric Retrospective Comparison Focusing on Pulmonary Toxicity. Cancers 2022, 14, 3226. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.; Ferini, G.; Lillo, S.; Brogna, A.; Chillar, F.; Ferrantelli, G.; Settineri, N.; Santacaterina, A.; Platania, A.; Leotta, S.; et al. Stereotactic boost on residual disease after external-beam irradiation in clinical stage III non-small cell lung cancer: Mature results of stereotactic body radiation therapy post radiation therapy (SBRTpostRT) study. La Radiol. Medica 2023, 128, 877–885. [Google Scholar] [CrossRef]
- Vadalà, R.E.; Santacaterina, A.; Sindoni, A.; Platania, A.; Arcudi, A.; Ferini, G.; Mazzei, M.M.; Marletta, D.; Rifatto, C.; Risoleti, E.V.I.; et al. Stereotactic body radiotherapy in non-operable lung cancer patients. Clin. Transl. Oncol. 2016, 18, 1158–1159. [Google Scholar] [CrossRef]
- Xu, T.; Wu, L.; Gandhi, S.; Jing, W.; Nguyen, Q.-N.; Chen, A.; Chang, J.Y.; Nurieva, R.; Sheshadri, A.; Altan, M.; et al. Treatment-related pulmonary adverse events induced by chemoradiation and Durvalumab affect survival in locally advanced non-small cell lung cancer. Radiother. Oncol. 2022, 176, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Coniac, S.; Outas, M.C.C.; Pirvu, E.E.; Patru, R.I.; Gainariu, E.; Aldea, C.; Iorga, P.G.; Ambroci, M.; Liscu, H.D.; Miron, A.-I.; et al. Challenges and limitations of endocrine toxicity evaluation in non-small cell lung cancer patients treated with immunotherapy—Retrospective study from tertiary-level hospital in Romania. Diagnostics 2023, 13, 1788. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Mazieres, J.; Reck, M.; Chouaid, C.; Bischoff, H.; Reinmuth, N.; Cove-Smith, L.; Mansy, T.; Cortinovis, D.; Migliorino, M.R.; et al. Durvalumab After Sequential Chemoradiotherapy in Stage III, Unresectable NSCLC: The Phase 2 PACIFIC-6 Trial. J. Thorac. Oncol. 2022, 17, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Faivre-Finn, C. Response to Letter to the Editor From Shaorong Yu and Jifeng Feng. J. Thorac. Oncol. 2024, 19, 174–175. [Google Scholar] [CrossRef]
- Yu, S.; Feng, J. Comment on “Durvalumab After Sequential Chemoradiotherapy in Stage III, Unresectable NSCLC: The Phase 2 PACIFIC-6 Trial”. J. Thorac. Oncol. 2024, 19, 173–174. [Google Scholar] [CrossRef]
- O’leary, C.; Naidoo, J. PACIFIC in the Real World. J. Thorac. Oncol. 2023, 18, 133–135. [Google Scholar] [CrossRef]
| Baseline Characteristics | ||||
|---|---|---|---|---|
| <66 Gy N = 110 (%) | >66 Gy N = 78 (%) | p-Value | ||
| Gender | male | 60 (55) | 54 (69) | 0.038 |
| female | 50 (45) | 24 (30) | ||
| Age (years) | median | 67 | 67 | 0.222 |
| range | 45–91 | 36–84 | ||
| Smoking status | never | 6 (5) | 7 (9) | 0.883 |
| ex | 65 (59) | 43 (55) | ||
| current | 39 (35) | 28 (36) | ||
| ECOG | 0–1 | 102 (93) | 74 (95) | 0.866 |
| 2–3 | 8 (7) | 4 (5) | ||
| UICC | IIIa | 33 (30) | 34 (44) | 0.061 |
| IIIb | 56 (51) | 27 (35) | ||
| IIIc | 21 (19) | 17 (22) | ||
| Histology | nonSCC | 63 (57) | 47 (60) | 0.793 |
| SCC | 47 (42) | 31 (40) | ||
| PDL-1 | <1% | 28 (25) | 15 (19) | 0.371 |
| >1% | 73 (66) | 57 (73) | ||
| unknown | 9 (8) | 6 (8) | ||
| CRT sequence | sCRT | 69 (63) | 61 (78) | 0.032 |
| cCRT | 33 (30) | 14 (18) | ||
| unknown | 8 (7) | 3 (4) | ||
| Durvalumab | yes | 57 (52) | 18 (23) | <0.001 |
| no | 53 (48) | 60 (77) | ||
| Tumour GTV (mL) | median | 78.6 | 19 | <0.001 |
| range | 1–784 | 1–282 | ||
| Local Relapses | ||
|---|---|---|
| All Patients N = 188 (%) | Durvalumab Patients N = 113 (%) | |
| Tumour (isolated) | 16 (9) | 9 (8) |
| Tumour + lymph nodes | 25 (13) | 15 (13) |
| Lymph nodes (isolated) | 5 (3) | 2 (2) |
| Baseline Characteristics | ||
|---|---|---|
| UVA | MVA | |
| Gender | 0.111 | n.s. |
| Age | 0.526 | n.s. |
| Smoking status | 0.116 | n.s. |
| ECOG | 0.400 | n.s. |
| UICC | 0.523 | n.s. |
| Histology | 0.001 | <0.001 |
| PDL-1 | 0.982 | n.s. |
| CRT sequence | 0.213 | n.s. |
| Durvalumab | 0.262 | n.s. |
| Tumour GTV | 0.152 | n.s. |
| Total radiation dose (>/<66 Gy) | 0.059 | n.s. |
| Toxicity | ||||
|---|---|---|---|---|
| Immunotherapy N = 130 (%) | No immunotherapy N = 58 (%) | p-Value | ||
| Oesophagitis | Grade 1 | 17 (13) | 7 (12) | 0.774 |
| Grade 2 | 43 (33) | 22 (38) | ||
| Grade 3 | 3 (2) | 1 (2) | ||
| Grade 4 | 0 (0) | 0 (0) | ||
| Grade 5 | 0 (0) | 0 (0) | ||
| Pneumonitis | Grade 1 | 18 (14) | 5 (9) | 0.741 |
| Grade 2 | 26 (20) | 12 (21) | ||
| Grade 3 | 1 (1) | 2 (2) | ||
| Grade 4 | 0 (0) | 1 (2) | ||
| Grade 5 | 1 (1) | 0 (0) | ||
| Haematologic | any grade | 2 (2) | 2 (3) | n.a. |
| Other | any grade | 30 (23) | 6 (10) | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zehentmayr, F.; Feurstein, P.; Ruznic, E.; Langer, B.; Grambozov, B.; Klebermass, M.; Hochreiter, A.; Purevdorj, A.; Gruber, G.; Minasch, D.; et al. Durvalumab Prolongs Overall Survival, Whereas Radiation Dose Escalation > 66 Gy Might Improve Long-Term Local Control in Unresectable NSCLC Stage III: Updated Analysis of the Austrian Radio-Oncological Lung Cancer Study Association Registry (ALLSTAR). Cancers 2025, 17, 1443. https://doi.org/10.3390/cancers17091443
Zehentmayr F, Feurstein P, Ruznic E, Langer B, Grambozov B, Klebermass M, Hochreiter A, Purevdorj A, Gruber G, Minasch D, et al. Durvalumab Prolongs Overall Survival, Whereas Radiation Dose Escalation > 66 Gy Might Improve Long-Term Local Control in Unresectable NSCLC Stage III: Updated Analysis of the Austrian Radio-Oncological Lung Cancer Study Association Registry (ALLSTAR). Cancers. 2025; 17(9):1443. https://doi.org/10.3390/cancers17091443
Chicago/Turabian StyleZehentmayr, Franz, Petra Feurstein, Elvis Ruznic, Brigitte Langer, Brane Grambozov, Marisa Klebermass, Alexandra Hochreiter, Ayurzana Purevdorj, Georg Gruber, Danijela Minasch, and et al. 2025. "Durvalumab Prolongs Overall Survival, Whereas Radiation Dose Escalation > 66 Gy Might Improve Long-Term Local Control in Unresectable NSCLC Stage III: Updated Analysis of the Austrian Radio-Oncological Lung Cancer Study Association Registry (ALLSTAR)" Cancers 17, no. 9: 1443. https://doi.org/10.3390/cancers17091443
APA StyleZehentmayr, F., Feurstein, P., Ruznic, E., Langer, B., Grambozov, B., Klebermass, M., Hochreiter, A., Purevdorj, A., Gruber, G., Minasch, D., Breitfelder, B., Steffal, C., Kirchhammer, K., Stranzl, H., Röder, F., & Dieckmann, K., on behalf of the ALLSTAR Group. (2025). Durvalumab Prolongs Overall Survival, Whereas Radiation Dose Escalation > 66 Gy Might Improve Long-Term Local Control in Unresectable NSCLC Stage III: Updated Analysis of the Austrian Radio-Oncological Lung Cancer Study Association Registry (ALLSTAR). Cancers, 17(9), 1443. https://doi.org/10.3390/cancers17091443








