Comparison of Weekly and Triweekly Cisplatin Regimens in the Treatment of Head and Neck Cancer: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Compliance with Chemotherapy Protocol
3.3. Compliance with Radiotherapy Protocol
3.4. Therapeutic Efficacy
3.5. Treatment-Related Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Rao, P.B.; Kumar, P.R.; Manam, S. Concurrent Chemoradiation with Weekly Cisplatin for the Treatment of Head and Neck Cancers: An Institutional Study on Acute Toxicity and Response to Treatment. Asian Pac. J. Cancer Prev. 2015, 16, 7331–7335. [Google Scholar] [CrossRef] [PubMed]
- Rucińska, M. Combined Radiotherapy and Chemotherapy. Nowotwory J. Oncol. 2022, 72, 319–325. [Google Scholar] [CrossRef]
- Bauml, J.M.; Vinnakota, R.; Anna Park, Y.-H.; Bates, S.E.; Fojo, T.; Aggarwal, C.; Limaye, S.; Damjanov, N.; Di Stefano, J.; Ciunci, C.; et al. Cisplatin Every 3 Weeks versus Weekly with Definitive Concurrent Radiotherapy for Squamous Cell Carcinoma of the Head and Neck. J. Natl. Cancer Inst. 2019, 111, 490–497. [Google Scholar] [CrossRef]
- Espeli, V.; Zucca, E.; Ghielmini, M.; Giannini, O.; Salatino, A.; Martucci, F.; Richetti, A. Weekly and 3-Weekly Cisplatin Concurrent with Intensity-Modulated Radiotherapy in Locally Advanced Head and Neck Squamous Cell Cancer. Oral. Oncol. 2012, 48, 266–271. [Google Scholar] [CrossRef]
- Gupta, T.; Kannan, S.; Ghosh-Laskar, S.; Agarwal, J.P. Concurrent Chemoradiotherapy with Cisplatin given Once-a-Week versus Every-Three Weekly in Head and Neck Squamous Cell Carcinoma: Non-Inferior, Equivalent, or Superior? Oral. Oncol. 2022, 134, 106130. [Google Scholar] [CrossRef]
- Jacinto, J.C.K.; Co, J.; Mejia, M.B.; Regala, E.E. The Evidence on Effectiveness of Weekly vs Triweekly Cisplatin Concurrent with Radiotherapy in Locally Advanced Head and Neck Squamous Cell Carcinoma (HNSCC): A Systematic Review and Meta-Analysis. Br. J. Radiol. 2017, 90, 20170442. [Google Scholar] [CrossRef]
- Kang, M.H.; Kang, J.H.; Song, H.-N.; Jeong, B.K.; Chai, G.Y.; Kang, K.; Woo, S.H.; Park, J.J.; Kim, J.P. Concurrent Chemoradiation with Low-Dose Weekly Cisplatin in Locally Advanced Stage IV Head and Neck Squamous Cell Carcinoma. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2015, 47, 441–447. [Google Scholar] [CrossRef]
- Mohamed, A.; Twardy, B.; Zordok, M.A.; Ashraf, K.; Alkhoder, A.; Schrapp, K.; Steuer, C.; Chen, Z.; Pakkala, S.; Pillai, R.; et al. Concurrent Chemoradiotherapy with Weekly versus Triweekly Cisplatin in Locally Advanced Squamous Cell Carcinoma of the Head and Neck: Comparative Analysis. Head. Neck 2019, 41, 1490–1498. [Google Scholar] [CrossRef]
- Al-Mamgani, A.; De RIdder, M.; Navran, A.; Klop, W.M.; de Boer, J.P.; Tesselaar, M.E. The Impact of Cumulative Dose of Cisplatin on Outcome of Patients with Head and Neck Squamous Cell Carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 3757–3765. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Noronha, V.; Patil, V.M.; Abhayankar, A.; Joshi, A.; Menon, N.S.; Prabhash, K. Optimal Cumulative Cisplatin Dose for Radio-Sensitization in Locally Advanced Head and Neck Cancer. J. Clin. Oncol. 2020, 38, e18553. [Google Scholar] [CrossRef]
- Ameri, A.; Norouzi, S.; Sourati, A.; Azghandi, S.; Novin, K.; Taghizadeh-Hesary, F. Randomized Trial on Acute Toxicities of Weekly vs Three-Weekly Cisplatin-Based Chemoradiation in Head and Neck Cancer. Cancer Rep. 2022, 5, e1425. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.; Sehrawat, A.; Mopidevi, T.R.; Parthasarthy, K.M.; Gupta, D.; Singh, A. Comparison of the Effect of Weekly Cisplatin Versus Three Weekly Cisplatin in Concurrent Chemoradiotherapy of Head and Neck Cancer: A Pilot Study. Indian J. Otolaryngol. Head. Neck Surg. 2024, 77, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Gemmete, J.J. Complications Associated with Selective High-Dose Intraarterial Cisplatin and Concomitant Radiation Therapy for Advanced Head and Neck Cancer. J. Vasc. Interv. Radiol. 2003, 14, 743–748. [Google Scholar] [CrossRef]
- Hunter, M.; Kellett, J.; Toohey, K.; D’Cunha, N.M.; Isbel, S.; Naumovski, N. Toxicities Caused by Head and Neck Cancer Treatments and Their Influence on the Development of Malnutrition: Review of the Literature. Eur. J. Investig. Health Psychol. Educ. 2020, 10, 935–949. [Google Scholar] [CrossRef]
- Muzumder, S.; Srikantia, N.; Udayashankar, A.H.; Kainthaje, P.B.; Sebastian, M.G.J. Burden of Acute Toxicities in Head-and-Neck Radiation Therapy: A Single-Institutional Experience. S. Asian J. Cancer 2019, 8, 120–123. [Google Scholar] [CrossRef]
- Ho, K.F.; Swindell, R.; Brammer, C.V. Dose Intensity Comparison between Weekly and 3-Weekly Cisplatin Delivered Concurrently with Radical Radiotherapy for Head and Neck Cancer: A Retrospective Comparison from New Cross Hospital, Wolverhampton, UK. Acta Oncol. 2008, 47, 1513–1518. [Google Scholar] [CrossRef]
- Schaeffers, A.W.M.A.; Devriese, L.A.; van Gils, C.H.; Dankbaar, J.W.; Voortman, J.; de Boer, J.P.; Slingerland, M.; Hendriks, M.P.; Smid, E.J.; Frederix, G.W.J.; et al. Low Dose Cisplatin Weekly versus High Dose Cisplatin Every Three Weeks in Primary Chemoradiotherapy in Head and Neck Cancer Patients with Low Skeletal Muscle Mass: The CISLOW-Study Protocol. PLoS ONE 2023, 18, e0294147. [Google Scholar] [CrossRef]
- Szturz, P.; Wouters, K.; Kiyota, N.; Tahara, M.; Prabhash, K.; Noronha, V.; Adelstein, D.; Van Gestel, D.; Vermorken, J.B. Low-Dose vs. High-Dose Cisplatin: Lessons Learned from 59 Chemoradiotherapy Trials in Head and Neck Cancer. Front. Oncol. 2019, 9, 86. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Weber, F.; Knapp, G.; Ickstadt, K.; Kundt, G.; Glass, Ä. Zero-Cell Corrections in Random-Effects Meta-Analyses. Res. Synth. Methods 2020, 11, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, N.; Tahara, M.; Mizusawa, J.; Kodaira, T.; Fujii, H.; Yamazaki, T.; Mitani, H.; Iwae, S.; Fujimoto, Y.; Onozawa, Y.; et al. Weekly Cisplatin plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. J. Clin. Oncol. 2022, 40, 1980–1990. [Google Scholar] [CrossRef]
- Lee, J.Y.; Sun, J.-M.; Oh, D.R.; Lim, S.H.; Goo, J.; Lee, S.-H.; Kim, S.-B.; Park, K.U.; Kim, H.-K.; Hong, D.S.; et al. Comparison of Weekly versus Triweekly Cisplatin Delivered Concurrently with Radiation Therapy in Patients with Locally Advanced Nasopharyngeal Cancer: A Multicenter Randomized Phase II Trial (KCSG-HN10-02). Radiother. Oncol. 2016, 118, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Mashhour, K.; Hashem, W. Cisplatin Weekly versus Every 3 Weeks Concurrently with Radiotherapy in the Treatment of Locally Advanced Head and Neck Squamous Cell Carcinomas: What Is the Best Dosing and Schedule? Asian Pac. J. Cancer Prev. 2020, 21, 799. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Choudhury, K.; Rashid, M.A. Concurrent Chemotherapy in Advanced Head and Neck Carcinoma A Prospective Randomized Trial. Bangladesh J. Otorhinolaryngol. 2011, 17, 88–95. [Google Scholar] [CrossRef]
- Nair, L.M.; Kumar, R.R.; Thomachan, K.C.; Rafi, M.; George, P.S.; Krishna, K.M.J.; Ramadas, K. Phase IIb Trial Comparing Two Concurrent Cisplatin Schedules in Locally Advanced Head and Neck Cancer. South. Asian J. Cancer 2017, 6, 64–68. [Google Scholar] [CrossRef]
- Nanda, R.; Katke, A.; Suneetha, N.; Thejaswini, B.; Pasha, T.; Jagannath, K.P.; Giri, G.V.; Babu, G.K. A Prospective Randomized Study Comparing Concurrent Chemoradiation with Weekly and 3 Weekly Cisplatin in Locally Advanced Oropharyngeal Carcinoma. S. Asian J. Cancer 2019, 8, 178–182. [Google Scholar] [CrossRef]
- Noronha, V.; Joshi, A.; Patil, V.M.; Agarwal, J.; Ghosh-Laskar, S.; Budrukkar, A.; Murthy, V.; Gupta, T.; D’Cruz, A.K.; Banavali, S.; et al. Once-a-Week versus Once-Every-3-Weeks Cisplatin Chemoradiation for Locally Advanced Head and Neck Cancer: A Phase III Randomized Noninferiority Trial. J. Clin. Oncol. 2018, 36, 1064–1072. [Google Scholar] [CrossRef]
- Panihar, C.; Rawat, S.; Singotia, L.; Raj, A.; Jain, R.K. A Prospective Randomised Comparative Study between Weekly Cisplatin versus Three Weekly Cisplatin with Radiotherapy in Unresectable Locally Advanced Head and Neck Cancer. Indian J. Otolaryngol. Head. Neck Surg. 2022, 74, 2670–2675. [Google Scholar] [CrossRef]
- Rawat, S.; Srivastava, H.; Ahlawat, P.; Pal, M.; Gupta, G.; Chauhan, D.; Tandon, S.; Khurana, R. Weekly versus Three-Weekly Cisplatin-Based Concurrent Chemoradiotherapy as Definitive Treatment in Head and Neck Cancer-Where Do We Stand? Gulf J. Oncol. 2016, 1, 6–11. [Google Scholar]
- SahOO, T.K.; Samanta, D.R.; Senapati, S.N.; Parida, K. A Comparative Study on Weekly versus Three Weekly Cisplatinum Based Chemoradiation in Locally Advanced Head and Neck Cancers. J. Clin. Diagn. Res. 2017, 11, XC07. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, M.; Bhasker, S.; Thakar, A.; Pramanik, R.; Biswas, A.; Kumar, A.; Sikka, K.; Singh, A.C.; Mallick, S.; et al. An Open-Label, Noninferiority Phase III RCT of Weekly versus Three Weekly Cisplatin and Radical Radiotherapy in Locally Advanced Head and Neck Squamous Cell Carcinoma (ConCERT Trial). J. Clin. Oncol. 2022, 40, 6004. [Google Scholar] [CrossRef]
- Tsan, D.-L.; Lin, C.-Y.; Kang, C.-J.; Huang, S.-F.; Fan, K.-H.; Liao, C.-T.; Chen, I.-H.; Lee, L.-Y.; Wang, H.-M.; Chang, J.T.C. The Comparison between Weekly and Three-Weekly Cisplatin Delivered Concurrently with Radiotherapy for Patients with Postoperative High-Risk Squamous Cell Carcinoma of the Oral Cavity. Radiat. Oncol. 2012, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Uygun, K.; Bilici, A.; Karagol, H.; Caloglu, M.; Cicin, I.; Aksu, G.; Fayda, M.; Uzunoglu, S. The Comparison of Weekly and Three-Weekly Cisplatin Chemotherapy Concurrent with Radiotherapy in Patients with Previously Untreated Inoperable Non-Metastatic Squamous Cell Carcinoma of the Head and Neck. Cancer Chemother. Pharmacol. 2009, 64, 601–605. [Google Scholar] [CrossRef]
- Gamez, M.E.; Blakaj, D.M.; Bhateja, P.; Custer, A.; Klamer, B.G.; Pan, J.; Gogineni, E.; Baliga, S.; Bonomi, M.R. Audiological Outcomes of Weekly vs. Triweekly Cisplatin in Head and Neck Cancer with Cochlear-Sparing Intensity-Modulated Radiation Therapy. Cancers 2024, 16, 2228. [Google Scholar] [CrossRef]
- Helfenstein, S.; Riesterer, O.; Meier, U.R.; Papachristofilou, A.; Kasenda, B.; Pless, M.; Rothschild, S.I. 3-Weekly or Weekly Cisplatin Concurrently with Radiotherapy for Patients with Squamous Cell Carcinoma of the Head and Neck–a Multicentre, Retrospective Analysis. Radiat. Oncol. 2019, 14, 32. [Google Scholar] [CrossRef]
- Ang, K.K. Concurrent Radiation Chemotherapy for Locally Advanced Head and Neck Carcinoma: Are We Addressing Burning Subjects? J. Clin. Oncol. 2004, 22, 4657–4659. [Google Scholar] [CrossRef]
- Szturz, P.; Wouters, K.; Kiyota, N.; Tahara, M.; Prabhash, K.; Noronha, V.; Castro, A.; Licitra, L.; Adelstein, D.; Vermorken, J.B. Weekly Low-Dose versus Three-Weekly High-Dose Cisplatin for Concurrent Chemoradiation in Locoregionally Advanced Non-Nasopharyngeal Head and Neck Cancer: A Systematic Review and Meta-Analysis of Aggregate Data. Oncologist 2017, 22, 1056–1066. [Google Scholar] [CrossRef]
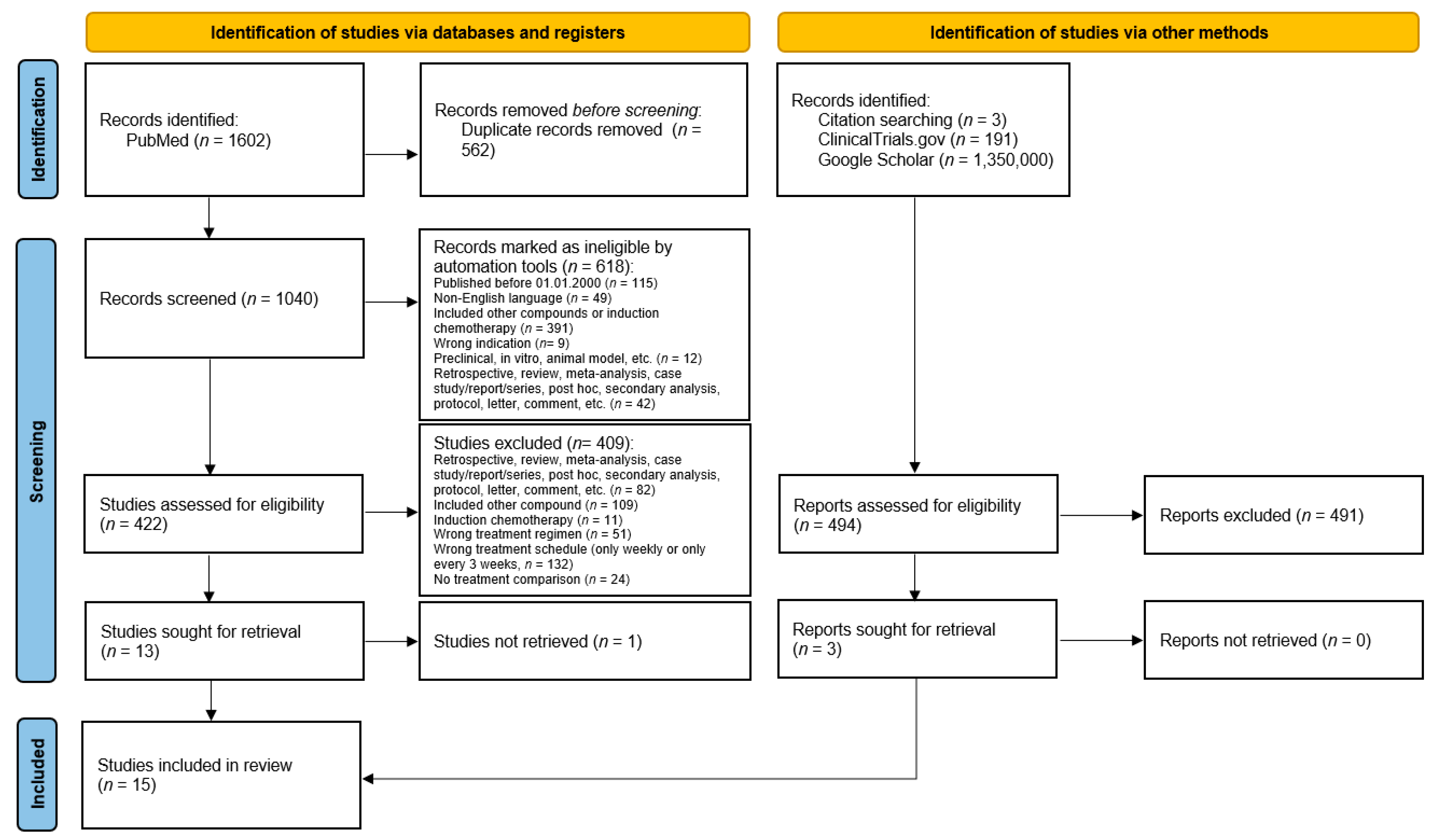


| Author, Year | Study Type/Phase | Randomized | Recruitment Dates | Country | Tumor Resection Status | Study Arms | Number of Patients (n) |
|---|---|---|---|---|---|---|---|
| Ameri A, 2021 [12] | Prospective | Yes | - | Iran | Mostly unresected | 40 mg/m2 Q1 | 39 |
| 100 mg/m2 Q3 | 38 | ||||||
| Chaturvedi A, 2024 [13] | Prospective | Yes | May 2016–June 2019 | India | Unresected | 35 mg/m2 Q1 | 26 |
| 100 mg/m2 Q3 | 25 | ||||||
| Kiyota N, 2022 [22] | Phase II/III | Yes | October 2012–December 2018 | Japan | Resected | 40 mg/m2 Q1 | 129 |
| 100 mg/m2 Q3 | 132 | ||||||
| Lee JY, 2016 [23] | Phase II | Yes | September 2009–December 2013 | South Korea | - | 40 mg/m2 Q1 | 53 |
| 100 mg/m2 Q3 | 56 | ||||||
| Mashhour K, 2020 [24] | Prospective | Yes | July 2016–July 2019 | Egypt | Both | 30 mg/m2 Q1 | 30 |
| 100 mg/m2 Q3 | 30 | ||||||
| Mitra D, 2011 [25] | Prospective | Yes | February 2010–January 2011 | India | Unresected | 30 mg/m2 Q1 | 30 |
| 100 mg/m2 Q3 | 30 | ||||||
| Nair LM, 2017 [26] | Phase IIb | Yes | June 2013–May 2014 | India | Unresected | 40 mg/m2 Q1 | 24 |
| 100 mg/m2 Q3 | 31 | ||||||
| Nanda R, 2020 [27] | Prospective | Yes | December 2010–January 2013 | India | Unresected | 40 mg/m2 Q1 | 29 |
| 100 mg/m2 Q3 | 31 | ||||||
| Noronha V, 2017 [28] | Phase III | Yes | 2013–2017 | India | Mostly resected | 30 mg/m2 Q1 | 150 |
| 100 mg/m2 Q3 | 150 | ||||||
| Panihar C, 2022 [29] | Prospective | Yes | December 2017–May 2019 | India | Unresected | 40 mg/m2 Q1 | 44 |
| 100 mg/m2 Q3 | 41 | ||||||
| Rawat S, 2016 [30] | Prospective | Yes | June 2013–March 2014 | India | Unresected | 35 mg/m2 Q1 | 30 |
| 100 mg/m2 Q3 | 30 | ||||||
| Sahoo TK, 2017 [31] | Prospective | Yes | November 2011–October 2012 | India | Unresected | 30 mg/m2 Q1 | 15 |
| 100 mg/m2 Q3 | 15 | ||||||
| Sharma A, 2022 [32] | Phase III | - | April 2018–January 2021 | India | Unresected | 40 mg/m2 Q1 | 132 |
| 100 mg/m2 Q3 | 132 | ||||||
| Tsan DL, 2012 [33] | Phase III | Yes | February 2008–August 2010 | Taiwan | Resected | 40 mg/m2 Q1 | 24 |
| 100 mg/m2 Q3 | 26 | ||||||
| Uygun K, 2009 [34] | Prospective | - | January 2002–December 2007 | Turkey | Unresected | 40 mg/m2 Q1 | 20 |
| 100 mg/m2 Q3 | 30 |
| Characteristic | Number of Studies [n] | Mean Value (95% CI) | p-Value | |
|---|---|---|---|---|
| Weekly | Triweekly | |||
| Chemotherapy completion [%] | 12 | 74.76% (65.35–84.16) | 72.29% (59.42–85.15) | 0.38 |
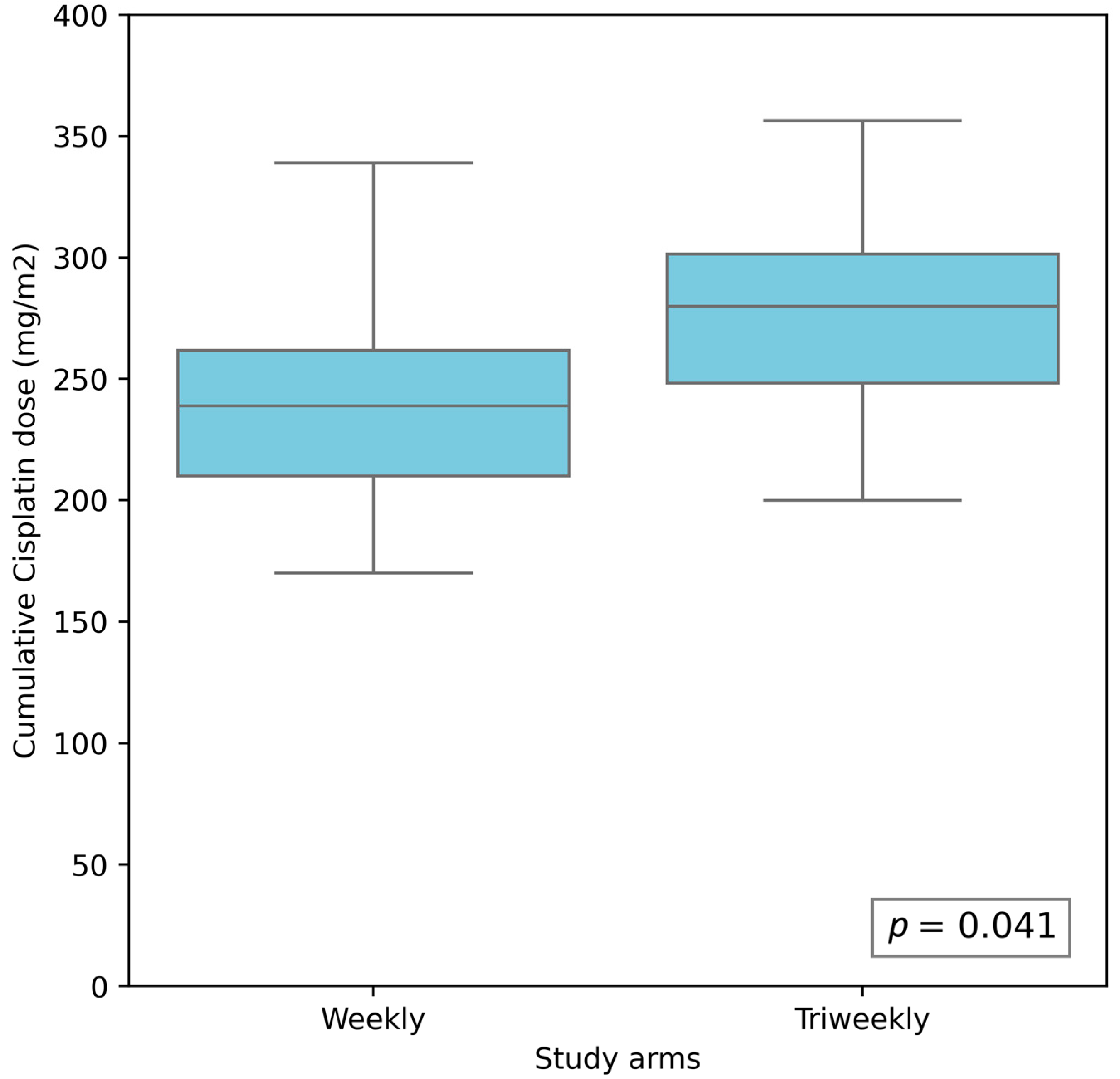
| Cumulative cisplatin dose [mg/m2] | 11 | 241.74 (213.94–269.55) | 287.52 (247.75–327.30) | 0.04 |
| Cumulative cisplatin dose ≥200 mg/m2 [%] | 7 | 70.64% (57.30–83.98) | 78.75% (63.36–94.14) | 0.23 |
| Characteristic | Number of Studies [n] | Mean Value (95% CI) [%] | p-Value | |
|---|---|---|---|---|
| Weekly | Triweekly | |||
| Complete response [%] | 11 | 63.18% (51.72–74.63) | 67.13% (55.50–78.76) | 0.32 |
| Partial response [%] | 7 | 30.34% (14.34–46.34) | 28.12% (11.66–44.57) | 0.43 |
| Locoregional control at 2 years [%] | 7 | 59.62% (49.36–69.89) | 66.94% (55.37–78.50) | 0.19 |
| Overall survival at 2 years [%] | 6 | 51.24% (31.68–70.79) | 49.47% (31.91–67.02) | 0.45 |
| Toxicity | Number of Studies [n] | Median (Min–Max) [%] | p-Value | |
|---|---|---|---|---|
| Weekly | Triweekly | |||
| Hematological | ||||
| Anemia | 11 | 73.37% (8.30–100.00) | 76.6% (22.50–100.00) | 0.34 |
| Leukopenia | 7 | 86.67% (20.27–100.00) | 95.00% (46.98–100.00) | 0.20 |
| Neutropenia | 10 | 66.42% (9.46–100.00) | 81.00% (30.87–100.00) | 0.19 |
| Thrombocytopenia | 10 | 46.50% (2.70–100.00) | 53.50% (2.70–100.00) | 0.44 |
| Non-hematological | ||||
| Dermatitis | 9 | 100.00% (61.49–100.00) | 100.00% (62.42–100.00) | 0.46 |
| Dysphagia | 7 | 95.83% (48.00–100.00) | 100.00% (58.00–100.00) | 0.44 |
| Mucositis | 9 | 100.00% (81.76–100.00) | 100.00% (90.60–100.00) | 0.35 |
| Nausea/vomiting | 9 | 93.37% (15.54–100.00) | 100.00% (29.53–100.00) | 0.33 |
| Renal toxicity | 4 | 3.75% (0.00–16.67) | 5.26% (0.00–9.68) | 0.41 |
| Significant weight loss (>10%) | 4 | 27.15% (11.49–43.60) | 40.70% (17.45–42.10) | 0.21 |
| Toxicity | Number of Studies [n] | Median (Min–Max) [%] | p-Value | |
|---|---|---|---|---|
| Weekly | Triweekly | |||
| Overall acute toxicities of grade ≥ 3 | 4 | 56.95% (40.00–71.62) | 67.05% (39.30–84.56) | 0.28 |
| Hematological | ||||
| Anemia | 11/10 * | 4.20% (0.00–26.70) | 6.64% (0.00–36.70) | 0.23 |
| Leukopenia | 7 | 20.00% (2.70–62.00) | 16.11% (0.00–55.00) | 0.46 |
| Neutropenia | 10 | 16.45% (1.35–35.00) | 18.05% (0.00–49.00) | 0.30 |
| Thrombocytopenia | 10 | 2.85% (0.00–7.50) | 2.66% (0.00–16.60) | 0.14 |
| Non-hematological | ||||
| Dermatitis | 10 | 13.79% (6.76–38.00) | 11.65% (3.20–64.00) | 0.48 |
| Dysphagia | 8 | 44.70% (0.00–85.00) | 35.61% (6.67–92.00) | 0.50 |
| Mucositis | 11 | 53.40% (15.54–85.00) | 46.70% (18.12–92.00) | 0.50 |
| Nausea/vomiting | 9 | 7.50% (1.35–20.80) | 13.00% (0.00–40.00) | 0.11 |
| Renal toxicity | 6 | 0.00% (0.00–5.00) | 0.00% (0.00–16.60) | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kloska, S.M.; Kloska, A. Comparison of Weekly and Triweekly Cisplatin Regimens in the Treatment of Head and Neck Cancer: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 1444. https://doi.org/10.3390/cancers17091444
Kloska SM, Kloska A. Comparison of Weekly and Triweekly Cisplatin Regimens in the Treatment of Head and Neck Cancer: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(9):1444. https://doi.org/10.3390/cancers17091444
Chicago/Turabian StyleKloska, Sylwester M., and Anna Kloska. 2025. "Comparison of Weekly and Triweekly Cisplatin Regimens in the Treatment of Head and Neck Cancer: A Systematic Review and Meta-Analysis" Cancers 17, no. 9: 1444. https://doi.org/10.3390/cancers17091444
APA StyleKloska, S. M., & Kloska, A. (2025). Comparison of Weekly and Triweekly Cisplatin Regimens in the Treatment of Head and Neck Cancer: A Systematic Review and Meta-Analysis. Cancers, 17(9), 1444. https://doi.org/10.3390/cancers17091444







