Simple Summary
In many malignancies, the lymph node (LN) status is a critical prognostic factor and determines the need for (neo-) adjuvant therapies. Current diagnostic methods often miss smaller LN metastases (LNM). Over the past decade, intraoperative fluorescence imaging (FI) has emerged, with multiple studies evaluating molecular-targeted fluorescence tracers. As tumor-targeted fluorescent tracers have expanded, these procedures are increasingly used for LNM detection, raising hopes of addressing challenges with occult LNMs. This systematic review evaluates 26 studies on molecular-targeted FI for LNM detection. The results showed significant variability in sensitivity and specificity in the intraoperative setting, while postoperative FI on formalin-fixed tissue blocks demonstrated high sensitivity (70–100%) and specificity (66–100%) for LNM detection. Overall, molecular-targeted FI offers a limited clinical benefit for in vivo detection, but could improve the accuracy and efficiency of postoperative pathological processing.
Abstract
Background/Objectives: Our objectives were to provide a comprehensive overview of clinical studies assessing the use of molecular-targeted fluorescence imaging (FI) for the detection of lymph node metastases (LNM), and to discuss the clinical applications, challenges, and limitations of using molecular-targeted FI for this purpose. Methods: The methods used included a systematic literature search that was conducted to identify articles on molecular-targeted FI of LNMs published before 12 January 2024. The selection process included fluorescent tracers, camera systems, target tissues, study types, populations, the number of resected LNs, clinical applications, fluorescence assessments, and diagnostic parameters. Results: A total of 10,263 studies were identified, of which 26 studies were included. For intraoperative imaging, both in vivo and ex vivo FI of LNMs were performed in these studies, of which the in vivo diagnostic accuracy of molecular-targeted FI for LNM detection was described in four out of the twenty-six studies. Trials evaluating the in vivo and ex vivo detection of LNMs reported significant variability in sensitivity and specificity. Postoperative molecular-targeted FI on formalin-fixed tissue blocks showed both high sensitivity (70–100%) and specificity (66–100%) in all studies for the detection of LNMs. Conclusions: The use of molecular-targeted FI has limited the clinical benefit for in vivo detection of LNMs; however, FI could improve the accuracy and efficiency of the postoperative pathological processing of LNMs.
1. Introduction
The lymphatic system plays a crucial role in the spread of solid tumors and is often the first site of metastasis, as cancer cells typically invade nearby lymph nodes (LN) before potentially spreading to other LNs through the lymphatic system and distant organs through the bloodstream []. In many malignancies, including colorectal, head and neck (HNC), melanoma, and breast cancer, the LN status is a vital prognostic factor for survival and determines the need for (neo-) adjuvant therapies [,,]. Therefore, pre-, intraoperative, and postoperative nodal staging is essential to determine the optimal treatment and the need for (neo-) adjuvant therapy. Diagnostic imaging modalities (e.g., CT, MRI, PET-CT, SPECT-CT, and ultrasound-guided fine needle aspiration) are used to adequately stage the regional status of solid tumors, but smaller LN metastases (LNM) are still being missed in 10–30% of cases []. Fibroblast activation protein inhibitor (FAPI) PET/CT has recently emerged as a novel imaging technique, demonstrating superior target-to-background ratios compared to [18F] FDG PET/CT across a range of solid tumors. This higher contrast may enhance the sensitivity and specificity for detecting lymph node metastases, thereby improving staging accuracy and potentially informing patient management and therapeutic planning []. Additionally, intraoperative LN staging could be performed through sampling or a sentinel node (SN) procedure [].
Over the past decade, a new era in the field of intraoperative imaging, using fluorescence imaging (FI), has emerged, as multiple clinical studies evaluating the use of molecular-targeted fluorescent tracers have been performed in recent years. Molecular-targeted fluorescent tracers, together with specialized camera systems, are essential tools in FI, that enable the precise intraoperative visualization of anatomical structures and pathological areas. These camera systems are able to detect the light emitted by the administered fluorescent tracer after excitation with a specific light source [,]. This provides the surgeon with real-time information on the location of the targeted cells. Applications of molecular-targeted FI have thus far mainly focused on the assessment of tumor resection margins and the detection of occult lesions. Non-targeted FI applications are more often used for the identification of vital structures (e.g., arteries, veins, and ureters) [,]. Two main application areas of molecular-targeted FI can be discerned, using either in vivo or ex vivo detection. In vivo assessment is defined as the utilization of FI in the patient during surgery. Conversely, ex vivo FI refers to the application of FI outside the patient to facilitate diagnostic evaluation and can be conducted either on the back-table during surgery, with the capacity to alter the surgical plan based on the imaging results, or postoperatively. With the growing variation of clinically available tumor-targeted fluorescent tracers, the scope of these intraoperative procedures has been increasingly expanded to include the detection of LNMs, raising expectations that it may provide a solution to the challenges associated with occult LNMs.
However, the different conjugates and fluorophores, the variety in NIR camera systems, and the differences in tissue optical properties require careful consideration in fluorescence-guided surgery []. NIR cameras differ in their sensitivity, resolution, and wavelength detection range, influencing the imaging efficacy. Additionally, tissue optical properties vary widely due to factors like composition, blood supply, and patient-specific characteristics (e.g., tissue vascularization, skin pigmentation), impacting light absorption, scattering, and emission. Consequently, ex vivo findings cannot be directly extrapolated to in vivo contexts.
This review is the first to summarize all clinical studies on molecular-targeted fluorescence imaging (FI) for detecting (occult) lymph node metastases (LNMs). Given the growing interest in novel FI tracers, there is a need for a structured framework to understand FI’s diverse applications in identifying LNMs, thus addressing the gaps in the current heterogeneous literature.
2. Materials and Methods
2.1. Literature Search and Study Selection
This systematic review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA) guidelines []. The PRISMA checklist is included in Appendix B. We conducted a comprehensive literature search in four major databases (PubMed, MEDLINE, Embase, and Cochrane) to identify articles published prior to 12 January 2024 that discussed the use of molecular-targeted fluorescence LN imaging. Our search strategy utilized various terms and synonyms related to (near-infrared) fluorescence, optical imaging, molecular imaging, LNs with or without metastases, surgery, tumor, and targeted imaging to ensure a thorough review of relevant articles. Based on the screened articles, we grouped the tracers into the following four categories: antibody-based, folate receptor alpha, molecular-targeted peptides, and hybrid tracers. The complete search strategy is available in Appendix C. The article selection process involved three independent researchers (H.G., B.Z., and L.M.) who identified all relevant articles. Our review excluded articles that did not correlate imaging with histopathology. A Clinical Trial Number was not applicable.
2.2. Data Extraction
The general study characteristics, such as details on the fluorescent tracer, camera system, target tissue, study type, study population, number of resected LNs, intended clinical application, histopathology, definition of a positive fluorescent signal (e.g., tumor-to-background ratio, TBR), and fluorescence assessment (in vivo and ex vivo), were extracted. Furthermore, diagnostic test parameters (sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV)) were extracted, if available, from the main text or Supplementary Materials. If these parameters were not previously described, they were calculated using standard formulas based on the values of true-positives (TP), false-positives (FP), true-negatives (TN), and false-negatives (FN), when available. Sensitivity was calculated as TP/(TP + FN), specificity as TN/(TN + FP), PPV as TP/(TP + FP), and NPV as TN/(TN + FN).
True-positive lymph nodes (LNs) were defined as those that were fluorescent and contained metastases. False-negative LNs, on the other hand, were non-fluorescent, yet still harbored metastases. True-negative LNs were non-fluorescent and did not contain any metastases, while false-positive LNs were benign nodes that exhibited a fluorescent signal. If multiple doses or test characteristics were studied, the optimal dose/setting outcomes were included in this review.
2.3. Quality Assessment
Two reviewers (L.M. and B.Z.) independently evaluated the quality and risk of bias of the included articles using the revised Quality Assessment of Diagnostic Accuracy Studies tool []. An overview of this risk assessment is available in Appendix A. In case of discrepancies in interpretation, a third reviewer (S.K.) was consulted to adjudicate the decision.
3. Results
3.1. Overview of Included Studies
The literature search resulted in a total of 10,263 studies, as reported in a flow diagram (Figure 1). After title and abstract screening, a total of 263 articles underwent a full-text review. In total, twenty-six studies reported on molecular-targeted fluorescence LN imaging, and were therefore included in the final analysis (Table 1) [,,,,,,,,,,,,,,,,,,,,,,,,,]. The included studies were divided into different types of clinical trials (type A, B, C, or D) according to their methodology, using the classification introduced by Lauwerends et al. []. This classification is based on how and when fluorescence-guided surgery was introduced into the standard of care (Figure A1, Appendix A). The results of the quality and risk of bias assessment of the studies are provided in Figure A2 (Appendix A). Furthermore, we will describe both the in vivo and ex vivo assessments separately to elucidate their distinct advantages and applications.
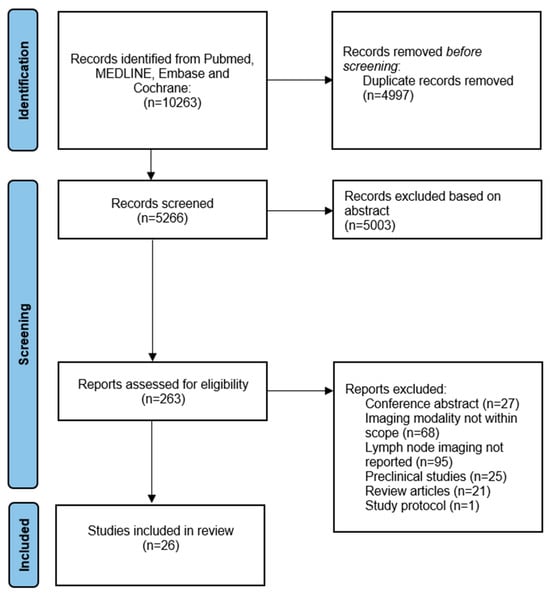
Figure 1.
Flow chart of study inclusions.

Table 1.
Overview on included studies.
3.2. In Vivo Assessment of Lymph Nodes
A total of fifteen studies discussed the in vivo application of molecular-targeted FI (Table 1). OTL38 contains a cyanine dye and targets the folate receptor alpha (FRα). Five studies evaluated the feasibility of in vivo LNMs detection using this dye in ovarian, endometrial, and gastric cancer, which also targets folate receptor alpha (FRα) [,,,,]. Boogerd et al. showed that targeting FRα resulted in the detection of all 16 LNMs out of the 22 resected LNs in cases of endometrial cancer, with a mean in vivo TBR of 6.3 (SD-4.5; range 3.2–14.1). Even LNMs located under a tissue layer of ±1 cm could be visualized using the Quest Artemis camera system. Furthermore, a total of 50 benign LNs were resected in this study, of which 17 showed a fluorescent signal (mean TBR 2.5; SD = 1.3; range 1.5–6.2), resulting in a sensitivity, specificity, PPV, and NPV of 100%, 70%, 48%, and 100%, respectively []. Subsequent studies observed a high (up to 100%) rate of false-positive fluorescent lesions using OTL38, which were mainly benign LNs (based on binding of OTL38 to folate receptor β, vastly present in activated intranodal macrophages), showing that fluorescence-positive lesions should be interpreted with caution [,,,].
The in vivo targeting of the folate receptor alpha (FRα) has also been reported in several other studies, of which Tummers et al. described the feasibility for detection of primary breast and ovarian cancer using EC17 (emission 520 nm), including the aim to detect LNMs in vivo. In this study, they found only one fluorescent LN, which turned out to be false-positive; the total of resected LNs was not described [].
[111In] In-DOTA-labetuzumab-IRDye800CW is a dual-labeled anti-carcinoembryonic antigen (anti-CEA) antibody conjugate. De Gooyer et al. demonstrated that deeper-seeded suspect lesions (e.g., retroperitoneal LNs) that were identified on SPECT imaging were not detectable with in vivo FI using [111In] this dye []; in all, 17 out of 28 (61%) malignant lesions could be detected with NIR-fluorescence imaging in the 2 mg group. In the 10 mg group, 95% (n = 16) of all malignant lesions could be visualized, and in the 50 mg group, all 25 (100%) malignant lesions could be detected. Additionally, an undetected retroperitoneal LNM was both visualized using SPECT-CT and FI prior to cytoreduction in one patient, and FI revealed four previously undetected LNMs, resulting in a change in treatment plan in two patients []. In both studies, no data on the fluorescence intensity of the LNs (such as Mean Fluorescence Intensity (MFI) or TBR) were provided, and the authors did not include information on the sensitivity, specificity, PPV, or NPV.
Additionally, in vivo targeting of the FRα has been reported in several studies, of which Tummers et al. described the feasibility of the detection of primary breast and ovarian cancer using EC17, including the aim to detect LNMs in vivo.
In this study, they found one fluorescent LN, which turned out to be false-positive; the total of resected LNs was not described. Three studies explored in vivo LN imaging during colorectal cancer surgery using a fluorescently labelled monoclonal antibody targeting the carcinoembryonic antigen receptor (SGM-101). Further assessment of the LNs was conducted ex vivo [,,]. In a subsequent pilot study, one fluorescent retroperitoneal-located LNM was detected in vivo, in addition to ten false-positive LNs. Information concerning the false-positive and false-negative fluorescent signals of the resected LNs was lacking in all of these studies [,,].
During robotic prostate cancer surgery using IS-002 (prostate-specific membrane antigen (PSMA)-targeted peptide-based fluorescent tracer), eight out of fourteen LNMs were detected in vivo, resulting in a sensitivity of 57.1% []. Similar results in prostate cancer patients were reported with another PSMA-targeted tracer, OTL78, which could detect four out of seven conglomerate LNMs in vivo, also resulting in a sensitivity of 57.1% []. Both studies showed a high number of false-positive LNs (81 out of 309, 26%) [] and conglomerates of LNs (13 out of 59, 22%)) [], leading to a relatively low PPV of 73.8% and 55.2%, respectively.
In a study on colorectal cancer, EMI-137, a c-MET-targeted, fluorescently labeled tracer was evaluated. No detectable fluorescent LN signal was observed in vivo in any participant (n = 9). Additionally, the systematic administration of EMI-137 and the fluorescent assessment using the Karl Storz® laparoscopic system did not facilitate the detection of LNMs in this trial [].
Further analysis of fifteen malignant nodes via immunohistochemistry revealed moderate to high c-MET expression in all samples. However, the c-MET expression did not correlate with the fluorescence signal observed on microscopy slides. Consequently, it remains challenging to draw definitive conclusions about the efficacy of EMI-137 in detecting LNMs through FI []. Two studies investigated the use of Bevacizumab (targeting the vascular endothelial growth factor α, VEGF-α) bound to either IRDye800CW or 800CW in the identification of primary breast cancer and locally advanced rectal cancer, respectively. As a secondary objective, the in vivo detection of LNMs was evaluated. A fluorescent signal was detected in the LNM of only one out of five patients with LNMs during breast cancer surgery.
Moreover, the fluorescent signal was evaluated alongside immunohistochemically staining in 104 LN tissue blocks, demonstrating no difference in VEGF-α expression between benign and LNMs [].
3.3. Ex Vivo Assessment of Lymph Nodes
Ex vivo assessment of LNs can be performed either intraoperatively or postoperatively. Ex vivo assessment during the surgical procedure allows for immediate clinical decision-making, potentially aiding the surgeon and directly benefiting the patient. Postoperative assessment, on the other hand, primarily assists the pathologist, contributing to the overall efficiency, efficacy, logistics, and cost-effectiveness of the process (see Figure 2). In this context, we will describe both approaches separately to elucidate their distinct advantages and applications. Eight studies examined the ex vivo use of molecular-targeted fluorescence imaging (FI) during surgery, while six studies focused on its ex vivo application postoperatively. Additionally, two studies covered both intraoperative and postoperative ex vivo applications (see Table 1).
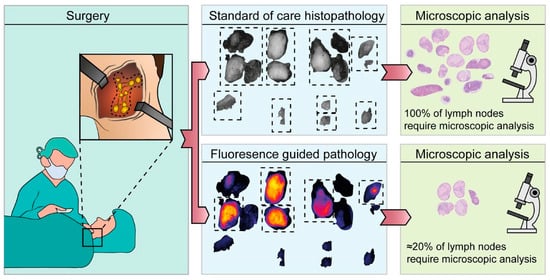
Figure 2.
Application of fluorescence molecular imaging postoperatively. This research was originally published in JNM by Vonk et al. Epidermal Growth Factor Receptor–Targeted Fluorescence Molecular Imaging for Postoperative Lymph Node Assessment in Patients with Oral Cancer. Journal of Nuclear Medicine May 2022, 63 (5) 672–678; DOI: https://doi.org/10.2967/jnumed.121.262530©SNMMI [].
3.3.1. Ex Vivo Assessment During the Surgical Procedure
Cetuximab-IRDye800CW and Panitumumab-IRDye800CW are fluorescent tracers that both target the endothelial growth factor receptor (EGFR) in HNC. The ex vivo identification of LNMs using Cetuximab-IRDye800CW demonstrated high sensitivity (97.2%) and specificity (92.7%). Furthermore, out of 471 resected LNs, 69 LNs were fluorescent, of which 35 were true-positive, and 34 were false-positive, resulting in a positive predictive value of 50.7%. Only one LNM was missed in these twelve patients (one false-negative) [].
In a colorectal cancer study, the CEA-targeting agent SGM-101 was used to identify colorectal cancer. Among thirty-seven patients who underwent surgery, eleven LNs were resected due to clinical suspicion, and metastases were confirmed in six of these nodes. Out of the six LNMs, five were detected ex vivo using FI, with a relatively low TBR ranging from 1.4 to 1.9 (true-positives). One LNM was non-fluorescent (false-negative), and details regarding the remaining resected LNs were not provided in the study []. SGM-101 was also utilized in thirteen patients with colorectal cancer and lung metastases.
Among these, two benign LNs were resected on white light suspicion in two patients, but they were fluorescence-negative (true-negatives). In another patient a lymphadenectomy was performed for preoperatively identified hilar LNMs, and three malignant LNs were fluorescent on the back-table (true-positives). Furthermore, three other non-fluorescent LNs were resected based on clinical suspicion for tumor involvement, and all three contained fibrosis without a tumor (true-negatives). Further details were not provided in the study report [].
In a study by Jonker et al., EMI-137 was evaluated for detecting LNMs using FI in papillary thyroid cancer (PTC). The fluorescence signal was assessed in 76 malignant and 340 benign lymph nodes (LNs), both ex vivo on fresh specimens and after pathological processing. If one or more lymph nodes within a level had a fluorescence intensity above the device-specific threshold, this was defined as a fluorescence-positive level. The tests showed an LN level-specific sensitivity (sensitivity specified per anatomical level stage) of 87.5%, a specificity of 26.3%, and an NPV of 83.3% for detecting levels containing PTC-positive LNs with micro-metastatic foci at gross examination. The authors concluded that if these LNs would be assessed ex vivo during surgery, this could have additional value for the surgeon to rule out the presence of nodal metastases in the central compartment, improving the selection of patients that may benefit from omitting a prophylactic central LN dissection, thus leading to a reduction in overtreatment and associated morbidity in the management of PTC [].
In pancreatic cancer, three studies assessed the use of FI to detect LNMs ex vivo during surgery. Two studies demonstrated that panitumumab-IRDye800CW and ex vivo FI could distinguish tumor-bearing LNs from negative ones based on a statistically significant difference in MFI [,]. In the first of these two studies, FI successfully identified LNMs. During surgery, tumor-bearing LNs could be identified with a mean TBR of 6.3 ± 0.82. Furthermore, tumor-bearing LNs (n = 29) could be detected with significantly higher MFI (0.06 ± 0.01) compared to tumor-negative (n = 78) LNs (0.02 ± 0.002) (p < 0.001) [].
In the second study, cetuximab-IRDye800 was tested in two dose cohorts (50 mg vs. 100 mg) across 144 lymph nodes (LNs) originating from seven patients. In the low-dose group, 72 LNs were evaluated, with 89% being tumor-negative and 11% being tumor-positive; in the high-dose group, 78% were tumor-negative and 22% were tumor-positive. Overall, tumor-positive LNs showed a significantly higher mean fluorescence intensity (MFI) (0.06 vs. 0.02; p < 0.001). This difference was more pronounced in the low-dose cohort (0.07 vs. 0.02; p < 0.001), while in the high-dose group, the MFI difference was not significant. The low-dose cohort showed high sensitivity and specificity, with a likelihood ratio of 4.6 for detecting tumor-positive LNs. In the high-dose cohort, both the sensitivity and specificity decreased, with a likelihood ratio of 1.3. Furthermore, of the seventeen LNs with an “occult” tumor (defined as tumor foci < 5 mm in size), fifteen were detected by fluorescence, and two were not, resulting in a sensitivity of 88%. The amount of tumors in the two non-fluorescent LNs was comparable to the fluorescent LNs (0.83 mm vs. 1.98 mm, p = 0.425), and the NIR FI enhanced the visualization of LNMs, and even small (≤ 2 mm) peritoneal metastases [].
In addition, ex vivo FI performed during surgery, as well as the postoperative and pathologic assessment of LNMs, showed a sensitivity between 88% and 100%, and a specificity between 32% and 78%, in favor of the low-dose [].
In breast cancer patients undergoing SN or axillary LN dissection, a ratiometric activatable peptide-based tracer (AVB-620) was evaluated ex vivo. Ratiometric measurements involve assessing AVB-620 uptake by comparing the fluorescence intensity in tumor tissue relative to adjacent non-tumor tissue, enabling a consistent and quantitative comparison of tracer accumulation. AVB-620 was administered either the day before surgery (12–20 h prior) or on the day of surgery (2–12 h prior). With day-of-surgery dosing, the average ex vivo TBR was 1.09 ± 0.18 compared to 0.59 ± 0.04 for adjacent tissue. It is of note that a minimum TBR of 1.5 is generally accepted to discriminate fluorescent lesions []. For day-prior dosing, the signal-to-background ratio (SBR) was 1.90 ± 0.18 compared to 1.17 ± 0.20 for adjacent tissue. Identified tumor regions of interest (ROIs) corresponded with surgeon-identified disease and pathology reports. Although the authors noted that positive LNs had a higher ratio than negative nodes, the number of positive nodes, and thus the detection efficacy outcome parameters, were not reported [].
In locally advanced rectal cancer, patients received an intravenous bolus injection of 4.5 mg of bevacizumab-800CW, a fluorescent tracer targeting VEGF-α, 2–3 days before surgery. Ex vivo FI of the LNs showed a fluorescence signal in two enlarged LNs, which proved to be tumor-positive. However, this study did not provide further details on the total number of imaged LNs and the number of true-/false-positive/negative LNs [].
Furthermore, two studies evaluated the feasibility of a hybrid fluorescent and radioactive targeted tracer in metastasized colorectal cancer undergoing cytoreductive surgery ([111In] In-DOTA-labetuzumab- IRDye800CW) and prostate cancer surgery ([18F]-BF3- Cy3-ACUPA) [,]. Aras et al. describe that, in one patient, four pelvic LNs were resected based on PET-imaging, and the ex vivo back-table FI showed that two LNs had a higher intensity and were confirmed to be LNMs. No further data about the resected LNs were described []. De Gooyer et al. demonstrated that the tracer is effective for sensitive imaging at doses of 10 or 50 mg, with a back-table TBR of 2.62 (SD 0.49) and 2.73 (SD 0.94), respectively. Preoperative imaging detected previously unidentified LNM in one patient, while intraoperative FI revealed additional LNMs in two patients. This multimodal imaging approach led to changes in the clinical strategy for three patients, supporting its potential to enhance surgical decision-making in cytoreductive procedures. Details on false-positive and false-negative results were not provided [].
3.3.2. Ex Vivo Assessment After the Surgical Procedure
Postoperative assessment of LNMs using FI could improve the timely and efficient assessment of LN dissection specimens by the pathologist. Furthermore, preoperative administration of fluorescent tracers for post-surgical pathology assessment may seem unconventional. Given the promising results from the growing number of studies using FI to detect primary tumor margins both in vivo and ex vivo, it could be expected that a significant number of patients will receive tumor-targeted tracers in the future. For many solid tumors, FI could serve as a potential tool in surgery for assessing primary tumor margins and aiding the postoperative evaluation of LNs during pathological assessment.
If a FI threshold—a specific fluorescent intensity value used to differentiate between benign and malignant LNs—could be found that secures a 100% NPV, the amount of LNs that need pathological assessment could potentially be reduced significantly, making pathology assessment less time-consuming and more efficient []. In addition, while direct visualization and palpation are currently considered the gold standard for pathological assessment in detecting and resecting LNs, this method is labor-intensive, can overlook smaller LNs, and is subject to high interobserver variability, potentially leading to missed occult metastases [].
In a study of oral cancer, 960 LNs were analyzed ex vivo, of which 34 (3.5%) were found to contain metastatic disease. Panitumumab-IRDye800CW demonstrated preferential localization to LNMs, as indicated by a higher fluorescent signal in these nodes compared to other LNs. The median MFI of LNMs was significantly higher than that of benign LNs (0.06 versus 0.02, p < 0.05) []. In addition, a selection of five LNs with the highest fluorescence intensity from individual specimens yielded a sensitivity of 100%, a specificity of 85.8%, and an NPV of 100% for detecting occult metastases, resulting in 100% accuracy in clinical neck staging within this study.
In the clinically node-positive (cN+) cohort, evaluating a selection of five LNs with the highest fluorescence intensity per patient demonstrated a sensitivity of 87.5%, a specificity of 93.2%, and an NPV of 99.1% for detecting LNMs []. Figure 3 provides an overview of both in and ex vivo fluorescence imaging of cervical lymph nodes, including the corresponding Hematoxylin and Eosin (H&E) slides. Although many benign lymph nodes exhibit fluorescence (Figure 3B), ranking the nodes based on mean fluorescence intensity reveals that metastatic lymph nodes display the highest fluorescence (Figure 3D), suggesting sufficient contrast for differentiation.
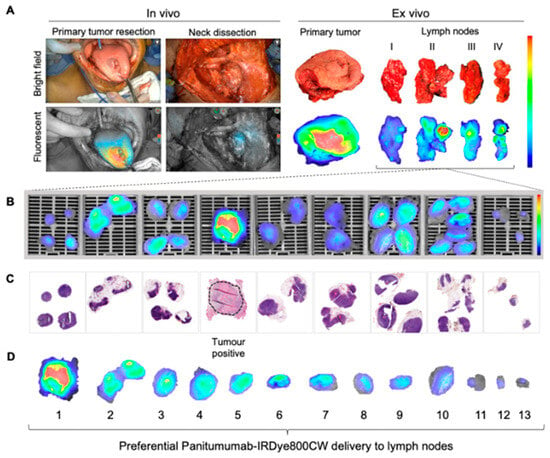
Figure 3.
Fluorescence imaging of cervical lymph nodes: (A) Intraoperative bright field and fluorescence imaging (in and ex vivo). (B) Postoperative ex vivo fluorescence imaging of formalin-fixed lymph node slides. (C) Corresponding H&E slides showing tumor status, with the most fluorescent LN being tumor-positive (dotted circle). (D) Ranking individual LNs by mean fluorescence intensity. This research was originally published in Theranostics by Krishnan et al. Metastatic and Sentinel Lymph Node Mapping Using Intravenously Delivered Panitumumab-IRDye800CW. Theranostics 2021;11:7188–7198 [].
Five studies performed postoperative FI of formalin-fixed paraffin-embedded (FFPE) tissue blocks on a microscopic level [,,,,]. Fluorescence-positivity was determined using variable definitions (thresholding the mean fluorescence intensity []); maximum fluorescence intensity []; TBR []; or combined mean FI and TBR [,,]). In case the TBR was used, the background was defined as either adjacent tissue [,,] or fibro-adipose tissue []. This procedure was detailed by Nishio et al. in a study involving 24 patients (1012 LNs analyzed) undergoing surgery for head and neck cancer following the systemic injection of Panitumumab-IRDYe800CW []. FI allowed for the identification of LNMs with a high sensitivity (85%) and specificity (94%), which could potentially reduce the number of LNs undergoing pathological processing and examination by 90%. These results were in concordance with another study, using Cetuximab-IRDye800CW, in 22 HNC patients (514 LNs analyzed), that demonstrated ex vivo detection of LNMs with a 100% sensitivity, 87% specificity, 49% PPV, and a 100% NPV using optimal mean FI thresholding. The authors concluded that this could serve as a selection tool for pathologists by reducing the amount of LNs necessitating microscopic examination by 77.4%, without overlooking LNMs. Importantly, in 7.5% of initially identified as false-positive LNs, tumor deposits were subsequently detected, underscoring instances missed by conventional histopathological analysis [].
In a colorectal cancer study, the feasibility of identifying LNMs ex vivo was investigated using an integrin-targeted fluorescent tracer (cRGD-ZW800-1). A total of 209 LNs were excised, of which 35 contained metastases. Ex vivo FI demonstrated a sensitivity and NPV of 100%, with a specificity and PPV of 87% and 33%, respectively. Since no false-negative LNs were reported, these findings indicate that the pathologist could selectively process only the fluorescent LNs, potentially reducing the workload of pathological examination by approximately 50% []. In addition, for patients with pancreatic cancer, the use of Cetuximab-IRDYe800CW demonstrated a significant difference in ex vivo mean fluorescence intensity (MFI) between tumor-positive and tumor-negative LNs (p < 0.001, with a dose-dependency of p = 0.03). The study’s microscopic detection of LNMs revealed high sensitivity (91–93%) but low to moderate specificity (35–66%).
Therefore, it is crucial that FI can even detect LNMs with minimal amounts of tumor, enhancing postoperative pathology analysis. Of the seventeen LNMs with an “occult” tumor (tumor foci < 5 mm in size), fifteen were detected by fluorescence, and two were not, resulting in a sensitivity of 88% []. In line with Cetuximab-IRDYe800CW, Panitumumab-IRDYe800CW showed a significantly higher TBR and MFI in LNMs, compared to benign LNs, in patients with pancreatic cancer.
However, they found a relatively lower sensitivity (68%) and higher specificity (92%). Of the 67 LNMs, 19 were not detected using FI, resulting in an NPV of 93% []. More recently, Stibbe et al. evaluated the use of OTL78 during prostate cancer surgery in 18 patients. Post hoc ex vivo imaging identified four (57%) of seven metastatic LN conglomerates. There were 21 false-positive LN clusters. OTL78 dose de-escalation and interval prolongation led to a lower false-positive LN cluster rate and a higher false-negative rate, in vivo and ex vivo. Post hoc gross-macroscopic and microscopic fluorescence imaging of individual LNs (n = 289) identified all nine LNMs with a median SBR of 3.5 (IQR 2.7–5.3) for gross-macroscopy and 8.8 (IQR 3.1–20.3) for microscopy. Because only tumor-positive and/or fluorescent microscopic slides were selected, requested, and imaged, specificity and false-positive rates were not calculated in this study [].
4. Discussion
The development of molecular-targeted FI for improved intraoperative visualization of primary tumors has led to significant interest in its potential for detecting LNMs. This article presents the first systematic review of clinical trials evaluating the use of molecular-targeted FI for perioperative in vivo and ex vivo detection of LNMs.
Clinical evaluations of molecular-targeted FI include both intraoperative and postoperative LN imaging. In the intraoperative setting, both in vivo and ex vivo FI techniques were employed. The use of fluorescent imaging and its diagnostic value requires more thorough reporting, as the diagnostic accuracy of in vivo molecular-targeted FI for detecting LNMs was reported in only four of the twenty-six studies reviewed [,,,].
Correspondingly, studies evaluating the detection of LNs report significant variability in sensitivity, specificity, PPV, and NPV. In some cases, data on LNs and LNMs were not reported, which underscores the lack of clear reporting. It is important to have all data available to correctly interpret the results. Furthermore, while most studies demonstrate high TBRs for LNMs, numerous false-positive LNs were also observed. For example, in benign LNs, the panitumumab-IRDye800CW signal appears to result from non-specific accumulation, demonstrated by a dose-dependent increase in fluorescent antibody-complex detection within lymphatic channels, despite the absence of EGFR expression []. Additionally, false-negative LNs were also noted, potentially due to several factors, such as the following: the absence of the targeting molecule in LNMs, the limited penetration depth of the NIR light for LNs covered by tissues thicker than 1 cm, or the limited camera sensitivity in detecting minimal fluorescence in small (micro-)metastases.
Importantly, ex vivo postoperative molecular-targeted FI on FFPE tissue blocks demonstrated both high sensitivity and specificity in all studies for detecting LNMs. High sensitivity is particularly clinically important for confidently ruling out the presence of metastases in LNs requiring pathological assessment. Additionally, intraoperative FI for detecting LNMs was primarily performed ex vivo on resected specimens, with fluorescence signals later correlated with histopathological findings, and less frequently conducted in vivo. Since most studies were exploratory, the diagnostic accuracy of FI for LN detection was reported in approximately one-third of the included studies, with only one study providing both in vivo and ex vivo test characteristics, which underlines the problem of reporting and extrapolating ex vivo data to in vivo purposes [].
The lack of detailed reporting on critical factors in scientific papers underscores the limited recognition of their importance, making our systematic review of this topic both crucial and highly relevant. The key issue driving this systematic review is the significant difference in imaging conditions between in vivo and ex vivo settings, which prevents the direct extrapolation of ex vivo findings to in vivo applications. Variations in tissue optical properties—such as composition, blood supply, vascularization, and skin pigmentation—combined with the limited penetration of NIR light, can greatly affect light absorption, scattering, and emission, influencing LN visualization in vivo []. Furthermore, other factors, such as the device sensitivity, the dose, and the pharmacological properties of the fluorescent agent, could be influential []. In contrast, ex vivo imaging allows for easier tissue manipulation and more standardized conditions. Recognizing these differences is essential for accurately interpreting the study results and evaluating LN visualization in vivo, highlighting the necessity of our comprehensive review.
Ultimately, intraoperative FI should serve as a tool for surgical decision-making in vivo, which requires reports on how the detection of LNMs could assist during surgical procedures. Our review indicates that FI using tumor-targeted tracers is not yet feasible in detecting (occult) LNMs in vivo, and thus the technique of optical imaging, because of its intrinsic limitations, may also not be the most suitable approach in this context. However, it does have an impact on the postoperative workflow, as it can reduce the workload of the pathologist by, for instance, prioritizing the assessment of higher-fluorescent LNs instead of all LNs, thereby creating a more efficient workflow. Only one study documented clinical decision-making based on the correlation of in vivo fluorescence with intraoperative assessment by the pathologist []. While the sensitivity for detecting LNMs was 80% and the specificity was 60%, these findings did not lead to changes in the surgical plan. Several clinical implications for using intraoperative FI for LN detection, both in vivo and ex vivo, can be proposed, but the current systems seem insufficient for detecting “occult” LNMs in vivo.
The current limitations of FI technology prevent it from replacing the SN procedure. While fluorescence imaging is a valuable tool for aiding in the detection of SNs, it is not yet capable of fully replacing the conventional approach due to the challenges associated with NIR camera systems. Light absorption and scattering in tissue reduce the depth at which fluorescence signals can be detected, limiting the visualization of deeper nodes. Additionally, variations in tissue properties (e.g., vascularization, pigmentation, variability in tissue types) can affect the fluorescence signal, leading to inconsistent results. Furthermore, the current fluorescence technique cannot distinguish between the SN and other LNs; typically, the first fluorescent lymph node is interpreted as the SN. These factors prevent the state-of-the-art FI from matching the accuracy and reliability of the SN procedure, currently making it a complementary rather than standalone method for intraoperative SN detection.
Theoretically, FLI could influence intraoperative decision-making in several ways, which are as follows:
Firstly, in vivo detection of occult LNMs could necessitate the abortion of the surgical procedure. For example, in colorectal cancer, detecting peritoneal lesions and LNs suspicious for metastases using FI and confirming them with fresh frozen sectioning could be critical in intraoperative decision-making, potentially leading to an open-and-close procedure. Such a finding would be a significant game-changer, as it directly impacts the surgical approach and patient management. However, while FI should serve as a tool for identifying LNMs, its state-of-the-art technology is not powerful enough to rule out occult LNMs [].
Secondly, real-time detection of LNMs could assist in guiding the extent of lymphadenectomy, potentially reducing the scope of surgery and associated morbidity. For instance, during thyroid surgery, FI could potentially reduce the number of resected benign LNs by more than 25%. However, its diagnostic accuracy needs to be evaluated in vivo in future studies, as this particular study was only conducted ex vivo [,,].
Lastly, another possible application of in vivo LN imaging could be to improve the tumor margin assessment in case of extra nodular extension of LNMs, which is associated with worse survival. FI could potentially help visualize margin delineation and could therefore be clinically relevant when the ingrowth extends beyond the nodal capsule [].
A possible solution to the limitations of FI for preoperative and intraoperative imaging could be optoacoustic imaging (OAI), which may improve imaging depth by several centimeters compared to the less than 1 cm depth achieved with FI. The principles of OAI are similar to those of FI, but OAI detects acoustic waves generated by the optical excitation of the fluorophore, rather than using NIR wavelengths. Therefore, combining FI and OAI could be beneficial for the non-invasive detection of LNMs. However, small tumor deposits in occult LNMs make detection with both FI and OAI challenging due to the reduced excitation of the fluorophore in these small deposits. This was demonstrated in a proof-of-concept study by Vonk et al., where they explored the use of multispectral optoacoustic tomography (MSOT) for the in vivo detection of LN metastases in oral cancer patients. The study highlighted the potential of MSOT in improving preoperative detection, as it allows for the visualization of both intrinsic tissue chromophores and administered contrast agents at depths of several centimeters, making it suitable for identifying early-stage metastases, though limitations related to signal intensity and fluorophore concentration were observed [,].
This review highlights a potential role for FI in the postoperative assessment of LNs. Pathological LNMs could be successfully distinguished from benign LNs through fluorescence assessment, as LNs can be preselected based on their MFI and TBR with high specificity. This could influence the LN assessment workflow, reducing the number of LNs that require pathological processing and histological examination. Furthermore, the high sensitivity suggests that analyzing a smaller number of fluorescent LNs could be more efficient and less labor-intensive, leading to better detection of LNMs, more accurate postoperative staging and treatment, and fewer missed LNMs compared to standard histopathologic analysis.
The studies included here reported various definitions for fluorescence-positive LNs, including subjective assessments by surgeons and more quantitative measures using thresholds (e.g., TBR, MFI, or a combination of both). Although studies have demonstrated that it is possible to distinguish between benign and malignant LNs using currently available semi-quantitative methods, the lack of consensus on standard fluorescence assessment restricts a fair comparison between clinical studies []. Therefore, future advancements in methods and techniques that support the accurate quantification of fluorophore concentrations in tissue, for instance, fluorescence lifetime imaging, could enhance the objective differentiation between tumor and non-tumor tissue [,].
5. Conclusions
While molecular-targeted fluorescence imaging (FI) has demonstrated the ability to detect LNMs using tumor-specific tracers, it has not yet shown in vivo benefits for the detection of LNMs, particularly in the context of occult metastases. Potential applications for intraoperative LN imaging have been described and suggested. However, FI’s effectiveness as a tool, combined with or without other techniques, and its impact on surgical planning, are infrequently reported. Nevertheless, the postoperative assessment of LNs using molecular-targeted FI has shown high accuracy, indicating its potential to distinguish pathologically confirmed LNMs from benign ones. This could potentially increase efficiency and reduce the need for extensive pathological processing and histological examination. Further research is warranted to fully elucidate the clinical utility of FI in the in vivo setting.
Author Contributions
Conceptualization: B.E.Z., S.W.R.D., L.v.M., H.A.G. and S.K.; methodology: B.E.Z., S.W.R.D., L.v.M. and H.A.G.; formal analysis: B.E.Z., S.W.R.D. and S.W.R.D.; investigation: B.E.Z., S.W.R.D. and L.v.M.; data curation: B.E.Z., S.W.R.D., L.v.M. and S.K.; writing—original draft preparation: B.E.Z., S.W.R.D., L.v.M. and S.K.; writing—review and editing: all authors; visualization: B.E.Z., S.W.R.D. and S.K.; supervision: S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CEA | Carcinoembryonic Antigen |
| CT | Computed Tomography |
| EGFR | Endothelial Growth Factor Receptor |
| FAPI | Fibroblast Activation Protein Inhibitor |
| FFPE | Formalin-Fixed Paraffin-Embedded |
| FI | Fluorescence Imaging |
| H&E | Hematoxylin and Eosin |
| HNC | Head and Neck Cancer |
| LN | Lymph Node |
| LNM | Lymph Node Metastasis |
| MFI | Mean Fluorescence Intensity |
| MRI | Magnetic Resonance Imaging |
| MSOT | Multispectral Optoacoustic Tomography |
| NIR | Near-Infrared |
| OAI | Optoacoustic Imaging |
| PET-CT | Positron Emission Tomography–Computed Tomography |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| PSMA | Prostate-Specific Membrane Antigen |
| SBR | Signal-to-Background Ratio |
| SN | Sentinel Node |
| SPECT-CT | Single Photon Emission Computed Tomography–Computed Tomography |
| TBR | Tumor-to-Background Ratio |
| VEGF-α | Vascular Endothelial Growth Factor Alpha |
Appendix A
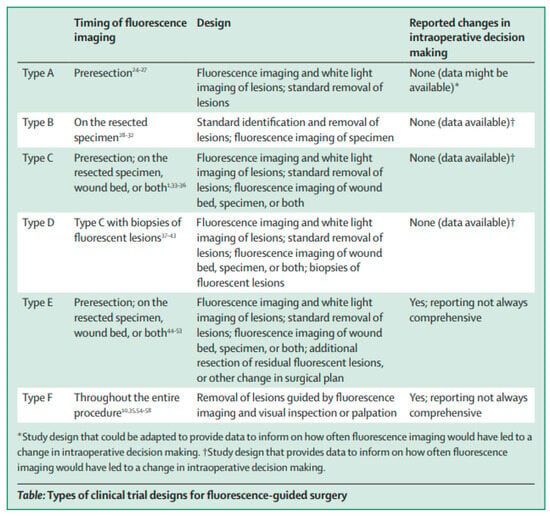
Figure A1.
Types of clinical trial designs for fluorescence-guided surgery. This research was originally published in The Lancet Oncology by Lauwerends et al. [].
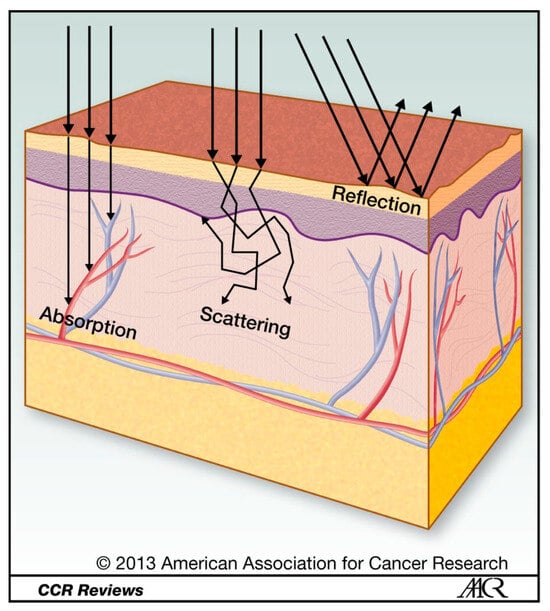
Figure A2.
Light propagation through tissue. Light travelling through tissue is subject to reflection, scattering, and absorption [].
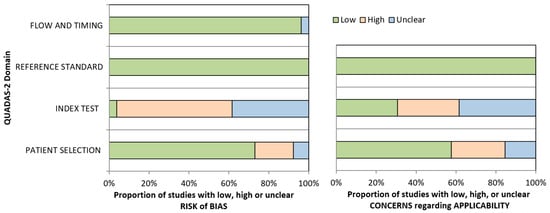
Figure A3.
Risk of bias overview as determined by two independent reviewers according to QUADAS-2 checklist.

Table A1.
Overview on the detailed search strategy.
Table A1.
Overview on the detailed search strategy.
| Database Searched | via | Years of Coverage | Records | Records After Duplicates Removed |
|---|---|---|---|---|
| Embase | Embase.com | 1971–Present | 3645 | 3600 |
| Medline ALL | Ovid | 1946–Present | 2942 | 617 |
| Web of Science Core Collection * | Web of Knowledge | 1975–Present | 3628 | 1021 |
| Cochrane Central Register of Controlled Trials | Wiley | 1992–Present | 48 | 28 |
| Total | 10,263 | 5266 | ||
* Science Citation Index Expanded (1975–present); Social Sciences Citation Index (1975–present); Arts & Humanities Citation Index (1975–present); Conference Proceedings Citation Index- Science (1990–present); Conference Proceedings Citation Index- Social Science & Humanities (1990–present); Emerging Sources Citation Index (2015–present).
Appendix B

| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Page 1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | Page 1–2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Page 3 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Page 3 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Page 3–4 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Page 3–4 |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | Page 22–25 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and, if applicable, details of automation tools used in the process. | Page 3–4 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from the study investigators, and, if applicable, details of automation tools used in the process. | Page 3–4 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and, if not, the methods used to decide which results to collect. | Page 3–4 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | Page 3–4 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and, if applicable, details of automation tools used in the process. | Page 22 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | Page 22 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | Page 3–4 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | Page 3–4 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | Page 3–4 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | Page 3–4 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among the study results (e.g., subgroup analysis, meta-regression). | Page 3–4 | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | Page 3–4 | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | Page 22 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | Page 22 |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Page 3–4 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | Page 5 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Page 6–10 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Page 22 |
| Results of individual studies | 19 | For all outcomes, present, for each study the (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Page 6–10 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | Page 22 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was completed, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | Page 6–10 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among the study results. | Page 6–10 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | Page 6–10 | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | Page 22 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | Page 22 |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Page 17–19 |
| 23b | Discuss any limitations of the evidence included in the review. | Page 17–19 | |
| 23c | Discuss any limitations of the review processes used. | Page 17–19 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | Page 17–19 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | N/A |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | N/A | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | N/A | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | N/A |
| Competing interests | 26 | Declare any competing interests of review authors. | N/A |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | N/A |
Appendix C
Embase.com
(‘molecular imaging’/de OR ‘near infrared fluorescence’/de OR ‘near infrared fluorescence imaging’/de OR photoacoustics/exp OR ((‘fluorescence imaging’/de OR ‘fluorescent dye’/de OR ‘fluorescence’/de OR ‘near infrared imaging system’/de) AND (tracer/de OR ‘antineoplastic monoclonal antibody’/exp)) OR ‘fluorescence guided resection’/de OR ‘fluorescence image guided surgery’/de OR (((molecul* OR tumor-target* OR receptor*-target* OR tumour-target* OR tumor-specific* OR receptor*-specific* OR tumour-specific* OR cancer-target* OR cancer-specific*) NEAR/6 (imag* OR tracer* OR fluorescen*)) OR ((near-infrared OR nearinfrared OR probe* OR guidan* OR guided* OR target*) NEAR/3 fluorescen*) OR ((near-infrared OR nearinfrared) NEAR/3 imag*) OR photoacoustic* OR photo-acoustic* OR ((cetuximab OR Girentuximab OR MabCAIX OR YY146 OR BIWA OR MN-14 OR SGM-101 OR Labetuzumab OR hM5A OR Cetuximab OR Panitumumab OR mAb-7-4 OR 323-A3 OR Trastuzumab OR Tocilizumab OR mAb62 OR D2B OR Bevacizumab OR DC101 OR ATN-658 OR TRC-105 OR ALT-836 OR A11-c-Mb OR anti-HER3-diabody OR A2cDb OR anti-CEA OR VB5-845d OR ssSM3E OR ScFvEGFR OR scFvD2B OR 3E8-scFv-Cys OR B9 OR 7D12 OR 7D12-9G6 OR 2Rs15d OR 11A1 OR 83v2Cys OR Z03115-Cys OR ZEGFR-1907 OR ZHER2 OR R01-MG OR Chlorotoxin OR cRGD OR NGR OR Ac-TZ14011 OR EGF OR TM1 OR G-pip-Sta-BBN OR GSG-Sta-BBN OR 6Ahx-Sta-BBN OR BBN-7-14-NH2 OR BBN-6-14-NH2 OR OTL78-DUPA- OR KuE OR Glu-urea-Lys-HBED-CC OR PSMA-1 OR AE105 OR ATF OR Hypoxyfluor-1 OR Z-360 OR OTL38 OR EC17 OR 2-DG OR BOEPL OR L-733 OR 060 OR YC-27 OR IY-IY OR alphavbeta* OR alpha-v-beta* OR ANXA2 OR CAIX OR CCK2i4svR OR CCK2R OR CD105-TF OR CD13 OR CD146 OR CD44v6 OR CEA OR CXCR4 OR EGFR OR EpCAM OR FR OR GLUT1 OR GRPR OR HER2 OR HER3 OR IL-10 OR Integrin* OR Kv-10-1 OR LHRH-R OR MMP-2 OR NK1R OR PSCA OR PSMA OR TAG-72 OR TrkC OR uPAR OR VEGF-A OR VEGFR-2) NEAR/6 (IRDye* OR fluoresc* OR zw800* OR bm104 OR FNIR-Z-759 OR Cy5 OR Dy676 OR XenoLight OR 770CF OR S0456 OR AF680 OR DyLight OR CH1055 OR CyAL-5-5b OR IRDye680RD OR AF750 OR IRDye650 OR NIR-830 OR LS288 OR ZWCC))):ab,ti,kw) AND (‘intraoperative period’/de OR ‘peroperative care’/de OR ‘preoperative period’/de OR ‘preoperative care’/de OR ‘postoperative period’/de OR ‘postoperative care’/de OR ‘surgical navigation system’/de OR ‘fluorescence guided resection’/de OR ‘fluorescence image guided surgery’/de OR (peroperat* OR preoperat* OR intraoperat* OR postoperat* OR per-operat* OR pre-operat* OR intra-operat* OR post-operat* OR persurg* OR presurg* OR intrasurg* OR postsurg* OR per-surg* OR pre-surg* OR intra-surg* OR post-surg* OR ((after OR before OR during OR guidan* OR guided* OR image-guid* OR decision* OR navigat* OR undergoing) NEAR/6 (resection OR dissection OR operation OR surgery OR surgical*))):Ab,ti,kw) AND (neoplasm/exp OR (cancer* OR tumor* OR tumour* OR neoplas* OR carcinom* OR metasta* OR malign*):ab,ti) NOT (‘brain tumor’/exp OR ‘central nervous system cancer’/mj OR neurosurgery/mj OR (brain OR cerebral* OR neurosurg* OR neuro*-surg* OR glioma):ti) NOT ([conference abstract]/lim AND [2000-2019]/py) NOT ([animals]/lim NOT [humans]/lim) AND [english]/lim
Medline ALL Ovid
(Molecular Imaging/OR ((Optical Imaging/OR Fluorescent Dyes/OR Fluorescence/) AND (Radioactive Tracers/OR exp Antineoplastic Agents, Immunological/)) OR (((molecul* OR tumor-target* OR receptor*-target* OR tumour-target* OR tumor-specific* OR receptor*-specific* OR tumour-specific* OR cancer-target* OR cancer-specific*) ADJ6 (imag* OR tracer* OR fluorescen*)) OR ((near-infrared OR nearinfrared OR probe* OR guidan* OR guided* OR target*) ADJ3 fluorescen*) OR ((near-infrared OR nearinfrared) ADJ3 imag*) OR photoacoustic* OR photo-acoustic* OR ((cetuximab OR Girentuximab OR MabCAIX OR YY146 OR BIWA OR MN-14 OR SGM-101 OR Labetuzumab OR hM5A OR Cetuximab OR Panitumumab OR mAb-7-4 OR 323-A3 OR Trastuzumab OR Tocilizumab OR mAb62 OR D2B OR Bevacizumab OR DC101 OR ATN-658 OR TRC-105 OR ALT-836 OR A11-c-Mb OR anti-HER3-diabody OR A2cDb OR anti-CEA OR VB5-845d OR ssSM3E OR ScFvEGFR OR scFvD2B OR 3E8-scFv-Cys OR B9 OR 7D12 OR 7D12-9G6 OR 2Rs15d OR 11A1 OR 83v2Cys OR Z03115-Cys OR ZEGFR-1907 OR ZHER2 OR R01-MG OR Chlorotoxin OR cRGD OR NGR OR Ac-TZ14011 OR EGF OR TM1 OR G-pip-Sta-BBN OR GSG-Sta-BBN OR 6Ahx-Sta-BBN OR BBN-7-14-NH2 OR BBN-6-14-NH2 OR OTL78-DUPA- OR KuE OR Glu-urea-Lys-HBED-CC OR PSMA-1 OR AE105 OR ATF OR Hypoxyfluor-1 OR Z-360 OR OTL38 OR EC17 OR 2-DG OR BOEPL OR L-733 OR 060 OR YC-27 OR IY-IY OR alphavbeta* OR alpha-v-beta* OR ANXA2 OR CAIX OR CCK2i4svR OR CCK2R OR CD105-TF OR CD13 OR CD146 OR CD44v6 OR CEA OR CXCR4 OR EGFR OR EpCAM OR FR OR GLUT1 OR GRPR OR HER2 OR HER3 OR IL-10 OR Integrin* OR Kv-10-1 OR LHRH-R OR MMP-2 OR NK1R OR PSCA OR PSMA OR TAG-72 OR TrkC OR uPAR OR VEGF-A OR VEGFR-2) ADJ6 (IRDye* OR fluoresc* OR zw800* OR bm104 OR FNIR-Z-759 OR Cy5 OR Dy676 OR XenoLight OR 770CF OR S0456 OR AF680 OR DyLight OR CH1055 OR CyAL-5-5b OR IRDye680RD OR AF750 OR IRDye650 OR NIR-830 OR LS288 OR ZWCC))).ab,ti,kf.) AND (Intraoperative Period/OR Preoperative Period/OR Preoperative Care/OR Postoperative Period/OR Postoperative Care/OR Surgical Navigation Systems/OR (peroperat* OR preoperat* OR intraoperat* OR postoperat* OR per-operat* OR pre-operat* OR intra-operat* OR post-operat* OR persurg* OR presurg* OR intrasurg* OR postsurg* OR per-surg* OR pre-surg* OR intra-surg* OR post-surg* OR ((after OR before OR during OR guidan* OR guided* OR image-guid* OR decision* OR navigat* OR undergoing) ADJ6 (resection OR dissection OR operation OR surgery OR surgical*))).ab,ti,kf.) AND (exp Neoplasms/OR (cancer* OR tumor* OR tumour* OR neoplas* OR carcinom* OR metasta* OR malign*).ab,ti.) NOT (exp Brain Neoplasms/OR * Neurosurgery/OR (brain OR cerebral* OR neurosurg* OR neuro*-surg* OR glioma).ti.) NOT (news OR congres* OR abstract* OR book* OR chapter* OR dissertation abstract*).pt. NOT (exp animals/NOT humans/) AND english.lg.
Web of Science
TS=(((((molecul* OR tumor-target* OR receptor*-target* OR tumour-target* OR tumor-specific* OR receptor*-specific* OR tumour-specific* OR cancer-target* OR cancer-specific*) NEAR/5 (imag* OR tracer* OR fluorescen*)) OR ((near-infrared OR nearinfrared OR probe* OR guidan* OR guided* OR target*) NEAR/2 fluorescen*) OR ((near-infrared OR nearinfrared) NEAR/2 imag*) OR photoacoustic* OR photo-acoustic*)) AND ((peroperat* OR preoperat* OR intraoperat* OR postoperat* OR per-operat* OR pre-operat* OR intra-operat* OR post-operat* OR persurg* OR presurg* OR intrasurg* OR postsurg* OR per-surg* OR pre-surg* OR intra-surg* OR post-surg* OR ((after OR before OR during OR guidan* OR guided* OR image-guid* OR decision* OR navigat* OR undergoing) NEAR/5 (resection OR dissection OR operation OR surgery OR surgical*)))) AND ((cancer* OR tumor* OR tumour* OR neoplas* OR carcinom* OR metasta* OR malign*)) NOT ((animal* OR rat OR rats OR mouse OR mice OR murine OR dog OR dogs OR canine OR cat OR cats OR feline OR rabbit OR cow OR cows OR bovine OR rodent* OR sheep OR ovine OR pig OR swine OR porcine OR veterinar* OR chick* OR zebrafish* OR baboon* OR nonhuman* OR primate* OR cattle* OR goose OR geese OR duck OR macaque* OR avian* OR bird* OR fish*) NOT (human* OR patient* OR women OR woman OR men OR man))) NOT TI=((brain OR cerebral* OR neurosurg* OR neuro*-surg* OR glioma)) AND LA=(English) NOT DT=(Meeting Abstract OR Meeting Summary)
Cochrane
(((molecul* OR tumor-target* OR receptor-target* OR tumour-target* OR tumor-specific* OR receptor* NEXT specific* OR tumour-specific* OR cancer-target* OR cancer-specific*) NEAR/6 (imag* OR tracer* OR fluorescen*)) OR photoacoustic* OR photo-acoustic*):ab,ti,kw AND ((peroperat* OR preoperat* OR intraoperat* OR postoperat* OR per-operat* OR pre-operat* OR intra-operat* OR post-operat* OR persurg* OR presurg* OR intrasurg* OR postsurg* OR per-surg* OR pre-surg* OR intra-surg* OR post-surg* OR ((after OR before OR during OR guidan* OR guided* OR image-guid* OR decision* OR navigat* OR undergoing) NEAR/6 (resection OR dissection OR operation OR surgery OR surgical*))):Ab,ti,kw) AND ((cancer* OR tumor* OR tumour* OR neoplas* OR carcinom* OR metasta* OR malign*):ab,ti) NOT ((brain OR cerebral* OR neurosurg* OR neuro* NEXT surg* OR glioma):ti)
References
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Mamelle, G.; Pampurik, J.; Luboinski, B.; Lancar, R.; Lusinchi, A.; Bosq, J. Lymph node prognostic factors in head and neck squamous cell carcinomas. Am. J. Surg. 1994, 168, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Soerjomataram, I.; Louwman, M.W.; Ribot, J.G.; Roukema, J.A.; Coebergh, J.W. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res. Treat. 2008, 107, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Sürücü, E.; Polack, B.D.; Demir, Y.; Durmuşoğlu, M.; Ekmekçi, S.; Sarıoğlu, S.; Çelik, A.O.; Ada, E.; Iİkiz, A.Ö. Dual-phase F-18 FDG PET-CT in staging and lymphoscintigraphy for detection of sentinel lymph nodes in oral cavity cancers. Clin. Imaging. 2015, 39, 781–786. [Google Scholar] [CrossRef] [PubMed]
- García Megías, I.; Almeida, L.S.; Calapaquí Terán, A.K.; Pabst, K.M.; Herrmann, K.; Giammarile, F.; Bolton, R.C.D. FAPI radiopharmaceuticals in nuclear oncology and theranostics of solid tumours: Are we nearer to surrounding the hallmarks of cancer? Ann. Nucl. Med. 2025. [Google Scholar] [CrossRef]
- Baldari, L.; Boni, L.; Cassinotti, E. Lymph node mapping with ICG near-infrared fluorescence imaging: Technique and results. Minim. Invasive Ther. Allied Technol. 2023, 32, 213–221. [Google Scholar] [CrossRef]
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef]
- Van Manen, L.; Handgraaf, H.J.M.; Diana, M.; Dijkstra, J.; Ishizawa, T.; Vahrmeijer, A.L.; Mieog, J.S.D. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J. Surg. Oncol. 2018, 118, 283–300. [Google Scholar] [CrossRef]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Mieog, J.S.D.; Achterberg, F.B.; Zlitni, A.; Hutteman, M.; Burggraaf, J.; Swijnenburg, R.J.; Gioux, S.; Vahrmeijer, A.L. Fundamentals and developments in fluorescence-guided cancer surgery. Nat. Rev. Clin. Oncol. 2022, 19, 9–22. [Google Scholar] [CrossRef]
- Keereweer, S.; Van Driel, P.B.; Snoeks, T.J.; Kerrebijn, J.D.; Baatenburg de Jong, R.J.; Vahrmeijer, A.L.; Sterenborg, H.J.C.M.; Löwik, C.W.G.M. Optical image-guided cancer surgery: Challenges and limitations. Clin. Cancer Res. 2013, 19, 3745–3754. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, M.J.; Akl, A.E.; Brennan, E.S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.; Rutjes, A.W.; Reitsma, J.B.; Bossuyt, P.M.; Kleijnen, J. The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med. Res. Methodol. 2003, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Hoogstins, C.E.S.; Tummers, Q.R.J.G.; Gaarenstroom, K.N.; De Kroon, C.D.; Trimbos, J.B.M.Z.; Bosse, T.; Smit, V.T.; Vuyk, J.; van de Velde, C.J.; Cohen, A.F.; et al. A novel tumor-specific agent for intraoperative near-infrared fluorescence imaging: A translational study in healthy volunteers and patients with ovarian cancer. Clin. Cancer Res. 2016, 22, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Tummers, Q.R.J.G.; Hoogstins, C.E.S.; Gaarenstroom, K.N.; de Kroon, C.D.; van Poelgeest, M.I.E.; Vuyk, J.; Bosse, T.; Smit, V.T.; van de Velde, C.J.; Cohen, A.F.; et al. Intraoperative imaging of folate receptor alpha positive ovarian and breast cancer using the tumor specific agent EC17. Oncotarget 2016, 7, 32144–32155. [Google Scholar] [CrossRef]
- Lamberts, L.E.; Koch, M.; De Jong, J.S.; Adams, A.L.L.; Glatz, J.; Kranendonk, M.E.G.; Van Scheltinga, A.G.T.; Jansen, L.; De Vries, J.; Hooge, M.N.L.-D.; et al. Tumor-specific uptake of fluorescent bevacizumab-IRDye800CW microdosing in patients with primary breast cancer: A phase I feasibility study. Clin. Cancer Res. 2017, 23, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.L.; Moore, L.S.; Tipirneni, K.; De Boer, E.; Stevens, T.M.; Hartman, Y.E.; Carroll, W.R.; Zinn, K.R.; Warram, J.M. Sensitivity and specificity of cetuximab-IRDye800CW to identify regional metastatic disease in head and neck cancer. Clin. Cancer Res. 2017, 23, 4744–4752. [Google Scholar] [CrossRef]
- Unkart, J.T.; Chen, S.L.; Wapnir, I.L.; Gonzalez, J.E.; Harootunian, A.; Wallace, A.M. Intraoperative Tumor Detection Using a Ratiometric Activatable Fluorescent Peptide: A First-in-Human Phase 1 Study. Ann. Surg. Oncol. 2017, 24, 3167–3173. [Google Scholar] [CrossRef]
- Boogerd, L.S.F.; Hoogstins, C.E.S.; Gaarenstroom, K.N.; de Kroon, C.D.; Beltman, J.J.; Bosse, T.; Stelloo, E.; Vuyk, J.; Low, P.S.; Burggraaf, J.; et al. Folate receptor-α targeted near-infrared fluorescence imaging in high-risk endometrial cancer patients: A tissue microarray and clinical feasibility study. Oncotarget 2018, 9, 791–801. [Google Scholar] [CrossRef]
- Boogerd, L.S.F.; Hoogstins, C.E.S.; Schaap, D.P.; Kusters, M.; Handgraaf, H.J.M.; van der Valk, M.J.M.; E Hilling, D.; A Holman, F.; Peeters, K.C.M.J.; Mieog, J.S.D.; et al. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: A dose-escalation pilot study. Lancet Gastroenterol. Hepatol. 2018, 3, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Tummers, W.S.; Miller, S.E.; Teraphongphom, N.T.; Gomez, A.; Steinberg, I.; Huland, D.M.; Hong, S.; Kothapalli, S.-R.; Hasan, A.; Ertsey, R.; et al. Intraoperative Pancreatic Cancer Detection using Tumor—Specific Multimodality Molecular Imaging. Ann. Surg. Oncol. 2018, 25, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Hoogstins, C.E.S.; Boogerd, L.S.F.; Gaarenstroom, K.N.; de Kroon, C.D.; Beltman, J.J.; Trimbos, J.B.M.Z.; Bosse, T.; Vuyk, J.; Low, P.S.; Burggraaf, J.; et al. Feasibility of folate receptor-targeted intraoperative fluorescence imaging during staging procedures for early ovarian cancer. Eur. J. Gynaecol. Oncol. 2019, 40, 203–208. [Google Scholar] [CrossRef]
- Nishio, N.; van den Berg, N.S.; van Keulen, S.; Martin, B.A.; Fakurnejad, S.; Teraphongphom, N.; Chirita, S.U.; Oberhelman, N.J.; Lu, G.; Horton, C.E.; et al. Optical molecular imaging can differentiate metastatic from benign lymph nodes in head and neck cancer. Nat. Commun. 2019, 10, 5044. [Google Scholar] [CrossRef]
- Randall, L.M.; Wenham, R.M.; Low, P.S.; Dowdy, S.C.; Tanyi, J.L. A phase II, multicenter, open-label trial of OTL38 injection for the intra-operative imaging of folate receptor-alpha positive ovarian cancer. Gynecol. Oncol. 2019, 155, 63–68. [Google Scholar] [CrossRef]
- Tummers, W.S.; Miller, S.E.; Teraphongphom, N.T.; van den Berg, N.S.; Hasan, A.; Longacre, T.A.; Fisher, G.A.; Bonsing, B.A.; Vahrmeijer, A.L.; Gambhir, S.S.; et al. Detection of visually occult metastatic lymph nodes using molecularly targeted fluorescent imaging during surgical resection of pancreatic cancer. HPB 2019, 21, 883–890. [Google Scholar] [CrossRef]
- De Jongh, S.J.; Tjalma, J.J.J.; Koller, M.; Linssen, M.D.; Vonk, J.; Dobosz, M.; Jorritsma-Smit, A.; Kleibeuker, J.H.; Hospers, G.A.; Havenga, K.; et al. Back-Table Fluorescence-Guided Imaging for Circumferential Resection Margin Evaluation Using Bevacizumab-800CW in Patients with Locally Advanced Rectal Cancer. J. Nucl. Med. 2020, 61, 655–661. [Google Scholar] [CrossRef]
- De Valk, K.S.; Deken, M.M.; Handgraaf, H.J.M.; Bhairosingh, S.S.; Bijlstra, O.D.; van Esdonk, M.J.; van Scheltinga, A.G.T.; Valentijn, A.R.P.; March, T.L.; Vuijk, J.; et al. First-in-Human Assessment of cRGD-ZW800-1, a Zwitterionic, Integrin-Targeted, Near-Infrared Fluorescent Peptide in Colon Carcinoma. Clin. Cancer Res. 2020, 26, 3990–3998. [Google Scholar] [CrossRef]
- Lu, G.; van den Berg, N.S.; Martin, B.A.; Nishio, N.; Hart, Z.P.; van Keulen, S.; Fakurnejad, S.; Chirita, S.U.; Raymundo, R.C.; Yi, G.; et al. Tumour-specific fluorescence-guided surgery for pancreatic cancer using panitumumab-IRDye800CW: A phase 1 single-centre, open-label, single-arm, dose-escalation study. Lancet Gastroenterol. Hepatol. 2020, 5, 753–764. [Google Scholar] [CrossRef]
- Aras, O.; Demirdag, C.; Kommidi, H.; Guo, H.; Pavlova, I.; Aygun, A.; Karayel, E.; Pehlivanoglu, H.; Yeyin, N.; Kyprianou, N.; et al. Small Molecule, Multimodal, [18F]-PET and Fluorescence Imaging Agent Targeting Prostate-Specific Membrane Antigen: First-in-Human Study. Clin. Genitourin. Cancer. 2021, 19, 405–416. [Google Scholar] [CrossRef]
- De Valk, K.S.; Deken, M.M.; Schaap, D.P.; Meijer, R.P.; Boogerd, L.S.; Hoogstins, C.E.; van der Valk, M.J.; Kamerling, I.M.; Bhairosingh, S.S.; Framery, B.; et al. Dose-Finding Study of a CEA-Targeting Agent, SGM-101, for Intraoperative Fluorescence Imaging of Colorectal Cancer. Ann. Surg. Oncol. 2021, 28, 1832–1844. [Google Scholar] [CrossRef]
- Krishnan, G.; van den Berg, N.S.; Nishio, N.; Juniper, G.; Pei, J.E.; Zhou, Q.; Lu, G.; Lee, Y.-J.; Ramos, K.; Iagaru, A.H.; et al. Metastatic and sentinel lymph node mapping using intravenously delivered Panitumumab-IRDye800CW. Theranostics 2021, 11, 7188–7198. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.D.; Predina, J.D.; Frenzel-Sulyok, L.G.; Low, P.S.; Singhal, S.; Roses, R.E. Intraoperative Molecular Imaging Utilizing a Folate Receptor-Targeted Near-Infrared Probe Can Identify Macroscopic Gastric Adenocarcinomas. Mol. Imaging Biol. 2021, 23, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.; de Wit, J.G.; Voskuil, F.J.; Tang, Y.H.; Hooghiemstra, W.T.; Linssen, M.D.; Broek, E.v.D.; Doff, J.J.; de Visscher, S.A.; Schepman, K.P.; et al. Epidermal growth factor receptor targeted fluorescence molecular imaging for postoperative lymph node assessment in patients with oral cancer. J. Nucl. Med. 2021, 63, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.R.; Khot, M.I.; Portal, C.; West, N.P.; Perry, S.L.; Maisey, T.I.; Tiernan, J.P.; Hughes, T.A.; Tolan, D.J.; Jayne, D.G. A novel fluorescent c-met targeted imaging agent for intra-operative colonic tumour mapping: Translation from the laboratory into a clinical trial. Surg. Oncol. 2022, 40, 101679. [Google Scholar] [CrossRef]
- De Gooyer, J.M.; Elekonawo, F.M.K.; Bremers, A.J.A.; Boerman, O.C.; Aarntzen, E.; de Reuver, P.R.; Nagtegaal, I.D.; Rijpkema, M.; de Wilt, J.H.W. Multimodal CEA-targeted fluorescence and radioguided cytoreductive surgery for peritoneal metastases of colorectal origin. Nat. Commun. 2022, 13, 2621. [Google Scholar] [CrossRef]
- Jonker, P.K.C.; Metman, M.J.H.; Sondorp, L.H.J.; Sywak, M.S.; Gill, A.J.; Jansen, L.; Links, T.P.; van Diest, P.J.; van Ginhoven, T.M.; Löwik, C.W.G.M.; et al. Intraoperative MET-receptor targeted fluorescent imaging and spectroscopy for lymph node detection in papillary thyroid cancer: Novel diagnostic tools for more selective central lymph node compartment dissection. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3557–3570. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.G.; van den Berg, N.S.; Antaris, A.L.; Xue, L.; Greenberg, S.; Rosenthal, J.W.; Muchnik, A.; Klaassen, A.; Simko, J.P.; Dutta, S.; et al. First-in-human Evaluation of a Prostate-specific Membrane Antigen-targeted Near-infrared Fluorescent Small Molecule for Fluorescence-based Identification of Prostate Cancer in Patients with High-risk Prostate Cancer Undergoing Robotic-assisted Prostatectomy. Eur. Urol. Oncol. 2023, 7, 63–72. [Google Scholar] [CrossRef]
- Stibbe, J.A.; de Barros, H.A.; Linders, D.G.J.; Bhairosingh, S.S.; Bekers, E.M.; van Leeuwen, P.J.; Low, P.S.; A Kularatne, S.; Vahrmeijer, A.L.; Burggraaf, J.; et al. First-in-patient study of OTL78 for intraoperative fluorescence imaging of prostate-specific membrane antigen-positive prostate cancer: A single-arm, phase 2a, feasibility trial. Lancet Oncol. 2023, 24, 457–467. [Google Scholar] [CrossRef]
- Meijer, R.P.J.; Galema, H.A.; Faber, R.A.; Bijlstra, O.D.; Maat, A.; Cailler, F.; Braun, J.; Keereweer, S.; Hilling, D.E.; Burggraaf, J.; et al. Intraoperative molecular imaging of colorectal lung metastases with SGM-101: A feasibility study. Eur. J. Nucl. Med. Mol. Imaging 2023, 51, 2970–2979. [Google Scholar] [CrossRef]
- Lauwerends, L.J.; van Driel, P.; Baatenburg de Jong, R.J.; Hardillo, J.A.U.; Koljenovic, S.; Puppels, G.; Mezzanotte, L.; Löwik, C.W.G.M.; Rosenthal, E.L.; Vahrmeijer, A.L.; et al. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. 2021, 22, e186–e195. [Google Scholar] [CrossRef]
- Azargoshasb, S.; Boekestijn, I.; Roestenberg, M.; KleinJan, G.H.; van der Hage, J.A.; van der Poel, H.G.; Rietbergen, D.D.D.; van Oosterom, M.N.; van Leeuwen, F.W.B. Quantifying the Impact of Signal-to-background Ratios on Surgical Discrimination of Fluorescent Lesions. Mol. Imaging Biol. 2023, 25, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Warram, J.M.; de Boer, E.; van Dam, G.M.; Moore, L.S.; Bevans, S.L.; Walsh, E.M.; Young, E.S.; Carroll, W.R.; Stevens, T.M.; Rosenthal, E.L. Fluorescence imaging to localize head and neck squamous cell carcinoma for enhanced pathological assessment. J. Pathol. Clin. Res. 2016, 2, 104–112. [Google Scholar] [CrossRef]
- Wreesmann, V.B.; Katabi, N.; Palmer, F.L.; Montero, P.H.; Migliacci, J.C.; Gönen, M.; Carlson, D.; Ganly, I.; Shah, J.P.; Ghossein, R.; et al. Influence of extracapsular nodal spread extent on prognosis of oral squamous cell carcinoma. Head Neck 2016, 38 (Suppl. 1), E1192–E1199. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, I.; Morscher, S.; Helfrich, I.; Hillen, U.; Leyh, J.; Burton, N.C.; Sardella, T.C.P.; Claussen, J.; Poeppel, T.D.; Bachmann, H.S.; et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci. Transl. Med. 2015, 7, 317ra199. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.; Kukacka, J.; Steinkamp, P.J.; de Wit, J.G.; Voskuil, F.J.; Hooghiemstra, W.T.R.; Bader, M.; Jüstel, D.; Ntziachristos, V.; van Dam, G.; et al. Multispectral optoacoustic tomography for in vivo detection of lymph node metastases in oral cancer patients using an EGFR-targeted contrast agent and intrinsic tissue contrast: A proof-of-concept study. Photoacoustics 2022, 26, 100362. [Google Scholar] [CrossRef]
- Pal, R.; Lwin, T.M.; Krishnamoorthy, M.; Collins, H.R.; Chan, C.D.; Prilutskiy, A.; Nasrallah, M.P.; Dijkhuis, T.H.; Shukla, S.; Kendall, A.L.; et al. Fluorescence lifetime of injected indocyanine green as a universal marker of solid tumours in patients. Nat. Biomed. Eng. 2023, 7, 1649–1666. [Google Scholar] [CrossRef]
- Valdes, P.A.; Angelo, J.P.; Choi, H.S.; Gioux, S. qF-SSOP: Real-time optical property corrected fluorescence imaging. Biomed. Opt. Express 2017, 8, 3597–3605. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).