Association Between CKAP4 Expression and Poor Prognosis in Patients with Bladder Cancer Treated with Radical Cystectomy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemistry and Scoring
2.3. Statistical Analyses
3. Results
3.1. Immunohistochemistry
3.2. Association of CKAP4 Expression with Clinicopathological Characteristics
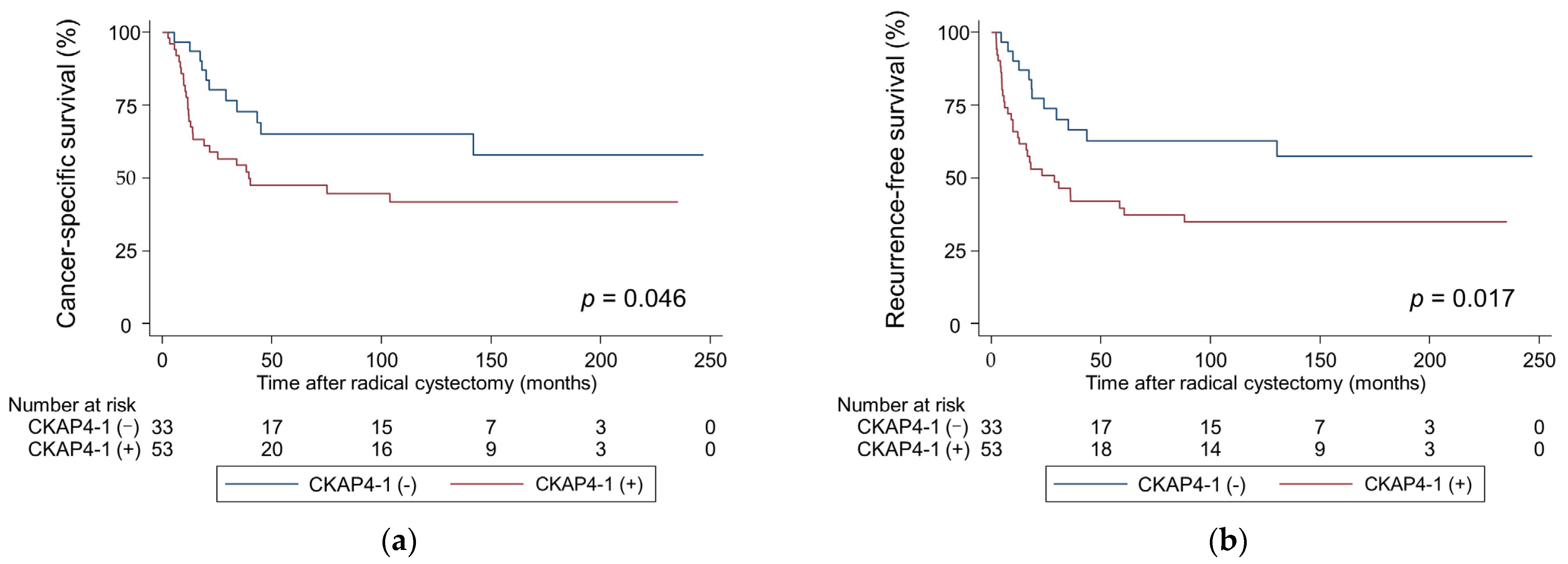
3.3. Survival Outcomes and CKAP4 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CKAP4 | Cytoskeleton-associated protein 4 |
| BCa | Bladder cancer |
| RC | Radical cystectomy |
| RFS | Recurrence-free survival |
| LN | Lymph node |
| MIBC | Muscle-invasive bladder cancer |
| NMIBC | Non-muscle-invasive bladder cancer |
| NAC | Neoadjuvant chemotherapy |
| AC | Adjuvant chemotherapy |
| LVI | Lymphovascular invasion |
| CAF | Cancer-associated fibroblast |
| CIS | Carcinoma in situ |
| SC | Salvage chemotherapy |
| TBS | Tris-buffered saline |
| CSS | Cancer-specific survival |
| myCAF | Myofibroblast-like cancer-associated fibroblast |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hautmann, R.E.; de Petriconi, R.C.; Pfeiffer, C.; Volkmer, B.G. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: Long-term results in 1100 patients. Eur. Urol. 2012, 61, 1039–1047. [Google Scholar] [CrossRef]
- Witjes, J.A.; Compérat, E.; Cowan, N.C.; De Santis, M.; Gakis, G.; Lebret, T.; Ribal, M.J.; Van der Heijden, A.G.; Sherif, A. EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur. Urol. 2014, 65, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Tokunaga, H.; Zhou, J.H.; Kim, J.H.; Ayala, G.E.; Benedict, W.F.; Lerner, S.P. p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J. Clin. Oncol. 2004, 22, 1014–1024. [Google Scholar] [CrossRef]
- Shariat, S.F.; Bolenz, C.; Godoy, G.; Fradet, Y.; Ashfaq, R.; Karakiewicz, P.I.; Isbarn, H.; Jeldres, C.; Rigaud, J.; Sagalowsky, A.I.; et al. Predictive value of combined immunohistochemical markers in patients with pT1 urothelial carcinoma at radical cystectomy. J. Urol. 2009, 182, 78–84; discussion 84. [Google Scholar] [CrossRef] [PubMed]
- Lotan, Y.; Bagrodia, A.; Passoni, N.; Rachakonda, V.; Kapur, P.; Arriaga, Y.; Bolenz, C.; Margulis, V.; Raj, G.V.; Sagalowsky, A.I.; et al. Prospective evaluation of a molecular marker panel for prediction of recurrence and cancer-specific survival after radical cystectomy. Eur. Urol. 2013, 64, 465–471. [Google Scholar] [CrossRef]
- Schweizer, A.; Ericsson, M.; Bächi, T.; Griffiths, G.; Hauri, H.P. Characterization of a novel 63 kDa membrane protein. Implications for the organization of the ER-to-Golgi pathway. J. Cell Sci. 1993, 104, 671–683. [Google Scholar] [CrossRef]
- Li, S.X.; Li, J.; Dong, L.W.; Guo, Z.Y. Cytoskeleton-associated protein 4, a promising biomarker for tumor diagnosis and therapy. Front. Mol. Biosci. 2020, 7, 552056. [Google Scholar] [CrossRef]
- Schweizer, A.; Rohrer, J.; Hauri, H.P.; Kornfeld, S. Retention of p63 in an ER-Golgi intermediate compartment depends on the presence of all three of its domains and on its ability to form oligomers. J. Cell Biol. 1994, 126, 25–39. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Wang, T.; Gan, X.; Chen, S.H.; He, Y.T.; Wang, Y.Q.; Zhang, K.H. Cytoskeleton-associated membrane protein 4 is upregulated in tumor tissues and is associated with clinicopathological characteristics and prognosis in hepatocellular carcinoma. Oncol. Lett. 2020, 19, 3889–3898. [Google Scholar] [CrossRef]
- Kajiwara, C.; Fumoto, K.; Kimura, H.; Nojima, S.; Asano, K.; Odagiri, K.; Yamasaki, M.; Hikita, H.; Takehara, T.; Doki, Y.; et al. p63-dependent Dickkopf3 expression promotes esophageal cancer cell proliferation via CKAP4. Cancer Res. 2018, 78, 6107–6120. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.M.; Geng, J.; Yan, Y.; Yao, X.; Liu, M. Overexpression of CKAP4 is associated with poor prognosis in clear cell renal cell carcinoma and functions via cyclin B signaling. J. Cancer 2017, 8, 4018–4026. [Google Scholar] [CrossRef]
- Kimura, H.; Yamamoto, H.; Harada, T.; Fumoto, K.; Osugi, Y.; Sada, R.; Maehara, N.; Hikita, H.; Mori, S.; Eguchi, H.; et al. CKAP4, a DKK1 receptor, is a biomarker in exosomes derived from pancreatic cancer and a molecular target for therapy. Clin. Cancer Res. 2019, 25, 1936–1947. [Google Scholar] [CrossRef]
- Sun, X.; Xie, L.; Qiu, S.; Li, H.; Zhou, Y.; Zhang, H.; Zhang, Y.; Zhang, L.; Xie, T.; Chen, Y. Elucidation of CKAP4-remodeled cell mechanics in driving metastasis of bladder cancer through aptamer-based target discovery. Proc. Natl. Acad. Sci. USA 2022, 119, e2110500119. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Tabata, K.; Hirayama, T.; Shimura, S.; Nishi, M.; Ishii, D.; Fujita, T.; Iwamura, M. Robot-assisted laparoscopic radical cystectomy is a safe and effective procedure for patients with bladder cancer compared to laparoscopic and open surgery: Perioperative outcomes of a single-center experience. Asian J. Surg. 2019, 42, 189–196. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ikeda, M.; Sato, Y.; Kuruma, H.; Kamata, Y.; Nishimori, T.; Tomonaga, T.; Nomura, F.; Egawa, S.; Iwamura, M. Loss of periplakin expression is associated with pathological stage and cancer-specific survival in patients with urothelial carcinoma of the urinary bladder. Biomed. Res. 2014, 35, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, K.; Nagashio, R.; Jiang, S.X.; Kuchitsu, Y.; Hachimura, K.; Ichinoe, M.; Igawa, S.; Fukuda, E.; Goshima, N.; Satoh, Y.; et al. Cytoskeleton-associated protein 4 is a novel serodiagnostic marker for lung cancer. Am. J. Pathol. 2018, 188, 1328–1333. [Google Scholar] [CrossRef]
- Nagoya, A.; Sada, R.; Kimura, H.; Yamamoto, H.; Morishita, K.; Miyoshi, E.; Morii, E.; Shintani, Y.; Kikuchi, A. CKAP4 is a potential exosomal biomarker and therapeutic target for lung cancer. Transl. Lung Cancer Res. 2023, 12, 408–426. [Google Scholar] [CrossRef]
- Akanda, M.R.; Ahn, E.J.; Kim, Y.J.; Salam, S.M.A.; Noh, M.G.; Kim, S.S.; Jung, T.Y.; Kim, I.Y.; Kim, C.H.; Lee, K.H.; et al. Different Expression and Clinical Implications of Cancer-Associated Fibroblast (CAF) Markers in Brain Metastases. J. Cancer 2023, 14, 464–479. [Google Scholar] [CrossRef]
- Saito, D.; Tadokoro, R.; Nagasaka, A.; Yoshino, D.; Teramoto, T.; Mizumoto, K.; Funamoto, K.; Kidokoro, H.; Miyata, T.; Tamura, K.; et al. Stiffness of primordial germ cells is required for their extravasation in avian embryos. IScience 2022, 25, 105629. [Google Scholar] [CrossRef]
- Innocenti, M. New insights into the formation and the function of lamellipodia and ruffles in mesenchymal cell migration. Cell Adh. Migr. 2018, 12, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Carmona, G.; Perera, U.; Gillett, C.; Naba, A.; Law, A.L.; Sharma, V.P.; Wang, J.; Wyckoff, J.; Balsamo, M.; Mosis, F.; et al. Lamellipodin promotes invasive 3D cancer cell migration via regulated interactions with Ena/VASP and SCAR/WAVE. Oncogene 2016, 35, 5155–5169. [Google Scholar] [CrossRef]
- Giralt, I.; Gallo-Oller, G.; Navarro, N.; Zarzosa, P.; Pons, G.; Magdaleno, A.; Segura, M.F.; Sábado, C.; Hladun, R.; Arango, D.; et al. Dickkopf-1 inhibition reactivates Wnt/β-catenin signaling in rhabdomyosarcoma, induces myogenic markers in vitro and impairs tumor cell survival in vivo. Int. J. Mol. Sci. 2021, 22, 12921. [Google Scholar] [CrossRef] [PubMed]
- Niida, A.; Hiroko, T.; Kasai, M.; Furukawa, Y.; Nakamura, Y.; Suzuki, Y.; Sugano, S.; Akiyama, T. DKK1, a negative regulator of Wnt signaling, is a target of the β-catenin/TCF pathway. Oncogene 2004, 23, 8520–8526. [Google Scholar] [CrossRef]
- Shinno, N.; Kimura, H.; Sada, R.; Takiguchi, S.; Mori, M.; Fumoto, K.; Doki, Y.; Kikuchi, A. Activation of the Dick-kopf1-CKAP4 pathway is associated with poor prognosis of esophageal cancer and anti-CKAP4 antibody may be a new therapeutic drug. Oncogene 2018, 37, 3471–3484. [Google Scholar] [CrossRef] [PubMed]
- Sada, R.; Yamamoto, H.; Matsumoto, S.; Harada, A.; Kikuchi, A. Newly developed humanized anti-CKAP4 antibody suppresses pancreatic cancer growth by inhibiting DKK1-CKAP4 signaling. Cancer Sci. 2024, 115, 3358–3369. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.H.; Hsieh, H.Y.; Wang, Y.H.; Chen, S.Y.; Tung, C.L.; Wu, J.D.; Lin, C.T.; Chan, M.W.Y.; Hsu, C.D.; Chang, D. High Dickkopf-1 expression is associated with poor prognosis in patients with advanced urothelial carcinoma. Exp. Ther. Med. 2010, 1, 893–898. [Google Scholar] [CrossRef]
- Gladka, M.M.; Molenaar, B.; de Ruiter, H.; van der Elst, S.; Tsui, H.; Versteeg, D.; Lacraz, G.P.A.; Huibers, M.M.H.; van Oudenaarden, A.; van Rooij, E. Single-cell sequencing of the healthy and diseased heart reveals cytoskeleton-associated protein 4 as a new modulater of fibroblasts activation. Circulation 2018, 138, 166–180. [Google Scholar] [CrossRef]
- Yang, D.; Liu, J.; Qian, H.; Zhuang, Q. Cancer-associated fibroblasts: From basic science to anticancer therapy. Exp. Mol. Med. 2023, 55, 1322–1332. [Google Scholar] [CrossRef]
- Du, Y.H.; Sui, Y.Q.; Cao, J.; Jiang, X.; Wang, Y.; Yu, J.; Wang, B.; Wang, X.Z.; Xue, B.X. Dynamic changes in myofibroblasts affect the carcinogenesis and prognosis of bladder cancer associated with tumor microenvironment remodeling. Front. Cell Dev. Biol. 2022, 10, 833578. [Google Scholar] [CrossRef]
- Thinyakul, C.; Sakamoto, Y.; Shimoda, M.; Liu, Y.; Thongchot, S.; Reda, O.; Nita, A.; Sakamula, R.; Sampattavanich, S.; Maeda, A.; et al. Hippo pathway in cancer cells induces NCAM1+αSMA+ fibroblasts to modulate tumor microenvironment. Commun. Biol. 2024, 7, 1343. [Google Scholar] [CrossRef] [PubMed]
- Lavie, D.; Ben-Shmuel, A.; Erez, N.; Scherz-Shouval, R. Cancer-associated fibroblasts in the single-cell era. Nat. Cancer 2022, 3, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Ofner, H.; Laukhtina, E.; Hassler, M.R.; Shariat, S.F. Blood-Based Biomarkers as Prognostic Factors of Recurrent Disease after Radical Cystectomy: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 5846. [Google Scholar] [CrossRef] [PubMed]
- Malinaric, R.; Mantica, G.; Monaco, L.L.; Mariano, F.; Leonardi, R.; Simonato, A.; der Merwe, A.V.; Terrone, C. The Role of Novel Bladder Cancer Diagnostic and Surveillance Biomarkers-What Should a Urologist Really Know? Int. J. Environ. Res. Public Health 2022, 19, 9648. [Google Scholar] [CrossRef]
- Tuffy, K.M.; Planey, S.L. Cytoskeleton-associated protein 4: Functions beyond the endoplasmic reticulum in physiology and disease. ISRN Cell Biol. 2012, 2012, 142313. [Google Scholar] [CrossRef][Green Version]
- Di Como, C.J.; Urist, M.J.; Babayan, I.; Drobnjak, M.; Hedvat, C.V.; Teruya-Feldstein, J.; Pohar, K.; Hoos, A.; Cordon-Cardo, C. p63 expression profiles in human normal and tumor tissues. Clin. Cancer Res. 2002, 8, 494–501. [Google Scholar] [CrossRef]
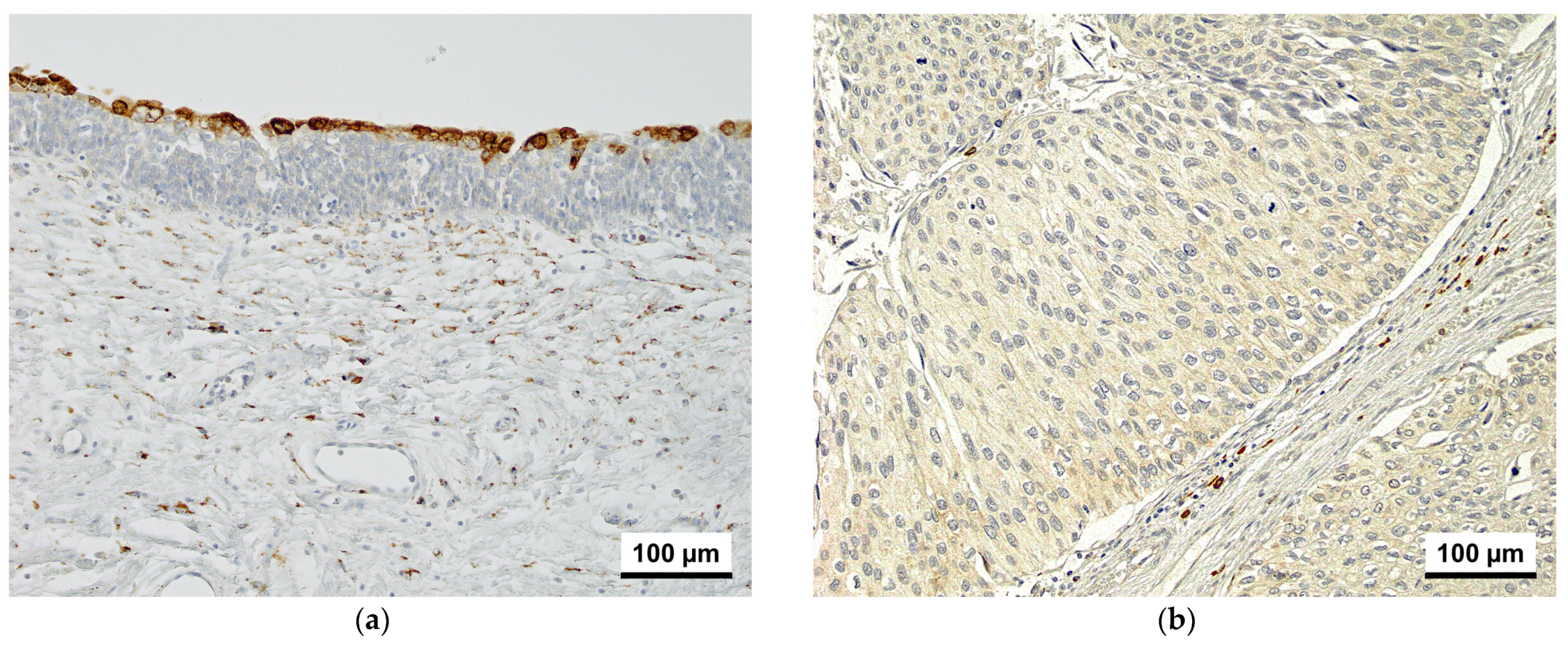
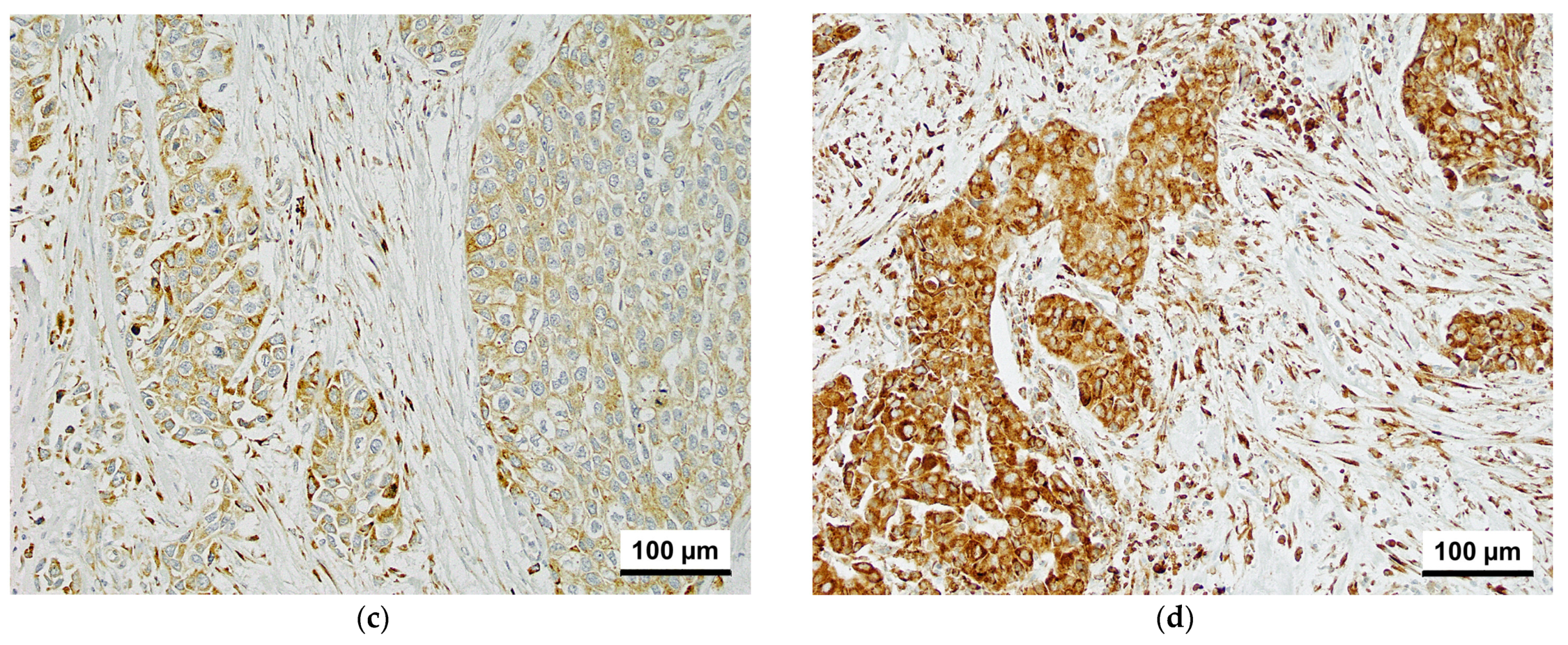

| Characteristics | CKAP4-1 | CKAP4-2 | |||||
|---|---|---|---|---|---|---|---|
| Total No. (%) | Negative (%) | Positive (%) | p-Value | Negative (%) | Positive (%) | p-Value | |
| Overall | 86 | 33 (38.4) | 53 (61.6) | 52 (60.5) | 34 (39.5) | ||
| Age, years | |||||||
| Median (IQR) | 65 (57–71) | 65 (56–71) | 64 (57–72) | 65 (57–70) | 64 (56–72) | ||
| <65 | 42 (48.8) | 15 (45.5) | 27 (50.9) | 0.66 | 24 (46.2) | 18 (52.9) | 0.66 |
| ≥65 | 44 (51.2) | 18 (54.6) | 26 (49.1) | 28 (53.9) | 16 (47.1) | ||
| Sex | |||||||
| Male | 67 (77.9) | 28 (84.9) | 39 (73.6) | 0.28 | 42 (80.8) | 25 (75.5) | 0.43 |
| Female | 19 (22.1) | 5 (15.2) | 14 (26.4) | 10 (19.2) | 9(26.7) | ||
| pT stage | |||||||
| pT ≤ 2 | 37 (43.0) | 20 (60.6) | 17 (32.1) | 0.014 | 28 (53.9) | 9 (26.5) | 0.015 |
| pT ≥ 3 | 49 (57.0) | 13 (39.4) | 36 (67.9) | 24 (46.2) | 25 (73.5) | ||
| Lymph node status | |||||||
| N0 | 61 (74.4) | 26 (83.9) | 35 (68.6) | 0.19 | 42 (84.0) | 19 (59.4) | 0.019 |
| N+ | 21 (25.6) | 5 (16.1) | 16 (31.4) | 8 (16.0) | 13 (40.6) | ||
| Pathological grade | |||||||
| G1–2 | 34 (39.5) | 16 (48.5) | 18 (34.0) | 0.25 | 28 (53.9) | 6 (17.6) | 0.001 |
| G3 | 52 (60.5) | 17 (51.5) | 35 (66.0) | 24 (46.2) | 28 (82.4) | ||
| LVI | |||||||
| Negative | 30 (37.0) | 16 (51.6) | 14 (28.0) | 0.037 | 25 (51.0) | 5 (15.6) | 0.002 |
| Positive | 51 (63.0) | 15 (48.4) | 36 (72.0) | 24 (49.0) | 27 (84.4) | ||
| Carcinoma in situ | |||||||
| Negative | 78 (90.7) | 31 (93.9) | 47 (88.7) | 0.70 | 47 (90.4) | 31 (91.2) | 1.000 |
| Positive | 8 (9.3) | 2 (6.1) | 6 (11.3) | 5 (9.6) | 3 (8.8) | ||
| Adjuvant chemotherapy | |||||||
| No | 65 (75.6) | 27 (81.8) | 38 (71.7) | 0.31 | 46 (88.5) | 19 (55.9) | 0.001 |
| Yes | 21 (24.4) | 6 (18.2) | 15 (28.3) | 6 (11.5) | 15 (44.1) | ||
| Salvage chemotherapy | |||||||
| No | 62 (72.1) | 26 (78.8) | 36 (67.9) | 0.32 | 38 (73.1) | 24 (70.6) | 0.81 |
| Yes | 24 (27.9) | 7 (21.2) | 17 (32.1) | 14 (26.9) | 10 (29.4) | ||
| Recurrence | |||||||
| No | 43 (50.0) | 21 (63.6) | 22 (41.5) | 0.075 | 29 (55.8) | 14 (41.2) | 0.27 |
| Yes | 43 (50.0) | 12 (36.4) | 31 (58.5) | 23 (44.2) | 20 (58.8) | ||
| Cancer-specific death | |||||||
| No | 48 (55.8) | 22 (66.7) | 26 (49.1) | 0.12 | 32 (61.5) | 16 (47.1) | 0.26 |
| Yes | 38 (44.2) | 11 (33.3) | 27 (50.9) | 20 (38.5) | 18 (52.9) | ||
| CKAP4-2 | |||||||
| Negative | 52 (60.5) | 24 (72.7) | 28 (52.8) | 0.075 | |||
| Positive | 34 (39.5) | 9 (27.3) | 25 (47.2) | ||||
| Cancer-Specific Survival | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Category | Univariate | Multivariate | ||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| CKAP4-1 | Positive | 2.01 | 0.99–4.06 | 0.050 | 1.84 | 0.87–3.90 | 0.11 |
| Negative | 1 | 1 | |||||
| CKAP4-2 | Positive | 1.74 | 0.91–3.30 | 0.089 | 1.24 | 0.56–2.73 | 0.58 |
| Negative | 1 | ||||||
| pT stage | pT ≥ 3 | 2.25 | 1.13–4.47 | 0.021 | 1.78 | 0.80–3.93 | 0.15 |
| pT ≤ 2 | 1 | 1 | |||||
| Pathological grade | G3 | 1.31 | 0.67–2.57 | 0.42 | 0.92 | 0.43–1.99 | 0.84 |
| G1–2 | 1 | 1 | |||||
| LN status | N+ | 3.01 | 1.52–5.94 | 0.001 | 2.11 | 0.95–4.71 | 0.066 |
| N0 | 1 | 1 | |||||
| CIS | Positive | 0.47 | 0.11–1.98 | 0.30 | 0.36 | 0.08–1.57 | 0.17 |
| Negative | 1 | 1 | |||||
| Recurrence-Free Survival | |||||||
| Variables | Category | Univariate | Multivariate | ||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| CKAP4-1 | Positive | 2.24 | 1.14–4.37 | 0.018 | 2.09 | 1.03–4.25 | 0.041 |
| Negative | 1 | 1 | |||||
| CKAP4-2 | Positive | 1.77 | 0.97–3.24 | 0.062 | 1.32 | 0.62–2.79 | 0.45 |
| Negative | 1 | 1 | |||||
| pT stage | pT ≥ 3 | 2.11 | 1.11–4.08 | 0.021 | 1.75 | 0.85–3.61 | 0.12 |
| pT ≤ 2 | 1 | 1 | |||||
| Pathological grade | G3 | 1.23 | 0.65–2.30 | 0.51 | 0.84 | 0.41–1.72 | 0.64 |
| G1–2 | 1 | 1 | |||||
| LN status | N+ | 2.71 | 1.42–5.16 | 0.002 | 1.85 | 0.88–3.87 | 0.10 |
| N0 | 1 | 1 | |||||
| CIS | Positive | 0.44 | 0.10–1.84 | 0.26 | 0.35 | 0.08–1.49 | 0.15 |
| Negative | 1 | 1 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsumata, H.; Koguchi, D.; Hirano, S.; Suzuki, A.; Yanagita, K.; Shimizu, Y.; Hirono, W.; Shimura, S.; Ikeda, M.; Tsumura, H.; et al. Association Between CKAP4 Expression and Poor Prognosis in Patients with Bladder Cancer Treated with Radical Cystectomy. Cancers 2025, 17, 1278. https://doi.org/10.3390/cancers17081278
Katsumata H, Koguchi D, Hirano S, Suzuki A, Yanagita K, Shimizu Y, Hirono W, Shimura S, Ikeda M, Tsumura H, et al. Association Between CKAP4 Expression and Poor Prognosis in Patients with Bladder Cancer Treated with Radical Cystectomy. Cancers. 2025; 17(8):1278. https://doi.org/10.3390/cancers17081278
Chicago/Turabian StyleKatsumata, Hiroki, Dai Koguchi, Shuhei Hirano, Anna Suzuki, Kengo Yanagita, Yuriko Shimizu, Wakana Hirono, Soichiro Shimura, Masaomi Ikeda, Hideyasu Tsumura, and et al. 2025. "Association Between CKAP4 Expression and Poor Prognosis in Patients with Bladder Cancer Treated with Radical Cystectomy" Cancers 17, no. 8: 1278. https://doi.org/10.3390/cancers17081278
APA StyleKatsumata, H., Koguchi, D., Hirano, S., Suzuki, A., Yanagita, K., Shimizu, Y., Hirono, W., Shimura, S., Ikeda, M., Tsumura, H., Ishii, D., Sato, Y., & Matsumoto, K. (2025). Association Between CKAP4 Expression and Poor Prognosis in Patients with Bladder Cancer Treated with Radical Cystectomy. Cancers, 17(8), 1278. https://doi.org/10.3390/cancers17081278







